Recent Developments in CaCO3 Nano-Drug Delivery Systems: Advancing Biomedicine in Tumor Diagnosis and Treatment
Abstract
1. Introduction
2. The Synthesis Methods for CaCO3 Nanoparticles
2.1. Chemical Precipitation Method
2.2. Gas Diffusion Method
2.3. Microemulsion Method
2.4. Bio-Based Preparation Method
3. The Effects and Mechanisms of CaCO3 Nanoparticles in Cancer Diagnosis and Treatment
3.1. Acidity Modulation
3.2. Calcium Overload
3.3. Facilitating Lysosome Escape and Intracellular Drug Release
3.4. Tumor Immunomodulation
3.5. Magnetic Resonance Imaging and Ultrasound Contrast Enhancers
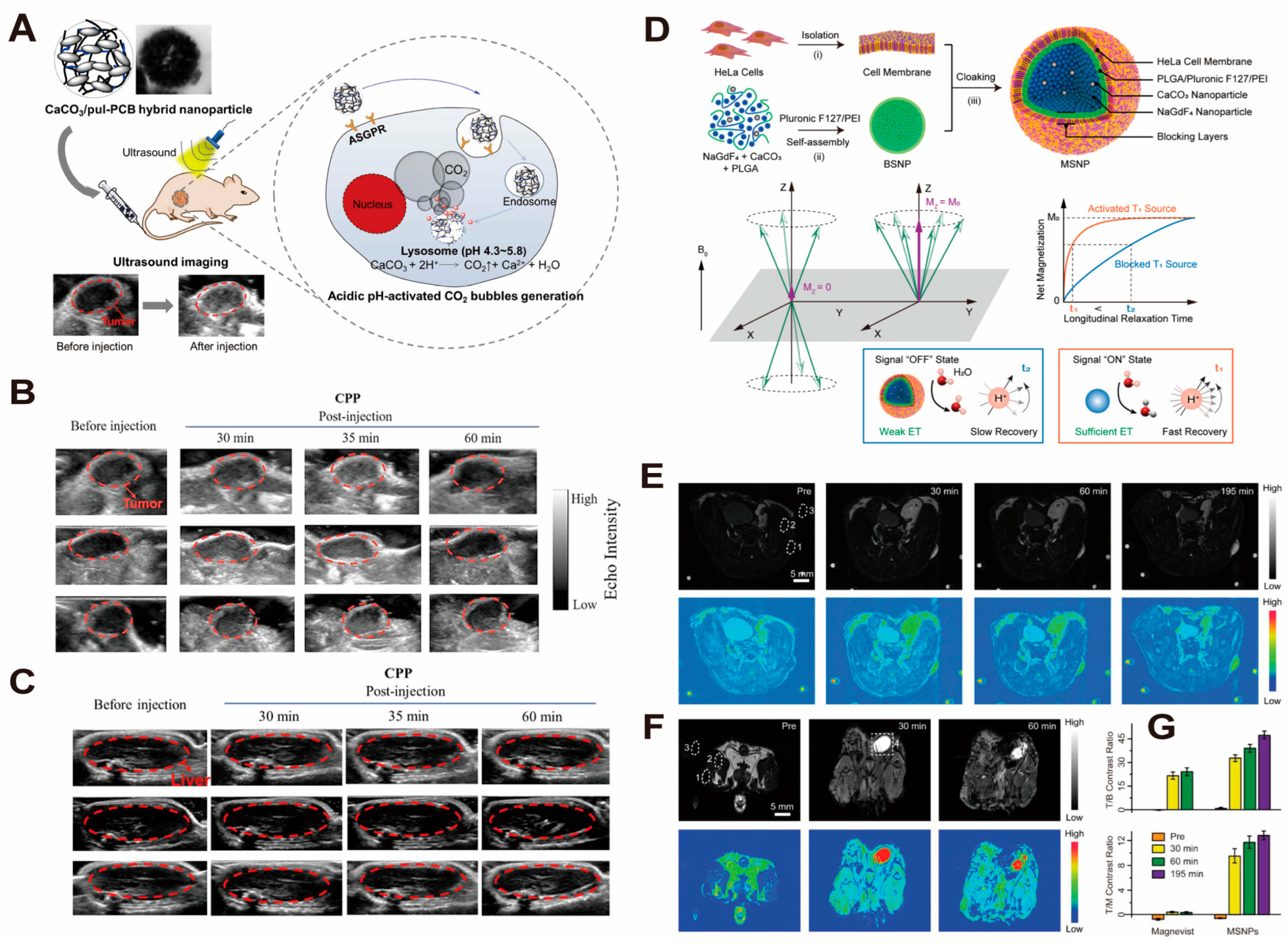
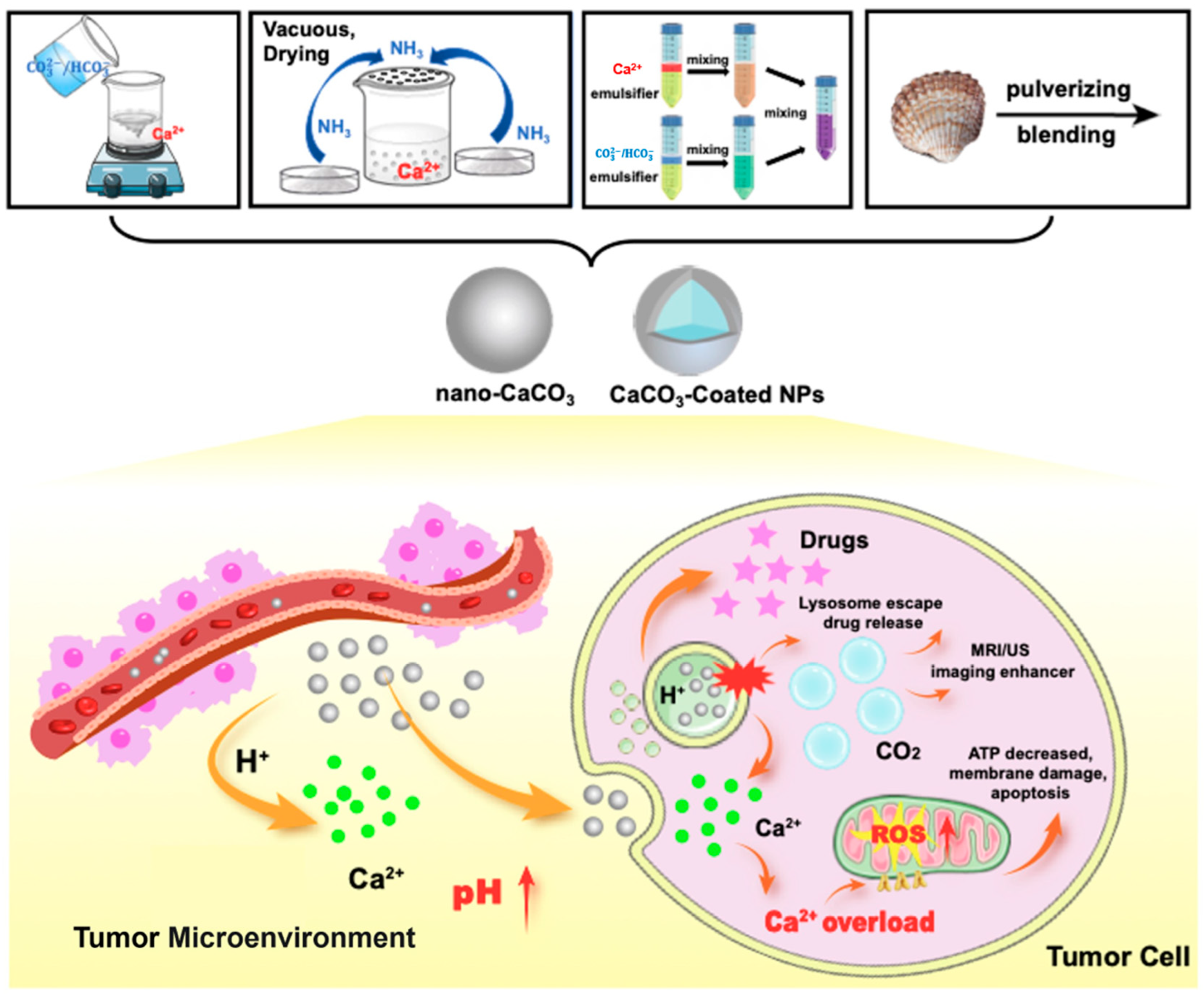
4. CaCO3-Based Nano-Drug Delivery Systems for Tumor Diagnosis and Treatment
4.1. Small-Molecule Drug Nano-Drug Delivery Systems
4.2. Biomolecular Drug Nano-Drug Delivery Systems
4.3. Surface Mineralization Nano-Drug Delivery Systems
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Fathi, N.; Rashidi, G.; Khodadadi, A.; Shahi, S.; Sharifi, S. STAT3 and apoptosis challenges in cancer. Int. J. Biol. Macromol. 2018, 117, 993–1001. [Google Scholar] [CrossRef]
- Lu, J.; Liu, X.; Liao, Y.-P.; Wang, X.; Ahmed, A.; Jiang, W.; Ji, Y.; Meng, H.; Nel, A.E. Retraction of “Breast Cancer Chemo-immunotherapy through Liposomal Delivery of an Immunogenic Cell Death Stimulus Plus Interference in the IDO-1 Pathway”. ACS Nano 2021, 15, 10735. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Li, H.J.; Chen, K.G.; Wang, Y.C.; Yang, X.Z.; Lian, Z.X.; Du, J.Z.; Wang, J. Spatial Targeting of Tumor-Associated Macrophages and Tumor Cells with a pH-Sensitive Cluster Nanocarrier for Cancer Chemoimmunotherapy. Nano Lett. 2017, 17, 3822–3829. [Google Scholar] [CrossRef] [PubMed]
- National Nanotechnology Initiative (NNI). National Nanotechnology Initiative. 2024. Available online: https://www.nano.gov/ (accessed on 1 February 2024).
