Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Microcapsules with α-Tocopherol
2.2.2. Characterization of α-Tocopherol-Loaded Microcapsules
2.2.3. Preparation of Hydrogels with Microencapsulated α-Tocopherol
2.2.4. Evaluation of the Compatibility of Microencapsulated α-Tocopherol with Carbomer Hydrogel
2.2.5. Evaluation of Topical Application Attributes of the Hydrogels with Microencapsulated α-Tocopherol
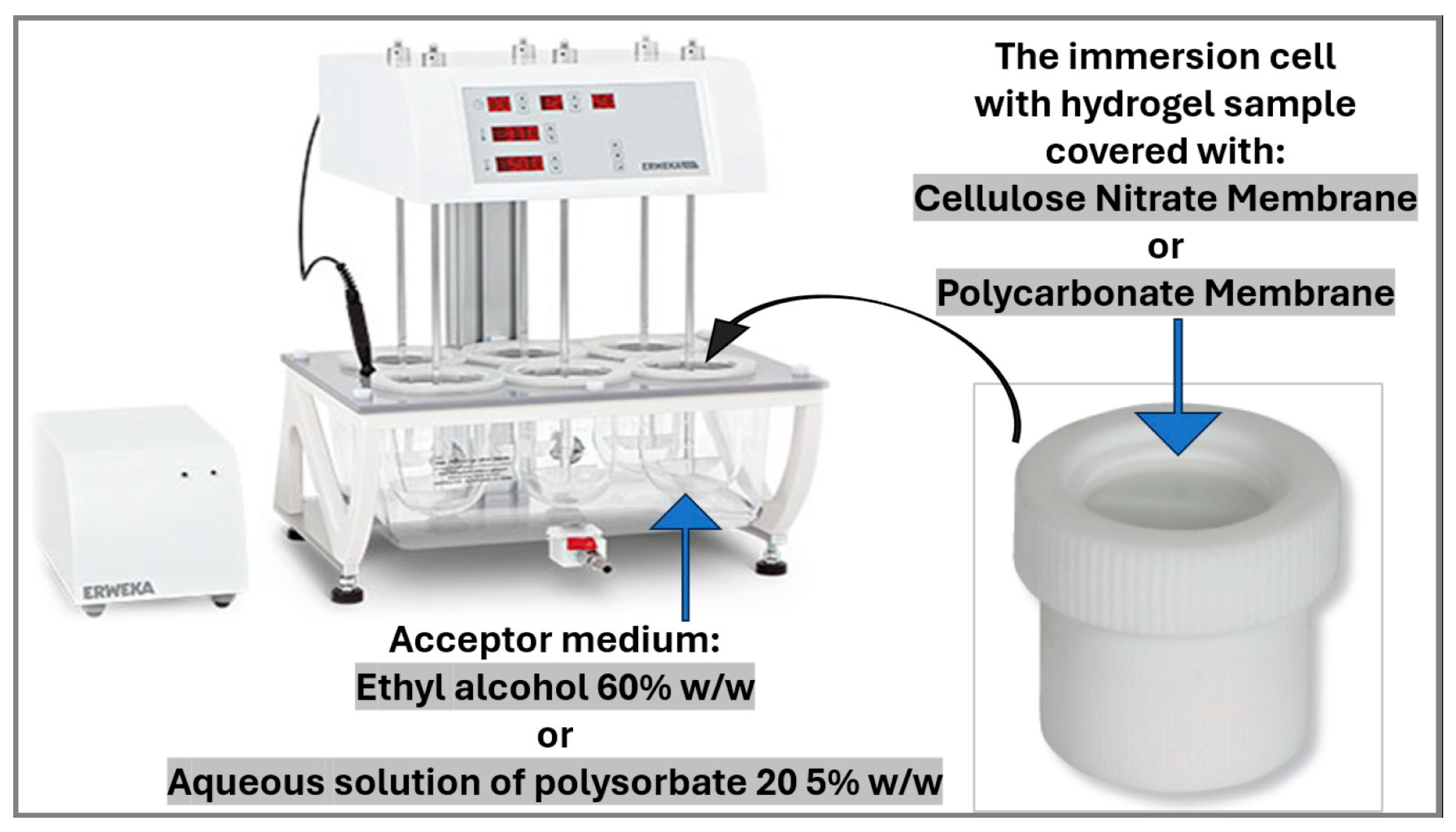
2.2.6. In Vitro Release Testing of the Hydrogels with Microencapsulated α-Tocopherol
3. Results
3.1. Influence of the Composition of the Microcapsule Wall on the Characteristics of α-Tocopherol-Loaded Microcapsules
3.2. Compatibility of Microencapsulated α-Tocopherol with Carbomer Hydrogel
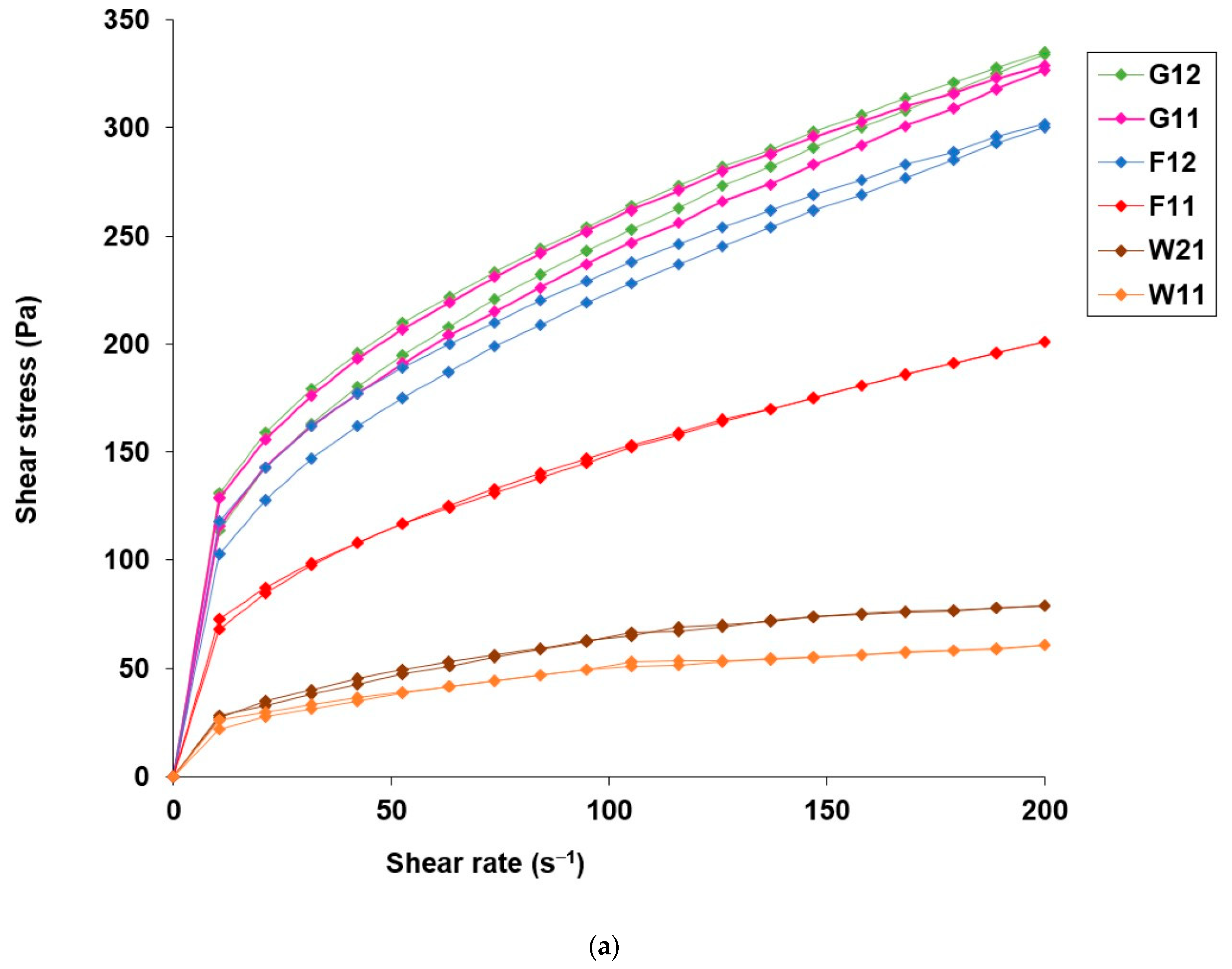
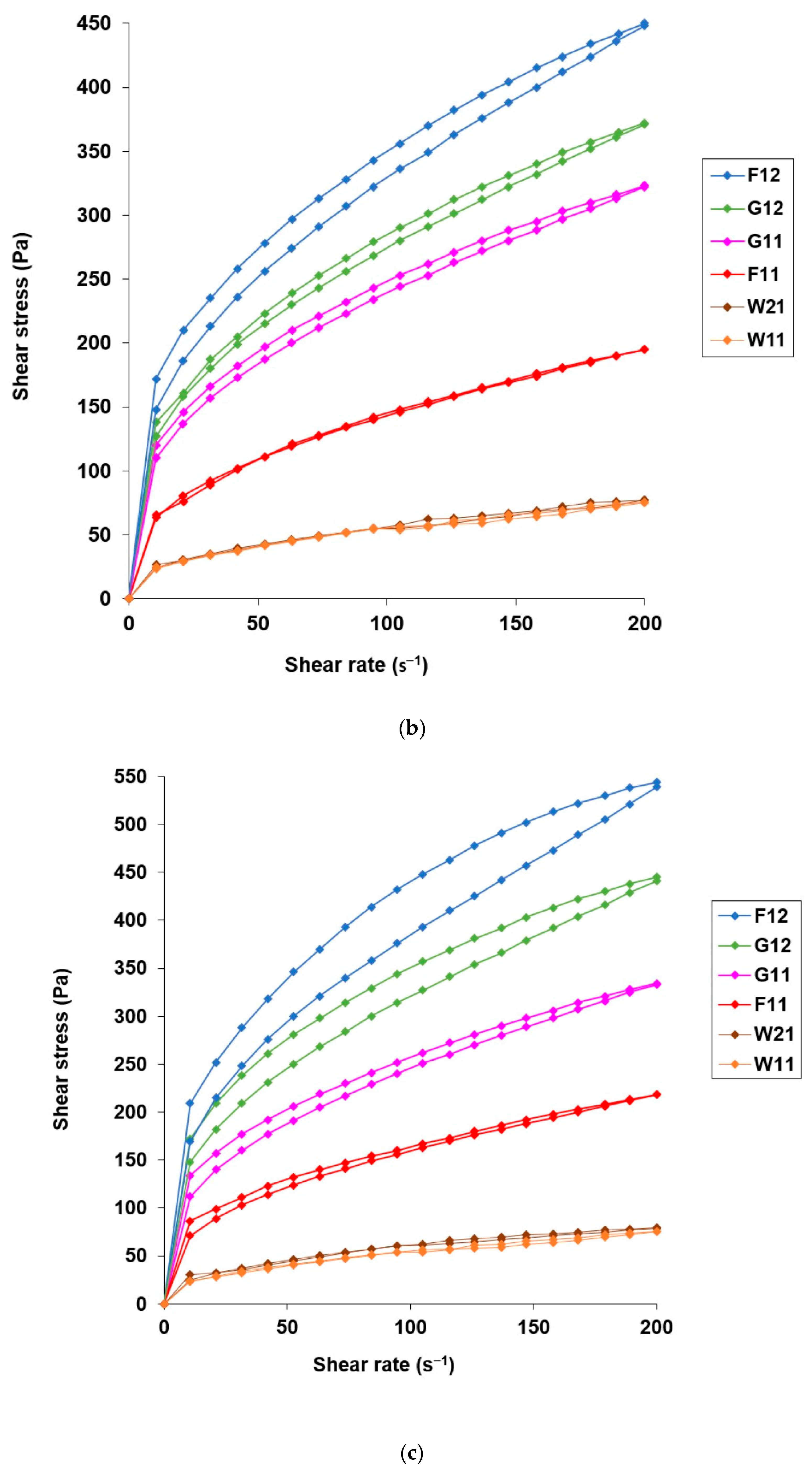
3.3. Application Attributes of Hydrogels with Microencapsulated α-Tocopherol
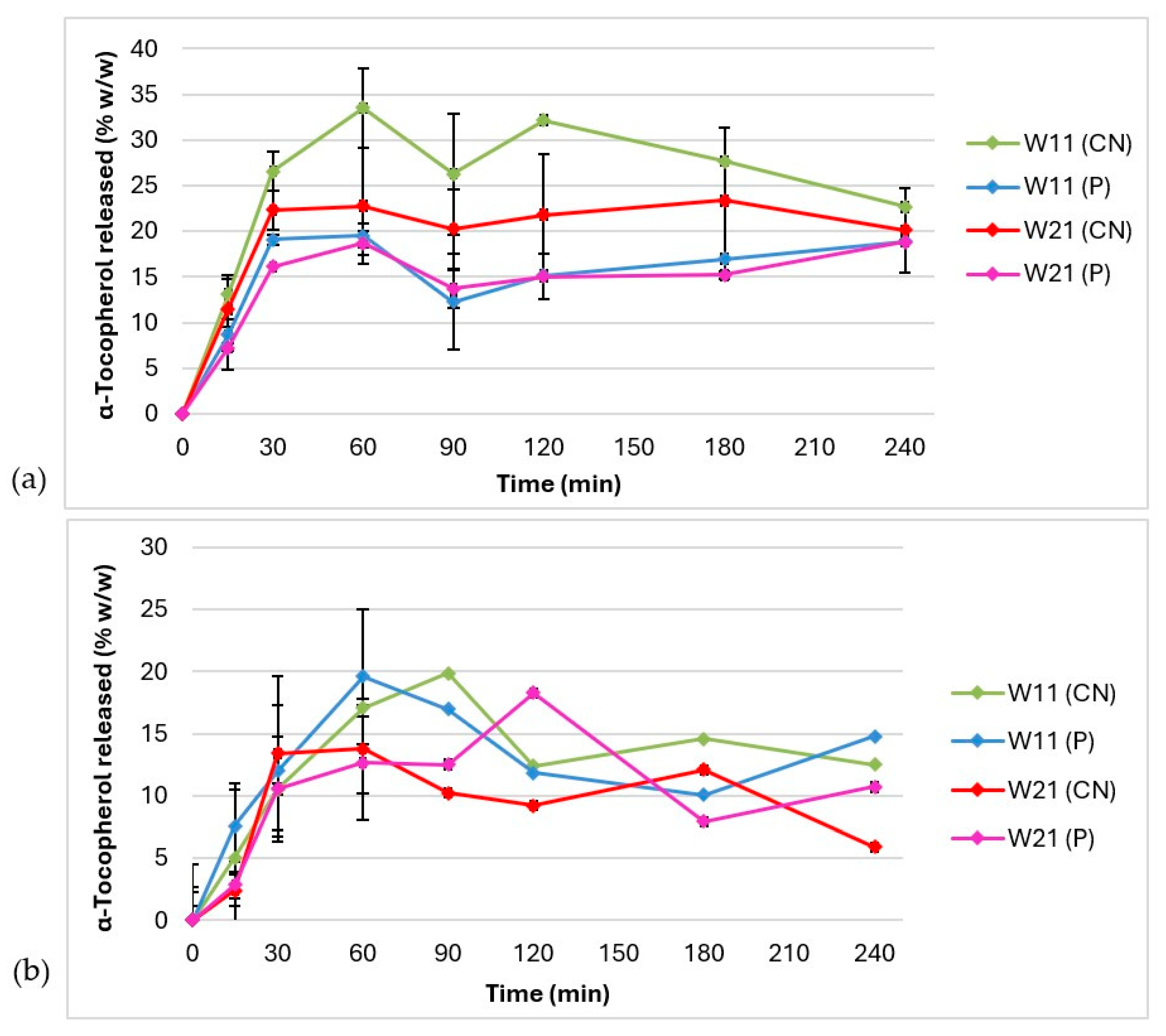
3.4. In Vitro Release of α-Tocopherol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallert, M.; Börmel, L.; Lorkowski, S. Inflammatory Diseases and Vitamin E—What Do We Know and Where Do We Go? Mol. Nutr. Food Res. 2021, 65, 2000097:1–2000097:16. