Vascular Graft Impregnation with a Fosfomycin/Oritavancin Combination to Prevent Early Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Antibiotic Stock Solution Preparation
2.2. Bacterial Strains and Culture Conditions
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays
2.4. Synergy Assays
2.5. Preparation of Fosfomycin/Oritavancin-Impregnated Vascular Graft
2.6. Antimicrobial Evaluation of Fosfomycin/Oritavancin-Impregnated Vascular Graft
3. Results
3.1. MIC and MBC Assays
3.2. Synergy Assays
3.3. Antimicrobial Evaluation of Fosfomycin/Oritavancin-Impregnated Vascular Graft
3.3.1. S. epidermidis
3.3.2. MRSA
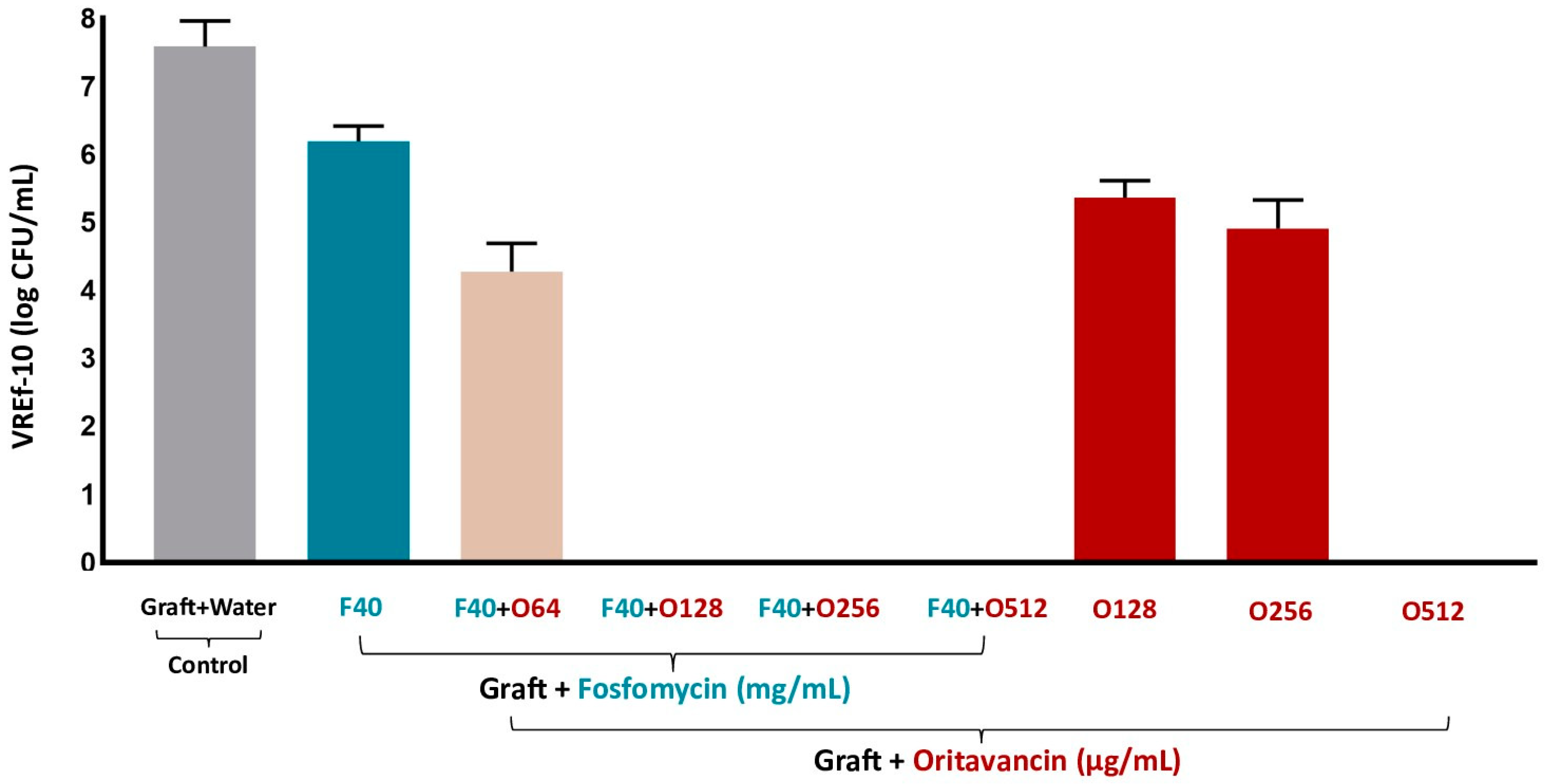
3.3.3. VREf-10
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gharamti, A.; Kanafani, Z.A. Vascular Graft Infections: An update. Infect. Dis. Clin. N. Am. 2018, 32, 789–809. [Google Scholar] [CrossRef]
- Antonello, R.M.; D’Oria, M.; Cavallaro, M.; Dore, F.; Cova, M.A.; Ricciardi, M.C.; Comar, M.; Campisciano, G.; Lepidi, S.; De Martino, R.R.; et al. Management of abdominal aortic prosthetic graft and endograft infections. A multidisciplinary update. J. Infect. Chemother. 2019, 25, 669–680. [Google Scholar] [CrossRef]
