Engineered Lubricative Lecithin-Based Electrospun Nanofibers for the Prevention of Postoperative Abdominal Adhesion
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Material Characterization Methods
2.3. In Vitro Test of Anti-Cell Adhesion
2.4. In Vivo Degradation Test
2.5. Evaluation of Cytocompatibility
2.6. Evaluation of Blood Compatibility
2.7. In Vivo Anti-Adhesion Test
2.7.1. Rat Abdominal Initial Adhesion Model
2.7.2. Rat Abdominal Recurrent Adhesion Model
3. Results and Discussion
3.1. Material Characterization
3.2. Evaluation of Anti-Adhesion Properties In Vitro
3.3. Evaluation of Biodegradability
3.4. Evaluation of Biocompatibility
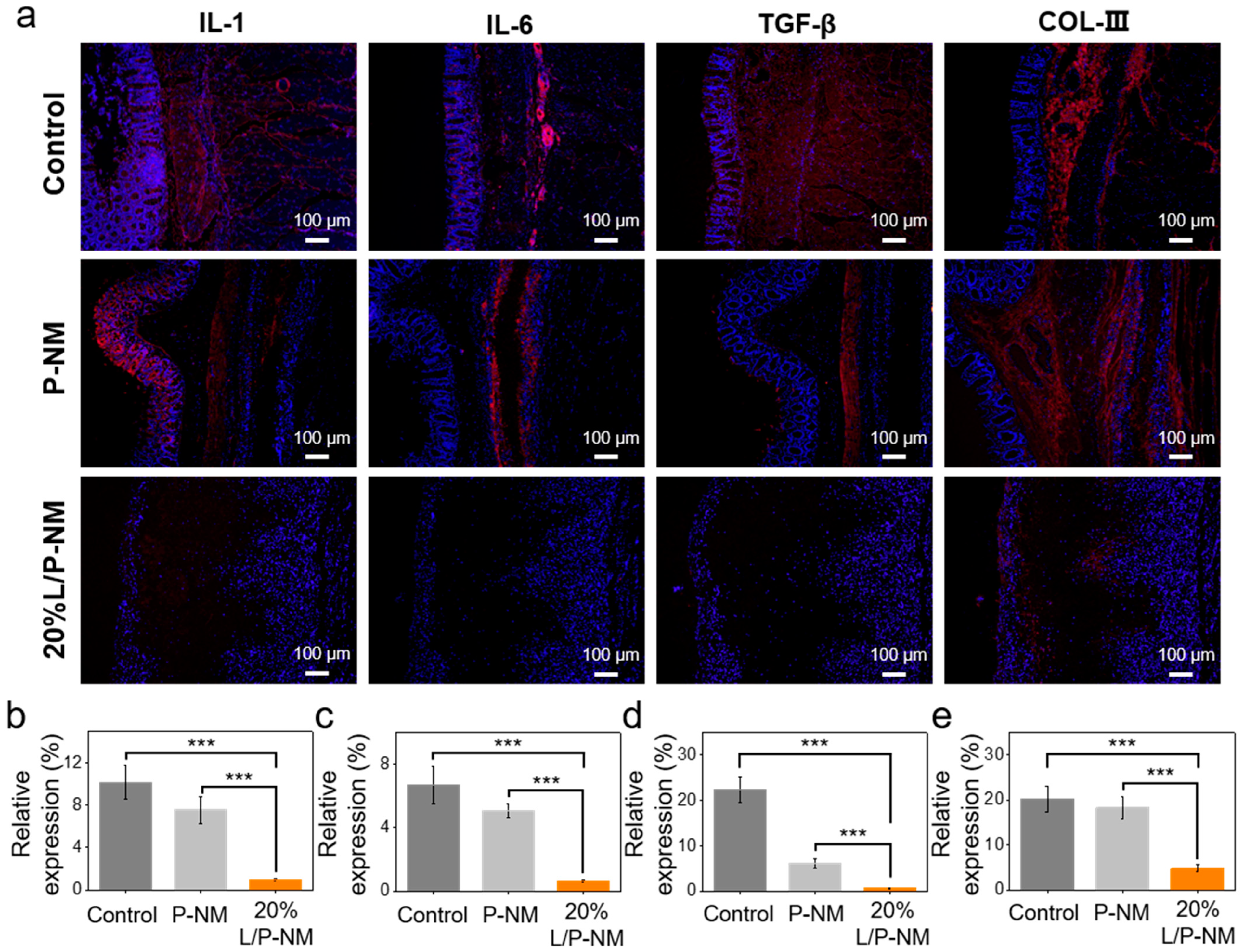
3.5. Evaluation of In Vivo Anti-Adhesion Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diamond, M.P.; Freeman, M.L. Clinical Implications of Postsurgical Adhesions. Hum. Reprod. Update 2001, 7, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.S.; Marshall, C.D.; Gulati, G.S.; Chinta, M.S.; Nguyen, A.; Salhotra, A.; Jones, R.E.; Burcham, A.; Lerbs, T.; Cui, L.; et al. Elucidating the Fundamental Fibrotic Processes Driving Abdominal Adhesion Formation. Nat. Commun. 2020, 11, 4061. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Yelorda, K.; Knowlton, L. Are Statins Associated With Reduced Risk of Adhesion-Related Complications After Abdominal Surgery? JAMA Netw. Open 2021, 4, e2037296. [Google Scholar] [CrossRef] [PubMed]
- Abdiev, A.; Mamatov, N.; Toktosunov, A.; Akeshov, A.; Kalikiri, S. Results of Repeat Operation for Early Adhesive Intestinal Obstruction. Biomedicine 2022, 42, 1272–1274. [Google Scholar] [CrossRef]
- Krielen, P.; Stommel, M.W.J.; Pargmae, P.; Bouvy, N.D.; Bakkum, E.A.; Ellis, H.; Parker, M.C.; Griffiths, E.A.; Van Goor, H.; Ten Broek, R.P.G. Adhesion-Related Readmissions after Open and Laparoscopic Surgery: A Retrospective Cohort Study (SCAR Update). Lancet 2020, 395, 33–41. [Google Scholar] [CrossRef]
- Tittel, A.; Treutner, K.H.; Titkova, S.; Öttinger, A.; Schumpelick, V. New Adhesion Formation after Laparoscopic and Conventional Adhesiolysis: A Comparative Study in the Rabbit. Surg. Endosc. 2001, 15, 44–46. [Google Scholar] [CrossRef]
- Catena, F.; Di Saverio, S.; Coccolini, F.; Ansaloni, L.; De Simone, B.; Sartelli, M.; Van Goor, H. Adhesive Small Bowel Adhesions Obstruction: Evolutions in Diagnosis, Management and Prevention? World J. Gastrointest. Surg. 2016, 8, 222. [Google Scholar] [CrossRef]
