1. Introduction
Oral liquid formulations are often deemed optimal for pediatric application, a viewpoint also supported by the European Medicines Agency (EMA). This preference primarily stems from their flexibility in dosing and adaptability, allowing drug dosage to be tailored according to the age and weight of each pediatric patient [
1]. Designing a pediatric oral formulation is challenging with respect to several issues, such as drug solubility and the selection of excipients, which greatly influence palatability. The palatability of a pharmaceutical formulation refers to its level of pleasantness or acceptability concerning taste, smell, and the overall sensory experience upon administration to patients. Oral pharmaceutical formulations are often unpalatable. Hence, it is often cited as a major cause of non-adherence, especially for geriatric and pediatric populations, who may have swallowing difficulties. An unpalatable formulation can heavily reduce the young patient’s compliance and, consequently, the effectiveness of the therapy [
2].
Various taste-masking strategies can be employed for this purpose, and it is important to account for children’s preferences. However, it is crucial to note that certain excipients might elicit adverse reactions in children, particularly when treating infants and neonates. The EMA [
1,
3] has issued guideline/reflection papers for the pediatric population, while the FDA (Food and Drug Administration) has created a set of guidance documents centered around pediatric patient-focused drug development. Additionally, taste-masking assessments were listed in the contents of the investigation of pediatric drug product development plan measures specified in the pediatric regulations [
4,
5].
Numerous taste-masking methods exist, yet none universally meet the needs of all drug types, posing a challenge in selecting suitable technology for various Active Pharmaceutical Ingredients (APIs). Taste masking approaches have been primarily categorized based on the mechanism of taste transmission. One approach involves blocking taste transmission pathways, employing methods such as flavoring agents and bitter inhibitors. Generally, the first choice is to use flavoring agents to mask the taste. After that, if the taste is still unacceptable, we should research other methods or a combination of them. The incorporation of flavors serves to mitigate the bitter taste of medicine by competing with the drugs to stimulate taste receptor cells. For example, sweeteners are found to be more effective in masking bitterness compared to acid agents [
6,
7]. Flavors are categorized as a type of excipient that inherently possesses a pleasant taste and odor. These flavoring agents are commonly classified into sweeteners, acidic flavors, and aromatic agents based on their taste. From the pharmaceutical industry’s point of view, adding flavors is a very attractive method because of its inherently enjoyable taste. There are numerous sweeteners in practical application, such as sucrose, lactose, aspartame, sucralose, mannitol, and saccharin sodium. Meanwhile, the acceptable daily intake (ADI) of sweeteners is also worthy of attention, especially for children. Other frequently used taste-masking techniques for oral formulations include lipophilic vehicles, cyclodextrin complexation, ion exchange resin, liposomes, microcapsules, and nanoemulsions [
7,
8].
CARV is a non-selective blocker of α- and β-adrenergic receptors used as an ‘off-label’ treatment for clinically treating cardiovascular diseases such as hypertension and congestive heart failure in the pediatric population. Additionally, EMA has included carvedilol on its list of pediatric and therapeutic requirements for cardiology. However, CARV has only been commercialized in an oral solid dosage form as a tablet; hence, the development of a pediatric-appropriate pharmaceutical formulation for CARV is necessary [
9]. We successfully developed a stable aqueous CARV liquid formulation suitable for pediatrics [
10]. However, optimization of this formulation is the next step, which includes taste assessment and a stability study in order to make CARV formulation more palatable. CARV dissolved in water exhibits an unpleasant bitter taste, which is challenging to mask, making the formulations less palatable. Therefore, palatability was selected as the criterion of choice for a new palatable age-adapted formulation.
CARV tablets are often manipulated prior to use in hospitals with the aim of improving patient compliance and adherence to prepare a CARV liquid suspension [
10]. The most common excipient used to prepare a suspension with triturated CARV tablets is a mixture of Ora-Sweet–Ora-Plus (1:1) or Ora-Blend. Some components of this mixture, such as sorbitol, sucrose, and saccharin, are not recommended in pediatrics [
11,
12]. Ora-sweet gave a sweet citrus-berry flavor to the formulation. Another example is SyrSpend SF PH4 (Fagron), a ready-to-use, all-in-one suspending and sweetening oral liquid vehicle containing cherry flavoring [
13].
Operto et al. [
14] developed two CARV liquid formulations for administration to pediatric. However, to improve the palatability of these formulations, a small volume of the formulation may be diluted in milk prior to administration. Combining a formulation with food or drinks, such as milk, to mask its unpleasant taste may be considered if it is proven that further improvement is not achievable. However, this can influence the pharmacokinetic behavior of the API [
3].
Furthermore, the development of CARV mini tablets could avoid the bitter, unpleasant taste of the API. Khan et al. [
15] developed a CARV orally disintegrating mini tablet (ODMT) appropriate for pediatrics, whose doses were 0.5 mg and 2 mg, compared to 3.125 mg as the lowest strength of CARV-marketed tablets. However, oral solutions remain the preferred choice for pediatrics, especially in neonates or children up to 4 years old, as well as for elderly patients who have swallowing difficulties [
3].