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, M.; Nguyen, M.H.L.; Marquardt, D. The antioxidant vitamin E as a membrane raft modulator: Tocopherols do not abolish lipid domains. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183189:1–183189:8. [Google Scholar] [CrossRef] [PubMed]
- Waghmode, M.; Kamble, M.; Shruti, A. Pharmaceutical profile of alpha-tocopherol—A brief review. Int. J. Pharm. Chem. Biol. Sci. 2012, 1, 1023–1039. [Google Scholar]
- CosIng—Cosmetics Ingredients. Ingredient: TOCOPHEROL. Available online: https://ec.europa.eu/growth/tools-databases/cosing/details/80273 (accessed on 5 March 2024).
- Sheskey, P.; Hancock, B.; Moss, G.; Goldfarb, D. (Eds.) Handbook of Pharmaceutical Excipients, 9th ed.; Pharmaceutical Press: London, UK, 2020; pp. 93–96. ISBN 978-08-5711-375-7. [Google Scholar]
- Ungurianu, A.; Zanfirescu, A.; Nitulescu, G.; Margina, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R. Vitamin E and wound healing: An evidence-based review. Int. Wound J. 2016, 13, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ashamalla, L.; Maurice, M.; Sidhom, K. Topical vitamin E in granuloma annulare. Int. J. Dermatol. 1998, 27, 348. [Google Scholar] [CrossRef]
