Acylation of Oleanolic Acid Oximes Effectively Improves Cytotoxic Activity in In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
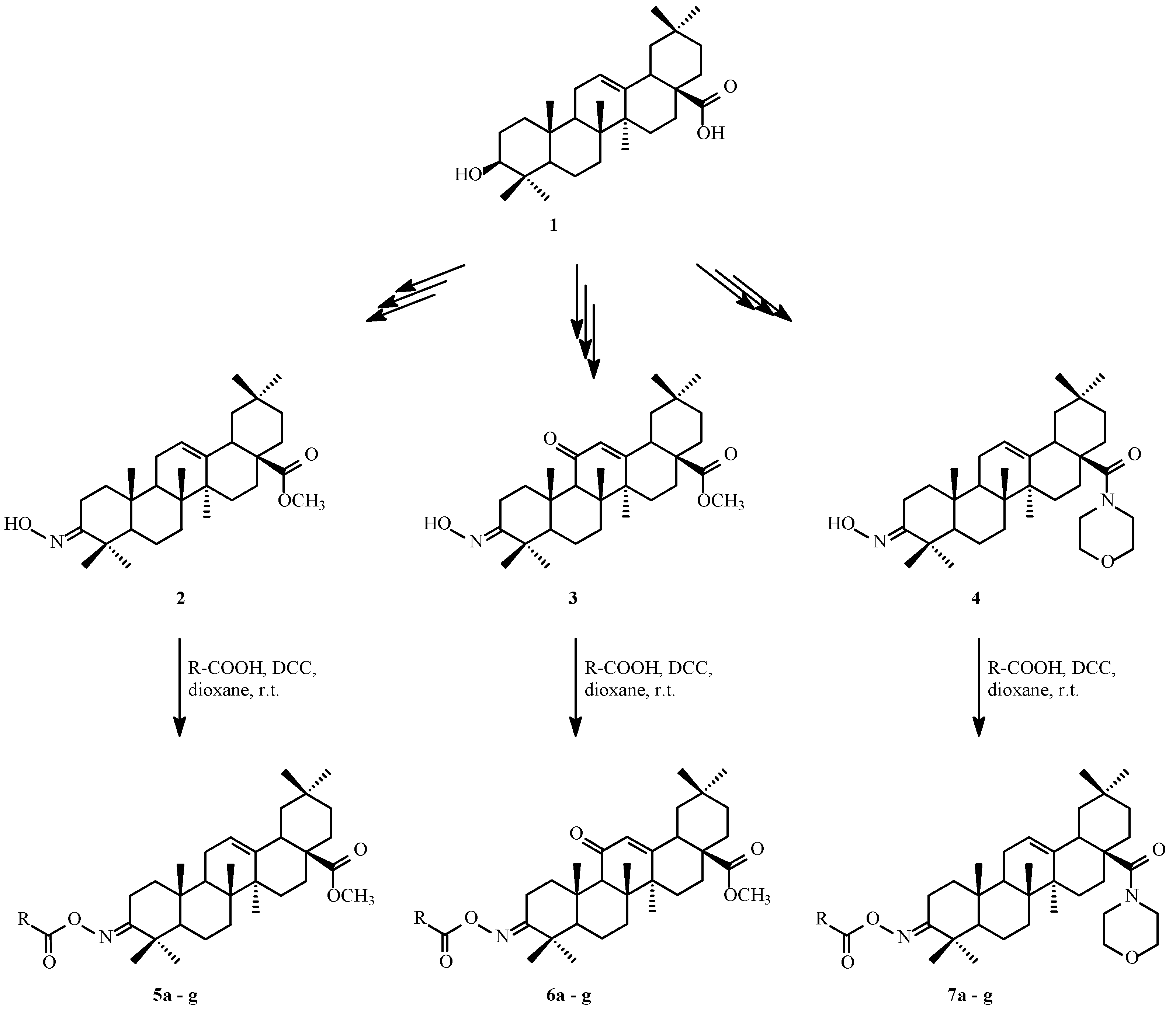
2.2. Synthesis of A-Ring Oleanolic Acid Derivatives 2–4, 5a–5g, 6a–6g, and 7a–7g
2.2.1. Synthesis of Oximes 2–4
2.2.2. Acylation of Oximes 2–4 with Carboxylic Acids
2.3. SAR Analysis
2.4. Cytotoxic Activity of A-Ring Oleanolic Acid Derivatives
2.4.1. MTT Assay
2.4.2. Apoptosis
2.5. Physicochemical Properties, Pharmacokinetics, and ADMETox Activity
3. Results
3.1. Synthesis of Cytotoxic Agents
3.2. SAR Analysis
3.3. Cytotoxic Activity of Acylated Oximes
3.3.1. In Vivo Assay
3.3.2. Selectivity Index
3.3.3. The Apoptosis Assay
3.4. Physicochemical Properties, Pharmacokinetics, and ADMETox Activity
4. Discussion
4.1. Synthesis
4.2. SAR Analysis
4.3. Biological Tests
4.4. ADMETox Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviation (In Alphabetical Order)
References
- Nirmala, M.J.; Samundeeswari, A.; Sankar, P.D. Natural plant resources in anti-cancer therapy—A review. Res. Plant Biol. 2011, 1, 1–14. [Google Scholar]
- Withers, S.T.; Keasling, J.D. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Biotechnol. 2007, 73, 980–990. [Google Scholar] [CrossRef]
- Patočka, J. Biologically active pentacyclic triterpenes and their current medicine. J. Appl. Biomed. 2003, 1, 7–12. [Google Scholar] [CrossRef]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef]
- Yeung, M.F.; Che, C.T. A review on the presence of oleanolic acid in natural products. Nat. Proda Medica 2009, 2, 77–290. [Google Scholar]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef]
- Ma, C.; Nakamura, N.; Hattori, M.; Kakuda, H.; Qiao, J.; Yu, H. Inhibitory effects on HIV-1 protease of constituents from the wood of Xanthoceras sorbifolia. J. Nat. Prod. 2000, 63, 238–242. [Google Scholar] [CrossRef]
- Tang, H.Q.; Hu, J.; Yang, L.; Tan, R.X. Terpenoids and flavonoids from Artemisia species. Planta Med. 2000, 66, 391–393. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Matsuda, H. Antidiabetogenic activity of oleanolic acid glycosides from medicinal foodstuffs. Biofactors 2000, 13, 231–237. [Google Scholar] [CrossRef]