The taste of a pharmaceutical oral liquid formulation can be quantitatively evaluated using a taste sensor as an electronic tongue (e-tongue) or qualitatively by taste panels. The e-tongue functions were akin to human gustatory sensation, capable of detecting tastes by changes in the electric charge density on the sensor’s membrane surface when exposed to taste substances. The electronic tongue provides an output indicating the taste quality and intensity of the tested formulations compared to predetermined references. Some researchers used an e-tongue for the development of a palatable pediatric formulation [
1,
16,
17].
However, human testing using a sensory questionnaire is acknowledged as the best method to assess the taste of a pharmaceutical formulation. Sensory results of taste assessment in human volunteers may be closer to the real, compared to sensory results using an e-tongue [
7]. It is evident that children as a target population are regarded as the most suitable panel for taste assessment of pediatric formulations because children experience different taste sensations than adults [
18]. Nevertheless, authorities recommend conducting palatability assessments involving children whenever feasible. When such assessments with children are not possible, taste screening by adult panels can serve as an alternative method, with the results examined for their applicability to children [
19]. At this stage of development, we deemed an initial screening by an adult testing panel to be acceptable. Regarding the palatability evaluation by healthy volunteers, each healthy adult volunteer was asked to evaluate their taste and odor perception using a questionnaire rating scale.
Considering these findings, the goal of this work was the development of an oral palatable solution of CARV for pediatric use, exploiting taste masking in association with different flavoring agents and a sweetener. Different combinations of flavorings and a sweetening agent were selected to prepare CARV solutions and were subject to a human taste assessment to evaluate their actual ability to mask the unpleasant taste of the CARV in comparison with the reference CARV solution [
10].
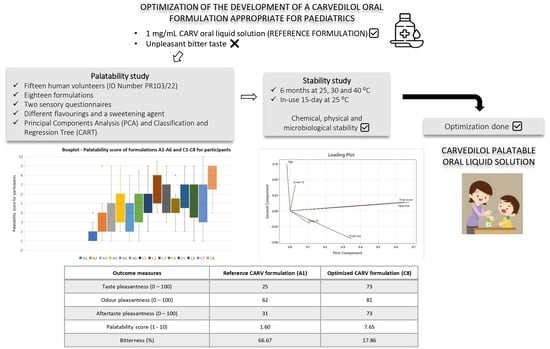
The aim of this research was to select the CARV solution with the highest palatability score and least bitterness level for healthy human volunteers. The highest palatability-rated CARV formulation for the participants was included in a stability study for 6 months and evaluated in terms of chemical, physical, and microbiological stability under 25, 30, and 40 °C. Additionally, a 15-day in-use stability study of this formulation was conducted at 25 °C.
4. Discussion
The next step to optimize the CARV liquid formulation we have developed for pediatrics [
10] was to make it more palatable in order to increase its acceptability in this population. Most APIs have an unpleasant taste when dissolved in water, so finding a method to mask each API’s taste is a challenge. CARV in solution also exhibits an unpleasant bitter taste; thus, the development of a palatable CARV liquid formulation was considered essential for children, especially those under 7 years old, since they are not able to swallow solid pharmaceutical forms [
30].
Acceptability of pediatric pharmaceutical formulations holds immense importance for children, given that treatment compliance heavily relies on an acceptable taste. Hence, ensuring adequate palatability of oral liquid formulations becomes an important factor in their acceptability, where flavors may be essential to achieve this objective [
1,
3]. Moreover, in enhancing the palatability of pediatric formulations, it is crucial to deliberate on excipients that are safe for this extremely young population, considering that this treatment could be administered very early in life, possibly even at birth [
31].
Different approaches might be utilized to obscure the unpleasant taste of APIs in pediatric oral dosage forms. In our case, sweeteners and flavors were added to CARV liquid formulations to mask the bitter taste of CARV. Syrups are commonly employed as carriers for compounded oral formulations. However, their high sucrose content makes them unsuitable for pediatric patients with diabetes and hereditary fructose intolerance. Moreover, sucrose alters dental plaque pH, leading to tooth enamel dissolution and contributing to dental cavities [
2]. Cherry and strawberry flavors, in combination with a high-intensity sweetener, may suit the United States and European pediatric market. Other typical flavors used in pediatric formulations include orange, vanilla, and grapefruit. For the selection of the most suitable flavor for a pediatric medication, the taste to cover (acid, alkaline, bitter, salty, or sweet) must be considered. To cover a bitter taste, most flavors used are cherry, chocolate, grapefruit, licorice, strawberry, peach, raspberry, and tutti-frutti [
1].
Palatability was selected as the criterion of choice for a new palatable age-adapted formulation, so we considered taste evaluation as a key step during the development of pediatric oral formulations. In our research, different flavorings, or a combination of two, in addition to a sweetening agent (sucralose), were chosen to create CARV palatable solutions. These formulations were then put through a taste test involving human adult volunteers to assess how effectively these formulations could cover up the unpleasant taste of CARV when compared to the reference CARV solution [
10]. This study aimed to identify the CARV solution that received the highest palatability score and the lowest bitterness level among all healthy human volunteers who participated in the study. One limitation of this study is that the human taste panel was conducted in healthy adults rather than children (who represent the target population) for a subsequent extrapolation of the results. As it is mentioned, for ethical reasons, conducting a clinical trial in children is quite complex [
19].
