Abstract
Background: Polytherapy in neonatal and pediatric patients requiring parenteral nutrition (PN) administration is a challenging task. Due to limited intravenous access, the Y-site administration of medication with PN admixtures is sometimes inevitable. Aim: This review aims to summarize the evidence on the compatibility of the Y-site of intravenous medications and PN admixtures in neonatal and pediatric settings. Methods: A literature review of the PubMed database was conducted. Articles published between January 1995 and November 2023 concerning the compatibility of intravenous medications in pediatric-dose PN admixtures or with intravenous lipid emulsions only were included. Studies concerning the compatibility/stability of the ingredients of PN admixtures and those concerning unapproved medications were excluded. Based on the methodology used, the quality of the research was assessed. Results: A total of fifteen studies were explored. Among fifty-five different drug substances assessed in the research reviewed, 56% (31/55) were found to be compatible, 13% (7/55) were assigned as incompatible, and for 31% (17/55), the data were ambiguous. None of the studies demonstrated an “A” grade (very high quality), and the grades “B”, “C”, and “D” were assigned to four, six, and five studies, respectively. The compatibility data are presented in two tables, the first concerning the simultaneous administration of medications with 2-in-1 PN formulations (without lipids) and the second, with 3-in-1 formulations (with lipids) and lipid emulsions. Conclusions: This review presents data on compatibilities between intravenously administered medications and PN mixtures intended for neonates and pediatric patients found in the PubMed database. It should be highlighted, however, that this work has some limitations. The clinical decisions on the simultaneous administration of intravenous medication with PN admixtures should be based not only on this review (including assessment of the quality of evidence) but also on manufacturer data, available electronic databases, and incompatibility data for PN admixtures dedicated to adult patients.
1. Introduction
Parenteral nutrition (PN) admixtures should be tailored to meet the specific nutritional needs of the child based on their age, weight, diagnosis, and growth requirements [1]. The complexity of PN admixture and the heightened risk of associated medication errors causing significant patient harm in acute care settings has resulted in this practice being classified as a high-alert medication by the Institute for Safety Medications Practices [2]. There are three main methods of conducting nutritional therapy in pediatric patients. Firstly, PN can be administered in the form of authorized pharmaceutical specialties provided as multi-chamber bags. These products are in line with current standards and meet the needs of a large group of preterm neonates, infants, young children, and adolescents [3,4]. Secondly, PN may be administered in the form of in-house compounded 3-in-1 formulations (containing amino acids, carbohydrates, and lipids as a source of macronutrients), either standardized or individualized, and prepared by hospital pharmacists. Thirdly, administration of a 2-in-1 PN solution (containing amino acids and carbohydrates as a source of macronutrients) and, concomitantly, intravenous lipid emulsion using two separate infusion lines may be conducted. Such an approach is recommended, especially for neonates and young children, due to the lower risk of lipid emulsion destabilization by the other components of the PN solution [5].
Pediatric patients often require the administration of other intravenous medications along with PN administration. Due to limited intravenous access, the use of Y-site administration in this group of patients is often inevitable. There are still gaps in the data regarding the compatibility of medications used in pediatric wards, which may concern up to 15% of the combinations used [6]. Potential complications of co-administration of incompatible medications include precipitation in infusion lines or central venous catheters, leading to infusion line occlusion. Administration of precipitate and large lipid droplets into the venous system can cause capillary embolization and local or systemic inflammatory responses, leading to venous thrombosis, chronic venous insufficiency, and even pulmonary embolism. Central venous catheter occlusion is the most common complication, occurring in up to 25% of patients. This situation can lead to a loss of ability to administer medications and PN through obstructed venous access [7,8]. The consequences of administering infusions with inadequate quality (the presence of precipitate) can be very serious for patients’ health and result in similar detrimental outcomes to administering lipid particles of considerable size, such as capillary embolization [9].
Administering two medications using one intravenous line requires special consideration and detailed analysis of the drug concentrations, infusion rates, diluents used for reconstitution, and proportions between components in the PN. Only such an approach can ensure the safety of therapy in such a special group of patients. This review aims to summarize the evidence on compatibility of the Y-site of intravenous medications and PN admixtures used in neonatal and pediatric settings. We also identify the methodology used to evaluate the compatibility and discuss the limitations of data extrapolation.
2. Methods
2.1. Research Methodology
The research methodology of this study involved searching data based on six different configurations of three-keyword-based sets. The keyword sequence used to search the database was the following: “parenteral nutrition” and “compatibility” or “Y-site” and “neonatal” or “neonates” or “pediatric” or “paediatric”. Figure 1 presents the flow diagram of the search methodology.