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Dreher, M.R.; Liu, W.; Michelich, C.R.; Dewhirst, M.W.; Yuan, F.; Chilkoti, A. Tumor Vascular Permeability, Accumulation, and Penetration of Macromolecular Drug Carriers. J. Natl. Cancer Inst. 2006, 98, 335–344. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Kumar, M.N.R. Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharm. Sci. 2000, 3, 234–258. [Google Scholar]
- Paciotti, G.F.; Kingston, D.G.I.; Tamarkin, L. Colloidal gold nanoparticles: A novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006, 67, 47–54. [Google Scholar] [CrossRef]
- Anglin, E.J.; Cheng, L.; Freeman, W.R.; Sailor, M.J. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 2008, 60, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Morales, M.A.; Sahoo, S.K.; Leslie-Pelecky, D.L.; Labhasetwar, V. Iron Oxide Nanoparticles for Sustained Delivery of Anticancer Agents. Mol. Pharm. 2005, 2, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.; Traykova, T.; Planell, J. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Avaro, J.T.; Ruiz-Agudo, C.; Landwehr, E.; Hauser, K.; Gebauer, D. Impurity-free amorphous calcium carbonate, a preferential material for pharmaceutical and medical applications. Eur. J. Miner. 2019, 31, 231–236. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Cartwright, J.H.E.; Checa, A.G.; Gale, J.D.; Gebauer, D.; Sainz-Díaz, C.I. Calcium carbonate polyamorphism and its role in biomineralization: How many amorphous calcium carbonates are there? Angew. Chem. Int. Ed. 2012, 51, 11960–11970. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, Y.; Guo, R. Amphiphilic Phosphoprotein-Controlled Formation of Amorphous Calcium Carbonate with Hierarchical Superstructure. Langmuir 2012, 28, 6097–6105. [Google Scholar] [CrossRef]
- Saharay, M.; Yazaydin, A.O.; Kirkpatrick, R.J. Dehydration-Induced Amorphous Phases of Calcium Carbonate. J. Phys. Chem. B 2013, 117, 3328–3336. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Y.; Wang, C.; Hao, Y.; Fan, Q.; Yang, Z.; Li, Q.; Feng, L.; Liu, Z. Tumor Microenvironment Modulating CaCO3-Based Colloidosomal Microreactors Can Generally Reinforce Cancer Immunotherapy. Adv. Mater. 2023, 35, e2308254. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; He, J.; Wang, J.; Li, Y.; Xu, Z.; Zhang, L.; Kang, Y.; Xue, P. Calcium carbonate-actuated ion homeostasis perturbator for oxidative damage-augmented Ca2+/Mg2+ interference therapy. Biomaterials 2023, 302, 122340. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, L.; Zhu, W.; Sun, X.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO3 nanoparticles as an ultra-sensitive tumor-pH-responsive nanoplatform enabling real-time drug release monitoring and cancer combination therapy. Biomaterials 2016, 110, 60–70. [Google Scholar] [CrossRef]
- Som, A.; Raliya, R.; Tian, L.; Akers, W.; Ippolito, J.E.; Singamaneni, S.; Biswas, P.; Achilefu, S. Monodispersed calcium carbonate nanoparticles modulate local pH and inhibit tumor growth in vivo. Nanoscale 2016, 8, 12639–12647. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Sharifi, S.; Ahmadian, E.; Eftekhari, A.; Adibkia, K.; Lotfipour, F. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin. Drug Deliv. 2019, 16, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Min, H.S.; Lee, H.J.; Park, D.J.; Yhee, J.Y.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Silvestre, O.F.; Chen, X.; et al. pH-controlled gas-generating mineralized nanoparticles: A theranostic agent for ultrasound imaging and therapy of cancers. ACS Nano 2015, 9, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, R.; Liu, Y.; Yi, Z.; Meng, X.; Zhang, J.; Tang, Z.; Yao, Z.; Liu, Y.; Liu, X.; et al. Calcium-Overload-Mediated Tumor Therapy by Calcium Peroxide Nanoparticles. Chem 2019, 5, 2171–2182. [Google Scholar] [CrossRef]
- Kamba, A.S.; Ismail, M.; Ibrahim, T.A.T.; Zakaria, Z.A.B. A pH-Sensitive, Biobased Calcium Carbonate Aragonite Nanocrystal as a Novel Anticancer Delivery System. BioMed Res. Int. 2013, 2013, 587451. [Google Scholar] [CrossRef]
- Islam, K.N.; Zuki, A.B.Z.; Ali, M.E.; Bin Hussein, M.Z.; Noordin, M.M.; Loqman, M.Y.; Wahid, H.; Hakim, M.A.; Hamid, S.B.A. Facile Synthesis of Calcium Carbonate Nanoparticles from Cockle Shells. J. Nanomater. 2012, 2012, 534010. [Google Scholar] [CrossRef]
- Ueno, Y.; Futagawa, H.; Takagi, Y.; Ueno, A.; Mizushima, Y. Drug-incorporating calcium carbonate nanoparticles for a new delivery system. J Control. Release 2005, 103, 93–98. [Google Scholar] [CrossRef]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.; Carregal-Romero, S.; Rejman, J.; Möhwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotechnol. 2015, 13, 53. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, W.; Yang, X.; Li, Y.; Hao, Y.; Zhang, H.; Hou, L.; Zhang, Z. pH/Ultrasound Dual-Responsive Gas Generator for Ultrasound Imaging-Guided Therapeutic Inertial Cavitation and Sonodynamic Therapy. Adv. Health Mater. 2018, 7, 1700957. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Hu, Q.; Wen, D.; Chen, Q.; Chen, G.; Lu, Y.; Wang, J.; Cheng, H.; Lu, W.; Gu, Z. A Dual-Bioresponsive Drug-Delivery Depot for Combination of Epigenetic Modulation and Immune Checkpoint Blockade. Adv. Mater. 2019, 31, e1806957. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Han, W.; Feng, L.; Wei, Z.; Liu, Y.; Zhang, H.; Zhang, S. pH-programmed responsive nanoplatform for synergistic cancer therapy based on single atom catalysts. Eur. J. Med. Chem. 2022, 233, 114236. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Yang, X.; Wang, B.; Cao, L.; Fang, Z.; Li, Z.; Liu, H.; Liang, X.-J.; Zhang, J.; Jin, Y. Biomineralized hydrogel DC vaccine for cancer immunotherapy: A boosting strategy via improving immunogenicity and reversing immune-inhibitory microenvironment. Biomaterials 2022, 288, 121722. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Ye, J.; Guo, Z.; Lu, J.; Xu, W.; Gao, X.; Huang, H.; Hu, R.; Mao, L.; Wei, Y.; et al. TME-responded Full-biodegradable nanocatalyst for mitochondrial calcium Overload-induced hydroxyl radical bursting cancer treatment. Chem. Eng. J. 2022, 438, 135372. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, L.; Li, D.; Lao, Y.-H.; Liu, D.; Li, M.; Ding, J.; Chen, X. Tumor microenvironment-responsive hyaluronate-calcium carbonate hybrid nanoparticle enables effective chemotherapy for primary and advanced osteosarcomas. Nano Res. 2018, 11, 4806–4822. [Google Scholar] [CrossRef]