- Sirovy, M.; Odlozilova, S.; Kotek, J.; Zajak, J.; Paral, J. Current Options for the Prevention of Postoperative Intra-Abdominal Adhesions. Asian J. Surg. 2024, 47, 77–82. [Google Scholar] [CrossRef]
- Liao, J.; Li, X.; Fan, Y. Prevention Strategies of Postoperative Adhesion in Soft Tissues by Applying Biomaterials: Based on the Mechanisms of Occurrence and Development of Adhesions. Bioact. Mater. 2023, 26, 387–412. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C.; Liang, Y.; Shi, J.; Yu, Q.; Liu, S.; Yu, D.; Liu, H. Advanced Postoperative Tissue Antiadhesive Membranes Enabled with Electrospun Nanofibers. Biomater. Sci. 2024, 12, 1643–1661. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, L.; Ma, L.; Sun, Y.; Yang, X. Controlled Hydrophobic Biosurface of Bacterial Cellulose Nanofibers through Self-Assembly of Natural Zein Protein. ACS Biomater. Sci. Eng. 2017, 3, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, X.; Zhao, L.; Hui, L.; Liu, D. Multilayer Amnion-PCL Nanofibrous Membrane Loaded with Celecoxib Exerts a Therapeutic Effect Against Tendon Adhesion by Improving the Inflammatory Microenvironment. Heliyon 2023, 9, e23214. [Google Scholar] [CrossRef] [PubMed]
- Raisi, A.; Dezfoulian, O.; Davoodi, F.; Taheri, S.; Ghahremani, S.A. Salvia Miltiorrhiza Hydroalcoholic Extract Inhibits Postoperative Peritoneal Adhesions in Rats. BMC Complement. Med. Ther. 2021, 21, 126. [Google Scholar] [CrossRef]
- Giannis, D.; Geropoulos, G.; Ziogas, I.A.; Gitlin, J.; Oropallo, A. The Anti-adhesive Effect of ANTI-VEGF Agents in Experimental Models: A Systematic Review. Wound Repair Regen. 2021, 29, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, M.; Guidoin, R.; Li, Y.; Wang, F.; Brochu, G.; Zhang, Z.; Wang, L. Potential of a Facile Sandwiched Electrospun Scaffold Loaded with Ibuprofen as an Anti-Adhesion Barrier. Mater. Sci. Eng. C 2021, 118, 111451. [Google Scholar] [CrossRef]
- Kheilnezhad, B.; Hadjizadeh, A. Ibuprofen-Loaded Electrospun PCL/PEG Nanofibrous Membranes for Preventing Postoperative Abdominal Adhesion. ACS Appl. Bio Mater. 2022, 5, 1766–1778. [Google Scholar] [CrossRef]
