The Role of Pharmacogenetic-Based Pharmacokinetic Analysis in Precise Breast Cancer Treatment
Abstract
1. Introduction
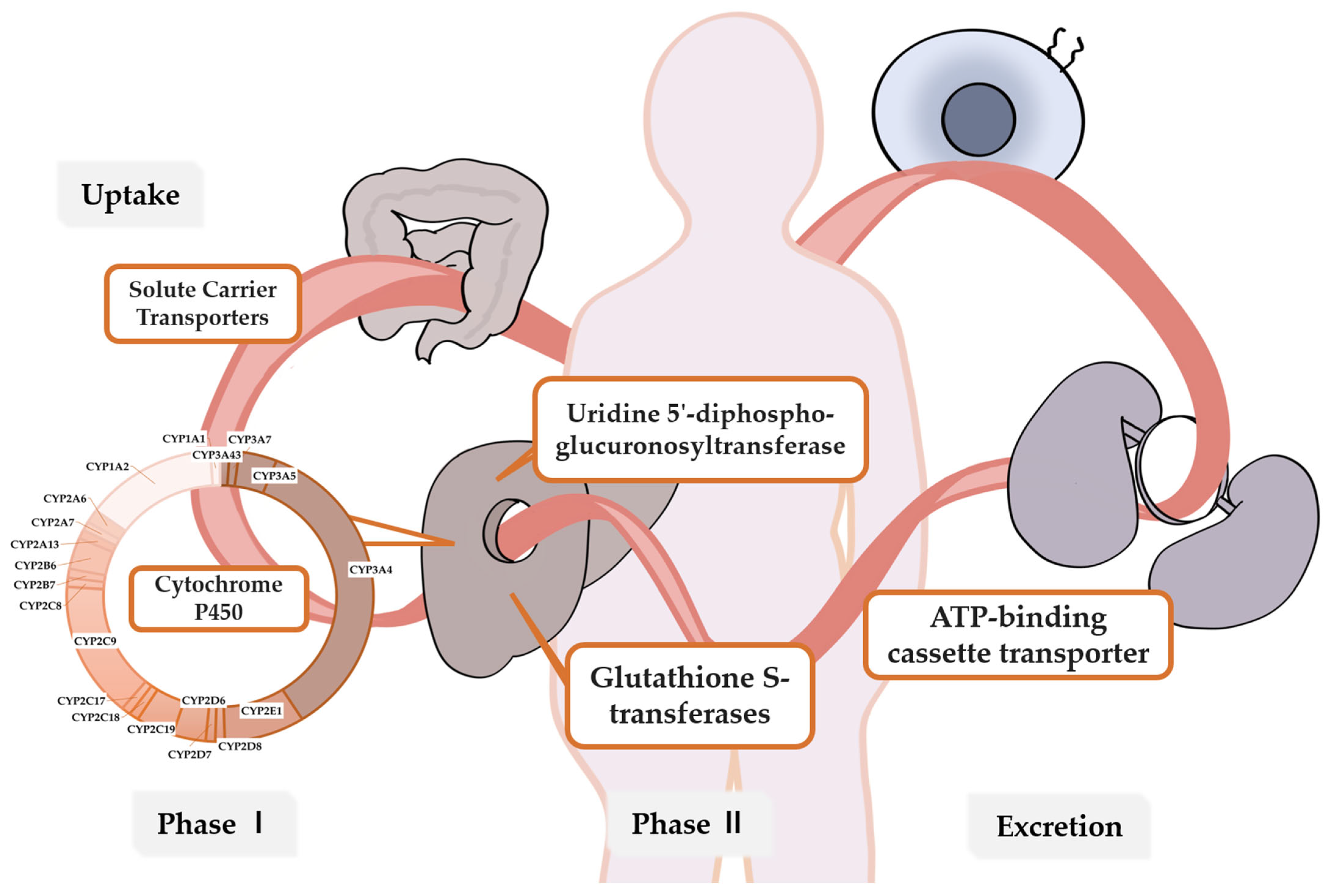
2. Metabolic Enzymes and Genes Related to Breast Cancer Drug Metabolism
3. Pharmacogenetic Variants and Breast Cancer Drugs
3.1. Endocrine Therapy
3.1.1. Tamoxifen
3.1.2. Aromatase Inhibitors (AIs)
3.1.3. Cyclin-Dependent Kinase (CDK) 4/6 Inhibitor
3.2. Chemotherapy
3.2.1. Taxanes
3.2.2. Cyclophosphamide (CTX)
3.2.3. Anthracyclines
3.3. Anti-HER2 Targeted Therapy
3.3.1. Monoclonal Antibodies
3.3.2. Tyrosine Kinase Inhibitors (TKIs)
3.3.3. Antibody-Drug Conjugate (ADC)
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, N.; Chad, M.A.; Lahkim, M.; Houari, A.; Dehbi, H.; Belmouden, A.; El Kadmiri, N. Risk factors for breast cancer in women: An update review. Med. Oncol. 2022, 39, 197. [Google Scholar] [CrossRef] [PubMed]
- Kolak, A.; Kamińska, M.; Sygit, K.; Budny, A.; Surdyka, D.; Kukiełka-Budny, B.; Burdan, F. Primary and secondary prevention of breast cancer. Ann. Agric. Environ. Med. 2017, 24, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, I.; Somerfield, M.R.; Achatz, M.I.; Boughey, J.C.; Curigliano, G.; Friedman, S.; Kohlmann, W.K.; Kurian, A.W.; Laronga, C.; Lynce, F.; et al. Germline Testing in Patients with Breast Cancer: ASCO-Society of Surgical Oncology Guideline. J. Clin. Oncol. 2024, 42, 584–604. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Hussain, S.A.; Ghori, Q.; Naeem, N.; Fazil, A.; Giri, S.; Sathian, B.; Mainali, P.; Al Tamimi, D.M. The spectrum of genetic mutations in breast cancer. Asian Pac. J. Cancer Prev. 2015, 16, 2177–2185. [Google Scholar] [CrossRef]
- Haidar, C.E.; Crews, K.R.; Hoffman, J.M.; Relling, M.V.; Caudle, K.E. Advancing Pharmacogenomics from Single-Gene to Preemptive Testing. Annu. Rev. Genom. Hum. Genet. 2022, 23, 449–473. [Google Scholar] [CrossRef]
- Cejalvo, J.M.; Martínez de Dueñas, E.; Galván, P.; García-Recio, S.; Burgués Gasión, O.; Paré, L.; Antolín, S.; Martinello, R.; Blancas, I.; Adamo, B.; et al. Intrinsic Subtypes and Gene Expression Profiles in Primary and Metastatic Breast Cancer. Cancer Res. 2017, 77, 2213–2221. [Google Scholar] [CrossRef]
- Al Sukhun, S.; Koczwara, B.; Temin, S.; Arun, B.K. Systemic Treatment of Patients with Metastatic Breast Cancer: ASCO Resource-Stratified Guideline Q and A. JCO Glob. Oncol. 2024, 10, e2300411. [Google Scholar] [CrossRef]
- Relling, M.V.; Evans, W.E. Pharmacogenomics in the clinic. Nature 2015, 526, 343–350. [Google Scholar] [CrossRef]
- Russell, L.E.; Schwarz, U.I. Variant discovery using next-generation sequencing and its future role in pharmacogenetics. Pharmacogenomics 2020, 21, 471–486. [Google Scholar] [CrossRef]
- Kolesar, J.; Peh, S.; Thomas, L.; Baburaj, G.; Mukherjee, N.; Kantamneni, R.; Lewis, S.; Pai, A.; Udupa, K.S.; Kumar An, N.; et al. Integration of liquid biopsy and pharmacogenomics for precision therapy of EGFR mutant and resistant lung cancers. Mol. Cancer 2022, 21, 61. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M.; Pirmohamed, M. Precision medicine in cardiovascular therapeutics: Evaluating the role of pharmacogenetic analysis prior to drug treatment. J. Intern. Med. 2024, 295, 583–598. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. In Table of Pharmacogenetic Associations [Section 1]; US Food and Drug Administration. Available online: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations (accessed on 16 August 2024).