- Berard, X.; Puges, M.; Pinaquy, J.-B.; Cazanave, C.; Stecken, L.; Bordenave, L.; Pereyre, S.; M’Zali, F. In Vitro Evidence of Improved Antimicrobial Efficacy of Silver and Triclosan Containing Vascular Grafts Compared with Rifampicin Soaked Grafts. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 424–432. [Google Scholar] [CrossRef]
- El Helou, O.C.; Berbari, E.F.; Marculescu, C.E.; El Atrouni, W.I.; Razonable, R.R.; Steckelberg, J.M.; Hanssen, A.D.; Osmon, D.R. Outcome of enterococcal prosthetic joint infection: Is combination systemic therapy superior to monotherapy? Clin. Infect. Dis. 2008, 47, 903–909. [Google Scholar] [CrossRef]
- Fitridge, R.; Thompson, M. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, Australia, 2011. [Google Scholar]
- Berger, P. Graft Infections After Surgical Aortic Reconstructions. Ph.D. Thesis, Universiteit Utrecht, Utrecht, The Netherlands, 2015. [Google Scholar]
- Lew, W.; Moore, W. Antibiotic-impregnated grafts for aortic reconstruction. Semin. Vasc. Surg. 2011, 24, 211–219. [Google Scholar] [CrossRef]
- Huang, F.; Sun, L.; Zheng, J. In vitro and in vivo characterization of a silk fibroin-coated polyester vascular prosthesis. Artif. Organs 2008, 32, 932–941. [Google Scholar] [CrossRef]
- He, E.; Serpelloni, S.; Alvear, P.; Rahimi, M.; Taraballi, F. Vascular Graft Infections: An Overview of Novel Treatments Using Nanoparticles and Nanofibers. Fibers 2022, 10, 12. [Google Scholar] [CrossRef]
