Controlling the Quality of Nanodrugs According to Their New Property—Radiothermal Emission
Abstract
1. Introduction
2. Materials and Methods
2.1. IFN Pharmaceuticals
2.2. VLP Vaccines
2.3. The Measurement of Intrinsic Radiothermal Emission
2.4. Nanoparticle Size Determination
2.5. The Application of the Technique in Preclinical Trials (Measurements of the T-Cell Response)
Cellular Response
2.6. Metrological Support
3. Results and Discussion
3.1. The Radiothermal Emission of IBPs
3.1.1. In-Lab Precision
3.1.2. Specificity
3.2. The Application of the Method in Preclinical Trials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Syroeshkin, A.V.; Petrov, G.V.; Taranov, V.V.; Pleteneva, T.V.; Koldina, A.M.; Gaydashev, I.A.; Kolyabina, E.S.; Galkina, D.A.; Sorokina, E.V.; Uspenskaya, E.V.; et al. Radiothermal Emission of Nanoparticles with a Complex Shape as a Tool for the Quality Control of Pharmaceuticals Containing Biologically Active Nanoparticles. Pharmaceutics 2023, 15, 966. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, M.; Sadhukhan, M.; Al-Hamdani, Y.S.; Hermann, J.; Tkatchenko, A. Coulomb Interactions between Dipolar Quantum Fluctuations in van Der Waals Bound Molecules and Materials. Nat. Commun. 2021, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Qi, W.; Wei, Y.; Ru, G.; Liu, W. High-Throughput Calculation of Interlayer van Der Waals Forces Validated with Experimental Measurements. Research 2022, 2022, 9765121. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yue, K.; Zhong, W.; Zhang, G.; Wang, L.; Wang, H.; Zhang, H.; Zhang, X. Ligand-Receptor Interaction in the Specific Targeting of Biomimetic Peptide Nanoparticles to Lysophosphatidylcholine. Int. J. Biol. Macromol. 2023, 227, 193–202. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Abrikosova, I.I.; Lifshitz, E.M. Molecular Attraction of Condensed Bodies. Phys.-Uspekhi 2015, 58, 906–924. [Google Scholar] [CrossRef]
- Dedkov, G.V.; Kyasov, A.A. On Thermal Vacuum Radiation of Nanoparticles and Their Ensembles. Phys. B Condens. Matter 2014, 433, 67–71. [Google Scholar] [CrossRef]
- Uspenskaya, E.V.; Pleteneva, T.V.; Syroeshkin, A.V.; Tarabrina, I.V. Preparation, Characterization and Studies of Physicochemical and Biological Properties of Drugs Coating Lactose in Fluidized Beds. Int. J. Appl. Pharm. 2020, 12, 272–278. [Google Scholar] [CrossRef]
