Sensitizing the Efficiency of ICIs by Neoantigen mRNA Vaccines for HCC Treatment
Abstract
:1. Current Role of Neoantigen mRNA Vaccine in HCC Treatment
2. Revelation on HCC Treatment Given by KEYNOTE-942 Trial
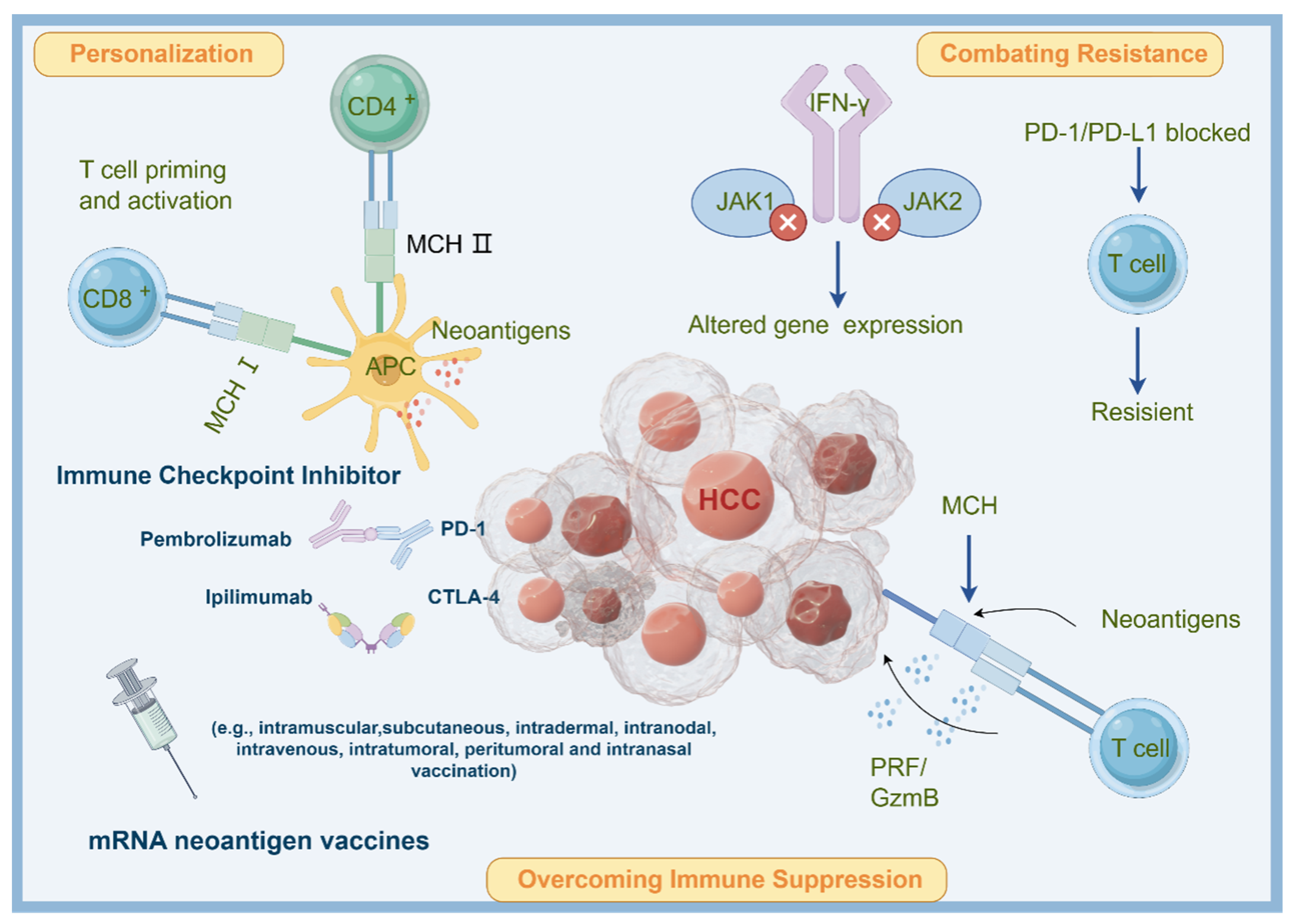
3. Conception of Combing Neoantigen mRNA Vaccine and ICIs for Treating HCC
3.1. Overcoming Immune Suppression
3.2. Personalization
3.3. Combating Resistance
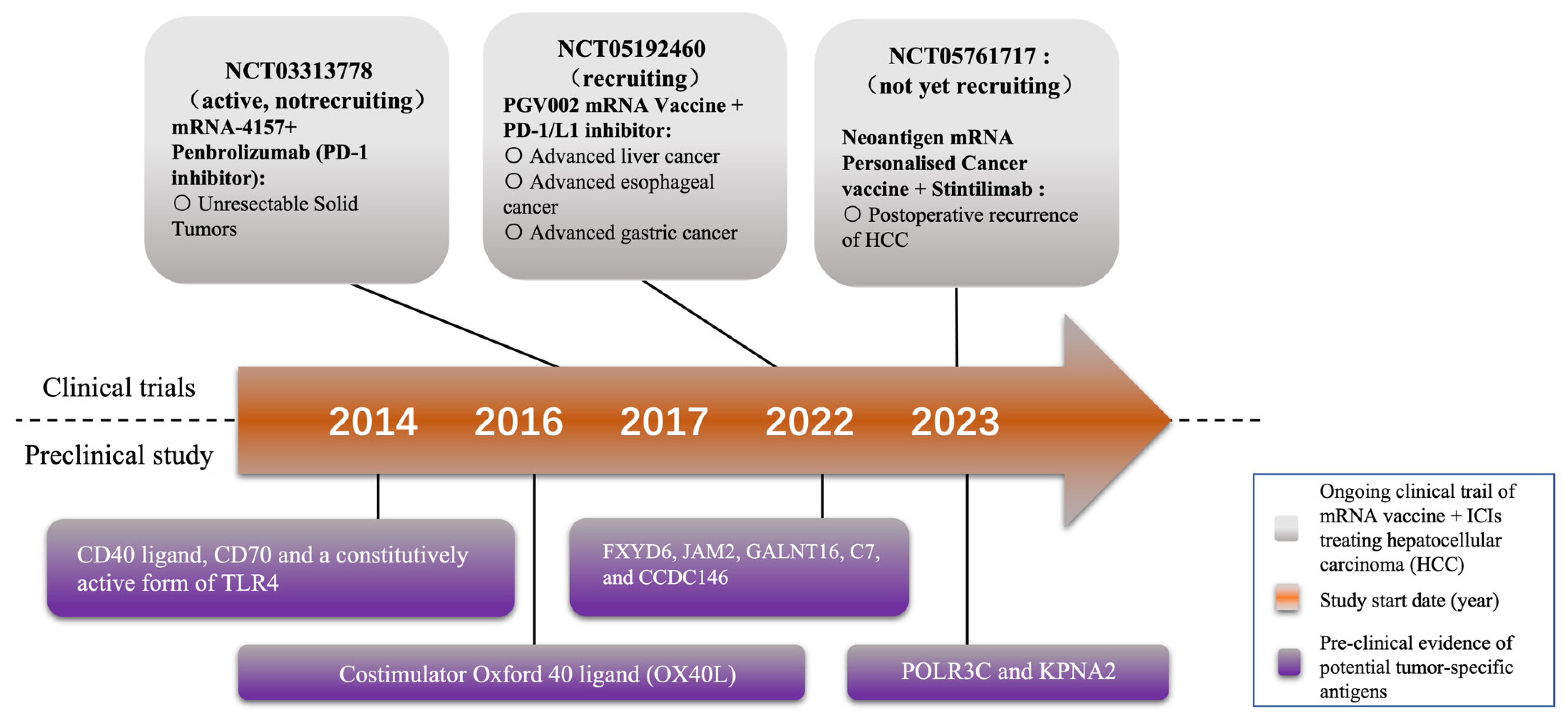
4. Current Research Stage of Applying Neoantigen mRNA Vaccine in HCC Treatment
4.1. Clinical Evidence of mRNA Vaccines Combined with ICIs in HCC Treatment
4.2. Potential Targets for Novel mRNA Vaccines
4.3. Novel Delivery for mRNA Vaccines
4.4. mRNA Vaccine for HCC Preventive Therapies
4.5. Potential Adverse Drug Reactions of mRNA Vaccines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sterner, E. Analyses of the 2023 Nobel Prize in Physiology or Medicine: Nucleoside Base Modifications and Effective mRNA Vaccines. Sci. Technol. Libr. 2023, 17, 709–719. [Google Scholar] [CrossRef]
- Offord, C.; Cohen, J. Award honors pair for mRNA work key to COVID-19 vaccines. Science 2023, 382, 22. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Robbins, P.F.; Tran, E.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Ivey, G.; Li, Y.F.; El-Gamil, M.; Lalani, A. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discov. 2019, 9, 1022–1035. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Esprit, A.; de Mey, W.; Bahadur Shahi, R.; Thielemans, K.; Franceschini, L.; Breckpot, K. Neo-antigen mRNA vaccines. Vaccines 2020, 8, 776. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Akbari, M.; Ghanaatian, M.; Roozbahani moghaddam, P.; Adelian, S.; Borjian Boroujeni, M.; Yazdani, E.; Ahmadi, A.; Hamblin, M.R. Development of neoantigens: From identification in cancer cells to application in cancer vaccines. Expert. Rev. Vaccines 2022, 21, 941–955. [Google Scholar] [CrossRef]
- Yang, X.; Chen, B.; Wang, Y.; Wang, Y.; Long, J.; Zhang, N.; Xue, J.; Xun, Z.; Zhang, L.; Cheng, J. Real-world efficacy and prognostic factors of lenvatinib plus PD-1 inhibitors in 378 unresectable hepatocellular carcinoma patients. Hepatol. Int. 2023, 17, 709–719. [Google Scholar] [CrossRef]
- Bidram, M.; Zhao, Y.; Shebardina, N.G.; Baldin, A.V.; Bazhin, A.V.; Ganjalikhany, M.R.; Zamyatnin Jr, A.A.; Ganjalikhani-Hakemi, M. mRNA-based cancer vaccines: A therapeutic strategy for the treatment of melanoma patients. Vaccines 2021, 9, 1060. [Google Scholar] [CrossRef]