- Chiu, A.; Kimball, A.B. Topical vitamins, minerals and botanical ingredients as modulators of environmental and chronological skin damage. Br. J. Dermatol. 2003, 149, 681–691. [Google Scholar] [CrossRef]
- Baumann, L.S.; Spencer, J. The effects of topical vitamin E on the cosmetic appearance of scars. Dermatol. Surg. 1999, 25, 311–315. [Google Scholar] [CrossRef]
- Keen, M.A.; Hassan, I. Vitamin E in dermatology. Indian Dermatol. Online J. 2016, 7, 311–315. [Google Scholar] [CrossRef]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in human skin: Organ-specific physiology and considerations for its use in dermatology. Mol. Asp. Med. 2007, 28, 646–667. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Koumentakou, I.; Hatzistamatiou, K.; Lykidou, S.; Barmpaxis, P.; Nikolaidis, N. Preparation and Investigation of the SPF and Antioxidant Properties of O/W and W/O Emulsions Containing Vitamins A, C and E for Cosmetic Applications. Cosmetics 2023, 10, 76. [Google Scholar] [CrossRef]
- Cosmile Europe. Ingredient. TOCOPHEROL. Available online: https://cosmileeurope.eu/inci/detail/16234/tocopherol/ (accessed on 5 March 2024).
- Zussman, J.; Ahdout, J.; Kim, J. Vitamins and photoaging: Do scientific data support their use? J. Am. Acad. Dermatol. 2010, 63, 507–525. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of tocopherols and tocotrienols as used in cosmetics. Int. J. Toxicol. 2018, 37, 61S–94S. [Google Scholar] [CrossRef] [PubMed]
- Personal Care Products Council. INCI. Available online: https://www.personalcarecouncil.org/resources/inci/ (accessed on 5 March 2024).
- Burton, G.W.; Ingold, K.U. Vitamine E: Application of the principles of physical organic chemistry to the exploration of its structure and function. Acc. Chem. Res. 1986, 19, 194–201. [Google Scholar] [CrossRef]
- Azzi, A.; Atkinson, J.; Ozer, N.K.; Manor, D.; Wallert, M.; Galli, F. Vitamin E discussion forum position paper on the revision of the nomenclature of vitamin E. Free Radic. Biol. Med. 2023, 207, 178–180. [Google Scholar] [CrossRef]
- Alpha-Tocopherol. 3 Chemical and Physical Properties. 3.2 Experimental Properties. 3.2.1 Physical Description. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alpha%2DTocopherol#section=Physical-Description (accessed on 5 March 2024).
