The Combination of Buparvaquone and ELQ316 Exhibit a Stronger Effect than ELQ316 and Imidocarb Against Babesia bovis In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds Tested
2.2. Babesia bovis In Vitro Culture
2.3. In Vitro Growth Inhibitory Assays
2.4. Post-Treatment Survival
2.5. Statistical Analysis
3. Results
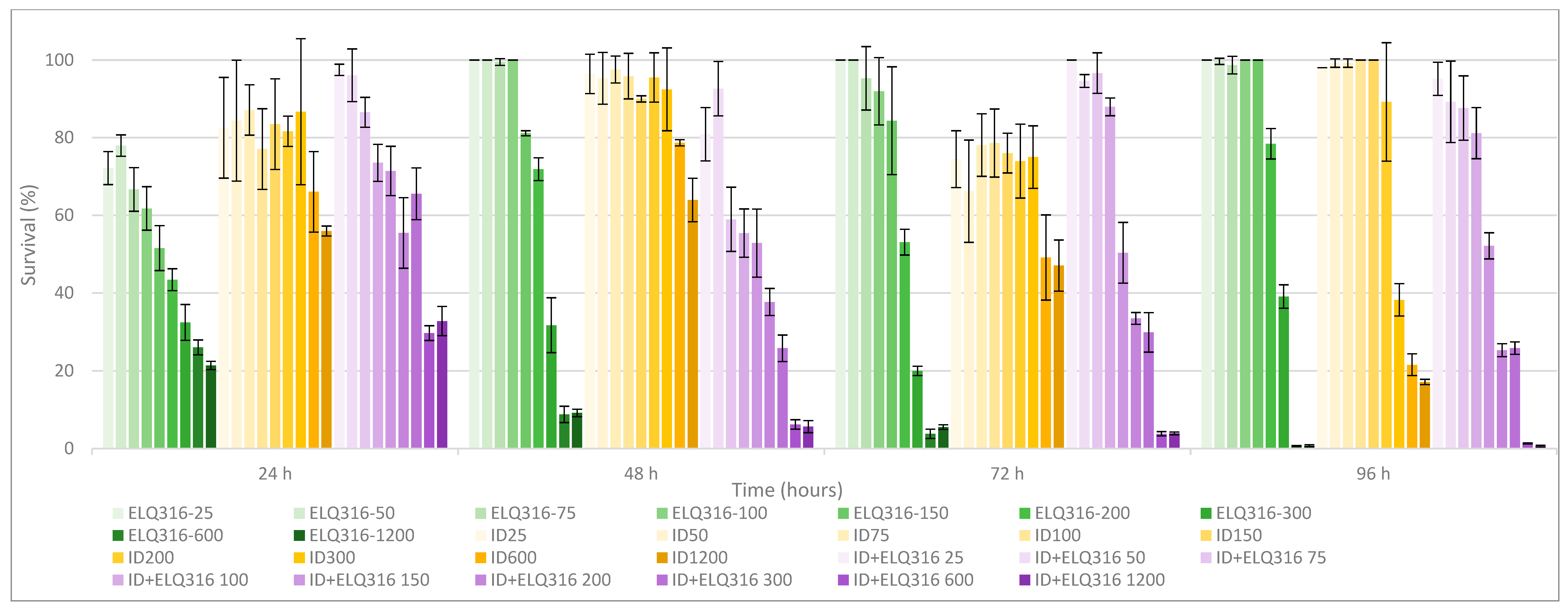
3.1. Comparative In Vitro Survival Effects of BPQ, ID, ELQ-316, BPQ+ ELQ-316, and ID+ ELQ-316 Combinations on B. bovis
3.2. Drug Potency
3.3. Time and Concentration of Drugs to Reach 0% Survival after Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
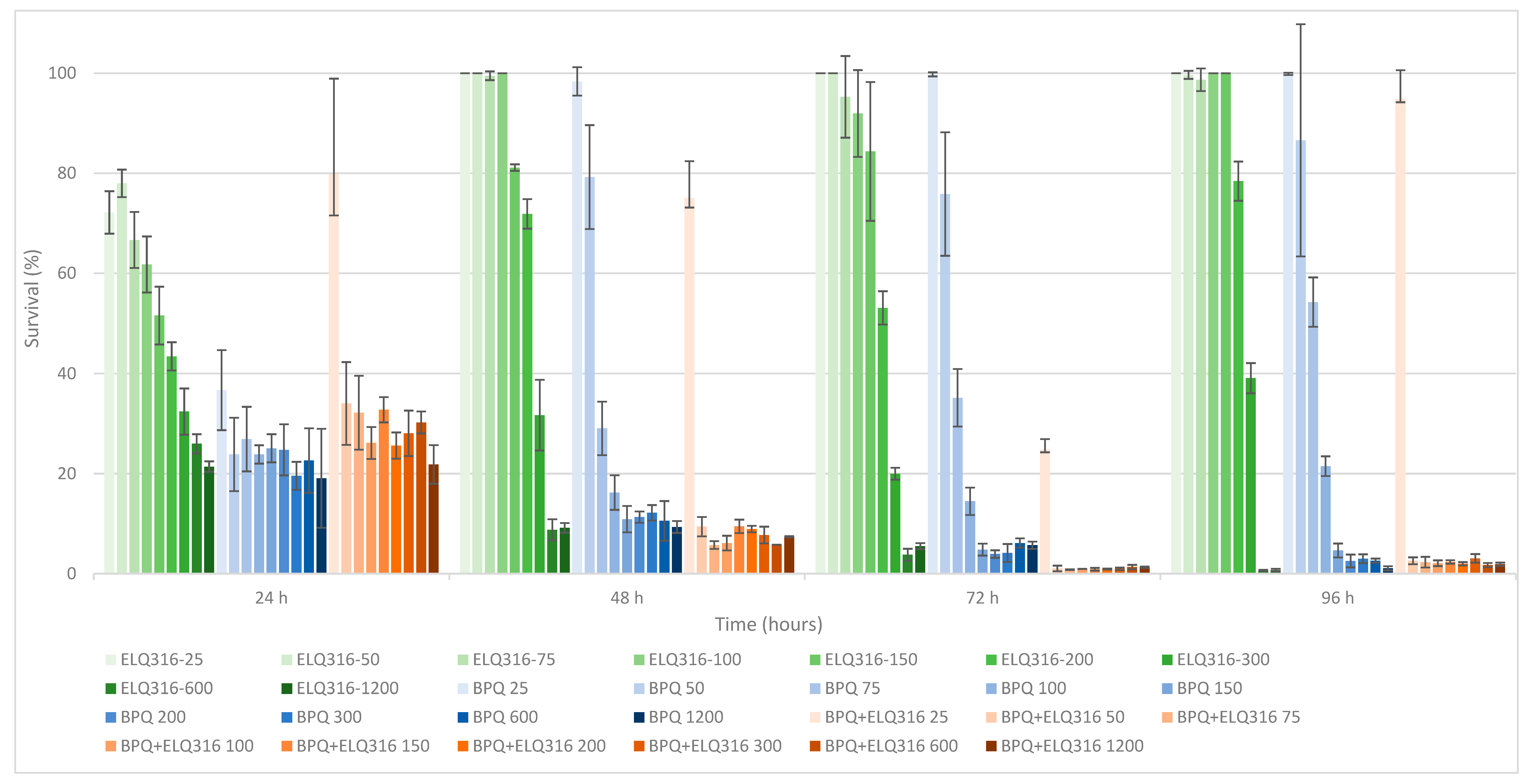
| Drug Treatment Concentration (nM) | BPQ | BPQ + ELQ-316 | p-Value | ||
|---|---|---|---|---|---|
| Mean (%) | CI 95% | Mean (%) | CI 95% | ||
| 25 | 99.87 | (99.78–99.96) | 97.31 | (96.16–98.46) | p = 0.72 |
| 50 | 86.6 | (77.56–95.64) | 2.9 | (2.8–3.00) | p < 0.05 |
| 75 | 54.25 | (52.33–56.17) | 2.31 | (1.89–2.73) | p = 0.10 |
| 100 | 21.5 | (20.74–22.26) | 2.1 | (1.87–2.33) | p = 0.10 |
| 150 | 4.64 | (4.1–5.18) | 2.37 | (2.24–2.50) | p = 0.10 |
| 200 | 2.55 | (2.05–3.05) | 1.99 | (1.86–2.12) | p = 0.70 |
| 300 | 3.01 | (2.67–3.35) | 3.06 | (2.73–3.39) | p = 0.90 |
| 600 | 2.62 | (2.51–2.73) | 1.72 | (1.60–1.84) | p < 0.05 |
| 1200 | 1.08 | (0.88–1.28) | 1.88 | (1.75–2.01) | p = 0.2 |
| Drug Treatment Concentration (nM) | ID | ELQ316 + ID | p-Value | ||
|---|---|---|---|---|---|
| Mean (%) | CI 95% | Mean (%) | CI 95% | ||
| 25 | 98.04 | N/A | 95.16 | (93.5–96.82) | p = 0.55 |
| 50 | 99.22 | (98.90–99.54) | 89.25 | (85.16–93.34) | p = 0.20 |
| 75 | 99.22 | N/A | 87.63 | (84.41–90.85) | p < 0.05 |
| 100 | 100 | N/A | 81.18 | (79.37–82.99) | p < 0.05 |
| 150 | 100 | N/A | 52.15 | (50.84–53.46) | p = 0.07 |
| 200 | 89.22 | (77.98–100) | 25.27 | (24.81–25.73) | p < 0.05 |
| 300 | 38.24 | (36.26–40.22) | 25.81 | (25.18–26.44) | p = 0.08 |
| 600 | 21.57 | (20.25–22.89) | 1.33 | (1.29–1.37) | p < 0.05 |
| 1200 | 17.16 | (16.83–17.49) | 0.59 | (0.49–0.69) | p < 0.05 |
References
