Nano-Drug Delivery Systems in Oral Cancer Therapy: Recent Developments and Prospective
Abstract
:1. Introduction
2. Targeting Methods for Nano-DDS in OC Therapy
2.1. Passive Targeting
2.2. Active Targeting
2.3. Immune Targeting
2.4. Magnetic Targeting
3. Nano-DDSs in OC Therapy
3.1. Polymeric NPs
| Nanocarrier | Active Agent | Assembly Method | Mechanism | Advantages | Ref. |
|---|---|---|---|---|---|
| PLGA | Resveratrol NP (Res) | PLGA encapsulated nano form of Res (Res-NP) | Res-NPs obstructed carcinogenesis and metastasis by inhibiting CXCL-12 and IL-6 production in vitro, in vivo, in ovo, and ex vivo systems | Reduced metastasis and angiogenic markers, ovo vascularization, intracellular NO production, matrix metalloproteinase expression, tubular formation, and representative CSCs and angiogenesis markers | [93] |
| 5-FU | 5-FU was conjugated with PLGA by ionic cross-linking, and α-tocopherol used as a functionalized surface moiety (α-t-FU-PLGA NPs) | 5-FU-induced apoptotic cell death | Higher apoptosis rate against OSCC and a robust inhibitory effect on SCC-15 cells after 96 h, establishing steady-state inhibition after 160 h incubation compared to 5-FU-PLGA NPs | [68] | |
| DOX (chemotherapy drug) and indocyanine green (ICG) (PTT agent) | PLGA NPs loaded with ICG and DOX and conjugated with chemokine SDF-1 | Anticancer effect of DOX and PTT effect mediated by ICG | Dual-killing effect on OSCC cells and l enhanced cellular uptake and cell apoptosis | [74] | |
| DTX | PLGA NPs encapsulating DTX | Anticancer effect of DTX | Sustained release of the drug and higher anti-proliferation efficiency against SCC-9 cells | [95] | |
| Poly (β-amino ester) (PBAE)/PLGA blended NPs | ICG (PDT PS and PTT agent) | PBAE/PLGA blended NPs co-loaded with ICG and Nrf2-siRNA, then encapsulated in cancer cell membrane from homologous OTSCC, thus obtaining M@PPI-siRNA | PTT effect mediated by ICG and PDT effect enhanced by Nrf2-siRNA | Reduced tumor cells’ resistance to oxidative stress and amplified anticancer effects of ICG-mediated PDT by maintaining intracellular ROS | [103] |
| PEG | Aggregation-induced emission photosensitizer 5 (AIEPS5) (PDT PS) | A PEG chain was linked onto AIEPS5, and anti-Her-2 nanobody (NB) was further utilized to achieve targeted delivery of AIEPS5-NPs-NB | Effect of AIEPS5-mediated PDT | Effective 1O2 generation capability, bright FR/NIR emission centered at 680 nm, and negligible dark toxicity | [72] |
| Graphene quantum dots (GQDs) (PDT PS) | GQDs as photosensitizers were conjugated to PEG to enhance solubility and blood circulation | High ROS from GQDs-mediated PDT toxicity killed tumor cells and triggered immune responses by releasing endogenous tumor antigens. | Low cytotoxicity, good solution stability, and strong endocytosis significantly increased host-immunity-related CD8+ T cells (cytotoxic T lymphocytes) and pro-inflammatory cytokines IFN-γ and TNF-α | [104] | |
| PEG-stabilized, PDPN antibody (PDPN Ab)- and DOX ((PDPN Ab)-AuNP-DOX) (Chemotherapy drug and PTT agent) | PEG-stabilized, PDPN antibody (PDPN Ab)- and DOX-conjugated AuNPs | Synergistic anticancer effects for combined chemotherapy and PTT against OC | Low toxicity, high drug-carrying capacity, and cell uptake efficiency, as well as enhanced anti-tumor efficacy combined with laser irradiation | [105] | |
| DOX (chemotherapy drug) and hematoporphyrin (HP) (PDT PS) | A prodrug of DOX, called RPTD, was synthesized via thioketal linkage and cRGD peptide modification and then used to prepare NPs to encapsulate hematoporphyrin photosensitizer (HP), and HP-loaded RPTD (RPTD/HP) NPs were formulated | Anticancer effect of DOX and PDT effect mediated by HP | Practical effects on inhibiting cell growth and inducing apoptosis, excellent tumor-targeting ability, and significantly inhibited tumor growth | [106] | |
| DOX | PEGylated DOX (PD) was first synthesized by the conjugation of DOX with bis-amino-terminated PEG via succinyl linkage, and then PD NPs were prepared by a modified nanoprecipitation method. After that, PD NPs were surface-modified with HN-1 to form HNPD NPs | Cytotoxicity of DOX | Higher cellular uptake and cytotoxicity than PD NPs in OSCC cells, significantly enhanced tumor-targeting and penetration efficiency compared with PD NPs, and inhibited tumor growth | [64] | |
| PDA | Black phosphorus nanosheets (BP NSs) (PTT agent) | PDA-modified BP NSs as basal material, polyacrylamide hydrochloride-dimethylmaleic acid (PAH-DMMA) charge reversal system for further surface modification (BP@PDA-PAH-DMMA) | Excellent photothermal properties and tumor enrichment ability of BP@PDA-PAH-DMMA | Enhanced uptake of OC cells, excellent photothermal properties and tumor enrichment ability, and a good killing effect on OC cells | [107] |
| BiVO4/Fe3O4@ PDA superparticles (SPs) (PTT agent) | BiVO4 and Fe3O4 NPs were first prepared, followed by their subsequent self-assembly into BiVO4/Fe3O4 SPs via the oil-in-water microemulsion route. After that, the as-prepared BiVO4/Fe3O4 SPs were covered by PDA | Photothermal effect mediated by Fe3O4 NPs | Improved the photothermal conversion capability, superior synergistic therapeutic efficacy on tumors | [108] | |
| DOX (chemotherapy drug) and terbium ion-doped hydroxyapatite (HATb) NP (PTT agent) | PDA encapsulated both HATb NP as a luminescent probe and anticancer DOX | HATb–PDA–DOX plus NIR treatment synergistically promoted the overproduction of ROS, cell cycle arrest, and increased cell apoptosis. | PH/NIR responsive release characteristics and a better antitumor effect on OSCC cells than chemotherapy or PTT alone through the overproduction of ROS, cell cycle arrest, and increased apoptosis | [109] | |
| PLA | CDDP and chloroquine (CQ) | PLA combined with CDDP/CQ-PLA NPs and PLA combined with CDDP NPs (CDDP-PLA NPs) | CDDP/CQ-PLA NPs reduced autophagy and enhanced ROS and apoptosis of CAL-27 cells | Good drug loading capacity and drug release, higher ROS and apoptosis rates and lower autophagy | [94] |
| Polyethylene glycol-polyethyleneimine | Wnt-1 siRNA (gene therapy drug) and Chlorin e6 (Ce6) (PDT PS) | Polyethylene glycol-polyethyleneimine-Ce6 (PEG-PEI-Ce6) NPs loading Wnt-1 small interfering RNA (siRNA) | PEG-PEI-Ce6 NP-mediated PDT inhibited cell growth and enhanced the cancer cell-killing effect | Inhibited Wnt/β-catenin signaling pathway and reduced the expression of Wnt-1, β-catenin, and vimentin, inhibited cell growth and significantly enhanced killing effect on cancer cells. | [110] |
| Fluorophore-modified poly(L-lysine) (PL-647) | Platinum prodrug | Hybrid nano-architectures (gold ultrasmall NPs linked by PL-647) enclosing a platinum prodrug and decorated with a customized peptide (Nas-cispt-tf2) | Anti-angiogenic and pro-apoptotic effects of platinum prodrug by the downregulation of the vascular endothelial growth factor gene and increased expression of cleaved caspase-3. | Increased accumulation of Nas-cispt-tf2, enhanced tumor volume reduction effect, increased expression of cleaved caspase-3 protein | [63] |
| γ-polyglutamic acid | Gefitinib (Gef) and Cur | γ-polyglutamic acid-coated NPs loaded with Gef and Cur (γ-PGA-Gef/Cur NPs) | Free Gef/Cur and γ-PGA-Gef/Cur NPs induced apoptotic cell death via caspase- and mitochondria-dependent pathways | Significantly reduced overall viability of SAS cells, significantly inhibited tumor size | [96] |
| Amphiphilic mPEG-PLA | Tetravalent platinum prodrug Pt (IV)-diazide (chemotherapy drug) and Ce6 (PDT PS) | Nanosize micelles self-assembled by amphiphilic mPEG-PLA, photosensitizer Ce6, and tetravalent platinum prodrug Pt (IV)-diazide | Irradiation activated Ce6 and photodecomposed to produce cytotoxic Pt (II), azidyl radical, and molecular oxygen. | Activated Ce6 upon laser irradiation and photodecomposed to produce cytotoxic Pt (II), azidyl radical, and molecular oxygen, producing a robust antitumor response, revealing its great potential to reverse hypoxia in chemo-photodynamic combination therapy. | [111] |
| H20-PLA@ PDA-PEG-FA NPs | DOX (chemotherapy drug) and PDA (PTT agent) | DOX-loaded polymeric NPs (DOX/H20-PLA@PDA NPs) were functionalized with amino-poly (ethylene glycol)-FA (H2N-PEG-FA) after coating them with PDA to form the targeting combination, DOX/H20-PLA@PDA-PEG-FA NPs. | Photothermal effect and the pH sensitivity of the PDA films; chemotherapy effect of DOX | Very effective therapeutic effect on OC, accelerated drug release in acidic TME under laser irradiation | [62] |
| PLGA-PEG nanocarriers | ATRA- PLGA- PEG-programmed death-ligand 1 (PD-L1) nanomedicines | ATRA-PLGA-PEG-PD-L1 nanomedicines were fabricated by loading ATRA into PLGA-PEG nanocarriers and modification using an anti-PD-L1 antibody | ATRA-PLGA-PEG-PD-L1 NPs inhibited proliferation and induced apoptosis in cancer cells | Rapid cellular uptake in DOK and CAL-27 cells, significantly inhibited proliferation and inducing apoptosis, specifically targeted tumor cells, enhanced anticancer activity and reduced side effects | [66] |
| FA-PEG-TK-PLGA NPs | DOX | PLGA, along with PEG, was used in the significant skeleton, and the ROS-responsive thioketone-containing (TK) was used for FA ligation to form FA-PEG-TK-PLGA NPs to load DOX | DOX-induced apoptotic cell death | Effective escape from endosomes, quick release of the entrapped DOX into the cytoplasm, induced apoptosis of OSCC cells | [56] |
| Poly (ethylene glycol)-poly (ε-caprolactone) copolymers (PEG-PCL) | ICG (PDT PS) and an organic compound (C3) (PTT agent) | C3 encapsulated in PEG-PCL with ICG to form hybrid NPs (PEGs-PCLs-C3s-ICG NPs) | PEGs-PCLs-C3s-ICG NPs simultaneously produced hyperthermia through C3 and produced ROS with 808snm laser irradiation at tumor sites | Better photothermal conversion stability, lower cytotoxicity, and a faster metabolic rate, which ensured the tumor elimination effect of PTT in vivo | [112] |
| Alginate hydrogel | CDDP (chemotherapy drug) and AuNPs (PTT agent) | A multifunctional nano platform comprising alginate hydrogel co-loaded with CDDP and AuNPs (abbreviated as ACA) | Tri-modal (thermo-chemo-radio) therapy effect | Induced a superior anticancer efficacy than mono- or bi-modality treatments, morphological features of KB cell injury and apoptosis | [113] |
| A self-destructive aliphatic polycarbonate | DOX (chemotherapy drug) and Ce6 (PDT PS) | DOX was conjugated to a self-destructive polymeric carrier through a ROS-sensitive pendant thioketal bond (PEG-PBC-TKDOX). Then, Ce6 was loaded through the π−π stacking interaction with DOX. | Anticancer effect of DOX and PDT effect mediated by Ce6 | Light stimulated Ce6 to produce cytotoxic ROS and spatiotemporally activated a cascade reaction to release the loaded drugs | [114] |
| Nano DOX-ICG MMP-responsive hydrogel (NDIMH) | DOX (chemotherapy drug) and ICG (PDT PS) | Nano DOX-ICG MMP-responsive hydrogel (NDIMH) | Anticancer effect of DOX and PTT effect mediated by ICG | Effectively inhibited viability, invasion, and metastasis of SCC-15 cells in vitro, exhibiting favorable synergistic antitumor efficacy and acceptable biosafety, significantly improving the retention of nanodrugs at the tumor site | [115] |
| Chitosan | Ursolic acid | Ursolic acid encapsulated with chitosan (UACNP) | The anti-lipid peroxidative/antioxidant properties of UACNP during DMBA-mediated oral tumor growth | Significant antitumor effects in the pre-initiation and post-initiation phases in experimental oral carcinogenesis in Syrian golden hamsters | [85] |
| LNCc (Cur lipid-core nanocapsules) | Hydrogel containing LNCc coated with chitosan | The formulations presented intrinsic cytotoxic activity | Significant reduction in cell viability of oral squamous cell lines in all test groups | [41] | |
| miR-144-source of macrophage-derived epolar [MEXO]/CA-miR-451a | Chitosan was prepared using the ionic cross-linking method, and biomimetic NPs coloaded with the miR-144/451a cluster were prepared using the uptake–efflux method | miR-144/451a cluster synergistically inhibited the proliferation, migration, and invasion of OSCC | Substantially reduced viability, migration, and invasion of OSCC. Calcium-binding protein 39 (CAB39) and migration inhibitory factor (MIF) expression in OSCC treated with miR-144-MEXO/CA-miR-451a NPs decreased significantly compared to the miR-144/451a group. | [86] | |
| GBAS gene plasmid DNA (shGBAS) (gene therapy drug) and 5-aminolevulinic acid (5-ALA) (PDT PS) | 5-ALA photosensitizer-loaded chitosan (CS) NPs were prepared using the ionic crosslinking method and further synthesized with the GBAS gene plasmid DNA (shGBAS) by electrostatic attraction (CS-ALA-shGBAS NPs) | Effect of 5-ALA mediated PDT and gene therapy effect of shGBAS | Good dispersion, stability, and hypotoxicity, showing a good mitochondrially targeted killing effect on OSCC in vitro and in vivo | [116] | |
