Levofloxacin and Ciprofloxacin Co-Crystals with Flavonoids: Solid-State Investigation for a Multitarget Strategy against Helicobacter pylori
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthethic Methodologies
2.1.1. Mechanochemical Synthesis
2.1.2. Slurry in Ethanol
2.1.3. Crystallization from Solution
2.2. Solid-State Characterization
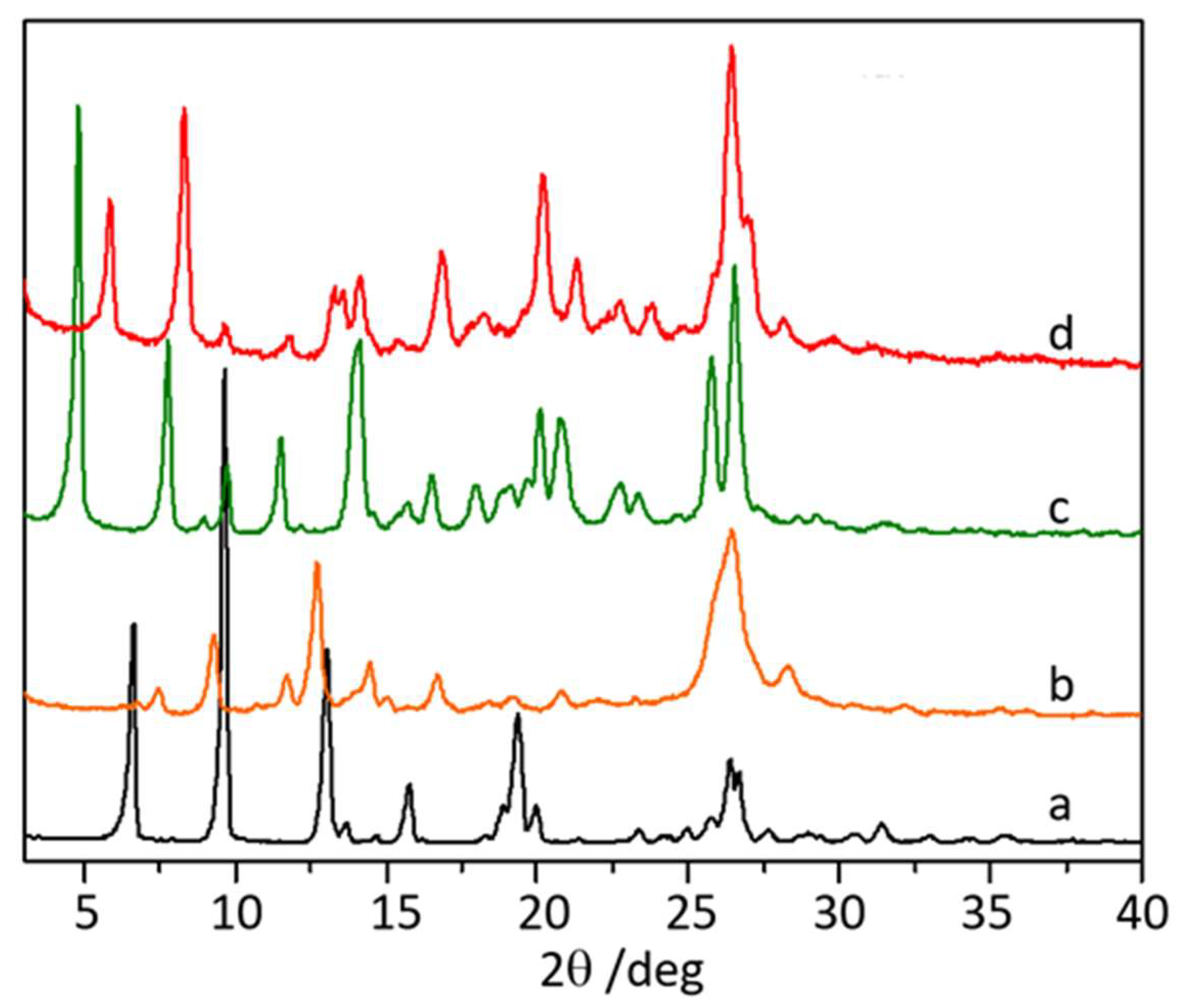
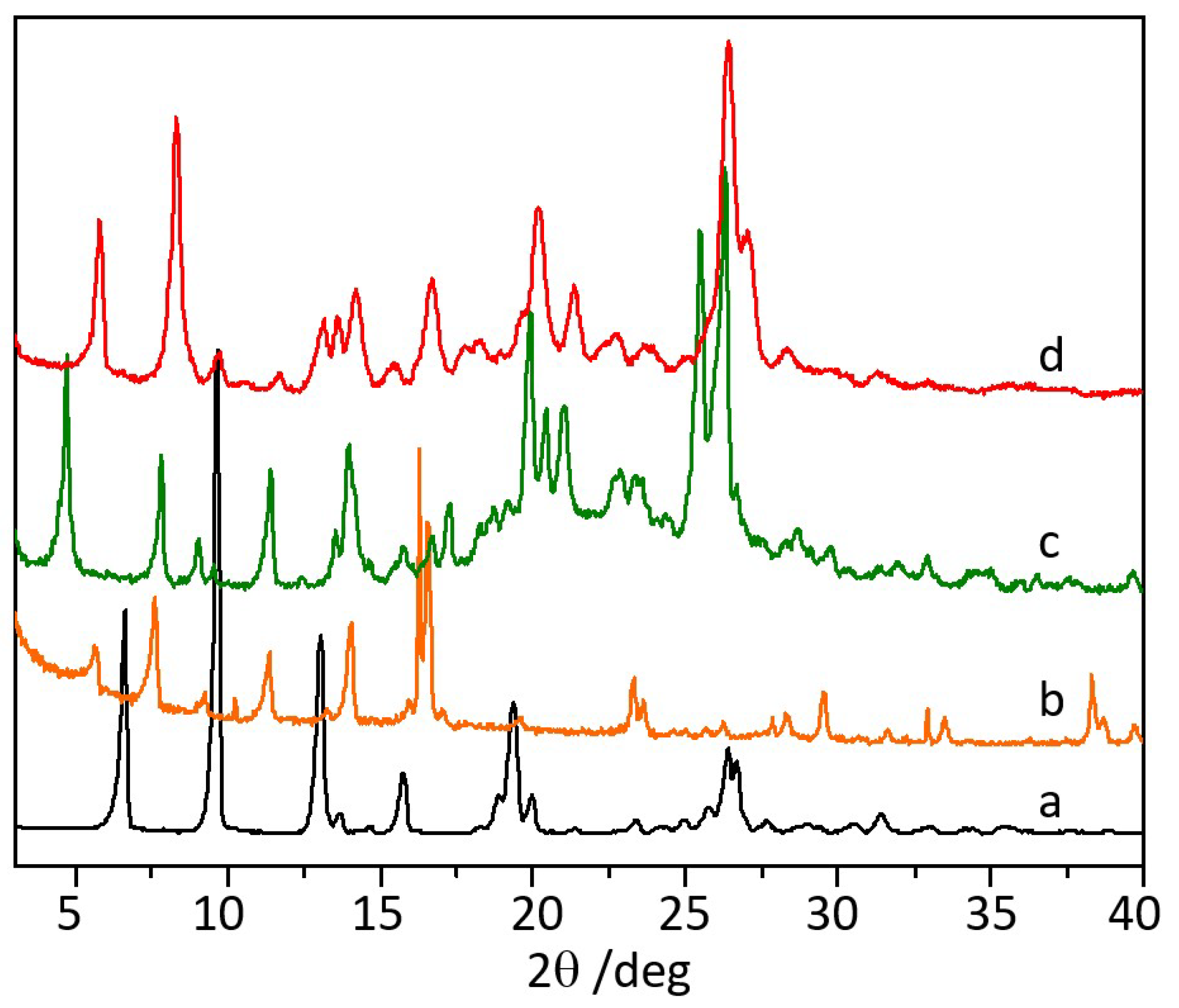
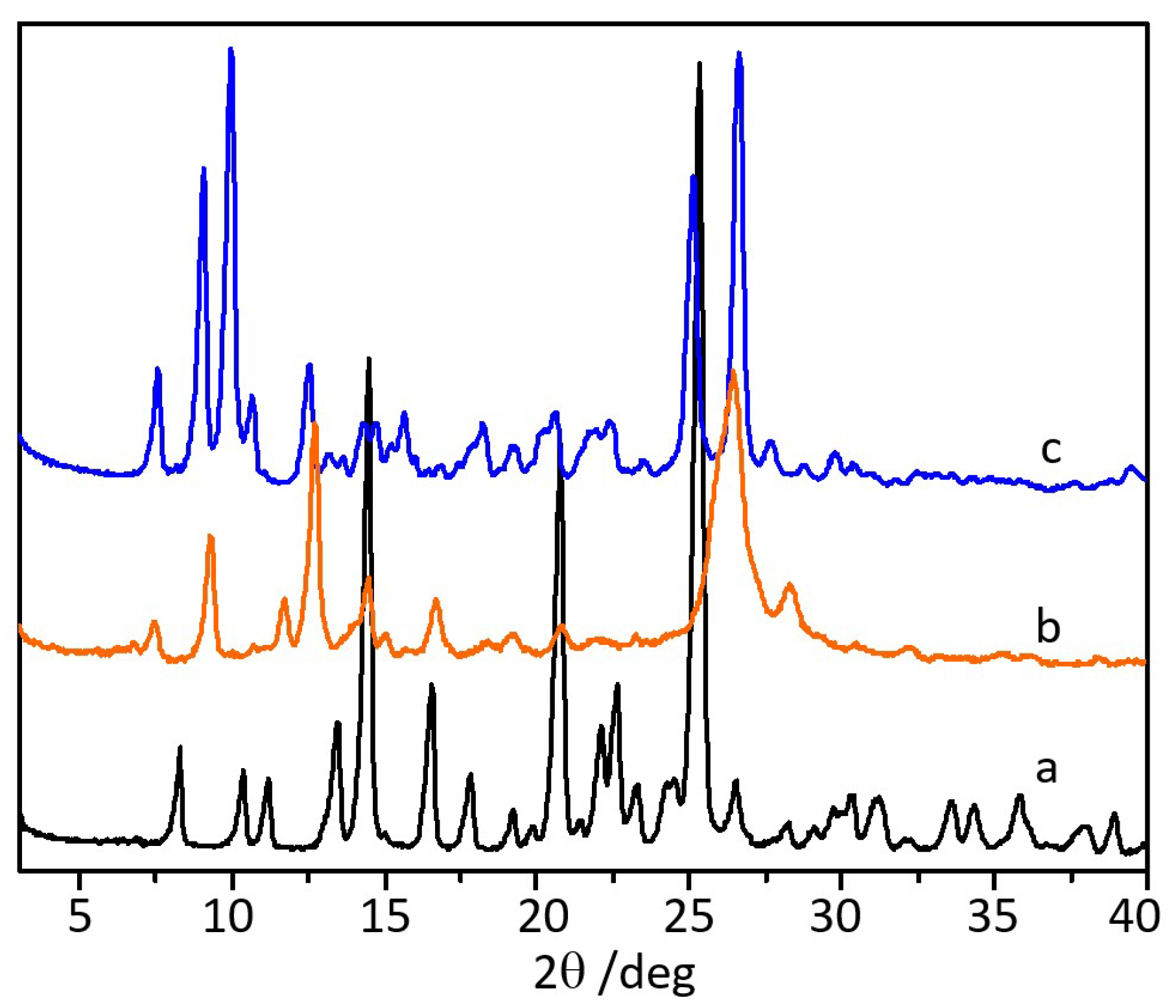
2.2.1. Powder X-ray Diffraction
2.2.2. Variable-Temperature Powder X-ray Diffraction (VT-PXRD)
2.2.3. Thermogravimetric Analysis (TGA)
2.3. Solution 1H NMR Characterization
2.4. Antimicrobial Activity Tests against Helicobacter pylori
3. Results and Discussion
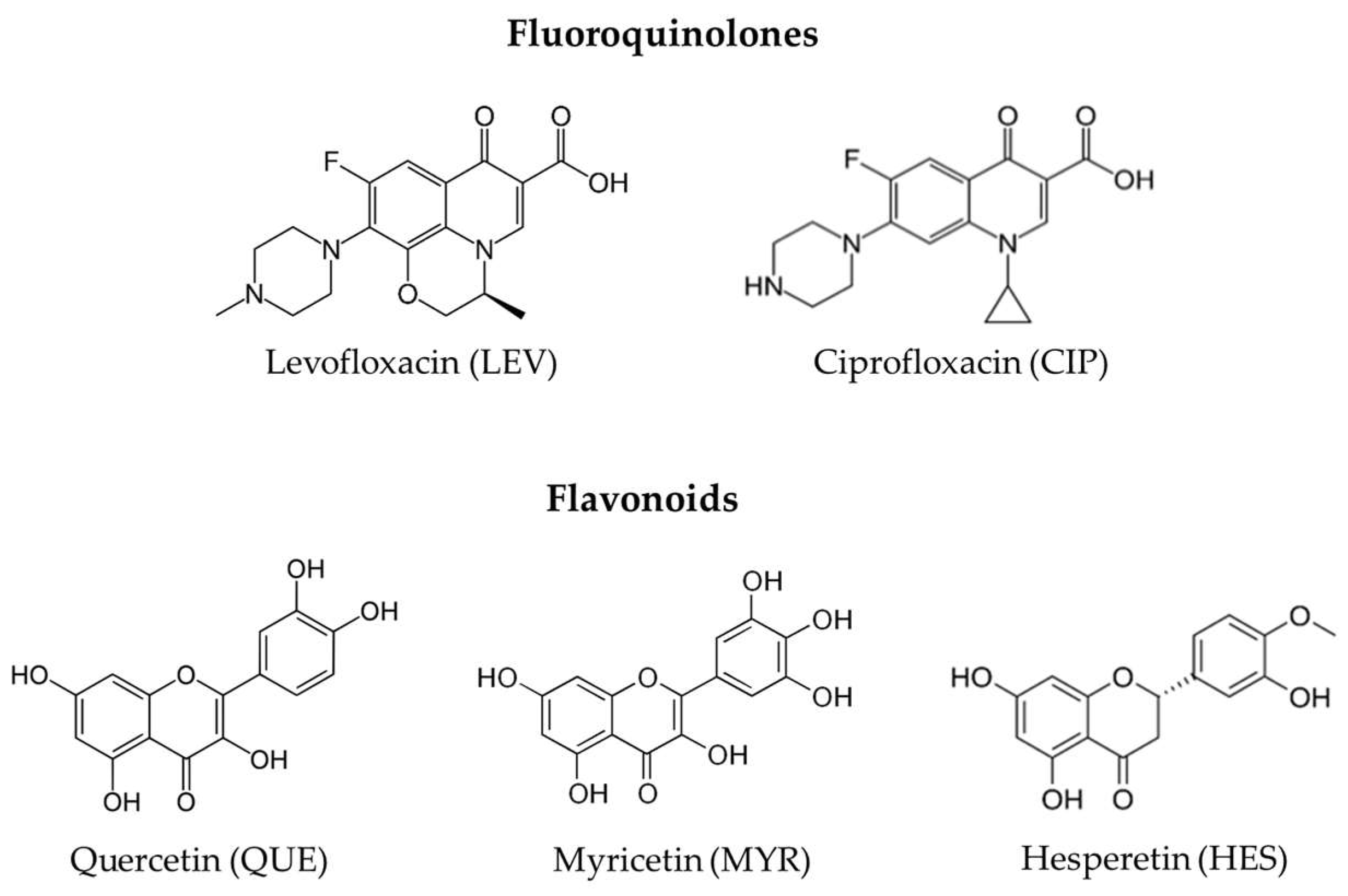
3.1. Co-Crystallization of Levofloxacin with Quercetin, Myricetin, and Hesperetin
3.2. Co-Crystallization of Ciprofloxacin with Quercetin
3.3. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, B.E.; Cohen, H.; Blaser, M.J. Helicobacter Pylori. Clin. Microbiol. Rev. 1997, 10, 720–741. [Google Scholar] [CrossRef]
- McColl, K.E.L. Clinical Practice. Helicobacter Pylori Infection. N. Engl. J. Med. 2010, 362, 1597–1604. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter Pylori Infection and Antibiotic Resistance—From Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guan, L.; Hu, B. Detection and Treatment of Helicobacter Pylori: Problems and Advances. Gastroenterol. Res. Pract. 2022, 2022, e4710964. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter Pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Nista, E.C.; Candelli, M.; Cremonini, F.; Cazzato, I.A.; Di Caro, S.; Gabrielli, M.; Santarelli, L.; Zocco, M.A.; Ojetti, V.; Carloni, E.; et al. Levofloxacin-Based Triple Therapy vs. Quadruple Therapy in Second-Line Helicobacter Pylori Treatment: A Randomized