Taste evaluation was made up of three tests. Test 1 and Test 2 were performed in order to find the best acceptable flavoring for the participants to mask the unpleasant taste of CARV, as well as the best acceptable concentration of sucralose. Flavoring agents used in Test 1 were the ones highly recommended by the EMA [
1], which explains why they were the starting point. However, the results of Test 1 revealed that these flavorings were not suitable for masking the unpleasant taste of CARV. Conversely, results from Test 2 showed the optimal concentration of sucralose. Due to these outcomes, Test 3 was conducted in a second session, which included formulations with the optimal concentration of sucralose and different flavoring agents or a combination of these.
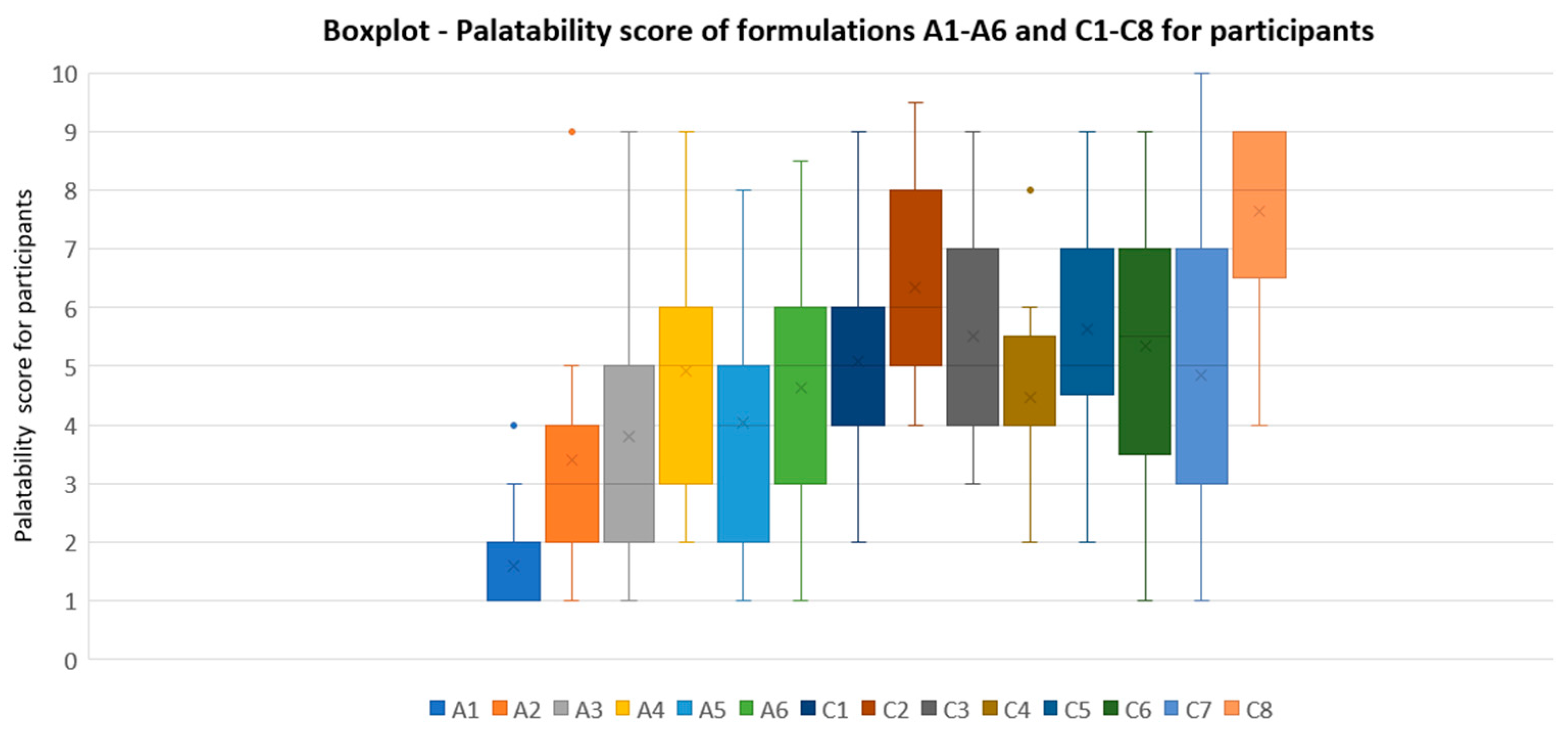
Formulations tested in Test 3 (C1 to C8) obtained significantly better results for participants compared to formulations tested in Test 1 (A1 to A6). Formulations closest to acceptability in Test 1 are A4 (strawberry 0.2% and sucralose 0.05%) and A6 (apple pear 0.2% and sucralose 0.05%), with scores of taste pleasantness and palatability for participants of 47/100 and 4.91/10, and 48/100 and 4.63/10, respectively. Nevertheless, these scores did not exceed our established acceptability limit.
Concerning sweetness-rated results for B1 to B4 formulations, B4 was the closest to the required optimal level (5/10). Therefore, the amount of sucralose in formulation B4 (0.20%) was chosen for formulations comprised in Test 3 (C1 to C8). It was not possible to increase the sucralose level in the formulation as it would exceed the allowable ADI [
21].