- Ryu, S.Y.; Oak, M.H.; Yoon, S.K.; Cho, D.I.; Yoo, G.S.; Kim, T.S.; Kim, K.M. Antiallergic and anti-inflammatory triterpenes from the herb of Prunella vulgaris. Planta Med. 2000, 66, 358–360. [Google Scholar] [CrossRef]
- Srivastava, P.; Kasoju, N.; Bora, U.; Chaturvedi, R. Accumulation of betulinic, oleanolic, and ursolic acids in in vitro cell cultures of Lantana camara L. and their significant cytotoxic effects on HeLa cell lines. Biotechnol. Bioproc. Eng. 2010, 15, 1038–1046. [Google Scholar] [CrossRef]
- Ovesná, Z.; Kozics, K.; Slameňová, D. Protective effects of ursolic acid and oleanolic acid in leukemic cells. Mut. Res. 2006, 600, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Chang, J.Y.; Kuo, C.C.; Chang, C.Y.; Kuo, Y.H. Cytotoxic triterpenes from the aerial roots of Ficus microcarpa. Phytochem 2005, 66, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Hoskeri, H.J.; Krishna, V.; Kumar, B.V.; Shridar, A.H.; Babu, K.R.; Sudarshana, M.S. In vivo prophylactic effects of oleanolic acid isolated from chloroform extract of Flaveria trinervia against ethanol induced liver toxicity in rats. Arch. Pharm. Res. 2012, 35, 1803–1810. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.G. Inhibition of cytochrome P450 2E1 expression by oleanolic acid: Hepatoprotective effects against carbon tetrachloride-induced hepatic injury. Toxicol Lett. 1999, 105, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kreppel, H.; Liu, J.; Choudhuri, S.; Klaassen, C.D. Oleanolic acid protects against cadmium hepatotoxicity by inducing metallothionein. J. Pharmacol. Exp. Ther. 1993, 266, 400–406. [Google Scholar] [PubMed]
- Reisman, S.A.; Aleksunes, L.M.; Klaassen, C.D. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem. Pharmacol. 2009, 77, 1273–1282. [Google Scholar] [CrossRef]