Trial. Aliment. Pharmacol. Ther. 2003, 18, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, S.; Franceschi, F.; Mariani, A.; Thompson, F.; Raimondo, D.; Masci, E.; Testoni, A.; La Rocca, E.; Gasbarrini, A. Second-Line Levofloxacin-Based Triple Schemes for Helicobacter Pylori Eradication. Dig. Liver Dis. 2009, 41, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Pérez-Aisa, Á.; Bermejo, F.; Castro-Fernández, M.; Almela, P.; Barrio, J.; Cosme, Á.; Modolell, I.; Bory, F.; Fernández-Bermejo, M.; et al. Second-Line Therapy with Levofloxacin after Failure of Treatment to Eradicate Helicobacter Pylori Infection: Time Trends in a Spanish Multicenter Study of 1000 Patients. J. Clin. Gastroenterol. 2013, 47, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Perna, F.; Zullo, A.; Ricci, C.; Hassan, C.; Morini, S.; Vaira, D. Levofloxacin-Based Triple Therapy for Helicobacter Pylori Re-Treatment: Role of Bacterial Resistance. Dig. Liver Dis. 2007, 39, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Drlica, K.; Malik, M. Fluoroquinolones: Action and Resistance. Curr. Top. Med. Chem. 2005, 3, 249–282. [Google Scholar] [CrossRef]
- Forsmark, C.E.; Mel Wilco, C.; Cello, J.P.; Margaretten, W.; Lee, B.; Sachdeeva, M.; Satow, J.; Sande, M.A. Ciprofloxacin in the Treatment of Helicobacter Pylori in Patients with Gastritis and Peptic Ulcer. J. Infect. Dis. 1990, 162, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Dresner, D.; Coyle, W.; Nemec, R.; Peterson, R.; Duntemann, T.; Lawson, J.M. Efficacy of Ciprofloxacin in the Eradication of Helicobacter Pylori. S. Med. J. 1996, 89, 775–778. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Helicobacter pylori. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100B-15.pdf (accessed on 13 January 2023).
- World Gastroenterology Organisation. Helicobacter Pylori. Available online: https://www.worldgastroenterology.org/guidelines/helicobacter-pylori (accessed on 13 January 2023).
