Design, Synthesis, and Antitumor Evaluation of an Opioid Growth Factor Bioconjugate Targeting Pancreatic Ductal Adenocarcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Selective Boc Protection of Gem
2.2. Peptide Synthesis
2.3. Conjugate Synthesis
2.4. Stability of OGF-Gem Conjugate and OGF
2.5. Cell Lines and Cell Cultures
2.6. MTT Assay
2.7. Organoid 3D Viability Assay
2.8. BrdU Assay
2.9. Cell Cycle Analysis
2.10. Cell Senescence and Flow Cytometry
2.11. Cell Senescence and Optical Microscopy
2.12. Preparation of Platelets from Whole-Blood and Pancreatic Cancer Cells
2.13. The Effect of the OGF-Gem Conjugate on TCIPA
2.14. LDH Release Assay
2.15. Statistical Analysis
3. Results
3.1. Selective Boc Protection of Gemcitabine
3.2. Synthesis and Stability Evaluation of the Conjugate Compounds
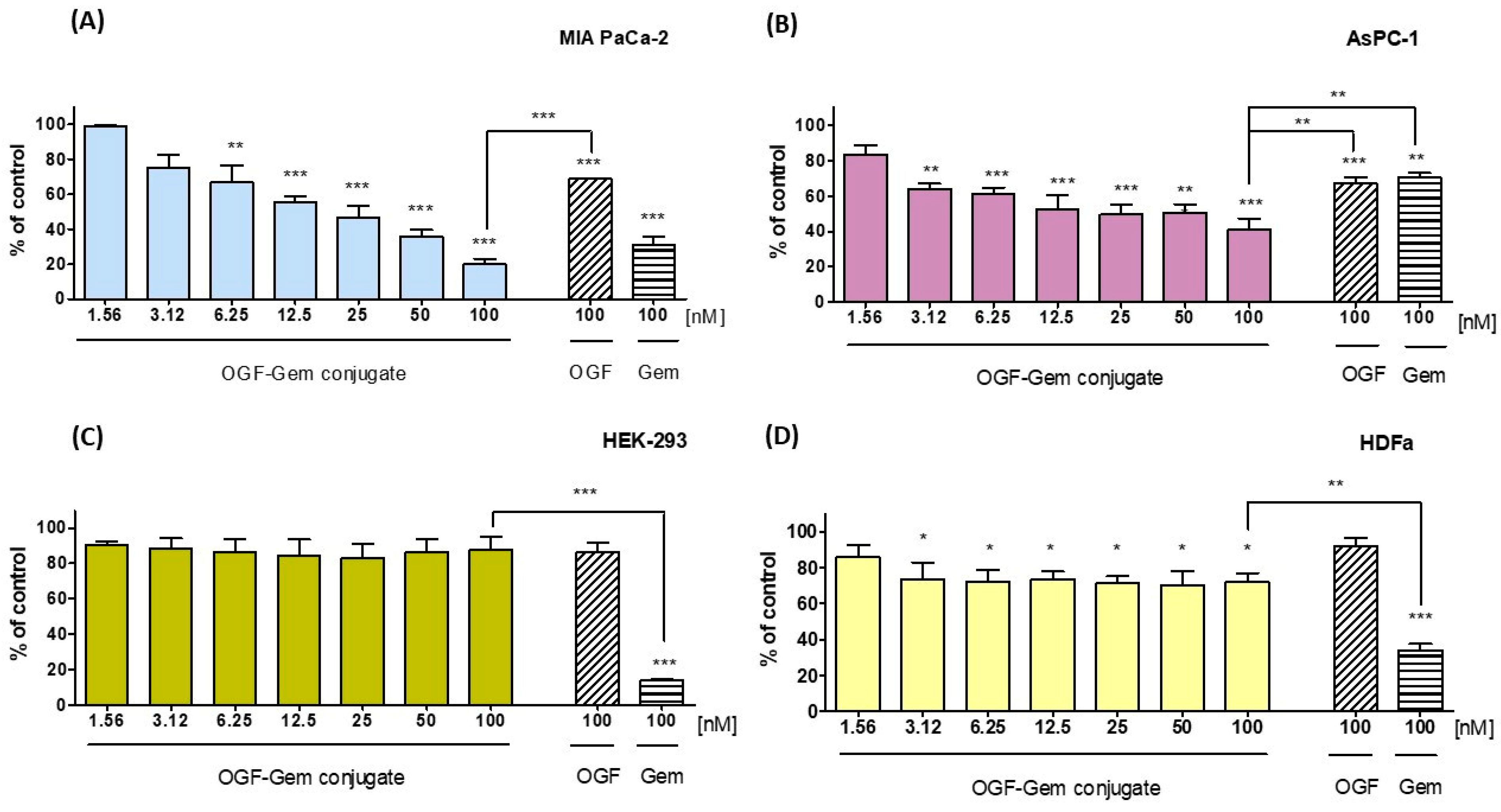
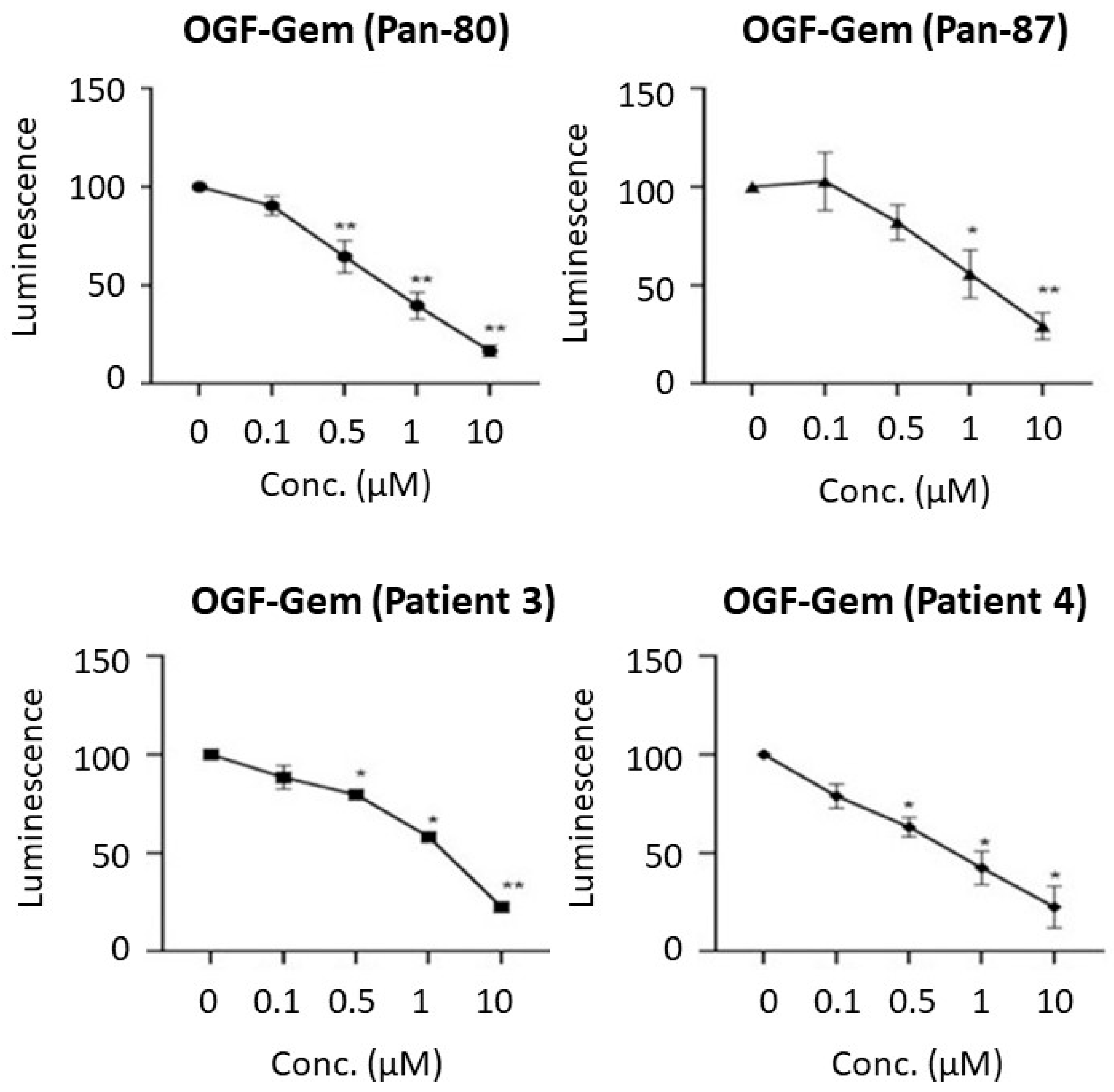
3.3. Assessment of the OGF-Gem Conjugate’s Impact on Cell Viability in a 2D Model and Its Effect on Pancreatic Cancer Organoids in a 3D Model
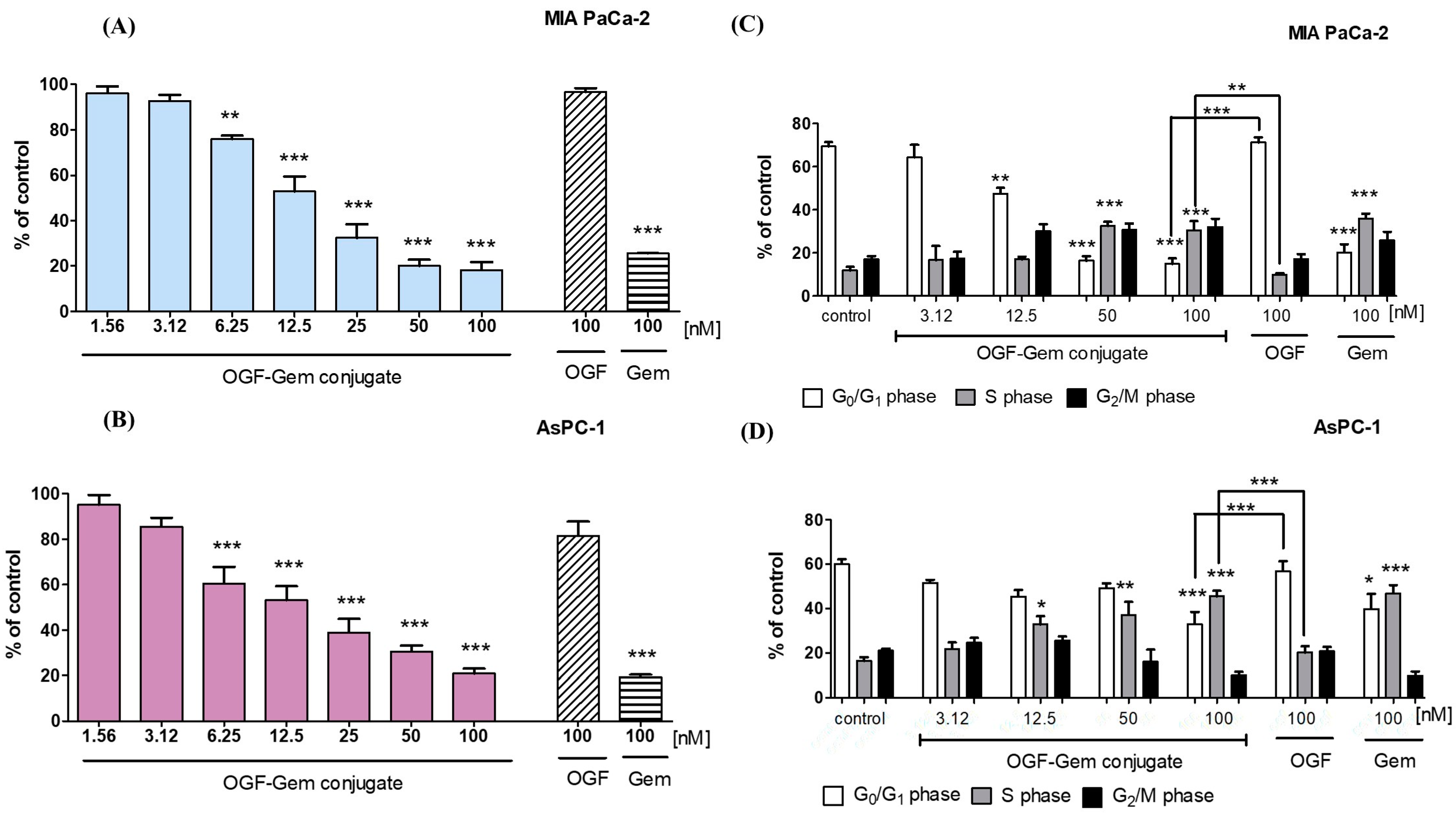
3.4. The Impact of OGF-Gem on Cell Proliferation and the Cell Cycle
3.5. The Impact of OGF-Gem on Cellular Senescence
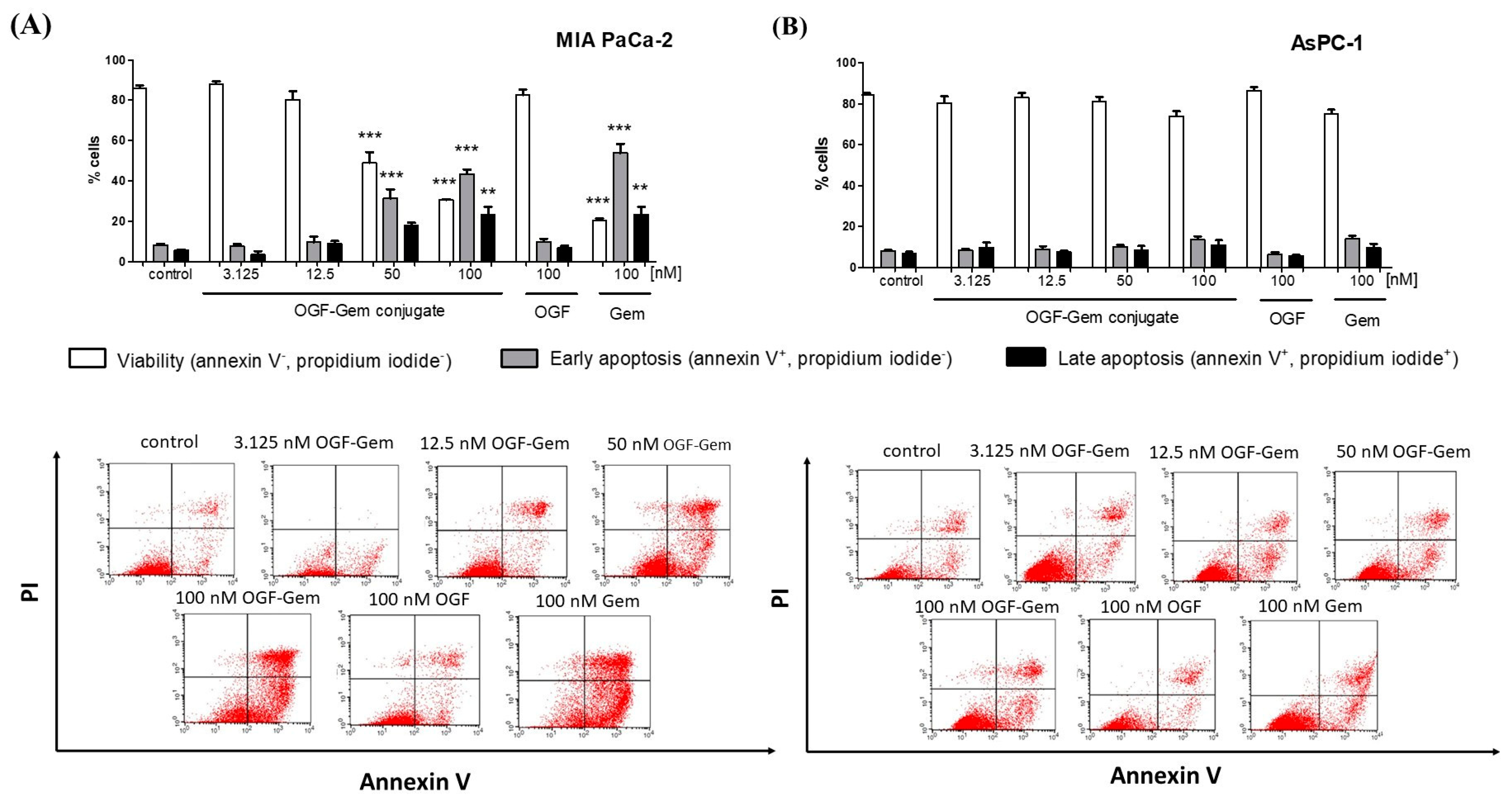
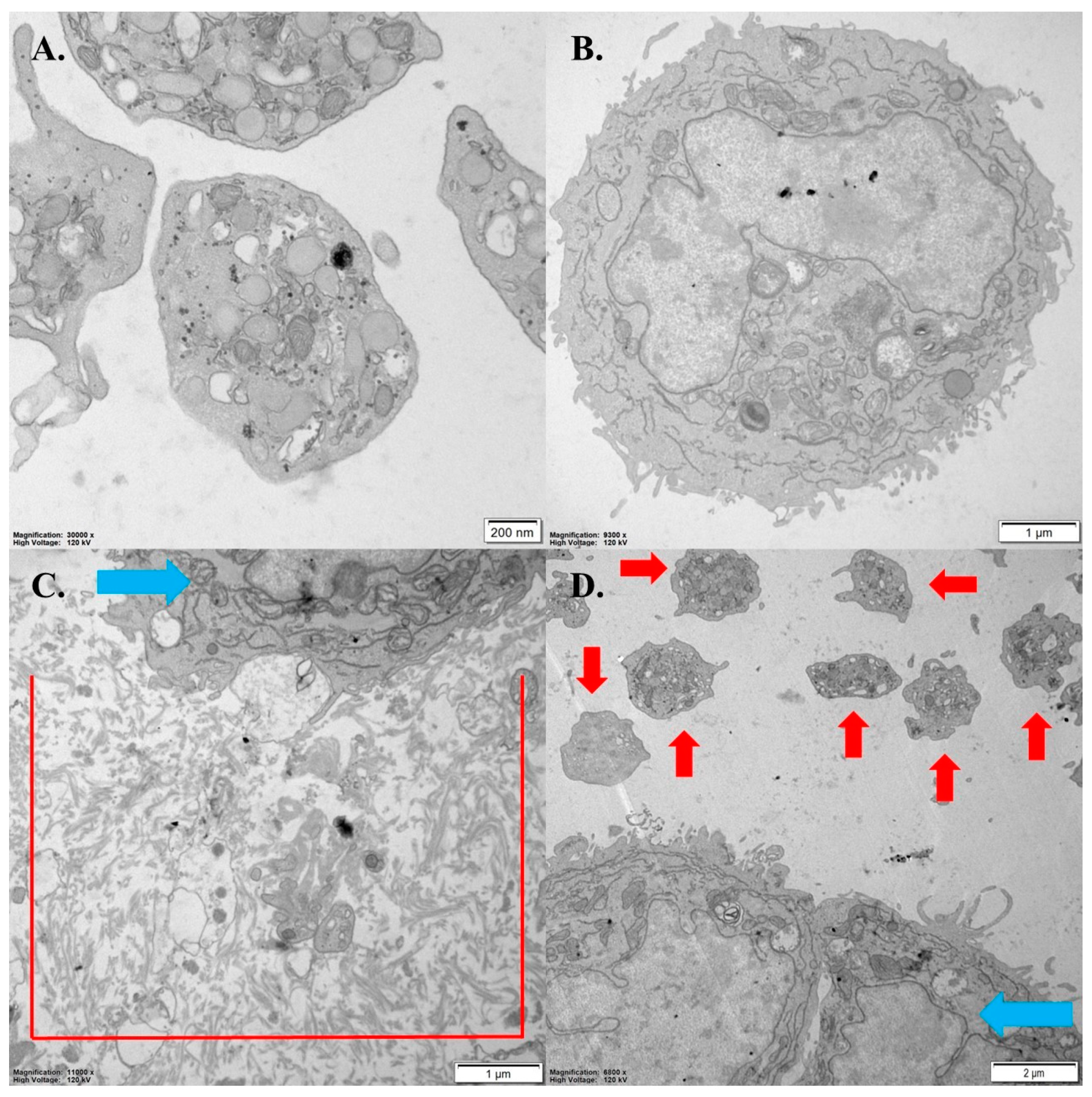
3.6. The Impact of OGF-Gem on Cell Apoptosis
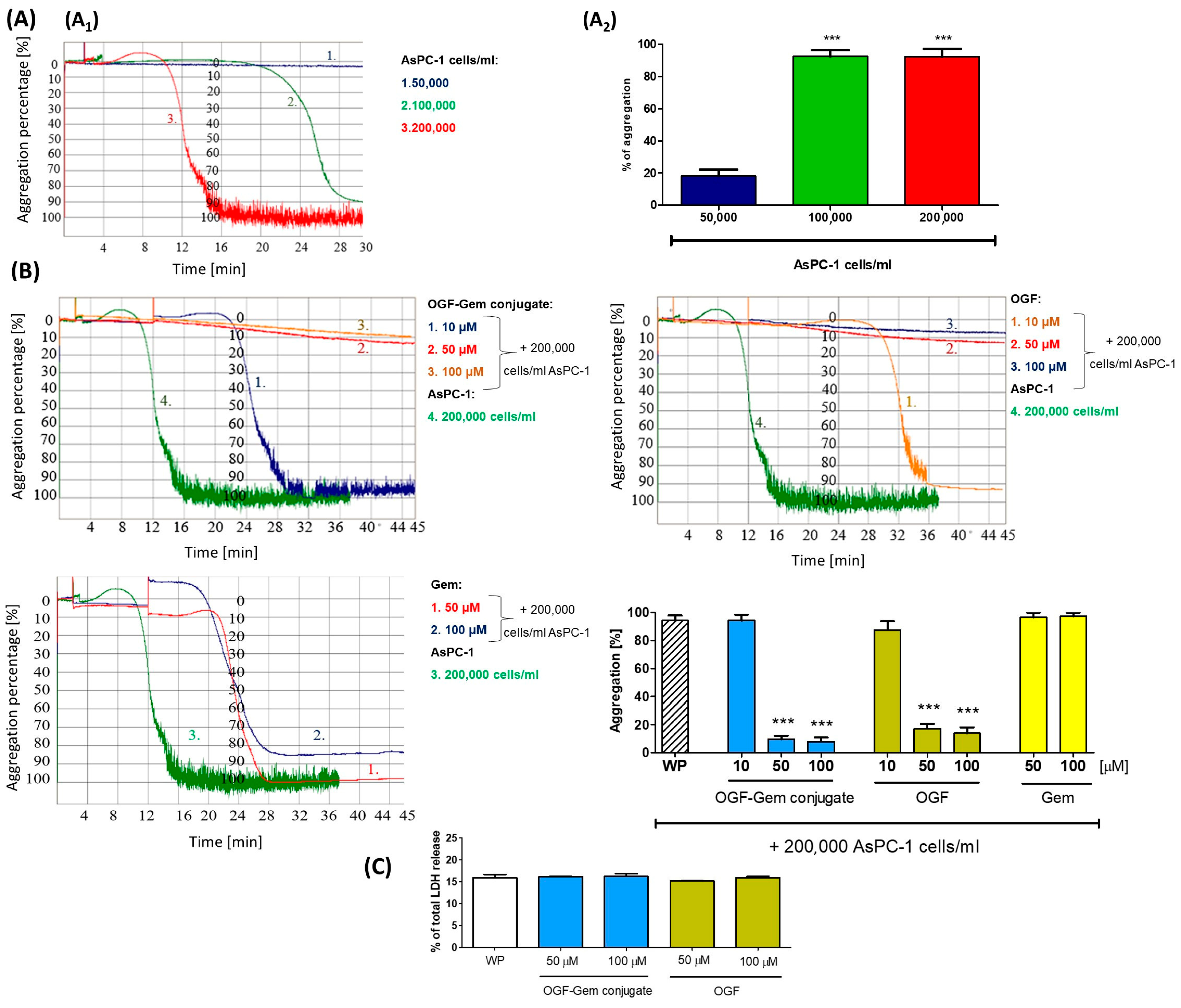
3.7. The Effect of OGF-Gem Conjugate on TCIPA
4. Discussion
4.1. Effect of OGF-Gem Conjugate on Cell Viability (2D Model) and Pancreatic Cancer Organoids (3D Model)
4.2. The Influence of OGF-Gem on the Proliferation of Cells and Cell Cycle
4.3. The Influence of OGF-Gem on Cell Senescence
4.4. The Influence of OGF-Gem on Cell Apoptosis
4.5. The Impact of OGF-Gem Conjugate on TCIPA
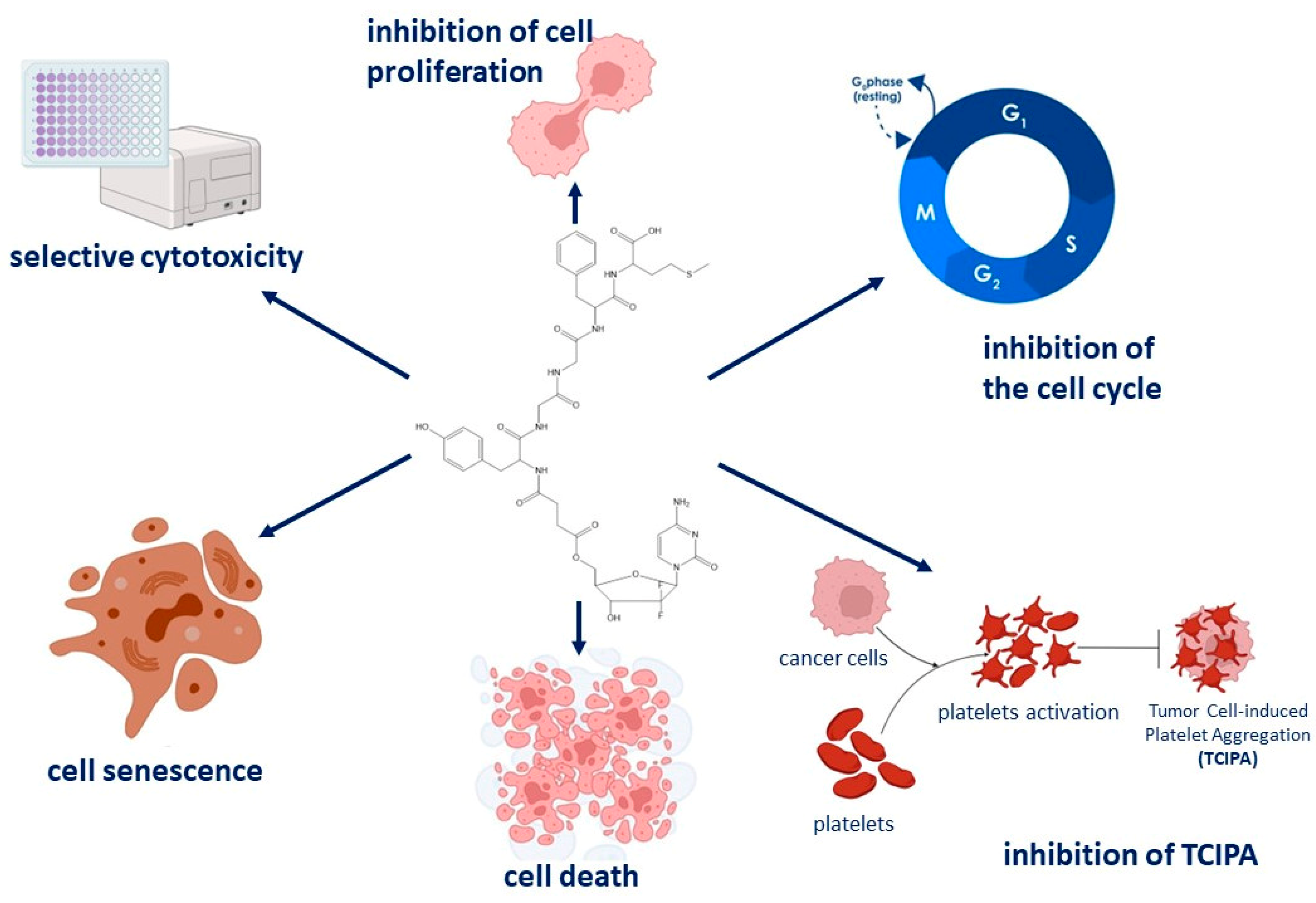
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory. 2022. Available online: https://gco.iarc.fr/ (accessed on 20 November 2022).
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Bures, J.; Kohoutova, D.; Skrha, J.; Bunganic, B.; Ngo, O.; Suchanek, S.; Skrha, P.; Zavoral, M. Diabetes Mellitus in Pancreatic Cancer: A Distinct Approach to Older Subjects with New-Onset Diabetes Mellitus. Cancers 2023, 15, 3669. [Google Scholar] [CrossRef] [PubMed]
- Mellenthin, C.; Balaban, V.D.; Dugic, A.; Cullati, S. Risk Factors for Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4684. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, M.; Śliwińska, A. The Link between Diabetes, Pancreatic Tumors, and miRNAs—New Players for Diagnosis and Therapy? Int. J. Mol. Sci. 2023, 24, 10252. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Sayyad, N.; Vrettos, E.I.; Karampelas, T.; Chatzigiannis, C.M.; Spyridaki, K.; Liapakis, G.; Tamvakopoulos, C.; Tzakos, A.G. Development of bioactive gemcitabine-D-Lys6-GnRH prodrugs with linker-controllable drug release rate and enhanced biopharmaceutical profile. Eur. J. Med. Chem. 2016, 166, 256–266. [Google Scholar] [CrossRef]
- Vrettos, E.I.; Karampelas, T.; Sayyad, N.; Kougioumtzi, A.; Syed, N.; Crook, T.; Murphy, C.; Tamvakopoulos, C.; Tzakos, A.G. Development of programmable gemcitabine-GnRH pro-drugs bearing linker controllable “click” oxime bond tethers and preclinical evaluation against prostate cancer. Eur. J. Med. Chem. 2021, 211, 113018. [Google Scholar] [CrossRef]
- Vrettos, E.I.; Mező, G.; Tzakos, A.G. On the design principles of peptide–drug conjugates for targeted drug delivery to the malignant tumor site. Beilstein J. Org. Chem. 2018, 14, 930–954. [Google Scholar] [CrossRef]
- Vrettos, E.I.; Tzakos, A.G. Construction of Peptide-Drug Conjugates for Selective Targeting of Malignant Tumor Cells. Methods Mol. Biol. 2021, 2207, 327–338. [Google Scholar] [CrossRef]
- Zagon, I.S.; McLaughlin, P.J. Opioid growth factor and the treatment of human pancreatic cancer: A review. World J. Gastroenterol. 2014, 20, 2218–2223. [Google Scholar] [CrossRef]
- Zagon, I.S.; McLaughlin, P.J. Opioid growth factor (OGF) inhibits anchorage-independent growth in human cancer cells. Int. J. Oncol. 2004, 24, 1443–1448. [Google Scholar]