- Pipano, E.; Hadani, A. Control of Bovine Babesiosis. In Malaria and Babesiosis; Ristic, M., Ambroise-Thomas, P., Kreier, J.P., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 263–303. [Google Scholar]
- Bock, R.; Jackson, L.; De Vos, A.; Jorgensen, W. Babesiosis of Cattle. Parasitology 2004, 129, S247–S269. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.S.; Sengupta, P.P.; Paramanandham, K.; Suresh, K.P.; Chamuah, J.K.; Rudramurthy, G.R.; Roy, P. Bovine Babesiosis: An Insight into the Global Perspective on the Disease Distribution by Systematic Review and Meta-Analysis. Vet. Parasitol. 2020, 283, 109136. [Google Scholar] [CrossRef] [PubMed]
- Asrar, R.; Farhan, H.R.; Sultan, D.; Ahmad, M.; Hassan, S.; Kalim, F.; Shakoor, A.; Muhammad Taimoor Ihsan, H.; Shahab, A.; Ali, W.; et al. Review Article Bovine Babesiosis; Review on Its Global Prevalence and Anticipatory Control for One Health. Continental Vet. J. 2022, 2, 42–49. [Google Scholar]
- Smith, R.D.; Evans, D.E.; Martins, J.R.; Ceresér, V.H.; Correa, B.L.; Cardozo, C.P.H.; Solari, M.A.; Nari, A. Babesiosis (Babesis bovis) Stability in Unstable Environments. Ann. N. Y. Acad. Sci. 2000, 916, 510–520. [Google Scholar] [CrossRef]
- Zintl, A.; Mcgrath, G.; O’grady, L.; Fanning, J.; Downing, K.; Roche, D.; Casey, M.; Gray, J.S. Changing Incidence of Bovine Babesiosis in Ireland. Ir. Vet. J. 2014, 67, 19. [Google Scholar] [CrossRef]
- Todorovic, R.A.; Viscaino, O.G.; Gonzalez, E.F.; Adams, L.G. Chemoprophylaxis (Imidocarb) against Babesia bigemina and Babesia argentina Infections. Am. J. Vet. Res. 1973, 34, 1153–1161. [Google Scholar]
- Vial, H.J.; Gorenflot, A. Chemotherapy against Babesiosis. Vet. Parasitol. 2006, 138, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Almazan, C.; Tipacamu, G.A.; Rodriguez, S.; Mosqueda, J.; Perez De Leon, A. Immunological Control of Ticks and Tick-Borne Diseases That Impact Cattle Health and Production. Front. Biosci. 2018, 23, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.A.; Rojas, C.; Figueroa, J.V. An Overview of Current Knowledge on in Vitro Babesia Cultivation for Production of Live Attenuated Vaccines for Bovine Babesiosis in Mexico. Front. Vet. Sci. 2020, 7, 364. [Google Scholar] [CrossRef]