- Alpha-Tocopherol. 3 Chemical and Physical Properties. 3.2 Experimental Properties. 3.2.3 Solubility. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alpha-Tocopherol#section=Solubility (accessed on 5 March 2024).
- Liu, S.; Liu, F.; Xue, Y.; Gao, Y. Evaluation on oxidative stability of walnut beverage emulsions. Food Chem. 2016, 203, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.; de Barros, D.P.C.; Fonseca, P.L. Design of multifunctional nanostructured lipid carriers enriched with α-tocopherol using vegetable oils. Ind. Crops Prod. 2018, 118, 149–159. [Google Scholar] [CrossRef]
- De Vaugelade, S.; Nicol, E.; Vujovic, S.; Bourcier, S.; Pirnay, S.; Bouchonnet, S. UV–vis degradation of α-tocopherol in a model system and in a cosmetic emulsion—Structural elucidation of photoproducts and toxicological consequences. J. Chromatogr. A 2017, 1517, 126–133. [Google Scholar] [CrossRef]
- Manyala, D.L.; Ghosh, M.; Dalai, S. Novel microemulsions formulated from sodium N-Lauroyl sarcosinate as nanocarrier for encapsulation of α-Tocopherol. J. Mol. Liq. 2023, 384, 122323:1–122323:9. [Google Scholar] [CrossRef]
- Pereira, G.G.; Detoni, C.B.; Balducci, A.G.; Rondelli, V.; Colombo, P.; Guterres, S.S.; Sonvico, F. Hyaluronate Nanoparticles Included in Polymer Films for the Prolonged Release of Vitamin E for the Management of Skin Wounds. Eur. J. Pharm. Sci. 2016, 83, 203–211. [Google Scholar] [CrossRef]
- Vaz, S.; Silva, R.; Amaral, M.H.; Martins, E.; Sousa Lobo, J.M.; Silva, A.C. Evaluation of the biocompatibility and skin hydration potential of vitamin E-loaded lipid nanosystems formulations: In vitro and human in vivo studies. Colloids Surf. B Biointerfaces 2019, 179, 242–249. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estervinho, B.N.; Rocha, F. The progress and application of vitamin E encapsulation—A review. Food Hydrocoll. 2021, 121, 106998:1–106998:22. [Google Scholar] [CrossRef]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application—A Review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Martins, E.; Poncelet, D.; Renard, D. A novel method of oil encapsulation in core-shell alginate microcapsules by dispersion-inverse gelation technique. React. Funct. Polym. 2017, 114, 49–57. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Lang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.-L.; Marian, E. Sodium Alginate-Natural Microencapsulation Material of Polymeric Microparticles. Int. J. Mol. Sci. 2022, 23, 12108. [Google Scholar] [CrossRef] [PubMed]
- Parente, J.F.; Sousa, V.I.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Biodegradable Polymers for Microencapsulation Systems. Adv. Polym. Technol. 2022, 2022, 4640379:1–4640379:43. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar] [PubMed]
- Sami El-banna, F.; Mahfouz, M.E.; Leporatti, S.; El-Kemary, M.; Hanafy, A.N.N. Chitosan as a Natural Copolymer with Unique Properties for the Development of Hydrogels. Appl. Sci. 2019, 9, 2193. [Google Scholar] [CrossRef]
- Thongngam, M.; Mc Clements, D.J. Influence of pH, ionic strength, and temperature on self-sssociation and interactions of sodium dodecyl sulfate in the absence and presence of chitosan. Langmuir 2005, 21, 79–86. [Google Scholar] [CrossRef]
- Onésippe, C.; Lagerge, S. Study of the complex formation between sodium dodecyl sulfate and chitosan. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 100–108. [Google Scholar] [CrossRef]
- Ren, Y.; Xie, H.; Liu, X.; Yang, F.; Yu, W.; Ma, X. Tuning the formation and stability of microcapsules by environmental conditions and chitosan structure. Int. J. Biol. Macromol. 2016, 91, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, J.; Conforto, E.; Chaigneau, C.; Vendeville, J.-E.; Maugard, T. Microencapsulation and controlled release of α-tocopherol by complex coacervation between pea protein and tragacanth gum: A comparative study with arabic and tara gums. Innov. Food Sci. Emerg. Technol. 2022, 77, 102951:1–102951:14. [Google Scholar] [CrossRef]
- Estevinho, B.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Wang, B.; Akanbi, T.O.; Agyei, D.; Holland, B.J.; Barrow, C.J. Coacervation Technique as an Encapsulation and Delivery Tool for Hydrophobic Biofunctional Compounds. In Role of Materials Science in Food Bioengineering; Grumezescu, A., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 235–261. ISBN 978-0-1281-1448-3. [Google Scholar]
- Hilton, J.; Dearman, R.J.; Harvey, P.; Evans, P.; Basketter, D.A.; Kimber, I. Estimation of relative skin sensitizing potency using the local lymph node assay: A comparison of formaldehyde with glutaraldehyde. Am. J. Contact Dermat. 1998, 9, 29–33. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) 2022/1181 of 8 July 2022 amending the preamble of Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. OJEU 2022, 184, L184/3–L184/4.
