Targeted PLGA–Chitosan Nanoparticles for NIR-Triggered Phototherapy and Imaging of HER2-Positive Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
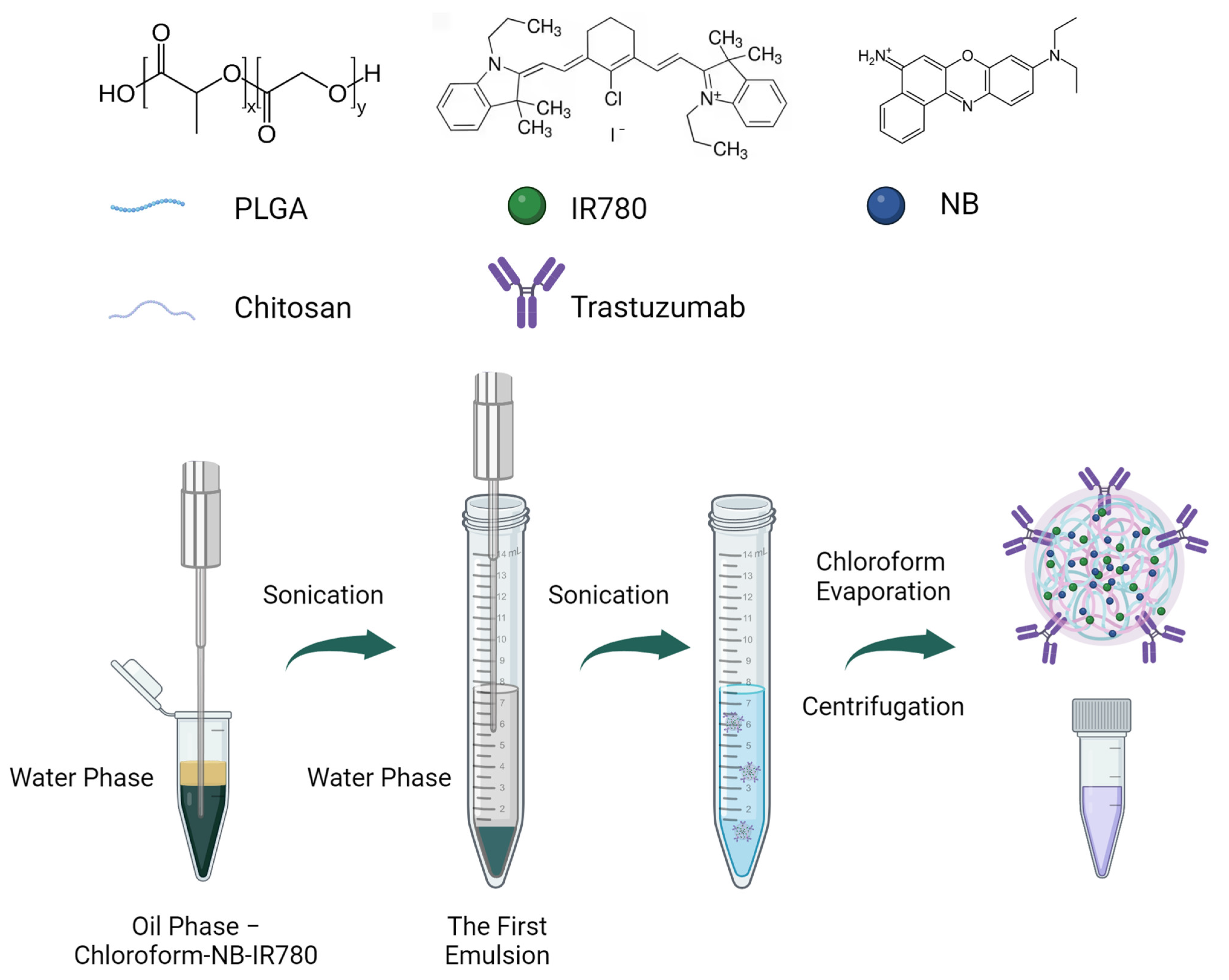
2.3. Nanoparticle Synthesis
2.4. Nanoparticle Characterization
2.5. NP Toxicity Studies
2.6. Flow Cytometry Binding Assay
2.7. ROS Generation
2.8. Study of Photothermal Properties
2.9. Phototherapy In Vitro
2.10. Tumor Bearing Mice
2.11. In Vivo Tumor Imaging
2.12. Confocal Microscopy
2.13. Statistical Analysis
3. Results
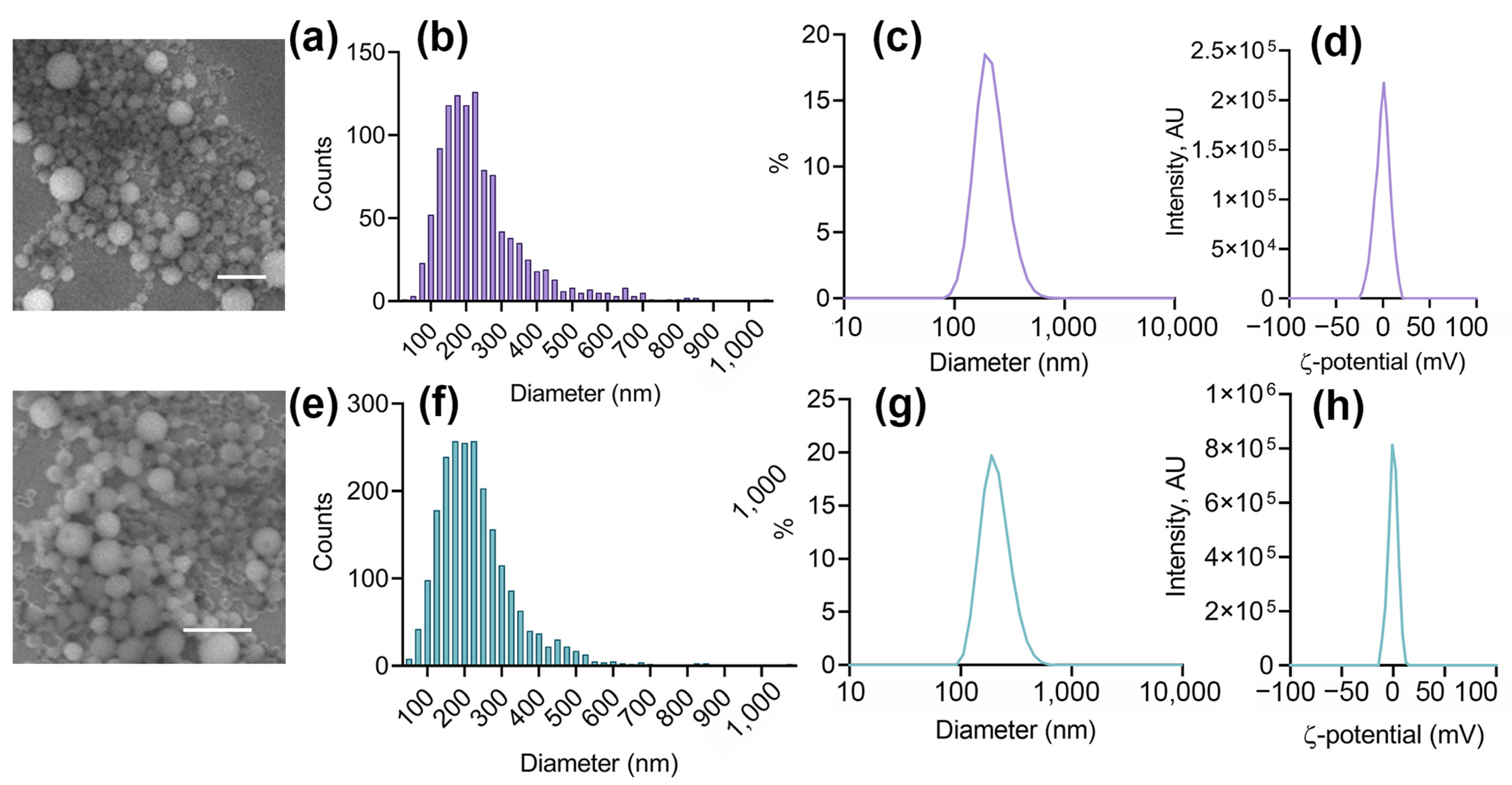
3.1. Nanoparticle Synthesis and Characterization
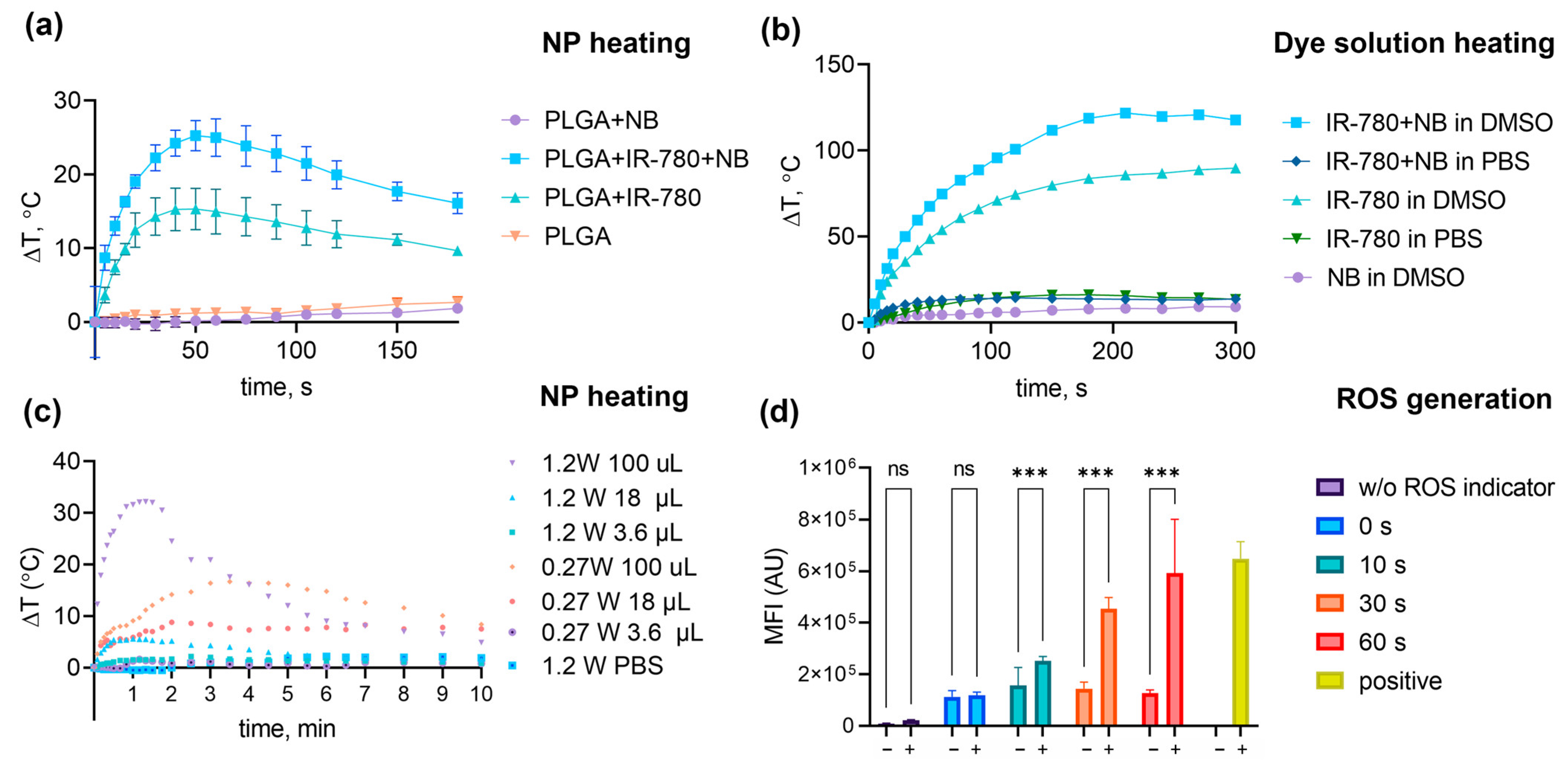
3.2. Study of Photothermal Properties
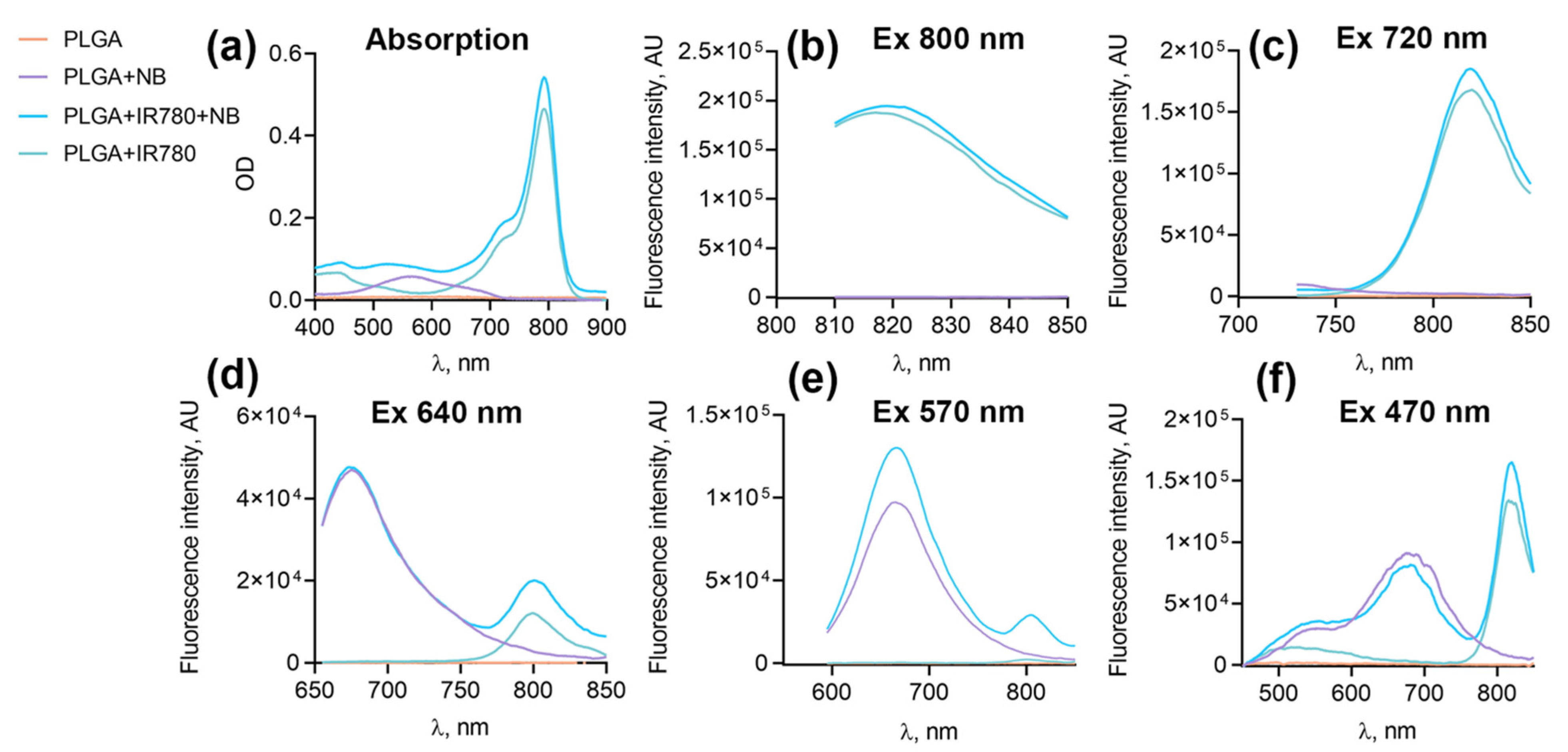
3.3. Study of the Photosensitizing Properties of Nanoparticles
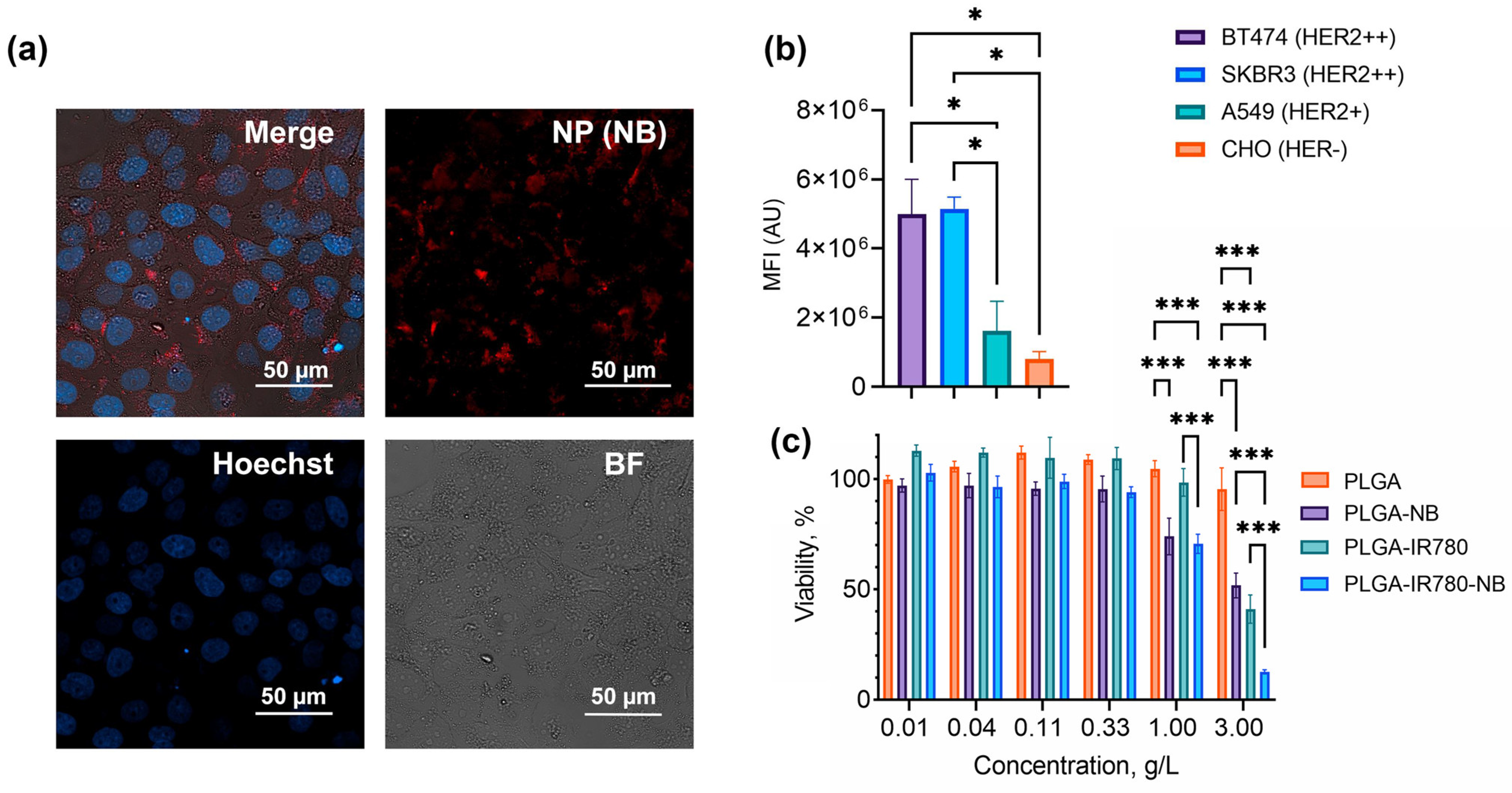
3.4. PLGA NP Interaction with Cells
3.5. Targeted Delivery of Dye-Loaded PLGA Nanoparticles to HER2-Positive Cells: Specificity Assays and Cytotoxicity Tests
3.6. NIR-Light Induced Phototherapy In Vitro
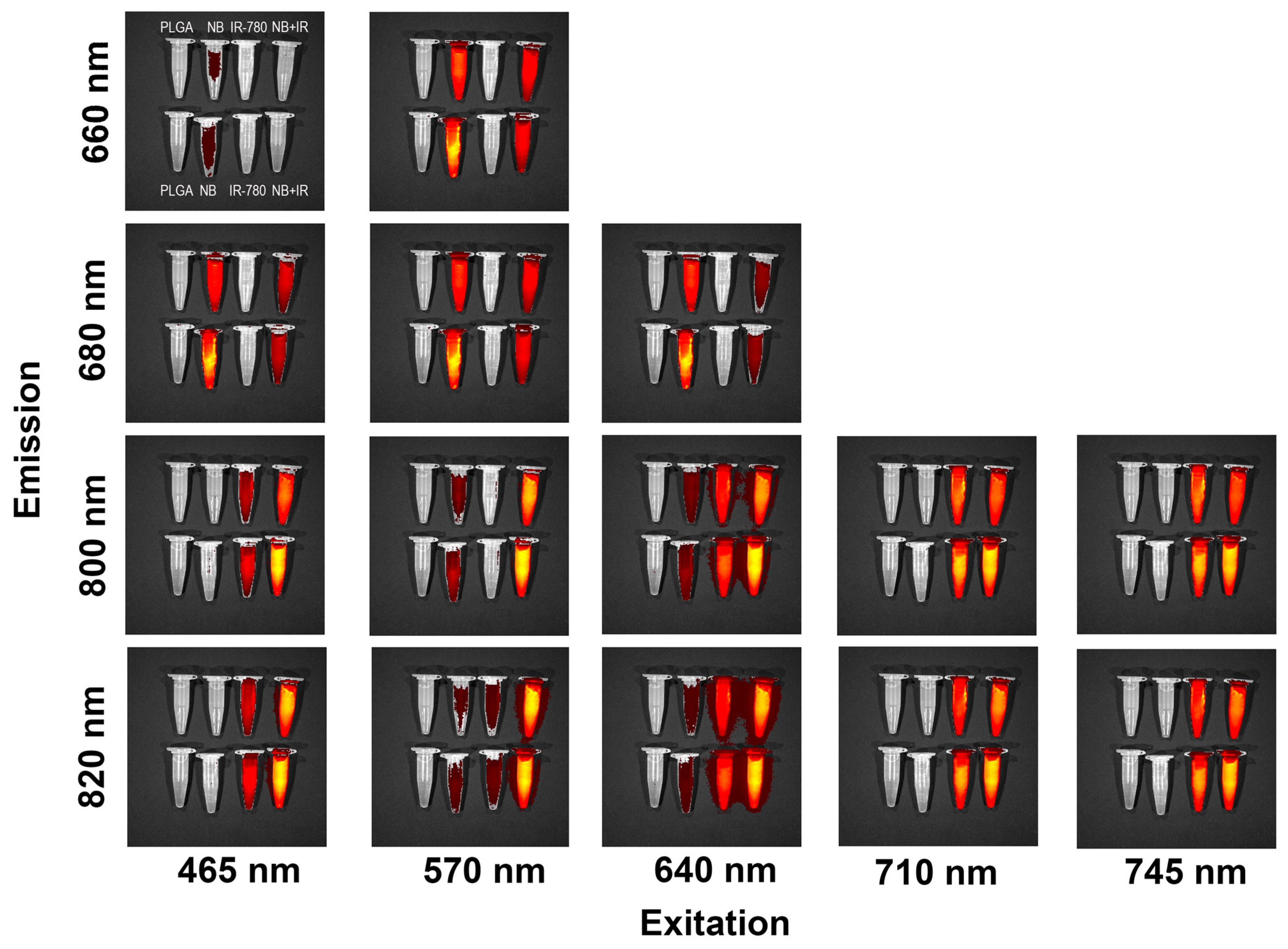
3.7. Imaging Properties of PLGA–IR-780–NB Nanoparticles
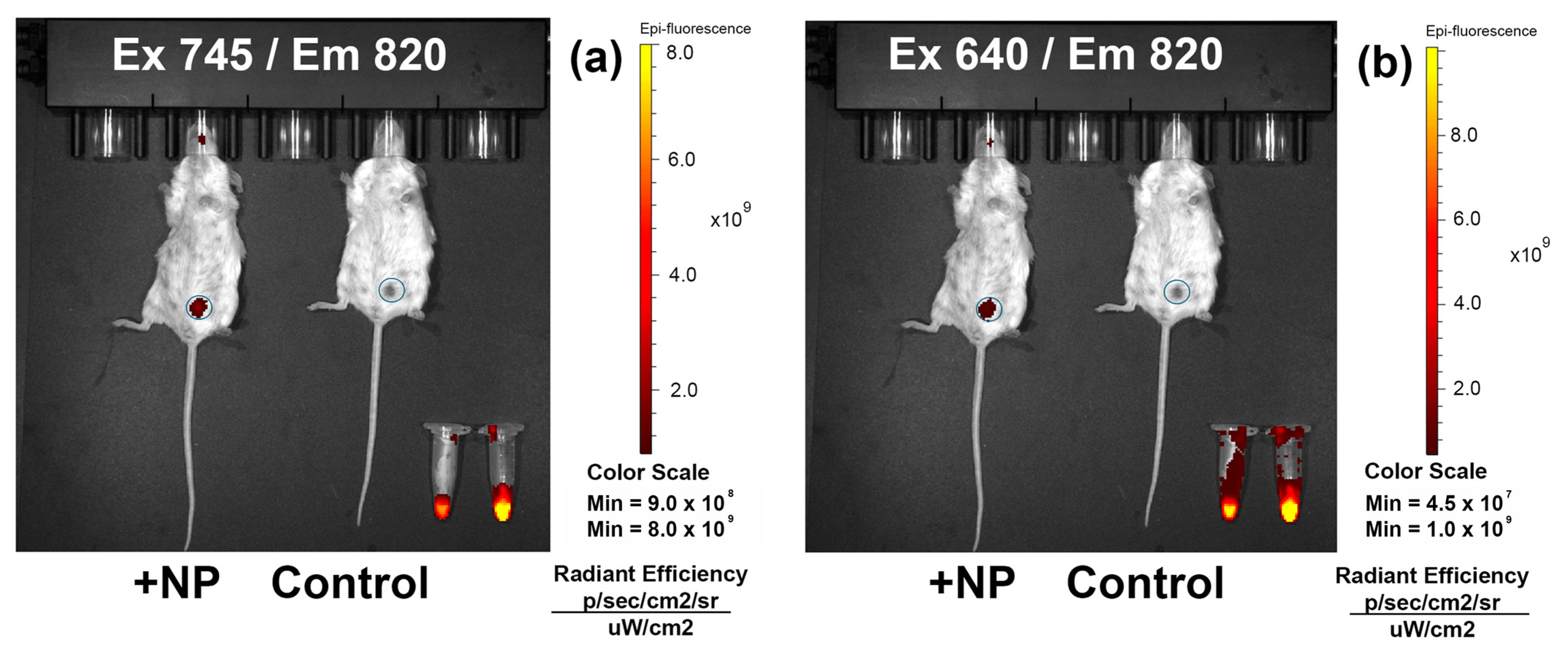
3.8. In Vivo Tumor Imaging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-Guided Surgery. Front. Oncol. 2017, 7, 314. [Google Scholar] [CrossRef]
- Lotan, Y.; Bivalacqua, T.J.; Downs, T.; Huang, W.; Jones, J.; Kamat, A.M.; Konety, B.; Malmström, P.-U.; McKiernan, J.; O’Donnell, M.; et al. Blue Light Flexible Cystoscopy with Hexaminolevulinate in Non-Muscle-Invasive Bladder Cancer: Review of the Clinical Evidence and Consensus Statement on Optimal Use in the USA—Update 2018. Nat. Rev. Urol. 2019, 16, 377–386. [Google Scholar] [CrossRef]
- Lakomkin, N.; Hadjipanayis, C.G. Fluorescence-Guided Surgery for High-Grade Gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef]