- Lan, X.; Wang, H.; Bai, J.; Miao, X.; Lin, Q.; Zheng, J.; Ding, S.; Li, X.; Tang, Y. Multidrug-Loaded Electrospun Micro/Nanofibrous Membranes: Fabrication Strategies, Release Behaviors and Applications in Regenerative Medicine. J. Control. Release 2021, 330, 1264–1287. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhai, W.; Zhang, Z.; Liu, Y.; Cheng, S.; Zhang, H. In-Situ Growth of Robust Superlubricated Nano-Skin on Electrospun Nanofibers for Post-Operative Adhesion Prevention. Nat. Commun. 2022, 13, 5056. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Sun, G.; Wen, S.; Deng, L.; Zhang, H.; Cui, W. Hydration-Enhanced Lubricating Electrospun Nanofibrous Membranes Prevent Tissue Adhesion. Research 2020, 2020, 4907185. [Google Scholar] [CrossRef]
- Bednarz, S.; Wesołowska-Piętak, A.; Konefał, R.; Świergosz, T. Persulfate Initiated Free-Radical Polymerization of Itaconic Acid: Kinetics, End-Groups and Side Products. Eur. Polym. J. 2018, 106, 63–71. [Google Scholar] [CrossRef]
- Jiang, X.; Han, J.; Cao, L.; Bao, Y.; Shi, J.; Zhang, J.; Ni, L.; Chen, J. A Facial Strategy for Catalyst and Reducing Agent Synchronous Separation for AGET ATRP Using Thiol-Grafted Cellulose Paper as Reducing Agent. Polymers 2017, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yao, D.; Rao, B.; Jian, L.; Chen, Y.; Hu, K.; Xia, Y.; Li, S.; Shen, Y.; Qin, A.; et al. The Structural Basis for the Phospholipid Remodeling by Lysophosphatidylcholine Acyltransferase 3. Nat. Commun. 2021, 12, 6869. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Ikeda, Y. Regulation of Membrane Phospholipid Biosynthesis in Mammalian Cells. Biochem. Pharmacol. 2022, 206, 115296. [Google Scholar] [CrossRef] [PubMed]
- Richey Levine, A.; Picoraro, J.A.; Dorfzaun, S.; LeLeiko, N.S. Emulsifiers and Intestinal Health: An Introduction. J. Pediatr. Gastroenterol. Nutr. 2022, 74, 314–319. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-Evaluation of Lecithins (E 322) as a Food Additive. EFSA J. 2017, 15, e04742. [Google Scholar] [CrossRef]
- Płaczek, M.; Wątróbska-Świetlikowska, D.; Stefanowicz-Hajduk, J.; Drechsler, M.; Ochocka, J.R.; Sznitowska, M. Comparison of the in Vitro Cytotoxicity among Phospholipid-Based Parenteral Drug Delivery Systems: Emulsions, Liposomes and Aqueous Lecithin Dispersions (WLDs). Eur. J. Pharm. Sci. 2019, 127, 92–101. [Google Scholar] [CrossRef]