- Azhar, M.; Gadahi, J.A.; Bhutto, B.; Tunio, S.; Vistro, W.A.; Tunio, H.; Bhutto, S.; Ram, T. Babesiosis: Current Status and Future Perspectives in Pakistan and Chemotherapy Used in Livestock and Pet Animals. Heliyon 2023, 9, e17172. [Google Scholar] [CrossRef]
- Mosqueda, J.; Olvera-Ramírez, A.; Aguilar-Tipacamú, G.; Cantó, G.J. Current Advances in Detection and Treatment of Babesiosis. Curr. Med. Chem. 2012, 19, 1504–1518. [Google Scholar] [CrossRef] [PubMed]
- Kuttler, K.L.; Aliu, Y.O. Chemotherapy of Babesiosis. In Malaria and Babesiosis; Ristic, M., Kreier, J.P., Eds.; Academic Press: New York, NY, USA, 1984; pp. 151–172. [Google Scholar]
- Silva, M.G.; Villarino, N.F.; Knowles, D.P.; Suarez, C.E. Assessment of Draxxin® (Tulathromycin) as an Inhibitor of in Vitro Growth of Babesia bovis, Babesia bigemina and Theileria equi. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 265–270. [Google Scholar] [CrossRef]
- Sears, K.; Knowles, D.; Dinkel, K.; Mshelia, P.W.; Onzere, C.; Silva, M.; Fry, L. Imidocarb Dipropionate Lacks Efficacy against Theileria Haneyi and Fails to Consistently Clear Theileria Equi in Horses Co-Infected with T. haneyi. Pathogens 2020, 9, 1035. [Google Scholar] [CrossRef]
- Silva, M.G.; Bastos, R.G.; Stone Doggett, J.; Riscoe, M.K.; Pou, S.; Winter, R.; Dodean, R.A.; Nilsen, A.; Suarez, C.E. Endochin-like Quinolone-300 and ELQ-316 Inhibit Babesia bovis, B. Bigemina, B. Caballi and Theileria equi. Parasit. Vectors 2020, 13, 606. [Google Scholar] [CrossRef]
- Müller, J.; Aguado-Martinez, A.; Manser, V.; Balmer, V.; Winzer, P.; Ritler, D.; Hostettler, I.; Solís, D.A.; Ortega-Mora, L.; Hemphill, A. Buparvaquone Is Active against Neospora Caninum in Vitro and in Experimentally Infected Mice. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 16–25. [Google Scholar] [CrossRef]
- Rizk, M.A.; El-Sayed, S.A.E.-S.; Igarashi, I. Diminazene Aceturate and Imidocarb Dipropionate-Based Combination Therapy for Babesiosis—A New Paradigm. Ticks Tick. Borne Dis. 2023, 14, 102145. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, N.M.; Lacy, P.A.; Villarino, N.F.; Doggett, J.S.; Riscoe, M.K.; Bastos, R.G.; Laughery, J.M.; Ueti, M.W.; Suarez, C.E. Comparative Efficacy of Buparvaquone and Imidocarb in Inhibiting the in Vitro Growth of Babesia Bovis. Front. Pharmacol. 2024, 15, 1407548. [Google Scholar] [CrossRef] [PubMed]