- Voelker, R. Lighting the Way for Improved Detection of Ovarian Cancer. JAMA 2022, 327, 27. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in Nanomaterials for Photodynamic Therapy Applications: Status and Challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past Is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Camorani, S.; Agnello, L.; Pedone, E.; Pirone, L.; Chesta, C.A.; Palacios, R.E.; Fedele, M.; Cerchia, L. Selective Photo-Assisted Eradication of Triple-Negative Breast Cancer Cells through Aptamer Decoration of Doped Conjugated Polymer Nanoparticles. Pharmaceutics 2022, 14, 626. [Google Scholar] [CrossRef]
- Arias-Ramos, N.; Ibarra, L.E.; Serrano-Torres, M.; Yagüe, B.; Caverzán, M.D.; Chesta, C.A.; Palacios, R.E.; López-Larrubia, P. Iron Oxide Incorporated Conjugated Polymer Nanoparticles for Simultaneous Use in Magnetic Resonance and Fluorescent Imaging of Brain Tumors. Pharmaceutics 2021, 13, 1258. [Google Scholar] [CrossRef]
- Zheng, Y.; Ling, Y.; Zhang, D.; Tan, C.; Zhang, H.; Yang, G.; Wang, H.; Ji, L.; Mao, Z. Regulating Tumor N6-Methyladenosine Methylation Landscape Using Hypoxia-Modulating OsSx Nanoparticles. Small 2021, 17, 2005086. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Huang, F.; Ma, Y.; Liang, G.; Peng, Z.; Guan, S.; Zhai, J. Tumor Microenvironment-Responsive Theranostic Nanoplatform for Guided Molecular Dynamic/Photodynamic Synergistic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 17392–17403. [Google Scholar] [CrossRef]
- Alamdari, S.G.; Amini, M.; Jalilzadeh, N.; Baradaran, B.; Mohammadzadeh, R.; Mokhtarzadeh, A.; Oroojalian, F. Recent Advances in Nanoparticle-Based Photothermal Therapy for Breast Cancer. J. Control. Release 2022, 349, 269–303. [Google Scholar] [CrossRef]
- Mallory, M.; Gogineni, E.; Jones, G.C.; Greer, L.; Simone, C.B. Therapeutic Hyperthermia: The Old, the New, and the Upcoming. Crit. Rev. Oncol. Hematol. 2016, 97, 56–64. [Google Scholar] [CrossRef]
- Jung, H.S.; Verwilst, P.; Sharma, A.; Shin, J.; Sessler, J.L.; Kim, J.S. Organic Molecule-Based Photothermal Agents: An Expanding Photothermal Therapy Universe. Chem. Soc. Rev. 2018, 47, 2280–2297. [Google Scholar] [CrossRef]
- Wang, M.; Liang, Y.; Liao, F.; Younis, M.R.; Zheng, Y.; Zhao, X.; Yu, X.; Guo, W.; Zhang, D.-Y. Iridium Tungstate Nanozyme-Mediated Hypoxic Regulation and Anti-Inflammation for Duplex Imaging Guided Photothermal Therapy of Metastatic Breast Tumors. ACS Appl. Mater. Interfaces 2022, 14, 56471–56482. [Google Scholar] [CrossRef]