- Syroeshkin, A.V.; Uspenskaya, E.V.; Pleteneva, T.V.; Morozova, M.A.; Maksimova, T.V.; Koldina, A.M.; Makarova, M.P.; Levitskaya, O.V.; Zlatskiy, I.A. Mechanochemical Activation of Pharmaceutical Substances as a Factor for Modification of Their Physical, Chemical and Biological Properties. Int. J. Appl. Pharm. 2019, 11, 118–123. [Google Scholar] [CrossRef]
- Martynenko, Y.V.; Ognev, L.I. Thermal Radiation from Nanoparticles. Tech. Phys. 2005, 50, 1522. [Google Scholar] [CrossRef]
- Pukhov, K.K.; Basiev, T.T.; Orlovskii, Y.V. Spontaneous Emission in Dielectric Nanoparticles. JETP Lett. 2008, 88, 12–18. [Google Scholar] [CrossRef]
- Petrov, G.V.; Taranov, V.V.; Syroeshkin, A.V. Express Method for Quality Control Of Products After The Fluidized Bed Aerosol Chamber By Detecting Radio Thermal Emission Of Nanoparticles Section A-Research Paper Express Method For Quality Control Of Products After The Fluidized Bed Aerosol Chamber By Detecting Radio Thermal Emission Of Nanoparticles. Chem. Bull. 2023, 12, 3035–3041. [Google Scholar] [CrossRef]
- Buonaguro, L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M. Developments in Virus-like Particle-Based Vaccines for Infectious Diseases and Cancer. Expert. Rev. Vaccines 2011, 10, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.; Gomes, A.; Vogel, M.; Bachmann, M. Interaction of Viral Capsid-Derived Virus-Like Particles (VLPs) with the Innate Immune System. Vaccines 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Akahata, W.; Yang, Z.-Y.; Andersen, H.; Sun, S.; Holdaway, H.A.; Kong, W.-P.; Lewis, M.G.; Higgs, S.; Rossmann, M.G.; Rao, S.; et al. A Virus-like Particle Vaccine for Epidemic Chikungunya Virus Protects Nonhuman Primates against Infection. Nat. Med. 2010, 16, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Petrov, G.V.; Gaidashev, I.A.; Syroeshkin, A.V. Physical and Chemical Characteristic of Aqueous Colloidal Infusions of Medicinal Plants Containing Humic Acids. Int. J. Appl. Pharm. 2024, 16, 76–82. [Google Scholar] [CrossRef]