- Kim, H.J.; Min, K.H.; Lee, H.J.; Hwang, Y.-S.; Lee, S.C. Fenton-like reaction performing mineralized nanocarriers as oxidative stress amplifying anticancer agents. J. Ind. Eng. Chem. 2019, 80, 829–837. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Lu, S.; Yang, L.; Yu, S.; Yang, H. CaCO3-Encapsulated Au Nanoparticles Modulate Macrophages toward M1-like Phenotype. ACS Appl. Bio Mater. 2021, 4, 3214–3223. [Google Scholar] [CrossRef]
- An, J.; Zhang, K.; Wang, B.; Wu, S.; Wang, Y.; Zhang, H.; Zhang, Z.; Liu, J.; Shi, J. Nanoenabled Disruption of Multiple Barriers in Antigen Cross-Presentation of Dendritic Cells via Calcium Interference for Enhanced Chemo-Immunotherapy. ACS Nano 2020, 14, 7639–7650. [Google Scholar] [CrossRef]
- Li, K.; Li, D.; Zhao, L.; Chang, Y.; Zhang, Y.; Cui, Y.; Zhang, Z. Calcium-mineralized polypeptide nanoparticle for intracellular drug delivery in osteosarcoma chemotherapy. Bioact. Mater. 2020, 5, 721–731. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, L.; Lv, F.; Li, W.; Li, Y.; Dong, Y. A Glycosylated Covalent Organic Framework Equipped with BODIPY and CaCO3 for Synergistic Tumor Therapy. Angew. Chem. Int. Ed. 2020, 59, 18042–18047. [Google Scholar] [CrossRef]
- Zhao, Q.; Gong, Z.; Li, Z.; Wang, J.; Zhang, J.; Zhao, Z.; Zhang, P.; Zheng, S.; Miron, R.J.; Yuan, Q.; et al. Target Reprogramming Lysosomes of CD8+ T Cells by a Mineralized Metal-Organic Framework for Cancer Immunotherapy. Adv. Mater. 2021, 33, e2100616. [Google Scholar] [CrossRef]
- Wang, P.; Xue, J.; Wu, S.; Pei, Y.; Xu, L.; Wang, Y. Cell-Friendly Isolation and pH-Sensitive Controllable Release of Circulating Tumor Cells by Fe3O4@CaCO3 Nanoplatform. Adv. Mater. Interfaces 2021, 8, 2101191. [Google Scholar] [CrossRef]
- Wang, S.; Ni, D.; Yue, H.; Luo, N.; Xi, X.; Wang, Y.; Shi, M.; Wei, W.; Ma, G. Exploration of Antigen Induced CaCO3 Nanoparticles for Therapeutic Vaccine. Small 2018, 14, e1704272. [Google Scholar] [CrossRef]
- Vidallon, M.L.P.; Douek, A.M.; Quek, A.; McLiesh, H.; Kaslin, J.; Tabor, R.F.; Bishop, A.I.; Teo, B.M. Gas-Generating, pH-Responsive Calcium Carbonate Hybrid Particles with Biomimetic Coating for Contrast-Enhanced Ultrasound Imaging. Part. Part. Syst. Charact. 2020, 37, 1900471. [Google Scholar] [CrossRef]
- Ju, Y.; Zhao, Y.; Guan, Q.; Yang, S.; Wang, W.; Yan, B.; Meng, Y.; Li, S.; Tang, P.; Mao, L.; et al. Amorphous Calcium Carbonate Cluster Nanospheres in Water-Deficient Organic Solvents. Angew. Chem. Int. Ed. 2022, 61, e202211254. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Z.; Li, M.; Qu, Q.; Ma, X.; Yu, S.H.; Zhao, Y. A Preloaded Amorphous Calcium Carbonate/Doxorubicin@Silica Nanoreactor for pH-Responsive Delivery of an Anticancer Drug. Angew. Chem. Int. Ed. 2015, 54, 919–922. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Wang, Y.; Liu, X.; Hu, F.; Sun, J.; Yuan, H. Lipase-Triggered Water-Responsive “Pandora’s Box” for Cancer Therapy: Toward Induced Neighboring Effect and Enhanced Drug Penetration. Adv. Mater. 2018, 30, e1706407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.K.; Li, C.X.; Wang, S.B.; Liu, T.; Song, X.L.; Yang, X.Q.; Feng, J.; Zhang, X.-Z. Tumor Starvation Induced Spatiotemporal Control over Chemotherapy for Synergistic Therapy. Small 2018, 14, e1803602. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wu, J.; He, M.; Hou, X.; Wang, Y.; Cai, X.; Xin, H.; Gao, F.; Chen, Y. Combined Cancer Chemo-Photodynamic and Photothermal Therapy Based on ICG/PDA/TPZ-Loaded Nanoparticles. Mol. Pharm. 2019, 16, 2172–2183. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Ma, H.; Yu, F.; Hu, F.; Yuan, H. Water-Responsive Hybrid Nanoparticles Codelivering ICG and DOX Effec-tively Treat Breast Cancer via Hyperthermia-aided DOX Functionality and Drug Penetration. Adv. Healthc. Mater. 2019, 8, e1801486. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Yu, F.; Lv, J.; Zhao, R.; Hu, F.; Yuan, H. Dual-Channel Theranostic System for Quantitative Self-Indication and Low-Temperature Synergistic Therapy of Cancer. Small 2021, 17, e2007953. [Google Scholar] [CrossRef]
- Wan, X.; Zhong, H.; Pan, W.; Li, Y.; Chen, Y.; Li, N.; Tang, B. Programmed Release of Dihydroartemisinin for Synergistic Cancer Therapy Using a CaCO3 Mineralized Metal–Organic Framework. Angew. Chem. Int. Ed. 2019, 58, 14134–14139. [Google Scholar] [CrossRef]
- Chang, M.; Hou, Z.; Jin, D.; Zhou, J.; Wang, M.; Wang, M.; Shu, M.; Ding, B.; Li, C.; Lin, J. Colorectal Tumor Microenvironment-Activated Bio-Decomposable and Metabolizable Cu2O@CaCO3 Nanocomposites for Synergistic Oncotherapy. Adv. Mater. 2020, 32, e2004647. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, L.; Hao, Y.; Li, Q.; Chen, M.; Yang, Z.; Zhao, H.; Liu, Z. Synthesis of CaCO3-Based Nanomedicine for Enhanced Sonodynamic Therapy via Amplification of Tumor Oxidative Stress. Chem 2020, 6, 1391–1407. [Google Scholar] [CrossRef]
- Dong, Z.; Hao, Y.; Li, Q.; Yang, Z.; Zhu, Y.; Liu, Z.; Feng, L. Metal-polyphenol-network coated CaCO3 as pH-responsive nanocarriers to enable effective intratumoral penetration and reversal of multidrug resistance for augmented cancer treatments. Nano Res. 2020, 13, 3057–3067. [Google Scholar] [CrossRef]
- Li, Y.; Gong, S.; Pan, W.; Chen, Y.; Liu, B.; Li, N.; Tang, B. A tumor acidity activatable and Ca2+-assisted immuno-nanoagent enhances breast cancer therapy and suppresses cancer recurrence. Chem. Sci. 2020, 11, 7429–7437. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, B.; Wang, L.; Zhou, F.; Smith, N.; Saunders, D.; Towner, R.A.; Song, J.; Qu, J.; Chen, W.R. Biodegradable pH-responsive amorphous calcium carbonate nanoparticles as immunoadjuvants for multimodal imaging and enhanced photoimmunotherapy. J. Mater. Chem. B 2020, 8, 8261–8270. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, S.; Song, H.; Yu, T.; Zheng, X.; Chu, Q.; Wang, Y. CaCO3 nanoparticles incorporated with KAE to enable amplified calcium overload cancer therapy. Biomaterials 2021, 277, 121080. [Google Scholar] [CrossRef]
- Xue, C.; Li, M.; Sutrisno, L.; Yan, B.; Zhao, Y.; Hu, Y.; Cai, K.; Zhao, Y.; Yu, S.; Luo, Z. Bioresorbable Scaffolds with Biocatalytic Chemotherapy and In Situ Microenvironment Modulation for Postoperative Tissue Repair. Adv. Funct. Mater. 2021, 31, 2008732. [Google Scholar] [CrossRef]