- Khakbaz, F.; Mirzaei, M.; Mahani, M. Lecithin Sensitized Thermo-Sensitive Niosome Using NIR-Carbon Dots for Breast Cancer Combined Chemo-Photothermal Therapy. J. Photochem. Photobiol. A Chem. 2023, 434, 114236. [Google Scholar] [CrossRef]
- Pal, K.; Mahato, P.; Singh, S.; Roy, P. NIR-Responsive 5-Fluorouracil Delivery Using Polydopamine Coated Polygonal CuS Nanoplates for Synergistic Chemo-Photothermal Therapy on Breast Cancer. J. Drug Deliv. Sci. Technol. 2023, 80, 104092. [Google Scholar] [CrossRef]
- Zhu, L.; Altman, M.B.; Laszlo, A.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med. Biol. 2019, 45, 1025–1043. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Zhang, E.; Luo, S.; Tan, X.; Shi, C. Preferential Accumulation of the near Infrared Heptamethine Dye IR-780 in the Mitochondria of Drug-Resistant Lung Cancer Cells. Biomaterials 2014, 35, 4116–4124. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Wang, J.; Yuan, A.; Sun, M.; Wu, J.; Hu, Y. Self-Assembled IR780-Loaded Transferrin Nanoparticles as an Imaging, Targeting and PDT/PTT Agent for Cancer Therapy. Sci. Rep. 2016, 6, 27421. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Yang, K.; Sheng, D.; Tan, B.; Wang, Z.; Ran, H.; Yi, H.; Zhong, Y.; Lin, H.; et al. Mitochondria-Targeted Artificial “Nano-RBCs” for Amplified Synergistic Cancer Phototherapy by a Single NIR Irradiation. Adv. Sci. 2018, 5, 1800049. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Li, Y.; Li, X.; Zhu, J.; Fan, L.; Yang, S. Exceptionally High Payload of the IR780 Iodide on Folic Acid-Functionalized Graphene Quantum Dots for Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 22332–22341. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Xiao, J.; Tan, X.; Zhu, Y.; Su, Y.; Cheng, T.; Shi, C. Sentinel Lymph Node Mapping by a Near-Infrared Fluorescent Heptamethine Dye. Biomaterials 2010, 31, 1911–1917. [Google Scholar] [CrossRef]
- Teng, C.W.; Huang, V.; Arguelles, G.R.; Zhou, C.; Cho, S.S.; Harmsen, S.; Lee, J.Y.K. Applications of Indocyanine Green in Brain Tumor Surgery: Review of Clinical Evidence and Emerging Technologies. Neurosurg. Focus 2021, 50, E4. [Google Scholar] [CrossRef]
