Core-Shell Microspheres Prepared Using Coaxial Electrostatic Spray for Local Chemotherapy of Solid Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PTX-CA/PLGA-MS
2.3. Characterization of PTX-CA/PLGA-MS
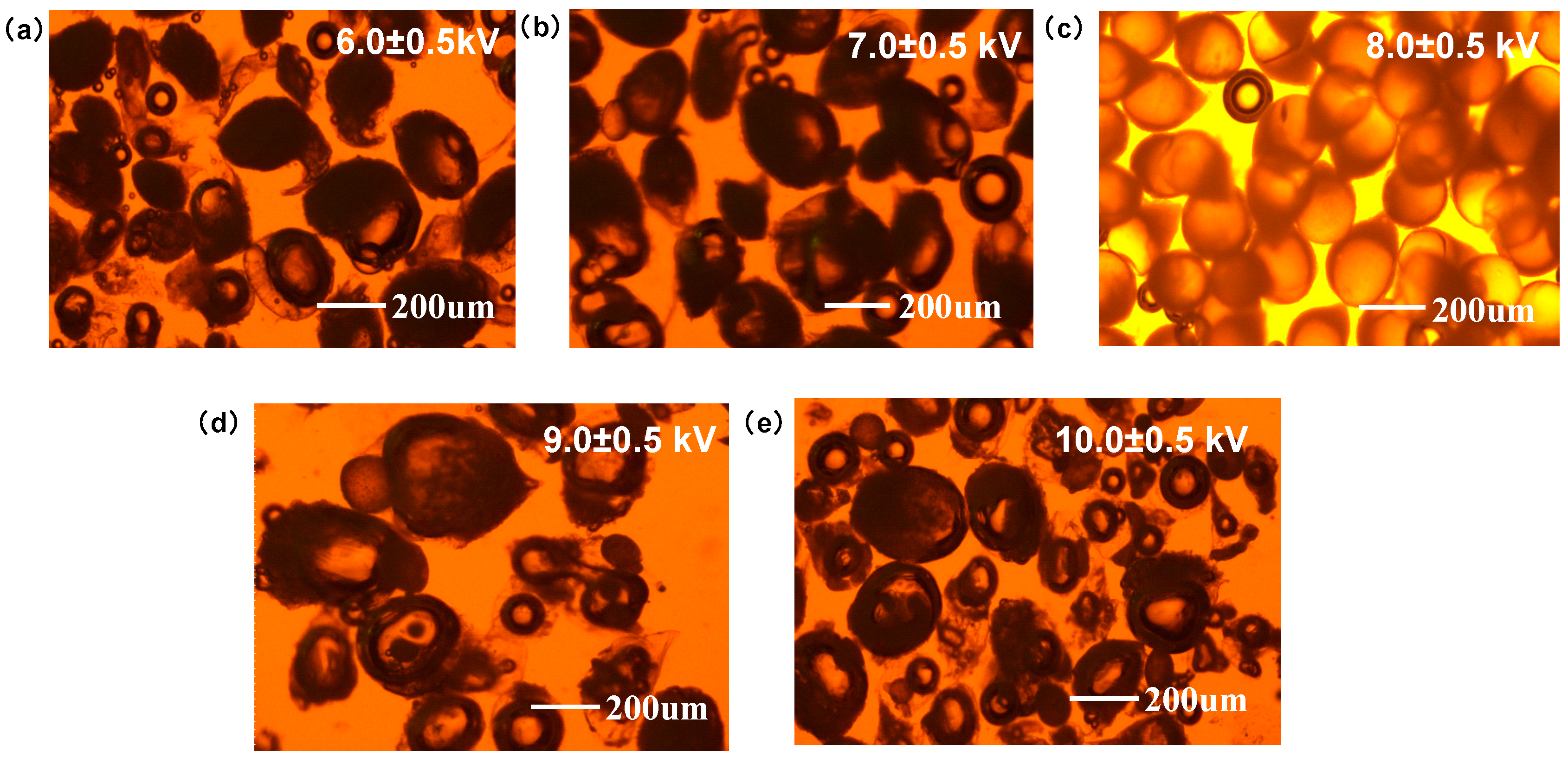
2.3.1. Appearance and Morphology
2.3.2. Particle Size and Structure
2.4. In Vitro Drug Release
2.5. In Vitro Cytotoxicity Evaluation
2.6. In Vivo Anti-Tumor Activity Evaluation
2.7. In Vivo Drug Release
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of PTX-CA/PLGA-MS
3.2. In Vitro Drug Release and Degradation
3.3. In Vitro Cytotoxicity Analysis
3.4. In Vivo Anti-Tumor Activity and Drug Release
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, K.; Han, Y.; Cho, W.C.; Zhu, H. The Rise of HumanStem Cell-derived Natural Killer Cells for Cancer Immunotherapy. Expert Opin. Biol. Ther. 2019, 19, 141–148. [Google Scholar] [CrossRef]
- Lamers, C.H.; Sleijfer, S.; van Steenbergen, S.; van Elzakker, P.; van Krimpen, B.; Groot, C.; Vulto, A.; den Bakker, M.; Oosterwijk, E.; Debets, R.; et al. Treatment of Metastatic Renal Cell Carcinoma with CAIX CAR-engineered T Cells: Clinical Evaluation and Management of On-target Toxicity. Mol. Ther. 2013, 21, 904912. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Yu, Y.; Shang, L.; Guo, J.; Wang, J.; Zhao, Y. Design of Capillary Microfluidics for Spinning Cell-laden Microfibers. Nat. Protoc. 2018, 13, 2557–2579. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Yildirimer, L.; Zhao, H.; Ding, R.; Wang, H.; Cui, W.; Weitz, D. Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Adv. Funct. Mater. 2016, 26, 28092819. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case Report of a Derious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2, 29528. [Google Scholar] [CrossRef]
- Okusaka, T.; Ueno, H.; Ikeda, M.; Morizane, C. Phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. Hepatol. Res. 2011, 41, 542–552. [Google Scholar] [CrossRef]
- Kim, B.K.; Hwang, S.J.; Park, J.B.; Park, H.J. Preparation and characterization of drug-loaded polymethacrylate microspheres by an emulsion solvent evaporation method. J. Microencapsul. 2002, 19, 811–822. [Google Scholar] [CrossRef]
- Kan, P.; Lin, X.Z.; Hsieh, M.F.; Chang, K.Y. Thermogelling emulsions for vascular embolization and sustained release of drugs. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Plackett, D.; Needham, D.; Burt, H.M. PLGA and PHBV microsphere formulations and solid-state characterization: Possible implications for local delivery of fusidic acid for the treatment and prevention of orthopaedic infections. Pharm. Res. 2009, 26, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Doria, C.; Liu, Y. Targeted therapies in the treatment of advanced hepatocellular carcinoma. Clin. Med. Insights Oncol. 2013, 7, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Takai, K.; Moriwaki, H. Strategy and mechanism for the prevention of hepatocellular carcinoma: Phosphorylated retinoid X receptor α is a critical target for hepatocellular carcinoma chemoprevention. Cancer Sci. 2009, 100, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Jia, J.; Cong, X.; Liu, Z.; Li, Q. N-Isopropylacrylamide-modified polyethylenimine-mediated miR-29a delivery to inhibit the proliferation and migration of lung cancer cells. Colloids Surf. B Biointerfaces 2021, 198, 111463. [Google Scholar] [CrossRef] [PubMed]