- Herten, M.; Idelevich, E.A.; Sielker, S.; Becker, K.; Scherzinger, A.S.; Osada, N.; Torsello, G.B.; Bisdas, T. Vascular Graft Impregnation with Antibiotics: The Influence of High Concentrations of Rifampin, Vancomycin, Daptomycin, and Bacteriophage Endolysin HY-133 on Viability of Vascular Cells. Med. Sci. Monit. Basic Res. 2017, 23, 250–257. [Google Scholar] [CrossRef]
- Cutting, K.; White, R.; Edmonds, M. The safety and efficacy of dressings with silver—Addressing clinical concerns. Int. Wound J. 2007, 4, 177–184. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Pawlak, J. In vitro activity of fosfomycin alone and in combination against Staphylococcus aureus with reduced susceptibility or resistance to methicillin, vancomycin, daptomycin or linezolid. J. Antimicrob. Chemother. 2022, 78, 238–241. [Google Scholar] [CrossRef]
- Falagas, M.E.; Vouloumanou, E.K.; Samonis, G.; Vardakasa, K.Z. Fosfomycin. Clin. Microbiol. Rev. 2016, 29, 321–347. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Mrozińska, Z. Biofunctionalization of textile materials. 2. Antimicrobial modification of poly(lactide) (PLA) nonwoven fabricsby fosfomycin. Polymers 2020, 12, 768. [Google Scholar] [CrossRef]