| Simvastatin (SIM) and Quercetin (QRC) | In situ gel (ISG) loaded with chitosan-coated SIM NPs doped with QRC (chitosan-coated SIM–QRC NPs) | Enhanced biological activity of SIM due to QRC | Slower drug release rate, significantly increased apoptosis mediated by caspase-3 and increased level of tumor suppressor protein, enhanced bioactivity of SIM | [87] | |
| Phloretin | Phloretin-loaded chitosan NPs (PhCsNPs) | PhCsNPs-mediated tumor cell apoptosis | Enhanced cellular uptake, sustainable release, and bioavailability of phloretin | [88] | |
| Fluorinated chitosan | Ce6 (PDT PS) | Fluorinated chitosan-Ce6 and catalase co-assemble to form stable NPs | Effect of Ce6-mediated PDT and intracellular H2O2 transformation into O2 under catalase catalysis | Significantly improved cross-membrane penetration capacity, better anticancer activity | [117] |
| PLGA-Chitosan Janus NPs (JNP) | IL-6 receptor antagonist, tocilizumab (TCZ) | TCZ loaded JNP | Antagonistic effect of TCZ on IL-6 and JNP enhanced oral keratinocyte internalization | Good mucosal penetration and significant xenograft inhibition and outperformed all control groups in inhibiting tumor cell proliferation, reducing tumor size, and reducing proto-oncogene ERG expression | [118] |
| Chitosan and PLGA-based NPs and polylactic acid fibers | 18-β-Glycyrrhetic Acid (GA) | GA coated with chitosan and PLGA (GA-NPs) and GA coated with polylactic acid fibers (GA-FBs) | Cytotoxicity of GA on PE/CA-PJ15 cells | Cytotoxic effect on PE/CA-PJ15 cells but had no cytotoxic effects on human gingival fibroblasts, increased sensitivity of cancer cells to ROS over-production | [119] |
| Catechol-modified chitosan/hyaluronic acid NPs | DOX | Catechol-modified chitosan/hyaluronic acid NPs loading DOX (DOX-NPs) | DOX-NPs were taken up, accumulated, and induced apoptosis in cells | Superior mucoadhesive capability, inhibited growth of HN22 cell lines with low IC50, more extensively taken up, accumulated, and induced apoptosis in cells than free DOX | [120] |
| Anionic protein-Chitosan-Ag3AuS2 hydrogel | Ag3AuS2 NPs (PTT agent) | Complexation of genetically engineered anionic protein (E72), chitosan, and Ag3AuS2 NP (termed as E72-Chitosan-Ag3AuS2) | Photothermal effect mediated by Ag3AuS2 NPs | Good biocompatibility and ultra-strong photothermal effect with no side effects on surrounding normal tissues and suppressed potential tumor recurrence | [121] |
| Sucrose esters | Ce6 (PDT PS) | The emulsion-solvent diffusion method was used to prepare the nano sucrose esters encapsulating Ce6 | Singlet oxygen generation and cytotoxicity to OSCC cells induced by Ce6-mediated PDT | Higher drug encapsulation efficiency and a faster drug release rate than pure Ce6, promoted cell uptake of Ce6, singlet oxygen production in vitro, and cytotoxicity to OSCC cells | [122] |
| Polymeric nanoemulsions | Methylene blue (MB) (PDT PS) | Polymeric nanoemulsions containing MB | Effect of MB-mediated PDT | Effectively reduced cell viability, a significant decline in viability for all fluences used, enhanced internalization of the adopted biological model, further reducing toxicity and reducing adverse reactions | [123] |
| Fucoidan (FU)/hyaluronic acid (HA) cross-linked zein (Zn) NPs | Fisetin (FS) | FS-loaded cross-linked Zn NPs (ZFH), which contained HA& FU | Significant cytotoxic action of ZFH and enhanced uptake of ZFH by tumor cells | Remarkable uptake by SCC-4 cells with a significant cytotoxic effect, significantly decreased OSCC-specific serum biomarkers and histological tumor grades, and increased caspase-3 levels | [69] |
| Hyaluronic acid (HA) | CDDP (chemotherapy drug) and TQTPA [4,4′-((6,7-bis(4-(hexyloxy)phenyl)-[1,2,5]thiadiazolo [3,4-g]quinoxaline-4,9-diyl)bis(thiophene-5,2-diyl))bis(N,N-diphenylaniline)] (PTT agent) | Multimodal NPs (NPs) loading TQTPA and CDDP (HT@CDDP) by hyaluronic acid. | Combined PTT effect with chemotherapy | Good tissue penetration quality and active targeting ability, outlined orthotopic tongue tumors and metastatic lymph nodes as small as 1 mm in nude mice by IR-808 under NIR exposure, biocompatibility and low systematic toxicity | [124] |
| A drug-mimicking peptide hydrogel named L-NILMDP | Cyclic dinucleotide (CDN) | L-NILMDP was loaded with immunotherapy agonist CDN | The combination of the L-NIL-MDP hydrogel with its inherent inducible nitric oxide synthase (iNOS) inhibition and the controlled release of stimulator of interferon genes (STING) agonist immunotherapy | 4- and 20-fold slower drug release rates than commercially available hydrogels, allowing the immune-mediated elimination of established treatment-resistant oral tumors with longer median survival than the untreated group | [125] |
| Cathepsin B-responsible NPs linear-dendritic mPEG5000-BMA4 | AKT inhibitor capivasertib (AZD5363) | Linear-dendritic mPEG5000-BMA4 encapsulated capivasertib | Capivasertib was a potent agent that sensitized radioresistant OSCC cells to irradiation (IR) | Greatly enhanced tumor cell suppression in 3D cell cultures and OSCC tumor shrinkage compared to IR alone | [126] |
| Oleanolic acid-conjugated human serum albumin NPs | DOX | HAS and oleanolic acid were conjugated to form self-assembled NPs that entrapped DOX (DOX@HSA-OA) | DOX@HSA-OA NPs-mediated DOX penetration, DNA damage, oxidative stress, and apoptosis-induction | Lower IC50 value than DOX against FaDuHTB-43 at various time points, higher apoptosis and cell cycle arrest (G2/M phase); the DOX@HSA-OA NPs-mediated DOX penetration and cell death/shrinkage were significant in FaDu-HTB-43 spheroids. | [89] |
| Human serum albumin-poly (Lactide)-conjugated self-assembly NPs | DTX | The DTX-loaded DTX@HSA-(PLA)2 NPs were prepared by the desolvation-self-assembly technique | The DTX@ HSA-(PLA)2 NPs treatment induced apoptotic marker expressions, cell-cycle arrest in the G2/M-phase, DNA damage, and mitochondrial depolarization | Greater penetration, resulting in the highest cytotoxic response in cancer cells grown in monolayers or spheroids compared to free DTX and DTX-loaded in HSA NPs, improved half-life of DTX, plasma residence time, and decreased clearance than free DTX. | [90] |
| HSA | CDDP (chemotherapy drug) and ICG (PDT PS and PTT agent) | Human serum albumin carrying ICG-CDDP NPs (HSA-ICG-CDDP NPs) | Anticancer effect of DDP and PDT and PTT effect mediated by Photosan-2 | Precisely being triggered to release DDP under NIR irradiation at 808 nm, more potent antitumor effects than the treatment with ICG, HSA-ICG, and DDP alone | [127] |
| Lactalbumin NPs | Genistein nano-formulation | Genistein-loaded lactalbumin NPs (GLNPs) were prepared by using the antisolvent precipitation method | GLNPs withdrew epigenetic transcriptional repression and selectively induced apoptosis in human OSCC | Selectively induced OSCC cell apoptosis compared with normal fibroblasts, causing the withdrawal of epigenetic transcription repression by simultaneously down-regulating polycomb group protein (PcG) and its subsequent targets, regulating EZH2 expression through proteasomal-mediated degradation and 3PK inhibition | [91] |
| Nano-cassava starch | Cordycepin (CS) | Cordycepin-loaded cassava starch NPs (CCSNPs) | CS had anti-oxidant properties capable of inhibiting the growth and promoting the death of cancer cells | Increased HSG proliferation, protein secretion, and salivary-specific gene, AMY, and AQP5 expression, protecting and clearing ROS by stimulating antioxidant genes in HSGs, inhibiting the growth of HSC-4 cells by stimulating ROS generation and reducing protein secretion | [92] |
3.2. Lipid-Based NPs (LBNPs)

| Nanocarrier | Active Agent | Assembly Method | Mechanism | Advantages | Ref. |
|---|---|---|---|---|---|
| Liposomes | Evodiamine (EVO) (chemotherapy drug) and ICG (PDT PS) | EI@Lipo (theragnostic nanoliposome system) encapsulated EVO, and ICG was fabricated using a typical thin-film spin evaporation process. | Chemo-antitumor effect and PDT effect of EI@Lipo encapsulating EVO and ICG and peroxidase-like catalytic activity of EVO | Theragnostic liposomes had a significant inhibitory effect on in situ tongue tumors by photodynamic combined chemotherapy. | [130] |
| DOX | Liposome-coated DOX and Doxil | Apoptotic effect on tumor cells of DOX and Doxil | Compared with DOX, Doxil had a higher apoptosis effect on CAL-27 cells, a higher elevation of caspase-3 levels, and a higher inhibition rate of C-Myc mRNA. | [131] | |
| CDDP (chemotherapy drug) and Photosan-2 (PDT PS) | Encapsulating CDDP into liposomes to form lipid-platinum-chloride NPs (LPC NPs) | Anticancer effect of CDDP and PDT effect mediated by Photosan-2 | PDT+LPC significantly reduced tumor volume. PDT+LPC or LPC treatment showed minimal side effects on renal damage compared to CDDP or the PDT+CDDP group. PDT+LPC prolonged tumor growth inhibition, thereby reducing the dose of chemotherapy drugs. | [161] | |
| DOX | Hybrid alginate/liposomes systems loading DOX | Cytotoxic effect on tumor cells of DOX | Alginate paste incorporating DOX-loaded liposomes presented similar release rates and was highly influential in promoting cancer cell death. | [162] | |
| MTX | MTX-loaded liposomes were prepared using the thin film hydration method. These liposomes were cast in the optimized mucoadhesive film to form MTX-entrapped liposomal film (M-LP-F7) | M-LP-F7 exerted a pro-oxidant effect in HSC-3 cells | Caused a significant decrease in the half-maximal inhibitory concentration of MTX on HSC-3 cells. Increased the apoptosis rate in HSC-3 cells by almost 3-fold. | [142] | |
| SLNs | HuR (ELAVL1, an RNA-binding protein) CRISPR and epirubicin | SLNs modified with pH-sensitive epidermal growth factor receptor (EGFR)-targeting and nucleus-directed peptides carrying HuR CRISPR and epirubicin | CRISPR/Cas9 suppressed proliferation, metastasis, and resistance in SAS cells. The cotreatment of epirubicin and HuR CRISPR in SAS cells further facilitated apoptosis/necroptosis/autophagy and caused cancer cell death | CRISPR/Cas9 successfully knocked out HuR and inhibited SAS cell proliferation, metastasis, and drug resistance. Epirubicin and HuR CRISPR worked together to further promote apoptosis/necrosis/autophagy of SAS cells, resulting in cancer cell death. Combined with HuR CRISPR NPs, epirubicin NPs’ anticancer effect and safety significantly improved in SAS tumor-bearing mice. | [71] |
| PTX and AA | FA-conjugated SLN loaded with PTX and AA | Cytotoxic effect of PTX and AA | Showed a biphasic drug release behavior. Had a higher efficiency when FA-conjugated PTX-loaded SLN and FA-conjugated AA-loaded SLN were combined compared to when used individually in vivo. | [58] | |
| ATRA | Phosphatidylethanolamine polyethylene glycol (PE–PEG) coated SLN loading ATRA | Chemotoxic effect of ATRA | The presence of PE–PEG improved active cell internalization of the NPs in SCC-25 cells and reduced the non-specific internalization mechanism. Delivery of ATRA into PE–PEG-coated SLNs increased their chemotoxic effect compared to non-coated SLNs. | [163] | |
| PTX, 5-FU, and AA | PTX, 5-FU, and AA entrapped SLNs | Cytotoxic effect on tumor cells of PTX, 5-FU, and AA | SLNs exhibited a biphasic nature of drug release both in vitro and in vivo. SLN loaded with PTX and SLN loaded with AA showed greater efficacy in the in vivo treatment of OSCC. | [164] | |
| NLCs | Pitavastatin (PV) and Pinus densiflora (Pd) oil | NLCs containing PV combined with Pd oil | Cytotoxicity of PV and Pd oil against HGF-1 | Had reasonable dissolution efficiency, good rheological properties, and vigorous cytotoxic activity against the HGF-1 cell line. | [132] |
| MET | MET encapsulated with NLC | Significant toxicity of GMSMET-NLCs in KB cells and an excellent inducing rise in ROS levels involved in ROS-mediated KB cell death | Achieved a MET release rate of up to 88% in 24 h. Showed significant cytotoxicity to KB OC cells with reduced IC50 values compared with the MET solution. Showed a substantial increase in intracellular ROS levels. | [133] | |
| Silymarin (SME) | SME was loaded in NLCs and further incorporated in mucoadhesive in situ gel (SME-NLCs-Plx/CP-ISG) | ROS generation potential and SME-NLCs-Plx/CP-ISG induced apoptosis at Sub-G0 phase owing to higher penetration of SME-NLCs | A sustained release effect for SMEs indicated enhanced oral mucosa retention. The IC50 value was significantly lower than that of SME-NLCs and plain SME. Had a higher inhibitory effect on human KB OC cells. | [165] | |
| QRC and piperine | QRC and piperine-enriched NLCs | Cell cycle arrest effect of QRC and piperine | Dual drug-loaded NLCs were more effective than the pure drug solution. Improved apoptosis in NLCs. Efficient distribution in various parts of the oral cavity through oral administration. | [166] | |