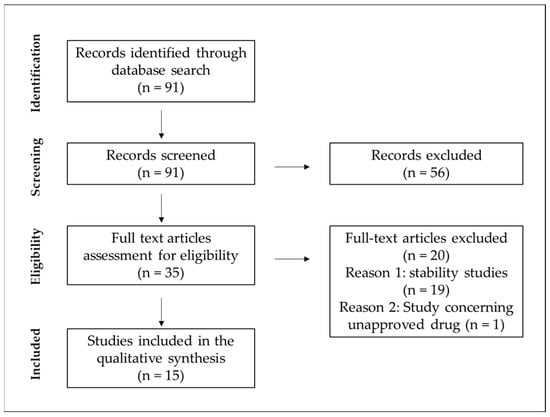
Figure 1.
Flow diagram of the search methodology.
One electronic database, PubMed, was searched by two independent researchers, where a total of 91 results were found from January 1995 up to November 2023. As the exclusion criteria, we adopted the studies concerning the compatibility/stability of drugs being added to PN admixtures but not administered simultaneously using the Y-site. The second criterion was studies investigating the Y-site compatibility of PN admixtures with unapproved medications. After removing reviews, clinical trials, case reports, and articles not written in English, the resulting database was analyzed by abstract screening for the exclusion criteria. Following their application, 16 records were obtained on the compatibility of intravenous medications in neonatal and pediatric doses with 3-in-1 PN, 2-in-1 PN, or intravenous lipid emulsions.
2.2. Research Quality Assessment
Inspired by the Stabilis 4.0 database (www.stabilis.org; accessed on 5 January 2024) grading system (that mainly concerns the quality of chemical stability research), for a better overview of the quality of the presented data, we developed a grading system for the assessment of the physicochemical compatibility of intravenous drugs with PN admixtures. This review used the following letter scale (A, B, C, or D) which scored the level of evidence and quality of the studies presented.
The A grade was assigned to studies presenting a very high evidence level. These studies evaluated both the chemical stability of the drug substance using the HPLC method, which allowed for the effective separation of the drug and its degradation products, and the physical compatibility using instrumental methods. The physical compatibility was assessed, in the case of lipid-containing formulations (3-in-1 PN emulsions or lipid emulsions), using two instrumental methods recommended by the United States Pharmacopeia in Chapter <729> (USP <729>): (i) dynamic light scattering or classical light scattering methods for determination of the mean droplet diameter (MDD) and (ii) measurement of the percentage of fat residing in globules larger than 5 µm (PFAT5) by light obscuration or light extinction methods or, in the case of lipid-free formulations (2-in-1 PN solutions), instrumental methods for both counting sub-visual particles and turbidity assessment.
The B grade was assigned to studies that presented a high level of evidence. These studies did not necessarily evaluate the chemical stability of the drug substance; however, a physical compatibility assessment was performed using instrumental methods including, in the case of lipid-containing formulations (3-in-1 PN emulsions or lipid emulsions), two methods recommended by the USP <729>: MDD evaluation using dynamic light scattering or classical light scattering methods and measurement of the PFAT5 by light obscuration or light extinction methods or, in the case of lipid-free formulations (2-in-1 PN solutions), instrumental methods for both counting sub-visual particles and turbidity assessment.
The C grade was assigned to studies that presented a medium level of evidence. These studies did not necessarily evaluate the chemical stability of the drug substance; however, a physical compatibility assessment was performed using instrumental methods, including, in the case of lipid-containing formulations (3-in-1 PN emulsions or lipid emulsions), at least one method recommended by the USP <729>: MDD evaluation using dynamic light scattering or classical light scattering methods or measurement of the PFAT5 by light obscuration or light extinction methods or, in the case of lipid-free formulations (2-in-1 PN solutions), at least one instrumental method for counting sub-visual particles or turbidity assessment.
The D grade was assigned to studies that presented a low level of evidence. These studies only evaluated chemical stability or physical compatibility, but the methodology used was not comprehensive.
