Foams Set a New Pace for the Release of Diclofenac Sodium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of the Formulations
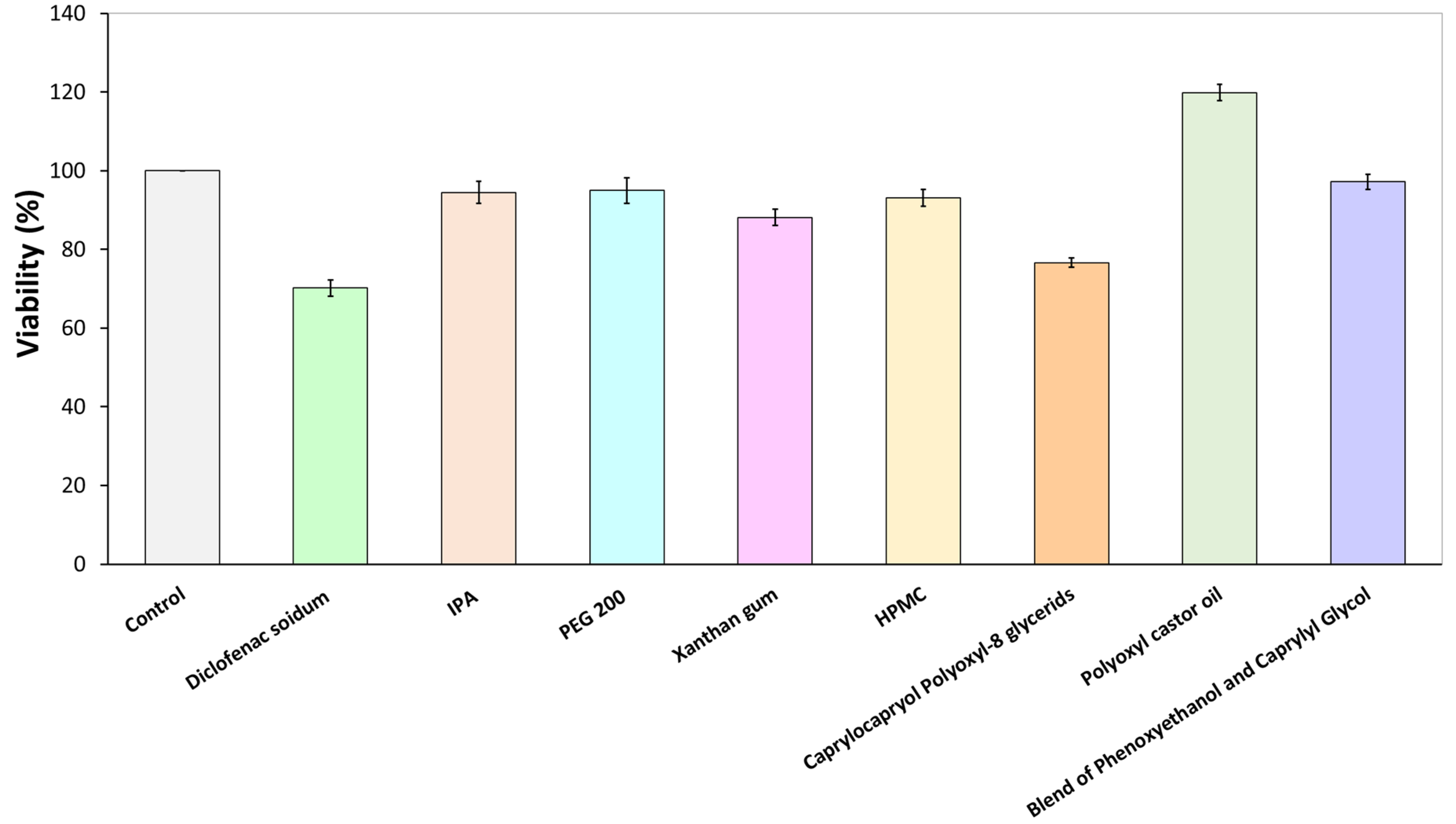
2.2.2. Citotoxicity Assay
2.2.3. Preformulation Studies of Foam Formula
2.2.3.1. Macroscopic Characterization of Foam Formula
2.2.3.2. Microscopic Characterization of Foam Formula
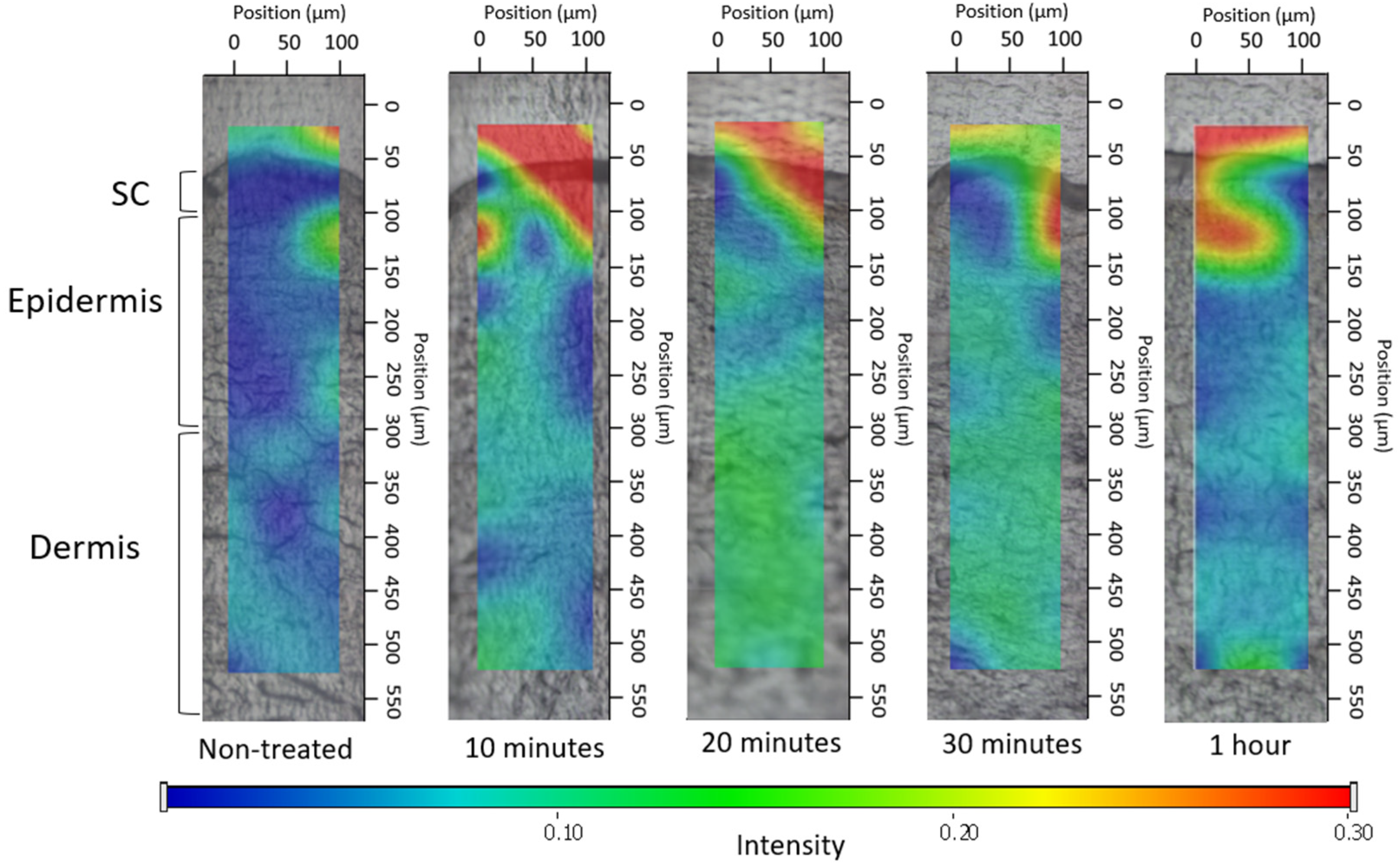
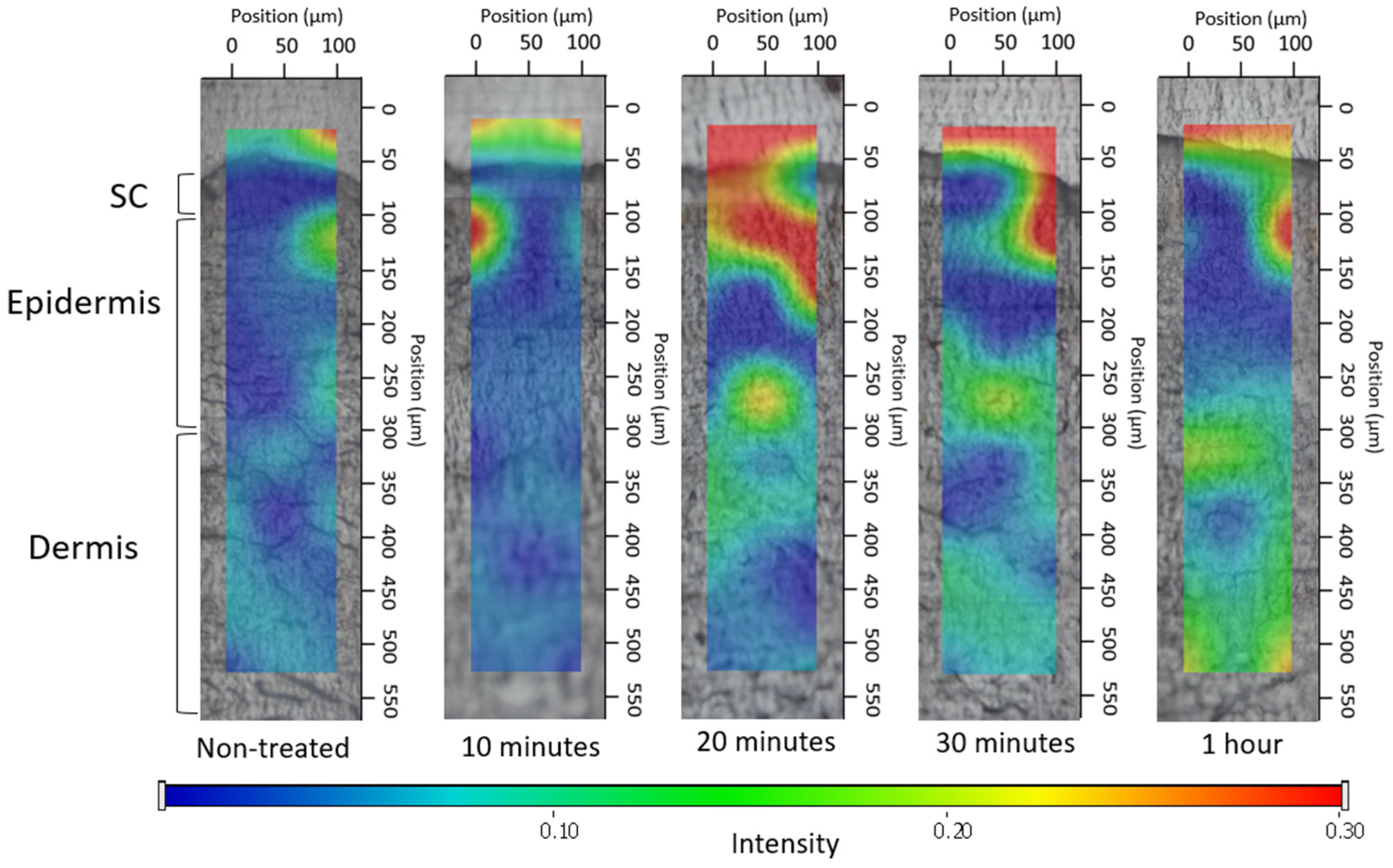
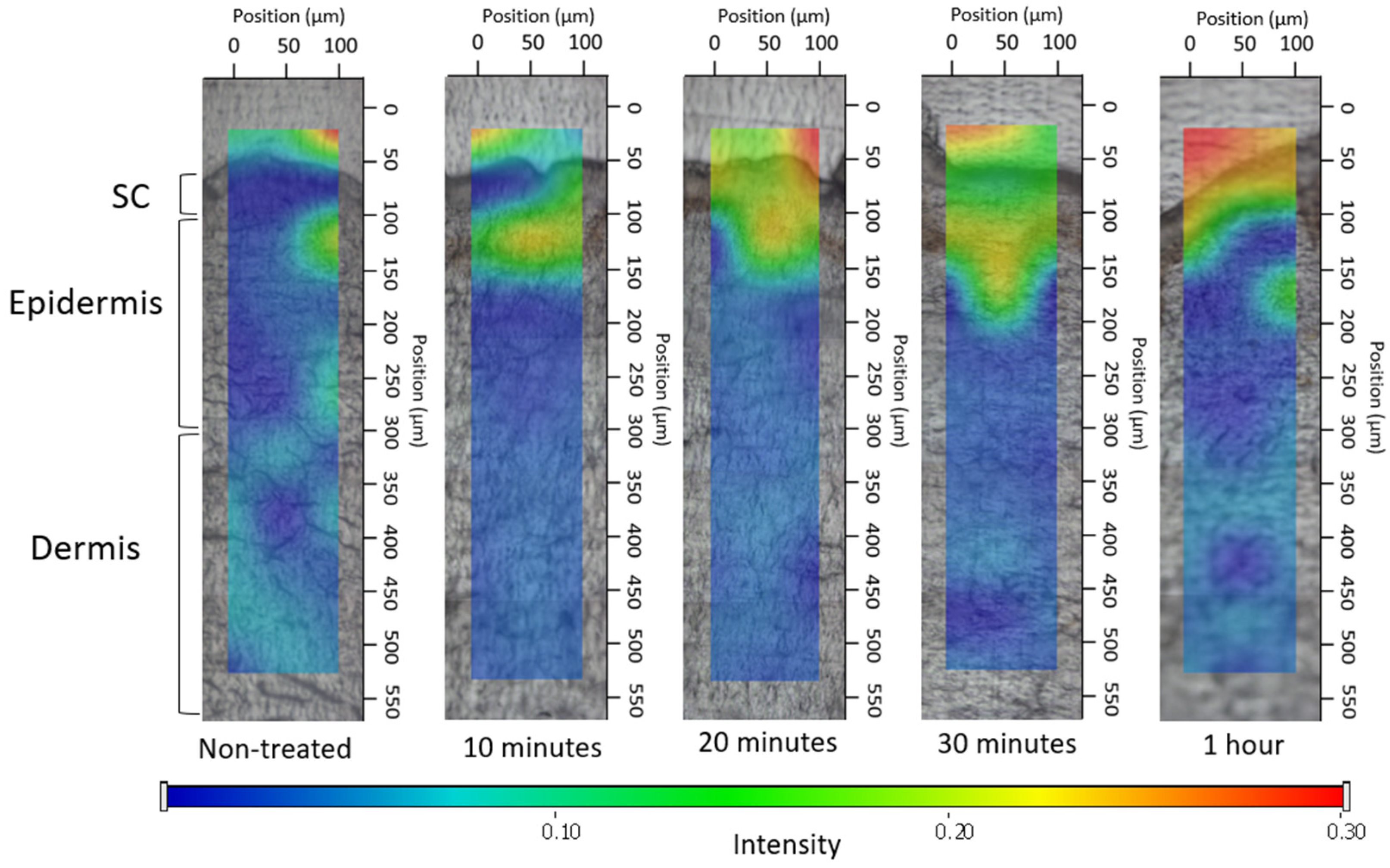
2.2.3.3. Ex Vivo Permeation through the Skin Using Fluorescent Microscope
2.2.4. Comparison of Physicochemical Properties of Foam Formula, Bulk Liquid, and Hydrogel
2.2.4.1. Rheological Measurements
2.2.4.2. Investigation of pH
2.2.5. Comparison of Biopharmaceutical Properties of Foam Formula, Bulk Liquid, and Hydrogel
2.2.5.1. In Vitro Drug Release and Permeation Tests (IVRT and IVPT) Using Franz Diffusion Cell System
2.2.5.2. Investigation of Ex Vivo Drug Permeation Using Raman Spectroscopy
2.2.6. Statistical Analysis
3. Results
3.1. Cytotoxicity Assay
3.2. Preformulation Studies of Foam Formula
3.2.1. Macroscopic Characterization of Foam Formula
3.2.2. Microscopic Characterization of Foam Formula
3.2.3. Ex Vivo Permeation through Fluorescent Microscope
3.3. Comparison of Physicochemical Properties of Foam Formula, Bulk Liquid, and Hydrogel
3.3.1. Rheological Measurements
3.3.2. Investigation of pH
3.4. Comparison of Biopharmaceutical Properties of Foam Formula, Bulk Liquid, and Hydrogel