- Antonello, R.M.; Principe, L.; Maraolo, A.E.; Viaggi, V.; Pol, R.; Fabbiani, M.; Montagnani, F.; Lovecchio, A.; Luzzati, R.; Di Bella, S. Fosfomycin as Partner Drug for Systemic Infection Management. A Systematic Review of Its Synergistic Properties from In Vitro and In Vivo Studies. Antibiotics 2020, 9, 500. [Google Scholar] [CrossRef]
- Lagatolla, C.; Mehat, J.W.; La Ragione, R.M.; Luzzati, R.; Di Bella, S. In Vitro and In Vivo Studies of Oritavancin and Fosfomycin Synergism against Vancomycin-Resistant Enterococcus faecium. Antibiotics 2022, 11, 1334. [Google Scholar] [CrossRef]
- Tran, T.T.; Villegas, S.G.; Aitken, S.L.; Butler-Wu, S.M.; Soriano, A.; Werth, B.J.; Munita, J.M. New Perspectives on Antimicrobial Agents: Long-Acting Lipoglycopeptides. Antimicrob. Agents Chemother. 2022, 66, e0261420. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Schweizer, F.; Karlowsky, J.A. Oritavancin: Mechanism of action. Clin. Infect. Dis. 2012, 54, 214–219. [Google Scholar] [CrossRef]
- The Medical Letter® on Drugs and Therapeutics. In Brief: Oritavancin (Kimyrsa) for Skin and Skin Structure Infections. 2021. Available online: https://secure.medicalletter.org/TML-article-1631f (accessed on 31 October 2022).
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved glycopeptide antibacterial drugs: Mechanism of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef]
- M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2021.