- Milinković Budinčić, J.; Petrović, L.; Đekić, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Study of vitamin E microencapsulation and controlled release from chitosan/sodium lauryl ether sulfate microcapsules. Carbohydr. Polym. 2021, 251, 116988:1–116988:8. [Google Scholar] [CrossRef]
- Schreiner, T.B.; Santamaria-Echart, A.; Barreiro, M.F. Saponin-based natural nanoemulsions as alpha-tocopherol delivery systems for dermal applications. J. Mol. Liq. 2023, 391, 123371:1–123371:12. [Google Scholar] [CrossRef]
- Olbińska, E.; Trela-Makowej, A.; Larysz, W.; Orzechowska, A.; Szymańska, R. The effect of α-tocopherol incorporated into different carriers on the oxidative stability of oil in water (O/W) emulsions. Colloids Surf. B Biointerface 2023, 230, 113536:1–113536:7. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Blanco, M.D.; Olmo, R.M.; Teijón, J.M. Hydrogels. In Encyclopedia of Pharmaceutical Technology, 3rd ed.; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; pp. 2021–2039. ISBN 978-0-8493-9396-9. [Google Scholar]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Jaworski, Z.; Spychaj, T.; Story, A.; Story, G. Carbomer microgels as model yield-stress fluids. Rev. Chem. Eng. 2022, 38, 881–919. [Google Scholar] [CrossRef]
- Lochhead, R.Y. The use of polymers in cosmetic products. In Cosmetic Science and Technology; Sakamoto, K., Lochhead, R.Y., Maibach, H.I., Yamashita, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–221. ISBN 978-0-12-802005-0. [Google Scholar]
- Carbopol® Ultrez 10 Polymer. Available online: https://www.lubrizol.com/en/Personal-Care/Products/Product-Finder/Products-Data/Carbopol-Ultrez-10-polymer (accessed on 5 March 2024).
- Milinković, J.; Petrović, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Interfacial and emulsifying properties of chitosan/sodium lauryl ether sulfate system. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 9–13. [Google Scholar] [CrossRef]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP 43-NF 38 Chapter <659> Packaging and Storage Requirements. In The United States Pharmacopoeia 43, The National Formulary 38; United States Pharmacopoeial Convention: Rockville, MD, USA, 2020; p. 6880. ISBN 978-1-936424-95-5. [Google Scholar]
- Djekic, L.; Martinović, M.; Dobričić, V.; Čalija, B.; Medarević, Đ.; Primorac, M. Comparison of the Effect of Bioadhesive Polymers on Stability and Drug Release Kinetics of Biocompatible Hydrogels for Topical Application of Ibuprofen. J. Pharm. Sci. 2019, 108, 1326–1333. [Google Scholar] [CrossRef]
- Shimamura, T.; Tairabune, T.; Kogo, T.; Ueda, H.; Numajiri, S.; Kobayashi, D.; Morimoto, Y. Investigation of the Release Test Method for the Topical Application of Pharmaceutical Preparations: Release Test of Cataplasm Including Nonsteroidal Anti-inflammatory Drugs Using Artificial Sweat. Chem. Pharm. Bull. 2004, 52, 167–171. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP 43-NF 38 chapter <1724> Semisolid Drug Products—Performance Tests. In The United States Pharmacopoeia 43, The National Formulary 38; United States Pharmacopoeial Convention: Rockville, MD, USA, 2020; pp. 8473–8484. ISBN 978-1-936424-95-5. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- Diaz, D.A.; Colgan, S.T.; Langer, C.S.; Bandi, N.T.; Likar, M.D.; Van Alstine, L. Dissolution Similarity Requirements: How Similar or Dissimilar Are the Global Regulatory Expectations? AAPS J. 2016, 18, 15–22. [Google Scholar] [CrossRef]
- Dejeu, I.L.; Vicaș, L.G.; Vlaia, L.L.; Jurca, T.; Mureșan, M.E.; Pallag, A.; Coneac, G.H.; Olariu, I.V.; Muț, A.M.; Bodea, A.S.; et al. Study for Evaluation of Hydrogels after the Incorporation of Liposomes Embedded with Caffeic Acid. Pharmaceuticals 2022, 15, 175. [Google Scholar] [CrossRef]
- United States Pharmacopeia. USP 43-NF 38 Chapter <1191> Stability Considerations in Dispensing Practice. In The United States Pharmacopoeia 43, The National Formulary 38; United States Pharmacopoeial Convention: Rockville, MD, USA, 2020; p. 8048. ISBN 978-1-936424-95-5. [Google Scholar]
- Allen, L.V. Gels. In The Art, Science, and Technology of Pharmaceutical Compounding, 6th ed.; Allen, L.V., Ed.; APhA PharmacyLibrary: Washington, DC, USA, 2020; ISBN 1-58212-357-8. [Google Scholar] [CrossRef]
- Lein, A.; Oussoren, C. Dermal. In Practical Pharmaceutics; Bouwman-Boer, Y., Fenton-May, V., Le Brun, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 229–263. ISBN 978-3-319-15813-6. [Google Scholar]
- Gutowski, I.A.; Lee, D.; de Bruyn, J.R.; Frisken, B.J. Scaling and mesostructure of Carbopol dispersions. Rheol. Acta 2012, 51, 441–450. [Google Scholar] [CrossRef]
- TDS-237 Neutralizing Carbopol and Pemulen Polymers in Aqueous and Hydroalcoholic Systems. Available online: https://www.academia.edu/42914025/TDS_237_Neutralizing_Carbopol_Pemulen_in_Aqueous_Hydroalcoholic_Systems_PH_1_ (accessed on 5 March 2024).