3.4.1. In Vitro Drug Release and Permeation Tests (IVRT and IVPT) Using Franz Diffusion Cell System
3.4.2. Investigation of Ex Vivo Drug Permeation Using Raman Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, T.M.; Mijaljica, D.; Townley, J.P.; Spada, F.; Harrison, I.P. Vehicles for Drug Delivery and Cosmetic Moisturizers: Review and Comparison. Pharmaceutics 2021, 13, 2012. [Google Scholar] [CrossRef]
- Daniels, R.; Knie, U. Galenics of Dermal Products—Vehicles, Properties and Drug Release. JDDG J. Dtsch. Dermatol. Ges. 2007, 5, 367–383. [Google Scholar] [CrossRef]
- Wang, M.; Marepally, S.K.; Vemula, P.K.; Xu, C. Chapter 5—Inorganic Nanoparticles for Transdermal Drug Delivery and Topical Application. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 57–72. ISBN 978-0-12-802926-8. [Google Scholar]
- Kis, N.; Gunnarsson, M.; Berkó, S.; Sparr, E. The Effects of Glycols on Molecular Mobility, Structure, and Permeability in Stratum Corneum. J. Control. Release 2022, 343, 755–764. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of Transdermal Drug Delivery via Synergistic Action of Chemicals. Biochim. Biophys. Acta BBA Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Alexander, A.; Dwivedi, S.; Ajazuddin; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for Breaking the Barriers of Drug Permeation through Transdermal Drug Delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Garg, A. An Insight of Techniques for the Assessment of Permeation Flux across the Skin for Optimization of Topical and Transdermal Drug Delivery Systems. J. Drug Deliv. Sci. Technol. 2021, 62, 102355. [Google Scholar] [CrossRef]