- Oguro, T.; Liu, J.; Klaassen, C.D.; Yoshida, T. Inhibitory effect of oleanolic acid on 12-O-tetradecanoylphorbol-13-acetate-induced gene expression in mouse skin. Toxicol. Sci. 1998, 45, 88–93. [Google Scholar] [CrossRef]
- Yan, S.L.; Huang, C.Y.; Wu, S.T.; Yin, M.C. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. Vitro 2010, 24, 842–848. [Google Scholar] [CrossRef]
- Paszel, A.; Rubiś, B.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Kaczmarek, M.; Hofmann, J.; Rybczyńska, M. The oleanolic acid derivative methyl 3,11-dioxoolean-12-en-28-oate targets multidrug resistance related to ABCB1. Pharm. Rep. 2011, 63, 1500–1517. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Bednarczyk-Cwynar, B.; Vasylenko, O.; Kazakova, O.; Zimenkovsky, B.; Zaprutko, L.; Lesyk, R. Synthesis of new potential anticancer agents based on 4-azolidinone and oleanane scaffolds. Med. Chem. Res. 2012, 21, 3568–3580. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Zaprutko, L.; Ruszkowski, P.; Hładoń, B. Anti-cancer effect of A-ring or/and C-ring modified oleanolic acid derivatives on KB, MCF-7 and HeLa cell lines. Org. Biomol. Chem. 2012, 10, 2201–2205. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Bobkiewicz-Hładoń, T.; Zaprutko, L. Oleanolic acid A-lactams inhibit the growth of HeLa, KB, MCF-7 and Hep-G2 cancer cell lines at micromolar concentrations. Anticancer Agents Med. Chem. 2016, 16, 579–592. [Google Scholar] [CrossRef]
- Available online: http://www.way2drug.com (accessed on 20 October 2023).
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.Y. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Poroikov, Y.V. Computer-aided prediction of biological activity spectra. In Design of Bioactive Compounds; BIOS Scientific Publishers: Oxford, UK, 1996; pp. 47–56. [Google Scholar]
- Bednarczyk-Cwynar, B.; Ruszkowski, P.; Jarosz, T.; Krukiewicz, K. Enhancing anticancer activity through the combination of bioreducing agents and triterpenes. Future Med. Chem. 2018, 10, 511–525. [Google Scholar] [CrossRef]
- Available online: https://admetmesh.scbdd.com (accessed on 30 October 2023).