- Kostina, L.V.; Filatov, I.E.; Eliseeva, O.V.; Latyshev, O.E.; Chernoryzh, Y.Y.; Yurlov, K.I.; Lesnova, E.I.; Khametova, K.M.; Cherepushkin, S.A.; Savochkina, T.E.; et al. Study of the Safety and Immunogenicity of VLP-Based Vaccine for the Prevention of Rotavirus Infection in Neonatal Minipig Model. Probl. Virol. 2023, 68, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Hadj Hassine, I.; Ben M’hadheb, M.; Almalki, M.A.; Gharbi, J. Virus-like Particles as Powerful Vaccination Strategy against Human Viruses. Rev. Med. Virol. 2024, 34, e2498. [Google Scholar] [CrossRef]
- Boix-Besora, A.; Gòdia, F.; Cervera, L. Gag Virus-like Particles Functionalized with SARS-CoV-2 Variants: Generation, Characterization and Recognition by COVID-19 Convalescent Patients’ Sera. Vaccines 2023, 11, 1641. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, Interferon-like Cytokines, and Their Receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- Moore, G.E. Interferon. Surg. Gynecol. Obstet. 1981, 153, 97–102. [Google Scholar]
- Krachmarova, E.; Ivanov, I.; Nacheva, G. Nucleic Acids in Inclusion Bodies Obtained from E. Coli Cells Expressing Human Interferon-Gamma. Microb. Cell Fact. 2020, 19, 139. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, J.; Yamazaki, S.; Hosoi, K.; Kimura, S.; Hanada, K.; Shtmazu, T.; Shtmizu, H. Characterization of E. Coli-Derived Recombinant Human Interferon-β as Compared with Fibroblast Human Interferon-β. J. Biochem. 1987, 101, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Stanton, G.J.; Weigent, D.A.; Fleischmann, W.R.; Dianzani, F.; Baron, S. Interferon Review. Investig. Radiol. 1987, 22, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.; Kirchner, H. Interferone Und Ihre Wirkungen. Oncol. Res. Treat. 1988, 11, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Ustinnikova, O.B.; Gaiderova, L.A.; Baykova, M.L.; Lobanova, T.N.; Scherbachenko, I.M.; Goloschapova, E.O.; Bondarev, V.P. Review of Methodological Approaches to Assessing the Quality of Medicines Based on Recombinant Interferons. Bioprep. Prev. Diagn. Treat. 2017, 17, 152–157. [Google Scholar]
- Bellucci, G.; Albanese, A.; Rizzi, C.; Rinaldi, V.; Salvetti, M.; Ristori, G. The Value of Interferon β in Multiple Sclerosis and Novel Opportunities for Its Anti-Viral Activity: A Narrative Literature Review. Front. Immunol. 2023, 14, 1161849. [Google Scholar] [CrossRef] [PubMed]