- Zagon, I.S.; Smith, J.P.; McLaughlin, P.J. Human pancreatic cancer cell proliferation in tissue culture is tonically inhibited by opioid growth factor. J. Oncol. 1999, 14, 577–661. [Google Scholar] [CrossRef]
- Smith, J.P.; Bingaman, S.I.; Mauger, D.T.; Harvey, H.H.; Demers, L.M.; Zagon, I.S. Opioid growth factor improves clinical benefit and survival in patients with advanced pancreatic cancer. Open Access J. Clin. Trials 2010, 2010, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Wang, X.; Meng, Y.; Shan, F. Prospective oncotarget for gynecological cancer: Opioid growth factor (OGF)-opioid growth factor receptor (OGFr) axis. Int. Immunopharmacol. 2019, 75, 105723. [Google Scholar] [CrossRef]
- Medina, C.; Harmon, S.; Inkielewicz, I.; Santos-Martinez, M.J.; Jones, M.; Cantwell, P.; Bazou, D.; Ledwidge, M.; Radomski, M.W.; Gilmer, J.F. Differential inhibition of tumour cell-induced platelet aggregation by the nicotinate aspirin prodrug (ST0702) and aspirin. Br. J. Pharmacol. 2012, 166, 938–949. [Google Scholar] [CrossRef]
- Roweth, H.G.; Battinelli, E.M. Lessons to learn from tumor-educated platelets. Blood 2021, 137, 3174–3180. [Google Scholar] [CrossRef]
- Ding, S.; Dong, X.; Song, X. Tumor educated platelet: The novel BioSource for cancer detection. Cancer Cell Int. 2023, 23, 91. [Google Scholar] [CrossRef]
- McNamee, N.; Rodriguez de la Fuente, L.; Santos-Martinez, M.J.; O’Driscoll, L. Proteomics profiling identifies extracellular vesicles’ cargo associated with tumour cell induced platelet aggregation. BMC Cancer 2022, 22, 1023. [Google Scholar] [CrossRef]
- Varkey, J.; Nicolaides, T. Tumor-Educated Platelets: A Review of Current and Potential Applications in Solid Tumors. Cureus 2021, 13, e19189. [Google Scholar] [CrossRef]
- Inkielewicz-Stepnia, I.; Mai, S. Pancreatic Cancer and Platelets Crosstalk: A Potential Biomarker and Target. Front. Cell Dev. Biol. 2021, 9, 749689. [Google Scholar] [CrossRef]
- Zw, Z.G.; Gallo, J.M. Selective Protection of 2′,2′-Difluorodeoxycytidine (Gemcitabine). J. Org. Chem. 1999, 64, 8319–8322. [Google Scholar] [CrossRef]
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Heldt, M.; Serocki, M.; Stupak, A.; Martynow, D.; Dębowski, D.; Agata Gitlin-Domagalska, A.; Lica, J.; et al. Conjugates of ciprofloxacin and levofloxacin with cell-penetrating peptide exhibit antifungal activity and mammalian cytotoxicity. Int. J. Mol. Sci. 2020, 21, 4696. [Google Scholar] [CrossRef]
- Ptaszyńska, N.; Gucwa, K.; Olkiewicz, K.; Łȩgowska, A.; Okońska, J.; Ruczyński, J.; Agata Gitlin-Domagalska, A.; Dębowski, D.; Milewski, S.; Rolka, K. Antibiotic-based conjugates containing antimicrobial HLopt2 peptide: Design, synthesis, antimicrobial and cytotoxic activities. ACS Chem. Biol. 2019, 14, 2233–2242. [Google Scholar]
- Biernacki, K.; Ciupak, O.; Daśko, M.; Rachon, J.; Flis, D.; Budka, J.; Inkielewicz-Stepniak, I.; Czaja, A.; Rak, J.; Demkowicz, S. Development of potent and effective SARS-CoV-2 main protease inhibitors based on maleimide analogs for the potential treatment of COVID-19. J. Enzym. Inhib. Med. Chem. 2024, 39, 2290910. [Google Scholar] [CrossRef]
- Kowalski, S.; Wyrzykowski, D.; Hac, S.; Rychlowski, M.; Radomski, M.W.; Inkielewicz-Stepniak, I. New oxidovanadium (IV) coordination complex containing 2-methylnitrilotriacetate ligands induces cell cycle arrest and autophagy in human pancreatic ductal adenocarcinoma cell lines. Int. J. Mol. Sci. 2019, 20, 261. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Sobczak, K.; Tomczyk, E.; Wojcik, M.; Inkielewicz-Stepniak, I. Assessment of Anti-Tumor potential and safety of application of Glutathione stabilized Gold Nanoparticles conjugated with Chemotherapeutics. Int. J. Med. Sci. 2020, 17, 824. [Google Scholar] [CrossRef]
- Geyer, M.; Schreyer, D.; Gaul, L.M.; Pfeffer, S.; Pilarsky, C.; Queiroz, K. A microfluidic-based PDAC organoid system reveals the impact of hypoxia in response to treatment. Cell Death Discov. 2023, 9, 20. [Google Scholar] [CrossRef]