- Yan, T.; Zhu, L.; Chen, J. Current advances and challenges in CAR T-Cell therapy for solid tumors: Tumor-associated antigens and the tumor microenvironment. Exp. Hematol. Oncol. 2023, 12, 14. [Google Scholar] [CrossRef]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles horizontal line from Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Bafaloukos, D.; Gazouli, I.; Koutserimpas, C.; Samonis, G. Evolution and Progress of mRNA Vaccines in the Treatment of Melanoma: Future Prospects. Vaccines 2023, 11, 636. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Kluger, H.; Olino, K. Educational Review: Clinical Application of Immune Checkpoint Blockade for the Treatment of Melanoma. Ann. Surg. Oncol. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Yang, C.W.; Chung, C.H.; Ho, C.L.; Chen, W.L.; Chien, W.C. The association between metabolic risk factors, nonalcoholic fatty liver disease, and the incidence of liver cancer: A nationwide population-based cohort study. Hepatol. Int. 2022, 16, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Scientific rationale for combination immunotherapy of hepatocellular carcinoma with anti-PD-1/PD-L1 and anti-CTLA-4 antibodies. Liver Cancer 2019, 8, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Li, J.; Hony, J.; Xiao, Z.; Wang, J.; Yao, M.; Liang, S.; Lu, L. CAXII inhibitors: Potential sensitizers for immune checkpoint inhibitors in HCC treatment. Front. Immunol. 2023, 14, 1052657. [Google Scholar] [CrossRef] [PubMed]
- Cafri, G.; Gartner, J.J.; Zaks, T.; Hopson, K.; Levin, N.; Paria, B.C.; Parkhurst, M.R.; Yossef, R.; Lowery, F.J.; Jafferji, M.S.; et al. mRNA vaccine-induced neoantigen-specific T cell immunity in patients with gastrointestinal cancer. J. Clin. Investig. 2020, 130, 5976–5988. [Google Scholar] [CrossRef]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.; et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef]
- Ruff, S.M.; Pawlik, T.M. Editorial on immune checkpoint inhibitors in the treatment of hepatocellular carcinoma. Immunotherapy 2023, 15, 1323–1326. [Google Scholar] [CrossRef]