- Chan, H.T.; Chin, Y.M.; Low, S.K. The Roles of Common Variation and Somatic Mutation in Cancer Pharmacogenomics. Oncol. Ther. 2019, 7, 1–32. [Google Scholar] [CrossRef]
- Filipski, K.K.; Mechanic, L.E.; Long, R.; Freedman, A.N. Pharmacogenomics in oncology care. Front. Genet. 2014, 5, 73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eichelbaum, M.; Ingelman-Sundberg, M.; Evans, W.E. Pharmacogenomics and individualized drug therapy. Annu. Rev. Med. 2006, 57, 119–137. [Google Scholar] [CrossRef]
- Matthaei, J.; Brockmöller, J.; Tzvetkov, M.V.; Sehrt, D.; Sachse-Seeboth, C.; Hjelmborg, J.B.; Möller, S.; Halekoh, U.; Hofmann, U.; Schwab, M.; et al. Heritability of metoprolol and torsemide pharmacokinetics. Clin. Pharmacol. Ther. 2015, 98, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Tremmel, R.; Zhou, Y.; Schwab, M.; Lauschke, V.M. Structural variation of the coding and non-coding human pharmacogenome. NPJ Genom. Med. 2023, 8, 24. [Google Scholar] [CrossRef]
- Huddart, R.; Fohner, A.E.; Whirl-Carrillo, M.; Wojcik, G.L.; Gignoux, C.R.; Popejoy, A.B.; Bustamante, C.D.; Altman, R.B.; Klein, T.E. Standardized Biogeographic Grouping System for Annotating Populations in Pharmacogenetic Research. Clin. Pharmacol. Ther. 2019, 105, 1256–1262. [Google Scholar] [CrossRef]
- Caudle, K.E.; Dunnenberger, H.M.; Freimuth, R.R.; Peterson, J.F.; Burlison, J.D.; Whirl-Carrillo, M.; Scott, S.A.; Rehm, H.L.; Williams, M.S.; Klein, T.E.; et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet. Med. 2017, 19, 215–223. [Google Scholar] [CrossRef]
- Zhang, F.; Finkelstein, J. Inconsistency in race and ethnic classification in pharmacogenetics studies and its potential clinical implications. Pharmgenomics Pers. Med. 2019, 12, 107–123. [Google Scholar] [CrossRef]
- Rodriguez-Antona, C.; Ingelman-Sundberg, M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006, 25, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Evers, R.; Kool, M.; Wijnholds, J. A family of drug transporters: The multidrug resistance-associated proteins. J. Natl. Cancer Inst. 2000, 92, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Q.; Yang, Y.; Cai, C.-Y.; Teng, Q.-X.; Cui, Q.; Lin, J.; Assaraf, Y.G.; Chen, Z.-S. Multidrug resistance proteins (MRPs): Structure, function and the overcoming of cancer multidrug resistance. Drug Resist. Updates 2021, 54, 100743. [Google Scholar] [CrossRef]
- Pérez-Ramírez, C.; Cañadas-Garre, M.; Molina, M.; Cabeza Barrera, J.; Faus-Dáder, M.J. Impact of single nucleotide polymorphisms on the efficacy and toxicity of EGFR tyrosine kinase inhibitors in advanced non-small cell lung cancer patients. Mutat. Res. Rev. Mutat. Res. 2019, 781, 63–70. [Google Scholar] [CrossRef]
- Slavin, T.P.; Banks, K.C.; Chudova, D.; Oxnard, G.R.; Odegaard, J.I.; Nagy, R.J.; Tsang, K.W.K.; Neuhausen, S.L.; Gray, S.W.; Cristofanilli, M.; et al. Identification of Incidental Germline Mutations in Patients with Advanced Solid Tumors Who Underwent Cell-Free Circulating Tumor DNA Sequencing. J. Clin. Oncol. 2018, 36, Jco1800328. [Google Scholar] [CrossRef] [PubMed]
- Cronin-Fenton, D.P.; Damkier, P.; Lash, T.L. Metabolism and transport of tamoxifen in relation to its effectiveness: New perspectives on an ongoing controversy. Future Oncol. 2014, 10, 107–122. [Google Scholar] [CrossRef]
- Osborne, C.K. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.B.; Swen, J.J.; Dezentje, V.O.; Moes, D.; Gelderblom, H.; Guchelaar, H.J. Clinical pharmacokinetics and pharmacogenetics of tamoxifen and endoxifen. Expert Rev. Clin. Pharmacol. 2019, 12, 523–536. [Google Scholar] [CrossRef]
- Stearns, V.; Johnson, M.D.; Rae, J.M.; Morocho, A.; Novielli, A.; Bhargava, P.; Hayes, D.F.; Desta, Z.; Flockhart, D.A. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer Inst. 2003, 95, 1758–1764. [Google Scholar] [CrossRef]
- Borges, S.; Desta, Z.; Li, L.; Skaar, T.C.; Ward, B.A.; Nguyen, A.; Jin, Y.; Storniolo, A.M.; Nikoloff, D.M.; Wu, L.; et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: Implication for optimization of breast cancer treatment. Clin. Pharmacol. Ther. 2006, 80, 61–74. [Google Scholar] [CrossRef]