- Zheng, C.; Song, Q.; Zhao, H.; Kong, Y.; Sun, L.; Liu, X.; Feng, Q.; Wang, L. A nanoplatform to boost multi-phases of cancer-immunity-cycle for enhancing immunotherapy. J. Control. Release 2021, 339, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Ding, B.; Jiang, Z.; Xu, W.; Li, G.; Ding, J.; Chen, X. Ultrasound-Augmented Mitochondrial Calcium Ion Overload by Calcium Nanomodulator to Induce Immunogenic Cell Death. Nano Lett. 2021, 21, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Ding, B.; Shi, R.; Jiang, Z.; Xu, W.; Li, G.; Ding, J.; Chen, X. A Multichannel Ca2+ Nanomodulator for Multilevel Mito-chondrial Destruction-Mediated Cancer Therapy. Adv. Mater. 2021, 33, e2007426. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Qin, R.; Xu, L.; Ma, X.; Ding, D.; Li, S.; Chen, L.; Liu, Y.; Sun, W.; Chen, H. Ion drugs for precise orthotopic tumor management by in situ the generation of toxic ion and drug pools. Theranostics 2022, 12, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Z.; Tan, M.; Liu, W.; Zhang, L.; Huang, J.; Cao, Y.; Li, P.; Wang, Z.; Wen, J.; et al. pH-Responsive Nano-particles for Enhanced Antitumor Activity by High-Intensity Focused Ultrasound Therapy Combined with Sonodynamic Therapy. Int. J. Nanomed. 2022, 17, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, C.; Dong, Z.; Hu, C.; Feng, L. Lipid-coated CaCO3-PDA nanoparticles as a versatile nanocarrier to enable pH-responsive dual modal imaging-guided combination cancer therapy. J. Mater. Chem. B 2022, 10, 4096–4104. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Liao, X.; Wu, W.; Feng, T.; Karges, J.; Lin, M.; Luo, H.; Chen, Y.; Chao, H. A pH-responsive iridium(iii) two-photon photosensitizer loaded CaCO3 nanoplatform for combined Ca2+ overload and photodynamic therapy. Inorg. Chem. Front. 2022, 9, 4171–4183. [Google Scholar] [CrossRef]
- Wu, J.; Cai, X.; Williams, G.R.; Meng, Z.; Zou, W.; Yao, L.; Hu, B.; Chen, Y.; Zheng, Y. 2D antimonene-integrated composite nanomedicine for augmented low-temperature photonic tumor hyperthermia by reversing cell thermoresistance. Bioact. Mater. 2022, 10, 295–305. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, J.; Mu, Y.; Foda, M.F.; Han, H. Activation of TRPV1 by capsaicin-loaded CaCO3 nanoparticle for tumor-specific therapy. Biomaterials 2022, 284, 121520. [Google Scholar] [CrossRef]
- Zhou, Y.; Jing, S.; Liu, S.; Shen, X.; Cai, L.; Zhu, C.; Zhao, Y.; Pang, M. Double-activation of mitochondrial permeability transition pore opening via calcium overload and reactive oxygen species for cancer therapy. J. Nanobiotechnol. 2022, 20, 188. [Google Scholar] [CrossRef]
- Zheng, P.; Ding, B.; Zhu, G.; Li, C.; Lin, J. Biodegradable Ca2+ Nanomodulators Activate Pyroptosis through Mitochondrial Ca2+ Overload for Cancer Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202204904. [Google Scholar] [CrossRef]
- Deng, G.; Wu, Y.; Song, Z.; Li, S.; Du, M.; Deng, J.; Xu, Q.; Deng, L.; Bahlol, H.S.; Han, H. Tea Polyphenol Liposomes Overcome Gastric Mucus to Treat Helicobacter Pylori Infection and Enhance the Intestinal Microenvironment. ACS Appl. Mater. Interfaces 2022, 14, 13001–13012. [Google Scholar] [CrossRef]
- Stawski, T.M.; Roncal-Herrero, T.; Fernandez-Martinez, A.; Matamoros-Veloza, A.; Kröger, R.; Benning, L.G. On demand” triggered crystallization of CaCO3 from solute precursor species stabilized by the water-in-oil microemulsion. Phys. Chem. Chem. Phys. 2018, 20, 13825–13835. [Google Scholar] [CrossRef]
- Shen, Y.; Xie, A.; Chen, Z.; Xu, W.; Yao, H.; Li, S.; Huang, L.; Wu, Z.; Kong, X. Controlled synthesis of calcium carbonate nanocrystals with multi-morphologies in different bicontinuous microemulsions. Mater. Sci. Eng. A 2007, 443, 95–100. [Google Scholar] [CrossRef]
- Jang, H.J.; Jeong, E.J.; Lee, K.Y. Carbon Dioxide-Generating PLG Nanoparticles for Controlled Anti-Cancer Drug Delivery. Pharm. Res. 2018, 35, 59. [Google Scholar] [CrossRef]
- Zhao, P.; Li, M.; Wang, Y.; Chen, Y.; He, C.; Zhang, X.; Yang, T.; Lu, Y.; You, J.; Lee, R.J.; et al. Enhancing anti-tumor efficiency in hepatocellular carcinoma through the autophagy inhibition by miR-375/sorafenib in lipid-coated calcium carbonate nanoparticles. Acta Biomater. 2018, 72, 248–255. [Google Scholar] [CrossRef]
- Chen, Y.; Du, Q.; Guo, Q.; Huang, J.; Liu, L.; Shen, X.; Peng, J. A W/O emulsion mediated film dispersion method for curcumin encapsulated pH-sensitive liposomes in the colon tumor treatment. Drug Dev. Ind. Pharm. 2019, 45, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.W.; Zhao, P.; Khan, A.; Raza, F.; Raza, S.M.; Sarfraz, M.; Chen, Y.; Li, M.; Yang, T.; Ma, X.; et al. Synergism of cisplatin-oleanolic acid co-loaded calcium carbonate nanoparticles on hepatocellular carcinoma cells for enhanced apoptosis and reduced hepatotoxicity. Int. J. Nanomed. 2019, 14, 3753–3771. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Zhang, J.; Li, X.; Shi, P.; Fu, P. High density lipoprotein coated calcium carbonate nanoparticle for chemotherapy of breast cancer. J. Biomater. Appl. 2019, 34, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Lin, X.; Zhuang, S.; Wu, Y.; Liu, Z. CaCO3 nanoparticles pH-sensitively induce blood coagulation as a potential strategy for starving tumor therapy. J. Mater. Chem. B 2020, 8, 1223–1234. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Z.; Dong, Z.; Gong, Y.; Hao, Y.; Tian, L.; Yang, X.; Liu, Z.; Feng, L. CaCO3-Assisted Preparation of pH-Responsive Immune-Modulating Nanoparticles for Augmented Chemo-Immunotherapy. Nano-Micro Lett. 2020, 13, 29. [Google Scholar] [CrossRef]
- Khan, M.W.; Zou, C.; Hassan, S.; Din, F.U.; Razak, M.Y.A.; Nawaz, A.; Alam, Z.; Wahab, A.; Bangash, S.A. Cisplatin and oleanolic acid Co-loaded pH-sensitive CaCO3 nanoparticles for synergistic chemotherapy. RSC Adv. 2022, 12, 14808–14818. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, X.; Zhu, Y.; Lv, X.; Wang, P.; Feng, L. pH-responsive nanomedicine co-encapsulated with Erlotinib and chlorin e6 can enable effective treatment of triple negative breast cancer via reprogramming tumor vasculature. Chem. Eng. J. 2022, 437, 135305. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, Y.; Sun, Q. Synthesis of precipitated calcium carbonate nanoparticles using a two-membrane system. Colloid J. 2004, 66, 745–750. [Google Scholar] [CrossRef]
- Hamidu, A.; Mokrish, A.; Mansor, R.; Razak, I.S.A.; Danmaigoro, A.; Jaji, A.Z.; Bakar, Z.A. Modified methods of nanoparticles synthesis in pH-sensitive nano-carriers production for doxorubicin delivery on MCF-7 breast cancer cell line. Int. J. Nanomed. 2019, 14, 3615–3627. [Google Scholar]