3. Results and Discussion
3.1. Compatibility Data
The literature review identified 15 research works on the Y-site compatibility of PN and other intravenous medications in neonatal and pediatric patients [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. We categorized them into two groups: those concerning a 2-in-1 PN solution, where the intravenous lipid emulsion was administered separately (Table 1), and those concerning compatibility studies of intravenous medications with 3-in-1 formulations either compounded by hospital pharmacists or authorized pharmaceutical specialties (Table 2). One study concerning the compatibility of intravenous medications with only an intravenous lipid emulsion was added to Table 2 [22]. A total of fifty-five different drug substances in concentrations used in neonatal and pediatric patients were explored. A total of 56% (31/55) were found to be compatible, 13% (7/55) were assigned as incompatible, and for 31% (17/55), the data were ambiguous. The result was considered ambiguous if the authors of the study included it in such a category or if several authors tested the same medication and obtained different results. The ambiguous data between authors was found for four drug solutions: aminophylline [14,17], ampicillin sodium [10,17,21,22,24], ceftazidime [17,21,24], and fosphenytoin sodium [13,21,24]. In the cases of acetazolamide sodium [17], acyclovir sodium [17], amiodarone [13], chlorothiazide sodium [17], pentobarbital sodium [13], phenobarbital sodium [13], and rifampicin [13], the results of the evaluated works indicated that concomitant administration of such medications with PN is contraindicated and can lead to therapy failure or serious consequences for patients’ health or life.

Table 1.
Compatibility studies of intravenous medications and 2-in-1 PN solutions (without lipids).

Table 2.
Compatibility studies of intravenous medications and 3-in-1 PN emulsions (with lipids).
3.2. Research Quality Evaluation
To determine the quality of the research, the method of determining the proportions between medications and PN admixtures, the sampling period, and the number of applied methods used for incompatibility identification were evaluated. Since the administration rates of both medications are crucial to properly evaluating the incompatibilities that may occur in clinical settings, this is an important parameter that should be considered when planning Y-site compatibility studies. To simulate clinical conditions, the proportion of medications coexisting in the infusion line should be determined concerning the extreme infusion rates of drug solutions being mixed. Such an approach was applied only by 40% (6/15) of the researchers [14,18,19,20,21,24]. Other researchers used the methodology introduced by the authors of the first works dealing with this topic, where only a 1:1 volume ratio was evaluated [25]. There are two known methods used for the assessment of compatibilities. The first is the static method, which is based on combining medications in tested ratios in a test tube. Such a method was used in all presented studies. The second is a dynamic method that involves sampling the combined medications infused by automatic pumps at a given rate and simulating their administration. Some authors preferred this method since it reflects clinical conditions and takes into account the possible influence of the infusion line material on the observed interactions [26,27]. There are also differences between authors in the sampling period. The most often used sampling periods were at the points 0 h and 4 h (60% (9/15) of analyzed works) [10,16,17,18,19,20,21,23,24]. However, 33% (5/15) of researchers collected their samples at more than four time points [12,13,15,17,23]. Nevertheless, the most important element in assessing the quality of conducted research evaluating a drug’s compatibility is not the sampling period but the type of methods used. To assess the quality of the studies presented in this review, we developed a four-grade letter scale (see Section 2) and assigned each study the appropriate letters: A, B, C, or D. None of the studies was assigned the A grade. Only four research groups applied five or more analytical methods to evaluate the compatibility between medications and PN admixtures and included both methods recommended by the USP for assessing injectable lipid emulsions, getting a B grade [19,20,21,24]. Those studies present a comprehensive approach and can be perceived as high-quality; however, since they are not assessing the chemical stability of the drugs, we could not assign them an A grade. Six studies were rated as being of medium quality (C grade) [10,12,13,18,22,23]. The remaining ones were based on selected methods and assigned with the D grade [11,14,15,16,17]. In some cases, the determination of particulate matter was made without the support of instrumental methods [14,15,16,17], which does not guarantee the detection of potential incompatibilities and indicates a poor quality of the research.
3.3. Compatibility Evaluation Methods
Determining the incompatibilities between intravenous medications and PN admixtures, either in a solution or an emulsion, is an important issue in ensuring safe therapy. The concomitant administration can be performed using a Y-site connector which is located in the lower part of the infusion line just before the distal end. Due to the short contact time of two medications administered in such a manner, which is less than 2 min, the chemical instability is considered at a low risk. Thus, it was rarely investigated (2 of the 15 assessed works) [11,23]. The HPLC method is much more often used in the stability assessment of PN admixture additives, e.g., vitamins [28,29]. Nevertheless, co-administration of medications with PN admixtures can affect the stability of the lipid emulsion or lead to changes in the drug substance’s ionic form [30]. The most common signs of drug–PN incompatibility are lipid emulsion breakdown manifested by pH or color changes and lipid emulsion phase separation, including creaming or coalescence processes [7,8,9]. Another important issue is the formation of a precipitate of active substances or excipients present in the drug dosage form. The combined administration of incompatible medications may also result in legal consequences for medical personnel and high costs of treatment of complications. Therefore, to ensure safe therapy, physicochemical compatibility is determined. A detailed analysis of the techniques used and the equipment applied by the scientists in the analyzed works is presented in Table 3 and Table 4.