- Cohen, R.B.; Twardowski, P.; Johnson, M.L.; Gillison, M.L.; Stein, M.N.; Vaishampayan, U.N.; Mcneil, L.; Shainheit, M.; Deoliveira, D.; Jain, M. GEN-009, a neoantigen vaccine containing ATLAS selected neoantigens, to generate broad sustained immunity against immunogenic tumor mutations and avoid inhibitory peptides. J. Clin. Oncol. 2020, 38, 3107. [Google Scholar] [CrossRef]
- Nogueira, C.; Kaufmann, J.K.; Lam, H.; Flechtner, J.B. Improving Cancer Immunotherapies through Empirical Neoantigen Selection. Trends Cancer 2018, 4, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Willimsky, G.; Schmidt, K.; Loddenkemper, C.; Gellermann, J.; Blankenstein, T. Virus-induced hepatocellular carcinomas cause antigen-specific local tolerance. J. Clin. Investig. 2013, 123, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 2014, 59, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, L.; Guan, A.; Yin, H.; Liu, M.; Mao, X.; Xu, H.; Zhao, H.; Lu, X.; Sang, X.; et al. Unique TP53 neoantigen and the immune microenvironment in long-term survivors of Hepatocellular carcinoma. Cancer Immunol. Immunother. 2021, 70, 667–677. [Google Scholar] [CrossRef]
- Stifter, K.; Dekhtiarenko, I.; Krieger, J.; Tissot, A.C.; Seufferlein, T.; Wagner, M.; Schirmbeck, R. A tumor-specific neoepitope expressed in homologous/self or heterologous/viral antigens induced comparable effector CD8+ T-cell responses by DNA vaccination. Vaccine 2020, 38, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Su, X.; Qiu, L.; Li, Z.; Li, X.; Dong, X.; Wei, F.; Zhou, Y.; Luo, L.; Chen, G.; et al. Personalized neoantigen vaccine prevents postoperative recurrence in hepatocellular carcinoma patients with vascular invasion. Mol. Cancer 2021, 20, 164. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Lu, L.; Ma, W.; Johnson, C.H.; Khan, S.A.; Irwin, M.L.; Pusztai, L. In silico designed mRNA vaccines targeting CA-125 neoantigen in breast and ovarian cancer. Vaccine 2023, 41, 2073–2083. [Google Scholar] [CrossRef]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Dolina, J.S.; Lee, J.; Brightman, S.E.; McArdle, S.; Hall, S.M.; Thota, R.R.; Zavala, K.S.; Lanka, M.; Ramamoorthy Premlal, A.L.; Greenbaum, J.A.; et al. Linked CD4+/CD8+ T cell neoantigen vaccination overcomes immune checkpoint blockade resistance and enables tumor regression. J. Clin. Investig. 2023, 133, e164258. [Google Scholar] [CrossRef]
- Raimondo, T.M.; Reed, K.; Shi, D.; Langer, R.; Anderson, D.G. Delivering the next generation of cancer immunotherapies with RNA. Cell 2023, 186, 1535–1540. [Google Scholar] [CrossRef]