- Jangili, P.; Kong, N.; Kim, J.H.; Zhou, J.; Liu, H.; Zhang, X.; Tao, W.; Kim, J.S. DNA-Damage-Response-Targeting Mitochon-dria-Activated Multifunctional Prodrug Strategy for Self-Defensive Tumor Therapy. Angew. Chem. Int. Ed. 2022, 61, e202117075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, D.; Guo, X.; Jin, L.; Zheng, J.; Wang, Y.; Chen, S.; Zheng, X.; Zeng, L.; Guo, M.; et al. Fighting Immune Cold and Reprogramming Immunosuppressive Tumor Microenvironment with Red Blood Cell Membrane-Camouflaged Nanobullets. ACS Nano 2020, 14, 17442–17457. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.-C.; Li, M.-H.; Zhao, Y.; Zhou, J.; Hu, Y.; Cai, K.-Y.; Zhao, Y.; Yu, S.-H.; Luo, Z. Tumor microenvironment-activatable Fe-doxorubicin preloaded amorphous CaCO3 nanoformulation triggers ferroptosis in target tumor cells. Sci. Adv. 2020, 6, eaax1346. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, Z.; Hao, Y.; Zhu, Y.; Ni, J.; Li, Q.; Liu, B.; Han, Y.; Yang, Z.; Wan, J.; et al. Coordination Polymer-Coated CaCO3 Reinforces Radiotherapy by Reprogramming the Immunosuppressive Metabolic Microenvironment. Adv. Mater. 2022, 34, e2106520. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, C.; Zhang, X.; Chen, G.; Hu, Q.; Li, H.; Wang, J.; Wen, D.; Zhang, Y.; Lu, Y.; et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 2019, 14, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Dai, X.; Liu, K.; Liu, Y.; Wu, J.; Wang, K.; Jiang, S.; Sun, F.; Wang, L.; Guo, B.; et al. A Self-Reinforcing Nanoplatform for Highly Effective Synergistic Targeted Combinatary Calcium-Overload and Photodynamic Therapy of Cancer. Adv. Healthc. Mater. 2023, 12, e2202424. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Zhivotovsky, B.; Nicotera, P. Regulation of cell death: The calcium–apoptosis link. Nat. Rev. Mol. Cell Biol. 2003, 4, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhou, J.; Chen, Z.; Luo, Q.; Xu, J.; Song, G. Tumor-Specific Expansion of Oxidative Stress by Glutathione Depletion and Use of a Fenton Nanoagent for Enhanced Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 30551–30565. [Google Scholar] [CrossRef]
- Ermak, G.; Davies, K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002, 38, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiao, A.; Li, Q.; Lv, X.; Wang, X.; Song, X.; Li, B.; Zhang, Y.; Dong, X. Mitochondrial Ca2+-overloading by oxygen/glutathione depletion-boosted photodynamic therapy based on a CaCO3 nanoplatform for tumor synergistic therapy. Acta Biomater. 2022, 137, 252–261. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xing, X.; Wang, X.; Wu, D.; Wu, W.; Guo, J.; Mitragotri, S. Nanocarrier-Mediated Cytosolic Delivery of Biopharmaceuticals. Adv. Funct. Mater. 2020, 30, 1910566. [Google Scholar] [CrossRef]
- Vermeulen, L.M.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. The proton sponge hypothesis: Fable or fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef]
- Mindell, J.A. Lysosomal Acidification Mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, M.; Wu, Y.; Dong, Z.; Zhu, Y.; Wang, C.; Li, Q.; Yang, Z.; Liu, Z.; Feng, L. CaCO3 based proton nanosponge to potentiate immune checkpoint blockade therapy by synergistically reversing tumor immunosuppression. Chem. Eng. J. 2023, 462, 142206. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, X.; Wang, B.; Tan, L.; Zhang, Y.; Jiao, Y. Light/gas cascade-propelled Janus micromotors that actively overcome sequential and multi-staged biological barriers for precise drug delivery. Chem. Eng. J. 2021, 408, 127897. [Google Scholar] [CrossRef]
- Bai, S.; Lan, Y.; Fu, S.; Cheng, H.; Lu, Z.; Liu, G. Connecting Calcium-Based Nanomaterials and Cancer: From Diagnosis to Therapy. Nano-Micro Lett. 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, M.; Zhao, L.; Lu, W.; Wu, S.; Zhang, K.; Liu, J.; Zhang, Z.; Shi, J. Boosting Tumor Immunotherapy by Bioactive Nanoparticles via Ca2+ Interference Mediated TME Reprogramming and Specific PD-L1 Depletion. Adv. Funct. Mater. 2022, 32, 2201275. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, K.; Wong, D.S.H.; Han, F.; Li, B.; Bian, L. Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials 2018, 178, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Dai, Z.; Tang, J.; Gu, Z.; Wang, Y.; Yang, Y.; Yang, Y.; Yu, C. Eliciting Immunogenic Cell Death via a Unitized Nanoinducer. Nano Lett. 2020, 20, 6246–6254. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, G.; Zheng, H.; Mu, D.; Chen, H.; Lu, Z.; He, P.; Zhang, Y.; Liu, C.; Lin, Z.; et al. Biomimetic nanoparticles blocking autophagy for enhanced chemotherapy and metastasis inhibition via reversing focal adhesion disassembly. J. Nanobiotechnol. 2021, 19, 447. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Liu, J.; Lin, G.; Xie, F.; Pang, X.; Pei, Y.; Cheng, Y.; Zhang, Y.; Lin, Z.; et al. Oxidative stress-driven DR5 upregulation restores TRAIL/Apo2L sensitivity induced by iron oxide nanoparticles in colorectal cancer. Biomaterials 2020, 233, 119753. [Google Scholar] [CrossRef]
- Guan, Y.H.; Wang, N.; Deng, Z.W.; Chen, X.G.; Liu, Y. Exploiting autophagy-regulative nanomaterials for activation of den-dritic cells enables reinforced cancer immunotherapy. Biomaterials 2022, 282, 121434. [Google Scholar] [CrossRef]
- Crawford, S.E.; Estes, M.K. Viroporin-mediated calcium-activated autophagy. Autophagy 2013, 9, 797–798. [Google Scholar] [CrossRef][Green Version]
- Casanova-Acebes, M.; Dalla, E.; Leader, A.M.; LeBerichel, J.; Nikolic, J.; Morales, B.M.; Brown, M.; Chang, C.; Troncoso, L.; Chen, S.T.; et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature 2021, 595, 578–584. [Google Scholar] [CrossRef]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the maintenance of homeostasis. Cell. Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, T.; Hu, R.; Zhu, R.; Li, C.; Ruan, Y.; Xie, X.; Li, Y. Next frontier in tumor immunotherapy: Macrophage-mediated immune evasion. Biomark. Res. 2021, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T.; et al. Chloroquine modulates antitumor immune response by resetting tumor-associated macrophages toward M1 phenotype. Nat. Commun. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xia, D.; Ni, Z.; Ou, T.; Wang, Y.; Zhang, H.; Mao, L.; Lin, K.; Xu, S.; Liu, J. Calcium silicate bioactive ceramics induce osteogenesis through oncostatin M. Bioact. Mater. 2021, 6, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Min, K.H.; Lee, H.J.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Lee, S.C. Photosensitizer-loaded bubble-generating mineralized nanoparticles for ultrasound imaging and photodynamic therapy. J. Mater. Chem. B 2016, 4, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, X.L.; Zhou, B.; Tian, J.; Luo, B.M.; Zhang, L.M. Acidic pH-Activated Gas-Generating Nanoparticles with Pullulan Decorating for Hepatoma-Targeted Ultrasound Imaging. ACS Appl. Mater. Interfaces 2019, 11, 22194–22205. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, W.; Liu, Z.; Guo, H.; Zhang, P. Smart responsive-calcium carbonate nanoparticles for dual-model cancer imaging and treatment. Ultrasonics 2020, 108, 106198. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Luo, Z.; Barth, N.D.; Meng, X.; Liu, H.; Bu, W.; All, A.; Vendrell, M.; Liu, X. In Vivo Tumor Visualization through MRI Off-On Switching of NaGdF4-CaCO3 Nanoconjugates. Adv. Mater. 2019, 31, e1901851. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Yu, A.; Wang, Y. Nano CaCO3 “Lysosomal Bombs” Enhance Chemotherapy Drug Efficacy via Rebalancing Tumor Intracellular pH. ACS Biomater. Sci. Eng. 2019, 5, 3398–3408. [Google Scholar] [CrossRef]