- Hasselmann, C. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- ISO 20645:2004; Textile Fabrics—Determination of Antibacterial Activity—Agar Diffusion Plate Test. ISO: Geneva, Switzerland, 2004.
- Xodo, A.; D’Oria, M.; Squizzato, F.; Antonello, M.; Grego, F.; Bonvini, S.; Milite, D.; Frigatti, P.; Cognolato, D.; Veraldi, G.F.; et al. Early and mid-term outcomes following open surgical conversion after failed endovascular aneurysm repair from the “Italian North-easT RegIstry of surgical Conversion AfTer Evar” (INTRICATE). J. Vasc. Surg. 2022, 75, 153–161. [Google Scholar] [CrossRef]
- Colacchio, E.C.; D’Oria, M.; Grando, B.; Garofalo, A.R.; D’Andrea, A.; Bassini, S.; Lepidi, S.; Antonello, M.; Ruaro, B. A systematic review of in-situ aortic reconstructions for abdominal aortic graft and endograft infections: Outcomes of currently available options for surgical replacement. Ann. Vasc. Surg. 2023, 95, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Chakfé, N.; Diener, H.; Lejay, A.; Assadian, O.; Berard, X.; Caillon, J.; Fourneau, I.; Glaudemans, A.W.J.M.; Koncar, I.; Lindholt, J.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Vascular Graft and Endograft Infections. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 339–384. [Google Scholar] [CrossRef]