- Belloli, C.; Crescenzo, G.; Lai, O.; Carofiglio, V.; Marang, O.; Ormas, P. Pharmacokinetics of Imidocarb Dipropionate in Horses after Intramuscular Administration. Equine Vet. J. 2002, 34, 625–629. [Google Scholar] [CrossRef]
- Mdachi, R.E.; Murilla, G.A.; Omukuba, J.N.; Cagnolati, V. Disposition of Diminazene Aceturate (Berenil®) in Trypanosome-Infected Pregnant and Lactating Cows. Vet. Parasitol. 1995, 58, 215–225. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Yamasaki, M.; Nakamura, K.; Sasaki, N.; Murakami, M.; Kumara, B.; Rajapakshage, W.; Ohta, H.; Maede, Y.; Takiguchi, M. Development and Characterization of a Strain of Babesia Gibsoni Resistant to Diminazene Aceturate In Vitro. J. Vet. Med. Sci. 2010, 72, 765–771. [Google Scholar] [CrossRef]
- Tuntasuvan, D.; Jarabrum, W.; Viseshakul, N.; Mohkaew, K.; Borisutsuwan, S.; Theeraphan, A.; Kongkanjana, N. Chemotherapy of Surra in Horses and Mules with Diminazene Aceturate. Vet. Parasitol. 2003, 110, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Pérez de León, A.A.; Strickman, D.A.; Knowles, D.P.; Fish, D.; Thacker, E.; de la Fuente, J.; Krause, P.J.; Wikel, S.K.; Miller, R.S.; Wagner, G.G.; et al. One Health Approach to Identify Research Needs in Bovine and Human Babesioses: Workshop Report. Parasit. Vectors 2010, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Pérez de Leon, A.A.; Teel, P.D.; Auclair, A.N.; Messenger, M.T.; Guerrero, F.D.; Schuster, G.; Miller, R.J. Integrated Strategy for Sustainable Cattle Fever Tick Eradication in USA Is Required to Mitigate the Impact of Global Change. Front. Physiol. 2012, 3, 195. [Google Scholar] [CrossRef]
- US. Food and Drug Administration. CVM-FDA FOI Summary for Original Approval of IMIZOL-NADA 141-071. 1997. Available online: https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/3635 (accessed on 28 October 2024).