- Kulkarni, V.S.; Shaw, C. Use of polymers and thickeners in semisolid and liquid formulations. In Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Kulkarni, V.S., Shaw, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 43–69. ISBN 978-0-12-801024-2. [Google Scholar]
- Saba, M.A.; Yosipovitch, G. Skin pH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wen, Y.; Chen, S.; Zhu, L.; Feng, R.; Song, Z. Paclitaxel skin delivery by micelles-embedded Carbopol 940 hydrogel for local therapy of melanoma. Int. J. Pharm. 2020, 587, 119626:1–119626:11. [Google Scholar] [CrossRef] [PubMed]
- Maslii, Y.; Ruban, O.; Kasparaviciene, G.; Kalveniene, Z.; Materiienko, A.; Ivanauskas, L.; Mazurkieviciute, A.; Kopustinskiene, D.M.; Bernatoniene, J. The Influence of pH Values on the Rheological, Textural and Release Properties of Carbomer Polacril® 40P-Based Dental Gel Formulation with Plant-Derived and Synthetic Active Components. Molecules 2020, 25, 5018. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Vyas, S.P. Carbopol/Chitosan Based pH Triggered In Situ Gelling System for Ocular Delivery of Timolol Maleate. Sci. Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Krieger, I.; Dougherthy, T. A mechanism for non-Newtonian flow in suspensions of rigid spheres. J. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Hui, X.; Anigbogu, A.; Singh, P.; Poblete, N.; Liu, P.; Maibach, H.I. Pharmacokinetic and local tissue disposition of [14C]sodium diclofenac following iontophoresis and systemic administration in rabbits. J. Pharm. Sci. 2001, 90, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Gabbanini, S.; Matera, R.; Beltramini, C.; Minghetti, A.; Valgimigli, L. Analysis of in vitro release through reconstructed human epidermis and synthetic membranes of multi-vitamins from cosmetic formulations. J. Pharm. Biomed. Anal. 2010, 52, 461–467. [Google Scholar] [CrossRef]
- Dreijer-van der Glas, S.; Hinrichs, W. Physical Chemistry. In Practical Pharmaceutics; Bouwman-Boer, Y., Fenton-May, V., Le Brun, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 357–382. ISBN 978-3-319-15813-6. [Google Scholar]
- Djekić, L.; Ćirić, A. Modeling of in vitro drug release from polymeric microparticle carriers. ARH Farm 2022, 72, 591–620. [Google Scholar] [CrossRef]


| Composition of the Microcapsule Wall | Moisture Content ± S.D. (% w/w) | Mean Diameter ± S.D. (µm) | EE ± S.D. (% w/w) | Hydrogel Label | Content of α-Tocopherol in Hydrogel (% w/w) |
|---|---|---|---|---|---|
| LMWC/SLES (mass ratio 2:1) without crosslinker | 1.27 ± 0.081 1 | 5.32 ± 0.252 1 | 73.17 ± 0.64 1 | W21 | 0.37 |
| LMWC/SLES (mass ratio 1:1) without crosslinker | 1.35 ± 0.180 | 4.86 ± 0.29 | 71.12 ± 0.98 | W11 | 0.36 |
| LMWC/SLES (mass ratio 2:1) crosslinked with glutaraldehyde (LMWC/SLES-to-glutaraldehyde mass ratio 1:2) | 1.30 ± 0.173 1 | 5.84 ± 0.256 1 | 99.50 ± 2.27 1 | G12 | 0.50 |
| LMWC/SLES (mass ratio 2:1) crosslinked with glutaraldehyde (LMWC/SLES-to-glutaraldehyde mass ratio 1:1) | 1.66 ± 0.376 1 | 5.04 ± 0.331 1 | 100.00 ± 3.55 1 | G11 | 0.50 |
| LMWC/SLES (mass ratio 2:1) crosslinked with formaldehyde (LMWC/SLES-to-formaldehyde mass ratio 1:2) | 1.76 ± 0.451 1 | 6.21 ± 0.242 1 | 82.30 ± 1.67 1 | F12 | 0.41 |
| LMWC/SLES (mass ratio 2:1) crosslinked with formaldehyde (LMWC/SLES-to-formaldehyde mass ratio 1:1) | 0.97 ± 0.312 1 | 6.25 ± 0.224 1 | 93.50 ± 1.08 1 | F11 | 0.47 |