Table 3.
Methods used for determining compatibility between medications and 2-in-1 PN solutions (without lipids).

Table 4.
Methods used for determining compatibility between medications and 3-in-1 PN emulsions (with lipids).
The analysis of studies whose authors undertook the problem of compatibility assessment between the intravenous medications and PN in the form of a solution allowed for distinguishing six scopes of interest: (i) the assessment of visible precipitation, color change, or gas production, (ii) analysis of the Tyndall beam effect, (iii) sub-visual particle counting, (iv) turbidity and (v) pH measurement, and (vi)drug concentration evaluation (Table 3). In the case of PN admixtures in the form of lipid emulsion, authors analyzed the appearance of visible precipitation, color change, and gas production, determined the particle size (MDD and PFAT5), and evaluated the changes in pH, osmolality, zeta potential, or drug concentration (Table 4). In the research methodology used to evaluate the compatibility of the PN and other intravenous drugs, the methods dedicated to PN admixtures both in the form of solution (2-in-1) and in the form of lipid emulsion (3-in-1), those dedicated uniquely to PN in the form of solution (2-in-1), and those dedicated uniquely to PN containing lipid emulsion (3-in-1) could be distinguished. Tests used for all types of PN included visual examination, pH, and drug concentration change evaluation. Additionally, for 2-in-1 PN admixtures uniquely, turbidity determination was preferred, and in the case of PN containing lipid emulsion zeta potential, osmolality and lipid emulsion particle size analyses were performed.
3.4. Limitations of Data Extrapolation
The special nutritional requirements of newborns and children who differ from adults have necessitated the development of PN ingredients dedicated to this special group of patients. Generally, infants and young children have higher protein requirements per unit of body weight compared to adults due to the period of rapid growth and tissue development in their bodies. The adequate supply of essential amino acids, including histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine, play a crucial role in their growth and development, and, as such, should be provided in proper amounts within PN. Moreover, some children and infants, especially preterm infants, due to illness or immaturity (this applies to premature infants), are unable to sufficiently, endogenously synthesize some essential amino acids, which in this condition become essential (conditionally essential). This concerns cysteine, tyrosine, glycine, arginine, proline, asparagine, and glutamine [31]. Nevertheless, the conditional indispensability of some of these amino acids is discussed [32,33]. Currently, few pediatric formulations of amino acid solutions are available on the market. In Figure 2, a comparison of the amino acid compositions of different products and their indications is provided.
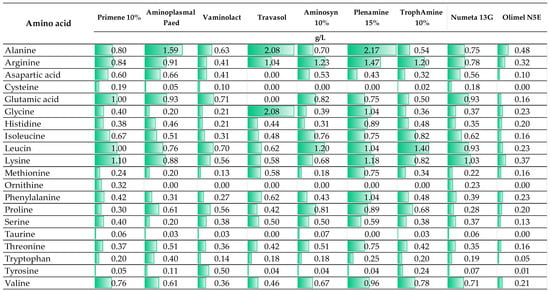
Figure 2.
The composition of intravenous amino acid solutions for the preparation of neonatal or pediatric PN (The presented data are based on the manufacturer information (SmPC)).
Interestingly, most amino acid solutions and commercially available PN admixtures in multi-chamber bags used in adult patients are registered from 2 years old. An example of such a preparation is Olimel N5E used by Staven et al. [24], in whose study the compatibility of intravenous medications was evaluated with two commercially available PN admixtures intended for central administration dedicated to neonates to 2 years (Numeta G16E) and children > 2 years (Olimel N5E). As shown in Figure 2, the quantitative composition of amino acids in Olimel N5E differed significantly from other presented preparations which are dedicated to patients from 1 day of life. As indicated in several works presented in this review, a change in qualitative and quantitative composition may determine a compatibility change [10,21,22]. This issue has not been well studied so far for amino acid solutions; however, it is already known that the stability of PN formulation depends on the amino acid solution used [34]; therefore, we cannot exclude the possibility that it does not affect compatibility with other medications. Other important components of compounded PN admixtures are glucose and electrolytes. These ingredients are provided mainly from a single preparation so their concentration in the final medication can be easily controlled. However, the role of phosphates (inorganic or organic) on the stability of PN admixture and therefore their impact on compatibility cannot be ignored. It is well known that PN formulations containing the organic source of phosphates are less prone to destabilization [35,36]. Therefore, drugs compatible with PN admixtures based on organic phosphates cannot be expected to behave in the same way when the phosphate source in PN admixtures is changed to inorganic. The analyzed works include research on compatibility with PN admixtures containing inorganic [10,11,12,13,14,15,16,17,22,23] and organic [18,19,20,21,24] phosphates.