- Bousman, C.A.; Stevenson, J.M.; Ramsey, L.B.; Sangkuhl, K.; Hicks, J.K.; Strawn, J.R.; Singh, A.B.; Ruaño, G.; Mueller, D.J.; Tsermpini, E.E.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A Genotypes and Serotonin Reuptake Inhibitor Antidepressants. Clin. Pharmacol. Ther. 2023, 114, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Desta, Z.; Stearns, V.; Ward, B.; Ho, H.; Lee, K.H.; Skaar, T.; Storniolo, A.M.; Li, L.; Araba, A.; et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst. 2005, 97, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Crews, K.R.; Monte, A.A.; Huddart, R.; Caudle, K.E.; Kharasch, E.D.; Gaedigk, A.; Dunnenberger, H.M.; Leeder, J.S.; Callaghan, J.T.; Samer, C.F.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6, OPRM1, and COMT Genotypes and Select Opioid Therapy. Clin. Pharmacol. Ther. 2021, 110, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Eklund, M.; Borgquist, S.; Hellgren, R.; Margolin, S.; Thoren, L.; Rosendahl, A.; Lång, K.; Tapia, J.; Bäcklund, M.; et al. Low-Dose Tamoxifen for Mammographic Density Reduction: A Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Eriksson, M.; Eliasson, E.; Grassmann, F.; Bäcklund, M.; Gabrielson, M.; Hammarström, M.; Margolin, S.; Thorén, L.; Wengström, Y.; et al. CYP2D6 genotype predicts tamoxifen discontinuation and drug response: A secondary analysis of the KARISMA trial. Ann. Oncol. 2021, 32, 1286–1293. [Google Scholar] [CrossRef]
- Vita, G.; Compri, B.; Matcham, F.; Barbui, C.; Ostuzzi, G. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst. Rev. 2023, 3, Cd011006. [Google Scholar] [CrossRef]
- Kelly, C.M.; Juurlink, D.N.; Gomes, T.; Duong-Hua, M.; Pritchard, K.I.; Austin, P.C.; Paszat, L.F. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: A population based cohort study. Bmj 2010, 340, c693. [Google Scholar] [CrossRef]
- Busby, J.; Mills, K.; Zhang, S.D.; Liberante, F.G.; Cardwell, C.R. Selective serotonin reuptake inhibitor use and breast cancer survival: A population-based cohort study. Breast Cancer Res. 2018, 20, 4. [Google Scholar] [CrossRef]
- Margolin, S.; Lindh, J.D.; Thorén, L.; Xie, H.; Koukel, L.; Dahl, M.L.; Eliasson, E. CYP2D6 and adjuvant tamoxifen: Possible differences of outcome in pre- and post-menopausal patients. Pharmacogenomics 2013, 14, 613–622. [Google Scholar] [CrossRef]
- Wang, H.; Ma, X.; Zhang, B.; Zhang, Y.; Han, N.; Wei, L.; Sun, C.; Sun, S.; Zeng, X.; Guo, H.; et al. Chinese breast cancer patients with CYP2D6*10 mutant genotypes have a better prognosis with toremifene than with tamoxifen. Asia Pac. J. Clin. Oncol. 2022, 18, e148–e153. [Google Scholar] [CrossRef]
- Napoli, N.; Rastelli, A.; Ma, C.; Yarramaneni, J.; Vattikutti, S.; Moskowitz, G.; Giri, T.; Mueller, C.; Kulkarny, V.; Qualls, C.; et al. Genetic polymorphism at Val80 (rs700518) of the CYP19A1 gene is associated with aromatase inhibitor associated bone loss in women with ER + breast cancer. Bone 2013, 55, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Straume, A.H.; Knappskog, S.; Lønning, P.E. Effect of CYP19 rs6493497 and rs7176005 haplotype status on in vivo aromatase transcription, plasma and tissue estrogen levels in postmenopausal women. J. Steroid Biochem. Mol. Biol. 2012, 128, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ghimenti, C.; Mello-Grand, M.; Regolo, L.; Zambelli, A.; Chiorino, G. Absence of the K303R estrogen receptor α mutation in breast cancer patients exhibiting different responses to aromatase inhibitor anastrozole neoadjuvant treatment. Exp. Ther. Med. 2010, 1, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, L.K.; Liu, Y.; Stearns, V.; Kadlubar, S.A.; Ramirez, J.; Jeter, S.; Shahverdi, K.; Ward, B.A.; Ogburn, E.; Ratain, M.J.; et al. In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br. J. Clin. Pharmacol. 2010, 70, 854–869. [Google Scholar] [CrossRef] [PubMed]
- Turkistani, A.; Marsh, S. Pharmacogenomics of third-generation aromatase inhibitors. Expert Opin. Pharmacother. 2012, 13, 1299–1307. [Google Scholar] [CrossRef]
- Precht, J.C.; Schroth, W.; Klein, K.; Brauch, H.; Krynetskiy, E.; Schwab, M.; Mürdter, T.E. The letrozole phase 1 metabolite carbinol as a novel probe drug for UGT2B7. Drug Metab. Dispos. Biol. Fate Chem. 2013, 41, 1906–1913. [Google Scholar] [CrossRef]
- Pfister, C.U.; Martoni, A.; Zamagni, C.; Lelli, G.; De Braud, F.; Souppart, C.; Duval, M.; Hornberger, U. Effect of age and single versus multiple dose pharmacokinetics of letrozole (Femara) in breast cancer patients. Biopharm. Drug Dispos. 2001, 22, 191–197. [Google Scholar] [CrossRef]
- Lønning, P.; Pfister, C.; Martoni, A.; Zamagni, C. Pharmacokinetics of third-generation aromatase inhibitors. Semin. Oncol. 2003, 30 (Suppl. S4), 23–32. [Google Scholar] [CrossRef]
- Desta, Z.; Kreutz, Y.; Nguyen, A.T.; Li, L.; Skaar, T.; Kamdem, L.K.; Henry, N.L.; Hayes, D.F.; Storniolo, A.M.; Stearns, V.; et al. Plasma letrozole concentrations in postmenopausal women with breast cancer are associated with CYP2A6 genetic variants, body mass index, and age. Clin. Pharmacol. Ther. 2011, 90, 693–700. [Google Scholar] [CrossRef]
- Nakajima, M.; Kuroiwa, Y.; Yokoi, T. Interindividual differences in nicotine metabolism and genetic polymorphisms of human CYP2A6. Drug Metab. Rev. 2002, 34, 865–877. [Google Scholar] [CrossRef]