| HEK293T cell exosomes | MicroRNA-34a | Cholesterol-modified microRNA-34a loaded into HEK293T cell exosomes by co-incubation | MicroRNA-34a-loaded exosomes led to significant inhibition of HN6 cell proliferation, migration, and invasion by down-regulating SATB2 expression | Absorbed by HN6 oral squamous carcinoma cells and significantly inhibited the proliferation, migration, and invasion of HN6 cells by down-regulating SATB2 expression. | [134] |
| Mesenchymal stem cell-derived exosomes | MiR-155 inhibitor | MiR-155 inhibitor-laded exosomes | MiR-155 inhibitor resulted in the upregulation of FOXO3a (Forkhead box O3-, a direct target of miRNA-155) and induction of the mesenchymal-to-epithelial transition with improved sensitization to CDDP | Pinned down the stem-cell-like behavior, reversed the epithelial-to-mesenchymal transition process, and enhanced drug sensitivity through up-regulation of FOXO3a in drug-resistant xenograft OC models. | [167] |
| Exosomes from normal fibroblasts transfected with Epstein–Barr Virus Induced-3 (EBI3) cDNA | siRNA of lymphocyte cytoplasmic protein 1 (LCP1) | Exosomes from normal fibroblasts transfected with Epstein–Barr Virus Induced-3 (EBI3) cDNA were electroporated with siRNA of lymphocyte cytoplasmic protein 1 (LCP1) as octExosomes | The silencing of LCP1 by siRNA suppressed both cancer cell growth and metastatic phenotypes | Able to transfer siLCP1 stably and efficiently into OSCC cells, LCP1 was downregulated in OSCC cells with octExosomes compared to their counterparts, thus having a significant tumor-suppressor effect in vitro and in vivo. | [135] |
| Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells (hBMSCs) | miR-101-3p | HBMSCs-derived exosomes loaded with miR-101-3p | HBMSCs-exosomes combined with miR-101-3p had an excellent therapeutic effect on OC by regulating collagen type X alpha one chain (COL10A1) | Inhibited OC progression. Tumorigenicity assay in nude mice confirmed the inhibitory effects of hBMSCs-derived exosomes, loaded with miR-101-3p, on OC. | [168] |
| Milk-exosome | DOX (chemotherapy drug) and Ce6 (PDT PS) | Milk-exosomes were conjugated to DOX by a pH-cleavable bond, anthracene endoperoxide derivative (EPT1) and Ce6 were also loaded (Exo@Dox–EPT1 NPs) | Chemo-antitumor effect of DOX and PDT effect mediated by Exo@Dox–EPT1 NPs | When the NPs accumulated at the tumor site, Ce6 produced plasmonic heat and accelerated ROS generation from EPT1 under NIR irradiation. Had synergistic effects of photochemistry, which could be triggered by acid TME and NIR. | [169] |
| NE | Cur | The Cur-NE formulation was prepared according to the interfacial prepolymer deposition and spontaneous nano-emulsification method | PI3K/Akt/mTOR suppression and miR-199a upregulation mediated by Cur-Nes | Had significant dose-dependent cytotoxicity on HSC-3 cells. Down-regulated PI3K/Akt/mTOR protein expression and up-regulated PI3K-targeting miR-199a expression in a dose- or time-dependent manner. Effectively counteracted the effects of miR-199a inhibitors on OSCC cell proliferation and cell cycle proliferation phase in a time-dependent manner. | [136] |
| PLC-NPs | SAB | SAB phospholipid complex loaded NPs (nano-SAB) | The blockade of Ki-67, PCNA, and cyclin D1 expression by nano-SAB | Compared with the free-SAB-treated group and 4NQO-exposed group, nano-SAB treatment could effectively inhibit the expression of Ki-67, proliferating cell nuclear antigen (PCNA), and cyclin D1 in high-risk dysplastic lesions. After four weeks of discontinuation, nano-SAB maintained low Ki-67, PCNA, and cyclin D1 expression levels. | [137] |
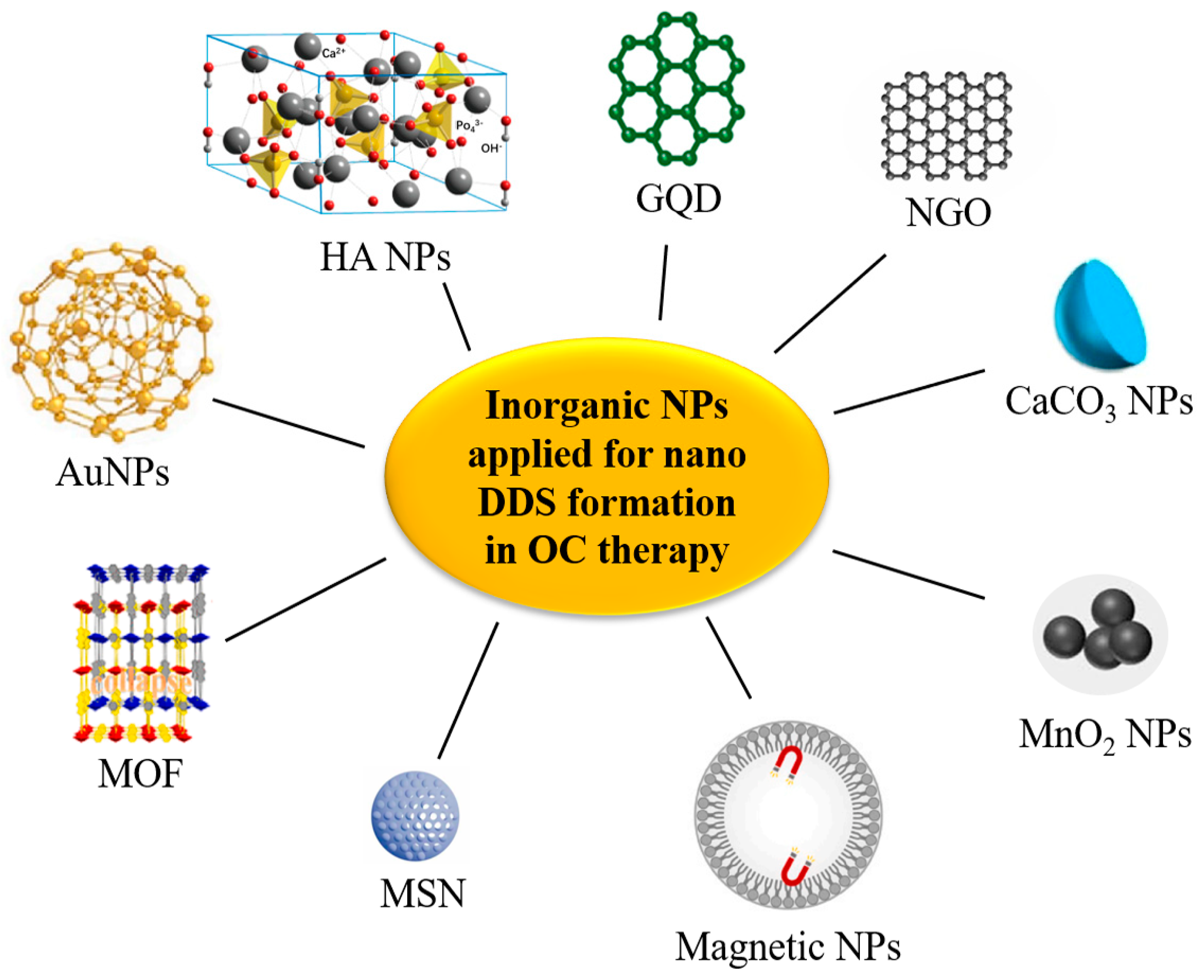
3.3. Inorganic NPs
| Nanocarrier | Active Agent | Assembly Method | Mechanism | Advantages | Ref. |
|---|---|---|---|---|---|
| AuNPs | A triple chemotherapy drug of DTX-CDDP-FU | Au NPs as a vehicle for the delivery of a triple chemotherapy drug of DTX-cisplatin-FU | Cytotoxicity of DTX-CDDP-FU | High loading efficacy of DTX, CDDP, and 5-FU, and had a controllable drug release profile at 24 h. High cytotoxicity on KB cells with lower half-maximal inhibitory concentrations than plain DTX-CDDP-FU. | [170] |
| PD-L1 specific antibodies (PD-L1-GNP) | PD-L1-specific antibodies conjugated to AuNPs (PD-L1-GNP) | PD-L1-GNP treatment induced SCC-25 cell apoptosis by inhibiting the function of the PD-L1 protein | Effectively inhibited the growth of SCC-25 cells but did not affect human immortalized keratinocytes (HaCaT). PD-L1-GNP-treated SCC-25 cells showed a phenotype with increased apoptotic proteins. | [67] | |
| DOX | Two nano-constructs conjugated to DOX through pH-sensitive and pH-resistant linkers (EDC/NHS coupling agents) | Cytotoxic effect of DOX. AuNPs enhanced the cytotoxic effect against cancer cells, inducing a significant cellular apoptosis | PH-resistant DOX NPs were more localized in the nuclei, inducing a 2-fold increase in the apoptotic effect compared with that of pH-sensitive DOX NPs. Higher tumor shrinkage and survival rates in animals treated with DOX pH-resistant AuNPs compared with pH-sensitive ones. | [171] | |
| Bilirubin | Folate-coated, bilirubin-conjugated gold (FGB) NPs | FGB nanoconjugate suppressed tumor growth in drug-resistant tumor cells by inducing apoptotic cell death | A more substantial inhibitory effect on the activity of multidrug-resistant KB-ChR-8-5 cells. The degree of ROS formation, DNA strand breakage, and apoptosis-induced morphological changes in P-gp-overexpressing drug-resistant cells induced by nanoconjugates were more significant than those induced by bilirubin alone. Had a more substantial inhibitory effect on tumor development in the KB-ChR-8-5 xenograft mouse model than bilirubin treatment alone. | [59] | |
| Gold hybrid NP | Quinacrine (QC) | PLGA-capped hybrid NPs containing QC and Au were formulated by using the oil/water single emulsion solvent evaporation technique (QAuNP) | QAuNP induced the DNA damage and re-replication stress and simultaneously down-regulated the critical repair and replication-related proteins | Excellent anti-CSC growth potential against SCC-9 CSCs and down-regulated representative CSC markers. Prolonged exposure to QAuNP induced S-phase arrest of SCC-9-CSC-like cells and prolonged the G2/M population, leading to cell re-replication and apoptosis. Resulted in the loss of DNA repair in CSCs. Led to excessive DNA damage and re-replication mediated replication stress and replication fork stalling. | [189] |
| FeAu bimetallic NPs (FeAu NPs) | MMP-1 antibody | Antibodies specific for MMP-1 conjugated to FeAu NPs | Synergistic effect of biomarker-specific antibodies and magnetic NP-induced hyperthermia | A 3.07-fold increase in uptake in HSC-3 cells compared to L929 (fibroblast) cells, which caused a 5-fold decrease in cell viability. | [70] |
| Zeolitic imidazolate framework-8 (ZIF-8) | USP30 inhibitor MF-094 | USP30 inhibitor MF-094 loaded in ZIF-8-PDA and polyethylene glycol-thioketal (PEGTK) to fabricate ZIF-8-PDA-PEGTK NPs | Targeting inhibition of USP30 by MF-094@NPs | USP30 regulated cell viability, glutamine consumption, and apoptosis of OSCC cells by regulating c-Myc deubiquitination. Targeting USP30 through the nano delivery system significantly increased its antitumor effect. | [172] |
| FeAu alloy@MOF nanostructures | DOX | DOX encapsulated with FeAu alloy@MOF nanostructures | AMF-induced hyperthermia and treatment effect of DOX | Superparamagnetic and magnetic hyperthermia behaviors and caused 90% death of HSC-3 cells, reducing the tumor size by 30-fold and tumor weight by 10-fold. | [173] |
| Metal-organic framework (IRMOF3)-Zn2+ | Disulfiram (DSF) | DSF incorporated in the metal-organic framework (IRMOF3)-Zn2+, FA) was subsequently loaded on the surface yielding IRMOF3 (IRMOF3-DSF-FA) for targeted therapy of tumors | DSF exerted an antitumor effect via targeted inhibition of ALDH1+ CSCs | High loading capacity, good biocompatibility, and strong cell uptake ability, providing metal ions, target tumor tissues, and inhibit ALDH1+ CSCs, significantly inhibiting CSCs and tumor growth without significant damage to vital organs during treatment. | [60] |
| Graphene Oxide (GO) @AlFu (aluminum fumarate) MOF | Saponins | Saponin-loaded nanostructure by modifying GO/reduced GO (rGO) with AlFu as MOF core–shell nanocomposite. | The anticancer effect of saponins on altering cell cycle proteins | The survival rate of OSCC cells treated with AlFu–GO–saponin was much lower than that of PDL cells, and the apoptosis of cells treated with AlFu–GO–saponin and AlFu–rGO–saponin was more than that of the untreated group. | [174] |
| PEGylated nano-graphene oxide | DOX (chemotherapy drug) and NGO (PTT agent) | PEGylated NGO linked DOX and fluorescently-labeled, FAP-targeted peptide chains via hydrogen bonding and π–π bonding. | The thermogenic effect of NPF@DOX simultaneously promoted the local release of DOX and apoptosis based on a pH-stimulated effect. | Exhibiting a high photothermal conversion efficiency under NIR radiation. FAP-targeted NPF@DOX in combination with PTT demonstrated better tumor suppression performance than either therapy alone. | [76] |
| ZIF-8 nanostructures composed of Zn(NO3)2·6H2O and 2-methylimidazole | DOX | MOFs coated with dental pulp mesenchymal stem cell (DPSC) membranes contained CXCR2 carried DOX to form MOF-DOX@DPSCM | Killing activity and induced apoptotic effect of MOFDOX@DPSCM and specific targeting effect of DPSC membranes | MOF@DPSCM was specific to OSCC and could induce CAL-27 cell death in vitro and block CAL-27 tumor growth in vivo. | [73] |
| Zinc-based MOFs (Zn4O(C8H5NO4)3, IRMOF-3) | DOX and celecoxib (Cel) | MOFs were integrated with thermosensitive hydrogels to devise an injectable implant, and DOX Cel was coloaded into the system (DOX/Cel/ MOFs@Gel) | Toxic effects against OC cells of DOX and Cel | Exhibited a high capacity for drug loading, stable and pH-responsive release of dual drugs, and enhanced toxic effects on KB and SCC-9 cells in vitro. Displayed outstanding tumor inhibition efficacy in vivo, inducing tumor apoptosis and regulating tumor angiogenesis. Had relatively low systemic toxicity and no apparent damage to other organs. | [175] |
| Metal-organic framework material PCN-224 | CQ (chemotherapy drug) and metal-organic framework material PCN-224 (PDT PS) | Autophagy-inhibiting photodynamic nano platform PCN-CQ@CCM coated with an OC cell membrane | PDT-generated ROS triggered the apoptosis pathway, as assessed by mitochondrial damage, and the released CQ further aggravated the ROS lethal pathway by effectively inhibiting the protective autophagic flux. | PCN-CQ@CCM could escape macrophage phagocytosis and adhere to tumor cells homologically, enhancing the retention and uptake of nanomaterials in the TME. After being activated with a 660 nm laser, the generated ROS triggered the apoptosis pathway through mitochondrial damage, and the released CQ further aggravated the ROS lethal pathway by effectively inhibiting the protective autophagic flux. | [190] |
| MSN | 5-FU | MSNs loaded with 5-FU (MSN-5-FU) were prepared first. Subsequently, the outer membrane vesicles (OMVs) of Escherichia coli were collected to wrap MSN-5-FU and prepare OMVs-MSN-5-FU. | The antitumor effect of 5-FU | The OMVs-MSN-5-FU DDS could slow the drug release rate, significantly inhibit OSCC cell proliferation, and regulate cancer cell metastasis to cervical lymph nodes. | [176] |
| MTH1 inhibitor (TH287) and multi-drug resistance protein 1 (MDR1) siRNA | TH287 and siRNA were loaded in a hyaluronic acid (HA)-based MSN | TH287 selectively inhibited the MTH1 protein in cells and MDR1 siRNA, inhibited or suppressed the gene expression of MDR1 in the cancer cells | Effectively controlled drug release and internalization in CAL-27 cells. The combination of TH287+MDR1 siRNA induced anticancer effects of tumor cells more effectively than TH287 alone. SiTMSN and HA-siTMSN significantly reduced tumor burden compared to untreated controls and free TH287. | [177] | |
| MDR1-siRNA and DOX | MSNs were modified by cationic polymer polyethylenimine (PEI) to obtain positive charges on the surface, which could enable the MSNP to carry MDR1-siRNA and DOX | MDR1-siRNA blocked MDR1 expression and DOX-induced apoptosis of cancer cells | Effectively reduced the expression of the MDR1 gene, induced apoptosis of KBV cells in vitro, and significantly reduced tumor size and slowed tumor growth in vivo compared to the control group. | [178] | |
| Light-responsive MSNs | DOX (chemotherapy drug) and IR820, a new green cyanine dye (PTT agent) | Incorporate light-responsive MSNs as DOX carriers into the IR820/methylcellulose hydrogel networks | IR820-mediated photothermal effects and MSNs achieved self-degradation-controlled DOX release via the cleavage of diselenide bonds induced by ROS | Through the combination of chemotherapy and PTT, long-lasting synergistic antitumor effects could be obtained both in vivo and in vitro with less toxicity. | [191] |