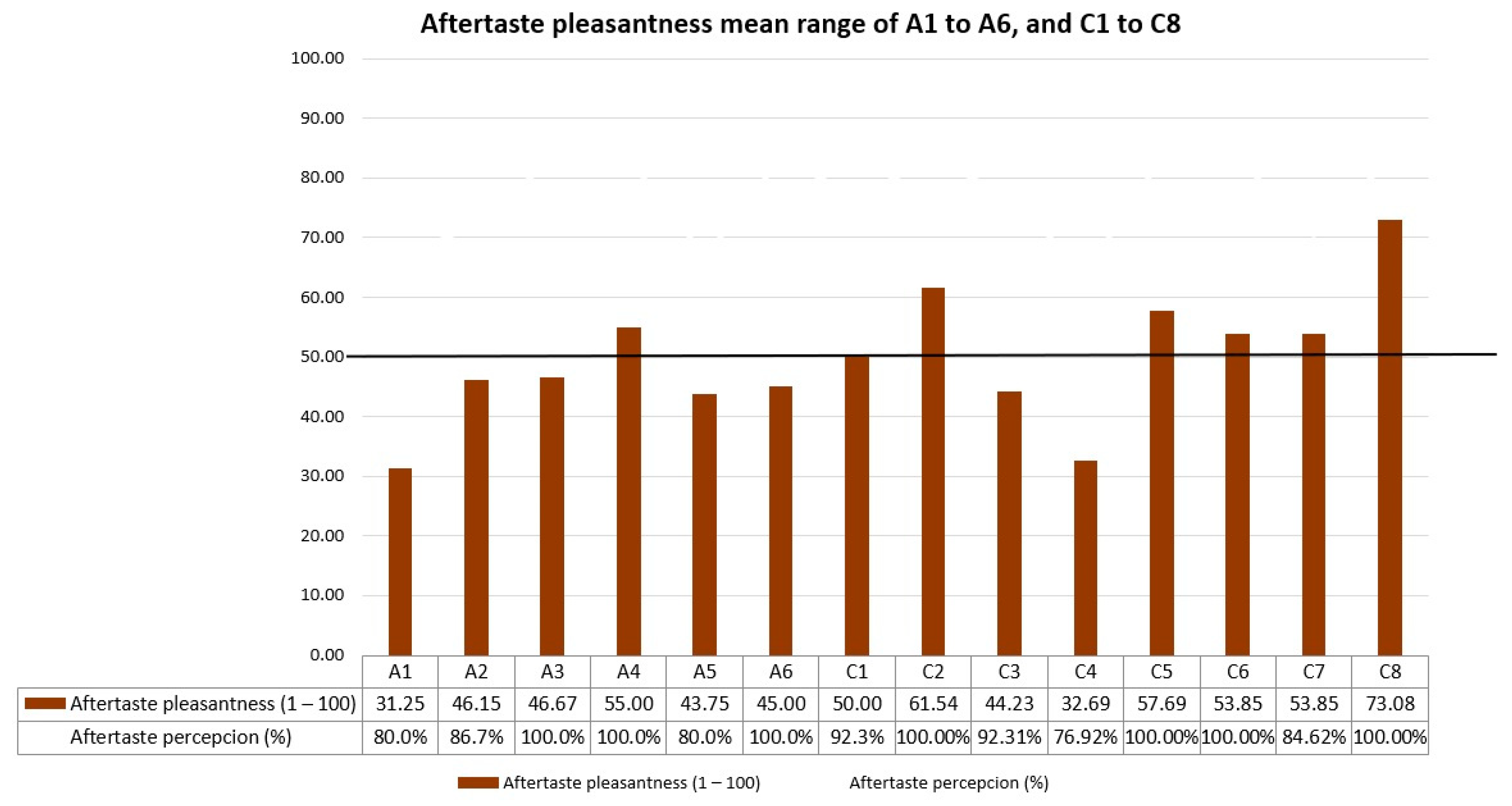
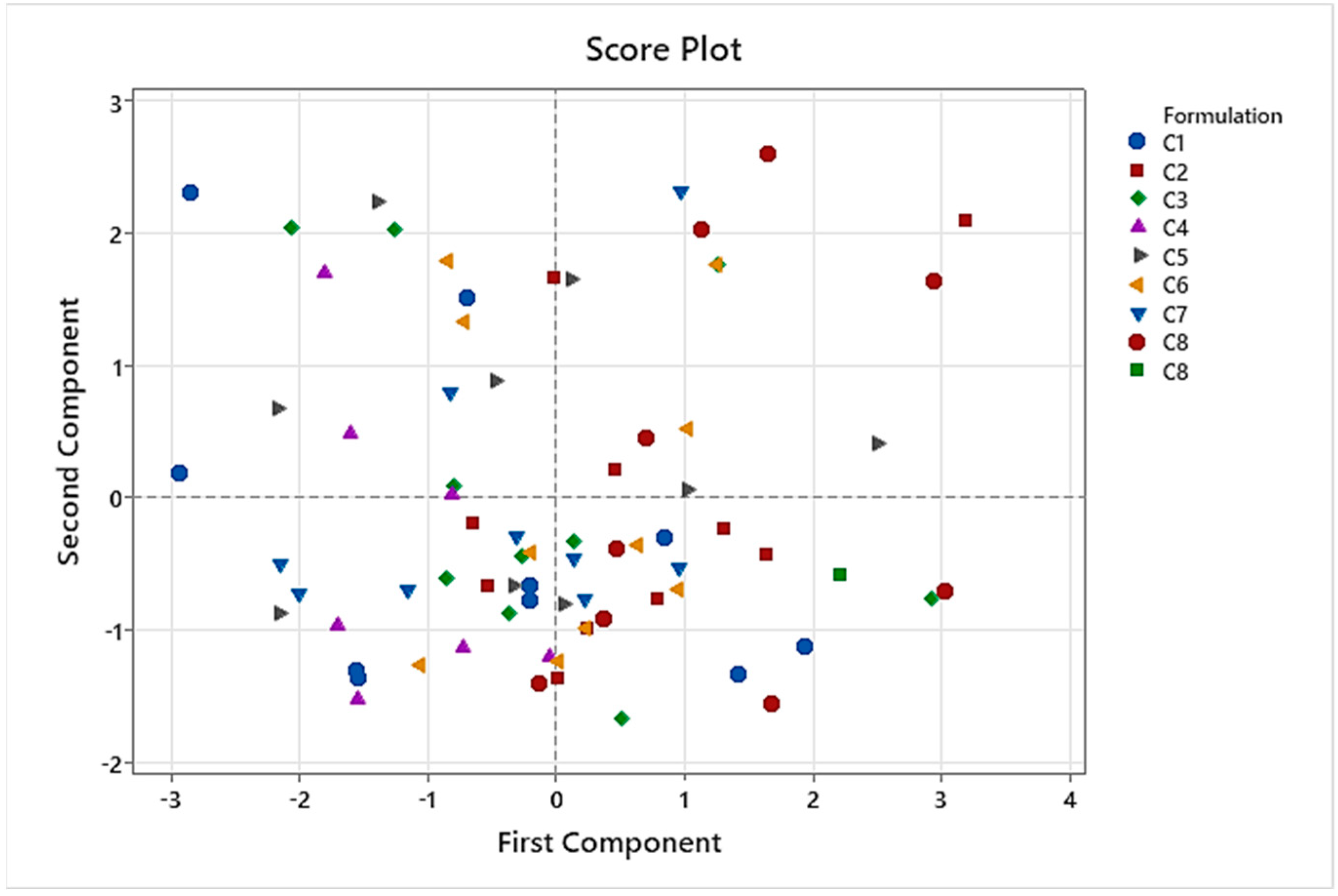
Referring to the palatability scores results of formulations in series C, one formula stood out for its high values in all the tests performed: C8 (lemon 0.15%-cola 0.05% flavorings, and 0.20% sucralose). Scores of C8 in the test were 73/100 for taste pleasantness, 81/100 for odor pleasantness, 73/100 for aftertaste pleasantness, and 7.65/10 for final palatability. All these scores exceeded our acceptability limits, making C8 the best palatability-rated formula among the three palatability tests conducted. Final palatability scores for participants increased from 1.6 (A1, reference CARV solution) to 7.65 (C8), exhibiting the taste-masking ability for C8.
The boxplot presented in
Figure 4 illustrates palatability scores for A1 to A6 and C1 to C8. This boxplot clearly demonstrates that all the formulations in series C with the incorporated improvements are better