- Abubakar, M.B.; Wei, K.; Gan, S.H. The influence of genetic polymorphisms on the efficacy and side effects of anastrozole in postmenopausal breast cancer patients. Pharm. Genom. 2014, 24, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Edavana, V.K.; Dhakal, I.B.; Williams, S.; Penney, R.; Boysen, G.; Yao-Borengasser, A.; Kadlubar, S. Potential role of UGT1A4 promoter SNPs in anastrozole pharmacogenomics. Drug Metab. Dispos. Biol. Fate Chem. 2013, 41, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Bojanic, K.; Kuna, L.; Bilic Curcic, I.; Wagner, J.; Smolic, R.; Kralik, K.; Kizivat, T.; Ivanac, G.; Vcev, A.; Wu, G.Y.; et al. Representation of CYP3A4, CYP3A5 and UGT1A4 Polymorphisms within Croatian Breast Cancer Patients’ Population. Int. J. Environ. Res. Public Health 2020, 17, 3692. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Sheehan, N.L. Understanding and preventing drug-drug and drug-gene interactions. Expert Rev. Clin. Pharmacol. 2014, 7, 533–544. [Google Scholar] [CrossRef]
- Buzdar, A.U.; Robertson, J.F.; Eiermann, W.; Nabholtz, J.M. An overview of the pharmacology and pharmacokinetics of the newer generation aromatase inhibitors anastrozole, letrozole, and exemestane. Cancer 2002, 95, 2006–2016. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, G.; Truica, C.I.; Baird, C.C.; Xia, Z.; Lazarus, P. Identification and Quantification of Novel Major Metabolites of the Steroidal Aromatase Inhibitor, Exemestane. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 1867–1878. [Google Scholar] [CrossRef] [PubMed]
- Hertz, D.L.; Kidwell, K.M.; Seewald, N.J.; Gersch, C.L.; Desta, Z.; Flockhart, D.A.; Storniolo, A.M.; Stearns, V.; Skaar, T.C.; Hayes, D.F.; et al. Polymorphisms in drug-metabolizing enzymes and steady-state exemestane concentration in postmenopausal patients with breast cancer. Pharm. J. 2017, 17, 521–527. [Google Scholar] [CrossRef]
- Teslenko, I.; Trudeau, J.; Luo, S.; Watson, C.J.W.; Chen, G.; Truica, C.I.; Lazarus, P. Influence of Glutathione-S-Transferase A1*B Allele on the Metabolism of the Aromatase Inhibitor, Exemestane, in Human Liver Cytosols and in Patients Treated with Exemestane. J. Pharmacol. Exp. Ther. 2022, 382, 327–334. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Q.; Zhang, P.; Hu, X.; Li, W.; Tong, Z.; Sun, T.; Teng, Y.; Wu, X.; Ouyang, Q.; et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: A randomized, phase 3 trial. Nat. Med. 2021, 27, 1904–1909. [Google Scholar] [CrossRef]
- Lu, Y.S.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Cardoso, F.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; et al. Updated Overall Survival of Ribociclib plus Endocrine Therapy versus Endocrine Therapy Alone in Pre- and Perimenopausal Patients with HR+/HER2- Advanced Breast Cancer in MONALEESA-7: A Phase III Randomized Clinical Trial. Clin. Cancer Res. 2022, 28, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M. GS01-12: MONARCH 3: Final Overall Survival Results with Abemaciclib-Based Therapy for Advanced Breast Cancer. In Proceedings of the 2023 San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 5–9 December 2023. [Google Scholar]
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; Del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig. New Drugs 2014, 32, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Tate, S.C.; Cai, S.; Ajamie, R.T.; Burke, T.; Beckmann, R.P.; Chan, E.M.; De Dios, A.; Wishart, G.N.; Gelbert, L.M.; Cronier, D.M. Semi-mechanistic pharmacokinetic/pharmacodynamic modeling of the antitumor activity of LY2835219, a new cyclin-dependent kinase 4/6 inhibitor, in mice bearing human tumor xenografts. Clin. Cancer Res. 2014, 20, 3763–3774. [Google Scholar] [CrossRef]
- O’Brien, N.; Conklin, D.; Beckmann, R.; Luo, T.; Chau, K.; Thomas, J.; Mc Nulty, A.; Marchal, C.; Kalous, O.; von Euw, E.; et al. Preclinical Activity of Abemaciclib Alone or in Combination with Antimitotic and Targeted Therapies in Breast Cancer. Mol. Cancer Ther. 2018, 17, 897–907. [Google Scholar] [CrossRef]
- Torres-Guzmán, R.; Calsina, B.; Hermoso, A.; Baquero, C.; Alvarez, B.; Amat, J.; McNulty, A.M.; Gong, X.; Boehnke, K.; Du, J.; et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 2017, 8, 69493–69507. [Google Scholar] [CrossRef] [PubMed]
- FDA Approves Abemaciclib for HR-Positive, HER2-Negative Breast Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer (accessed on 16 August 2024).
- Summary of Product Characteristics Palbociclib. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance (accessed on 16 August 2024).
- Summary of Product Characteristics Ribociclib (Kisqali). Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali (accessed on 16 August 2024).