- Bednarczyk-Cwynar, B.; Partyka, D.; Zaprutko, L. Simple amides of oleanolic acid as effective penetration enhancers. PLoS ONE 2015, 10, e01228557. [Google Scholar] [CrossRef]
- Giuliani, C.; Bucci, I.; Napolitano, G. The role of the transcription factor nuclear factor-kappa B in thyroid autoimmunity and cancer. Front. Endocrinol. 2018, 9, 471–478. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
- Valderrama, J.A.; Delgado, V.; Sepúlveda, S.; Benites, J.; Theoduloz, C.; Calderon, P.B.; Muccioli, G.G. Synthesis and cytotoxic activity on human cancer cells of novel isoquinolinequinone-amino acid derivatives. Molecules 2016, 21, 1199. [Google Scholar] [CrossRef]
- Lopes, M.S.; de Andrade Sena, C.F.; Silva, B.L.; de Souza, C.M.; Ramos, J.P.; Cassali, G.D.; de Souza-Fagundes, E.M.; Alves, R.J.; de Oliveira, M.C.; de Oliveira, R.B. Synthesis of nitroaromatic compounds as potential anticancer agents. Anticancer Agents Med. Chem. 2015, 15, 206–216. [Google Scholar] [CrossRef]
- Kovacic, P.; Osuna, J.A. Mechanisms of anti-cancer agents: Emphasis on oxidative stress and electron transfer. Curr. Pharm. Des. 2000, 6, 277–309. [Google Scholar] [CrossRef]

| Comp. No. | 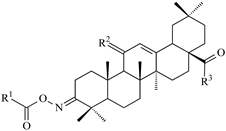 Compound Structure | IC50 [μM] (±s) | ||||||
|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | HeLa | KB | MCF-7 | A-549 | HDF | |
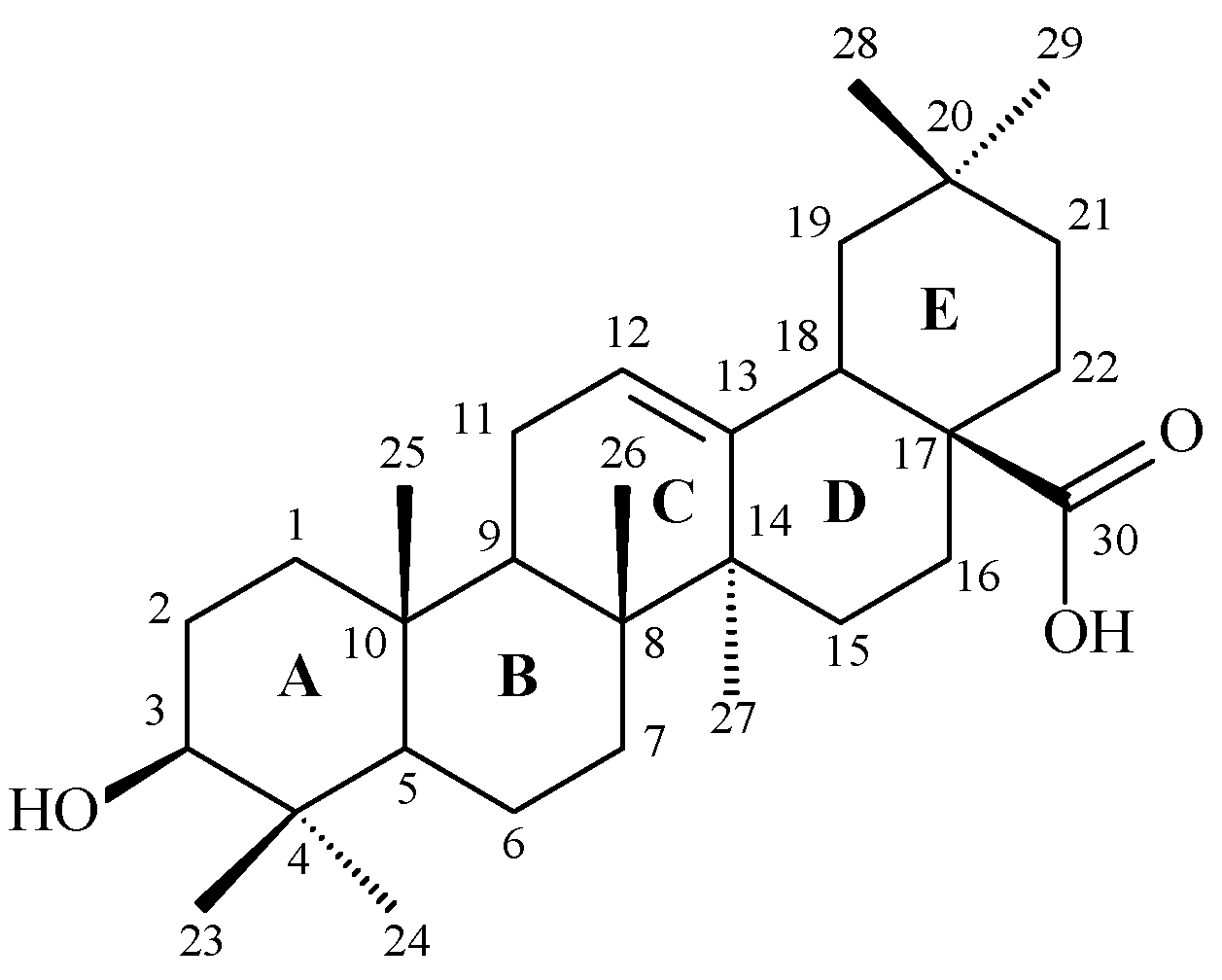
| 1 (OA) | --- | H2 | OH | 11.82 * (0.19) | 14.93 * (0.12) | 13.95 * (0.11) | 8.79 (0.28) | 17.89 (0.33) |
| 2 | H | H2 | OMe | 13.23 * (0.70) | 12.40 * (0.80) | 15.30 * (1.60) | 13.00 (0.07) | 21.14 (0.39) |
| 5a | CH3- | 11.08 (0.16) | 11.39 (0.26) | 10.53 (1.19) | 10.60 (0.04) | 29.04 (0.01) | ||
| 5b | Cl-CH2- | 19.84 (0.06) | 18.44 (0.71) | 18.03 (0.03) | 19.79 (0.09) | 37.02 (0.16) | ||
| 5c | CH3-CH2- | 38.06 (0.11) | 38.75 (0.28) | 38.12 (0.33) | 36.17 (0.62) | 39.92 (0.21) | ||