- Adams, L.; Corrier, D.; Williams, J. A Study of the Toxicity of Imidocarb Dipropionate in Cattle. Res. Vet. Sci. 1980, 28, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Coldham, N.G.; Moore, A.S.; Sivapathasundaram, S.; Sauer, M.J. Lmidocarb Depletion from Cattle Liver and Mechanism of Retention in Isolated Bovine Hepatocytes. Analyst 1994, 119, 2549–2552. [Google Scholar] [CrossRef]
- Tang, Y.; Yu, N.; Liu, C.; Han, M.; Wang, H.; Chen, X.; Kang, J.; Li, X.; Liu, Y. Residue Depletion of Imidocarb in Bovine Tissues by UPLC-MS/MS. Animals 2023, 13, 104. [Google Scholar] [CrossRef]
- Fray, M.; Pudney, M. Site of Action of the Antimalarial Hydroxynaphthoquinone, 2-[Trans-4-(40chlorophenyl)Cyclohexyl]-3-Hydroxy-1,4,-Naphthoquinone (566c80). Biochem. Pharmacol. 1992, 40, 914–919. [Google Scholar] [CrossRef]
- Hudson, A.T.; Randall, A.W.; Fry, M.; Ginger, C.D.; Hill, B.; Latter, V.S.; McHardy, N.; Williams, R.B. Novel Anti-Malarial Hydroxynpahthoquinones with Potent Broad Spectrum Anti-Protozoal Activity. Parasitology 1985, 90, 45–55. [Google Scholar] [CrossRef]
- Sharifiyazdi, H.; Namazi, F.; Oryan, A.; Shahriari, R.; Razavi, M. Point Mutations in the Theileria Annulata Cytochrome b Gene Is Associated with Buparvaquone Treatment Failure. Vet. Parasitol. 2012, 187, 431–435. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Chaouch, M.; Ajroud, K.; Darghouth, M.A.; BenAbderrazak, S. Sequence Polymorphism of Cytochrome b Gene in Theileria annulata Tunisian Isolates and Its Association with Buparvaquone Treatment Failure. PLoS ONE 2015, 10, e0129678. [Google Scholar] [CrossRef]
- Carter, P. Animal Health Assessment of the Efficacy of Buparvaquone for the Treatment of “Benign” Bovine Theileriosis; Project Code: B.AHE.0048; Tick Fever Centre Department of Employment, Economic Development and Innovation; Meat & Livestock Australia Limited: North Sydney, Australia, 2011; ISBN 9781741916591. [Google Scholar]
- Müller, J.; Aguado-Martínez, A.; Manser, V.; Wong, H.N.; Haynes, R.K.; Hemphill, A. Repurposing of Antiparasitic Drugs: The Hydroxy-Naphthoquinone Buparvaquone Inhibits Vertical Transmission in the Pregnant Neosporosis Mouse Model. Vet. Res. 2016, 47, 32. [Google Scholar] [CrossRef] [PubMed]
- Muraguri, G.R.; Ngumi, P.N.; Wesonga, D.; Ndungu, S.G.; Wanjohi, J.M.; Bang, K.; Fox, A.; Dunne, J.; McHardy, N. Clinical Efficacy and Plasma Concentrations of Two Formulations of Buparvaquone in Cattle Infected with East Coast Fever (Theileria Parva Infection). Res. Vet. Sci. 2006, 81, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Bailey, G. Buparvaquone Tissue Residue Study; Meat & Livestock Australia Limited: Sydney, Australia, 2013; ISBN 9781925045475. [Google Scholar]
- Wilkie, G.M.; Kirvar, E.; Thomas, E.M.; Sparagano, O.; Brown, C.G.D. Stage-Specific Activity in Vitro on the Theileria Infection Process of Serum from Calves Treated Prophylactically with Buparvaquone. Vet. Parasitol. 1998, 80, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Goud, S.K.; Vijayakumar, K.; Davis, J.; Tresamol, P.V.; Ravishankar, C.; Devada, K. Efficacy of Different Treatment Regimens against Oriental Theileriosis in Naturally Infected Cattle. Indian J. Vet. Med. 2021, 40, 14–19. [Google Scholar]