- Parsa, M.; Trybala, A.; Malik, D.J.; Starov, V. Foam in Pharmaceutical and Medical Applications. Curr. Opin. Colloid Interface Sci. 2019, 44, 153–167. [Google Scholar] [CrossRef]
- Trybala, A.; Koursari, N.; Johnson, P.; Arjmandi-Tash, O.; Starov, V. Interaction of Liquid Foams with Porous Substrates. Curr. Opin. Colloid Interface Sci. 2019, 39, 212–219. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, P.K.; Malviya, R. Eco Friendly Pharmaceutical Packaging Material. World Appl. Sci. J. 2011, 14, 1703–1716. [Google Scholar]
- Gennari, C.G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Medicated Foams and Film Forming Dosage Forms as Tools to Improve the Thermodynamic Activity of Drugs to Be Administered Through the Skin. Curr. Drug Deliv. 2019, 16, 461–471. [Google Scholar] [CrossRef]
- Farkas, D.; Kállai-Szabó, N.; Sárádi-Kesztyűs, Á.; Lengyel, M.; Magramane, S.; Kiss, É.; Antal, I. Investigation of Propellant-Free Aqueous Foams as Pharmaceutical Carrier Systems. Pharm. Dev. Technol. 2021, 26, 253–261. [Google Scholar] [CrossRef]
- Purdon, C.H.; Haigh, J.M.; Surber, C.; Smith, E.W. Foam Drug Delivery in Dermatology: Beyond the Scalp. Am. J. Drug Deliv. 2003, 1, 71–75. [Google Scholar] [CrossRef]
- Maimouni, I.; Cejas, C.M.; Cossy, J.; Tabeling, P.; Russo, M. Microfluidics Mediated Production of Foams for Biomedical Applications. Micromachines 2020, 11, 83. [Google Scholar] [CrossRef]