- Bot, F.; Cossuta, D.; O’Mahony, J.A. Inter-Relationships between Composition, Physicochemical Properties and Functionality of Lecithin Ingredients. Trends Food Sci. Technol. 2021, 111, 261–270. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Dong, Y.; Kampf, N.; Schilt, Y.; Cao, W.; Raviv, U.; Klein, J. Dehydration Does Not Affect Lipid-Based Hydration Lubrication. Nanoscale 2022, 14, 18241–18252. [Google Scholar] [CrossRef]
- Klein, J. Hydration Lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; She, J.; Zhang, Q.; Hu, Q.; Li, D.; Wu, H.; Ye, X.; Diao, R.; Shi, X.; et al. An Anti-Fibroblast Adhesion and Anti-Inflammatory Hydrogel Film Combined with VEGF for Intrauterine Adhesion Prevention. Chem. Eng. J. 2023, 466, 143144. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Sheu, C.; Chen, C.-H.; Chen, S.-H.; Jose, G.; Kuo, C.-Y.; Chen, J.-P. Multi-Functional Electrospun Antibacterial Core-Shell Nanofibrous Membranes for Prolonged Prevention of Post-Surgical Tendon Adhesion and Inflammation. Acta Biomater. 2018, 72, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zeng, Y.; Meng, Y.; Li, Y.; Wang, L. GelMA and Aliphatic Polyesters Janus Nanofibrous Membrane with Lubrication/Anti-Fibroblast Barrier Functions for Abdominal Adhesion Prevention. Eur. Polym. J. 2022, 178, 111499. [Google Scholar] [CrossRef]
- Shalem, A.; Yehezkeli, O.; Fishman, A. Enzymatic Degradation of Polylactic Acid (PLA). Appl. Microbiol. Biotechnol. 2024, 108, 413. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New Advances in the Biodegradation of Poly(lactic) Acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Meng, L.; Liu, X.; Liu, L.; Hong, Q.; Cheng, Y.; Gao, F.; Chen, J.; Zhang, Q.; Pan, C. Comparative Investigation of the Corrosion Behavior and Biocompatibility of the Different Chemical Conversion Coatings on the Magnesium Alloy Surfaces. Metals 2022, 12, 1644. [Google Scholar] [CrossRef]
- Ruiz-Esparza, G.U.; Wang, X.; Zhang, X.; Jimenez-Vazquez, S.; Diaz-Gomez, L.; Lavoie, A.-M.; Afewerki, S.; Fuentes-Baldemar, A.A.; Parra-Saldivar, R.; Jiang, N.; et al. Nanoengineered Shear-Thinning Hydrogel Barrier for Preventing Postoperative Abdominal Adhesions. Nano-Micro Lett. 2021, 13, 212. [Google Scholar] [CrossRef]
- Erdi, M.; Saruwatari, M.S.; Rozyyev, S.; Acha, C.; Ayyub, O.B.; Sandler, A.D.; Kofinas, P. Controlled Release of a Therapeutic Peptide in Sprayable Surgical Sealant for Prevention of Postoperative Abdominal Adhesions. ACS Appl. Mater. Interfaces 2023, 15, 14089–14098. [Google Scholar] [CrossRef]