- Yang, S. mRNA Vaccines in Cancer Immunotherapy: Clinical Benefits, Limitations, and Future. In Proceedings of the International Conference on Biological Engineering and Medical Science (ICBioMed2022), Oxford, UK, 7–13 November 2022; SPIE: St. Bellingham, WA, USA, 2023; Volume 12611. [Google Scholar]
- Wang, Y.-S.; Kumari, M.; Chen, G.-H.; Hong, M.-H.; Yuan, J.; Tsai, J.-L.; Wu, H.-C. mRNA-based vaccines and therapeutics: An in-depth survey of current and upcoming clinical applications. J. Biomed. Sci. 2023, 30, 84. [Google Scholar] [CrossRef]
- Han, R. The potential of mRNA vaccine in HCC treatment. Int. J. Immunother. Cancer Res. 2023, 9, 008–012. [Google Scholar]
- Niemi, J.V.L.; Sokolov, A.V.; Schiöth, H.B. Neoantigen Vaccines; Clinical Trials, Classes, Indications, Adjuvants and Combinatorial Treatments. Cancers 2022, 14, 5163. [Google Scholar] [CrossRef]
- Donhauser, L.V.; Veloso de Oliveira, J.; Schick, C.; Manlik, W.; Styblova, S.; Lutzenberger, S.; Aigner, M.; Philipp, P.; Robert, S.; Gandorfer, B.; et al. Responses of patients with cancer to mRNA vaccines depend on the time interval between vaccination and last treatment. J. Immunother. Cancer 2023, 11, e007387. [Google Scholar] [CrossRef]
- Salehi-Sangani, G.; Mohebali, M.; Jajarmi, V.; Khamesipour, A.; Bandehpour, M.; Mahmoudi, M.; Zahedi-Zavaram, H. Immunization against Leishmania major infection in BALB/c mice using a subunit-based DNA vaccine derived from TSA, LmSTI1, KMP11, and LACK predominant antigens. Iran. J. Basic. Med. Sci. 2019, 22, 1493–1501. [Google Scholar] [CrossRef]
- Ghaffarifar, F.; Jorjani, O.; Sharifi, Z.; Dalimi, A.; Hassan, Z.M.; Tabatabaie, F.; Khoshzaban, F.; Hezarjaribi, H.Z. Enhancement of immune response induced by DNA vaccine cocktail expressing complete LACK and TSA genes against Leishmania major. APMIS 2013, 121, 290–298. [Google Scholar] [CrossRef]
- Fu, J.; Chen, F.; Lin, Y.; Gao, J.; Chen, A.; Yang, J. Discovery and characterization of tumor antigens in hepatocellular carcinoma for mRNA vaccine development. J. Cancer Res. Clin. Oncol. 2023, 149, 4047–4061. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Zhao, B.; Zheng, Y.; Zhuang, Q.; Liao, N.; Wang, P.; Cai, Z.; Zhang, D.; Zeng, Y.; et al. Remodeling Tumor-Associated Neutrophils to Enhance Dendritic Cell-Based HCC Neoantigen Nano-Vaccine Efficiency. Adv. Sci. 2022, 9, e2105631. [Google Scholar] [CrossRef]