- Mantripragada, S. A Lipid Based Depot (DepoFoam Technology) for Sustained Release Drug Delivery. Prog. Lipid Res. 2002, 41, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Mantripragada, S.B.; Howell, S.B. Sustained-Release Drug Delivery with DepoFoam. In Drug Delivery Systems in Cancer Therapy; Brown, D.M., Ed.; Cancer Drug Discovery and Development; Humana Press: Totowa, NJ, USA, 2004; pp. 247–262. ISBN 978-1-59259-427-6. [Google Scholar]
- Salisbury, A.-M.; Mullin, M.; Foulkes, L.; Chen, R.; Percival, S.L. Controlled-Release Iodine Foam Dressings Demonstrate Broad-Spectrum Biofilm Management in Several in Vitro Models. Int. Wound J. 2022, 19, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Casiraghi, A.; Selmin, F.; Minghetti, P. Supersaturation as a Tool for Skin Penetration Enhancement. Curr. Pharm. Des. 2015, 21, 2733–2744. [Google Scholar] [CrossRef]
- Zhao, Y.; Brown, M.B.; Jones, S.A. Pharmaceutical Foams: Are They the Answer to the Dilemma of Topical Nanoparticles? Nanomedicine Nanotechnol. Biol. Med. 2010, 6, 227–236. [Google Scholar] [CrossRef]
- Kumar, M.; Thakur, A.; Mandal, U.K.; Thakur, A.; Bhatia, A. Foam-Based Drug Delivery: A Newer Approach for Pharmaceutical Dosage Form. AAPS PharmSciTech 2022, 23, 244. [Google Scholar] [CrossRef]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef]