- Marziniak, M.; Meuth, S. Current Perspectives on Interferon Beta-1b for the Treatment of Multiple Sclerosis. Adv. Ther. 2014, 31, 915–931. [Google Scholar] [CrossRef]
- Shearer, W.T.; Kline, M.W.; Abramson, S.L.; Fenton, T.; Starr, S.E.; Douglas, S.D. Recombinant Human Gamma Interferon in Human Immunodeficiency Virus-Infected Children: Safety, CD4+-Lymphocyte Count, Viral Load, and Neutrophil Function (AIDS Clinical Trials Group Protocol 211). Clin. Diagn. Lab. Immunol. 1999, 6, 311–315. [Google Scholar] [CrossRef]
- Cherepushkin, S.A.; Tsibezov, V.V.; Yuzhakov, A.G.; Latyshev, O.E.; Alekseev, K.P.; Altayeva, E.G.; Khametova, K.M.; Vorkunova, G.K.; Yuzhakova, K.A.; Grebennikova, T.V. Synthesis and Characterization of Human Rotavirus A (Reoviridae: Sedoreovirinae: Rotavirus: Rotavirus) Virus-like Particles. Probl. Virol. 2021, 66, 55–64. [Google Scholar] [CrossRef]
- Yang, S.; Ye, Y.-H.; Zang, J.; Pei, Y.; Xia, Y.; Zhang, J. Direct Observation Brownian Motion of Individual Nanoparticles in Water Using Microsphere-Assisted Microscopy. Opt. Lett. 2021, 46, 3099. [Google Scholar] [CrossRef]
- Minoura, I.; Katayama, E.; Sekimoto, K.; Muto, E. One-Dimensional Brownian Motion of Charged Nanoparticles along Microtubules: A Model System for Weak Binding Interactions. Biophys. J. 2010, 98, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Kurteva, E.; Vasilev, G.; Tumangelova-Yuzeir, K.; Ivanova, I.; Ivanova-Todorova, E.; Velikova, T.; Kyurkchiev, D. Interferon-Gamma Release Assays Outcomes in Healthy Subjects Following BNT162b2 MRNA COVID-19 Vaccination. Rheumatol. Int. 2022, 42, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, P.; Sanz, J.C.; San Román, J.; Pérez-Abeledo, M.; Carretero, M.; Megías, G.; Viñuela-Prieto, J.M.; Ramos, B.; Canora, J.; Martínez-Peromingo, F.J.; et al. A Pilot Study for the Evaluation of an Interferon Gamma Release Assay (IGRA) To Measure T-Cell Immune Responses after SARS-CoV-2 Infection or Vaccination in a Unique Cloistered Cohort. J. Clin. Microbiol. 2022, 60, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-Like Particles: Revolutionary Platforms for Developing Vaccines Against Emerging Infectious Diseases. Front. Microbiol. 2022, 12, 790121. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Jailani, A.A.K.; Mandal, B. Exigency of Plant-Based Vaccine against COVID-19 Emergence as Pandemic Preparedness. Vaccines 2023, 11, 1347. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Couillard, J.; Trépanier, S.; Poulin, J.-F.; Yassine-Diab, B.; Guy, B.; Ward, B.J.; Landry, N. Immunogenicity and Safety of a Quadrivalent Plant-Derived Virus like Particle Influenza Vaccine Candidate—Two Randomized Phase II Clinical Trials in 18 to 49 and ≥50 Years Old Adults. PLoS ONE 2019, 14, e0216533. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.R. The Role of Structure in the Biology of Interferon Signaling. Front. Immunol. 2020, 11, 606489. [Google Scholar] [CrossRef]
- Crosse, K.M.; Monson, E.A.; Beard, M.R.; Helbig, K.J. Interferon-Stimulated Genes as Enhancers of Antiviral Innate Immune Signaling. J. Innate Immun. 2018, 10, 85–93. [Google Scholar] [CrossRef]
- Bayer, M.E.; Blumberg, B.S.; Werner, B. Particles Associated with Australia Antigen in the Sera of Patients with Leukaemia, Down’s Syndrome and Hepatitis. Nature 1968, 218, 1057–1059. [Google Scholar] [CrossRef]
- Isaacs, A. Interferon. Sci. Am. 1961, 204, 51–57. [Google Scholar] [CrossRef]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Young, H.A. Interferons: Success in Anti-Viral Immunotherapy. Cytokine Growth Factor. Rev. 2014, 25, 369–376. [Google Scholar] [CrossRef]
- Ruther, U.; Nunnensiek, C.; Muller, H.A.; Bader, H.; May, U.; Jipp, P. Interferon Alpha (IFN Alpha 2a) Therapy for Herpes Virus-Associated Inflammatory Bowel Disease (Ulcerative Colitis and Crohn’s Disease). Hepatogastroenterology 1998, 45, 691–699. [Google Scholar] [PubMed]
- Bigley, N.J. Complexity of Interferon-γ Interactions with HSV-1. Front. Immunol. 2014, 5, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madsen, C. The Innovative Development in Interferon Beta Treatments of Relapsing-remitting Multiple Sclerosis. Brain Behav. 2017, 7, e00696. [Google Scholar] [CrossRef] [PubMed]
- Ed-driouch, C.; Chéneau, F.; Simon, F.; Pasquier, G.; Combès, B.; Kerbrat, A.; Le Page, E.; Limou, S.; Vince, N.; Laplaud, D.; et al. Multiple Sclerosis Clinical Decision Support System Based on Projection to Reference Datasets. Ann. Clin. Transl. Neurol. 2022, 9, 1863–1873. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Lin, F.; Wu, L.; Tan, L.; Lu, L.; Xie, X.; Zhang, Y.; Bao, Y.; Ma, Y.; Huang, X.; et al. The Prevalence and the Effect of Interferon -γ in the Comorbidity of Rheumatoid Arthritis and Depression. Behav. Brain Res. 2023, 439, 114237. [Google Scholar] [CrossRef]