- Brasseur, P.; Lecoublet, S.; Kapel, N.; Favennec, L.; Ballet, J.J. In Vitro Evaluation of Drug Susceptibilities of Babesia Divergens Isolates. Antimicrob. Agents Chemother. 1998, 42, 818. [Google Scholar] [CrossRef]
- Hasheminasab, S.S.; Moradi, P.; Wright, I. A Four Year Epidemiological and Chemotherapy Survey of Babesiosis and Theileriosis, and Tick Vectors in Sheep, Cattle and Goats in Dehgolan, Iran. Ann. Parasitol. 2018, 64, 43–48. [Google Scholar] [CrossRef]
- Shah, N.D.; Bhikane, A.U.; Jadhav, R.K.; Chavhan, S.G.; Mohan, A. Therapeutic Management of Babesiosis Alone and Its Mixed Infection with Theileriosis in Cattle. Indian J. Vet. Med. Vol. 2019, 39, 26–31. [Google Scholar]
- McConnell, E.V.; Bruzual, I.; Pou, S.; Winter, R.; Dodean, R.A.; Smilkstein, M.J.; Krollenbrock, A.; Nilsen, A.; Zakharov, L.N.; Riscoe, M.K.; et al. Targeted Structure–Activity Analysis of Endochin-like Quinolones Reveals Potent Qi and Qo Site Inhibitors of Toxoplasma Gondii and Plasmodium Falciparum Cytochrome Bc1 and Identifies ELQ-400 as a Remarkably Effective Compound against Acute Experimental Toxoplasmosis. ACS Infect. Dis. 2018, 4, 1574–1584. [Google Scholar] [CrossRef]
- Fisher, N.; Meunier, B.; Biagini, G.A. The Cytochrome Bc1 Complex as an Antipathogenic Target. FEBS Lett. 2020, 594, 2935–2952. [Google Scholar] [CrossRef]
- Lawres, L.A.; Garg, A.; Kumar, V.; Bruzual, I.; Forquer, I.P.; Renard, I.; Virji, A.Z.; Boulard, P.; Rodriguez, E.X.; Allen, A.J.; et al. Radical Cure of Experimental Babesiosis in Immunodeficient Mice Using a Combination of an Endochin-like Quinolone and Atovaquone. J. Exp. Med. 2016, 213, 1307–1318. [Google Scholar] [CrossRef]
- Nilsen, A.; Miley, G.P.; Forquer, I.P.; Mather, M.W.; Katneni, K.; Li, Y.; Pou, S.; Pershing, A.M.; Stickles, A.M.; Ryan, E.; et al. Discovery, Synthesis, and Optimization of Antimalarial 4(1H)-Quinolone-3-Diarylethers. J. Med. Chem. 2014, 57, 3818–3834. [Google Scholar] [CrossRef] [PubMed]
- Goff, W.L.; Johnson, W.C.; Cluff, C.W. Babesia Bovis Immunity: In Vitro and in Vivo Evidence for IL-10 Regulation of IFN-γ and INOS. Ann. N. Y. Acad. Sci. 1998, 849, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.G.; Ristic, M. Babesia Bovis: Continuous Cultivation in a Microaerophilous Stationary Phase Culture. Science 1980, 207, 1218–1220. [Google Scholar] [CrossRef]
- Mehlhorn, H. Babesiacidal Drugs. In Encyclopedia of Parasitology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–11. [Google Scholar]
- Doggett, J.S.; Nilsen, A.; Forquer, I.; Wegmann, K.W.; Jones-Brando, L.; Yolken, R.H.; Bordón, C.; Charman, S.A.; Katneni, K.; Schultz, T.; et al. Endochin-like Quinolones Are Highly Efficacious against Acute and Latent Experimental Toxoplasmosis. Proc. Natl. Acad. Sci. USA 2012, 109, 15936–15941. [Google Scholar] [CrossRef] [PubMed]
- Painter, H.J.; Morrisey, J.M.; Mather, M.W.; Vaidya, A.B. Specific Role of Mitochondrial Electron Transport in Blood-Stage Plasmodium Falciparum. Nature 2007, 446, 88–91. [Google Scholar] [CrossRef]