- Xue, P.; Hou, M.; Sun, L.; Li, Q.; Zhang, L.; Xu, Z.; Kang, Y. Calcium-carbonate packaging magnetic polydopamine nanoparticles loaded with indocyanine green for near-infrared induced photothermal/photodynamic therapy. Acta Biomater. 2018, 81, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cao, W.; Cheng, J.; Fan, S.; Pan, S.; Wang, L.; Niu, J.; Pan, Y.; Liu, Y.; Sun, X.; et al. Human natural killer cells for targeting delivery of gold nanostars and bimodal imaging directed photothermal/photodynamic therapy and immunotherapy. Cancer Biol. Med. 2019, 16, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Z.; Qiu, Y.; Li, J.; Yang, J.; Li, J. Biomineralization of Aggregation-Induced Emission-Active Photosensitizers for pH-Mediated Tumor Imaging and Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 5566–5574. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xue, Y.; Zhang, Y.; Lu, S.; Wu, J.; Yang, L.; Yang, H.; Yu, S. Mitochondrial Targeting Strategy for Enhanced Photothermal Cancer Therapy. ChemNanoMat 2021, 7, 457–466. [Google Scholar] [CrossRef]
- Tan, H.; Liu, Y.; Hou, N.; Cui, S.; Liu, B.; Fan, S.; Yu, G.; Han, C.; Zheng, D.; Li, W.; et al. Tumor microenvironment pH-responsive pentagonal gold prism-based nanoplatform for multimodal imaging and combined therapy of castration-resistant prostate cancer. Acta Biomater. 2022, 141, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhu, Y.; Xu, S.; Xu, G.; Xiong, R.; Sun, X.; Liu, C. A nanoplatform with tumor-targeted aggregation and drug-specific release characteristics for photodynamic/photothermal combined antitumor therapy under near-infrared laser irradiation. Nanoscale 2020, 12, 11497–11509. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, B.; Dai, X.; Zhao, N.; Xu, F.-J. Biomineralized calcium carbonate nanohybrids for mild photothermal heating-enhanced gene therapy. Biomaterials 2021, 274, 120885. [Google Scholar] [CrossRef]
- Ding, X.-L.; Liu, M.-D.; Cheng, Q.; Guo, W.-H.; Niu, M.-T.; Huang, Q.-X.; Zeng, X.; Zhang, X.-Z. Multifunctional liquid metal-based nanoparticles with glycolysis and mitochondrial metabolism inhibition for tumor photothermal therapy. Biomaterials 2022, 281, 121369. [Google Scholar] [CrossRef]
- Han, Y.; Dong, Z.; Wang, C.; Li, Q.; Hao, Y.; Yang, Z.; Zhu, W.; Zhang, Y.; Liu, Z.; Feng, L. Ferrous ions doped calcium carbonate nanoparticles potentiate chemotherapy by inducing ferroptosis. J. Control. Release 2022, 348, 346–356. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Shen, F.; Dong, Z.; Hao, Y.; Chen, Y.; Liu, Z.; Feng, L. Lipid-Coated CaCO3 Nanoparticles as a Versatile pH-Responsive Drug Delivery Platform to Enable Combined Chemotherapy of Breast Cancer. ACS Appl. Bio Mater. 2022, 5, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, M.; Zhao, H.; Liu, Y.; Gao, N.; Jing, J.; Zhang, X. A dual-functional biomimetic-mineralized nanoplatform for glucose detection and therapy with cancer cells in vitro. J. Mater. Chem. B 2021, 9, 3885–3891. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Y.; Yan, B.-B.; Dong, L.; Lu, Y.; Yu, S.-H. Calcium carbonate-doxorubicin@silica-indocyanine green nanospheres with photo-triggered drug delivery enhance cell killing in drug-resistant breast cancer cells. Nano Res. 2018, 11, 3385–3395. [Google Scholar] [CrossRef]
- Wang, C.; Yu, F.; Liu, X.; Chen, S.; Wu, R.; Zhao, R.; Hu, F.; Yuan, H. Cancer-Specific Therapy by Artificial Modulation of Intracellular Calcium Concentration. Adv. Healthc. Mater. 2021, 10, e2001166. [Google Scholar] [CrossRef]
- Sheng, Y.; Gao, J.; Yin, Z.-Z.; Kang, J.; Kong, Y. Dual-drug delivery system based on the hydrogels of alginate and sodium carboxymethyl cellulose for colorectal cancer treatment. Carbohydr. Polym. 2021, 269, 118325. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, W.; Tang, J.; Xu, Z.P. Devising new lipid-coated calcium phosphate/carbonate hybrid nanoparticles for controlled release in endosomes for efficient gene delivery. J. Mater. Chem. B 2017, 5, 7194–7203. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, K.; Xie, L.; Li, K.; Zhang, W.; Xi, Z.; Wang, X.; Xia, M.; Xu, L. Construction of calcium carbonate-liposome dual-film coated mesoporous silica as a delayed drug release system for antitumor therapy. Colloids Surf. B Biointerfaces 2022, 212, 112357. [Google Scholar] [CrossRef]
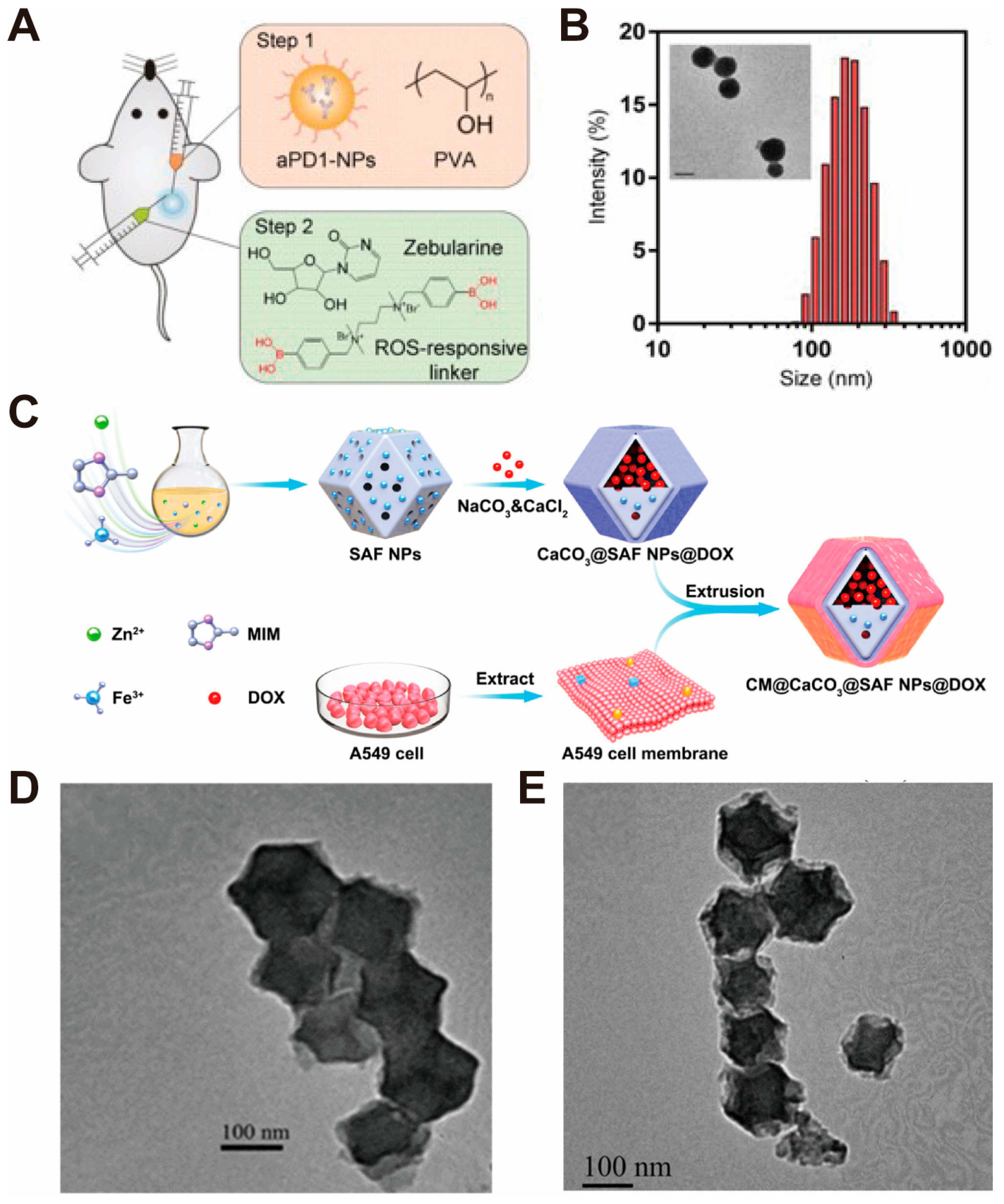
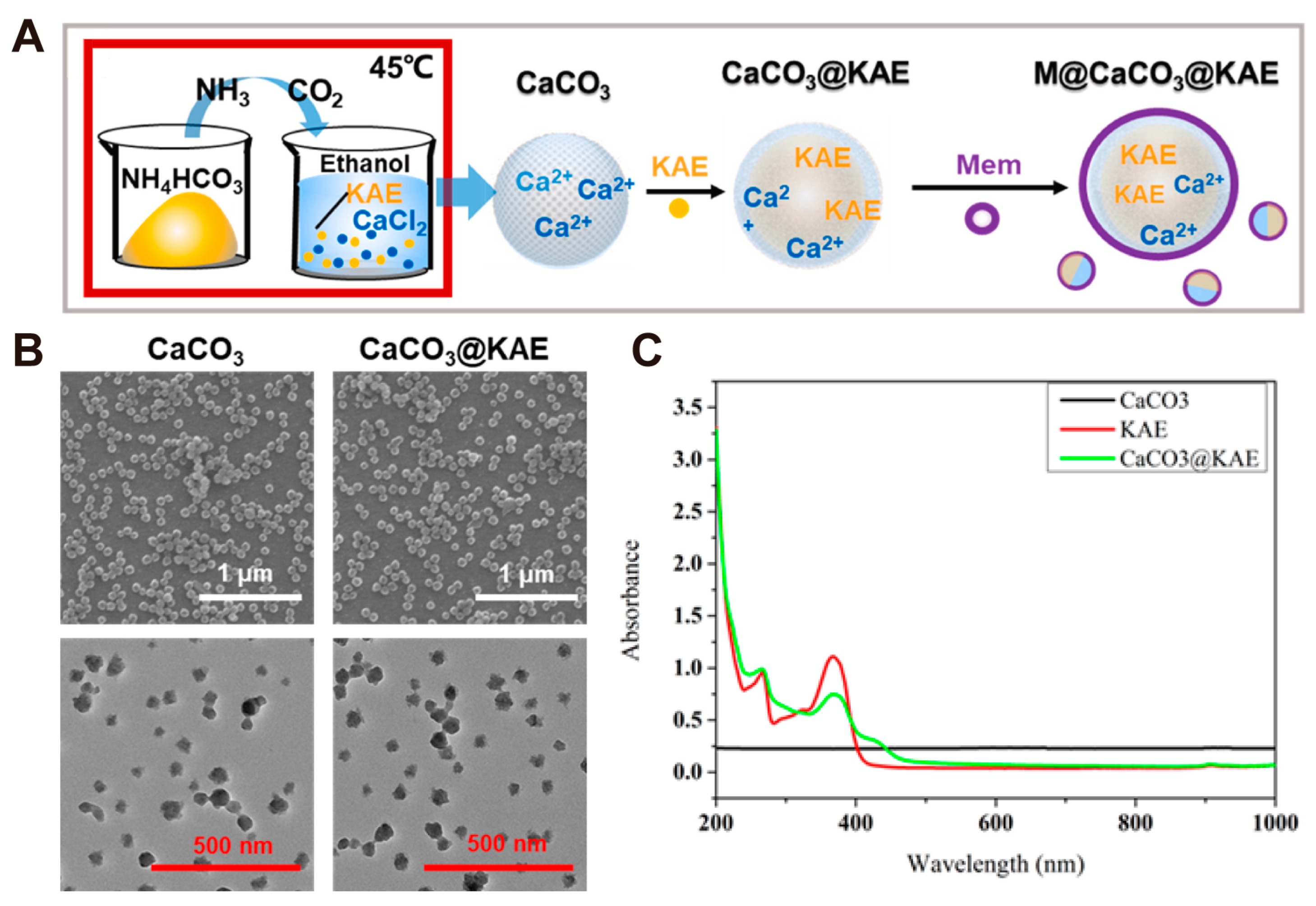


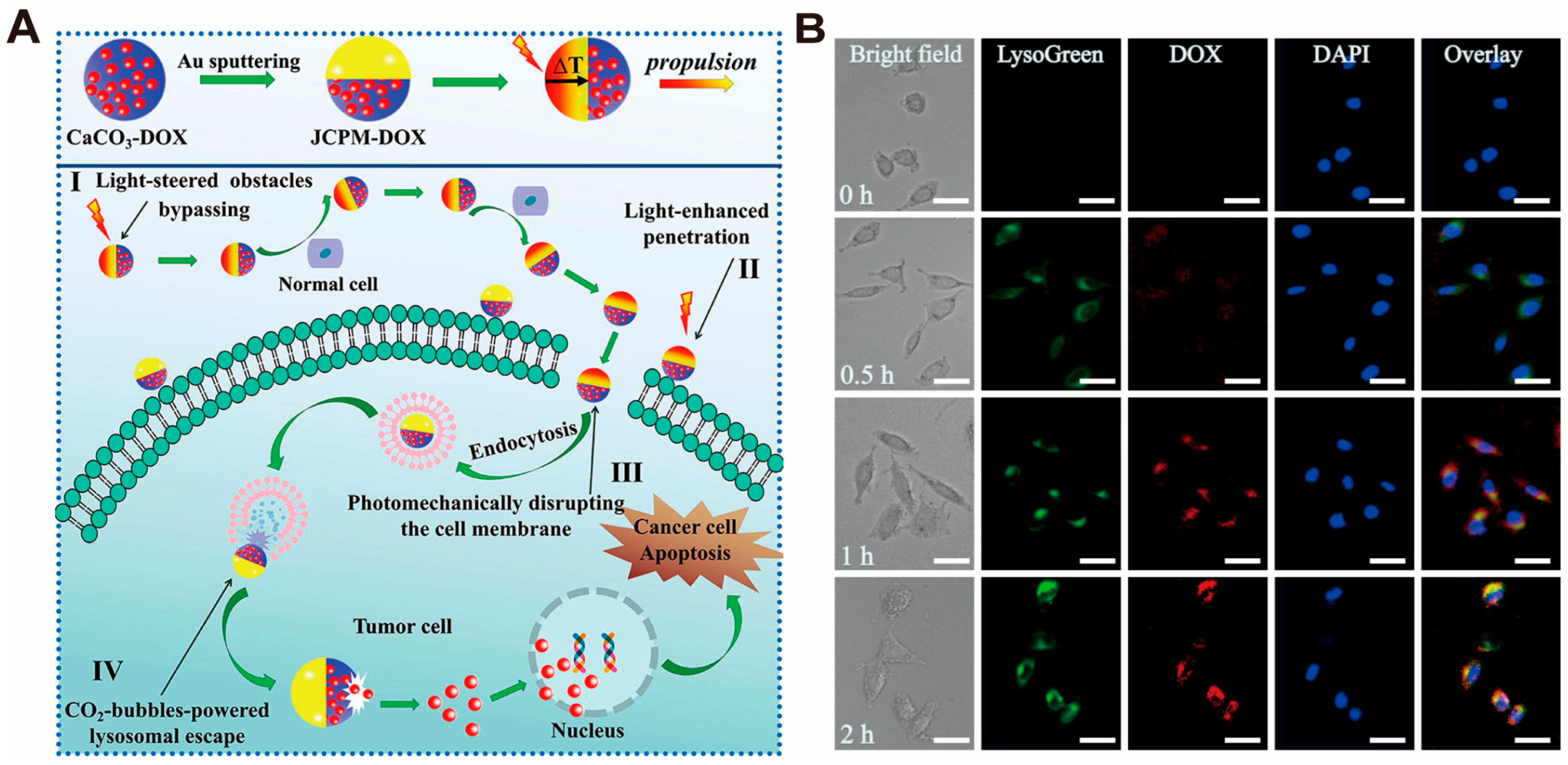
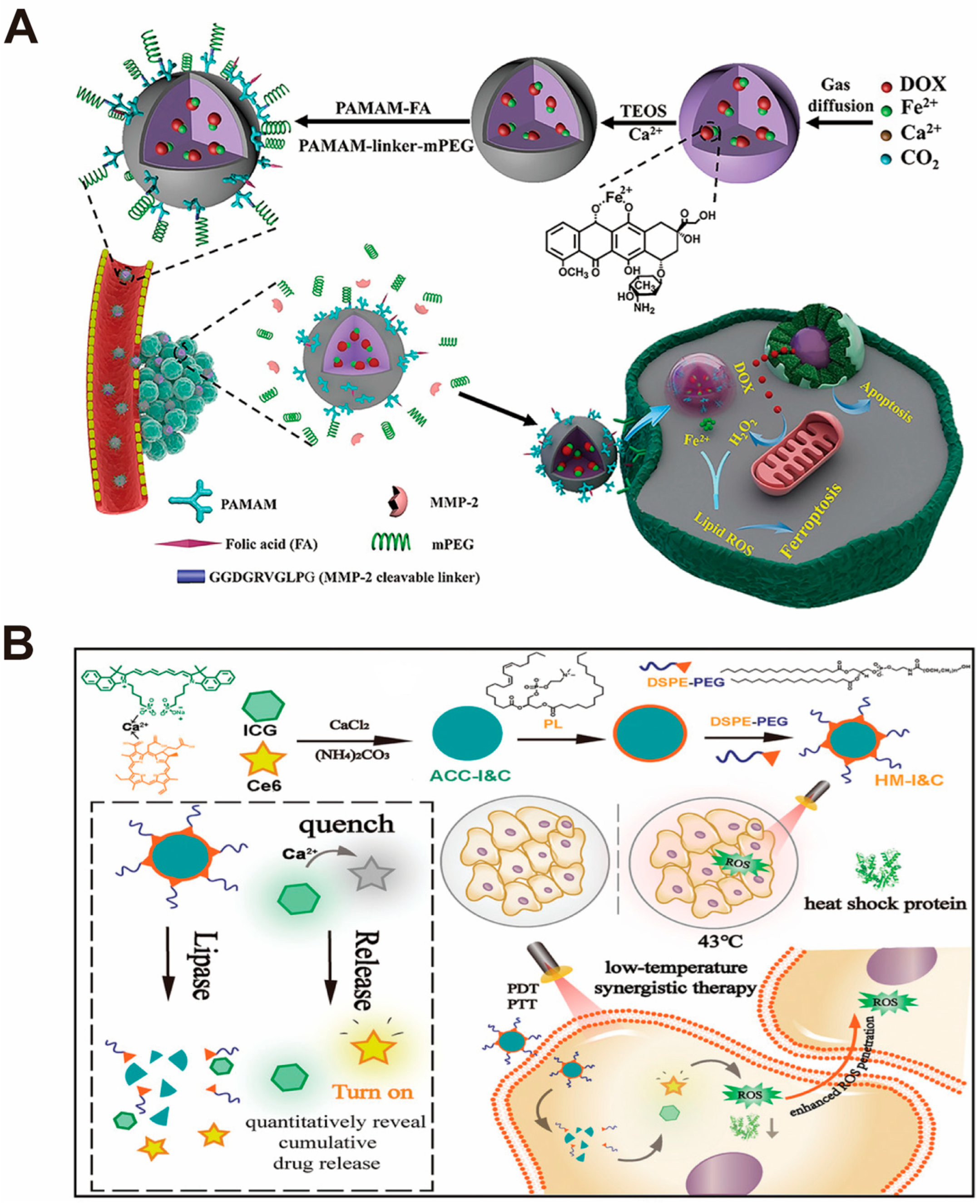


| CaCO3-Based Nano-Drug Delivery System | Sources of Ca2+ and CO32− | Modifier/Template/Core | Particle Sizes | Reference |
|---|---|---|---|---|
| HMME/MCC-HA | CaCl2 and Na2CO3 solution | soluble starch solution | # 264.5 ± 4.7 nm | [33] |
| Zeb-aPD1-NPs-Gel | CaCl2 and Na2CO3 solution | PEG-P(Glu) | #* ~100 nm (aPD1-NPs) | [34] |
| CM@CaCO3@SAF NPs | CaCl2 and Na2CO3 solution | SAF NPs@DOX | #* 150~160 nm (CaCO3@SAF NPs) #* ~200 nm (CM@CaCO3@SAF NPs) | [35] |
| SH@FP@CaCO3 vaccine | CaCl2 and Na2CO3 solution | silk fibroin solution + acetic acid solution | — | [36] |
| ACC@Cu2O-TPP NCs | anhydrous CaCl2 and Dimethyl carbonate (DMC) | — | # 93.38 nm (ACC NPs) # 210.398 nm (ACC@Cu2O-TPP NCs) | [37] |
| HA-DOX/CaCO3 | (Ca(NO3)2·4H2O and Na2CO3 solution | Low-molecular-weight sodium hyaluronate | # 88.5 nm | [38] |
| Cu/SOD-MNPs | CaCl2 and Na2CO3 solution | PEG-PAsp | * 220 nm | [39] |
| Au@CaCO3 NPs | CaCl2 and Na2CO3 solution | AuNPs | * 32 nm | [40] |
| HOCN (OVA@CaCO3) | CaCl2 and Na2CO3 solution | Ovalbumin (OVA) | # 250 nm | [41] |
| CaNP/DOX | (Ca(NO3)2 and Na2CO3 solution | Mpeg-b-PGA | * 150.3 ± 8.6 nm (TEM) # 103.0 ± 7.5 nm (DLS) | [42] |
| CaCO3@COF-BODIPY-2I@GAG | CaCl2 and NH4HCO3 solution | COF-BODIPY-2I | #* 180.4 nm (CaCO3@COF-BODIPY-2I) #* 319.4 nm (CaCO3@COF-BODIPY-2I@GAG) | [43] |
| LYS-NPs | CaCl2 and Na2CO3 solution | ZIF-8 NPs | # 270.6 nm | [44] |
| MNCa⊕ | CaCl2 and Na2CO3 solution | MN⊕ | * ~120 nm | [45] |