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A Review on Antimicrobial Botanicals, Phytochemicals and Natural Resistance Modifying Agents from Apocynaceae Family: Possible Therapeutic Approaches against Multidrug Resistance in Pathogenic Microorganisms. Drug Resist. Updat. 2020, 51, 100695. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical Co-Crystals: An Overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duggirala, N.K.; Perry, M.L.; Almarsson, Ö.; Zaworotko, M.J. Pharmaceutical Co-crystals: Along the Path to Improved Medicines. Chem. Commun. 2015, 52, 640–655. [Google Scholar] [CrossRef]
- Fiore, C.; Baraghini, A.; Shemchuk, O.; Sambri, V.; Morotti, M.; Grepioni, F.; Braga, D. Inhibition of the Antibiotic Activity of Cephalosporines by Co-Crystallization with Thymol. Cryst. Growth Des. 2022, 22, 1467–1475. [Google Scholar] [CrossRef]
- Shemchuk, O.; d’Agostino, S.; Fiore, C.; Sambri, V.; Zannoli, S.; Grepioni, F.; Braga, D. Natural Antimicrobials Meet a Synthetic Antibiotic: Carvacrol/Thymol and Ciprofloxacin Co-crystals as a Promising Solid-State Route to Activity Enhancement. Cryst. Growth Des. 2020, 20, 6796–6803. [Google Scholar] [CrossRef]
- Kosalec, I.; Rai, M. Natural Antimicrobials: An Introduction. In Promising Antimicrobials from Natural Products; Springer: Cham, Switzerland, 2022; pp. 3–13. [Google Scholar] [CrossRef]
- Rai, M.; Kosalec, I. Promising Antimicrobials from Natural Products; Springer: Dordrecht, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Havsteen, B.H. The Biochemistry and Medical Significance of the Flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Lalani, S.; Poh, C.L. Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71). Viruses 2020, 12, 184. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K.; et al. Antioxidant Properties of Quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hisaka, T.; Sakai, H.; Sato, T.; Goto, Y.; Nomura, Y.; Fukutomi, S.; Fujita, F.; Mizobe, T.; Nakashima, O.; Tanigawa, M.; et al. Quercetin Suppresses Proliferation of Liver Cancer Cell Lines in Vitro. Anticancer Res 2020, 40, 4695–4700. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fang, L.; Liao, J.; Li, L.; Yao, W.; Xiong, Z.; Zhou, X. Investigation of the Anti-Cancer Effect of Quercetin on HepG2 Cells in Vivo. PLoS ONE 2017, 12, e0172838. [Google Scholar] [CrossRef] [PubMed]
- González-Segovia, R.; Quintanar, J.L.; Salinas, E.; Ceballos-Salazar, R.; Aviles-Jiménez, F.; Torres-López, J. Effect of the Fl Avonoid Quercetin on Infl Ammation and Lipid Peroxidation Induced by Helicobacter Pylori in Gastric Mucosa of Guinea Pig. J. Gastroenterol. 2008, 43, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Wang, Q.; Bao, Y.; Chao, Y. Anti-Rheumatic Effect of Quercetin and Recent Developments in Nano Formulation. RSC Adv. 2021, 11, 7280–7293. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An Insight into Anticancer, Antioxidant, Antimicrobial, Antidiabetic and Anti-Inflammatory Effects of Quercetin: A Review. Polym. Bull. 2022, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial Activity of Flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Lenard, N.; Henagan, T.M.; Lan, T.; Nguyen, A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Chong, Y.; Kim, M.K. Myricetin: Biological Activity Related to Human Health. Appl. Biol. Chem. 2016, 59, 259–269. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, X.M.; Wang, S.J.; Yang, Y.; Cao, Y.L. Analgesic Activity of Myricetin Isolated from Myrica Rubra Sieb. et Zucc. Leaves. Arch. Pharmacal. Res. 2009, 32, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.K.; Jung, I.; Kim, M.E.; Bae, S.K.; Lee, J.S. Anti-Cancer Activity of Myricetin against Human Papillary Thyroid Cancer Cells Involves Mitochondrial Dysfunction–Mediated Apoptosis. Biomed. Pharmacother. 