- Liao, L.Z.; Chen, Z.C.; Wang, S.S.; Liu, W.B.; Zhao, C.L.; Zhuang, X.D. NLRP3 inflammasome activation contributes to the pathogenesis of cardiocytes aging. Aging 2021, 13, 20534. [Google Scholar] [CrossRef]
- Pilch, J.; Kowalik, P.; Bujak, P.; Nowicka, A.M.; Augustin, E. Quantum dots as a good carriers of unsymmetrical bisacridines for modulating cellular uptake and the biological response in lung and colon cancer cells. Nanomaterials 2021, 11, 462. [Google Scholar] [CrossRef]
- Hajtuch, J.; Santos-Martinez, M.J.; Wojcik, M.; Tomczyk, E.; Jaskiewicz, M.; Kamysz, W.; Narajczyk, M.; Inkielewicz-Stepniak, I. Lipoic acid-coated silver nanoparticles: Biosafety potential on the vascular microenvironment and antibacterial properties. Front. Pharmacol. 2022, 12, 733743. [Google Scholar] [CrossRef]
- Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Medina, C.; Radomski, M.W. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int. J. Nanomed. 2014, 9, 1677–1687. [Google Scholar] [CrossRef]
- Medina, C.; Jurasz, P.; Santos-Martinez, M.J.; Jeong, S.S.; Mitsky, T.; Chen, R.; Radomski, M.W. Platelet aggregation-induced by caco-2 cells: Regulation by matrix metalloproteinase-2 and adenosine diphosphate. J. Pharmacol. Exp. Ther. 2006, 317, 739–745. [Google Scholar] [CrossRef]
- Carreno, E.A.; Alberto, A.V.P.; de Souza, C.A.M.; de Mello, H.L.; Henriques-Pons, A.; Anastacio Alves, L. Considerations and technical pitfalls in the employment of the MTT assay to evaluate photosensitizers for photodynamic therapy. Appl. Sci. 2021, 11, 2603. [Google Scholar] [CrossRef]
- Dominijanni, A.J.; Devarasetty, M.; Forsythe, S.D.; Votanopoulos, K.I.; Soker, S. Cell Viability Assays in Three-Dimensional Hydrogels: A Comparative Study of Accuracy. Tissue Eng. Part C Methods 2021, 27, 401–410. [Google Scholar] [CrossRef]
- Sharma, R.B.; Darko, C.; Zheng, X.; Gablaski, B.; Alonso, L.C. DNA damage does not cause BrdU labeling of mouse or human β-cells. Diabetes 2019, 68, 975–987. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Cheng, H.; Fan, Z.; Huang, Q.; Lu, Y.; Fan, K.; Luo, G.; Jin, K.; Wang, Z.; et al. Novel agents for pancreatic ductal adenocarcinoma: Emerging therapeutics and future directions. J. Hematol. Oncol. 2018, 11, 14. [Google Scholar] [CrossRef]
- Zagon, I.S.; Verderame, M.F.; Hankins, J.; McLaughlin, P.J. Overexpression of the opioid growth factor receptor potentiates growth inhibition in human pancreatic cancer cells. Int. J. Oncol. 2007, 30, 775–783. [Google Scholar] [CrossRef][Green Version]
- Karampelas, T.; Skavatsou, E.; Argyros, O.; Fokas, D.; Tamvakopoulos, C. Gemcitabine based peptide conjugate with improved metabolic properties and dual mode of efficacy. Mol. Pharm. 2017, 14, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Jaglowski, J.R.; Verderame, M.F.; Smith, J.P.; Leure-duPree, A.E.; McLaughlin, P.J. Combination chemotherapy with gemcitabine and biotherapy with opioid growth factor (OGF) enhances the growth inhibition of pancreatic adenocarcinoma. Cancer Chemother. Pharmacol. 2005, 56, 510–520. [Google Scholar] [CrossRef]
- Gou, S.; Yao, X.; Jiang, Q.; Wang, K.; Zhang, Y.; Peng, H.; Tang, J.; Yang, W. Dihydroartemisinin-Loaded Magnetic Nanoparticles for Enhanced Chemodynamic Therapy. Front. Pharmacol. 2020, 11, 226. [Google Scholar] [CrossRef]
- Khaled, Y.S.; Wright, K.; Melcher, A.; Jayne, D. Anti-cancer effects of oncolytic viral therapy combined with photodynamic therapy in human pancreatic cancer cell lines. Lancet 2015, 385 (Suppl. S1), S56. [Google Scholar] [CrossRef]
- Lu, C.H.; Lin, S.H.; Hsieh, C.H.; Chen, W.T.; Chao, C.Y. Enhanced anticancer effects of low-dose curcumin with non-invasive pulsed electric field on PANC-1 cells. Onco-Targets Ther. 2018, 11, 4723–4732. [Google Scholar] [CrossRef]