- Smith, N.F.; Acharya, M.R.; Desai, N.; Figg, W.D.; Sparreboom, A. Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol. Ther. 2005, 4, 815–818. [Google Scholar] [CrossRef]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2021, 54, 100742. [Google Scholar] [CrossRef]
- Hjorth, C.F.; Damkier, P.; Stage, T.B.; Feddersen, S.; Hamilton-Dutoit, S.; Rørth, M.; Ejlertsen, B.; Lash, T.L.; Ahern, T.P.; Sørensen, H.T.; et al. Single-nucleotide polymorphisms and the effectiveness of taxane-based chemotherapy in premenopausal breast cancer: A population-based cohort study in Denmark. Breast Cancer Res. Treat. 2022, 194, 353–363. [Google Scholar] [CrossRef]
- Bahadur, N.; Leathart, J.B.; Mutch, E.; Steimel-Crespi, D.; Dunn, S.A.; Gilissen, R.; Houdt, J.V.; Hendrickx, J.; Mannens, G.; Bohets, H.; et al. CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6alpha-hydroxylase activity in human liver microsomes. Biochem. Pharmacol. 2002, 64, 1579–1589. [Google Scholar] [CrossRef]
- Hertz, D.L.; Motsinger-Reif, A.A.; Drobish, A.; Winham, S.J.; McLeod, H.L.; Carey, L.A.; Dees, E.C. CYP2C8*3 predicts benefit/risk profile in breast cancer patients receiving neoadjuvant paclitaxel. Breast Cancer Res. Treat. 2012, 134, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, Y.; Ando, A.; Takamura, Y.; Taguchi, T.; Tamaki, Y.; Noguchi, S. Prediction of response to docetaxel by CYP3A4 mRNA expression in breast cancer tissues. Int. J. Cancer 2002, 97, 129–132. [Google Scholar] [CrossRef]
- Tran, A.; Jullien, V.; Alexandre, J.; Rey, E.; Rabillon, F.; Girre, V.; Dieras, V.; Pons, G.; Goldwasser, F.; Tréluyer, J.M. Pharmacokinetics and toxicity of docetaxel: Role of CYP3A, MDR1, and GST polymorphisms. Clin. Pharmacol. Ther. 2006, 79, 570–580. [Google Scholar] [CrossRef]
- Kim, K.P.; Ahn, J.H.; Kim, S.B.; Jung, K.H.; Yoon, D.H.; Lee, J.S.; Ahn, S.H. Prospective evaluation of the drug-metabolizing enzyme polymorphisms and toxicity profile of docetaxel in Korean patients with operable lymph node-positive breast cancer receiving adjuvant chemotherapy. Cancer Chemother. Pharmacol. 2012, 69, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Hertz, D.L.; Roy, S.; Motsinger-Reif, A.A.; Drobish, A.; Clark, L.S.; McLeod, H.L.; Carey, L.A.; Dees, E.C. CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann. Oncol. 2013, 24, 1472–1478. [Google Scholar] [CrossRef]
- Bosó, V.; Herrero, M.J.; Santaballa, A.; Palomar, L.; Megias, J.E.; de la Cueva, H.; Rojas, L.; Marqués, M.R.; Poveda, J.L.; Montalar, J.; et al. SNPs and taxane toxicity in breast cancer patients. Pharmacogenomics 2014, 15, 1845–1858. [Google Scholar] [CrossRef]
- Abraham, J.E.; Guo, Q.; Dorling, L.; Tyrer, J.; Ingle, S.; Hardy, R.; Vallier, A.L.; Hiller, L.; Burns, R.; Jones, L.; et al. Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with Paclitaxel. Clin. Cancer Res. 2014, 20, 2466–2475. [Google Scholar] [CrossRef]
- Kim, H.J.; Im, S.A.; Keam, B.; Ham, H.S.; Lee, K.H.; Kim, T.Y.; Kim, Y.J.; Oh, D.Y.; Kim, J.H.; Han, W.; et al. ABCB1 polymorphism as prognostic factor in breast cancer patients treated with docetaxel and doxorubicin neoadjuvant chemotherapy. Cancer Sci. 2015, 106, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Kim, J.O.; Kang, D.R.; Shin, J.Y.; Zhang, X.H.; Oh, J.E.; Park, J.Y.; Kim, K.A.; Kang, J.H. Genetic Variations of Drug Transporters Can Influence on Drug Response in Patients Treated with Docetaxel Chemotherapy. Cancer Res. Treat. 2015, 47, 509–517. [Google Scholar] [CrossRef]
- Jabir, R.S.; Ho, G.F.; Annuar, M.; Stanslas, J. Association of Allelic Interaction of Single Nucleotide Polymorphisms of Influx and Efflux Transporters Genes with Nonhematologic Adverse Events of Docetaxel in Breast Cancer Patients. Clin. Breast Cancer 2018, 18, e1173–e1179. [Google Scholar] [CrossRef]
- Gréen, H.; Söderkvist, P.; Rosenberg, P.; Mirghani, R.A.; Rymark, P.; Lundqvist, E.A.; Peterson, C. Pharmacogenetic studies of Paclitaxel in the treatment of ovarian cancer. Basic. Clin. Pharmacol. Toxicol. 2009, 104, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Mysona, D.; Dorr, K.; Ward, A.; Shaver, E.; Rungruang, B.; Ghamande, S. Pharmacogenetics as a predictor chemotherapy induced peripheral neuropathy in gynecologic cancer patients treated with Taxane-based chemotherapy. Gynecol. Oncol. 2023, 168, 114–118. [Google Scholar] [CrossRef]
- Hertz, D.L.; Roy, S.; Jack, J.; Motsinger-Reif, A.A.; Drobish, A.; Clark, L.S.; Carey, L.A.; Dees, E.C.; McLeod, H.L. Genetic heterogeneity beyond CYP2C8*3 does not explain differential sensitivity to paclitaxel-induced neuropathy. Breast Cancer Res. Treat. 2014, 145, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, S.; La Verde, N.; Rota, S.; Casazza, G.; Montrasio, C.; Cheli, S.; Cona, M.S.; Dalu, D.; Fasola, C.; Ferrario, S.; et al. Single nucleotide polymorphisms to predict taxanes toxicities and effectiveness in cancer patients. Pharm. J. 2021, 21, 491–497. [Google Scholar] [CrossRef]
- Gudur, R.A.; Bhosale, S.J.; Gudur, A.K.; Kale, S.R.; More, A.L.; Datkhile, K.D. The Effect of CYP2C19*2 (rs4244285) and CYP17 (rs743572) SNPs on Adriamycin and Paclitaxel based Chemotherapy Outcomes in Breast Cancer Patients. Asian Pac. J. Cancer Prev. 2024, 25, 1977–1986. [Google Scholar] [CrossRef]
- Chew, S.C.; Lim, J.; Singh, O.; Chen, X.; Tan, E.H.; Lee, E.J.; Chowbay, B. Pharmacogenetic effects of regulatory nuclear receptors (PXR, CAR, RXRα and HNF4α) on docetaxel disposition in Chinese nasopharyngeal cancer patients. Eur. J. Clin. Pharmacol. 2014, 70, 155–166. [Google Scholar] [CrossRef]