- Barcelos, J.M.; Hayasaki, T.G.; de Santana, R.C.; Lima, E.M.; Mendanha, S.A.; Bakuzis, A.F. Photothermal Properties of IR-780-Based Nanoparticles Depend on Nanocarrier Design: A Comparative Study on Synthetic Liposomes and Cell Membrane and Hybrid Biomimetic Vesicles. Pharmaceutics 2023, 15, 444. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Ma, Y.; Liu, W.; Zhao, H.; Di, J.; Fan, Z.; Yin, Y.; Zheng, Y.; Xi, R.; et al. Synergistic Release of Photothermal Molecules from Nanocarriers Induced by Light and Hyperthermia Benefits Efficient Anticancer Phototherapy. Anal. Chem. 2022, 94, 17160–17168. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile Red and Nile Blue: Applications and Syntheses of Structural Analogues. Chem.-Eur. J. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Lin, C.W.; Shulok, J.R.; Kirley, S.D.; Cincotta, L.; Foley, J.W. Lysosomal Localization and Mechanism of Uptake of Nile Blue Photosensitizers in Tumor Cells. Cancer Res. 1991, 51, 2710–2719. [Google Scholar]
- Lin, C.-W.; Shulok, J.R.; Kirley, S.D.; Cincotta, L.; Foley, J.W. Nile Blue Derivatives as Lysosomotropic Photosensitizers; Dougherty, T.J., Ed.; SPIE: Bellingham, WA, USA, 1991; pp. 216–227. [Google Scholar]
- Cincotta, L.; Foley, J.W.; Cincotta, A.H. Novel red absorbing benzo[a]phenoxazinium and benzo[a]phenothiazinium photosensitizers: In vitro evaluation. Photochem. Photobiol. 1987, 46, 751–758. [Google Scholar] [CrossRef]
- Fiedorowicz, M.; Galindo, J.R.; Julliard, M.; Mannoni, P.; Chanon, M. Efficient photodynamic action of victoria blue bo against the human leukemic cell lines k-562 and tf-1. Photochem. Photobiol. 1993, 58, 356–361. [Google Scholar] [CrossRef]
- Hung, H.-I.; Klein, O.J.; Peterson, S.W.; Rokosh, S.R.; Osseiran, S.; Nowell, N.H.; Evans, C.L. PLGA Nanoparticle Encapsulation Reduces Toxicity While Retaining the Therapeutic Efficacy of EtNBS-PDT in vitro. Sci. Rep. 2016, 6, 33234. [Google Scholar] [CrossRef]
- Nowell, N.H.; Hung, H.-I.; Austin, L.A.; Evans, C.L. EtNBS in Photodynamic Therapy. In Handbook of Photodynamic Therapy; World Scientific: Singapore, 2016; pp. 365–399. [Google Scholar]