rated than the initial ones in series A. Additionally, C8 was the formulation with lower variability results between the participants, exhibiting the minimum QCD among all samples studied of 0.16.
These improvements were the increase in sucralose from 0.05% (series A formulations) to 0.20% (series C formulations), in addition to new flavorings, such as lemon and cola flavors, or a combination of two agents. The high palatability score of C8 for the participants could be explained by the synergistic effect of combining sucralose with these sweetening agents, in addition to the specific concentration of these.
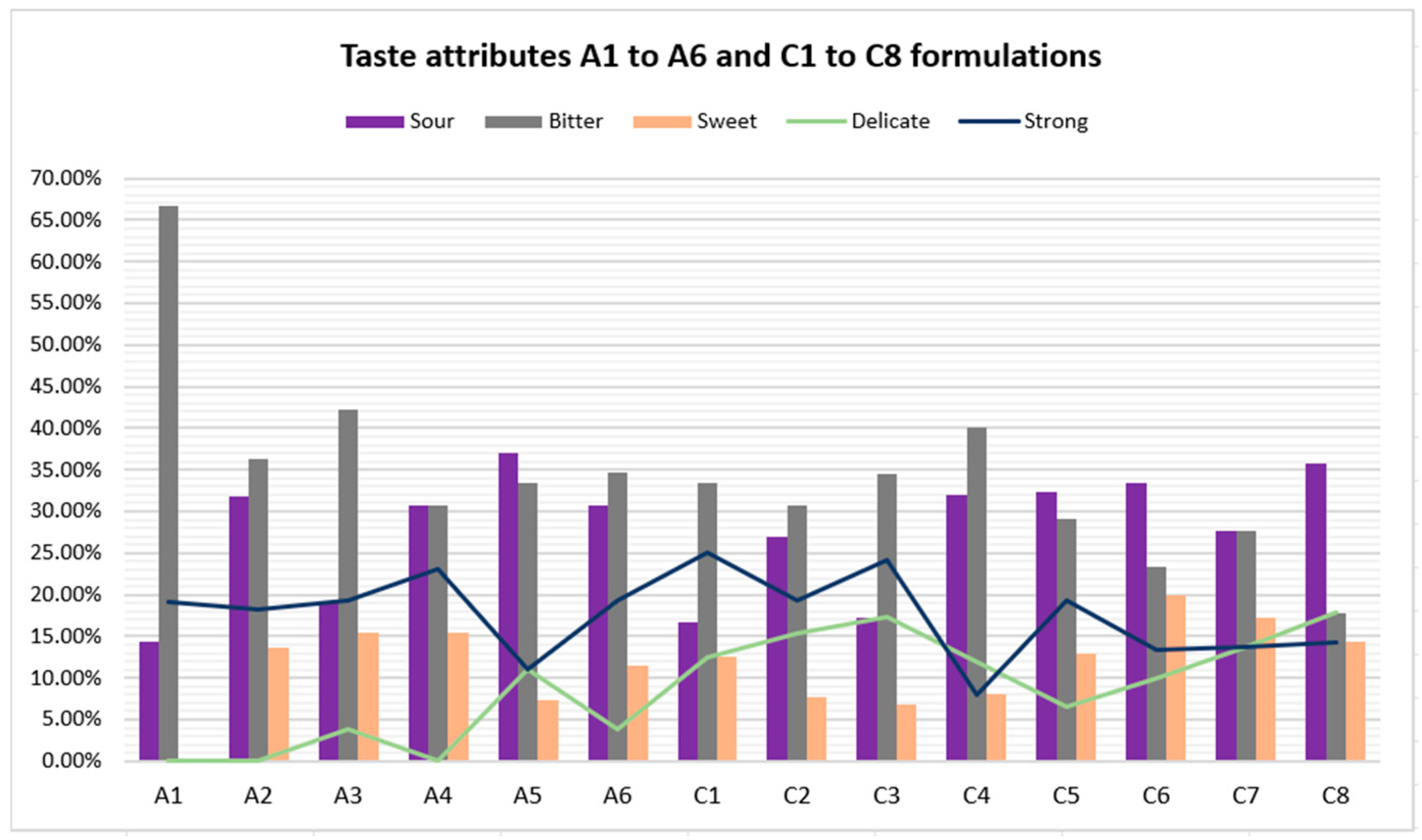
Furthermore, formulations in series C obtained better bitterness results. Once again, formulation C8 topped the rankings, as the bitterness level decreased from 66.67% (A1 reference CARV solution) to 17.86% (C8). Concerning the sourness level, C8 achieved a percentage of 35.78%. Despite having a high