- Tomavo, S.; Boothroydt, J.C. Interconnection between Organellar Functions, Development and Drug Resistance in the Protozoan Parasite, Toxoplasma Gondii. Int. J. Parasitol. 1995, 25, 1293–1299. [Google Scholar] [CrossRef]
- Nugraha, A.B.; Tuvshintulga, B.; Guswanto, A.; Tayebwa, D.S.; Rizk, M.A.; Gantuya, S.; El-Saber Batiha, G.; Beshbishy, A.M.; Sivakumar, T.; Yokoyama, N.; et al. Screening the Medicines for Malaria Venture Pathogen Box against Piroplasm Parasites. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 84–90. [Google Scholar] [CrossRef]
- Chiu, J.E.; Renard, I.; Pal, A.C.; Singh, P.; Vydyam, P.; Thekkiniath, J.; Kumar, M.; Gihaz, S.; Pou, S.; Winter, R.W.; et al. Effective Therapy Targeting Cytochrome Bc1prevents Babesia Erythrocytic Development and Protects from Lethal Infection. Antimicrob. Agents Chemother. 2021, 65, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, N.; Balmer, V.; Müller, J.; Müller, N.; Winter, R.; Pou, S.; Nilsen, A.; Riscoe, M.; Francisco, S.; Leitao, A.; et al. Activities of Endochin-Like Quinolones Against in Vitro Cultured Besnoitia Besnoiti Tachyzoites. Front. Vet. Sci. 2020, 7, 96. [Google Scholar] [CrossRef]
- Alday, P.H.; Bruzual, I.; Nilsen, A.; Pou, S.; Winter, R.; Mamoun, C.B.; Riscoe, M.K.; Doggett, J.S. Genetic Evidence for Cytochrome b Qi Site Inhibition by 4(1H)-Quinolone-3-Diarylethers and Antimycin in Toxoplasma Gondii. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Nilsen, A.; Lacrue, A.N.; White, K.L.; Forquer, I.P.; Cross, R.M.; Marfurt, J.; Mather, M.W.; Delves, M.J.; Shackleford, D.M.; Saenz, F.E.; et al. Quinolone-3-Diarylethers: A New Class of Drugs for a New Era of Malaria Eradication. Sci. Transl. Med. 2013, 5, 177ra37. [Google Scholar] [CrossRef] [PubMed]
- Miley, G.P.; Pou, S.; Winter, R.; Nilsen, A.; Li, Y.; Kelly, J.X.; Stickles, A.M.; Mather, M.W.; Forquer, I.P.; Pershing, A.M.; et al. ELQ-300 Prodrugs for Enhanced Delivery and Single-Dose Cure of Malaria. Antimicrob. Agents Chemother. 2015, 59, 5555–5560. [Google Scholar] [CrossRef]
- McDougall, S.; Hillerton, J.E.; Pegram, D. Concentrations of Buparvaquone in Milk and Tissue of Dairy Cows. N. Z. Vet. J. 2016, 64, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Tallarida, R.J. Drug Synergism and Dose-Effect Data Analysis. In Drug Synergism and Dose-Effect Data Analysis; Chapman and Hall/CRC: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Winter, R.; Kelly, J.X.; Smilkstein, M.J.; Hinrichs, D.; Koop, D.R.; Riscoe, M.K. Optimization of Endochin-like Quinolones for Antimalarial Activity. Exp. Parasitol. 2011, 127, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Proma, F.H.; Shourav, M.K.; Choi, J. Post-Antibiotic Effect of Ampicillin and Levofloxacin to Escherichia coli and Staphylococcus aureus Based on Microscopic Imaging Analysis. Antibiotics 2020, 9, 458. [Google Scholar] [CrossRef]
- Mazuz, M.L.; Golenser, J.; Fish, L.; Haynes, R.K.; Wollkomirsky, R.; Leibovich, B.; Shkap, V. Artemisone Inhibits in Vitro and in Vivo Propagation of Babesia Bovis and B. Bigemina Parasites. Exp. Parasitol. 2013, 135, 690–694. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. |