- Janko, M.R.; Hubbard, G.; Back, M.; Shah, S.K.; Pomozi, E.; Szeberin, Z.; DeMartino, R.; Wang, L.J.; Crofts, S.; Belkin, M.; et al. In-Situ Bypass is Associated with Superior Reinfection-free Survival Compared to Extra-Anatomic Bypass for the Management of Secondary Aortic Graft Infections Without Enteric Involvement. J. Vasc. Surg. 2022, 76, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Wyss, T.R.; Giardini, M.; Sorelius, K. Infective Native Aortic Aneurysms: A Delphi Consensus Document on Treatment, Follow-Up, and Definition of Cure. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.; Baxter, M.; Wong, M.; Mirzanejad, Y.; Lee, A.; Dhami, R.; Kosar, J.; Werry, D.; Irfan, N.; Tessier, J.F.; et al. Real-life experience with IV fosfomycin in Canada: Results from the Canadian LEadership on Antimicrobial Real-life usage (CLEAR) registry. J. Glob. Antimicrob. Resist. 2023, 33, 171–176. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Wang, J.; Cai, Y. Efficacy and safety of oritavancin for the treatment of acute bacterial skin and skin-structure infections: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2021, 25, 380–389. [Google Scholar] [CrossRef]
- Redell, M.; Sierra-Hoffman, M.; Assi, M.; Bochan, M.; Chansolme, D.; Gandhi, A.; Sheridan, K.; Soosaipillai, I.; Walsh, T.; Massey, J. The CHROME Study, a Real-world Experience of Single- and Multiple-Dose Oritavancin for Treatment of Gram-Positive Infections. Open Forum Infect. Dis. 2019, 6, ofz479. [Google Scholar] [CrossRef]