On the contrary, vitamins and trace elements are ingredients provided in a complex pharmaceutical formulation, providing a panel of active ingredients. Several preparations of vitamins and trace elements indicated for pediatric patients are available on the pharmaceutical market, including Infuvite Pediatric, Peditrace, Soluvit N Infant, and Vitlipid N Infant. Scientists agree that the results obtained for a specific PN composition should not be extrapolated, especially when the obtained results would be transferred to different preparations of intravenous medications or PN formulations containing a different pharmaceutical preparation (e.g., another amino acid preparation) or higher concentrations of individual ingredients (e.g., electrolytes) [37,38,39,40].
Since there are multiple ways of providing PN therapy, in the literature concerning Y-site compatibility, PN is defined in various ways, either as 2-in-1 PN in the form of solution or as 3-in-1 PN admixture in the form of lipid emulsion. Lipid emulsion is a critical component of PN. It is prone to many destabilization factors. Due to their ability to neutralize repellent electrostatic interactions between lipid droplets, exposure to acidic conditions, especially at pH below 5.5, and high concentrations of calcium and magnesium ions are the main known destabilizers of lipid emulsions [41]. Infusing unstable lipid emulsions separately or as an ingredient of a PN with droplets that exceed the internal diameter of the pulmonary microvasculature increases the risk of embolic syndrome [42]. As shown in several studies, the type of lipid emulsion can affect compatibility [43,44,45]. This is a result of different oil compositions between preparations and, in some cases, various types or concentrations of excipients. A comparison of intravenous lipid emulsions used to prepare neonatal or pediatric PN is shown in Figure 3.
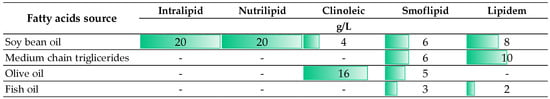
Figure 3.
The oil phase compositions of intravenous lipid emulsions used for the preparation of neonatal or pediatric PN (The presented data are based on the manufacturer information (SmPC)).
Those differences are the reason why the data obtained by the authors for PN admixtures prepared based on one type of intravenous lipid emulsion should not be extrapolated to PN admixtures prepared using other preparations. Extrapolation of compatibility results should be avoided. Nevertheless, for safety reasons, it is advisable and necessary to extrapolate the results regarding incompatibility, regardless of whether the incompatibility was demonstrated for preparations intended for adults or children. It is also advisable to check the compatibility data not only in the available literature but also in electronic databases, e.g., Micromedex, Lexicomp, Medscape, or Drugs.com [46].
These data extrapolation limitations point to the need for continued research to improve infusion therapy safety in neonates and children requiring parenteral nutrition administration.
4. Limitations
The presented review study has some limitations. Firstly, we searched only the PubMed database, omitting ScienceDirect, Scopus, Web of Science, and Google Scholar, and we did not present the compatibility data provided by the producers in the SmPC, making it possible that we overlooked some important incompatibility data. We justify this lack by the fact that the data from the manufacturer would have been difficult to assess in terms of the quality of the study because none of the PN manufacturers provided their methodology based on which they determined the incompatibility of individual drugs with PN. In this study, we decided to analyze only data from scientific research publications with a known methodology. Secondly, we did not analyze electronic databases, e.g., Micromedex, Lexicomp, Medscape, or Drugs.com. Moreover, data available in the scientific literature present data obtained on request, which also limits the clinical relevance of our study. Thirdly, the analysis of the source of financing of the studies showed that two of the presented studies were financed by the producer of the medications being studied [16,17], which could have affected the published results and suggests a bias in their data analyses. The other reviewed studies had no financing support [10,11,14,15,22,23] or were funded by regional or national institutions [12,13,18,19,20,21,24]. Nevertheless, we believe that the presented manuscript will help in determining incompatibilities between drugs and PN admixtures dedicated to neonatal and pediatric patients, as well as the appropriate research methodology and areas (medications) for which there is a need for further research.
5. Conclusions
This review presents data on compatibilities between intravenously administered medications and PN mixtures for neonates and pediatric patients found in the PubMed database. Research concerning compatibility studies was characterized by various evidence quality assessments, and none of the studies gained an A grade (very high quality). Among a total of fifty-five different drug substances assessed in the research reviewed, 56% (31/55) were found to be compatible, 13% (7/55) were assigned as incompatible, and for 31% (17/55), the data were ambiguous. It should be highlighted, however, that this work has some limitations. The clinical decisions on the simultaneous administration of intravenous medication with PN admixtures should be based not only on this review (including assessment of the evidence quality) but also on the manufacturer data, available electronic databases, and incompatibility data known for PN admixtures dedicated to adult patients.