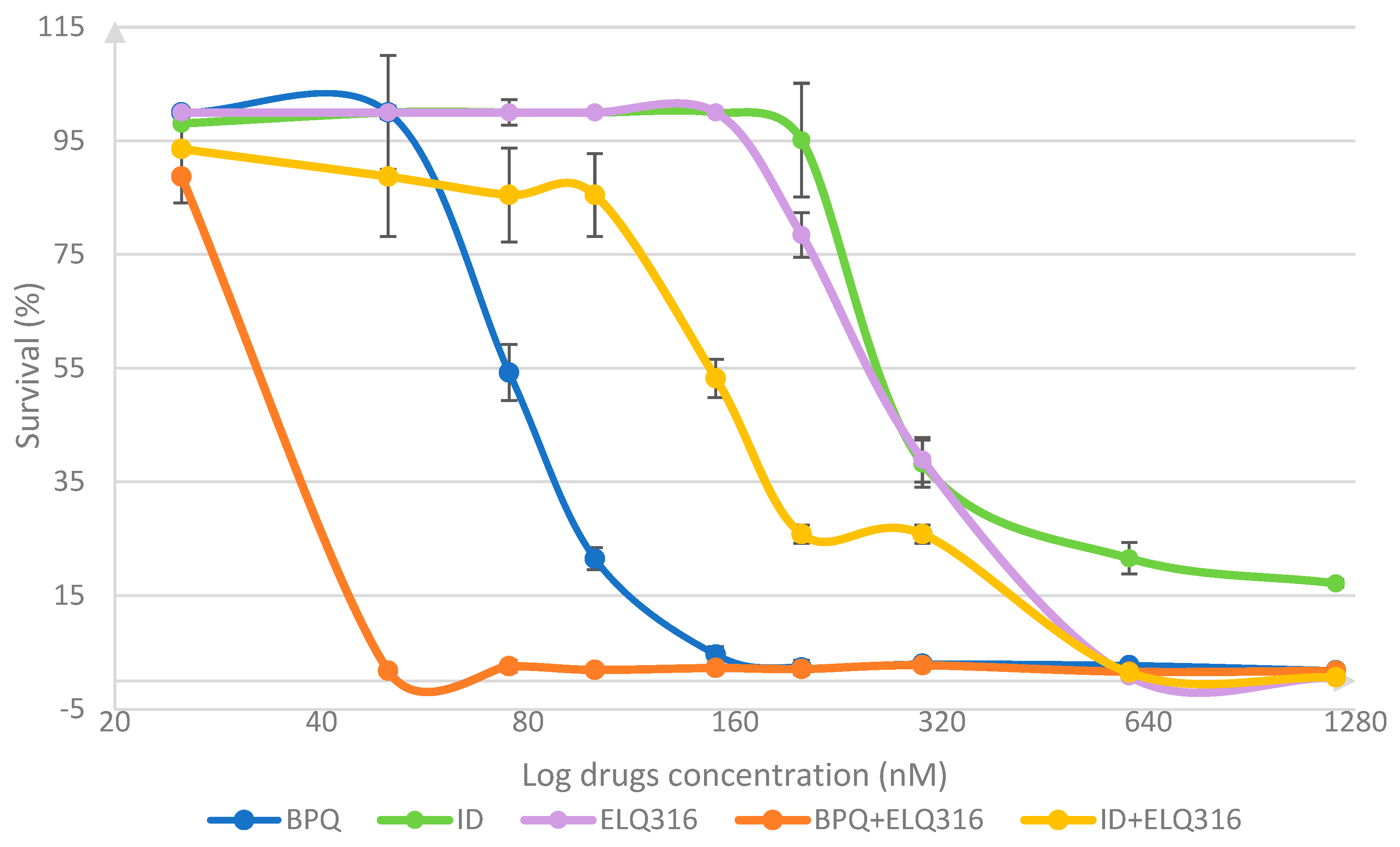
| Drug Treatment Concentration (nM) | BPQ | ID | ELQ316 | |||
|---|---|---|---|---|---|---|
| Mean (%) | CI 95% | Mean (%) | CI 95% | Mean (%) | CI 95% | |
| 25 | 99.87 | (99.78–99.96) | 98.04 | N/A | 100 | N/A |
| 50 | 86.6 | (77.56–95.64) | 99.22 | (98.90–99.54) | 99.67 | (99.45–99.89) |
| 75 | 54.25 a | (52.33–56.17) | 99.22 b | N/A | 98.69 b | (97.81–99.57) |
| 100 | 21.5 a | (20.74–22.26) | 100 a,b | N/A | 100 b | N/A |
| 150 | 4.64 a | (4.1–5.18) | 100 a,b | N/A | 100 b | N/A |
| 200 | 2.55 a | (2.05–3.05) | 89.22 b | (77.98–100) | 78.43 a,b | (76.9–79.96) |
| 300 | 3.01 a | (2.67–3.35) | 38.24 a,b | (36.26–40.22) | 39.1 b | (38.19–40.01) |
| 600 | 2.62 a | (2.51–2.73) | 21.57 a | (20.25–22.89) | 0.65 b | (0.62–0.68) |
| 1200 | 1.08 | (0.88–1.28) | 17.16 | (16.83–17.49) | 0.75 | (0.65–0.85) |
| Drug | IC50% (nM) | IC99% (nM) | ||
|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | |
| BPQ + ELQ-316 | 31.21 | (15.06–68.48) | 78 | (51.68–117.6) |
| BPQ | 77.06 | (70.16–86.01) | 186 | (97.53–276.9) |
| ID + ELQ-316 | 197 | (129.0–311.2) | 899 | (789.2–1009) |
| ID | 635.1 | (280.9–2119) | 2390 | N/A |
| ELQ-316 | 654.9 | (362.3–1411) | 731 | (511.1–894.4) |
| Single Drugs and Combination Treatments | Time Post-Treatment Without Drug (h) | ||||
|---|---|---|---|---|---|
| 24 | 48 | 72 | 96 | 120 | |
| Control | N/A | ||||
| BPQ | 200 to 1200 | 150 to 1200 | |||
| BPQ + ELQ-316 | 200 to 1200 | 100 to 1200 | 50 to 1200 | ||
| ELQ-316 | 600 to 1200 | ||||
| ID + ELQ-316 | 600 to 1200 | 300 to 1200 | |||
| ID | 1200 | 300 to 1200 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardillo, N.M.; Villarino, N.F.; Lacy, P.A.; Riscoe, M.K.; Doggett, J.S.; Ueti, M.W.; Chung, C.J.; Suarez, C.E. The Combination of Buparvaquone and ELQ316 Exhibit a Stronger Effect than ELQ316 and Imidocarb Against Babesia bovis In Vitro. Pharmaceutics 2024, 16, 1402. https://doi.org/10.3390/pharmaceutics16111402
Cardillo NM, Villarino NF, Lacy PA, Riscoe MK, Doggett JS, Ueti MW, Chung CJ, Suarez CE. The Combination of Buparvaquone and ELQ316 Exhibit a Stronger Effect than ELQ316 and Imidocarb Against Babesia bovis In Vitro. Pharmaceutics. 2024; 16(11):1402. https://doi.org/10.3390/pharmaceutics16111402
Chicago/Turabian StyleCardillo, Natalia M., Nicolas F. Villarino, Paul A. Lacy, Michael K. Riscoe, Joseph Stone Doggett, Massaro W. Ueti, Chungwon J. Chung, and Carlos E. Suarez. 2024. "The Combination of Buparvaquone and ELQ316 Exhibit a Stronger Effect than ELQ316 and Imidocarb Against Babesia bovis In Vitro" Pharmaceutics 16, no. 11: 1402. https://doi.org/10.3390/pharmaceutics16111402
APA StyleCardillo, N. M., Villarino, N. F., Lacy, P. A., Riscoe, M. K., Doggett, J. S., Ueti, M. W., Chung, C. J., & Suarez, C. E. (2024). The Combination of Buparvaquone and ELQ316 Exhibit a Stronger Effect than ELQ316 and Imidocarb Against Babesia bovis In Vitro. Pharmaceutics, 16(11), 1402. https://doi.org/10.3390/pharmaceutics16111402