- Ni, L.; Lu, J. Interferon Gamma in Cancer Immunotherapy. Cancer Med. 2018, 7, 4509–4516. [Google Scholar] [CrossRef]
- Burke, J.D.; Young, H.A. IFN-γ: A Cytokine at the Right Time, Is in the Right Place. Semin. Immunol. 2019, 43, 101280. [Google Scholar] [CrossRef]
- Kostina, L.V.; Zaberezhnyy, A.D.; Grebennikova, T.V.; Antipova, N.V.; Aliper, T.I.; Nepoklonov, E.A. Vaccines against Avian Influenza in Poultry. Probl. Virol. 2017, 62, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Arunachalam, P.S.; Andreani, G.; Golden, N.; Fontenot, J.; Aye, P.P.; Röltgen, K.; Lehmicke, G.; Gobeil, P.; Dubé, C.; et al. Safety, Immunogenicity, and Protection Provided by Unadjuvanted and Adjuvanted Formulations of a Recombinant Plant-Derived Virus-like Particle Vaccine Candidate for COVID-19 in Nonhuman Primates. Cell Mol. Immunol. 2022, 19, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Sharifzadeh, M.; Mottaghi-Dastjerdi, N.; Soltany Rezae Raad, M. A Review of Virus-Like Particle-Based SARS-CoV-2 Vaccines in Clinical Trial Phases. Iran. J. Pharm. Res. 2022, 21, e127042. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.N.; Yadav, S.; Shire, S.J.; Kalonia, D.S. Dipole-Dipole Interaction in Antibody Solutions: Correlation with Viscosity Behavior at High Concentration. Pharm. Res. 2014, 31, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Rieser, J.; Ciampini, M.A.; Rudolph, H.; Kiesel, N.; Hornberger, K.; Stickler, B.A.; Aspelmeyer, M.; Delić, U. Tunable Light-Induced Dipole-Dipole Interaction between Optically Levitated Nanoparticles. Science (1979) 2022, 377, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Pohl, C.; Polimeni, M.; Indrakumar, S.; Streicher, W.; Peters, G.H.J.; Nørgaard, A.; Lund, M.; Harris, P. Electrostatics Drive Oligomerization and Aggregation of Human Interferon Alpha-2a. J. Phys. Chem. B 2021, 125, 13657–13669. [Google Scholar] [CrossRef] [PubMed]
- Fukuzumi, S.; Ohkubo, K.; D’Souza, F.; Sessler, J.L. Supramolecular Electron Transfer by Anion Binding. Chem. Commun. 2012, 48, 9801. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Sun, C.; Zhang, X. Microbubble-Enhanced Water Activation by Cold Plasma. SSRN Electron. J. 2022, 446, 137318. [Google Scholar] [CrossRef]