- Avelar Júnior, J.T.D.; Lima-Batista, E.; Castro Junior, C.J.; Pimenta, A.M.D.C.; Dos Santos, R.G.; Souza-Fagundes, E.M.; De Lima, M.E. LyeTxI-b, a synthetic peptide derived from a spider venom, is highly active in triple-negative breast cancer cells and acts synergistically with cisplatin. Front. Mol. Biosci. 2022, 9, 876833. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Z.; Liu, X.; Liu, Q.; Wu, M.; Yao, X.; Cui, C.; Li, H.; Song, C.; Liu, D.; et al. Cytotoxicity evaluation of chloroquine and hydroxychloroquine in multiple cell lines and tissues by dynamic imaging system and physiologically based pharmacokinetic model. Front. Pharmacol. 2020, 11, 574720. [Google Scholar] [CrossRef] [PubMed]
- Sersenová, D.; Machala, Z.; Repiská, V.; Gbelcová, H. Selective Apoptotic Effect of Plasma Activated Liquids on Human Cancer Cell Lines. Molecules 2021, 26, 4254. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, Z.; Long, W.; Yan, Y.; Li, Y.; Fu, T.; Liu, Y.; Zhao, Z.; Tan, W.; Stang, P.J. Self-assembled Pt (II) metallacycles enable precise cancer combination chemotherapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2202255119. [Google Scholar] [CrossRef]
- Abolhasani, A.; Heidari, F.; Raminfar, R.; Mousavi, S.; Abolhasani, H. In Vitro Cytotoxicity Evaluation of Steviosid on Cancerous Liver (Hep G2), Colon (HT29), Breast (MCF7) cells and Normal Kidney Cell (Hek293) in Comparison with Cisplatin. QOM Univ. Med. Sci. J. 2020, 14, 26–34. [Google Scholar] [CrossRef]
- McLaughlin, P.J.; Zagon, I.S. The opioid growth factor-opioid growth factor receptor axis: Homeostatic regulator of cell proliferation and its implications for health and disease. Biochem. Pharmacol. 2012, 84, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Paproski, R.J.; Yao, S.Y.; Favis, N.; Evans, D.; Young, J.D.; Cass, C.E.; Zemp, R.J. Human concentrative nucleoside transporter 3 transfection with ultrasound and microbubbles in nucleoside transport deficient HEK293 cells greatly increases gemcitabine uptake. PLoS ONE 2013, 8, e56423. [Google Scholar] [CrossRef][Green Version]
- Goldenberg, D.; Zagon, I.S.; Fedok, F.; Crist, H.S.; McLaughlin, P.J. Expression of opioid growth factor (OGF)-OGF receptor (OGFr) axis in human nonmedullary thyroid cancer. Thyroid 2008, 18, 1165–1170. [Google Scholar] [CrossRef]
- Cheng, F.; McLaughlin, P.J.; Verderame, M.F.; Zagon, I.S. The OGF-OGFr axis utilizes the p21 pathway to restrict progression of human pancreatic cancer. Mol. Cancer 2008, 7, 5. [Google Scholar] [CrossRef]
- Zagon, I.S.; Hytrek, S.D.; Smith, J.P.; McLaughlin, P.J. Opioid growth factor (OGF) inhibits human pancreatic cancer transplanted into nude mice. Cancer Lett. 1997, 112, 167–175. [Google Scholar] [CrossRef]
- Donahue, R.N.; McLaughlin, P.J.; Zagon, I.S. Under-expression of the opioid growth factor receptor promotes progression of human ovarian cancer. Exp. Biol. Med. 2012, 237, 167–177. [Google Scholar] [CrossRef]
- Belltall, A.; Zúñiga-Trejos, S.; Garrido-Cano, I.; Eroles, P.; Argente-Navarro, M.P.; Buggy, D.J.; Díaz-Cambronero, O.; Mazzinari, G. Solid Tumor Opioid Receptor Expression and Oncologic Outcomes: Analysis of the Cancer Genome Atlas and Genotype Tissue Expression Project. Front. Oncol. 2022, 12, 801411. [Google Scholar] [CrossRef]
- Zagon, I.S.; McLaughlin, P.J. Identification of opioid peptides regulating proliferation of neurons and glia in the developing nervous system. Brain Res. 1991, 542, 318–323. [Google Scholar] [CrossRef]
- Saiki, Y.; Hirota, S.; Horii, A. Attempts to remodel the pathways of gemcitabine metabolism: Recent approaches to overcoming tumours with acquired chemoresistance. Cancer Drug Resist. 2020, 3, 819–831. [Google Scholar] [CrossRef]
- Aier, I.; Varadwaj, P.K. Understanding the mechanism of cell death in gemcitabine resistant pancreatic ductal adenocarcinoma: A systems biology approach. Curr. Genom. 2019, 20, 483–490. [Google Scholar] [CrossRef]
- Amrutkar, M.; Vethe, N.T.; Verbeke, C.S.; Aasrum, M.; Finstadsveen, A.V.; Sántha, P.; Gladhaug, I.P. Differential gemcitabine sensitivity in primary human pancreatic cancer cells and paired stellate cells is driven by heterogenous drug uptake and processing. Cancers 2020, 12, 3628. [Google Scholar] [CrossRef]