- Mejias, J.C.; Roy, K. In-vitro and in-vivo characterization of a multi-stage enzymeresponsive nanoparticle-in-microgel pulmonary drug delivery system. J. Control. Release 2019, 316, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Mohyeldin, S.M.; Elgindy, N.A. Rifampicin-carbohydrate spraydried nanocomposite: A futuristic multiparticulate platform for pulmonary delivery. Int. J. Nanomed. 2019, 14, 9089–9112. [Google Scholar] [CrossRef]
- Hittinger, M.; Juntke, J.; Kletting, S.; Schneider-Daum, N.; de Souza Carvalho, C.; Lehr, C.M. Preclinical safety and efficacy models for pulmonary drug delivery of antimicrobials with focus on in vitro models. Adv. Drug Deliv. Rev. 2015, 85, 44–56. [Google Scholar] [CrossRef]
- Wei, D.; Zhongxiong, Z.; Rong, L. Primary study on preparation ofcarboplatina-loaded PLGA microphere for intratumoral injection. Ultrasound Clin. Med. 2003, 5, 1–3. [Google Scholar]
- Pilcer, G.; Wauthoz, N.; Amighi, K. Lactose characteristics and the generation of the aerosol. Adv. Drug Deliv. Rev. 2012, 64, 233–256. [Google Scholar] [CrossRef]
- Wu, D.; Wang, C.; Yang, J.; Wang, H.; Han, H.; Zhang, A.; Yang, Y.; Li, Q. Improving the intracellular drug concentration in lung cancer treatment through the codelivery of doxorubicin and miR-519c mediated by porous PLGA microparticle. Mol. Pharm. 2016, 13, 3925–3933. [Google Scholar] [CrossRef] [PubMed]
- Touzeau, C.; Maciag, P.; Amiot, M.; Moreau, P. Targeting Bcl-2 for the treatment of multiple myeloma. Leukemia 2018, 32, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Shive, M.S. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 2012, 64, 72–82. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhu, R.; Wang, X.; Wang, H.; Xu, Z.; Wang, Y.; Quan, D.; Shen, L. Core-Shell Microspheres Prepared Using Coaxial Electrostatic Spray for Local Chemotherapy of Solid Tumors. Pharmaceutics 2024, 16, 45. https://doi.org/10.3390/pharmaceutics16010045
Zhang X, Zhu R, Wang X, Wang H, Xu Z, Wang Y, Quan D, Shen L. Core-Shell Microspheres Prepared Using Coaxial Electrostatic Spray for Local Chemotherapy of Solid Tumors. Pharmaceutics. 2024; 16(1):45. https://doi.org/10.3390/pharmaceutics16010045
Chicago/Turabian StyleZhang, Xiaowei, Rundong Zhu, Xingzhi Wang, Hao Wang, Zushun Xu, Yongan Wang, Dongqin Quan, and Liao Shen. 2024. "Core-Shell Microspheres Prepared Using Coaxial Electrostatic Spray for Local Chemotherapy of Solid Tumors" Pharmaceutics 16, no. 1: 45. https://doi.org/10.3390/pharmaceutics16010045
APA StyleZhang, X., Zhu, R., Wang, X., Wang, H., Xu, Z., Wang, Y., Quan, D., & Shen, L. (2024). Core-Shell Microspheres Prepared Using Coaxial Electrostatic Spray for Local Chemotherapy of Solid Tumors. Pharmaceutics, 16(1), 45. https://doi.org/10.3390/pharmaceutics16010045