| Multifunctional hyaluronic acid (HA) modified gold nanorods/mesoporous silica-based NPs | DOX hydrochloride (chemotherapy drug) and gold NPs (PTT agent) | Multifunctional HA-modified gold nanorods/mesoporous silica-based NPs loaded with DOX hydrochloride (DOX-AuNRs@mSiO2-HA) | Chemo-antitumor effect of DOX and PDT effect mediated by DOX-AuNRs@mSiO2-HA | Had excellent photothermal conversion efficiency in PTT. Combined chemotherapy-PTT therapy’s therapeutic effect was better than chemotherapy or PTT alone. NPs injected into CAL-27 tumor-bearing mice combined with NIR laser irradiation could accumulate rapidly in tumor sites and achieve complete tumor ablation without significant side effects on normal tissues. | [192] |
| Hollow mesoporous MnO2 nano-shells | DTX and CDDP | Hollow mesoporous MnO2 (H-MnO2) nano-shells were loaded with DTX and CDDP to form H-MnO2-PEG/TP nano-shells | H-MnO2-PEG/TP nano-shells decomposed in the acidic TME, releasing the loaded drugs and simultaneously attenuated tumor hypoxia and hypoxia-inducible factor-1α (HIF-1α) expression by inducing endogenous tumor hydrogen peroxide (H2O2) decomposition. | The proliferation, colony formation, and migration of CAL-27 and SCC-7 cells in the H-MnO2-PEG/TP group were significantly decreased, apoptosis was enhanced, and HIF-1α expression was down-regulated compared with the control group. The ratio of tumor uptake to normal organs in the H-MnO2-PEG/TP group was significantly higher than in the group without the NP, and tumor growth was partially delayed. | [180] |
| Fe3O4 Magnetic NPs | Therapeutic siRNAs targeting B-cell lymphoma-2 (BCL2) and Baculoviral IAP repeat-containing 5 (BIRC5) | Polyethyleneimine (PEI)-modified magnetic Fe3O4 NPs loading BCL2 and BIRC5 | RNA interference triggered by siRNA | The NPs blocked siRNA in a concentration-dependent manner, and the NP-delivered siRNAs targeting BCL2 and BIRC5 significantly inhibited the viability and migration of Ca9-22 cells. | [179] |
| TiO2 NPs | A siRNA targeting HIF-1α (gene therapy drug) and ruthenium-based photosensitizer (Ru) (PDT PS) | TiO2@Ru@siRNA constructed from a ruthenium-based photosensitizer (Ru) modified-TiO2 NPs (NPs) loaded with siRNA of HIF-1α | TiO2@Ru@siRNA elicited photodynamic effects, which caused lysosomal damage, HIF-1α gene silencing, and OSCC cell elimination. TiO2@Ru@siRNA reshaped the immune microenvironment by downregulating key immunosuppressive factors, upregulating immune cytokines, and activating CD4+ and CD8+ T lymphocytes. | Inducing a photodynamic effect under visible light irradiation, effectively causing lysosome damage, HIF-1α gene silencing, and OSCC cell elimination. TiO2@Ru@siRNA-mediated PDT could significantly inhibit tumor growth and progression and enhance cancer immunity. | [193] |
| Fe3SO4 Magnetic NPs | CDDP | FA-mediated CDDP Fe3SO4 magnetic NPs (FA-CDDP-MNPs) | Cytotoxic effect of CDDP | A more significant inhibitory effect on OSCC than CDDP alone. Due to the introduction of FA, the targeting of FA-CDDPMNPs was improved, and the cytotoxicity was reduced. | [61] |
| CaCO3 NPs (CCNPs) | CDDP and Chrysin | Layer-by-layer of poly [di(sodium carboxyphenoxy)phosphazene] (PDCPP) and poly (diallyldimethyl ammonium chloride) (PDADMAC) deposited on the CaCO3 NPs (CCNPs) surface to form multi-layer NPs (MLNPs). CDDP and Chrysin were incorporated in the porous mineralized CaCO3 NP core and a polymeric shell | Cytotoxic effect of CDDP and chrysin-activated ROS production | Improved cytotoxic potential of MLNPs. Chrysin activated ROS production and eventually led to mitochondrial dysfunction. Buccal pouch carcinoma in the hamster model was significantly reduced. Dual-drug loaded MLNPs achieved 92% regressions of tumor volume compared to MLNPs loaded with CDDP alone. | [181] |
| Nanoscale graphene oxide (NGO) | DOX | PEG functionalized NGO carrying DOX, modified by tumor-specific peptide (HN-1) (DOX@NGO-PEG-HN-1) | Cytotoxic effect of DOX and targeting effect of HN-1 on OSCC | Significantly higher cellular uptakes and cytotoxicity in CAL-27 and SCC-25 cells when compared to free DOX. HN-1 showed considerable tumor-targeting and competition inhibition phenomena. | [65] |
| GQDs | Pt | A DDS based on Pt-loaded, polyethylene glycol-modified graphene quantum dots (GPt) via chemical oxidation and covalent reaction | GPt enhanced Pt accumulation in cells, which led to a notable increase of S-phase cell cycle arrest and apoptosis of OSCC cells | OSCC cells were sensitized to GPt under both normoxia and hypoxia conditions. GPt enhanced the accumulation of Pt in OSCC cells, resulting in a significant increase in S-phase cell cycle arrest and apoptosis of OSCC cells under normoxic and hypoxic conditions. GPt had a more substantial inhibitory effect on tumor growth and less systemic drug toxicity than free CDDP. | [182] |
| Highly-dispersive calcined HA NPs (nano-SHAP) | Zoledronic acid (ZA), CDDP, and carboplatin | Nano-SHAP was dissolved in distilled water, after which each drug was added and suspended | Antitumor activity of ZA, CDDP, and carboplatin | Nano-SHAP alone did not affect the proliferation of any cell line until a concentration of 1 μg/mL was reached. ZA-bearing nano-SHAP inhibited cell proliferation better than ZA alone. CDDP and carboplatin-bearing nano-SHAP had the same effect as these drugs alone | [183] |
3.4. Other Types of Nano-DDSs
3.5. Clinical Applications of Nano-DDSs in OC Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sachdeva, A.; Dhawan, D.; Jain, G.K.; Yerer, M.B.; Collignon, T.E.; Tewari, D.; Bishayee, A. Novel Strategies for the Bioavailability Augmentation and Efficacy Improvement of Natural Products in Oral Cancer. Cancers 2022, 15, 268. [Google Scholar] [CrossRef] [PubMed]
- Nor, J.E.; Gutkind, J.S. Head and Neck Cancer in the New Era of Precision Medicine. J. Dent. Res. 2018, 97, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Chu, T.H.; Tsai, K.W.; Hsieh, S.; Kung, M.L. Phytochemically Derived Zingerone Nanoparticles Inhibit Cell Proliferation, Invasion and Metastasis in Human Oral Squamous Cell Carcinoma. Biomedicines 2022, 10, 320. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Mendoza, A.; Olivares-Nino, G.; Diaz-Baez, D.; Lafaurie, G.I.; Perdomo, S.J. Chemopreventive and Anti-tumor Potential of Natural Products in Oral Cancer. Nutr. Cancer 2022, 74, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.; Rathod, M.; Patel, M.M. Insight on nano drug delivery systems with targeted therapy in treatment of oral cancer. Nanomedicine 2023, 49, 102662. [Google Scholar] [CrossRef] [PubMed]
- Mummudi, N.; Agarwal, J.P.; Chatterjee, S.; Mallick, I.; Ghosh-Laskar, S. Oral Cavity Cancer in the Indian Subcontinent—Challenges and Opportunities. Clin. Oncol. R. Coll. Radiol. 2019, 31, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Peacock, Z.S.; Pogrel, M.A.; Schmidt, B.L. Exploring the reasons for delay in treatment of oral cancer. J. Am. Dent. Assoc. 2008, 139, 1346–1352. [Google Scholar] [CrossRef]
- Wessels, R.; De Roose, S.; De Bruyckere, T.; Eghbali, A.; Jacquet, W.; De Rouck, T.; Cosyn, J. The Mucosal Scarring Index: Reliability of a new composite index for assessing scarring following oral surgery. Clin. Oral. Investig. 2019, 23, 1209–1215. [Google Scholar] [CrossRef]
- Desai, K. Polymeric drug delivery systems for intraoral site-specific chemoprevention of oral cancer. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1383–1413. [Google Scholar] [CrossRef]
- Furness, S.; Glenny, A.M.; Worthington, H.V.; Pavitt, S.; Oliver, R.; Clarkson, J.E.; Macluskey, M.; Chan, K.K.; Conway, D.I. Interventions for the treatment of oral cavity and oropharyngeal cancer: Chemotherapy. Cochrane Database Syst. Rev. 2010, 12, CD006386. [Google Scholar]
- Nandini, D.B.; Rao, R.S.; Hosmani, J.; Khan, S.; Patil, S.; Awan, K.H. Novel therapies in the management of oral cancer: An update. Dis. Mon. 2020, 66, 101036. [Google Scholar] [CrossRef] [PubMed]
- Lin, A. Radiation Therapy for Oral Cavity and Oropharyngeal Cancers. Dent. Clin. N. Am. 2018, 62, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, N.; Muthukrishnan, A. Oral mucositis and microbial colonization in oral cancer patients undergoing radiotherapy and chemotherapy: A prospective analysis in a tertiary care dental hospital. J. Investig. Clin. Dent. 2019, 10, e12454. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, G.; Arcuri, F.; Agostini, T.; Innocenti, M.; Raffaini, M.; Spinelli, G. Classification of tongue cancer resection and treatment algorithm. J. Surg. Oncol. 2018, 117, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhou, H.; Cheng, L.; Wang, Y.; Liu, F.; Wang, S. The application of mesoporous silica nanoparticles as a drug delivery vehicle in oral disease treatment. Front. Cell Infect. Microbiol. 2023, 13, 1124411. [Google Scholar] [CrossRef] [PubMed]
- Hartner, L. Chemotherapy for Oral Cancer. Dent. Clin. N. Am. 2018, 62, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Rawat, P.; Singh, P.K. Adverse drug reactions of anticancer drugs derived from natural sources. Food Chem. Toxicol. 2019, 123, 522–535. [Google Scholar] [CrossRef]
- Mitra, A.K.; Agrahari, V.; Mandal, A.; Cholkar, K.; Natarajan, C.; Shah, S.; Joseph, M.; Trinh, H.M.; Vaishya, R.; Yang, X.; et al. Novel delivery approaches for cancer therapeutics. J. Control Release 2015, 219, 248–268. [Google Scholar] [CrossRef]
- Calixto, G.; Bernegossi, J.; Fonseca-Santos, B.; Chorilli, M. Nanotechnology-based drug delivery systems for treatment of oral cancer: A review. Int. J. Nanomed. 2014, 9, 3719–3735. [Google Scholar] [CrossRef]
- Fan, H.Y.; Zhu, Z.L.; Zhang, W.L.; Yin, Y.J.; Tang, Y.L.; Liang, X.H.; Zhang, L. Light stimulus responsive nanomedicine in the treatment of oral squamous cell carcinoma. Eur. J. Med. Chem. 2020, 199, 112394. [Google Scholar] [CrossRef]
- Fan, H.Y.; Yu, X.H.; Wang, K.; Yin, Y.J.; Tang, Y.J.; Tang, Y.L.; Liang, X.H. Graphene quantum dots (GQDs)-based nanomaterials for improving photodynamic therapy in cancer treatment. Eur. J. Med. Chem. 2019, 182, 111620. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for Head and Neck Squamous Cell Carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Valdez, J.A.; Brennan, M.T. Impact of Oral Cancer on Quality of Life. Dent. Clin. N. Am. 2018, 62, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Zhang, X.Q.; Liu, Q.; Zhang, J.; Zhou, G. Nanotechnology: A promising method for oral cancer detection and diagnosis. J. Nanobiotechnol. 2018, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Marcazzan, S.; Varoni, E.M.; Blanco, E.; Lodi, G.; Ferrari, M. Nanomedicine, an emerging therapeutic strategy for oral cancer therapy. Oral. Oncol. 2018, 76, 1–7. [Google Scholar] [CrossRef]
- Zhu, Y.; Wen, L.M.; Li, R.; Dong, W.; Jia, S.Y.; Qi, M.C. Recent advances of nano-drug delivery system in oral squamous cell carcinoma treatment. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9445–9453. [Google Scholar]
- Yu, C.; Li, L.; Wang, S.; Xu, Y.; Wang, L.; Huang, Y.; Hieawy, A.; Liu, H.; Ma, J. Advances in nanomaterials for the diagnosis and treatment of head and neck cancers: A review. Bioact. Mater. 2023, 25, 430–444. [Google Scholar] [CrossRef]
- Ding, Z.; Sigdel, K.; Yang, L.; Liu, Y.; Xuan, M.; Wang, X.; Gu, Z.; Wu, J.; Xie, H. Nanotechnology-based drug delivery systems for enhanced diagnosis and therapy of oral cancer. J. Mater. Chem. B 2020, 8, 8781–8793. [Google Scholar] [CrossRef]
- Essawy, M.M.; Mohamed, M.M.; Raslan, H.S.; Rafik, S.T.; Awaad, A.K.; Ramadan, O.R. The theranostic potentialities of bioavailable nanocurcumin in oral cancer management. BMC Complement. Med. Ther. 2022, 22, 309. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, G.K.; Gaddam, S.A.; Kotakadi, V.S.; Gunti, H.; Palithya, S.; Penchalaneni, J.; Challagundla, V.N. Green Fabrication of silver nanoparticles by leaf extract of Byttneria Herbacea Roxb and their promising therapeutic applications and its interesting insightful observations in oral cancer. Artif. Cells Nanomed. Biotechnol. 2023, 51, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Halkai, K.R.; Halkai, R.; Patil, S.; Alawadi, J.; Alawadhi, W.S.; Marukala, N.R.; Mohammad, A.N.; Indi, S. Evaluation of cytotoxic effects of fungal origin nanosilver particles on oral cancer cell lines: An in vitro study. J. Cancer Res. Ther. 2022, 18, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Lee, C.H.; Lin, M.S.; Chi, C.W.; Chen, Y.J.; Wang, G.S.; Liao, K.W.; Chiu, L.P.; Wu, S.H.; Huang, D.M.; et al. ZnO Nanoparticles Induced Caspase-Dependent Apoptosis in Gingival Squamous Cell Carcinoma through Mitochondrial Dysfunction and p70S6K Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 1612. [Google Scholar] [CrossRef]