- Coyne, C.P.; Narayanan, L. Gemcitabine-(5′-phosphoramidate)-[anti-IGF-1R]: Molecular design, synthetic organic chemistry reactions, and antineoplastic cytotoxic potency in populations of pulmonary adenocarcinoma (A549). Chem. Biol. Drug Des. 2016, 89, 379–399. [Google Scholar] [CrossRef]
- Ciccolini, J.; Serdjebi, C.; Peters, G.J.; Giovannetti, E. Pharmacokinetics and pharmacogenetics of Gemcitabine as a mainstay in adult and pediatric oncology: An EORTC-PAMM perspective. Cancer Chemother. Pharmacol. 2016, 78, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mini, E.; Nobili, S.; Caciagli, B.; Landini, I.; Mazzei, T. Cellular pharmacology of gemcitabine. Ann. Oncol. 2006, 17 (Suppl. S5), v7–v12. [Google Scholar] [CrossRef]
- Cheng, F.; McLaughlin, P.J.; Banks, W.A.; Zagon, I.S. Internalization of the opioid growth factor, [Met5]-enkephalin, is dependent on clathrin-mediated endocytosis for downregulation of cell proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R774–R785. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Verderame, M.F.; McLaughlin, P.J. The biology of the opioid growth factor receptor (OGFr). Brain Res. Brain Res. Rev. 2002, 38, 351–376. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Shan, F. Interaction of opioid growth factor (OGF) and opioid antagonist and their significance in cancer therapy. Int. Immunopharmacol. 2019, 75, 105785. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Tofani, L.B.; Abriata, J.P.; Luiz, M.T.; Marchetti, J.M.; Swiech, K. Establishment and characterization of an in vitro 3D ovarian cancer model for drug screening assays. Biotechnol. Prog. 2020, 36, e3034. [Google Scholar] [CrossRef]
- Hou, S.; Tiriac, H.; Sridharan, B.P.; Scampavia, L.; Madoux, F.; Seldin, J.; Souza, G.R.; Watson, D.; Tuveson, D.; Spicer, T.P. Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening. SLAS Discov. 2018, 23, 574–584. [Google Scholar] [CrossRef]
- Hong, J.; Jin, J.O.; Chen, W.Y.; Poggi, A.; Cheong, J.H. Emerging roles and mechanisms of stromal cells in carcinomas at the molecular level. Front. Immunol. 2022, 13, 1025838. [Google Scholar] [CrossRef]
- Mun, J.Y.; Leem, S.H.; Lee, J.H.; Kim, H.S. Dual relationship between stromal cells and immune cells in the tumor microenvironment. Front. Immunol. 2022, 13, 864739. [Google Scholar] [CrossRef]
- Li, C.; Jin, B.; Sun, H.; Wang, Y.; Zhao, H.; Sang, X.; Yang, H.; Mao, Y. Exploring the function of stromal cells in cholangiocarcinoma by three-dimensional bioprinting immune microenvironment model. Front. Immunol. 2022, 13, 941289. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Radhakrishnan, P. Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol. Cancer 2019, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Forciniti, S.; Dalla Pozza, E.; Greco, M.R.; Amaral Carvalho, T.M.; Rolando, B.; Ambrosini, G.; Carmona-Carmona, C.A.; Pacchiana, R.; Di Molfetta, D.; Donadelli, M.; et al. Extracellular matrix composition modulates the responsiveness of differentiated and stem pancreatic cancer cells to lipophilic derivate of gemcitabine. Int. J. Mol. Sci. 2021, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Fontoura, J.C.; Viezzer, C.; Dos Santos, F.G.; Ligabue, R.A.; Weinlich, R.; Puga, R.D.; Antonow, D.; Severino, P.; Bonorino, C. Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Mater. Sci. Eng. C 2020, 107, 110264. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Lee, J.M.; Mhawech-Fauceglia, P.; Lee, N.; Parsanian, L.C.; Lin, Y.G.; Gayther, S.A.; Lawrenson, K. A three-dimensional microenvironment alters protein expression and chemosensitivity of epithelial ovarian cancer cells in vitro. Lab. Investig. 2013, 93, 528–542. [Google Scholar] [CrossRef]
- Härmä, V.; Virtanen, J.; Mäkelä, R.; Happonen, A.; Mpindi, J.P.; Knuuttila, M.; Kohonen, P.; Lötjönen, J.; Kallioniemi, O.; Nees, M. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS ONE 2010, 5, e10431. [Google Scholar] [CrossRef]