sourness percentage, it did not cause unpleasantness among the participants, given that C8 is the highest-rated formulation among the fourteen. Hence, the bitterness level of CARV formulations studied significantly decreases with an increase in the amount of sucralose in the formulation and even more with an appropriate combination of sucralose and one or two flavoring agents.
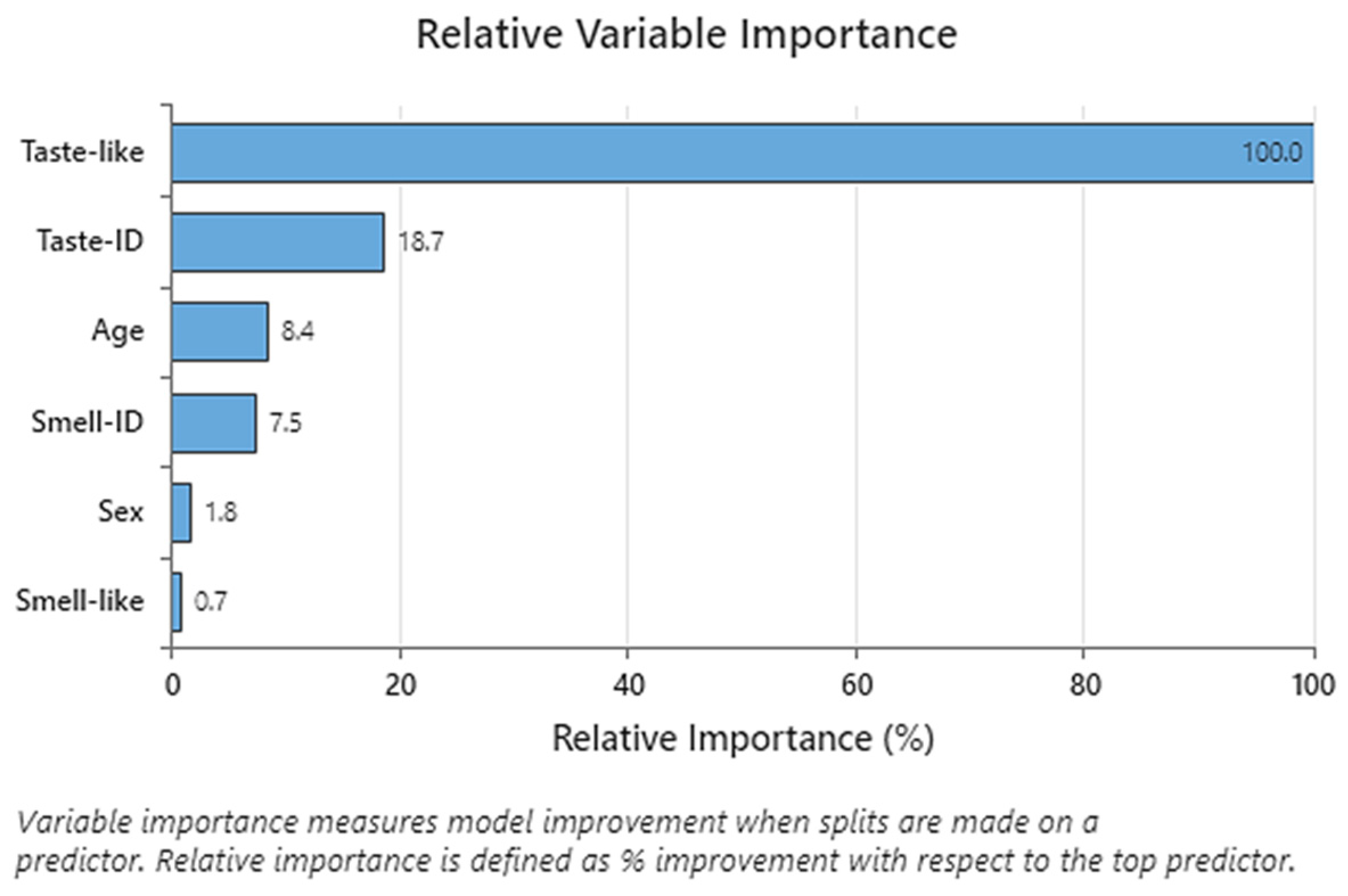
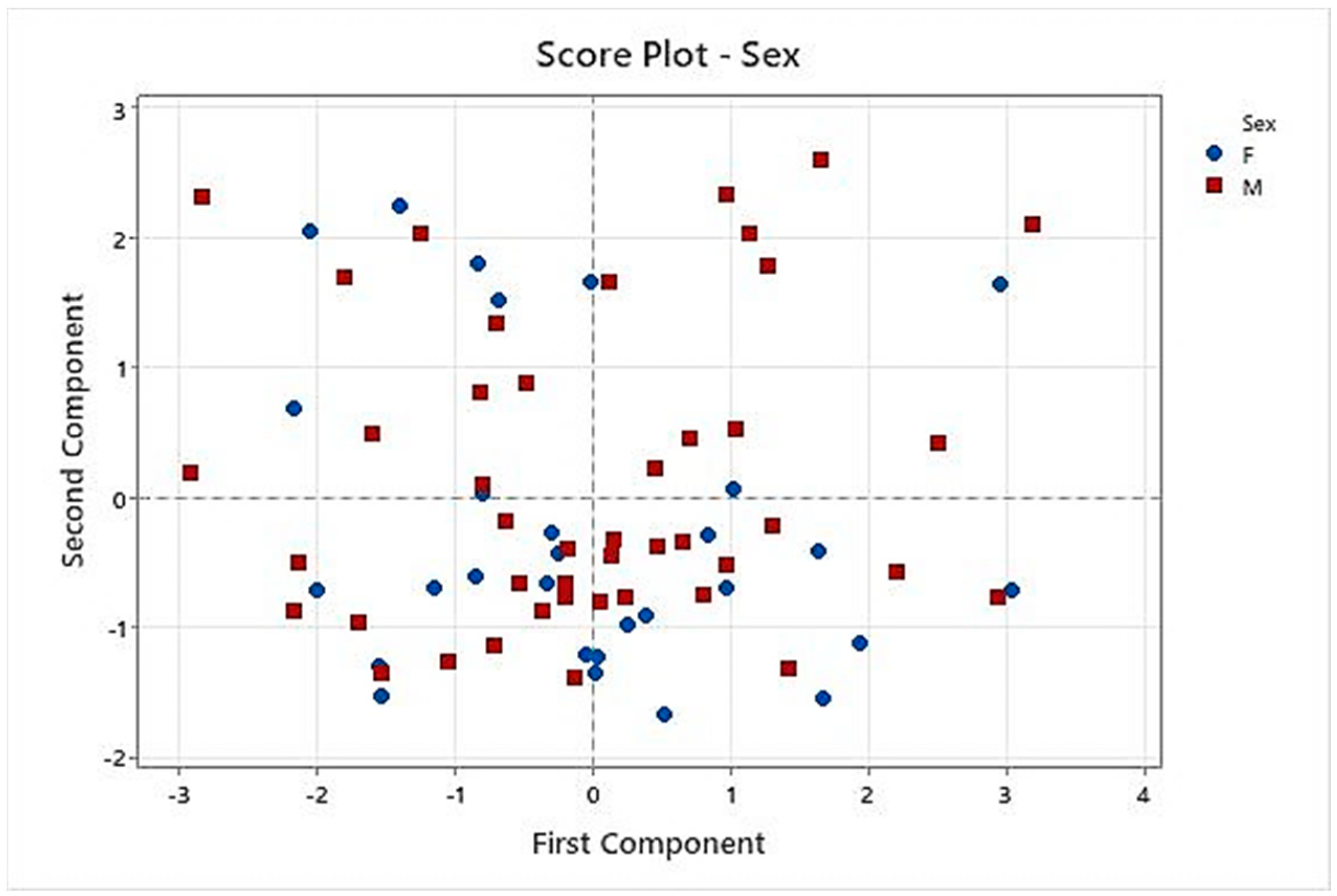
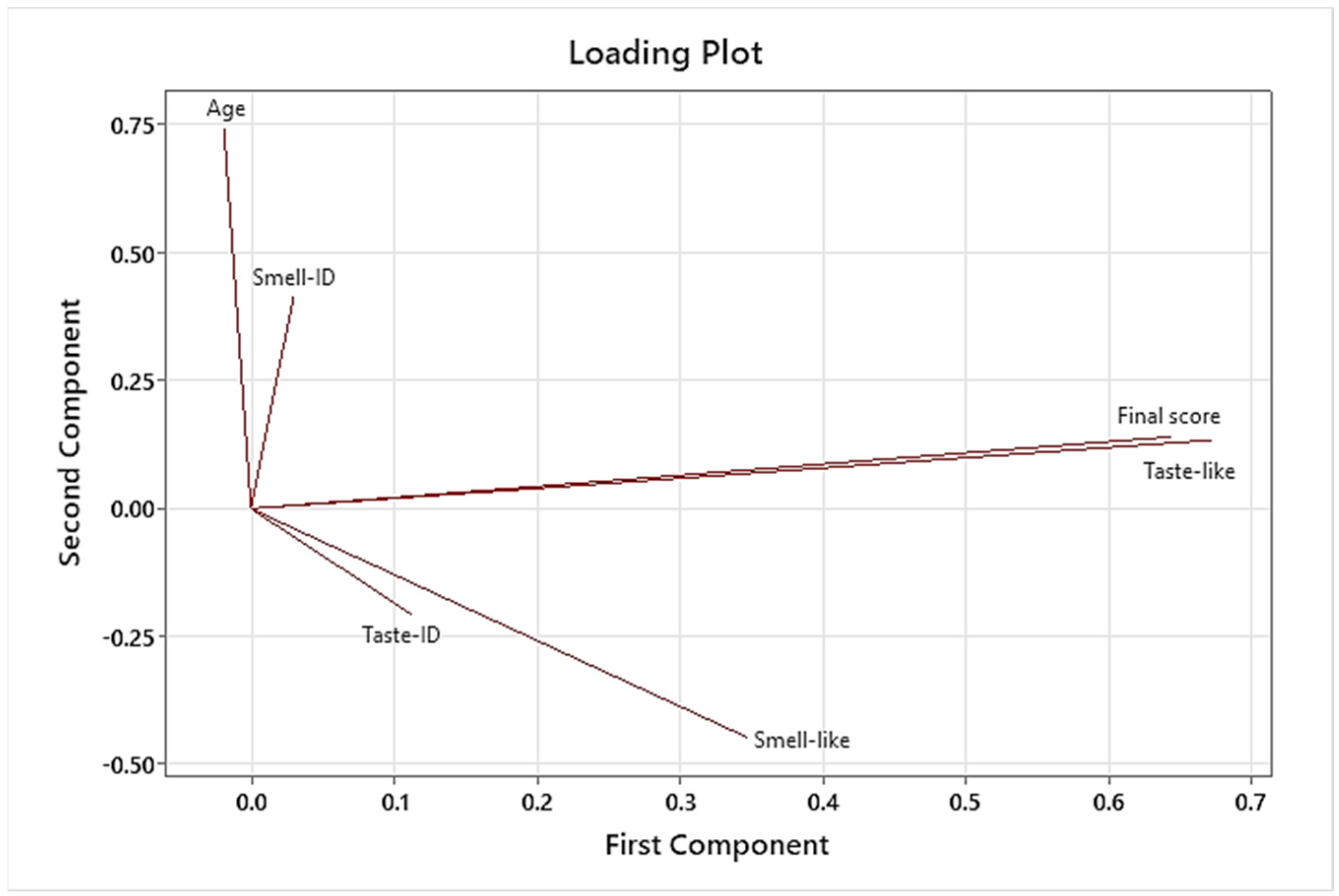
CART analysis performed on C1 to C8 formulations showed that taste pleasantness was highly related to the final palatability score, so we especially considered these variables for the taste assessment. Results of PCA demonstrated that C8 had the best results for these two variables because C8 spots were presented in the quadrants influenced by taste pleasantness and final palatability score. Another conclusion of PCA was that the results of the taste assessment we performed were not affected by gender in this study.