- Balmaceda, C.M. Evolving Guidelines in the Use of Topical Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Osteoarthritis. BMC Musculoskelet. Disord. 2014, 15, 27. [Google Scholar] [CrossRef]
- Heyneman, C.A.; Lawless-Liday, C.; Wall, G.C. Oral versus Topical NSAIDs in Rheumatic Diseases. Drugs 2000, 60, 555–574. [Google Scholar] [CrossRef]
- Hoc, D.; Haznar-Garbacz, D. Foams as Unique Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2021, 167, 73–82. [Google Scholar] [CrossRef]
- Falusi, F.; Budai-Szűcs, M.; Csányi, E.; Berkó, S.; Spaits, T.; Csóka, I.; Kovács, A. Investigation of the Effect of Polymers on Dermal Foam Properties Using the QbD Approach. Eur. J. Pharm. Sci. 2022, 173, 106160. [Google Scholar] [CrossRef]
- Kis, N.; Kovács, A.; Budai-Szűcs, M.; Erős, G.; Csányi, E.; Berkó, S. The Effect of Non-Invasive Dermal Electroporation on Skin Barrier Function and Skin Permeation in Combination with Different Dermal Formulations. J. Drug Deliv. Sci. Technol. 2022, 69, 103161. [Google Scholar] [CrossRef]
- Ali, S.M.; Yosipovitch, G. Skin pH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Honari, G.; Andersen, R.; Maibach, H.L. Sensitive Skin Syndrome; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-3735-7. [Google Scholar]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on Wound-Healing: A New Perspective for Wound-Therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Cao, Y.; Zou, Y. Dermal-Epidermal Separation by Heat. Methods Mol. Biol. Clifton NJ 2020, 2109, 23–25. [Google Scholar] [CrossRef]