- Stopa, K.B.; Kusiak, A.A.; Szopa, M.D.; Ferdek, P.E.; Jakubowska, M.A. Pancreatic cancer and its microenvironment—Recent advances and current controversies. Int. J. Mol. Sci. 2020, 21, 3218. [Google Scholar] [CrossRef]
- Greene, M.K.; Johnston, M.C.; Scott, C.J. Nanomedicine in pancreatic cancer: Current status and future opportunities for overcoming therapy resistance. Cancers 2021, 13, 6175. [Google Scholar] [CrossRef]
- Szweda, R.; Trzebicka, B.; Dworak, A.; Otulakowski, L.; Kosowski, D.; Hertlein, J.; Haladjova, E.; Rangelov, S.; Szweda, D. Smart polymeric nanocarriers of Met-enkephalin. Biomacromolecules 2016, 17, 2691–2700. [Google Scholar] [CrossRef]
- Olajubutu, O.; Ogundipe, O.D.; Adebayo, A.; Adesina, S.K. Drug Delivery Strategies for the Treatment of Pancreatic Cancer. Pharmaceutics 2023, 15, 1318. [Google Scholar] [CrossRef] [PubMed]
- Shoari, A.; Tooyserkani, R.; Tahmasebi, M.; Löwik, D.W. Delivery of various cargos into cancer cells and tissues via cell-penetrating peptides: A review of the last decade. Pharmaceutics 2021, 13, 1391. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Inkielewicz-Stepniak, I. Graphene Oxide Nanoparticles and Organoids: A Prospective Advanced Model for Pancreatic Cancer Research. Int. J. Mol. Sci. 2024, 25, 1066. [Google Scholar] [CrossRef]
- Kimbrough, C.W.; Khanal, A.; Zeiderman, M.; Khanal, B.R.; Burton, N.C.; McMasters, K.M.; Vickers, S.; Grizzle, W.E.; McNally, L.R. Targeting acidity in pancreatic adenocarcinoma: Multispectral optoacoustic tomography detects pH-low insertion peptide probes in vivo. Clin. Cancer Res. 2015, 21, 4576–4585. [Google Scholar] [CrossRef] [PubMed]
- Weerakkody, D.; Moshnikova, A.; Thakur, M.S.; Moshnikova, V.; Daniels, J.; Engelman, D.M.; Andreev, O.A.; Reshetnyak, Y.K. Family of pH (low) insertion peptides for tumor targeting. Proc. Natl. Acad. Sci. USA 2013, 110, 5834–5839. [Google Scholar] [CrossRef]
- Locasale, J.W.; Cantley, L.C. Altered metabolism in cancer. BMC Biol. 2010, 8, 88. [Google Scholar] [CrossRef]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in pancreatic cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Zhao, X.; Cao, L.; Karnad, A.; Kumar, A.P.; Freeman, J.W. Gemcitabine resistance of pancreatic cancer cells is mediated by IGF1R dependent upregulation of CD44 expression and isoform switching. Cell Death Dis. 2022, 13, 682. [Google Scholar] [CrossRef]
- Cheng, F.; McLaughlin, P.J.; Verderame, M.F.; Zagon, I.S. The OGF–OGFr axis utilizes the p16INK4a and p21WAF1/CIP1 pathways to restrict normal cell proliferation. Mol. Biol. Cell 2009, 20, 319–327. [Google Scholar] [CrossRef]
- Cheng, F.; Zagon, I.S.; Verderame, M.F.; McLaughlin, P.J. The opioid growth factor (OGF)–OGF receptor axis uses the p16 pathway to inhibit head and neck cancer. Cancer Res. 2007, 67, 10511–10518. [Google Scholar] [CrossRef] [PubMed]
- Zagon, I.S.; Roesener, C.D.; Verderame, M.F.; Ohlsson-Wilhelm, B.M.; Levin, R.J.; McLaughlin, P.J. Opioid growth factor regulates the cell cycle of human neoplasias. Int. J. Oncol. 2000, 17, 1053–1114. [Google Scholar] [CrossRef]
- Gradiz, R.; Silva, H.C.; Carvalho, L.; Botelho, M.F.; Mota-Pinto, A. MIA PaCa-2 and PANC-1—Pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci. Rep. 2016, 6, 21648. [Google Scholar] [CrossRef]
- Nakamura, N.; Sugimoto, H.; Ogata, T.; Hiraoka, K.; Yoda, H.; Sang, M.; Sang, M.; Zhu, Y.; Yu, M.; Shimozato, O.; et al. Improvement of gemcitabine sensitivity of p53-mutated pancreatic cancer MiaPaCa-2 cells by RUNX2 depletion-mediated augmentation of TAp73-dependent cell death. Oncogenesis 2016, 5, e233. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.L.; González-Hernández, J.; Coursen, J.D.; Shea, J.E.; Ngatia, J.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. Phenotype and genotype of pancreatic cancer cell lines. Pancreas 2010, 39, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Zińczuk, J.; Zaręba, K.; Guzińska-Ustymowicz, K.; Kędra, B.; Kemona, A.; Pryczynicz, A. p16, p21, and p53 proteins play an important role in development of pancreatic intraepithelial neoplastic. Ir. J. Med. Sci. 2018, 187, 629–637. [Google Scholar] [CrossRef]