- Coglitore, D. Nanoparticle Dynamics in Simple Fluids; The University of Liverpool: Liverpool, UK, 2016. [Google Scholar]
- Jin, S.-E.; Jin, J.E.; Hwang, W.; Hong, S.W. Photocatalytic Antibacterial Application of Zinc Oxide Nanoparticles and Self-Assembled Networks under Dual UV Irradiation for Enhanced Disinfection. Int. J. Nanomed. 2019, 14, 1737–1751. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Sathishkumar, P.; Albormani, O.; Murugesan, S.; Kandasamy, M.; Selvaraj, M.; Suresh, S.; Kumar, S.K.; Contreras, D.; Váldes, H.; et al. Silver Nanoparticles Modified ZnO Nanocatalysts for Effective Degradation of Ceftiofur Sodium under UV–Vis Light Illumination. Chemosphere 2023, 313, 137515. [Google Scholar] [CrossRef]
- Zilker, M.; Sörgel, F.; Holzgrabe, U. A Systematic Review of the Stability of Finished Pharmaceutical Products and Drug Substances beyond Their Labeled Expiry Dates. J. Pharm. Biomed. Anal. 2019, 166, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Kutty, R.G.; Bevry, M.; Hoffmann, P.; Abourashed, E.A. Determination of Albuterol and Montelukast Post-Expiry Drug Strength by HPLC. Heliyon 2022, 8, e10104. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhin, O.V.; Ponezheva, Z.B.; Kupchenko, A.N.; Shuvalov, A.N.; Guseva, T.S.; Parshina, O.V.; Malinovskaya, V.V.; Akimkin, V.G. Clinical and Interferon-Modulating Efficacy of a Combination of Rectal and Topical Dosage Forms of Interferon-A2b in Acute Respiratory Infections. Ter. Arkh 2018, 90, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Frontiera, R.R. Intermolecular Forces Dictate Vibrational Energy Transfer in Plasmonic–Molecule Systems. ACS Nano 2022, 16, 847–854. [Google Scholar] [CrossRef]
- Dong, X.; Wang, S.; Yu, P.; Yang, F.; Zhao, J.; Wu, L.-Z.; Tung, C.-H.; Wang, J. Ultrafast Vibrational Energy Transfer through the Covalent Bond and Intra- and Intermolecular Hydrogen Bonds in a Supramolecular Dimer by Two-Dimensional Infrared Spectroscopy. J. Phys. Chem. B 2020, 124, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, B.L.; Pellicane, A.J.; Tyring, S.K. Vaccine Immunology. Dermatol. Ther. 2009, 22, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Hagan, T.; Gerritsen, B.; Tomalin, L.E.; Fourati, S.; Mulè, M.P.; Chawla, D.G.; Rychkov, D.; Henrich, E.; Miller, H.E.R.; Diray-Arce, J.; et al. Transcriptional Atlas of the Human Immune Response to 13 Vaccines Reveals a Common Predictor of Vaccine-Induced Antibody Responses. Nat. Immunol. 2022, 23, 1788–1798. [Google Scholar] [CrossRef]