| 5d | C6H5- | 84.92 (0.11) | 83.18 (0.37) | 83.29 (0.01) | 73.94 (0.99) | 113.02 (0.54) | ||
| 5e | 3′-NO2-C6H4- | 20.14 (0.51) | 23.91 (0.19) | 21.84 (0.74) | 21.08 (0.31) | 37.96 (0.37) | ||
| 5f | 4′-NO2-C6H4- | 62.02 (0.63) | 60.74 (0.48) | 61.36 (0.41) | 69.93 (0.19) | 132.19 (0.03) | ||
| 5g | 3′,5′-di-NO2-C6H3- | 4.19 (0.09) | 4.61 (0.33) | 5.18 (0.59) | 4.82 (0.61) | 6.22 (0.39) | ||
| 3 | H | O2 | OMe | 125.17 (0.80) | 60.27 (0.40) | 48.42 (0.40) | 63.02 (0.03) | 60.95 (0.12) |
| 6a | CH3- | 27.45 (0.94) | 27.91 (0.02) | 28.03 (0.002) | 28.16 (0.18) | 49.05 (0.08) | ||
| 6b | Cl-CH2- | 61.03 (0.38) | 60.99 (0.25) | 64.12 (0.08) | 61.77 (0.18) | 88.01 (0.40) | ||
| 6c | CH3-CH2- | 24.02 (0.07) | 24.82 (0.19) | 23.02 (0.94) | 24.16 (0.31) | 49.04 (0.27) | ||
| 6d | C6H5- | 28.43 (0.13) | 27.21 (0.07) | 27.50 (0.07) | 27.83 (0.08) | 41.99 (0.02) | ||
| 6e | 3′-NO2-C6H4- | 71.18 (0.94) | 70.01 (0.16) | 74.98 (0.71) | 74.03 (0.02) | 97.03 (0.13) | ||
| 6f | 4′-NO2-C6H4- | 28.30 (0.44) | 26.29 (0.04) | 26.01 (0.71) | 28.11 (0.62) | 37.17 (0.01) | ||
| 6g | 3′,5′-di-NO2-C6H3- | 6.11 (0.39) | 8.98 (0.12) | 6.62 (0.19) | 5.39 (0.05) | 7.27 (0.21) | ||
| 4 | H | 8.72 (0.20) | 8.72 (0.70) | 7.42 (0.60) | 7.29 (0.19) | 13.92 (0.06) | ||
| 7a | CH3- | 82.19 (0.38) | 81.49 (0.30) | 81.03 (0.18) | 84.92 (0.03) | 116.03 (0.81) | ||
| 7b | Cl-CH2- | 19.24 (0.14) | 19.83 (0.79) | 15.63 (0.12) | 19.34 (0.22) | 39.05 (0.39) | ||
| 7c | CH3-CH2- | H2 | OMor | 142.59 (0.56) | 149.74 (0.04) | 152.88 (0.14) | 147.81 (0.19) | 179.41 (0.05) |
| 7d | C6H5- | 45.27 (0.15) | 45.92 (0.41) | 41.74 (0.09) | 46.82 (0.27) | 66.17 (0.02) | ||
| 7e | 3′-NO2-C6H4- | 12.05 (0.07) | 13.88 (0.14) | 10.18 (0.17) | 13.82 (0.14) | 28.02 (0.16) | ||
| 7f | 4′-NO2-C6H4- | 60.04 (0.22) | 61.52 (0.11) | 57.16 (0.38) | 62.94 (0.77) | 85.13 (0.02) | ||