- Seo, Y.H.; Joo, Y.E.; Choi, S.K.; Rew, J.S.; Park, C.S.; Kim, S.J. Prognostic significance of p21 and p53 expression in gastric cancer. Korean J. Intern. Med. 2003, 18, 98–103. [Google Scholar] [CrossRef]
- Ivanova, D.; Bakalova, R.; Lazarov, D.; Gadjeva, V.; Zhelev, Z. The impact of reactive oxygen species on anticancer therapeutic strategies. Adv. Clin. Exp. Med. 2013, 22, 899–908. [Google Scholar]
- Hamed, S.S.; Straubinger, R.M.; Jusko, W.J. Pharmacodynamic modeling of cell cycle and apoptotic effects of gemcitabine on pancreatic adenocarcinoma cells. Cancer Chemother. Pharmacol. 2003, 72, 553–563. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, J.; Zhao, C.; Guo, Y.; Wang, S.; Shen, F.; Xing, X.; Luo, Y. To control or to be controlled? Dual roles of CDK2 in DNA damage and DNA damage response. DNA Repair. 2020, 85, 102702. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and regulation of cellular senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Müllers, E.; Silva Cascales, H.; Burdova, K.; Macurek, L.; Lindqvist, A. Residual Cdk1/2 activity after DNA damage promotes senescence. Aging Cell 2017, 16, 575–584. [Google Scholar] [CrossRef]
- Song, Y.; Baba, T.; Li, Y.-Y.; Furukawa, K.; Tanabe, Y.; Matsugo, S.; Sasaki, S.; Mukaida, N. Gemcitabine-induced CXCL8 expression counteracts its actions by inducing tumor neovascularization. Biochem. Biophys. Res. Commun. 2015, 458, 341–346. [Google Scholar] [CrossRef]
- Wyld, L.; Bellantuono, I.; Tchkonia, T.; Morgan, J.; Turner, O.; Foss, F.; George, J.; Danson, S.; Kirkland, J.L. Senescence and cancer: A review of clinical implications of senescence and senotherapies. Cancers 2020, 12, 2134. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Pardo, R.; Ré, A.L.; Archange, C.; Ropolo, A.; Papademetrio, D.L.; Gonzalez, C.D.; Alvarez, E.M.; Iovanna, J.L.; Vaccaro, M.I. Gemcitabine induces the VMP1-mediated autophagy pathway to promote apoptotic death in human pancreatic cancer cells. Pancreatology 2010, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nikoletopoulou, V.; Markaki, M.; Palikaras, K.; Tavernarakis, N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta 2013, 1833, 3448–3459. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gil, M.; Turri, B.; Gabriele, M.; Pucci, L.; Agnarelli, A.; Lai, M.; Freer, G.; Pistello, M.; Vignali, R.; Batistoni, R.; et al. Protopine/gemcitabine combination induces cytotoxic or cytoprotective effects in cell type-specific and dose-dependent manner on human cancer and normal cells. Pharmaceuticals 2021, 14, 90. [Google Scholar] [CrossRef]
- Nuevemann, D.; Christgen, M.; Ungefroren, H.; Kalthoff, H. Stable expression of temperature-sensitive p53: A suitable model to study wild-type p53 function in pancreatic carcinoma cells. Oncol. Rep. 2006, 16, 575–579. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Melvin, W.S.; Bekaii-Saab, T.S.; Chen, C.S.; Muscarella, P. A structurally optimized celecoxib derivative inhibits human pancreatic cancer cell growth. J. Gastrointest. Surg. 2006, 10, 207–214. [Google Scholar] [CrossRef]
- Waissi, W.; Amé, J.C.; Mura, C.; Noël, G.; Burckel, H. Gemcitabine-based chemoradiotherapy enhanced by a PARP inhibitor in pancreatic cancer cell lines. Int. J. Mol. Sci. 2021, 22, 6825. [Google Scholar] [CrossRef] [PubMed]