Author Contributions
Conceptualization, A.G.; methodology, A.G.; investigation, A.G. and M.O.; data curation, A.G., T.P. and M.O.; writing—original draft preparation, A.G.; writing—review and editing, A.G. and M.O.; visualization, A.G.; supervision, M.O.; project administration, A.G.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Centre for Research and Development, Grant No. LIDER/17/0092/L-12/20/NCBR/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Groh-Wargo, S.; Barr, S.M. Parenteral Nutrition. Clin. Perinatol. 2022, 49, 355–379. [Google Scholar] [CrossRef] [PubMed]
- Institute for Safe Medication Practices. [High Alert Drug] Institute for Safe Medication Practices List of High-Alert Medications in Acute Care Settings; Institute for Safe Medication Practices: Buttler Pike, PA, USA, 2018. [Google Scholar]
- Riskin, A.; Picaud, J.-C.; Shamir, R.; ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Standard versus individualized parenteral nutrition. Clin. Nutr. 2018, 37, 2409–2417. [Google Scholar] [CrossRef]
- Koletzko, B.; Bhutta, Z.A.; Cai, W.; Cruchet, S.; El Guindi, M.; Fuchs, G.J.; Goddard, E.A.; van Goudoever, J.B.; Quak, S.H.; Kulkarny, B.; et al. Compositional requirements of follow-up formula for use in infancy: Recommendations of an international expert group coordinated by the Early Nutrition Academy. Ann. Nutr. Metab. 2013, 62, 44–54. [Google Scholar] [CrossRef]
- Blackmer, A.B.; Partipilo, M.L. Three-in-one parenteral nutrition in neonates and pediatric patients: Risks and benefits. Nutr. Clin. Pract. 2015, 30, 337–343. [Google Scholar] [CrossRef]
- Häni, C.; Vonbach, P.; Fonzo-Christe, C.; Russmann, S.; Cannizzaro, V.; Niedrig, D. Evaluation of Incompatible Co-administration of Continuous Intravenous Infusions in a Pediatric/Neonatal Intensive Care Unit. J. Pediatr. Pharmacol. Ther. 2019, 24, 479–488. [Google Scholar] [CrossRef]
- Benlabed, M.; Perez, M.; Gaudy, R.; Genay, S.; Lannoy, D.; Barthélémy, C.; Odou, P.; Lebuffe, G.; Decaudin, B. Clinical implications of intravenous drug incompatibilities in critically ill patients. Anaesth. Crit. Care Pain Med. 2019, 38, 173–180. [Google Scholar] [CrossRef]
- Leopoldino, R.W.; Costa, H.T.; Costa, T.X.; Martins, R.R.; Oliveira, A.G. Potential drug incompatibilities in the neonatal intensive care unit: A network analysis approach. BMC Pharmacol. Toxicol. 2018, 19, 83. [Google Scholar] [CrossRef]
- Hörmann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control Release 2016, 223, 85–98. [Google Scholar] [CrossRef]
- Ross, E.L.; Petty, K.; Salinas, A.; Her, C.; Carpenter, J.F. Physical compatibility of medications with concentrated neonatal and pediatric parenteral nutrition: A simulated Y-site drug compatibility study. JPEN J. Parenter. Enteral. Nutr. 2023, 47, 372–381. [Google Scholar] [CrossRef]
- Campbell, A.L.; Petrovski, M.; Senarathna, S.G.; Mukadam, N.; Strunk, T.; Batty, K.T. Compatibility of pentoxifylline and parenteral medications. Arch. Dis. Child 2020, 105, 395–397. [Google Scholar] [CrossRef]
- Greenhill, K.; Hornsby, E.; Gorman, G. Investigations of Physical Compatibilities of Commonly Used Intravenous Medications with and without Parenteral Nutrition in Pediatric Cardiovascular Intensive Care Unit Patients. Pharmaceuticals 2019, 12, 67. [Google Scholar] [CrossRef]
- Fox, L.M.; Wilder, A.G.; Foushee, J.A. Physical compatibility of various drugs with neonatal total parenteral nutrient solution during simulated Y-site administration. Am. J. Health Syst. Pharm. 2013, 70, 520–524. [Google Scholar] [CrossRef]
- Sykes, R.; McPherson, C.; Foulks, K.; Wade, J.; Gal, P. Aminophylline compatibility with neonatal total parenteral nutrition. J. Pediatr. Pharmacol. Ther. 2008, 13, 76–79. [Google Scholar] [CrossRef]