- Helmedag, M.J.; Heise, D.; Eickhoff, R.M.; Schmitz, S.M.; Mechelinck, M.; Emonts, C.; Bolle, T.; Gries, T.; Neumann, U.P.; Klink, C.D.; et al. Ultra-Fine Polyethylene Hernia Meshes Improve Biocompatibility and Reduce Intraperitoneal Adhesions in IPOM Position in Animal Models. Biomedicines 2022, 10, 1294. [Google Scholar] [CrossRef]
- Wang, R.; Guo, T.; Li, J. Mechanisms of Peritoneal Mesothelial Cells in Peritoneal Adhesion. Biomolecules 2022, 12, 1498. [Google Scholar] [CrossRef]
- Pitenis, A.A.; Urueña, J.M.; Hart, S.M.; O’Bryan, C.S.; Marshall, S.L.; Levings, P.P.; Angelini, T.E.; Sawyer, W.G. Friction-Induced Inflammation. Tribol. Lett. 2018, 66, 81. [Google Scholar] [CrossRef]







| Organs | Normal | P-NM | 20%L/P-NM |
|---|---|---|---|
| Heart | 0.350 ± 0.020 | 0.354 ± 0.020 | 0.349 ± 0.022 |
| Liver | 3.060 ± 0.281 | 3.095 ± 0.237 | 3.026 ± 0.297 |
| Spleen | 0.245 ± 0.027 | 0.231 ± 0.032 | 0.234 ± 0.024 |
| Lung | 0.468 ± 0.021 | 0.459 ± 0.021 | 0.467 ± 0.020 |
| Kidney | 0.783 ± 0.033 | 0.794 ± 0.024 | 0.790 ± 0.030 |
| Organs | Normal | P-NM | 20%L/P-NM |
|---|---|---|---|
| Heart | 0.362 ± 0.026 | 0.359 ± 0.021 | 0.357 ± 0.022 |
| Liver | 3.087 ± 0.221 | 3.051 ± 0.293 | 3.126 ± 0.265 |
| Spleen | 0.248 ± 0.023 | 0.245 ± 0.036 | 0.254 ± 0.025 |
| Lung | 0.488 ± 0.018 | 0.484 ± 0.017 | 0.493 ± 0.021 |
| Kidney | 0.790 ± 0.051 | 0.795 ± 0.054 | 0.793 ± 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lin, H.; Li, J.; Wang, Y. Engineered Lubricative Lecithin-Based Electrospun Nanofibers for the Prevention of Postoperative Abdominal Adhesion. Pharmaceutics 2024, 16, 1562. https://doi.org/10.3390/pharmaceutics16121562
Li J, Lin H, Li J, Wang Y. Engineered Lubricative Lecithin-Based Electrospun Nanofibers for the Prevention of Postoperative Abdominal Adhesion. Pharmaceutics. 2024; 16(12):1562. https://doi.org/10.3390/pharmaceutics16121562
Chicago/Turabian StyleLi, Junhan, Hao Lin, Jinghua Li, and Yi Wang. 2024. "Engineered Lubricative Lecithin-Based Electrospun Nanofibers for the Prevention of Postoperative Abdominal Adhesion" Pharmaceutics 16, no. 12: 1562. https://doi.org/10.3390/pharmaceutics16121562
APA StyleLi, J., Lin, H., Li, J., & Wang, Y. (2024). Engineered Lubricative Lecithin-Based Electrospun Nanofibers for the Prevention of Postoperative Abdominal Adhesion. Pharmaceutics, 16(12), 1562. https://doi.org/10.3390/pharmaceutics16121562