| OVA@NP | CaCl2 and Na2CO3 solution | Ovalbumin (OVA) | * ~500 nm (OVA@NP) * ~30 nm (CaCO3) | [46] |
| PDA/BSA/CaCO3 Hybrid Particles | CaCl2·2H2O and Na2CO3·2H2O solution | dopamine hydrochloride (PDA) and BSA | # 572 nm | [47] |
| CaCO3-Based Nano-Drug Delivery System | Sources of Ca2+ and CO32− | Conditions (Medium and Temperature and Time) | Particle Sizes | Reference |
|---|---|---|---|---|
| MS/ACC–DOX NPs | CaCl2 and (NH4)2CO3 | ethanol and 25 °C and 2–3 days | #* ~80 nm (ACC–DOX NPs) #* ~100 nm (MS/ACC–DOX NPs) | [50] |
| CaCO3-TPZ@GOD@HA (AC-TGH) NPs | CaCl2 and NH4HCO3 | anhydrous ethanol and 30 °C and 60 h | * ~80 nm (AC-T NPs) # 161 nm (AC-TGH NPs) | [51] |
| TPZ@CaCO3-PDA-ICG-TPGS/TPGS-RGD nanoparticles (ICG-PDA-TPZ NPs) | CaCl2·6H2O and NH4HCO3 | ethanol and 24 h | # 104.7 ± 1.3 nm (TPZ@CaCO3 nanoparticles) # 178.5 ± 1.8 nm (TPZ@CaCO3-PDA-ICG-TPGS/TPGS-RGD nanoparticles) | [52] |
| PL/ACC-DOX&ICG | CaCl2 and (NH4)2CO3 | anhydrous ethanol and 30 °C and 48 h | * ~80 nm (ACC-DOX&ICG) * ~100 nm (PL/ACC-DOX&ICG) | [53] |
| HM-I&C | CaCl2 and (NH4)2CO3 | anhydrous ethanol and 25 °C and 2–3 days | # ~100 nm | [54] |
| NMOF@DHA@CaCO3 | CaCl2·2H2O and NH4HCO3 | ethanol and room temperature and 24 h | # 382 ± 23 nm in length # 182 ± 37 nm in width | [55] |
| Cu2O@CaCO3@HA (CCH) | CaCl2 and NH4HCO3 | ethanol and room temperature and 8 h | # ~180 nm (average hydrodynamic diameter) * 167.6 nm (physical particle size) | [56] |
| BSO-TCPP-Fe@CaCO3-PEG | CaCl2·2H2O and NH4HCO3 | ethanol and 24 h | # 193.4 ± 2.4 nm (DLS) * 125.2 ± 7.7 nm (TEM) | [57] |
| DOX/GA–Fe@CaCO3-PEG | CaCl2·2H2O and NH4HCO3 | ethanol and 24 h | * ~106.3 nm (CaCO3 NPs) * ~109.2 nm (GA–Fe@CaCO3) | [58] |
| CaCO3@IDOi@PEG@PEI@CpG (CaIPC) nanoparticles | CaCl2·2H2O and NH4HCO3 | ethanol and 24 h | * 55–65 nm | [59] |
| IMQ@ACC(Mn)–ICG/PEG nanoparticles | CaCl2 and NH4HCO3 | ethanol and room temperature and 2 days | * ~43 nm (ACC(Mn)-ICG NPs) # ~72.4 nm (ACC(Mn)–ICG/PEG NPs) | [60] |
| M@CaCO3@KAE nanoparticles | CaCl2 and NH4HCO3 | ethanol and 45 °C and 4 d | #* ~100 nm | [61] |
| ACC@Fe2+/BLM-CaSi-GP | CaCl2 and NH4HCO3 | anhydrous ethanol and 30 °C and 36 h | * 78.8 nm (ACC@Fe2+/BLM) # ~100 nm (ACC@Fe2+/BLM-CaSi) | [62] |
| Mn/CaCO3@PL/SLC NPs | CaCl2 and NH4HCO3 | anhydrous ethanol and 40 °C and 24 h | * ~138 nm (CaCO3 NPs) #* ~183 nm (Mn/CaCO3@PL/SLC NPs) | [63] |
| PEGCaCUR | CaCl2·2H2O and NH4HCO3 | ethanol and 40 °C and 10 h | * ~140 nm | [64] |
| PEGCaNMCUR+CDDP | CaCl2·2H2O and NH4HCO3 | ethanol and 40 °C and 18 h | CaNMCUR+CDDP 211 nm (TEM)/252 nm (DLS) CaNMCUR 198 nm (TEM)/200 nm (DLS) CaNMCDDP 127 nm (TEM)/130 nm (DLS) CaNM 121 nm (TEM)/127 nm (DLS) | [65] |
| Mn:CaCO3-DEX | CaCl2·2H2O and NH4HCO3 | ethanol and room temperature and 8 h | * 150.4 ± 20.2 nm (CaCO3-DEX) | [66] |
| Ca@H NPs | CaCl2·2H2O and NH4HCO3 | ethanol and 48 h | ~200 nm (TEM) 216.2 ± 46.09 nm (DLS) | [67] |
| DiR-DOX-Gd@pCaCO3-PEG | CaCl2·2H2O and NH4HCO3 | anhydrous ethanol and 40 °C and 24 h | # 156.5 nm (DiR-DOX@pCaCO3-PEG) | [68] |
| IrCOOH–CaCO3@PEG | CaCl2·2H2O and NH4HCO3 | ethanol and 30 °C and 24 h | # 126.73 ± 0.65 nm (CaCO3) # 168.76 ± 3.73 nm (IrCOOH–CaCO3) # 188.75 ± 3.79 nm (IrCOOH–CaCO3@PEG) | [69] |
| G/A@CaCO3-PEG | CaCl2·2H2O and NH4HCO3 | anhydrous ethanol and 40 °C and 12 h | * 108 nm (CaCO3 NPs) # 114 ± 4.8 nm (G/A@CaCO3) # 126 ± 6.3 nm (G/A@CaCO3-PEG) | [70] |
| CaCO3@CAP-PEG nanoparticle | CaCl2·2H2O and NH4HCO3 | ethanol and 40 °C and 24 h | CaCO3@CAP ~40 nm (TEM)/45 nm (DLS) | [71] |
| O2-FeCOF@CaCO3@FA (OFCCF) | CaCl2·2H2O and NH4HCO3 | ethanol and 40 °C and 8 h | * 200~250 nm | [72] |
| CaNMs | CaCl2·2H2O and NH4HCO3 | ethanol and 40 °C and 10 h | * ~200 nm | [73] |
| CaCO3-Based Nano-Drug Delivery System | Sources of Ca2+ and CO32− | Emulsion Principle | Water Phase and Oil Phase | Particle Sizes | Reference |
|---|---|---|---|---|---|
| mCNPs | CaCl2 and Na2CO3 | W1/O/W2 double emulsion method | W1 phase: CaCl2 (27.7% w/v) Oil phase: PLG (5% w/v, Dichloromethane) W2 phase: PVA (4% w/v) + Na2CO3 (1.06% w/v) | # ~200 nm | [77] |
| miR-375/Sf-LCC NPs | CaCl2 and Na2CO3 | W/O reverse microemulsion method | Water phase: Water Oil phase: Cyclohexane/Igepal CO-520 (71:29, v/v) | # 100.7 ± 12.1 nm | [78] |
| lipid/CaCO3/curcumin (LCC) | CaCl2 and Na2CO3 | W/O reverse microemulsion method | Water phase: Water Oil phase: Dichloromethane | # 155.3 nm | [79] |
| CDDP/OA-LCC NPs | CaCl2 and Na2CO3 | W/O reverse microemulsion method | Water phase: Water Oil phase: Cyclohexane/Igepal CO-520 (71:29, v/v) | * 206 ± 15 nm | [80] |
| HDL/CC/DOX NPs | CaCl2 and Na2CO3 | W/O reverse microemulsion method | Water phase: Water Oil phase: Cyclohexane/Triton X-100/n-hexanol (v/v: 70/20/10) | # 68.2 ± 3.9 nm | [81] |
| DOX@CaCO3 NPs | CaCl2 and Na2CO3 | W/O reverse microemulsion method | Water phase: Water Oil phase: n-hexane + n-butyl alcohol + CTAB | # 70.6 nm ± 0.9 nm | [82] |
| DNCaNPs | CaCl2 and NaHCO3 | W1/O/W2 double emulsion method | W1 phase: Water Oil phase: DCM (PLGA + PLGA-PEG + Anlg919) W2 phase: PVA (1 wt%) | * ~100 nm | [83] |
| CDDP/OA-LCC NPs | CaCl2 and Na2CO3 | W/O reverse microemulsion method | Water phase: Water Oil phase: Cyclohexane/Igepal CO-520 (71:29, v/v) | # 217 ± 20 nm | [84] |
| ECCaNPs | CaCl2 and NaHCO3 | W1/O/W2 double emulsion method | W1 phase: Water Oil phase: DCM (PLGA + PLGA-PEG + Erlotinib) W2 phase: PVA (1 wt%) | #* ~100 nm | [85] |
| CaCO3-Based Nano-Drug Delivery System | Types of Nano-Drug Delivery Systems | Particle Loading | Function of CaCO3 | Therapeutic Strategy | Reference |
|---|---|---|---|---|---|
| Zeb-aPD1-NPs-Gel | Biomolecular Drug | Anti-PD1 antibody (aPD1) | · pH-responsive drug carrier | · Drug therapy · Immunotherapy | [34] |
| aCD47@CaCO3 | Biomolecular Drug | Anti-CD47 antibody (aPD47) | · A release reservoir of drug · A proton scavenger | · Immunotherapy | [92] |
| CM@CaCO3@SAF NPs | Surface Mineralization | SAF NPs@DOX | · In situ mineralized therapeutic agent · Calcium ion supplier · pH-responsive drug carrier | · Calcium ion interference therapy · Chemotherapy · Chemodynamic therapy | [35] |
| Au@CaCO3 NPs | Surface Mineralization | AuNPs | · Encapsulating agent | · Therapy based on macrophage activation | [40] |
| LYS-NPs | Surface Mineralization | ZIF-8 NPs | · In situ mineralizer · Calcium ion supplier | · T cell immunotherapy | [44] |
| MNCa⊕ | Surface Mineralization | positive-charged Fe3O4 nanoparticle (MN⊕) | · pH-responsive mineralizer | · Circulating tumor cell capture agent | [45] |
| DSA/CC-DOX NPs | Small-Molecule Drug | Doxorubicin (DOX) | · pH-responsive drug carrier | · Chemotherapy | [122] |