2017, 91, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Wang, H.R.; Wang, Y.I.; Zhai, Z.H.; Wang, L.W.; Li, L.; Zhang, C.; Tang, L. Myricetin Attenuated Diabetes-Associated Kidney Injuries and Dysfunction via Regulating Nuclear Factor (Erythroid Derived 2)-like 2 and Nuclear Factor-ΚB Signaling. Front. Pharmacol. 2019, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-Tumor Effects and Associated Molecular Mechanisms of Myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef]
- Gordon, M.H.; Roedig-Penman, A. Antioxidant Activity of Quercetin and Myricetin in Liposomes. Chem. Phys. Lipids. 1998, 97, 79–85. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin Bioactive Effects: Moving from Preclinical Evidence to Potential Clinical Applications. BMC Complement. Med. Ther. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Srimathi Priyanga, K.; Vijayalakshmi, K. Investigation of Antioxidant Potential of Quercetin and Hesperidin: An in Vitro Approach. Asian J. Pharm. Clin. Res. 2017, 10, 83–86. [Google Scholar] [CrossRef]
- González, A.; Casado, J.; Lanas, Á. Fighting the Antibiotic Crisis: Flavonoids as Promising Antibacterial Drugs Against Helicobacter Pylori Infection. Front. Cell. Infect. Microbiol. 2021, 11, 671. [Google Scholar] [CrossRef]
- González, A.; Salillas, S.; Velázquez-Campoy, A.; Espinosa Angarica, V.; Fillat, M.F.; Sancho, J.; Lanas, Á. Identifying Potential Novel Drugs against Helicobacter Pylori by Targeting the Essential Response Regulator HsrA. Sci. Rep. 2019, 9, 11294. [Google Scholar] [CrossRef]