- Jing, D.; Jiang, N.; Wang, F.; Mao, C.; Han, S.; Ho, P.Y.; Xiao, W.; Li, Y.; Li, J.J.; Zhang, L.; et al. Nanoradiosensitizer with good tissue penetration and enhances oral cancer radiotherapeutic effect. Biomaterials 2022, 289, 121769. [Google Scholar] [CrossRef]
- Chen, M.H.; Chen, M.H.; Li, C.Y.; Tung, F.I.; Chen, S.Y.; Liu, T.Y. Using Gold-Nanorod-Filled Mesoporous Silica Nanobeads for Enhanced Radiotherapy of Oral Squamous Carcinoma. Nanomaterials 2021, 11, 2235. [Google Scholar] [CrossRef]
- Chen, L.; Kong, Q.; Tian, M.; Zhang, Q.; Xia, C.; Deng, C. Zn0.4Mg0.6Fe2O4 nanoenzyme: A novel chemo-sensitizer for the chemotherapy treatment of oral squamous cell carcinoma. Nanoscale Adv. 2023, 5, 851–860. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 569–578. [Google Scholar] [CrossRef]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, J.; Yang, Y.; Liang, H.; Jia, H.; Li, D. Current Trends of Targeted Drug Delivery for Oral Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 618931. [Google Scholar] [CrossRef]
- Ortega, A.; Da, S.A.; Da, C.L.; Zatta, K.C.; Onzi, G.R.; Da, F.F.; Guterres, S.S.; Paese, K. Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma. Drug Deliv. Transl. Res. 2023, 13, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Ying, N.; Liu, S.; Zhang, M.; Cheng, J.; Luo, L.; Jiang, J.; Shi, G.; Wu, S.; Ji, J.; Su, H.; et al. Nano delivery system for paclitaxel: Recent advances in cancer theranostics. Colloids Surf. B Biointerfaces 2023, 228, 113419. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, M.; Golusinska-Kardach, E.; Golusinski, W.; Florek, E. Polydopamine-Based Material and Their Potential in Head and Neck Cancer Therapy-Current State of Knowledge. Int. J. Mol. Sci. 2023, 24, 4890. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, e1901223. [Google Scholar] [CrossRef]
- Pastore, C. Size-dependent nano-bio interactions. Nat. Nanotechnol. 2021, 16, 1052. [Google Scholar] [CrossRef]
- Pattipeiluhu, R.; Arias-Alpizar, G.; Basha, G.; Chan, K.; Bussmann, J.; Sharp, T.H.; Moradi, M.A.; Sommerdijk, N.; Harris, E.N.; Cullis, P.R.; et al. Anionic Lipid Nanoparticles Preferentially Deliver mRNA to the Hepatic Reticuloendothelial System. Adv. Mater. 2022, 34, e2201095. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Li, J.Q.; Wang, Z.Z.; Dong, D.W.; Qi, X.R. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 2014, 35, 5226–5239. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, R.; Shevtsov, M.; Gao, H. When imaging meets size-transformable nanosystems. Adv. Drug Deliv. Rev. 2022, 183, 114176. [Google Scholar] [CrossRef]
- Cheng, X.; Li, H.; Ge, X.; Chen, L.; Liu, Y.; Mao, W.; Zhao, B.; Yuan, W.E. Tumor-Microenvironment- Responsive Size-Shrinkable Drug-Delivery Nanosystems for Deepened Penetration Into Tumors. Front. Mol. Biosci. 2020, 7, 576420. [Google Scholar] [CrossRef] [PubMed]
- Das, R.P.; Gandhi, V.V.; Singh, B.G.; Kunwar, A. Passive and Active Drug Targeting: Role of Nanocarriers in Rational Design of Anticancer Formulations. Curr. Pharm. Des. 2019, 25, 3034–3056. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Liang, Y.; Miao, L.; Guo, W.; Chao, Y.; He, H.; Zhang, Y.; Yang, J.; Wu, C.; Yin, T.; et al. Improved tumor tissue penetration and tumor cell uptake achieved by delayed charge reversal nanoparticles. Acta Biomater. 2017, 62, 157–166. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Xu, M.; Yang, D. Current trends of targeted therapy for oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2022, 148, 2169–2186. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Huang, Q.; Chen, J.; Song, X.; Chen, Z.; Huang, M.; Xu, P.; Zhang, J. Tumor-targeting photodynamic therapy based on folate-modified polydopamine nanoparticles. Int. J. Nanomed. 2019, 14, 6799–6812. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, M.; Zhao, L.; Li, Q. A new biosafe reactive oxygen species responsive nanoplatform for targeted oral squamous cell carcinoma therapy. Mater. Express 2019, 9, 1076–1081. [Google Scholar] [CrossRef]
- Yin, H.; Wang, H.; Li, Z.; Shu, D.; Guo, P. RNA Micelles for the Systemic Delivery of Anti-miRNA for Cancer Targeting and Inhibition without Ligand. ACS Nano 2019, 13, 706–717. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Medhi, S. Effectual nanotherapy against oral squamous cell carcinoma. Drug Dev. Ind. Pharm. 2021, 47, 711–724. [Google Scholar] [CrossRef]
- Rathinaraj, P.; Muthusamy, G.; Prasad, N.R.; Gunaseelan, S.; Kim, B.; Zhu, S. Folate-Gold-Bilirubin Nanoconjugate Induces Apoptotic Death in Multidrug-Resistant Oral Carcinoma Cells. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 285–296. [Google Scholar] [CrossRef]
- Cui, J.; Li, W.; Bu, W.; Liu, J.; Chen, X.; Li, X.; Liu, C.; Meng, L.; Chen, M.; Sun, H.; et al. Folic acid-modified disulfiram/Zn-IRMOF3 nanoparticles for oral cancer therapy by inhibiting ALDH1A1+ cancer stem cells. Biomater. Adv. 2022, 139, 213038. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Zhang, L.; Liu, X.Q.; Xu, L.P.; Liu, J.J. Folic acid mediated cisplatin magnetic nanodrug targeting in the treatment of oral squamous cell carcinoma. Mater. Express 2021, 11, 1299–1305. [Google Scholar] [CrossRef]
- Yin, X.; Li, Z.; Zhang, Y.; Zeng, X.; Wang, Q.; Liang, Z. Polydopamine surface-modified hyperbranched polymeric nanoparticles for synergistic chemo/photothermal therapy of oral cancer. Front. Bioeng. Biotechnol. 2023, 11, 1174014. [Google Scholar] [CrossRef] [PubMed]
- Mapanao, A.K.; Sarogni, P.; Santi, M.; Menicagli, M.; Gonnelli, A.; Zamborlin, A.; Ermini, M.L.; Voliani, V. Pro-apoptotic and size-reducing effects of protein corona-modulating nano-architectures enclosing platinum prodrug in in vivo oral carcinoma. Biomater. Sci. 2022, 10, 6135–6145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, G.; Li, Z.; Shi, S.; Chen, B.; Li, C.; Zhang, L.; Wang, Y. PEGylated doxorubicin nanoparticles mediated by HN-1 peptide for targeted treatment of oral squamous cell carcinoma. Int. J. Pharm. 2017, 525, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Du, J.; Wang, X.; Duan, A.; Gao, R.; Liu, J.; Li, B. Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy. Sci. Rep. 2021, 11, 1725. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.Q.; Tang, M.X.; Liu, Q.; Zhou, G. Anti-PD-L1-modified and ATRA-loaded nanoparticles for immuno-treatment of oral dysplasia and oral squamous cell carcinoma. Nanomedicine 2020, 15, 951–968. [Google Scholar] [CrossRef]
- Choi, B.; Choi, J.H.; Kim, U.K.; Hwang, D.S.; Kim, G.C. Gold nanoparticles conjugated with programmed death-ligand 1 antibodies induce apoptosis of SCC-25 oral squamous cell carcinoma cells via programmed death-ligand 1/signal transducer and transcription 3 pathway. Arch. Oral. Biol. 2021, 125, 105085. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, S.; Mohammad, S.; Ahmad, I. Development of alpha-tocopherol surface-modified targeted delivery of 5-fluorouracil-loaded poly-D, L-lactic-co-glycolic acid nanoparticles against oral squamous cell carcinoma. J. Cancer Res. Ther. 2019, 15, 480–490. [Google Scholar] [CrossRef]
- Moustafa, M.A.; El-Refaie, W.M.; Elnaggar, Y.; El-Mezayen, N.S.; Awaad, A.K.; Abdallah, O.Y. Fucoidan/hyaluronic acid cross-linked zein nanoparticles loaded with fisetin as a novel targeted nanotherapy for oral cancer. Int. J. Biol. Macromol. 2023, 241, 124528. [Google Scholar] [CrossRef]
- Tsai, M.T.; Sun, Y.S.; Keerthi, M.; Panda, A.K.; Dhawan, U.; Chang, Y.H.; Lai, C.F.; Hsiao, M.; Wang, H.Y.; Chung, R.J. Oral Cancer Theranostic Application of FeAu Bimetallic Nanoparticles Conjugated with MMP-1 Antibody. Nanomaterials 2021, 12, 61. [Google Scholar] [CrossRef]
- Wang, C.S.; Chang, C.H.; Tzeng, T.Y.; Lin, A.M.; Lo, Y.L. Gene-editing by CRISPR-Cas9 in combination with anthracycline therapy via tumor microenvironment-switchable, EGFR-targeted, and nucleus-directed nanoparticles for head and neck cancer suppression. Nanoscale Horiz. 2021, 6, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, L.; Duan, Y.; Xu, S.; Shen, L.; Zhu, T.; Hou, L.; Meng, X.; Liu, B. Nanobody modified high-performance AIE photosensitizer nanoparticles for precise photodynamic oral cancer therapy of patient-derived tumor xenograft. Biomaterials 2021, 274, 120870. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Chen, Y.; Bu, W.; Meng, L.; Wang, C.; Jin, N.; Chen, Y.; Ren, C.; Zhang, K.; Sun, H. Modification of Metal-Organic Framework Nanoparticles Using Dental Pulp Mesenchymal Stem Cell Membranes to Target Oral Squamous Cell Carcinoma. J. Colloid. Interface Sci. 2021, 601, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Feng, J.; Qiu, L.; Gao, Z.; Li, P.; Pang, L.; Zhang, Z. SDF-1-loaded PLGA nanoparticles for the targeted photoacoustic imaging and photothermal therapy of metastatic lymph nodes in tongue squamous cell carcinoma. Int. J. Pharm. 2019, 554, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Palomares, E.; Garcia-Hevia, L.; Galan-Vidal, J.; Gandarillas, A.; Garcia-Reija, F.; Sanchez-Iglesias, A.; Liz-Marzan, L.M.; Valiente, R.; Fanarraga, M.L. Shiga Toxin-B Targeted Gold Nanorods for Local Photothermal Treatment in Oral Cancer Clinical Samples. Int. J. Nanomed. 2022, 17, 5747–5760. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, C.; Wan, C.; Liu, T.; Zhang, R.; Du, J.; Wang, X.; Jiao, X.; Gao, R.; Li, B. A Targeted and pH-Responsive Nano-Graphene Oxide Nanoparticle Loaded with Doxorubicin for Synergetic Chemo-Photothermal Therapy of Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2023, 18, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Huo, M.; Wang, L.; Li, J.; Chen, Y.; Xiong, P. Photonic hyperthermal and sonodynamic nanotherapy targeting oral squamous cell carcinoma. J. Mater. Chem. B 2020, 8, 9084–9093. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar] [CrossRef]
- Xie, X.; Feng, Y.; Zhang, H.; Su, Q.; Song, T.; Yang, G.; Li, N.; Wei, X.; Li, T.; Qin, X.; et al. Remodeling tumor immunosuppressive microenvironment via a novel bioactive nanovaccines potentiates the efficacy of cancer immunotherapy. Bioact. Mater. 2022, 16, 107–119. [Google Scholar] [CrossRef]
- Lubek, J.E. Head and Neck Cancer Research and Support Foundations. Oral. Maxillofac. Surg. Clin. N. Am. 2018, 30, 459–469. [Google Scholar] [CrossRef]
- Grillone, A.; Ciofani, G. Magnetic Nanotransducers in Biomedicine. Chemistry 2017, 23, 16109–16114. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Carreiro, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Perez-Aranda, M.; Martinez, G.; Merinero, M.; Arguelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Li, Z.; Ho, W.; Bai, X.; Li, F.; Chen, Y.J.; Zhang, X.Q.; Xu, X. Nanoparticle depots for controlled and sustained gene delivery. J. Control Release 2020, 322, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Karthik, M.; Manoharan, S.; Muralinaidu, R. Ursolic acid-loaded chitosan nanoparticles suppress 7,12-dimethylbenz(a)anthracene-induced oral tumor formation through their antilipid peroxidative potential in golden Syrian hamsters. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 3061–3074. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Qiu, Y.; Liu, X.; Huang, F. Biomimetic Nanosystems for the Synergistic Delivery of miR-144/451a for Oral Squamous Cell Carcinoma. Balk. Med. J. 2022, 39, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Naveen, N.R. In Situ Gel Loaded with Chitosan-Coated Simvastatin Nanoparticles: Promising Delivery for Effective Anti-Proliferative Activity against Tongue Carcinoma. Mar. Drugs 2020, 18, 201. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, M.G.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef]
- Kumbham, S.; Paul, M.; Itoo, A.; Ghosh, B.; Biswas, S. Oleanolic acid-conjugated human serum albumin nanoparticles encapsulating doxorubicin as synergistic combination chemotherapy in oropharyngeal carcinoma and melanoma. Int. J. Pharm. 2022, 614, 121479. [Google Scholar] [CrossRef]
- Kumbham, S.; Ghosh, A.; Ghosh, B.; Biswas, S. Human serum albumin-poly(Lactide)-conjugated self-assembly NPs for targeted docetaxel delivery and improved therapeutic efficacy in oral cancer. Int. J. Biol. Macromol. 2022, 222, 1287–1303. [Google Scholar] [CrossRef]
- Dev, A.; Sardoiwala, M.N.; Kushwaha, A.C.; Karmakar, S.; Choudhury, S.R. Genistein nanoformulation promotes selective apoptosis in oral squamous cell carcinoma through repression of 3PK-EZH2 signalling pathway. Phytomedicine 2021, 80, 153386. [Google Scholar] [CrossRef] [PubMed]
- Kaokaen, P.; Jaiboonma, A.; Chaicharoenaudomrung, N.; Kunhorm, P.; Janebodin, K.; Noisa, P.; Jitprasertwong, P. Cordycepin-loaded Nanoparticles from Cassava Starch Promote the Proliferation of Submandibular Gland Cells and Inhibit the Growth of Oral Squamous Carcinoma Cells. Nutr. Cancer 2021, 73, 2014–2029. [Google Scholar] [CrossRef]