| Fe3O4@PDA@CaCO3/ICG (FPCI) NPs | Surface Mineralization | Fe3O4@PDA and ICG | · pH-responsive drug carrier | · Photothermal therapy · Photodynamic therapy | [123] |
| GNS@CaCO3/Ce6-NK | Small-Molecule Drug and Surface Mineralization | Chlorin e6 (Ce6) and Gold nanostars (GNS) | · pH-responsive mineralizer | · Fluorescence imaging and Photoacoustic imaging · Photothermal/photodynamic therapy · Immunotherapy | [124] |
| CaCO3-DOX NPs | Small-Molecule Drug | Doxorubicin (DOX) | · CO2-releasing agent · pH-responsive drug carrier | · Ultrasound imaging · Fluorescence imaging · Chemotherapy | [120] |
| BSA/AIEgen@CaCO3 | Small-Molecule Drug | 1-methyl-4-(4-(1,2,2-triphenylvinyl) styryl) quinolinium iodide (TPE-Qu+) | · pH-responsive mineralizer | · Photodynamic therapy | [125] |
| Bi2S3@CaCO3 NRs | Surface Mineralization | Bi2S3 nanorods | · pH-responsive mineralizer | · Photothermal therapy | [126] |
| PGP/CaCO3@IR820/DTX-HA | Small-Molecule Drug and Surface Mineralization | IR820 and Docetaxel (DTX) and Pentagonal gold prisms (PGPs) | · pH-responsive drug carrier | · Photothermal therapy · Photodynamic therapy · Chemotherapy | [127] |
| Fe@CaCO3/ICG | Small-Molecule Drug and Surface Mineralization | Fe3O4 NPs and IR820 and ICG | · pH-responsive mineralizer | · Photothermal therapy · Photodynamic therapy | [128] |
| Alg-CaCO3-PDA-PGED (ACDP) | Surface Mineralization | Alginate (Alg) micelles | · pH-responsive gene carrier | · Mild hyperthermia-enhanced gene therapy · Ultrasound imaging · Photoacoustic imaging | [129] |
| LMGC NPs | Surface Mineralization | LMG NPs | · pH-responsive mineralizer · Calcium ion supplier | · ATP generation inhibition · Photothermal therapy | [130] |
| CaCO3-TPZ@GOD@HA (AC-TGH) NPs | Small-Molecule and Biomolecular Drug | Glucose oxidase (GOD) and Tirapazamine (TPZ) | · pH-responsive drug carrier | · Tumor starvation therapy · Chemotherapy | [51] |
| TPZ@CaCO3-PDA-ICG-TPGS/TPGS-RGD (ICG-PDA-TPZ NPs) | Small-Molecule Drug | Tirapazamine (TPZ) | · pH-responsive drug carrier | · Photothermal therapy · Photodynamic therapy · Chemotherapy | [52] |
| PL/ACC-DOX&ICG | Small-Molecule Drug | Indocyanine green (ICG) and Doxorubicin (DOX) | · pH-responsive drug carrier | · Near-infrared (NIR) imaging · Photothermal therapy · Chemotherapy | [53] |
| NMOF@DHA@CaCO3 | Surface Mineralization | NMOF@DHA | · pH-responsive mineralizer · Calcium ion supplier | · Ca2+-DHA-mediated oncosis therapy · Photodynamic therapy | [55] |
| Cu2O@CaCO3@HA (CCH) | Surface Mineralization | Hollow mesoporous Cu2O | · pH-responsive mineralizer · Calcium ion supplier | · Photothermal therapy · Photodynamic therapy · Chemodynamic therapy · Calcium-overload-mediated therapy · Immunotherapy | [56] |
| CaCO3@IDOi@PEG@PEI@CpG (CaIPC) nanoparticles | Small-Molecule and Biomolecular Drug | Cytosine-phosphate-guanosine oligonucleotides (CpG ODNs) and IDO inhibitor INCB24360 (IDOi) | · pH-responsive drug carrier · Calcium ion supplier | · T cell immunotherapy | [59] |
| IMQ@ACC(Mn)–ICG/PEG nanoparticles | Small-Molecule Drug | Indocyanine green (ICG) and Imiquimod (IMQ) | · pH-responsive drug carrier | · Photoimmunotherapy | [60] |
| M@CaCO3@KAE nanoparticles | Small-Molecule Drug | Kaempferol-3-O-rutinoside (KAE) | · pH-responsive drug carrier · Calcium ion supplier | · Calcium overload tumor therapy | [61] |
| ACC@Fe2+/BLM-CaSi-GP | Small-Molecule Drug | Fe2+ and Bleomycin (BLM) | · pH-responsive drug carrier · Proton scavengers | · Postoperative management of melanoma | [62] |
| Ca@H NPs | Small-Molecule Drug | Hematoporphyrin monomethyl ether (HMME) | · pH-responsive drug carrier · CO2-releasing agent | · High-intensity focused ultrasound · Sonodynamic therapy · Photoacoustic (PA) imaging | [67] |
| IrCOOH–CaCO3@PEG | Small-Molecule Drug | IrCOOH (Ir(III) complexs) | · pH-responsive drug carrier · Calcium ion supplier | · Calcium overload tumor therapy · Two-photon photodynamic therapy | [69] |
| G/A@CaCO3-PEG | Biomolecular Drug and Surface Mineralization | 2D antimonene quantum dots (AQDs) and glucose oxidase (GOD) | · pH-responsive drug carrier | · Low-temperature photothermal therapy | [70] |
| CaCO3@CAP-PEG nanoparticle | Small-Molecule Drug | Capsaicin | · pH-responsive drug carrier · Calcium ion supplier | · Calcium overload tumor therapy | [71] |
| O2-FeCOF@CaCO3@FA (OFCCF) | Surface Mineralization | FeCOF | · pH-responsive mineralizer · Calcium ion supplier | · Photodynamic therapy · Calcium overload tumor therapy | [72] |
| PGFCaCO3-PEG | Small-Molecule Drug | Gallic acid (GA) and Fe2+ and Pt(IV)-SA | · pH-responsive drug carrier | · Ferroptosis · Chemotherapy | [131] |
| miR-375/Sf-LCC NPs | Small-Molecule and Biomolecular Drug | miR-375 and Sorafenib | · pH-responsive gene carrier · pH-responsive drug carrier | · Gene therapy · Chemotherapy | [78] |
| CDDP/OA-LCC NPs | Small-Molecule Drug | Cisplatin and oleanolic acid | · pH-responsive drug carrier | · Combination chemotherapy | [80] |
| HDL/CC/DOX NPs | Small-Molecule Drug | Doxorubicin (DOX) | · pH-responsive drug carrier | · Chemotherapy | [81] |
| DOX@CaCO3 NPs | Small-Molecule Drug | Doxorubicin (DOX) | · pH-responsive drug carrier | · Chemotherapy · Starving tumor therapy | [82] |
| DNCaNPs | Small-Molecule Drug | Doxorubicin (DOX) and alkylated NLG919 (Anlg919) | · pH-responsive drug carrier | · Chemo-immunotherapy | [83] |
| ECCaNPs | Small-Molecule Drug | Erlotinib and chlorin e6 (Ce6) | · pH-responsive drug carrier | · Chemotherapy · Photodynamic therapy | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.; Akhtar, M.; Li, Y.; Ji, M.; Huang, R. Recent Developments in CaCO3 Nano-Drug Delivery Systems: Advancing Biomedicine in Tumor Diagnosis and Treatment. Pharmaceutics 2024, 16, 275. https://doi.org/10.3390/pharmaceutics16020275
Lin C, Akhtar M, Li Y, Ji M, Huang R. Recent Developments in CaCO3 Nano-Drug Delivery Systems: Advancing Biomedicine in Tumor Diagnosis and Treatment. Pharmaceutics. 2024; 16(2):275. https://doi.org/10.3390/pharmaceutics16020275
Chicago/Turabian StyleLin, Chenteng, Muhammad Akhtar, Yingjie Li, Min Ji, and Rongqin Huang. 2024. "Recent Developments in CaCO3 Nano-Drug Delivery Systems: Advancing Biomedicine in Tumor Diagnosis and Treatment" Pharmaceutics 16, no. 2: 275. https://doi.org/10.3390/pharmaceutics16020275
APA StyleLin, C., Akhtar, M., Li, Y., Ji, M., & Huang, R. (2024). Recent Developments in CaCO3 Nano-Drug Delivery Systems: Advancing Biomedicine in Tumor Diagnosis and Treatment. Pharmaceutics, 16(2), 275. https://doi.org/10.3390/pharmaceutics16020275