- Dice, J.E. Physical Compatibility of Alprostadil with Commonly Used IV Solutions and Medications in the Neonatal Intensive Care Unit. J. Pediatr. Pharmacol. Ther. 2006, 11, 233–236. [Google Scholar] [CrossRef]
- Veltri, M.A.; Conner, K.G. Physical compatibility of milrinone lactate injection with intravenous drugs commonly used in the pediatric intensive care unit. Am. J. Health Syst. Pharm. 2002, 59, 452–454. [Google Scholar] [CrossRef]
- Veltri, M.; Lee, C.K. Compatibility of neonatal parenteral nutrient solutions with selected intravenous drugs. Am. J. Health Syst. Pharm. 1996, 53, 2611–2613. [Google Scholar] [CrossRef]
- Tomczak, S.; Chmielewski, M.; Szkudlarek, J.; Jelińska, A. Antiemetic Drugs Compatibility Evaluation with Paediatric Parenteral Nutrition Admixtures. Pharmaceutics 2023, 15, 2143. [Google Scholar] [CrossRef]
- Nilsson, N.; Storesund, I.; Tho, I.; Nezvalova-Henriksen, K. Co-administration of drugs with parenteral nutrition in the neonatal intensive care unit—Physical compatibility between three components. Eur. J. Pediatr. 2022, 181, 2685–2693. [Google Scholar] [CrossRef]
- Nezvalova-Henriksen, K.; Nilsson, N.; Østerberg, C.T.; Staven Berge, V.; Tho, I. Y-Site Physical Compatibility of Numeta G13E with Drugs Frequently Used at Neonatal Intensive Care. Pharmaceutics 2020, 12, 677. [Google Scholar] [CrossRef] [PubMed]
- Staven, V.; Wang, S.; Grønlie, I.; Tho, I. Physical stability of an all-in-one parenteral nutrition admixture for preterm infants upon mixing with micronutrients and drugs. Eur. J. Hosp. Pharm. Sci. Pract. 2020, 27, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.L.; Salinas, A.; Petty, K.; Her, C.; Carpenter, J.F. Compatibility of medications with intravenous lipid emulsions: Effects of simulated Y-site mixing. Am. J. Health Syst. Pharm. 2020, 77, 1980–1985. [Google Scholar] [CrossRef]
- Garcia, J.; Garg, A.; Song, Y.; Fotios, A.; Andersen, C.; Garg, S. Compatibility of intravenous ibuprofen with lipids and parenteral nutrition, for use as a continuous infusion. PLoS ONE 2018, 13, e0190577. [Google Scholar] [CrossRef]
- Staven, V.; Iqbal, H.; Wang, S.; Grønlie, I.; Tho, I. Physical compatibility of total parenteral nutrition and drugs in Y-site administration to children from neonates to adolescents. J. Pharm. Pharmacol. 2017, 69, 448–462. [Google Scholar] [CrossRef]
- Thompson, D.F.; Allen, L.V.; Desai, S.R.; Rao, P.S. Compatibility of furosemide with aminoglycoside admixtures. Am. J. Hosp. Pharm. 1985, 42, 116–119. [Google Scholar] [CrossRef]
- Campos-Baeta, Y.; Saavedra-Mitjans, M.; Garin, N.; Cardenete, J.; Cardona, D.; Riera, P. Physicochemical Compatibility of Dexmedetomidine with Parenteral Nutrition. Nutr. Clin. Pract. 2020, 35, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, M.M.; Molina, A.; Navarro, L.; Grau, L.; Pujol, M.D.; Cardenete, J.; Cardona, D.; Riera, P. Physicochemical Compatibility of Amiodarone with Parenteral Nutrition. J. Parenter. Enter. Nutr. 2019, 43, 298–304. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Jelińska, A.; Ogrodowczyk, M. Stability of high-dose thiamine in parenteral nutrition for treatment of patients with Wernicke’s encephalopathy. Clin. Nutr. 2020, 39, 2929–2932. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Dettlaff, K.; Jelińska, A.; Ogrodowczyk, M. Stability studies of parenteral nutrition with a high dose of vitamin C. J. Oncol. Pharm. Pract. 2020, 26, 1894–1902. [Google Scholar] [CrossRef]
- Gostyńska, A.; Stawny, M.; Dettlaff, K.; Jelińska, A. The Interactions between Ciprofloxacin and Parenteral Nutrition Admixtures. Pharmaceutics 2019, 12, 27. [Google Scholar] [CrossRef]