- Evans, C.L.; Abu-Yousif, A.O.; Park, Y.J.; Klein, O.J.; Celli, J.P.; Rizvi, I.; Zheng, X.; Hasan, T. Killing Hypoxic Cell Populations in a 3D Tumor Model with EtNBS-PDT. PLoS ONE 2011, 6, e23434. [Google Scholar] [CrossRef]
- Lin, C.W.; Shulok, J.R.; Wong, Y.K.; Schanbacher, C.F.; Cincotta, L.; Foley, J.W. Photosensitization, Uptake, and Retention of Phenoxazine Nile Blue Derivatives in Human Bladder Carcinoma Cells. Cancer Res. 1991, 51, 1109–1116. [Google Scholar]
- Brufsky, A. Trastuzumab-Based Therapy for Patients With HER2-Positive Breast Cancer. Am. J. Clin. Oncol. 2010, 33, 186–195. [Google Scholar] [CrossRef]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab Emtansine: Mechanisms of Action and Drug Resistance. Breast Cancer Res. 2014, 16, 3378. [Google Scholar] [CrossRef]
- Colzani, B.; Pandolfi, L.; Hoti, A.; Iovene, P.A.; Natalello, A.; Avvakumova, S.; Colombo, M.; Prosperi, D. Investigation of Antitumor Activities of Trastuzumab Delivered by PLGA Nanoparticles. Int. J. Nanomed. 2018, 13, 957–973. [Google Scholar] [CrossRef]
- Spreen, H.; Barth, C.; Keuter, L.; Mulac, D.; Humpf, H.-U.; Langer, K. Tuning the Protein Corona of PLGA Nanoparticles: Characterization of Trastuzumab Adsorption Behavior and Its Cellular Interaction with Breast Cancer Cell Lines. J. Drug Deliv. Sci. Technol. 2022, 74, 103543. [Google Scholar] [CrossRef]
- Prasad, P.N.; Shipunova, V.O.; Kabashin, A.v.; Komedchikova, E.N.; Kotelnikova, P.A.; Zelepukin, I.v.; Schulga, A.A.; Proshkina, G.M.; Shramova, E.I.; Kutscher, H.L.; et al. Dual Regioselective Targeting the Same Receptor in Nanoparticle-Mediated Combination Immuno/Chemotherapy for Enhanced Image-Guided Cancer Treatment. ACS Nano 2020, 14, 12781–12795. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Sogomonyan, A.S.; Zelepukin, I.V.; Nikitin, M.P.; Deyev, S.M. Plga Nanoparticles Decorated with Anti-her2 Affibody for Targeted Delivery and Photoinduced Cell Death. Molecules 2021, 26, 3955. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Komedchikova, E.N.; Kotelnikova, P.A.; Nikitin, M.P.; Deyev, S.M. Targeted Two-Step Delivery of Oncotheranostic Nano-PLGA 2 for HER2-Positive Tumor Imaging and Therapy In Vivo: Improved Effectiveness Compared to One-Step Strategy. Pharmaceutics 2022, 2022, 833. [Google Scholar] [CrossRef]