- Chadha, K.; Karan, M.; Bhalla, Y.; Chadha, R.; Khullar, S.; Mandal, S.; Vasisht, K. Co-crystals of Hesperetin: Structural, Pharmacokinetic, and Pharmacodynamic Evaluation. Cryst. Growth Des. 2017, 17, 2386–2405. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial Action of Propolis and Some of Its Components: The Effects on Growth, Membrane Potential and Motility of Bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, D.; Scarlato, V.; Vannini, A. Targeting of Regulators as a Promising Approach in the Search for Novel Antimicrobial Agents. Microorganisms 2022, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, A.; Pelliciari, S.; Musiani, F.; Chiappori, F.; Roncarati, D.; Scarlato, V. Definition of the Binding Architecture to a Target Promoter of HP1043, the Essential Master Regulator of Helicobacter Pylori. Int. J. Mol. Sci. 2021, 22, 7848. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Roncarati, D.; D’Agostino, F.; Antoniciello, F.; Scarlato, V. Insights into the Orchestration of Gene Transcription Regulators in Helicobacter Pylori. Int. J. Mol. Sci. 2022, 23, 13688. [Google Scholar] [CrossRef]
- Tan, D.; Loots, L.; Friščić, T. Towards medicinal mechanochemistry: Evolution of milling from pharmaceutical solid form screening to the synthesis of active pharmaceutical ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. [Google Scholar] [CrossRef]
- Sekhon, B.S. Drug-Drug Co-Crystals. DARU J. Pharm. Sci. 2012, 20, 45. [Google Scholar] [CrossRef]
- Bordignon, S.; Vioglio, P.C.; Priola, E.; Voinovich, D.; Gobetto, R.; Nishiyama, Y.; Chierotti, M.R. Engineering Codrug Solid Forms: Mechanochemical Synthesis of an Indomethacin–Caffeine System. Cryst. Growth Des. 2017, 17, 5744–5752. [Google Scholar] [CrossRef]
- Byrn, S.R.; Pfeiffer, R.R.; Stephenson, G.; Grant, D.J.W.; Gleason, W.B. Solid-State Pharmaceutical Chemistry. Chem. Mater. 1994, 6, 1148–1158. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure–Activity Relationship: An Update Review. Phytother Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]