- Helsby, N.A.; Burns, K.E. Molecular mechanisms of genetic variation and transcriptional regulation of CYP2C19. Front. Genet. 2012, 3, 206. [Google Scholar] [CrossRef]
- Helsby, N.A.; Yong, M.; van Kan, M.; de Zoysa, J.R.; Burns, K.E. The importance of both CYP2C19 and CYP2B6 germline variations in cyclophosphamide pharmacokinetics and clinical outcomes. Br. J. Clin. Pharmacol. 2019, 85, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, N.; Miyazaki, M.; Toide, K.; Sawamura, Y.; Kamataki, T. A single nucleotide polymorphism of CYP2b6 found in Japanese enhances catalytic activity by autoactivation. Biochem. Biophys. Res. Commun. 2001, 281, 1256–1260. [Google Scholar] [CrossRef]
- Hofmann, M.H.; Blievernicht, J.K.; Klein, K.; Saussele, T.; Schaeffeler, E.; Schwab, M.; Zanger, U.M. Aberrant splicing caused by single nucleotide polymorphism c.516G>T [Q172H], a marker of CYP2B6*6, is responsible for decreased expression and activity of CYP2B6 in liver. J. Pharmacol. Exp. Ther. 2008, 325, 284–292. [Google Scholar] [CrossRef]
- Helsby, N.A.; Tingle, M.D. Which CYP2B6 variants have functional consequences for cyclophosphamide bioactivation? Drug Metab. Dispos. Biol. Fate Chem. 2012, 40, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Haroun, F.; Al-Shaar, L.; Habib, R.H.; El-Saghir, N.; Tfayli, A.; Bazarbachi, A.; Salem, Z.; Shamseddine, A.; Taher, A.; Cascorbi, I.; et al. Effects of CYP2B6 genetic polymorphisms in patients receiving cyclophosphamide combination chemotherapy for breast cancer. Cancer Chemother. Pharmacol. 2015, 75, 207–214. [Google Scholar] [CrossRef]
- Gor, P.P.; Su, H.I.; Gray, R.J.; Gimotty, P.A.; Horn, M.; Aplenc, R.; Vaughan, W.P.; Tallman, M.S.; Rebbeck, T.R.; DeMichele, A. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: A retrospective cohort study. Breast Cancer Res. 2010, 12, R26. [Google Scholar] [CrossRef]
- Martis, S.; Mei, H.; Vijzelaar, R.; Edelmann, L.; Desnick, R.J.; Scott, S.A. Multi-ethnic cytochrome-P450 copy number profiling: Novel pharmacogenetic alleles and mechanism of copy number variation formation. Pharm. J. 2013, 13, 558–566. [Google Scholar] [CrossRef]
- Bray, J.; Sludden, J.; Griffin, M.J.; Cole, M.; Verrill, M.; Jamieson, D.; Boddy, A.V. Influence of pharmacogenetics on response and toxicity in breast cancer patients treated with doxorubicin and cyclophosphamide. Br. J. Cancer 2010, 102, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, S.; Agarwal, G.; Lal, P.; Mittal, B. Significant role of CYP450 genetic variants in cyclophosphamide based breast cancer treatment outcomes: A multi-analytical strategy. Clin. Chim. Acta 2014, 434, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Kaur, R.P.; Ludhiadch, A.; Shafi, G.; Vashista, R.; Kumar, R.; Munshi, A. Association of CYP2C19*2 and ALDH1A1*1/*2 variants with disease outcome in breast cancer patients: Results of a global screening array. Eur. J. Clin. Pharmacol. 2018, 74, 1291–1298. [Google Scholar] [CrossRef]
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and other anthracyclines in cancers: Activity, chemoresistance and its overcoming. Mol. Aspects Med. 2023, 93, 101205. [Google Scholar] [CrossRef]
- Mordente, A.; Meucci, E.; Silvestrini, A.; Martorana, G.E.; Giardina, B. New developments in anthracycline-induced cardiotoxicity. Curr. Med. Chem. 2009, 16, 1656–1672. [Google Scholar] [CrossRef]
- Siebel, C.; Lanvers-Kaminsky, C.; Würthwein, G.; Hempel, G.; Boos, J. Bioanalysis of doxorubicin aglycone metabolites in human plasma samples-implications for doxorubicin drug monitoring. Sci. Rep. 2020, 10, 18562. [Google Scholar] [CrossRef]
- Voon, P.J.; Yap, H.L.; Ma, C.Y.; Lu, F.; Wong, A.L.; Sapari, N.S.; Soong, R.; Soh, T.I.; Goh, B.C.; Lee, H.S.; et al. Correlation of aldo-ketoreductase (AKR) 1C3 genetic variant with doxorubicin pharmacodynamics in Asian breast cancer patients. Br. J. Clin. Pharmacol. 2013, 75, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, M.B.; Pituskin, E.; Damaraju, S.; Bies, R.R.; Vos, L.J.; Prado, C.M.; Kuzma, M.; Scarfe, A.G.; Clemons, M.; Tonkin, K.; et al. A Uridine Glucuronosyltransferase 2B7 Polymorphism Predicts Epirubicin Clearance and Outcomes in Early-Stage Breast Cancer. Clin. Breast Cancer 2016, 16, e131–e133. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.; Bardin, C.; Rigal, C.; Anthony, B.; Rousseau, R.; Dutour, A. Anti-MDR1 siRNA restores chemosensitivity in chemoresistant breast carcinoma and osteosarcoma cell lines. Anticancer. Res. 2011, 31, 2813–2820. [Google Scholar] [PubMed]
- Yin, W.; Xiang, D.; Wang, T.; Zhang, Y.; Pham, C.V.; Zhou, S.; Jiang, G.; Hou, Y.; Zhu, Y.; Han, Y.; et al. The inhibition of ABCB1/MDR1 or ABCG2/BCRP enables doxorubicin to eliminate liver cancer stem cells. Sci. Rep. 2021, 11, 10791. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, S.; Mittal, R.D.; Mittal, B. The effect of ABCB1 polymorphisms on the outcome of breast cancer treatment. Pharmgenomics Pers. Med. 2016, 9, 47–58. [Google Scholar] [CrossRef]
- Turiján-Espinoza, E.; Ruíz-Rodríguez, V.M.; Uresti-Rivera, E.E.; Martínez-Leija, E.; Zermeño-Nava, J.J.; Guel-Pañola, A.; Romano-Moreno, S.; Vargas-Morales, J.M.; Portales-Pérez, D.P. Clinical utility of ABCB1 and ABCG2 genotyping for assessing the clinical and pathological response to FAC therapy in Mexican breast cancer patients. Cancer Chemother. Pharmacol. 2021, 87, 843–853. [Google Scholar] [CrossRef]