- Sogomonyan, A.S.; Deyev, S.M.; Shipunova, V.O. Internalization-Responsive Poly(Lactic-Co-Glycolic Acid) Nanoparticles for Image-Guided Photodynamic Therapy against HER2-Positive Breast Cancer. ACS Appl. Nano Mater. 2023, 6, 11402–11415. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Kovalenko, V.L.; Kotelnikova, P.A.; Sogomonyan, A.S.; Shilova, O.N.; Komedchikova, E.N.; Zvyagin, A.V.; Nikitin, M.P.; Deyev, S.M. Targeting Cancer Cell Tight Junctions Enhances PLGA-Based Photothermal Sensitizers’ Performance In Vitro and In Vivo. Pharmaceutics 2021, 14, 43. [Google Scholar] [CrossRef]
- Lunt, S.J.; Kalliomaki, T.M.; Brown, A.; Yang, V.X.; Milosevic, M.; Hill, R.P. Interstitial Fluid Pressure, Vascularity and Metastasis in Ectopic, Orthotopic and Spontaneous Tumours. BMC Cancer 2008, 8, 2. [Google Scholar] [CrossRef]
- Okano, M.; Oshi, M.; Butash, A.; Okano, I.; Saito, K.; Kawaguchi, T.; Nagahashi, M.; Kono, K.; Ohtake, T.; Takabe, K. Orthotopic Implantation Achieves Better Engraftment and Faster Growth Than Subcutaneous Implantation in Breast Cancer Patient-Derived Xenografts. J. Mammary Gland Biol. Neoplasia 2020, 25, 27–36. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjugate Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Danhier, F. To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Jensen, B.V.; Johansen, J.S.; Price, P.A. High Levels of Serum HER-2/Neu and YKL-40 Independently Reflect Aggressiveness of Metastatic Breast Cancer. Clin. Cancer Res. 2003, 9, 4423–4434. [Google Scholar]
- Guo, F.; Yu, M.; Wang, J.; Tan, F.; Li, N. Smart IR780 Theranostic Nanocarrier for Tumor-Specific Therapy: Hyperthermia-Mediated Bubble-Generating and Folate-Targeted Liposomes. ACS Appl. Mater. Interfaces 2015, 7, 20556–20567. [Google Scholar] [CrossRef]
- Lin, T.; Yuan, A.; Zhao, X.; Lian, H.; Zhuang, J.; Chen, W.; Zhang, Q.; Liu, G.; Zhang, S.; Chen, W.; et al. Self-Assembled Tumor-Targeting Hyaluronic Acid Nanoparticles for Photothermal Ablation in Orthotopic Bladder Cancer. Acta Biomater. 2017, 53, 427–438. [Google Scholar] [CrossRef]