- Binz, E.; Berchtold, S.; Beil, J.; Schell, M.; Geisler, C.; Smirnow, I.; Lauer, U.M. Chemovirotherapy of pancreatic adenocarcinoma by combining oncolytic vaccinia virus GLV-1h68 with nab-paclitaxel plus gemcitabine. Mol. Ther. Oncolytics 2017, 6, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, M.C.; Schmid, R.M.; Häcker, G. Analysis of the cytochrome c-dependent apoptosis apparatus in cells from human pancreatic carcinoma. Br. J. Cancer 2002, 86, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, E.; Burchell, J.M.; Dazzi, F.; Sarker, D.; Beatson, R. Apoptosis in the Pancreatic Cancer Tumor Microenvironment—The Double-Edged Sword of Cancer-Associated Fibroblasts. Cells 2021, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- Shannon, H.E.; Fishel, M.L.; Xie, J.; Gu, D.; McCarthy, B.P.; Riley, A.A.; Sinn, A.L.; Silver, J.M.; Peterman, K.; Kelley, M.R.; et al. Longitudinal bioluminescence imaging of primary versus abdominal metastatic tumor growth in orthotopic pancreatic tumor models in NSG Mice. Pancreas 2015, 44, 64. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haschemi, R.; Gockel, L.M.; Bendas, G.; Schlesinger, M. A combined activity of thrombin and p-selectin is essential for platelet activation by pancreatic Cancer cells. Int. J. Mol. Sci. 2021, 22, 3323. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Batra, S.K. Pancreatic cancer metastasis: Are we being pre-EMTed? Curr. Pharm. Des. 2015, 21, 1249–1255. [Google Scholar] [CrossRef]
- Chen, X.; Liu, F.; Xue, Q.; Weng, X.; Xu, F. Metastatic pancreatic cancer: Mechanisms and detection. Oncol. Rep. 2021, 46, 231. [Google Scholar] [CrossRef]
- Lim, M.; Park, S.; Jeong, H.O.; Park, S.H.; Kumar, S.; Jang, A.; Lee, S.; Kim, D.U.; Cho, Y.K. Circulating tumor cell clusters are cloaked with platelets and correlate with poor prognosis in unresectable pancreatic cancer. Cancers 2021, 13, 5272. [Google Scholar] [CrossRef]
- Wang, H.; Gao, J.; Bai, M.; Liu, R.; Li, H.; Deng, T.; Zhou, L.; Han, R.; Ge, S.; Huang, D.; et al. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets 2014, 25, 382–387. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, X.; Dong, S.; Han, F.; He, R.; Zhou, W. Challenges and opportunities associated with platelets in pancreatic cancer. Front. Oncol. 2022, 12, 850485. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumor metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Lucotti, S.; Muschel, R.J. Platelets and Metastasis: New Implications of an Old Interplay. Front. Oncol. 2020, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, M. Role of platelets and platelet receptors in cancer metastasis. J. Hematol. Oncol. 2018, 11, 125. [Google Scholar] [CrossRef]
- Zarà, M.; Canobbio, I.; Visconte, C.; Canino, J.; Torti, M.; Guidetti, G.F. Molecular mechanisms of platelet activation and aggregation induced by breast cancer cells. Cell. Signal. 2018, 48, 45–53. [Google Scholar] [CrossRef]



| Cell Line | IC50 ± SD | logIC50 ± SD |
|---|---|---|
| MIA PaCa-2 | 17.63 ± 2.334 | 1.25 ± 0.37 |
| AsPC-1 | 27.44 ± 9.161 | 1.44 ± 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Budka, J.; Debowski, D.; Mai, S.; Narajczyk, M.; Hac, S.; Rolka, K.; Vrettos, E.I.; Tzakos, A.G.; Inkielewicz-Stepniak, I. Design, Synthesis, and Antitumor Evaluation of an Opioid Growth Factor Bioconjugate Targeting Pancreatic Ductal Adenocarcinoma. Pharmaceutics 2024, 16, 283. https://doi.org/10.3390/pharmaceutics16020283
Budka J, Debowski D, Mai S, Narajczyk M, Hac S, Rolka K, Vrettos EI, Tzakos AG, Inkielewicz-Stepniak I. Design, Synthesis, and Antitumor Evaluation of an Opioid Growth Factor Bioconjugate Targeting Pancreatic Ductal Adenocarcinoma. Pharmaceutics. 2024; 16(2):283. https://doi.org/10.3390/pharmaceutics16020283
Chicago/Turabian StyleBudka, Justyna, Dawid Debowski, Shaoshan Mai, Magdalena Narajczyk, Stanislaw Hac, Krzysztof Rolka, Eirinaios I. Vrettos, Andreas G. Tzakos, and Iwona Inkielewicz-Stepniak. 2024. "Design, Synthesis, and Antitumor Evaluation of an Opioid Growth Factor Bioconjugate Targeting Pancreatic Ductal Adenocarcinoma" Pharmaceutics 16, no. 2: 283. https://doi.org/10.3390/pharmaceutics16020283
APA StyleBudka, J., Debowski, D., Mai, S., Narajczyk, M., Hac, S., Rolka, K., Vrettos, E. I., Tzakos, A. G., & Inkielewicz-Stepniak, I. (2024). Design, Synthesis, and Antitumor Evaluation of an Opioid Growth Factor Bioconjugate Targeting Pancreatic Ductal Adenocarcinoma. Pharmaceutics, 16(2), 283. https://doi.org/10.3390/pharmaceutics16020283