- Madrid-Paredes, A.; Cañadas-Garre, M.; Sánchez-Pozo, A.; Expósito-Ruiz, M.; Calleja-Hernández, M. ABCB1 gene polymorphisms and response to chemotherapy in breast cancer patients: A meta-analysis. Surg. Oncol. 2017, 26, 473–482. [Google Scholar] [CrossRef]
- Ebaid, N.F.; Abdelkawy, K.S.; Shehata, M.A.; Salem, H.F.; Magdy, G.; Hussein, R.R.S.; Elbarbry, F. Effects of pharmacogenetics on pharmacokinetics and toxicity of doxorubicin in Egyptian breast cancer patients. Xenobiotica 2024, 54, 160–170. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.; Lei, T.; Liu, J.; Zhang, S.; Wu, N.; Sun, B.; Wang, M. Drug resistance gene expression and chemotherapy sensitivity detection in Chinese women with different molecular subtypes of breast cancer. Cancer Biol. Med. 2020, 17, 1014–1025. [Google Scholar] [CrossRef]
- Zeng, X.; Morgenstern, R.; Nyström, A.M. Nanoparticle-directed sub-cellular localization of doxorubicin and the sensitization breast cancer cells by circumventing GST-mediated drug resistance. Biomaterials 2014, 35, 1227–1239. [Google Scholar] [CrossRef]
- Tulsyan, S.; Chaturvedi, P.; Agarwal, G.; Lal, P.; Agrawal, S.; Mittal, R.D.; Mittal, B. Pharmacogenetic influence of GST polymorphisms on anthracycline-based chemotherapy responses and toxicity in breast cancer patients: A multi-analytical approach. Mol. Diagn. Ther. 2013, 17, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sugishita, M.; Imai, T.; Kikumori, T.; Mitsuma, A.; Shimokata, T.; Shibata, T.; Morita, S.; Inada-Inoue, M.; Sawaki, M.; Hasegawa, Y.; et al. Pharmacogenetic association between GSTP1 genetic polymorphism and febrile neutropenia in Japanese patients with early breast cancer. Breast Cancer 2016, 23, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wu, H.; Liu, D.; Li, L.; Li, J.; Wang, Q.; Ye, M.; Huang, Q.; Yu, Z.; Zhang, J. GSTP1 c.313A > G mutation is an independent risk factor for neutropenia hematotoxicity induced by anthracycline-/paclitaxel-based chemotherapy in breast cancer patients. World J. Surg. Oncol. 2022, 20, 212. [Google Scholar] [CrossRef]
- Rocca, A.; Andreis, D.; Fedeli, A.; Maltoni, R.; Sarti, S.; Cecconetto, L.; Pietri, E.; Schirone, A.; Bravaccini, S.; Serra, P.; et al. Pharmacokinetics, pharmacodynamics and clinical efficacy of pertuzumab in breast cancer therapy. Expert. Opin. Drug Metab. Toxicol. 2015, 11, 1647–1663. [Google Scholar] [CrossRef]
- Richard, S.; Selle, F.; Lotz, J.P.; Khalil, A.; Gligorov, J.; Soares, D.G. Pertuzumab and trastuzumab: The rationale way to synergy. An. Acad. Bras. Ciências 2016, 88 (Suppl. S1), 565–577. [Google Scholar] [CrossRef]
- von Arx, C.; De Placido, P.; Caltavituro, A.; Di Rienzo, R.; Buonaiuto, R.; De Laurentiis, M.; Arpino, G.; Puglisi, F.; Giuliano, M.; Del Mastro, L. The evolving therapeutic landscape of trastuzumab-drug conjugates: Future perspectives beyond HER2-positive breast cancer. Cancer Treat. Rev. 2023, 113, 102500. [Google Scholar] [CrossRef]
- Cocca, M.; Bedognetti, D.; La Bianca, M.; Gasparini, P.; Girotto, G. Pharmacogenetics driving personalized medicine: Analysis of genetic polymorphisms related to breast cancer medications in Italian isolated populations. J. Transl. Med. 2016, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.L.; Chaiyakunapruk, N.; Tassaneeyakul, W.; Arunmanakul, P.; Nathisuwan, S.; Lee, S.W.H. Roles of pharmacogenomics in non-anthracycline antineoplastic-induced cardiovascular toxicities: A systematic review and meta-analysis of genotypes effect. Int. J. Cardiol. 2019, 280, 190–197. [Google Scholar] [CrossRef]
- Sondermann, P.; Huber, R.; Oosthuizen, V.; Jacob, U. The 3.2-A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature 2000, 406, 267–273. [Google Scholar] [CrossRef]
- Varchetta, S.; Gibelli, N.; Oliviero, B.; Nardini, E.; Gennari, R.; Gatti, G.; Silva, L.S.; Villani, L.; Tagliabue, E.; Ménard, S.; et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007, 67, 11991–11999. [Google Scholar] [CrossRef]
- Tamura, K.; Shimizu, C.; Hojo, T.; Akashi-Tanaka, S.; Kinoshita, T.; Yonemori, K.; Kouno, T.; Katsumata, N.; Ando, M.; Aogi, K.; et al. FcγR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann. Oncol. 2011, 22, 1302–1307. [Google Scholar] [CrossRef]
- Musolino, A.; Naldi, N.; Bortesi, B.; Pezzuolo, D.; Capelletti, M.; Missale, G.; Laccabue, D.; Zerbini, A.; Camisa, R.; Bisagni, G.; et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 2008, 26, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Musolino, A.; Naldi, N.; Dieci, M.V.; Zanoni, D.; Rimanti, A.; Boggiani, D.; Sgargi, P.; Generali, D.G.; Piacentini, F.; Ambroggi, M.; et al. Immunoglobulin G fragment C receptor polymorphisms and efficacy of preoperative chemotherapy plus trastuzumab and lapatinib in HER2-positive breast cancer. Pharm. J. 2016, 16, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Betting, D.J.; Stern, H.M.; Quinaux, E.; Stinson, J.; Seshagiri, S.; Zhao, Y.; Buyse, M.; Mackey, J.; Driga, A.; et al. Analysis of Fcγ receptor IIIa and IIa polymorphisms: Lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin. Cancer Res. 2012, 18, 3478–3486. [Google Scholar] [CrossRef]
- Madrid-Paredes, A.; Cañadas-Garre, M.; Sánchez-Pozo, A.; Segura-Pérez, A.M.; Chamorro-Santos, C.; Vergara-Alcaide, E.; Castillo-Portellano, L.; Calleja-Hernández, M. ABCB1 C3435T gene polymorphism as a potential biomarker of clinical outcomes in HER2-positive breast cancer patients. Pharmacol. Res. 2016, 108, 111–118. [Google Scholar] [CrossRef]
- Sarah, A.; Dondi, E.; De Francia, S. Tyrosine kinase inhibitors: The role of pharmacokinetics and pharmacogenetics. Expert. Opin. Drug Metab. Toxicol. 2023, 19, 733–739. [Google Scholar] [CrossRef]