- Dong, L.Q.; Peng, L.H.; Ma, L.J.; Liu, D.B.; Zhang, S.; Luo, S.Z.; Rao, J.H.; Zhu, H.W.; Yang, S.X.; Xi, S.J.; et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J. Hepatol. 2020, 72, 896–908. [Google Scholar] [CrossRef]
- Reparaz, D.; Ruiz, M.; Llopiz, D.; Silva, L.; Vercher, E.; Aparicio, B.; Egea, J.; Tamayo-Uria, I.; Hervas-Stubbs, S.; Garcia-Balduz, J.; et al. Neoantigens as potential vaccines in hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e003978. [Google Scholar] [CrossRef]
- Liu, C.; Shao, J.; Dong, Y.; Xu, Q.; Zou, Z.; Chen, F.; Yan, J.; Liu, J.; Li, S.; Liu, B.; et al. Advanced HCC Patient Benefit from Neoantigen Reactive T Cells Based Immunotherapy: A Case Report. Front. Immunol. 2021, 12, 685126. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, L.; Tian, Y.; Ji, X.; Hu, Q.; Zhou, B.; Zhenyu, D.; Heng, X.; Yang, L. Cholesterol-modified DP7 enhances the effect of individualized cancer immunotherapy based on neoantigens. Biomaterials 2020, 241, 119852. [Google Scholar] [CrossRef]
- Li, Y.F.; Hou, Q.Q.; Zhao, S.; Chen, X.; Tang, M.; Li, L. Identification of tumor-specific neoantigens and immune clusters of hepatocellular carcinoma for mRNA vaccine development. J. Cancer Res. Clin. Oncol. 2023, 149, 623–637. [Google Scholar] [CrossRef]
- Aerts, M.; Benteyn, D.; Van Vlierberghe, H.; Thielemans, K.; Reynaert, H. Current status and perspectives of immune-based therapies for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 253–261. [Google Scholar] [CrossRef]
- Van Lint, S.; Goyvaerts, C.; Maenhout, S.; Goethals, L.; Disy, A.; Benteyn, D.; Pen, J.; Bonehill, A.; Heirman, C.; Breckpot, K.; et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012, 72, 1661–1671. [Google Scholar] [CrossRef]
- Van Lint, S.; Wilgenhof, S.; Heirman, C.; Corthals, J.; Breckpot, K.; Bonehill, A.; Neyns, B.; Thielemans, K. Optimized dendritic cell-based immunotherapy for melanoma: The TriMix-formula. Cancer Immunol. Immunother. 2014, 63, 959–967. [Google Scholar] [CrossRef]
- Alburquerque-Gonzalez, B.; Lopez-Abellan, M.D.; Luengo-Gil, G.; Montoro-Garcia, S.; Conesa-Zamora, P. Design of Personalized Neoantigen RNA Vaccines against Cancer Based on Next-Generation Sequencing Data. Methods Mol. Biol. 2022, 2547, 165–185. [Google Scholar] [CrossRef]
- Huang, P.; Deng, H.; Zhou, Y.; Chen, X. The roles of polymers in mRNA delivery. Matter 2022, 5, 1670–1699. [Google Scholar] [CrossRef]