| 7g | 3′,5′-di-NO2-C6H3- | 6.24 (0.02) | 6.01 (0.39) | 7.27 (0.05) | 6.52 (0.38) | 9.16 (0.03) | ||
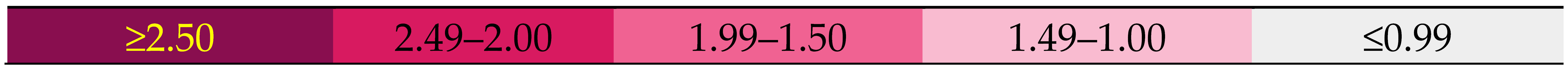
| Comp. No. | 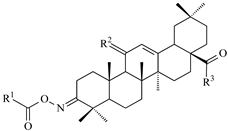 Compound structure | Selectivity Index | |||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | HeLa | KB | MCF-7 | A-549 | |
| 1 (OA) | --- | H2 | OH | 1.51 | 1.20 | 1.28 | 2.03 |
| 2 | H | H2 | OMe | 1.60 | 1.70 | 1.38 | 1.62 |
| 5a | CH3- | 2.62 | 2.55 | 2.76 | 2.74 | ||
| 5b | Cl-CH2- | 1.87 | 2.01 | 2.05 | 1.87 | ||
| 5c | CH3-CH2- | 1.05 | 1.03 | 1.05 | 1.10 | ||
| 5d | C6H5- | 1.33 | 1.36 | 1.36 | 1.53 | ||
| 5e | 3′-NO2-C6H4- | 1.88 | 1.59 | 1.74 | 1.80 | ||
| 5f | 4′-NO2-C6H4- | 2.13 | 2.18 | 2.15 | 1.89 | ||
| 5g | 3′,5′-di-NO2-C6H3- | 1.48 | 1.35 | 1.20 | 1.29 | ||
| 3 | H | O2 | OMe | 0.49 | 1.01 | 1.26 | 0.97 |
| 6a | CH3- | 1.79 | 1.76 | 1.75 | 1.74 | ||
| 6b | Cl-CH2- | 1.44 | 1.44 | 1.37 | 1.42 | ||
| 6c | CH3-CH2- | 2.04 | 1.97 | 2.13 | 2.03 | ||
| 6d | C6H5- | 1.48 | 1.54 | 1.53 | 1.51 | ||
| 6e | 3′-NO2-C6H4- | 1.36 | 1.39 | 1.29 | 1.31 | ||
| 6f | 4′-NO2-C6H4- | 1.31 | 1.41 | 1.43 | 1.32 | ||
| 6g | 3′,5′-di-NO2-C6H3- | 1.19 | 0.81 | 1.10 | 1.34 | ||
| 4 | H | 1.60 | 1.60 | 1.88 | 1.91 | ||
| 7a | CH3- | 1.41 | 1.42 | 1.43 | 1.37 | ||
| 7b | Cl-CH2- | 2.03 | 1.97 | 2.50 | 2.02 | ||
| 7c | CH3-CH2- | H2 | OMor | 1.27 | 1.20 | 1.17 | 1.21 |
| 7d | C6H5- | 1.46 | 1.44 | 1.58 | 1.41 | ||
| 7e | 3′-NO2-C6H4- | 2.32 | 2.02 | 2.75 | 2.03 | ||
| 7f | 4′-NO2-C6H4- | 1.42 | 1.38 | 1.49 | 1.35 | ||
| 7g | 3′,5′-di-NO2-C6H3- | 1.47 | 1.52 | 1.26 | 1.40 | ||
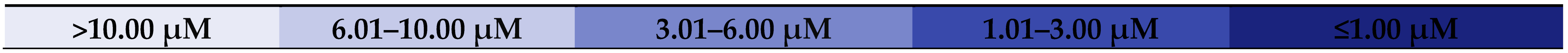
| Comp. No. | Cell Line, IC50 [μM] | Compound Structure, Position, and Type of Substituent | Lit. | ||||
|---|---|---|---|---|---|---|---|
| HeLa | MCF-7 | KB | at the C-17 | at the C-12 | at the C-3 | ||
| 1 (OA) | 11.82 | 13.95 | 14.93 | -COOH | double bond (C12–C-13) | -OH | [22] |