- Pradhan, R.; Paul, S.; Das, B.; Sinha, S.; Dash, S.R.; Mandal, M.; Kundu, C.N. Resveratrol nanoparticle attenuates metastasis and angiogenesis by deregulating inflammatory cytokines through inhibition of CAFs in oral cancer by CXCL-12/IL-6-dependent pathway. J. Nutr. Biochem. 2023, 113, 109257. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Yan, W.; Chen, Y. Antitumor effect of poly lactic acid nanoparticles loaded with cisplatin and chloroquine on the oral squamous cell carcinoma. Aging 2020, 13, 2593–2603. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, M.; Kumar, R.; Belz, J.; Shanker, R.; Dwivedi, P.D.; Sridhar, S.; Singh, S.P. Synthesis and in vitro studies of PLGA-DTX nanoconjugate as potential drug delivery vehicle for oral cancer. Int. J. Nanomed. 2018, 13, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.C.; Chueh, F.S.; Hsiao, Y.T.; Cheng, Z.Y.; Lien, J.C.; Liu, K.C.; Peng, S.F.; Chung, J.G. Gefitinib and curcumin-loaded nanoparticles enhance cell apoptosis in human oral cancer SAS cells in vitro and inhibit SAS cell xenografted tumor in vivo. Toxicol. Appl. Pharmacol. 2019, 382, 114734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.M.; Nie, S.C.; Xu, Z.Y.; Fan, Y.R.; Jiao, M.N.; Miao, H.J.; Liang, S.X.; Yan, Y.B. Advanced Polymeric Nanoagents for Oral Cancer Theranostics: A Mini Review. Front. Chem. 2022, 10, 927595. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.S.; Xian, J.W.; Li, Q.; Chan, C.W.; Leung, S.; To, K. Biodegradable Thermosensitive PLGA-PEG-PLGA Polymer for Non-irritating and Sustained Ophthalmic Drug Delivery. AAPS J. 2019, 21, 59. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, H.T.; Yang, W.F.; Li, Y.F.; Liu, C. Evaluation of METase-pemetrexed-loaded PEG-PLGA nanoparticles modified with anti-CD133-scFV for treatment of gastric carcinoma. Biosci. Rep. 2018, 38, BSR20171001. [Google Scholar] [CrossRef]
- Liang, C.; Li, N.; Cai, Z.; Liang, R.; Zheng, X.; Deng, L.; Feng, L.; Guo, R.; Wei, B. Co-encapsulation of magnetic Fe3O4 nanoparticles and doxorubicin into biocompatible PLGA-PEG nanocarriers for early detection and treatment of tumours. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4211–4221. [Google Scholar] [CrossRef]
- Chaves, P.D.; Ourique, A.F.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C. Carvedilol-loaded nanocapsules: Mucoadhesive properties and permeability across the sublingual mucosa. Eur. J. Pharm. Biopharm. 2017, 114, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ait, S.H.; Mabroum, H.; Lahcini, M.; Oudadesse, H.; Barroug, A.; Ben, Y.H.; Noukrati, H. Manufacturing methods, properties, and potential applications in bone tissue regeneration of hydroxyapatite-chitosan biocomposites: A review. Int. J. Biol. Macromol. 2023, 243, 125150. [Google Scholar]
- Shi, S.R.; Wang, Y.; Wang, B.B.; Chen, Q.; Wan, G.Y.; Yang, X.Y.; Zhang, J.; Zhang, L.Y.; Li, C.Y.; Wang, Y.S. Homologous-targeting biomimetic nanoparticles for photothermal therapy and Nrf2-siRNA amplified photodynamic therapy against oral tongue squamous cell carcinoma. Chem. Eng. J. 2020, 388, 124268. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Yi, C.; Chen, G.; Li, Y.; Zhou, Y.; Chen, G.; Li, Y.; He, Y.; Yu, D. Host Immune Response Triggered by Graphene Quantum-Dot-Mediated Photodynamic Therapy for Oral Squamous Cell Carcinoma. Int. J. Nanomed. 2020, 15, 9627–9638. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, J.; Zhu, B.; Xu, Q. Development of a multifunctional gold nanoplatform for combined chemo-photothermal therapy against oral cancer. Nanomedicine 2020, 15, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, L.; Zhu, M.; Wan, G.; Li, C.; Zhang, J.; Wang, Y.; Wang, Y. Reactive Oxygen Species-Responsive Nanoparticles Based on PEGlated Prodrug for Targeted Treatment of Oral Tongue Squamous Cell Carcinoma by Combining Photodynamic Therapy and Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 29260–29272. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Zhang, Y.; Yang, Y.; Zhou, X.; Peng, W.; Liang, Z.; Zeng, X.; Wang, Q.; Gao, N. Charge-reversal nanomedicine based on black phosphorus for the development of A Novel photothermal therapy of oral cancer. Drug Deliv. 2021, 28, 700–708. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G.; Kang, T.T.; Liu, S.W.; Wang, L.; Zou, H.Y.; Chong, Y.; Liu, Y. BiVO4/Fe3O4@polydopamine superparticles for tumor multimodal imaging and synergistic therapy. J. Nanobiotechnol. 2021, 19, 90. [Google Scholar] [CrossRef]
- Gu, M.; Jiang, L.; Hao, L.; Lu, J.; Liu, Z.; Lei, Z.; Li, Y.; Hua, C.; Li, W.; Li, X. A novel theranostic nanoplatform for imaging-guided chemo-photothermal therapy in oral squamous cell carcinoma. J. Mater. Chem. B 2021, 9, 6006–6016. [Google Scholar] [CrossRef]
- Ma, C.; Shi, L.; Huang, Y.; Shen, L.; Peng, H.; Zhu, X.; Zhou, G. Nanoparticle delivery of Wnt-1 siRNA enhances photodynamic therapy by inhibiting epithelial-mesenchymal transition for oral cancer. Biomater. Sci. 2017, 5, 494–501. [Google Scholar] [CrossRef]
- Paul, M.; Itoo, A.M.; Ghosh, B.; Biswas, S. Hypoxia alleviating platinum(IV)/chlorin e6-based combination chemotherapeutic-photodynamic nanomedicine for oropharyngeal carcinoma. J. Photochem. Photobiol. B 2023, 238, 112627. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Cheng, X.; Chen, M.; Liu, C.; Zhao, P.; Huang, W.; He, J.; Zhou, Z.; Miao, L. Hypotoxic and Rapidly Metabolic PEG-PCL-C3-ICG Nanoparticles for Fluorescence-Guided Photothermal/Photodynamic Therapy against OSCC. ACS Appl. Mater. Interfaces 2017, 9, 31509–31518. [Google Scholar] [CrossRef] [PubMed]
- Alamzadeh, Z.; Beik, J.; Mirrahimi, M.; Shakeri-Zadeh, A.; Ebrahimi, F.; Komeili, A.; Ghalandari, B.; Ghaznavi, H.; Kamrava, S.K.; Moustakis, C. Gold nanoparticles promote a multimodal synergistic cancer therapy strategy by co-delivery of thermo-chemo-radio therapy. Eur. J. Pharm. Sci. 2020, 145, 105235. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhai, Y.; Ye, H.; Lv, Q.; Sun, B.; Luo, C.; Jiang, Q.; Zhang, H.; Xu, Y.; Jing, Y.; et al. High Co-loading Capacity and Stimuli-Responsive Release Based on Cascade Reaction of Self-Destructive Polymer for Improved Chemo-Photodynamic Therapy. ACS Nano 2019, 13, 7010–7023. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Fu, Z.G.; Li, W.; Li, Y.X.; Zhao, L.S.; Wen, L.; Zhang, J.J.; Wen, N. The synthesis and application of nano doxorubicin-indocyanine green matrix metalloproteinase-responsive hydrogel in chemophototherapy for head and neck squamous cell carcinoma. Int. J. Nanomed. 2019, 14, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Liu, H. Co-delivery of chitosan nanoparticles of 5-aminolevulinic acid and shGBAS for improving photodynamic therapy efficacy in oral squamous cell carcinomas. Photodiagnosis Photodyn. Ther. 2021, 34, 102218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Shi, L.; Ma, C.; Xu, L.; Yang, J.; Zhou, G.; Zhu, X.; Shen, L. Fluorinated chitosan-mediated intracellular catalase delivery for enhanced photodynamic therapy of oral cancer. Biomater. Sci. 2021, 9, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Habibi, N.; Bissonnette, C.; Pei, P.; Wang, D.; Chang, A.; Raymond, J.E.; Lahann, J.; Mallery, S.R. Mucopenetrating Janus Nanoparticles For Field-Coverage Oral Cancer Chemoprevention. Pharm. Res. 2023, 40, 749–764. [Google Scholar] [CrossRef]
- Cacciotti, I.; Chronopoulou, L.; Palocci, C.; Amalfitano, A.; Cantiani, M.; Cordaro, M.; Lajolo, C.; Calla, C.; Boninsegna, A.; Lucchetti, D.; et al. Controlled release of 18-beta-glycyrrhetic acid by nanodelivery systems increases cytotoxicity on oral carcinoma cell line. Nanotechnology 2018, 29, 285101. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Catechol-modified chitosan/hyaluronic acid nanoparticles as a new avenue for local delivery of doxorubicin to oral cancer cells. Colloids Surf. B Biointerfaces 2020, 196, 111279. [Google Scholar] [CrossRef]
- Su, J.; Lu, S.; Jiang, S.; Li, B.; Liu, B.; Sun, Q.; Li, J.; Wang, F.; Wei, Y. Engineered Protein Photo-Thermal Hydrogels for Outstanding In Situ Tongue Cancer Therapy. Adv. Mater. 2021, 33, e2100619. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Heng, P.; Chan, L.W. Improvement in dissolution rate and photodynamic efficacy of chlorin e6 by sucrose esters as drug carrier in nanosuspension formulation: Optimisation and in vitro characterisation. J. Pharm. Pharmacol. 2018, 70, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Do, A.S.; Amantino, C.F.; De Annunzio, S.R.; de Paula, A.V.; Fontana, C.R.; Primo, F.L. Advanced methylene blue—Nanoemulsions for in vitro photodynamic therapy on oral and cervical human carcinoma. Lasers Med. Sci. 2022, 37, 3443–3450. [Google Scholar]
- Wang, Y.; Zhang, W.; Sun, P.; Cai, Y.; Xu, W.; Fan, Q.; Hu, Q.; Han, W. A Novel Multimodal NIR-II Nanoprobe for the Detection of Metastatic Lymph Nodes and Targeting Chemo-Photothermal Therapy in Oral Squamous Cell Carcinoma. Theranostics 2019, 9, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.G.; Dharmaraj, N.; Lopez-Silva, T.L.; Venzor, J.R.; Pogostin, B.H.; Sikora, A.G.; Hartgerink, J.D.; Young, S. Biomaterial-Facilitated Immunotherapy for Established Oral Cancers. ACS Biomater. Sci. Eng. 2021, 7, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lang, L.; Lam, T.; Chen, A.; Jensen, C.; Duncan, L.; Kong, F.C.; Kurago, Z.B.; Shay, C.; Teng, Y. Circumventing AKT-Associated Radioresistance in Oral Cancer by Novel Nanoparticle-Encapsulated Capivasertib. Cells 2020, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, D.; Pan, J.; Xia, C.; Fan, L.; Pu, Y.; Zhang, Q.; Ni, Y.H.; Wang, J.; Hu, Q. A near infrared light-triggered human serum albumin drug delivery system with coordination bonding of indocyanine green and cisplatin for targeting photochemistry therapy against oral squamous cell cancer. Biomater. Sci. 2019, 7, 5270–5282. [Google Scholar] [CrossRef]
- El, M.S.; Rodriguez-Nogales, C.; Blanco-Prieto, M.J. Oral lipid nanomedicines: Current status and future perspectives in cancer treatment. Adv. Drug Deliv. Rev. 2021, 173, 238–251. [Google Scholar]
- Agrawal, S.; Garg, A.; Varshney, V. Recent Updates on Applications of Lipid-Based Nanoparticles for Site-Specific Drug Delivery. Pharm. Nanotechnol. 2022, 10, 24–41. [Google Scholar] [CrossRef]
- Wei, Z.; Zou, H.; Liu, G.; Song, C.; Tang, C.; Chen, S.; Zhang, G.; Ran, J.; Wang, Y.; Yin, X.; et al. Peroxidase-mimicking evodiamine/indocyanine green nanoliposomes for multimodal imaging-guided theranostics for oral squamous cell carcinoma. Bioact. Mater. 2021, 6, 2144–2157. [Google Scholar] [CrossRef]
- El-Hamid, E.; Gamal-Eldeen, A.M.; Sharaf, E.A. Liposome-coated nano doxorubicin induces apoptosis on oral squamous cell carcinoma CAL-27 cells. Arch. Oral. Biol. 2019, 103, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Felimban, R.I.; Tayeb, H.H.; Chaudhary, A.G.; Felemban, M.A.; Alnadwi, F.H.; Ali, S.A.; Alblowi, J.A.; ALfayez, E.; Bukhary, D.; Alissa, M.; et al. Utilization of a nanostructured lipid carrier encapsulating pitavastatin-Pinus densiflora oil for enhancing cytotoxicity against the gingival carcinoma HGF-1 cell line. Drug Deliv. 2023, 30, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Shete, M.B.; Deshpande, A.S.; Shende, P.K. Nanostructured lipid carrier-loaded metformin hydrochloride: Design, optimization, characterization, assessment of cytotoxicity and ROS evaluation. Chem. Phys. Lipids 2023, 250, 105256. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Meng, Y.; Wang, B.; Wang, C.; Hou, C.; Zhu, Q.; Tang, Y.; Ye, J. In vitro experimental study on the formation of microRNA-34a loaded exosomes and their inhibitory effect in oral squamous cell carcinoma. Cell Cycle 2022, 21, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Kase, Y.; Uzawa, K.; Wagai, S.; Yoshimura, S.; Yamamoto, J.I.; Toeda, Y.; Okubo, M.; Eizuka, K.; Ando, T.; Nobuchi, T.; et al. Engineered exosomes delivering specific tumor-suppressive RNAi attenuate oral cancer progression. Sci. Rep. 2021, 11, 5897. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, J.; Zhang, C.; Bao, Z.; Wu, L. Curcumin nanoemulsions inhibit oral squamous cell carcinoma cell proliferation by PI3K/Akt/mTOR suppression and miR-199a upregulation: A preliminary study. Oral. Dis. 2022, 29, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, Z.; Zhu, L.; Li, H.; Wu, L. Chemopreventive efficacy of salvianolic acid B phospholipid complex loaded nanoparticles against experimental oral carcinogenesis: Implication of sustained drug release. Ann. Transl. Med. 2022, 10, 244. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, J.; Kong, L.; Yu, Y.; Hu, Q.; Yang, T.; Wang, Y.; Tu, K.; Qiao, Q.; Qin, X.; et al. Immunogenic Hybrid Nanovesicles of Liposomes and Tumor-Derived Nanovesicles for Cancer Immunochemotherapy. ACS Nano 2021, 15, 3123–3138. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Insights into Lipid-Based Delivery Nanosystems of Protein-Tyrosine Kinase Inhibitors for Cancer Therapy. Pharmaceutics 2022, 14, 2706. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, X.; Chen, X. Polymer-Modified Liposomes for Drug Delivery: From Fundamentals to Applications. Pharmaceutics 2022, 14, 778. [Google Scholar] [CrossRef]