- Iarikov, D.; Wassel, R.; Farley, J.; Nambiar, S. Adverse Events Associated with Fosfomycin Use: Review of the Literature and Analyses of the FDA Adverse Event Reporting System Database. Infect. Dis. Ther. 2015, 4, 433–458. [Google Scholar] [CrossRef]
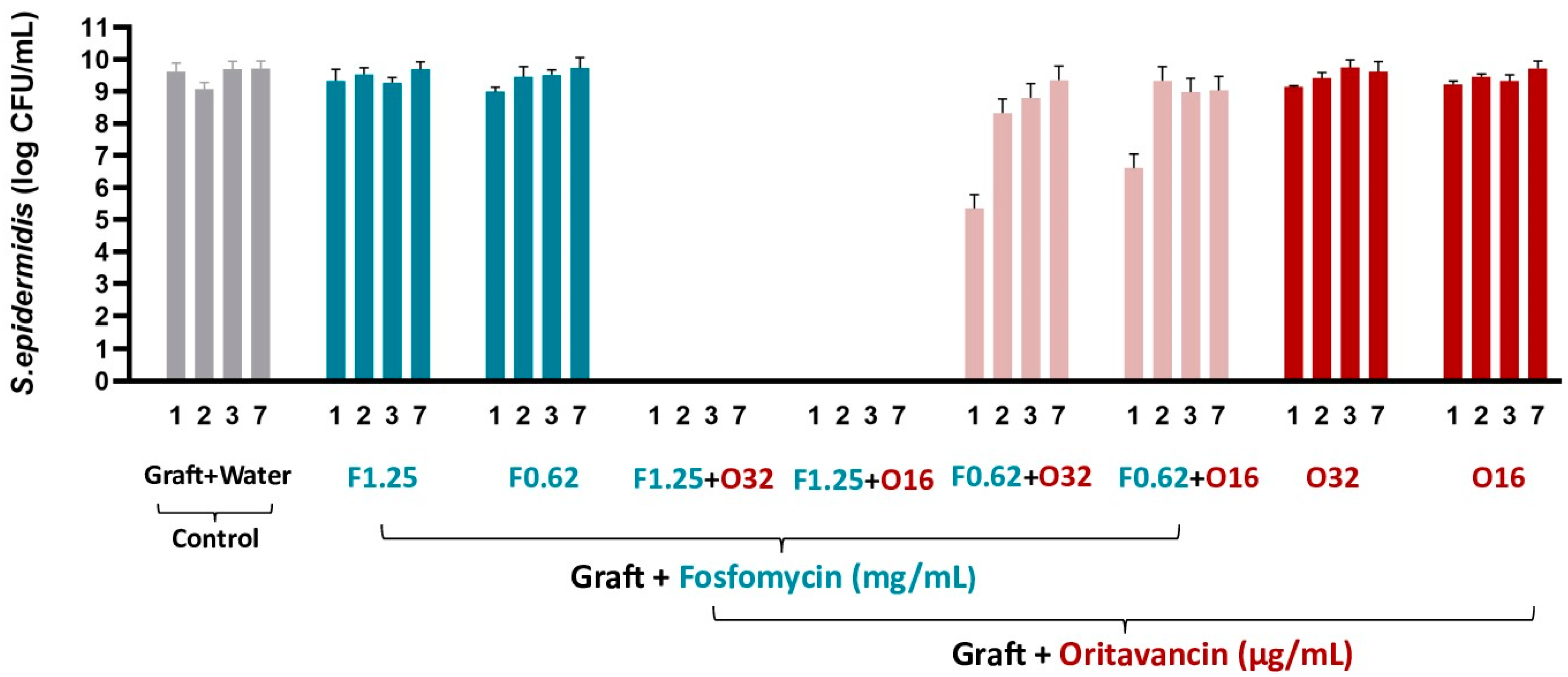
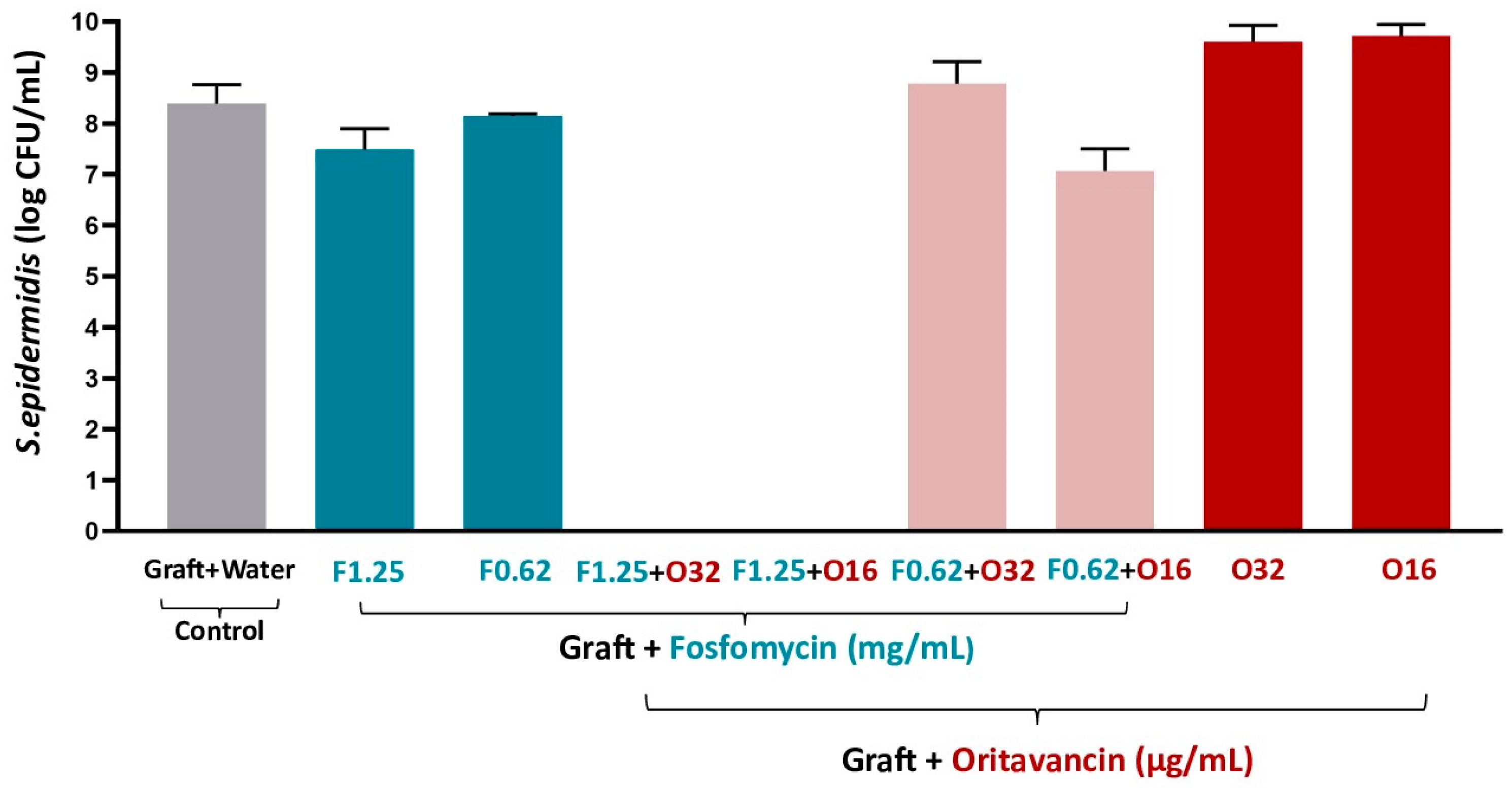

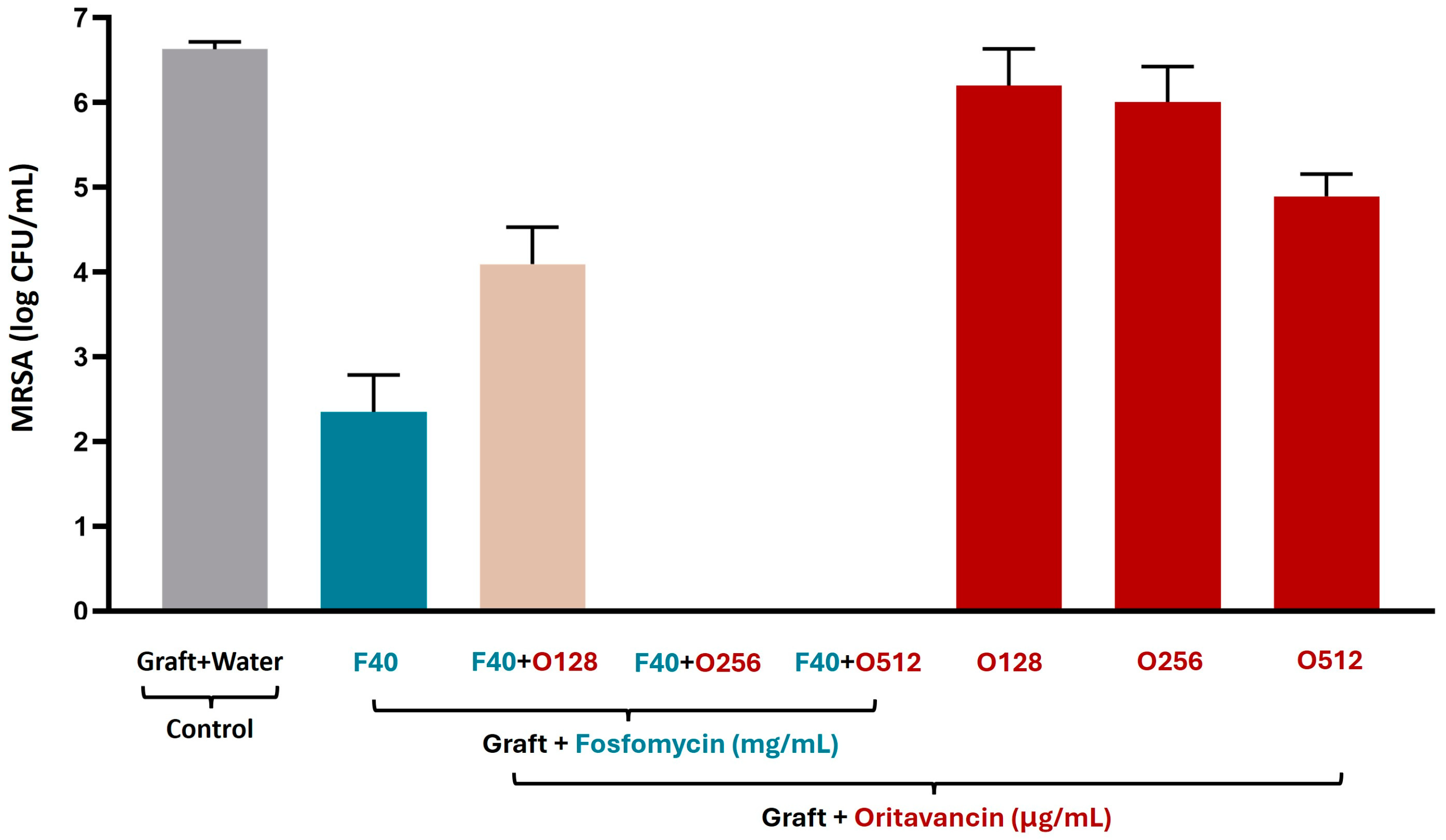
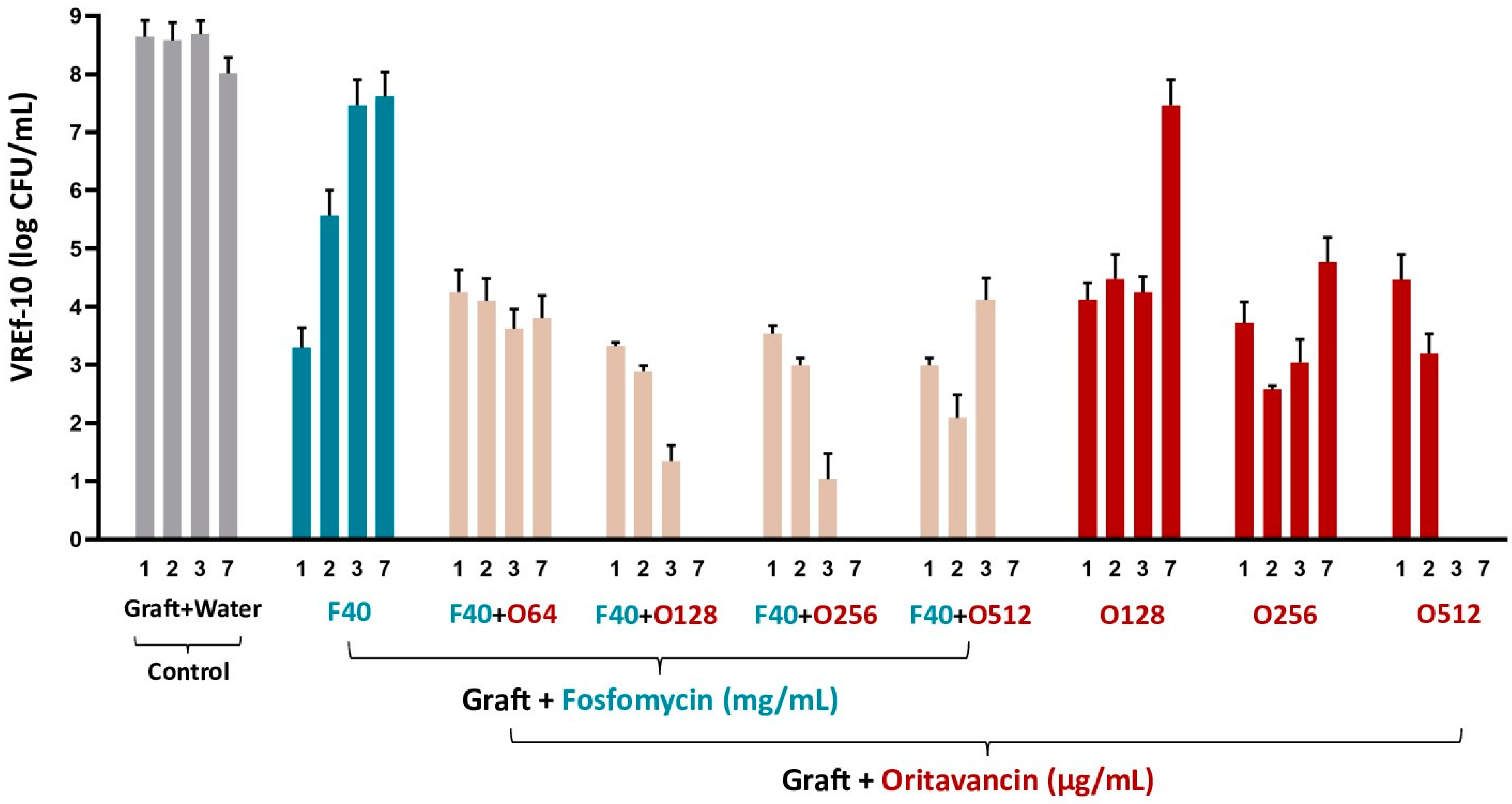
| Antibiotic Solutions | S. epidermidis | MRSA and VREf-10 |
|---|---|---|
| Fosfomycin (mg/mL) | 1.25/0.625 | 40 |
| + | + | + |
| Oritavancin (μg/mL) | 16/32/64 | 64/128/265/512 |
| Antimicrobial Agent | S. epidermidis (ATCC 35984) | MRSA (ATCC 33591) | VREf-10 (Clinical Strin) | |
|---|---|---|---|---|
| Fosfomycin | MIC | 0.5–1 | 16–32 | 128 |
| MBC | 2 | >128 | >512 | |
| Oritavancin | MIC | 1–2 | 0.5 | 0.5 |
| MBC | 1–2 | 0.5–1 | >2 |
| S. epidermidis (ATCC 35984) | MRSA (ATCC 33591) | VREf-10 (Clinical Strin) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC Alone | MIC in Combination | FICI | MIC Alone | MIC in Combination | FICI | MIC Alone | MIC in Combination | FICI | ||||||
| F | O | F | O | F | O | F | O | F | O | F | O | |||
| 8 | 0.5 | 2 | 0.008–0.016 | 0.266–0.282 | 8 | 0.250 | 2 | 0.062 | 0.498 | 128 | 0.25 | 32 | 0.062 | 0.5 |