| 3 | 5.03 | >10 | 6.19 | -COOCH3 | =O | =O | [22] |
| 4 | 1.80 | 1.60 | 1.74 | -COOCH3 | =O | =NOH | [22] |
| 6 | 7.38 | >10 | 8.51 | -COOCH3 | =O | CH3COO- | [22] |
| 7 | 1.34 | >10 | 2.06 | -COOCH3 | =NOH | CH3COO- | [22] |
| 8b | 9.19 | 7.26 | 9.42 | -COOCH3 | =NOC(O)CH2Cl | CH3COO- | [22] |
| 8c | 4.41 | 3.76 | 4.90 | -COOCH3 | =NOC(O)CH2Br | CH3COO- | [22] |
| 8d | 1.87 | 2.13 | 0.72 | -COOCH3 | =NOC(O)CH2CH3 | CH3COO- | [22] |
| 8e | 9.84 | 9.28 | >10 | -COOCH3 | =NOC(O)C6H4-(o-NO2) | CH3COO- | [22] |
| 13 | 3.93 | 2.69 | 3.93 | -COOCH3 | double bond (C12–C-13) | A-lactam system | [23] |
| 17 | 2.81 | 1.61 | 3.42 | -COOCH3 | double bond (C12–C-13) and =O at the C-11 | A-lactam system | [23] |
| 19 | 8.72 | 7.42 | 8.72 | -C(O)Morph | double bond (C12–C-13) | =NOH | [23] |
| 21 | 1.48 | 2.41 | 1.48 | -C(O)Morph | double bond (C12–C-13) | A-lactam system | [23] |
| 6 | 4.19 | 5.18 | 4.61 | -COOCH3 | double bond (C12–C-13) | =NOC(O)-C6H3-(3,5-di-NO2) | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk-Cwynar, B.; Ruszkowski, P. Acylation of Oleanolic Acid Oximes Effectively Improves Cytotoxic Activity in In Vitro Studies. Pharmaceutics 2024, 16, 86. https://doi.org/10.3390/pharmaceutics16010086
Bednarczyk-Cwynar B, Ruszkowski P. Acylation of Oleanolic Acid Oximes Effectively Improves Cytotoxic Activity in In Vitro Studies. Pharmaceutics. 2024; 16(1):86. https://doi.org/10.3390/pharmaceutics16010086
Chicago/Turabian StyleBednarczyk-Cwynar, Barbara, and Piotr Ruszkowski. 2024. "Acylation of Oleanolic Acid Oximes Effectively Improves Cytotoxic Activity in In Vitro Studies" Pharmaceutics 16, no. 1: 86. https://doi.org/10.3390/pharmaceutics16010086
APA StyleBednarczyk-Cwynar, B., & Ruszkowski, P. (2024). Acylation of Oleanolic Acid Oximes Effectively Improves Cytotoxic Activity in In Vitro Studies. Pharmaceutics, 16(1), 86. https://doi.org/10.3390/pharmaceutics16010086