- Khodaverdi, H.; Zeini, M.S.; Moghaddam, M.M.; Vazifedust, S.; Akbariqomi, M.; Tebyaniyan, H. Lipid-based Nanoparticles for the Targeted Delivery of Anticancer Drugs: A Review. Curr. Drug Deliv. 2022, 19, 1012–1033. [Google Scholar] [PubMed]
- Jin, B.Z.; Dong, X.Q.; Xu, X.; Zhang, F.H. Development and in vitro evaluation of mucoadhesive patches of methotrexate for targeted delivery in oral cancer. Oncol. Lett. 2018, 15, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Salah, E.; Abouelfetouh, M.M.; Pan, Y.; Chen, D.; Xie, S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf. B Biointerfaces 2020, 196, 111305. [Google Scholar] [CrossRef] [PubMed]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Poonia, N.; Kharb, R.; Lather, V.; Pandita, D. Nanostructured lipid carriers: Versatile oral delivery vehicle. Future Sci. OA 2016, 2, FSO135. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, J.; Amin, S. Nanostructured Lipid Carriers: A Novel Platform for Chemotherapeutics. Curr. Drug Deliv. 2016, 13, 4–26. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef]
- Tomita, R.; Sasabe, E.; Tomomura, A.; Yamamoto, T. Macrophage-derived exosomes attenuate the susceptibility of oral squamous cell carcinoma cells to chemotherapeutic drugs through the AKT/GSK-3beta pathway. Oncol. Rep. 2020, 44, 1905–1916. [Google Scholar]
- Debnath, K.; Heras, K.L.; Rivera, A.; Lenzini, S.; Shin, J.W. Extracellular vesicle-matrix interactions. Nat. Rev. Mater. 2023, 8, 390–402. [Google Scholar] [CrossRef]
- Baig, M.S.; Roy, A.; Rajpoot, S.; Liu, D.; Savai, R.; Banerjee, S.; Kawada, M.; Faisal, S.M.; Saluja, R.; Saqib, U.; et al. Tumor-derived exosomes in the regulation of macrophage polarization. Inflamm. Res. 2020, 69, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Vats, R.; Yadav, P.; Bhardwaj, R. Exosomics in oral cancer diagnosis, prognosis, and therapeutics—An emergent and imperative non-invasive natural nanoparticle-based approach. Crit. Rev. Oncol. Hematol. 2022, 178, 103799. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; You, H.H.; Zhou, S.H. The value of exosomes in patients with laryngeal or hypopharyngeal cancer. Scienceasia 2021, 47, 57–63. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Liu, Y.; Xu, W.; Zhu, X. Exosomal Non-coding RNAs-Mediated Crosstalk in the Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 646864. [Google Scholar] [CrossRef]
- Wei, X.; Liu, S.; Cao, Y.; Wang, Z.; Chen, S. Polymers in Engineering Extracellular Vesicle Mimetics: Current Status and Prospective. Pharmaceutics 2023, 15, 1496. [Google Scholar] [CrossRef]
- Elkateb, H.; Cauldbeck, H.; Niezabitowska, E.; Hogarth, C.; Arnold, K.; Rannard, S.; McDonald, T.O. High drug loading solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsions for the dual drug delivery of the HIV drugs darunavir and ritonavir. JCIS Open 2023, 11, 100087. [Google Scholar] [CrossRef]
- Fricker, G.; Kromp, T.; Wendel, A.; Blume, A.; Zirkel, J.; Rebmann, H.; Setzer, C.; Quinkert, R.O.; Martin, F.; Muller-Goymann, C. Phospholipids and lipid-based formulations in oral drug delivery. Pharm. Res. 2010, 27, 1469–1486. [Google Scholar] [CrossRef]
- Khan, J.; Alexander, A.; Ajazuddin; Saraf, S.; Saraf, S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Control Release 2013, 168, 50–60. [Google Scholar] [CrossRef]
- Gusti-Ngurah-Putu, E.P.; Huang, L.; Hsu, Y.C. Effective Combined Photodynamic Therapy with Lipid Platinum Chloride Nanoparticles Therapies of Oral Squamous Carcinoma Tumor Inhibition. J. Clin. Med. 2019, 8, 2112. [Google Scholar] [CrossRef] [PubMed]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; Abu, E.I.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Arana, L.; Bayon-Cordero, L.; Sarasola, L.I.; Berasategi, M.; Ruiz, S.; Alkorta, I. Solid Lipid Nanoparticles Surface Modification Modulates Cell Internalization and Improves Chemotoxic Treatment in an Oral Carcinoma Cell Line. Nanomaterials 2019, 9, 464. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Sahu, B.P.; Haloi, J.; Laloo, D.; Barooah, P.; Keppen, C.; Deka, M.; Medhi, S. Combinatorial therapeutic approach for treatment of oral squamous cell carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Shete, M.B.; Deshpande, A.S.; Shende, P. Enhancement of in-vitro anti-oral cancer activities of silymarin using dispersion of nanostructured lipid carrier in mucoadhesive in-situ gel. Int. J. Pharm. 2023, 636, 122860. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, V.S.; Gawali, B.; Saha, P.; Naidu, V.; Murty, U.S.; Banerjee, S. Quercetin and piperine enriched nanostructured lipid carriers (NLCs) to improve apoptosis in oral squamous cellular carcinoma (FaDu cells) with improved biodistribution profile. Eur. J. Pharmacol. 2021, 909, 174400. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, A.A.; Gondaliya, P.; Mali, M.; Pawar, A.; Bhat, P.; Khairnar, A.; Arya, N.; Kalia, K. MiR-155 Inhibitor-Laden Exosomes Reverse Resistance to Cisplatin in a 3D Tumor Spheroid and Xenograft Model of Oral Cancer. Mol. Pharm. 2021, 18, 3010–3025. [Google Scholar] [CrossRef]
- Xie, C.; Du, L.Y.; Guo, F.; Li, X.; Cheng, B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol. Cell Biochem. 2019, 458, 11–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, Q.; Yin, H.; Xia, C.; Pu, Y.; He, Z.; Hu, Q.; Wang, J.; Wang, Y. Milk-exosome based pH/light sensitive drug system to enhance anticancer activity against oral squamous cell carcinoma. RSC Adv. 2020, 10, 28314–28323. [Google Scholar] [CrossRef]
- Khamaikawin, W.; Locharoenrat, K. Evaluation of a docetaxel-cisplatin-fluorouracil-Au complex in human oral carcinoma cell line. Artif. Cells Nanomed. Biotechnol. 2023, 51, 148–157. [Google Scholar] [CrossRef]
- Essawy, M.M.; El-Sheikh, S.M.; Raslan, H.S.; Ramadan, H.S.; Kang, B.; Talaat, I.M.; Afifi, M.M. Function of gold nanoparticles in oral cancer beyond drug delivery: Implications in cell apoptosis. Oral. Dis. 2021, 27, 251–265. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Liu, S.; Guo, B.; Xu, S.; He, Y.; Liu, L. MF-094 nanodelivery inhibits oral squamous cell carcinoma by targeting USP30. Cell Mol. Biol. Lett. 2022, 27, 107. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, U.; Tseng, C.L.; Wu, P.H.; Liao, M.Y.; Wang, H.Y.; Wu, K.C.; Chung, R.J. Theranostic doxorubicin encapsulated FeAu alloy@metal-organic framework nanostructures enable magnetic hyperthermia and medical imaging in oral carcinoma. Nanomedicine 2023, 48, 102652. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Ghahramani, Y.; Azhdari, R.; Yousefi, K.; Gholami, A.; Fallahi, N.F.; Vijayakameswara, R.N.; Omidifar, N.; Chiang, W.H. Antiproliferative and Apoptotic Effects of Graphene Oxide @AlFu MOF Based Saponin Natural Product on OSCC Line. Pharmaceuticals 2022, 15, 1137. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhong, Y.; Yang, L.; Jiang, Y.; Ren, F. A multifunctional MOF-based nanohybrid as injectable implant platform for drug synergistic oral cancer therapy. Chem. Eng. J. 2020, 390, 124446. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Z.; Xu, J. Effects of Biofilm Nano-Composite Drugs OMVs-MSN-5-FU on Cervical Lymph Node Metastases From Oral Squamous Cell Carcinoma. Front. Oncol. 2022, 12, 881910. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.L.; Li, Y.; Zhao, L.M.; Su, L.W.; Ding, G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic acid-based mesoporous silica nanoparticles for oral cancers treatment. Colloids Surf. B Biointerfaces 2019, 173, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, X.; Zhang, K.; Sun, B.; Wang, L.; Meng, L.; Liu, Q.; Zheng, C.; Yang, B.; Sun, H. Codelivery of doxorubicin and MDR1-siRNA by mesoporous silica nanoparticles polymerpolyethylenimine to improve oral squamous carcinoma treatment. Int. J. Nanomed. 2018, 13, 187–198. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Q.; Chen, J.; Wang, Z.; Xin, H.; Zhang, D. Efficient Delivery of Therapeutic siRNA by Fe3O4 Magnetic Nanoparticles into Oral Cancer Cells. Pharm. 2019, 11, 615. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Liang, S.Y.; Zhao, T.C.; Chen, X.Z.; Cao, X.K.; Qi, M.; Huang, Y.Y.; Ju, W.T.; Yang, M.; Zhu, D.W.; et al. Overcoming chemotherapy resistance using pH-sensitive hollow MnO2 nanoshells that target the hypoxic tumor microenvironment of metastasized oral squamous cell carcinoma. J. Nanobiotechnol. 2021, 19, 157. [Google Scholar] [CrossRef]
- Mehnath, S.; Arjama, M.; Rajan, M.; Annamalai, G.; Jeyaraj, M. Co-encapsulation of dual drug loaded in MLNPs: Implication on sustained drug release and effectively inducing apoptosis in oral carcinoma cells. Biomed. Pharmacother. 2018, 104, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yin, X.; Cai, Y.; Xu, W.; Song, C.; Wang, Y.; Zhang, J.; Kang, A.; Wang, Z.; Han, W. Antitumor effect of a Pt-loaded nanocomposite based on graphene quantum dots combats hypoxia-induced chemoresistance of oral squamous cell carcinoma. Int. J. Nanomed. 2018, 13, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Kutsuna, T.; Kurohara, K.; Shimizu, K.; Tomeoku, A.; Arai, N. Evaluation of a New Hydroxyapatite Nanoparticle as a Drug Delivery System to Oral Squamous Cell Carcinoma Cells. Anticancer. Res. 2018, 38, 6715–6720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.J. Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications. Chem. Rev. 2019, 119, 1666–1762. [Google Scholar] [CrossRef]
- Tan, Q.; Zhao, S.; Xu, T.; Wang, Q.; Zhang, M.; Yan, L.; Chen, X.; Lan, M. Inorganic nano-drug delivery systems for crossing the blood–brain barrier: Advances and challenges. Coord. Chem. Rev. 2023, 494, 215344. [Google Scholar] [CrossRef]
- Mohan, B.; Dhiman, D.; Virender; Mehak; Priyanka; Sun, Q.; Jan, M.; Singh, G.; Raghav, N. Metal-organic frameworks (MOFs) structural properties and electrochemical detection capability for cancer biomarkers. Microchem. J. 2023, 193, 108956. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ramasamy, T.; Kim, S.Y.; Kim, J.; Ku, S.K.; Youn, Y.S.; Kim, J.R.; Jeong, J.H.; Choi, H.G.; Yong, C.S.; et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic co-delivery of axitinib and celastrol in multi-targeted cancer therapy. Acta Biomater. 2016, 39, 94–105. [Google Scholar] [CrossRef]
- Choi, J.Y.; Gupta, B.; Ramasamy, T.; Jeong, J.H.; Jin, S.G.; Choi, H.G.; Yong, C.S.; Kim, J.O. PEGylated polyaminoacid-capped mesoporous silica nanoparticles for mitochondria-targeted delivery of celastrol in solid tumors. Colloids Surf. B Biointerfaces 2018, 165, 56–66. [Google Scholar] [CrossRef]
- Hembram, K.C.; Dash, S.R.; Das, B.; Sethy, C.; Chatterjee, S.; Bindhani, B.K.; Kundu, C.N. Quinacrine Based Gold Hybrid Nanoparticles Caused Apoptosis through Modulating Replication Fork in Oral Cancer Stem Cells. Mol. Pharm. 2020, 17, 2463–2472. [Google Scholar] [CrossRef]
- Dai, H.; Yan, H.; Dong, F.; Zhang, L.; Du, N.; Sun, L.; Li, N.; Yu, G.; Yang, Z.; Wang, Y.; et al. Tumor-targeted biomimetic nanoplatform precisely integrates photodynamic therapy and autophagy inhibition for collaborative treatment of oral cancer. Biomater. Sci. 2022, 10, 1456–1469. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, F.; Huang, N.; Li, J.; Wu, C.; Tan, B.; Liu, Y.; Li, L.; Yang, C.; Shao, D.; et al. Near-infrared light-responsive hybrid hydrogels for the synergistic chemo-photothermal therapy of oral cancer. Nanoscale 2021, 13, 17168–17182. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Liu, S.; Lan, Y.; Qiu, T.; Zhou, M.; Gao, W.; Huang, W.; Ge, L.; Zhang, J. Combined Photothermotherapy and Chemotherapy of Oral Squamous Cell Carcinoma Guided by Multifunctional Nanomaterials Enhanced Photoacoustic Tomography. Int. J. Nanomed. 2021, 16, 7373–7390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Wang, W.J.; Zhang, C.Y.; Ling, Y.Y.; Hong, X.J.; Su, Q.; Li, W.G.; Mao, Z.W.; Cheng, B.; Tan, C.P.; et al. Ru(II)-modified TiO2 nanoparticles for hypoxia-adaptive photo-immunotherapy of oral squamous cell carcinoma. Biomaterials 2022, 289, 121757. [Google Scholar] [CrossRef] [PubMed]
- Fazli, B.; Irani, S.; Bardania, H.; Moosavi, M.S.; Rohani, B. Prophylactic effect of topical (slow-release) and systemic curcumin nano-niosome antioxidant on oral cancer in rat. BMC Complement. Med. Ther. 2022, 22, 109. [Google Scholar] [CrossRef]
- Shi, S.; Kong, N.; Feng, C.; Shajii, A.; Bejgrowicz, C.; Tao, W.; Farokhzad, O.C. Drug Delivery Strategies for the Treatment of Metabolic Diseases. Adv. Healthc. Mater. 2019, 8, e1801655. [Google Scholar] [CrossRef]
- Seleci, D.A.; Seleci, M.; Walter, J.G.; Stahl, F.; Scheper, T. Niosomes as Nanoparticular Drug Carriers: Fundamentals and Recent Applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 15 November 2023).