- Maibach, Y.Z.; Howard, I. Dermal-Epidermal Separation Methods: Research Implications. In Percutaneous Absorption; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-429-20297-1. [Google Scholar]
- Szoleczky, R.; Budai-Szűcs, M.; Csányi, E.; Berkó, S.; Tonka-Nagy, P.; Csóka, I.; Kovács, A. Analytical Quality by Design (AQbD) Approach to the Development of In Vitro Release Test for Topical Hydrogel. Pharmaceutics 2022, 14, 707. [Google Scholar] [CrossRef] [PubMed]
- Bayan, M.F.; Chandrasekaran, B.; Alyami, M.H. Development and Characterization of Econazole Topical Gel. Gels 2023, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Binder, L.; SheikhRezaei, S.; Baierl, A.; Gruber, L.; Wolzt, M.; Valenta, C. Confocal Raman Spectroscopy: In Vivo Measurement of Physiological Skin Parameters—A Pilot Study. J. Dermatol. Sci. 2017, 88, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ji, C.; Miao, M.; Yang, K.; Luo, Y.; Hoptroff, M.; Collins, L.Z.; Janssen, H.-G. Ex-Vivo Measurement of Scalp Follicular Infundibulum Delivery of Zinc Pyrithione and Climbazole from an Anti-Dandruff Shampoo. J. Pharm. Biomed. Anal. 2017, 143, 26–31. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Novel In Vitro Investigational Methods for Modeling Skin Permeation: Skin PAMPA, Raman Mapping. Pharmaceutics 2020, 12, 803. [Google Scholar] [CrossRef] [PubMed]
- ANSI/AAMI/ISO 10993-5:2009/(R)2014; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. AAMI: Arlington, VA, USA, 2009; ISBN 978-1-57020-355-8.