| 8 | 1 | 0.016 | 0.157 | 0.125 | 1–2 | 0.031 | 0.373–0.498 | |||||||
| 4 | 1 | 0.008–0.062 | 0.266–0.374 | 0.062 | 2 | 0.008 | 0.379 | |||||||
| 4 | 0.5 | 0.031 | 0.187 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, I.; Di Bella, S.; D’Oria, M.; Lagatolla, C.; Martins, M.C.L.; Monteiro, C. Vascular Graft Impregnation with a Fosfomycin/Oritavancin Combination to Prevent Early Infection. Pharmaceutics 2024, 16, 1348. https://doi.org/10.3390/pharmaceutics16111348
Cruz I, Di Bella S, D’Oria M, Lagatolla C, Martins MCL, Monteiro C. Vascular Graft Impregnation with a Fosfomycin/Oritavancin Combination to Prevent Early Infection. Pharmaceutics. 2024; 16(11):1348. https://doi.org/10.3390/pharmaceutics16111348
Chicago/Turabian StyleCruz, Inês, Stefano Di Bella, Mario D’Oria, Cristina Lagatolla, M. Cristina L. Martins, and Cláudia Monteiro. 2024. "Vascular Graft Impregnation with a Fosfomycin/Oritavancin Combination to Prevent Early Infection" Pharmaceutics 16, no. 11: 1348. https://doi.org/10.3390/pharmaceutics16111348
APA StyleCruz, I., Di Bella, S., D’Oria, M., Lagatolla, C., Martins, M. C. L., & Monteiro, C. (2024). Vascular Graft Impregnation with a Fosfomycin/Oritavancin Combination to Prevent Early Infection. Pharmaceutics, 16(11), 1348. https://doi.org/10.3390/pharmaceutics16111348