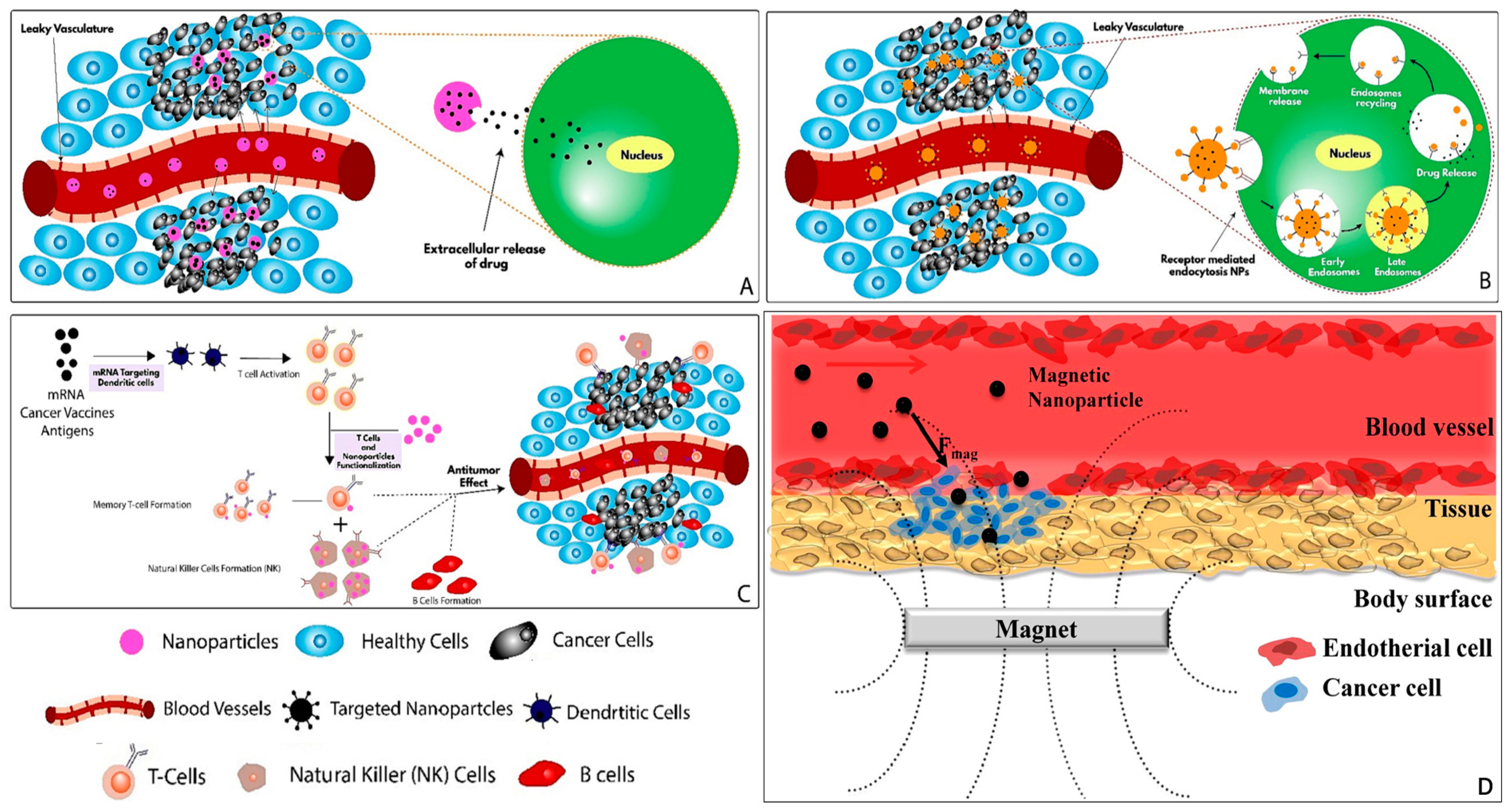
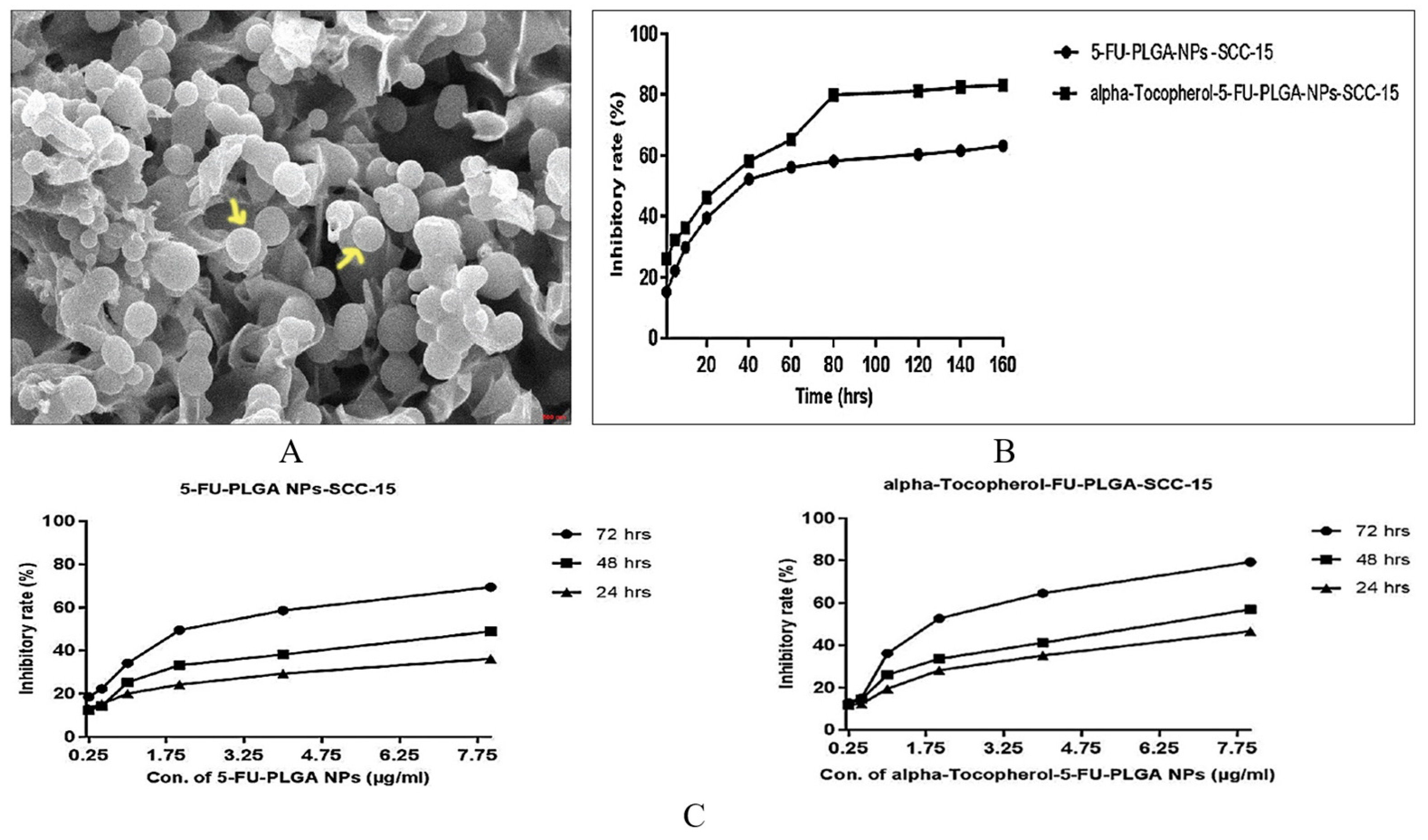
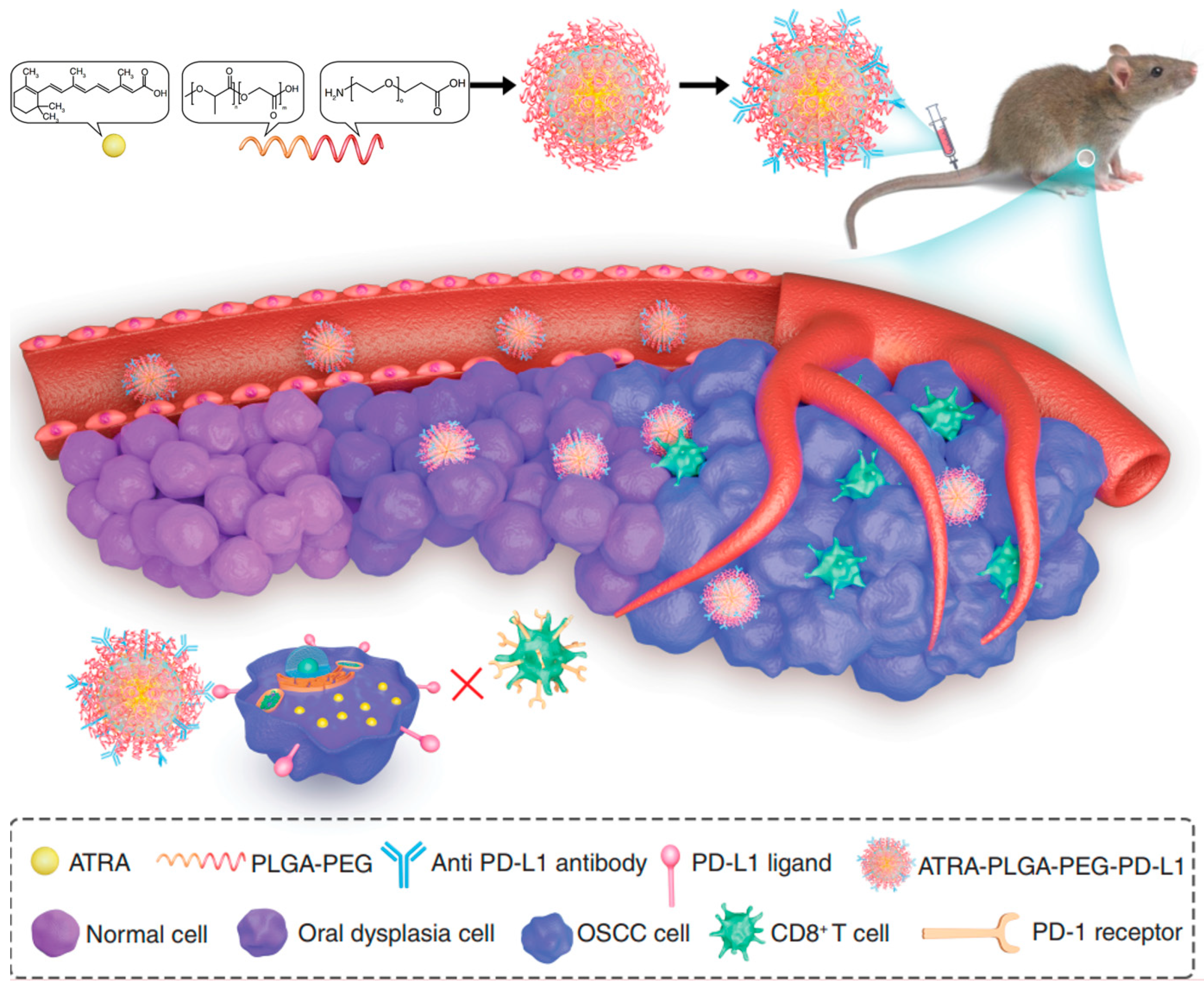
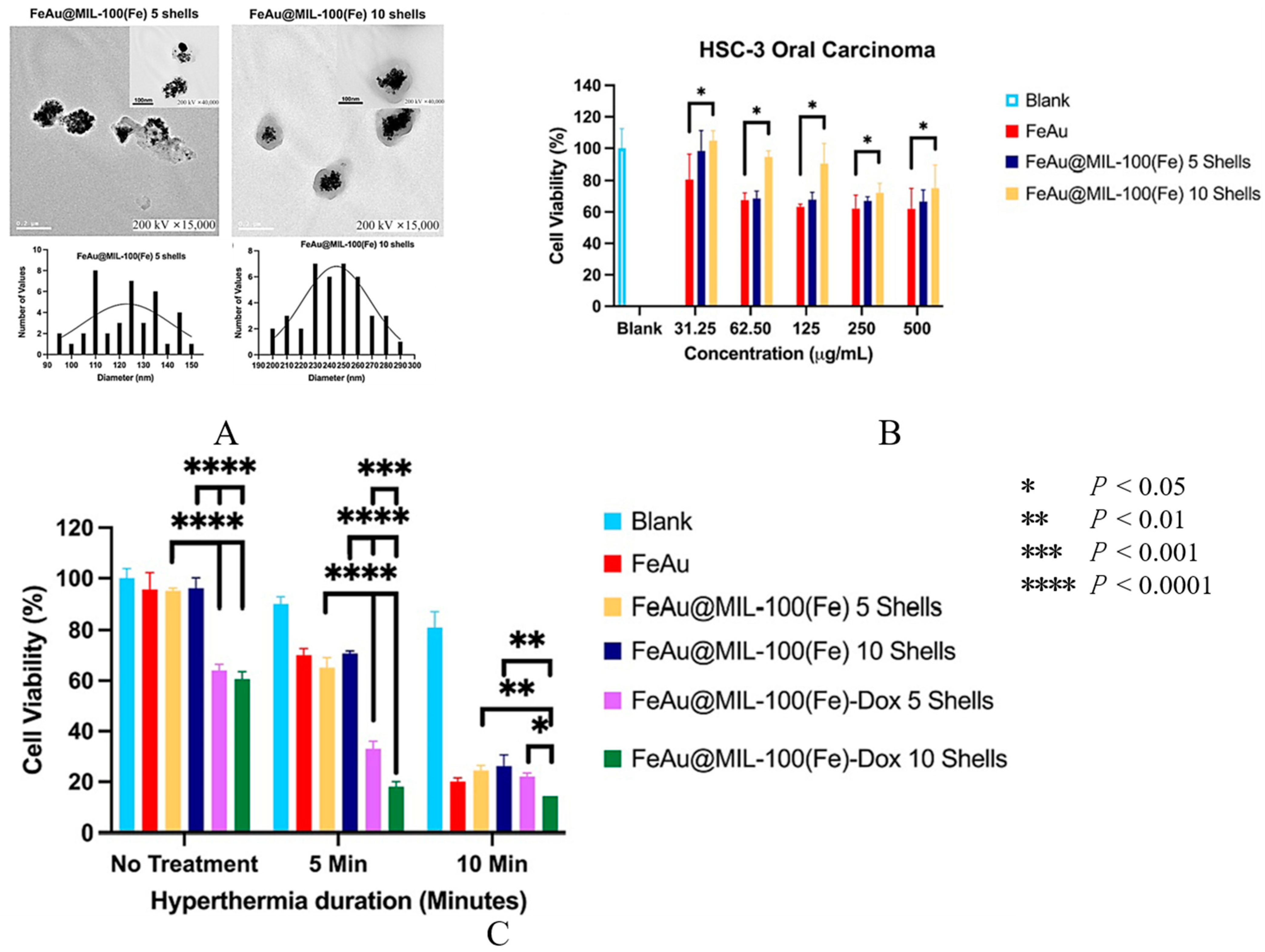

| Ligand | Target Receptor | Ref. |
|---|---|---|
| Folic acid (FA) | FA receptor | [56,57,58,59,60,61,62] |
| Protein corona-modulating Tf2 peptide | Transferrin receptor (TfR) | [63] |
| HN-1, a 12-amino acid peptide | HN-1 receptor | [64,65] |
| Anti Programmed death-ligand 1 (PD-L1) antibody | PD-L1 | [66,67] |
| α-tocopherol | α-tocopherol receptor | [68] |
| Fucoidan | Scavenger-A receptors (SR-A), L-selectin, Toll-like receptors, and PD-L1 | [69] |
| Antibodies specific for matrix metalloproteinase-1 (MMP-1) | MMP-1 | [70] |
| pH-sensitive H-peptide | Epidermal growth factor receptor (EGFR) | [71] |
| Anti-Her-2 (human epidermal growth factor receptor 2) nanobody | Her-2 | [72] |
| Dental pulp mesenchymal stem cell (DPSC), which expresses the CXCL8 binding receptor, CXCR2 | Chemokine CXCL8 | [73] |
| Chemokine SDF-1 | CXC chemokine receptor 4 (CXCR4) | [74] |
| Shiga Toxin-B | Globotriaosylceramide receptor (GB3) | [75] |
| Fibroblast activation protein (FAP)-targeted peptide chains | FAP | [76] |
| AE105 (H-Asp-Cha-Phe-(d)Ser-(d)Arg-Tyr-Leu-Trp-SerOH) peptide | Urokinase plasminogen activator receptor (uPAR) | [77] |
| Nanocarrier | Active Agent | Assembly Method | Mechanism | Advantages | Ref. |
|---|---|---|---|---|---|
| RNA Micelles | Anti-miRNA | The phi29 packaging RNA three-way junction(pRNA-3WJ) was used as a scaffold to construct micelles. An oligo with 8 nt locked nucleic acid (LNA) complementary to the seed region of microRNA21(miR21) was included in the micelles as an interference molecule for cancer inhibition | Internalization of anti-miRNA inhibited the function of oncogenic miR21, enhanced the expression of the pro-apoptotic factor, and induced cell apoptosis | The RNA micelles carrying anti-miR21 showed strong binding and internalization to cancer cells, inhibited the function of oncogenic miR21, enhanced the expression of the pro-apoptotic factors, and induced cell apoptosis. Animal experiments revealed effective tumor targeting and inhibition of RNA micelles in xenograft models. | [57] |
| Nano-niosomes | Cur | Niosomes containing Cur were prepared by the thin-film hydration method | Cur counteracted the effects of 4-NQO to cause cancer in the oral tissue through its antioxidant properties | The use of injectable Cur niosomes significantly prevented the development of severe forms of dysplasia. According to the results obtained from the culture medium of KB OC cells, Cur-loaded niosomes were found to be significantly effective in inhibiting the growth and necrosis of cancer cells compared with free Cur. | [194] |
| ClinicalTrials.Gov Identifier | Type of Cancer | Nanocarrier | Active Ingredients | Phase | Recruitment Status |
|---|---|---|---|---|---|
| NCT05456022 | Tongue Squamous Cell Carcinoma Cell Line | PLGA-PEG NPs | QRC | Phase Ⅱ | Not yet recruiting |
| NCT01847326 | Squamous Cell Carcinoma of the Hypopharynx; Squamous Cell Carcinoma of the Lip and Oral Cavity | HSA NPs | PTX | Phase I | Active, not recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wu, Y.; Du, H.; Li, Z.; Bai, X.; Wu, Y.; Li, H.; Zhou, M.; Cao, Y.; Chen, X. Nano-Drug Delivery Systems in Oral Cancer Therapy: Recent Developments and Prospective. Pharmaceutics 2024, 16, 7. https://doi.org/10.3390/pharmaceutics16010007
Zhang Y, Wu Y, Du H, Li Z, Bai X, Wu Y, Li H, Zhou M, Cao Y, Chen X. Nano-Drug Delivery Systems in Oral Cancer Therapy: Recent Developments and Prospective. Pharmaceutics. 2024; 16(1):7. https://doi.org/10.3390/pharmaceutics16010007
Chicago/Turabian StyleZhang, Yun, Yongjia Wu, Hongjiang Du, Zhiyong Li, Xiaofeng Bai, Yange Wu, Huimin Li, Mengqi Zhou, Yifeng Cao, and Xuepeng Chen. 2024. "Nano-Drug Delivery Systems in Oral Cancer Therapy: Recent Developments and Prospective" Pharmaceutics 16, no. 1: 7. https://doi.org/10.3390/pharmaceutics16010007
APA StyleZhang, Y., Wu, Y., Du, H., Li, Z., Bai, X., Wu, Y., Li, H., Zhou, M., Cao, Y., & Chen, X. (2024). Nano-Drug Delivery Systems in Oral Cancer Therapy: Recent Developments and Prospective. Pharmaceutics, 16(1), 7. https://doi.org/10.3390/pharmaceutics16010007