- Ashtikar, M.A.; Verma, D.D.; Fahr, A. Confocal Microscopy for Visualization of Skin Penetration. In Percutaneous Penetration Enhancers Drug Penetration Into/Through the Skin: Methodology and General Considerations; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin, Heidelberg, 2017; pp. 255–281. ISBN 978-3-662-53270-6. [Google Scholar]
- Proksch, E. pH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]





| Hydrogel | Foam/Foam Bulk Liquid | |
|---|---|---|
| Diclofenac sodium (g) | 1 | 1 |
| PEG 200 | + | − |
| IPA | + | − |
| HPMC | + | − |
| Xanthan gum | − | + |
| Caprylocaproyl Polyoxyl-8 glycerides | + | + |
| Polyoxyl castor oil | + | + |
| Blend of Phenoxyethanol and Caprylyl Glycol | + | + |
| Purified water | up to 100 g | up to 100 g |
| Polymer-and-API-Free Foam | Xanthan-Gum-Containing Foam | Xanthan-Gum-and-DS-Containing Foam | |
|---|---|---|---|
| Foam expansion (FE, %) | 172 ±15.8 | 134 ± 1.9 | 120 ± 0.7 |
| Foam volume stability (FVS, %) | 14 ± 1.8 | 100 ±0.0 | 98 ± 0.0 |
| Foam liquid stability (FLS, %) | 36 ± 2.0 | 0 ± 0.0 | 1.5 ± 0.2 |
| Viscosity (mPas) | |
|---|---|
| Bulk liquid | 46.99 ± 0.80 |
| Liquid film (after the foam decay) | 58.68 ± 1.07 |
| Hydrogel | 378.78 ± 4.39 |
| pH | |
|---|---|
| Bulk liquid/Foam | 7.86 ± 0.11 |
| Hydrogel | 7.29 ± 0.37 |
| Formulation | R2 | n | k |
|---|---|---|---|
| Bulk liquid | 0.9094 | 0.6105 | 4.9352 |
| Foam | 0.9372 | 0.6243 | 9.8115 |
| Hydrogel | 0.9880 | 0.8033 | 0.9284 |
| J (µg/cm2/h) | R2 | |
|---|---|---|
| Bulk liquid | 0.866 | 0.9811 |
| Foam | 5.838 | 0.9921 |
| Hydrogel | 1.65 | 0.9173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falusi, F.; Berkó, S.; Budai-Szűcs, M.; Veréb, Z.; Kovács, A. Foams Set a New Pace for the Release of Diclofenac Sodium. Pharmaceutics 2024, 16, 287. https://doi.org/10.3390/pharmaceutics16020287
Falusi F, Berkó S, Budai-Szűcs M, Veréb Z, Kovács A. Foams Set a New Pace for the Release of Diclofenac Sodium. Pharmaceutics. 2024; 16(2):287. https://doi.org/10.3390/pharmaceutics16020287
Chicago/Turabian StyleFalusi, Fanni, Szilvia Berkó, Mária Budai-Szűcs, Zoltán Veréb, and Anita Kovács. 2024. "Foams Set a New Pace for the Release of Diclofenac Sodium" Pharmaceutics 16, no. 2: 287. https://doi.org/10.3390/pharmaceutics16020287
APA StyleFalusi, F., Berkó, S., Budai-Szűcs, M., Veréb, Z., & Kovács, A. (2024). Foams Set a New Pace for the Release of Diclofenac Sodium. Pharmaceutics, 16(2), 287. https://doi.org/10.3390/pharmaceutics16020287