- Prolla, I.; Tomlinson, C.; Pencharz, P.B.; Porto, B.; Elango, R.; Courtney-Martin, G. Amino acid requirements of total parenteral nutrition (TPN) fed neonates: A narrative review of current knowledge and the basis for a new amino acid solution in neonatal nutrition. Pediatr. Med. 2022, 5, 29. [Google Scholar] [CrossRef]
- Riedijk, M.A.; van Beek, R.H.T.; Voortman, G.; de Bie, H.M.A.; Dassel, A.C.M.; van Goudoever, J.B. Cysteine: A conditionally essential amino acid in low-birth-weight preterm infants? Am. J. Clin. Nutr. 2007, 86, 1120–1125. [Google Scholar] [CrossRef]
- Pencharz, P.B.; House, J.D.; Wykes, L.J.; Ball, R.O. What are the essential amino acids for the preterm and term infant? In Recent Developments in Infant Nutrition: Scheveningen 29 November–2 December 1995; Bindels, J.G., Goedhart, A.C., Visser, H.K.A., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 278–296. [Google Scholar] [CrossRef]
- Unger, N.; Holzgrabe, U. Stability and assessment of amino acids in parenteral nutrition solutions. J. Pharm. Biomed. Anal. 2018, 147, 125–139. [Google Scholar] [CrossRef]
- Wang, H.-J.; Hsieh, Y.-T.; Liu, L.-Y.; Huang, C.-F.; Lin, S.-C.; Tsao, P.-N.; Chou, H.-C.; Yen, T.-A.; Chen, C.-Y. Use of sodium glycerophosphate in neonatal parenteral nutrition solutions to increase calcium and phosphate compatibility for preterm infants. Pediatr. Neonatol. 2020, 61, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Bouchoud, L.; Fonzo-Christe, C.; Sadeghipour, F.; Bonnabry, P. Maximizing calcium and phosphate content in neonatal parenteral nutrition solutions using organic calcium and phosphate salts. JPEN J. Parenter. Enteral. Nutr. 2010, 34, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.; Neyer, P.; Schönenberger, K.A.; Saxer, C.; Bernasconi, L.; Stanga, Z.; Huber, A.; Hammerer-Lercher, A.; Mühlebach, S. Physicochemical Stability and Compatibility Testing of Voriconazole in All-in-One Parenteral Nutrition Admixtures. Pharmaceutics 2021, 13, 1447. [Google Scholar] [CrossRef] [PubMed]
- Staven, V.; Wang, S.; Grønlie, I.; Tho, I. Development and evaluation of a test program for Y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr. J. 2016, 15, 29. [Google Scholar] [CrossRef]
- Trissel, L.A. Everything in a compatibility study is important. Am. J. Health Syst. Pharm. 1996, 53, 2990. [Google Scholar] [CrossRef]
- Mühlebach, S. Basics in clinical nutrition: Drugs and nutritional admixtures. E-SPEN Eur. E-J. Clin. Nutr. Metab. 2009, 4, e134–e136. [Google Scholar] [CrossRef]
- Driscoll, D.F. Clinical issues regarding the use of total nutrient admixtures. DICP Ann. Pharmacother. 1990, 24, 296–303. [Google Scholar] [CrossRef]
- Driscoll, D.F. Physicochemical assessment of total nutrient admixture stability and safety: Quantifying the risk. Nutr. Burbank 1997, 13, 166–167. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Dettlaff, K.; Jelińska, A.; Główka, E.; Ogrodowczyk, M. Effect of Lipid Emulsion on Stability of Ampicillin in Total Parenteral Nutrition. Nutrients 2019, 11, 559. [Google Scholar] [CrossRef] [PubMed]
- Stawny, M.; Gostyńska, A.; Nadolna, M.; Jelińska, A. Safe Practice of Y-Site Drug Administration: The Case of Colistin and Parenteral Nutrition. Pharmaceutics 2020, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Gostyńska, A.; Piwowarczyk, L.; Nadolna, M.; Jelińska, A.; Dettlaff, K.; Ogrodowczyk, M.; Popielarz-Brzezinska, M.; Stawny, M. Toward Safe Pharmacotherapy: The Interplay between Meropenem and Parenteral Nutrition Admixtures. Antibiotics 2021, 10, 217. [Google Scholar] [CrossRef]
- Pehlivanli, A.; Eren-Sadioglu, R.; Aktar, M.; Eyupoglu, S.; Sengul, S.; Keven, K.; Erturk, S.; Basgut, B.; Ozcelikay, A.T. Potential drug-drug interactions of immunosuppressants in kidney transplant recipients: Comparison of drug interaction resources. Int. J. Clin. Pharm. 2022, 44, 651–662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).