- Kim, C.H.; Lee, S.G.; Kang, M.J.; Lee, S.; Choi, Y.W. Surface modification of lipid-based nanocarriers for cancer cell-specific drug targeting. J. Pharm. Investig. 2017, 47, 203–227. [Google Scholar] [CrossRef]
- Kai, M.P. Development and Applications of a Cisplatin-Containing Hydrogel Nanoparticle; North Carolina State University: Raleigh, NC, USA, 2014. [Google Scholar]
- Kim, P.S.; Lee, P.P.; Levy, D. Modeling regulation mechanisms in the immune system. J. Theor. Biol. 2007, 246, 33–69. [Google Scholar] [CrossRef] [PubMed]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-based nanoparticles for delivery of vaccine adjuvants and antigens: Toward multicomponent vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic nanotechnology toward personalized vaccines. Adv. Mater. 2020, 32, 1901255. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z.; et al. Combining p53 mRNA nanotherapy with immune checkpoint blockade reprograms the immune microenvironment for effective cancer therapy. Nat. Commun. 2022, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liao, R.; Wang, G.; Yang, B.H.; Luo, X.; Varki, N.M.; Qiu, S.J.; Ren, B.; Fu, W.; Feng, G.S. Preventive Inhibition of Liver Tumorigenesis by Systemic Activation of Innate Immune Functions. Cell Rep. 2017, 21, 1870–1882. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G. Worse than the disease? Reviewing some possible unintended consequences of the mRNA vaccines against COVID-19. Int. J. Vaccine Theory Pract. Res. 2021, 2, 38–79. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A.; Lau, G.K.; Zheng, M.-H.; Ji, D.; Abd-Elsalam, S.; Hwang, J.; Qi, X.; Cua, I.H.; Suh, J.I. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol. Int. 2020, 14, 690–700. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: Current evidence and suggested approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Selvaraj, G.; Kaliamurthi, S.; Peslherbe, G.H.; Wei, D.-Q. Are the allergic reactions of COVID-19 vaccines caused by mRNA constructs or nanocarriers? Immunological insights. Interdiscip. Sci. Comput. Life Sci. 2021, 13, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Hervé, C.; Laupèze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Chan, S.L.; Meyer, T.; Reig, M.; El-Khoueiry, A.; Galle, P.R. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 2020, 72, 320–341. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Dagnew, A.F.; Rausch, D.; Herve, C.; Zahaf, T.; Levin, M.J.; Schuind, A.; Group, Z.-S. Efficacy and serious adverse events profile of the adjuvanted recombinant zoster vaccine in adults with pre-existing potential immune-mediated diseases: A pooled post hoc analysis on two parallel randomized trials. Rheumatology 2021, 60, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Kosten, T.; Owens, S.M. Immunotherapy for the treatment of drug abuse. Pharmacol. Ther. 2005, 108, 76–85. [Google Scholar] [CrossRef]
- Montomoli, E.; Piccirella, S.; Khadang, B.; Mennitto, E.; Camerini, R.; De Rosa, A. Current adjuvants and new perspectives in vaccine formulation. Expert Rev. Vaccines 2011, 10, 1053–1061. [Google Scholar] [CrossRef]
- Koornstra, R.H.; Peters, M.; Donofrio, S.; van den Borne, B.; de Jong, F.A. Management of fatigue in patients with cancer—A practical overview. Cancer Treat. Rev. 2014, 40, 791–799. [Google Scholar] [CrossRef]

| Year | NCT Number | Study Status | Phase | Cancer Type | Number of Patients | Ref. |
|---|---|---|---|---|---|---|
| 2022 | NCT05192460 | Recruiting | N/A | Gastric Cancer Esophageal Cancer Liver Cancer | 30 | [34] |
| 2023 | NCT05761717 | Not yet recruiting | N/A | Postoperative Hepatocellular Carcinoma | 67 | [35] |
| 2023 | NCT05738447 | Recruiting | I | Liver Cancer Hepatocellular Carcinoma | 9 | [36] |
| 2018 | NCT03480152 | Terminated (slow accrual) | I/II | Melanoma Colon Cancer Gastrointestinal Cancer Genitourinary Cancer Hepatocellular Cancer | 5 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, R.; Wang, Y.; Lu, L. Sensitizing the Efficiency of ICIs by Neoantigen mRNA Vaccines for HCC Treatment. Pharmaceutics 2024, 16, 59. https://doi.org/10.3390/pharmaceutics16010059
Han R, Wang Y, Lu L. Sensitizing the Efficiency of ICIs by Neoantigen mRNA Vaccines for HCC Treatment. Pharmaceutics. 2024; 16(1):59. https://doi.org/10.3390/pharmaceutics16010059
Chicago/Turabian StyleHan, Rui, Yuqian Wang, and Lingeng Lu. 2024. "Sensitizing the Efficiency of ICIs by Neoantigen mRNA Vaccines for HCC Treatment" Pharmaceutics 16, no. 1: 59. https://doi.org/10.3390/pharmaceutics16010059
APA StyleHan, R., Wang, Y., & Lu, L. (2024). Sensitizing the Efficiency of ICIs by Neoantigen mRNA Vaccines for HCC Treatment. Pharmaceutics, 16(1), 59. https://doi.org/10.3390/pharmaceutics16010059