| MIC [µg/mL] (MBC) | ||
|---|---|---|
| Compounds | H. pylori Strains | |
| G27 | 26695 | |
| LEV2·HES | 1 (2) | 0.5 (1) |
| 2LEV + HES | 1 (2) | 0.5 (1) |
| LEV·MYR | 4 (8) | 2 (4) |
| LEV + MYR | 4 (8) | 4 (8) |
| LEV·QUE | 1 (2) | 1 (2) |
| LEV + QUE | 1 (2) | 1 (2) |
| LEV | 1 (2) | 0.5 (1) |
| CIP·QUE | 2 (4) | 1 (2) |
| CIP + QUE | 2 (4) | 2 (2) |
| CIP | 2 (2) | 1 (2) |
| HES | 128 (256) | 128 (256) |
| MYR | 256 (512) | 256 (512) |
| QUE | 512 (512) | 256 (512) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, C.; Antoniciello, F.; Roncarati, D.; Scarlato, V.; Grepioni, F.; Braga, D. Levofloxacin and Ciprofloxacin Co-Crystals with Flavonoids: Solid-State Investigation for a Multitarget Strategy against Helicobacter pylori. Pharmaceutics 2024, 16, 203. https://doi.org/10.3390/pharmaceutics16020203
Fiore C, Antoniciello F, Roncarati D, Scarlato V, Grepioni F, Braga D. Levofloxacin and Ciprofloxacin Co-Crystals with Flavonoids: Solid-State Investigation for a Multitarget Strategy against Helicobacter pylori. Pharmaceutics. 2024; 16(2):203. https://doi.org/10.3390/pharmaceutics16020203
Chicago/Turabian StyleFiore, Cecilia, Federico Antoniciello, Davide Roncarati, Vincenzo Scarlato, Fabrizia Grepioni, and Dario Braga. 2024. "Levofloxacin and Ciprofloxacin Co-Crystals with Flavonoids: Solid-State Investigation for a Multitarget Strategy against Helicobacter pylori" Pharmaceutics 16, no. 2: 203. https://doi.org/10.3390/pharmaceutics16020203
APA StyleFiore, C., Antoniciello, F., Roncarati, D., Scarlato, V., Grepioni, F., & Braga, D. (2024). Levofloxacin and Ciprofloxacin Co-Crystals with Flavonoids: Solid-State Investigation for a Multitarget Strategy against Helicobacter pylori. Pharmaceutics, 16(2), 203. https://doi.org/10.3390/pharmaceutics16020203