- Uthaman, S.; Mathew, A.P.; Park, H.J.; Lee, B.-I.; Kim, H.-S.; Huh, K.M.; Park, I.-K. IR 780-Loaded Hyaluronic Acid Micelles for Enhanced Tumor-Targeted Photothermal Therapy. Carbohydr. Polym. 2018, 181, 1–9. [Google Scholar] [CrossRef]
- Seok, H.-Y.; Sanoj Rejinold, N.; Lekshmi, K.M.; Cherukula, K.; Park, I.-K.; Kim, Y.-C. CD44 Targeting Biocompatible and Biodegradable Hyaluronic Acid Cross-Linked Zein Nanogels for Curcumin Delivery to Cancer Cells: In Vitro and in Vivo Evaluation. J. Control. Release 2018, 280, 20–30. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, B.; Akakuru, O.U.; Yao, C.; Sun, S.; Chen, L.; Ren, W.; Wu, A.; Huang, P. Hsp90 Inhibitor-Loaded IR780 Micelles for Mitochondria-Targeted Mild-Temperature Photothermal Therapy in Xenograft Models of Human Breast Cancer. Cancer Lett. 2021, 500, 41–50. [Google Scholar] [CrossRef]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical Translation of Gold Nanoparticles. Drug Deliv. Transl. Res. 2023, 13, 378–385. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold Nanoshell-Localized Photothermal Ablation of Prostate Tumors in a Clinical Pilot Device Study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Yang, N.; Shi, Y.; Ge, W.; Wang, W.; Huang, W.; Song, X.; Dong, X. Phase-Change Materials Based Nanoparticles for Controlled Hypoxia Modulation and Enhanced Phototherapy. Adv. Funct. Mater. 2019, 29, 1906805. [Google Scholar] [CrossRef]
- Yuan, A.; Qiu, X.; Tang, X.; Liu, W.; Wu, J.; Hu, Y. Self-Assembled PEG-IR-780-C13 Micelle as a Targeting, Safe and Highly-Effective Photothermal Agent for in Vivo Imaging and Cancer Therapy. Biomaterials 2015, 51, 184–193. [Google Scholar] [CrossRef]
- Rajendrakumar, S.; Chang, N.-C.; Mohapatra, A.; Uthaman, S.; Lee, B.-I.; Tsai, W.; Park, I.-K. A Lipophilic IR-780 Dye-Encapsulated Zwitterionic Polymer-Lipid Micellar Nanoparticle for Enhanced Photothermal Therapy and NIR-Based Fluorescence Imaging in a Cervical Tumor Mouse Model. Int. J. Mol. Sci. 2018, 19, 1189. [Google Scholar] [CrossRef]