- van Erp, N.P.; Gelderblom, H.; Guchelaar, H.J. Clinical pharmacokinetics of tyrosine kinase inhibitors. Cancer Treat. Rev. 2009, 35, 692–706. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, Z.E.; Li, B.; Li, F. Recent advances in metabolism and toxicity of tyrosine kinase inhibitors. Pharmacol. Ther. 2022, 237, 108256. [Google Scholar] [CrossRef] [PubMed]
- Bissada, J.E.; Truong, V.; Abouda, A.A.; Wines, K.J.; Crouch, R.D.; Jackson, K.D. Interindividual Variation in CYP3A Activity Influences Lapatinib Bioactivation. Drug Metab. Dispos. Biol. Fate Chem. 2019, 47, 1257–1269. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Li, Y.; He, X.; Bryant, M.; Qin, X.; Li, F.; Seo, J.E.; Guo, X.; Mei, N.; et al. The involvement of hepatic cytochrome P450s in the cytotoxicity of lapatinib. Toxicol. Sci. 2023, 197, 69–78. [Google Scholar] [CrossRef]
- Breslin, S.; Lowry, M.C.; O’Driscoll, L. Neratinib resistance and cross-resistance to other HER2-targeted drugs due to increased activity of metabolism enzyme cytochrome P4503A4. Br. J. Cancer 2017, 116, 620–625. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Oitate, M.; Hagihara, K.; Shiozawa, H.; Furuta, Y.; Ogitani, Y.; Kuga, H. Pharmacokinetics of trastuzumab deruxtecan (T-DXd), a novel anti-HER2 antibody-drug conjugate, in HER2-positive tumour-bearing mice. Xenobiotica 2020, 50, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, C.M.; Yamaguchi, A.; Anami, Y.; Xiong, W.; Otani, Y.; Lee, J.; Ueno, N.T.; Zhang, N.; An, Z.; Tsuchikama, K. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat. Commun. 2021, 12, 3528. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Trastuzumab emtansine. An inadequately assessed combination of two cytotoxic drugs. Prescrire Int. 2014, 23, 289. [Google Scholar]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody-Drug Conjugate, [fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M.; Lauschke, V.M. Individualized Pharmacotherapy Utilizing Genetic Biomarkers and Novel In Vitro Systems As Predictive Tools for Optimal Drug Development and Treatment. Drug Metab. Dispos. 2024, 52, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Lauschke, V.M.; Ingelman-Sundberg, M. Prediction of drug response and adverse drug reactions: From twin studies to Next Generation Sequencing. Eur. J. Pharm. Sci. 2019, 130, 65–77. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, M.H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
| Drug Class | Enzyme/Genetic Variant | Effect on Pharmacokinetics |
|---|---|---|
| Tamoxifen (SERM) | CYP2D6 | Enhanced-function allele increases symptoms and discontinuation rates; the reduced-function allele diminishes tamoxifen metabolism and efficacy. |
| Aromatase Inhibitors (AIs) | CYP19A1 | Associated with baseline aromatase activity |
| CYP2A6 | Reduced-function allele elevates letrozole plasma concentrations. | |
| UGT1A4 and UGT2B7 | Affect drug conjugation and clearance. | |
| GSTA1 *B*B | inhibited metabolism | |
| CDK 4/6 Inhibitors | CYP3A4 | Higher risk of toxicity with strong CYP3A4 inhibitors |
| Taxanes | CYP2C8*3 | Higher remission rates in neoadjuvant treatment, with possible increased toxicity. |
| CYP3A4 | Reduced mRNA plasma level is associated with the docetaxel response rates | |
| ABCB1 | Increased risk of neutropenia and diarrhea | |
| SLCO1B1 521T>C | Decreased risk of mortality | |
| Cyclophosphamide (CTX) | CYP2B6 516G>T and A785A>G | Poorer overall survival (OS) |
| CYP2C19*2 | Related to an increased risk of adverse reactions (AEs) | |
| Anthracyclines | CYP2C19*2 | Increased drug-induced AEs |
| UGT2B7 161 C>T | Higher epirubicin elimination, lower risk of leukopenia | |
| ABCB1 3435 C>T | Better OS but increased risk of diarrhea and neutropenia | |
| SLC22A16 T>C | Increased risk of diarrhea and neutropenia | |
| GSTM1 and GSTT1 deletions | Decreased recurrence and mortality rates. | |
| GSTP1 313A>G | Increased risk of hematological toxicity | |
| Monoclonal Antibodies | HER2 1173A>G | Increased risk of trastuzumab-induced cardiac toxicity |
| FCGR2A 519A>G | Associated with reduced trastuzumab efficacy | |
| FCGR3A 559T>G | Higher pCR rates with trastuzumab plus lapatinib in neoadjuvant therapy | |
| ABCB1 3435 C>T | Increased resistance to chemotherapy/trastuzumab regimens | |
| Tyrosine Kinase Inhibitors (TKIs) | CYP3A4 | Increased risk of lapatinib-induced hepatotoxic toxicity and associated with resistance in neratinib-resistant cells |
| CYP3A5, CYP3A7 | May reduce lapatinib cytotoxicity and DNA damage | |
| Antibody-drug conjugate (ADCs) | Unknown | Needs further exploration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Xiong, H. The Role of Pharmacogenetic-Based Pharmacokinetic Analysis in Precise Breast Cancer Treatment. Pharmaceutics 2024, 16, 1407. https://doi.org/10.3390/pharmaceutics16111407
Wu X, Xiong H. The Role of Pharmacogenetic-Based Pharmacokinetic Analysis in Precise Breast Cancer Treatment. Pharmaceutics. 2024; 16(11):1407. https://doi.org/10.3390/pharmaceutics16111407
Chicago/Turabian StyleWu, Xinyu, and Huihua Xiong. 2024. "The Role of Pharmacogenetic-Based Pharmacokinetic Analysis in Precise Breast Cancer Treatment" Pharmaceutics 16, no. 11: 1407. https://doi.org/10.3390/pharmaceutics16111407
APA StyleWu, X., & Xiong, H. (2024). The Role of Pharmacogenetic-Based Pharmacokinetic Analysis in Precise Breast Cancer Treatment. Pharmaceutics, 16(11), 1407. https://doi.org/10.3390/pharmaceutics16111407