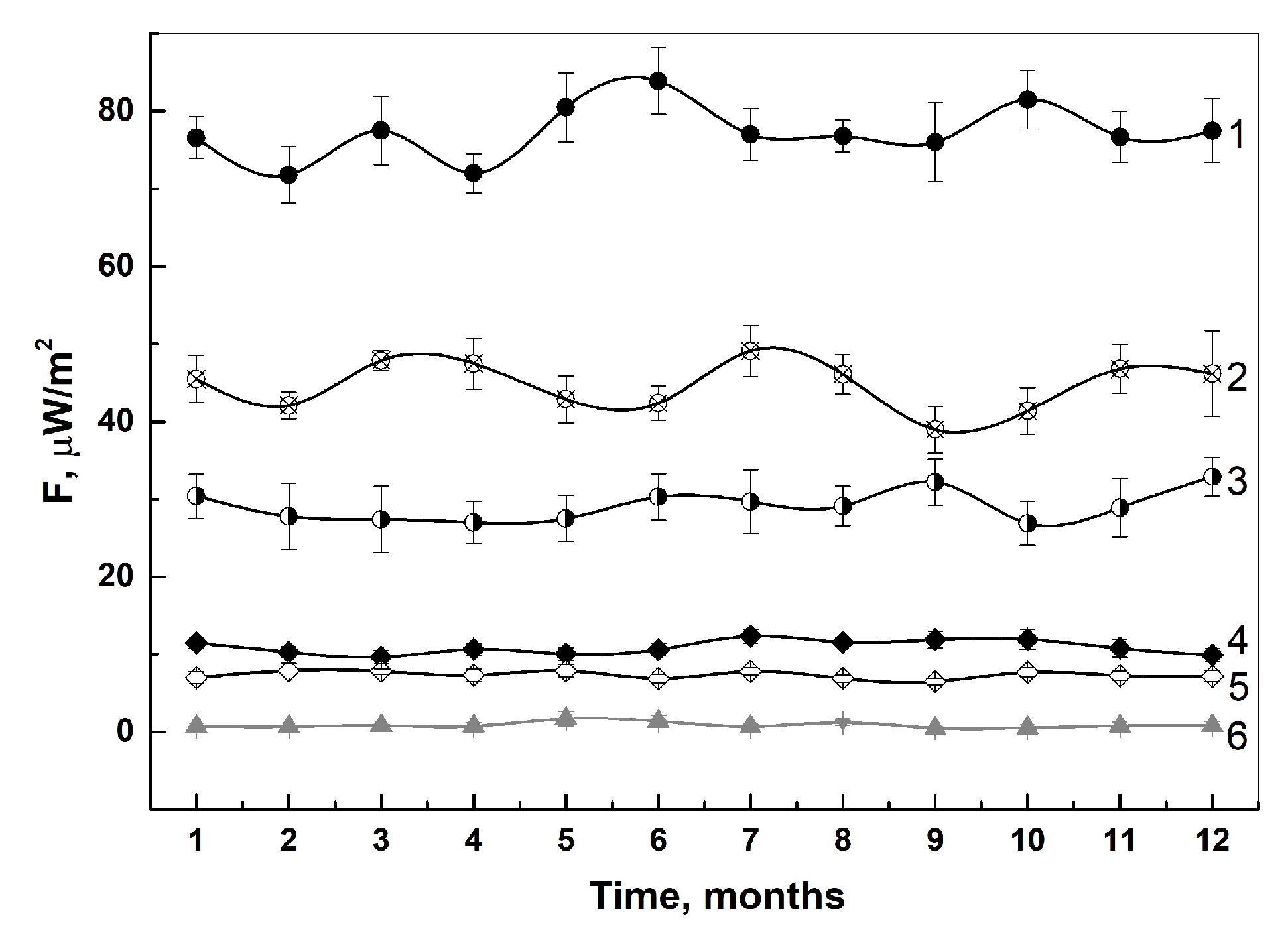
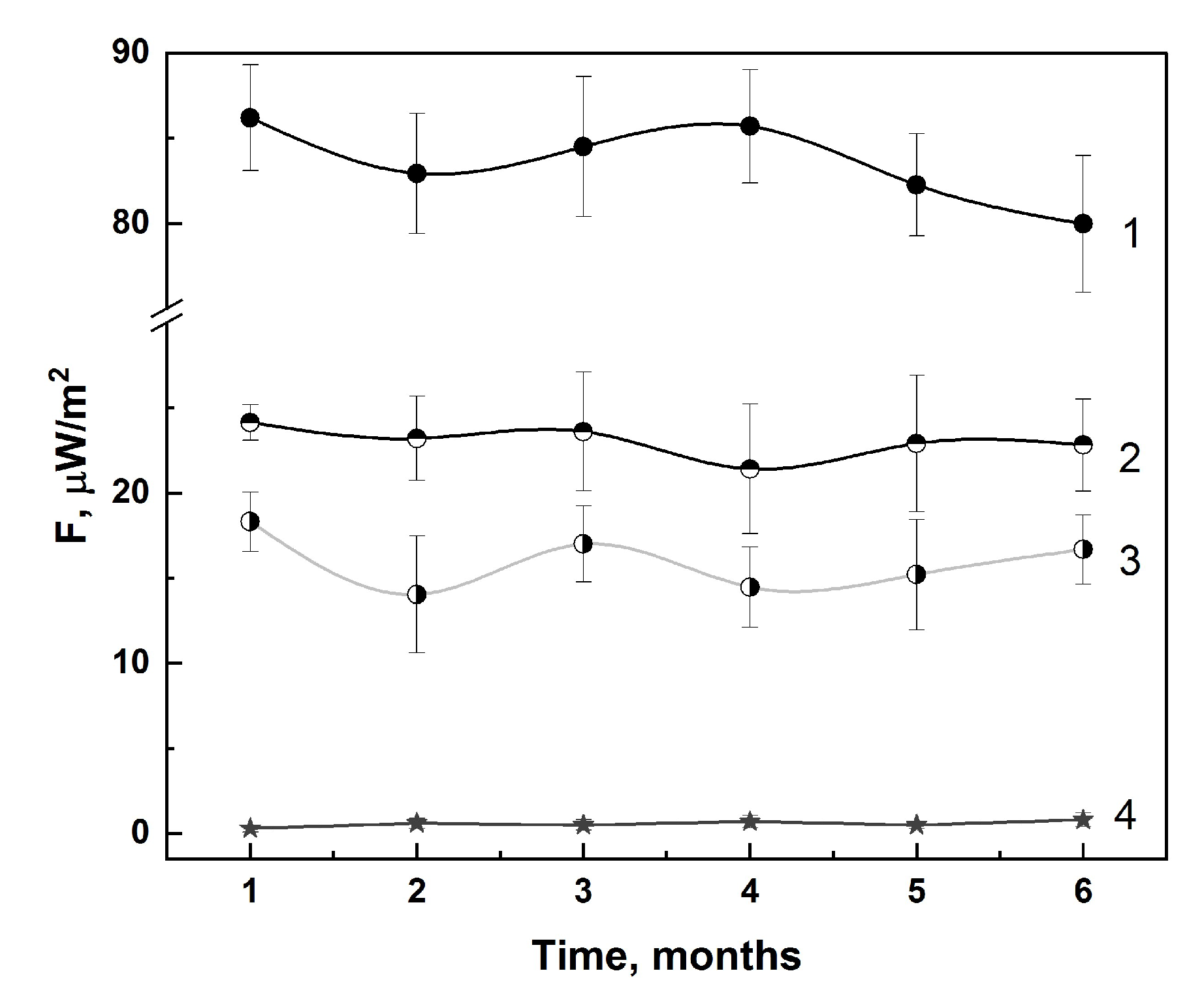
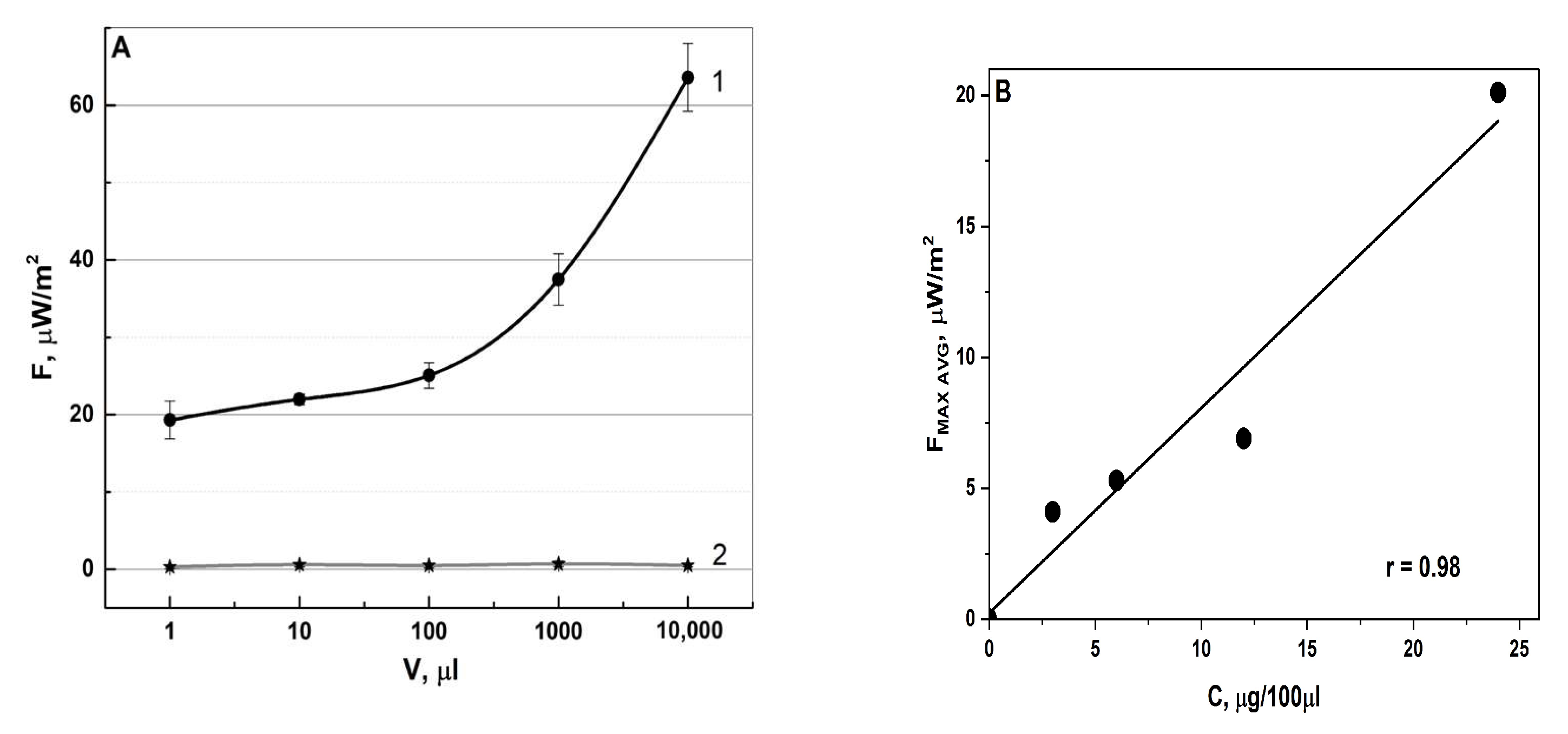


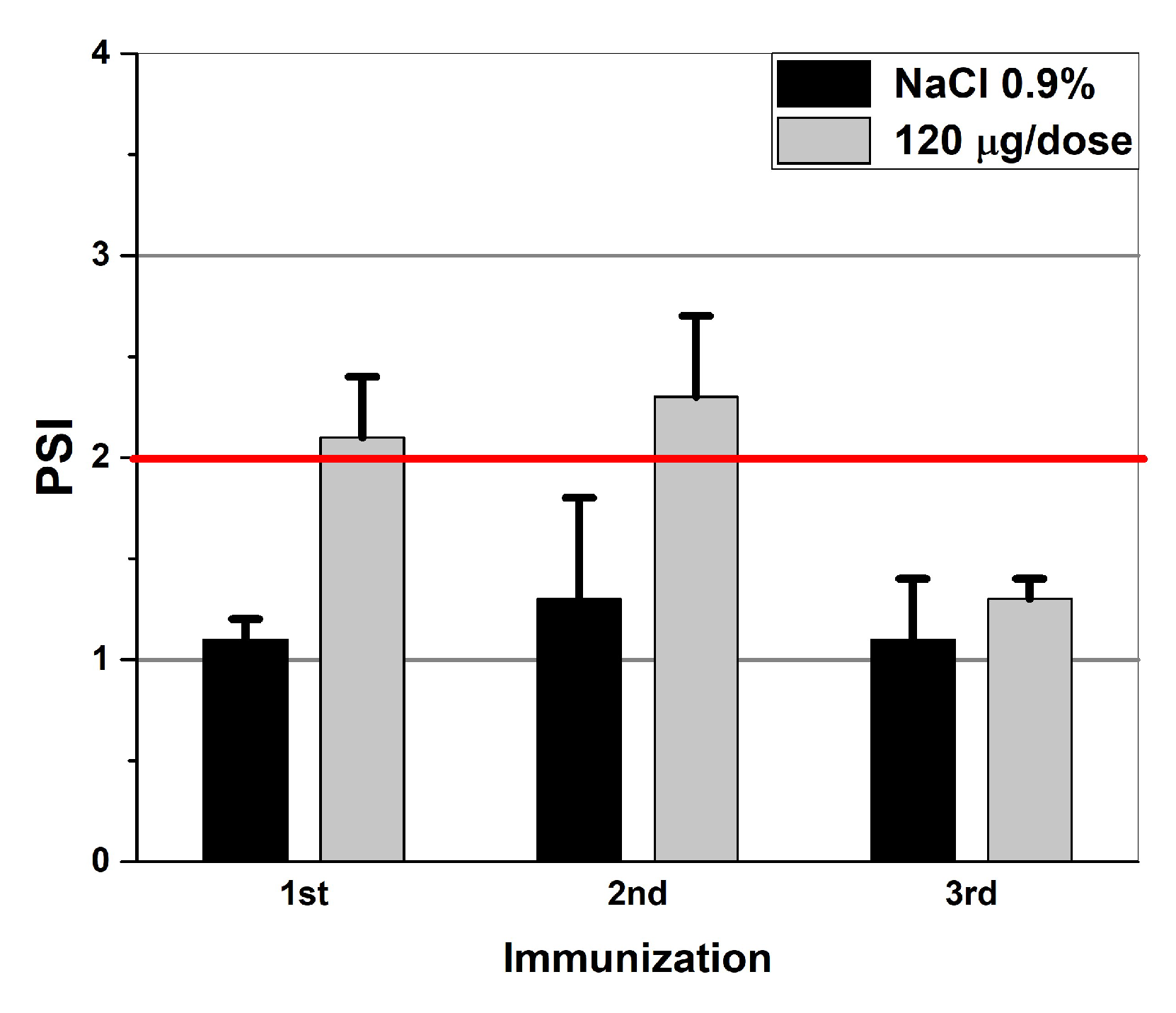
| Specimens | IFN-α2b | IFN-β1a | IFN-γ | VLP-SARS |
|---|---|---|---|---|
| F ± Sr, μW/m2 | ||||
| Proper shelf life | 89 ± 4 | 41 ± 3 | 31 ± 3 | 13 ± 1 |
| Expired | 55 ± 3 | 21 ± 3 | 19 ± 3 | 9 ± 2 |
| Artificially aged | 31 ± 4 | 19 ± 3 | 20 ± 3 | 4 ± 1 |
| Specimens | IFN-α2b | IFN-β1a | IFN-γ | VLP-SARS |
|---|---|---|---|---|
| D ± Sr, nm | ||||
| Proper shelf life | 190 ± 37 | 220 ± 40 | 295 ± 65 | 190 ± 22 |
| Artificially aged | 615 ± 71 | 531 ± 68 | 712 ± 60 | 220 ± 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, G.V.; Galkina, D.A.; Koldina, A.M.; Grebennikova, T.V.; Eliseeva, O.V.; Chernoryzh, Y.Y.; Lebedeva, V.V.; Syroeshkin, A.V. Controlling the Quality of Nanodrugs According to Their New Property—Radiothermal Emission. Pharmaceutics 2024, 16, 180. https://doi.org/10.3390/pharmaceutics16020180
Petrov GV, Galkina DA, Koldina AM, Grebennikova TV, Eliseeva OV, Chernoryzh YY, Lebedeva VV, Syroeshkin AV. Controlling the Quality of Nanodrugs According to Their New Property—Radiothermal Emission. Pharmaceutics. 2024; 16(2):180. https://doi.org/10.3390/pharmaceutics16020180
Chicago/Turabian StylePetrov, Gleb V., Daria A. Galkina, Alena M. Koldina, Tatiana V. Grebennikova, Olesya V. Eliseeva, Yana Yu. Chernoryzh, Varvara V. Lebedeva, and Anton V. Syroeshkin. 2024. "Controlling the Quality of Nanodrugs According to Their New Property—Radiothermal Emission" Pharmaceutics 16, no. 2: 180. https://doi.org/10.3390/pharmaceutics16020180
APA StylePetrov, G. V., Galkina, D. A., Koldina, A. M., Grebennikova, T. V., Eliseeva, O. V., Chernoryzh, Y. Y., Lebedeva, V. V., & Syroeshkin, A. V. (2024). Controlling the Quality of Nanodrugs According to Their New Property—Radiothermal Emission. Pharmaceutics, 16(2), 180. https://doi.org/10.3390/pharmaceutics16020180