| pH | |||
|---|---|---|---|
| Sample | 48 h | 1 Month | 2 Months |
| Blank hydrogel | 7.22 | 7.17 | 7.20 |
| W21 | 6.44 | 6.51 | 6.60 |
| W11 | 6.41 | 6.48 | 6.53 |
| F12 | 6.68 | 6.77 | 6.82 |
| F11 | 6.60 | 6.63 | 6.71 |
| G12 | 6.83 | 6.87 | 6.88 |
| G11 | 6.58 | 6.64 | 6.68 |
| 48 h | 1 Month | 2 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | ηmax (Pa∙s) | ηmin (Pa∙s) | H (Pa/s) | ηmax (Pa∙s) | ηmin(Pa∙s) | H (Pa/s) | ηmax (Pa∙s) | ηmin (Pa∙s) | H (Pa/s) |
| Blank hydrogel | 16.4 ± 0.14 | 2.41 ± 0.01 | 2614.99 | 18.7 ± 0.32 | 4.41 ± 0.06 | 2934.09 | 20.5 ± 0.16 | 3.38 ± 0.07 | 3512.08 |
| W21 | 2.54 ± 0.05 | 0.62 ± 0.05 | 7.51 | 2.25 ± 0.34 | 0.51 ± 0.09 | 6.36 | 2.71 ± 0.51 | 0.61 ± 0.02 | 8.33 |
| W11 | 2.18 ± 0.06 | 0.54 ± 0.01 | 6.17 | 2.22 ± 0.07 | 0.54 ± 0.01 | 5.88 | 2.34 ± 0.14 | 0.49 ± 0.03 | 6.24 |
| F12 | 11.2 ± 0.42 | 1.51 ± 0.02 | 1933.9 | 15.4 ± 0.37 | 2.15 ± 0.04 | 2985.64 | 19.8 ± 0.75 | 2.72 ± 0.06 | 8319.54 |
| F11 | 6.9 ± 0.69 | 1.01 ± 0.03 | 8.11 | 6.2 ± 0.08 | 0.98 ± 0.02 | 91.88 | 8.12 ± 0.31 | 1.09 ± 0.02 | 1049.11 |
| G12 | 12.45 ± 0.49 | 1.67 ± 0.01 | 2045.86 | 13.1 ± 0.65 | 1.86 ± 0.07 | 1552.81 | 16.3 ± 0.54 | 2.22 ± 0.07 | 4810.33 |
| G11 | 12.15 ± 0.35 | 1.65 ± 0.02 | 2481.12 | 11.4 ± 0.29 | 1.61 ± 0.02 | 1533.52 | 12.7 ± 0.41 | 1.67 ± 0.03 | 2227.38 |
| Sample | Φ (mm) ± S.D. | t (min) |
|---|---|---|
| Blank hydrogel | 19.5 ± 0.5 | >5 |
| W21 | 30.7 ± 1.5 | 1–2 |
| W11 | 43.3 ± 2.5 | <1 |
| F12 | 28.7 ± 2.5 | 2–5 |
| F11 | 39.7 ± 1.2 | 2–5 |
| G12 | 36.7 ± 0.6 | 2–5 |
| G11 | 38.3 ± 1.5 | 2–5 |
| Acceptor Medium | Ethyl Alcohol 60% w/w | Polysorbate 20 5% w/w | ||
|---|---|---|---|---|
| Samples | f1 | f2 | f1 | f2 |
| W11 (CN) vs. W11 (P) | 39.25 | 47.36 | 18.12 | 65.24 |
| W21 (CN) vs. W21 (P) | 26.29 | 61.73 | 32.72 | 66.90 |
| W11 (CN) vs. W21 (CN) | 21.92 | 58.76 | 33.39 | 64.87 |
| W11 (P) vs. W21 (P) | 26.29 | 61.73 | 32.54 | 66.03 |
| W11 (CN) vs. W21 (P) | 42.45 | 46.20 | 30.71 | 56.98 |
| W21 (CN) vs. W11 (P) | 22.19 | 64.41 | 52.82 | 57.74 |
| Membrane | Cellulose Nitrate | Polycarbonate | ||
|---|---|---|---|---|
| Samples | f1 | f2 | f1 | f2 |
| W11 (ethyl alcohol 60% w/w) vs. W11 (polysorbate 20 5% w/w) | 49.45 | 46.15 | 24.69 | 60.03 |
| W21 (ethyl alcohol 60% w/w) vs. W21 (polysorbate 20 5% w/w) | 52.82 | 48.99 | 34.08 | 58.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đekić, L.; Milinković Budinčić, J.; Stanić, D.; Fraj, J.; Petrović, L. Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study. Pharmaceutics 2024, 16, 628. https://doi.org/10.3390/pharmaceutics16050628
Đekić L, Milinković Budinčić J, Stanić D, Fraj J, Petrović L. Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study. Pharmaceutics. 2024; 16(5):628. https://doi.org/10.3390/pharmaceutics16050628
Chicago/Turabian StyleĐekić, Ljiljana, Jelena Milinković Budinčić, Dušanka Stanić, Jadranka Fraj, and Lidija Petrović. 2024. "Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study" Pharmaceutics 16, no. 5: 628. https://doi.org/10.3390/pharmaceutics16050628
APA StyleĐekić, L., Milinković Budinčić, J., Stanić, D., Fraj, J., & Petrović, L. (2024). Carbomer Hydrogels with Microencapsulated α-Tocopherol: Focus on the Biocompatibility of the Microcapsules, Topical Application Attributes, and In Vitro Release Study. Pharmaceutics, 16(5), 628. https://doi.org/10.3390/pharmaceutics16050628