These results suggested that sucralose, as a sweetener, in addition to lemon and cola as flavoring agents, would effectively minimize the bitterness of CARV in its solution state. The achieved masking of CARV’s unpleasant taste properties presents a tangible advantage for its oral administration, especially in pediatric use, thereby enhancing children’s compliance with the pharmaceutical formulation.
Furthermore, through a stability study according to ICH guidelines [
24], C8 has proven to be physically, chemically, and microbiologically stable after 6 months of storage at 25, 30, and 40 °C. Moreover, in-use stability results of C8 were confirmed for 15 days at 25 °C. We utilized Minitab 21.0 to analyze the CARV assay results (%) from three stability batches of C8_P2 over a 6-month period to estimate the formulation’s shelf life. The correlation observed between CARV assay (%) and time formed a straight line with a zero slope (~100% adjustment), suggesting no substantial variation in CARV assay (%) throughout the study duration. Consequently, these findings were employed to extrapolate a stability duration of 12 months, aligning with guidelines from ICH Q1(a). Simultaneously, C8_P2 continues undergoing a long-term stability study for 24 months at 25 and 30 °C to verify these results. All data accomplished the upper and lower specifications.
The C8_P2 formulation herein developed, a 1 mg/mL sweetened and flavored oral CARV aqueous formulation, had good stability according to the CARV assay (%), pH, and appearance results and satisfied the requirements of European Pharmacopoeia. It can be concluded that we developed an optimized CARV 1 mg/mL palatable solution, especially interesting for patients with swallowing difficulty in elderly populations, in addition to challenges in acceptability as pediatric patients [
1].