- Chiang, W.-L.; Ke, C.-J.; Liao, Z.-X.; Chen, S.-Y.; Chen, F.-R.; Tsai, C.-Y.; Xia, Y.; Sung, H.-W. Pulsatile Drug Release from PLGA Hollow Microspheres by Controlling the Permeability of Their Walls with a Magnetic Field. Small 2012, 8, 3584–3588. [Google Scholar] [CrossRef]
- Zahn, D.; Weidner, A.; Nosrati, Z.; Wöckel, L.; Dellith, J.; Müller, R.; Saatchi, K.; Häfeli, U.O.; Dutz, S. Temperature Controlled Camptothecin Release from Biodegradable Magnetic PLGA Microspheres. J. Magn. Magn. Mater. 2019, 469, 698–703. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, B.; Zheng, C.; Ji, R.; Ren, X.; Guo, F.; Sun, S.; Shi, J.; Zhang, H.; Zhang, Z.; et al. The Tumor-Targeting Core–Shell Structured DTX-Loaded PLGA@Au Nanoparticles for Chemo-Photothermal Therapy and X-Ray Imaging. J. Control. Release 2015, 220, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Zelepukin, I.V.; Griaznova, O.Y.; Shevchenko, K.G.; Ivanov, A.V.; Baidyuk, E.V.; Serejnikova, N.B.; Volovetskiy, A.B.; Deyev, S.M.; Zvyagin, A.V. Flash Drug Release from Nanoparticles Accumulated in the Targeted Blood Vessels Facilitates the Tumour Treatment. Nat. Commun. 2022, 13, 6910. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotelnikova, P.A.; Shipunova, V.O.; Deyev, S.M. Targeted PLGA–Chitosan Nanoparticles for NIR-Triggered Phototherapy and Imaging of HER2-Positive Tumors. Pharmaceutics 2024, 16, 9. https://doi.org/10.3390/pharmaceutics16010009
Kotelnikova PA, Shipunova VO, Deyev SM. Targeted PLGA–Chitosan Nanoparticles for NIR-Triggered Phototherapy and Imaging of HER2-Positive Tumors. Pharmaceutics. 2024; 16(1):9. https://doi.org/10.3390/pharmaceutics16010009
Chicago/Turabian StyleKotelnikova, Polina A., Victoria O. Shipunova, and Sergey M. Deyev. 2024. "Targeted PLGA–Chitosan Nanoparticles for NIR-Triggered Phototherapy and Imaging of HER2-Positive Tumors" Pharmaceutics 16, no. 1: 9. https://doi.org/10.3390/pharmaceutics16010009
APA StyleKotelnikova, P. A., Shipunova, V. O., & Deyev, S. M. (2024). Targeted PLGA–Chitosan Nanoparticles for NIR-Triggered Phototherapy and Imaging of HER2-Positive Tumors. Pharmaceutics, 16(1), 9. https://doi.org/10.3390/pharmaceutics16010009






