An Intravenous Pharmacokinetic Study of Cannabidiol Solutions in Piglets through the Application of a Validated Ultra-High-Pressure Liquid Chromatography Coupled to Tandem Mass Spectrometry Method for the Simultaneous Quantification of CBD and Its Carboxylated Metabolite in Plasma
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation and Method
2.3. Preparation of Standard Solutions
2.4. Calibration, Validation and QC Samples
2.5. Sample Preparation
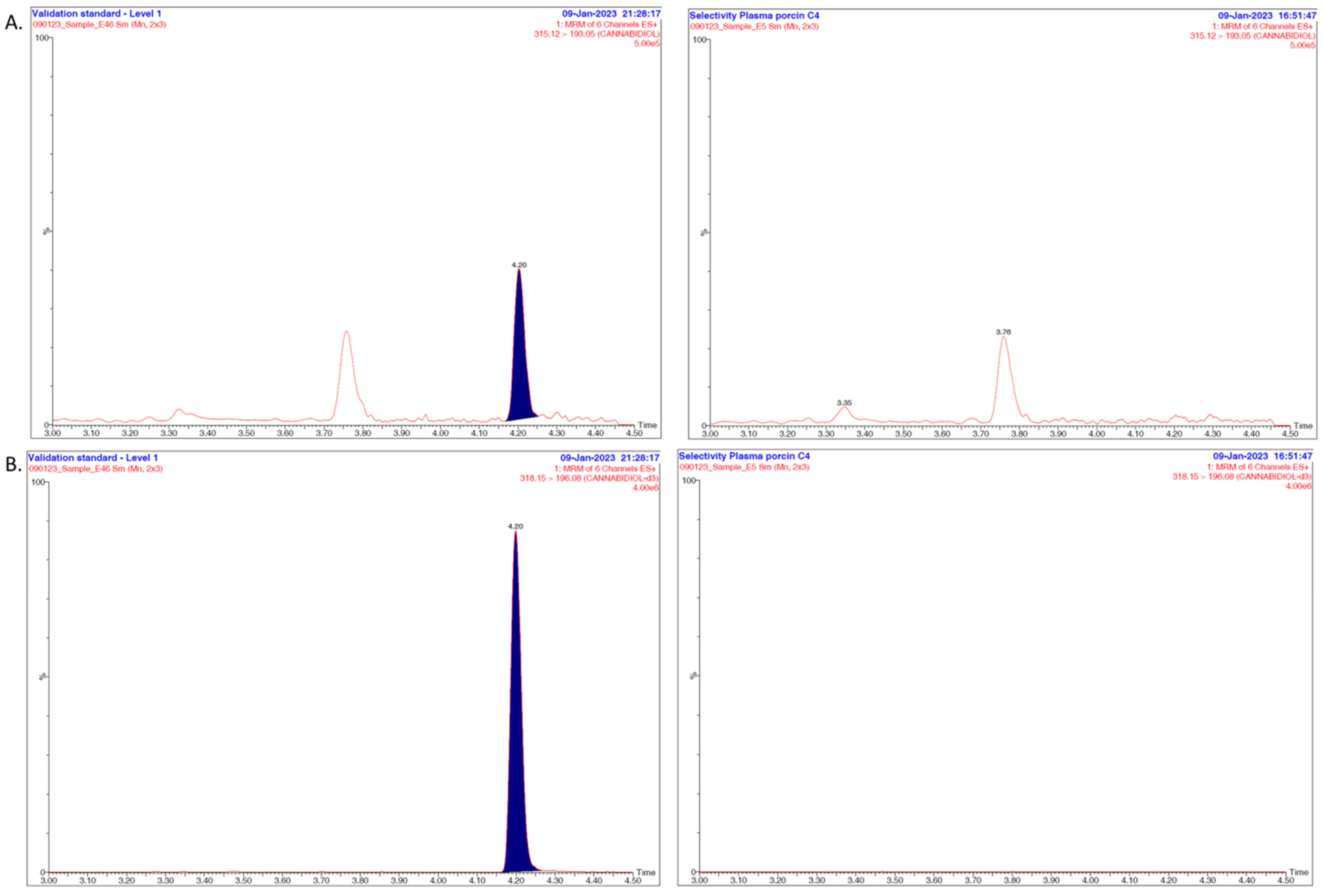
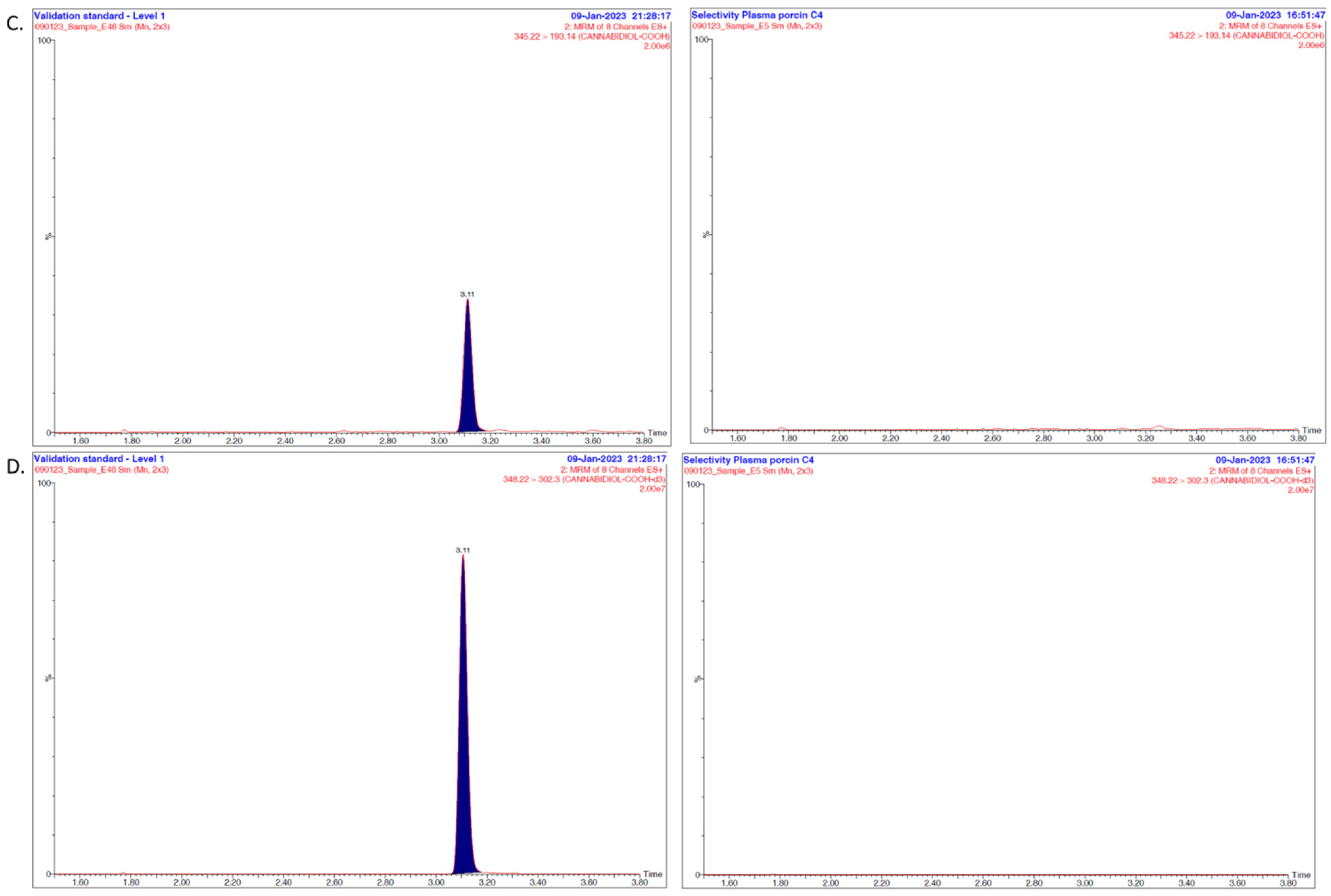
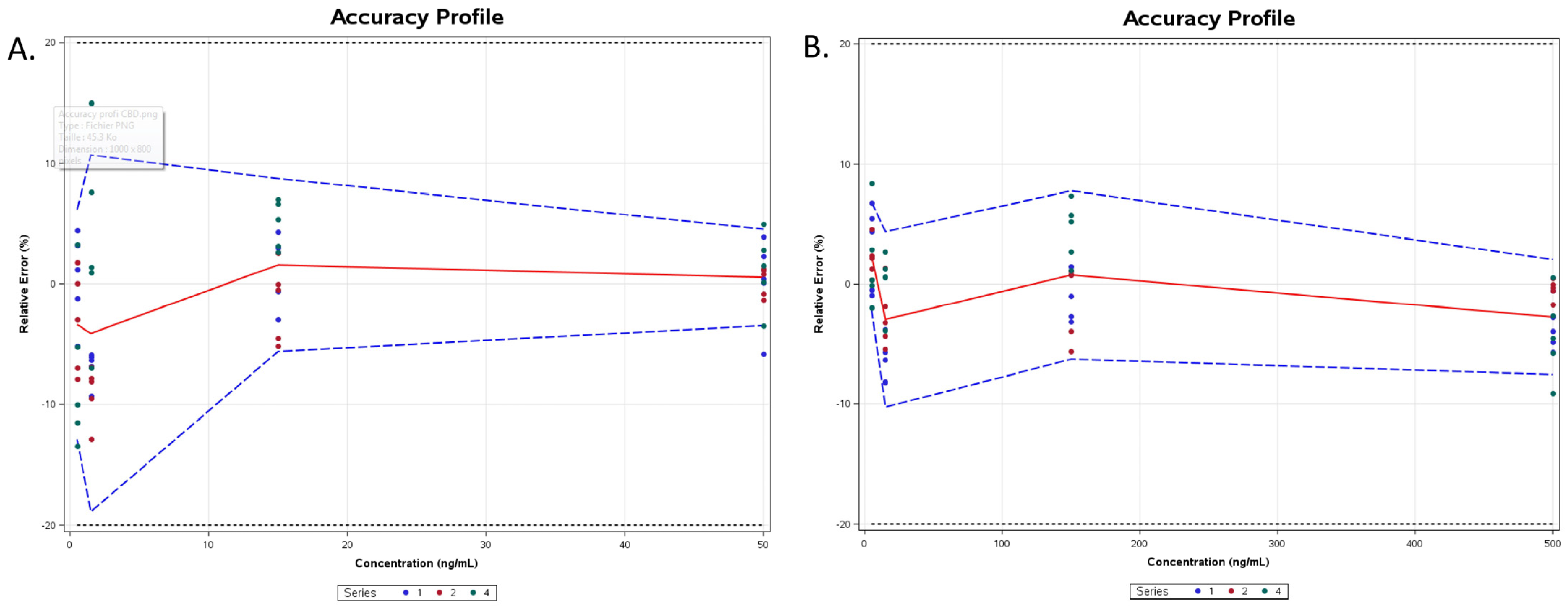
2.6. Method Validation
2.7. Data Treatment
2.8. Application of the Method
2.8.1. In Vivo Study
2.8.2. In Vivo Data Treatment
3. Results and Discussion
3.1. Overall Discussion of the Bioanalytical Method
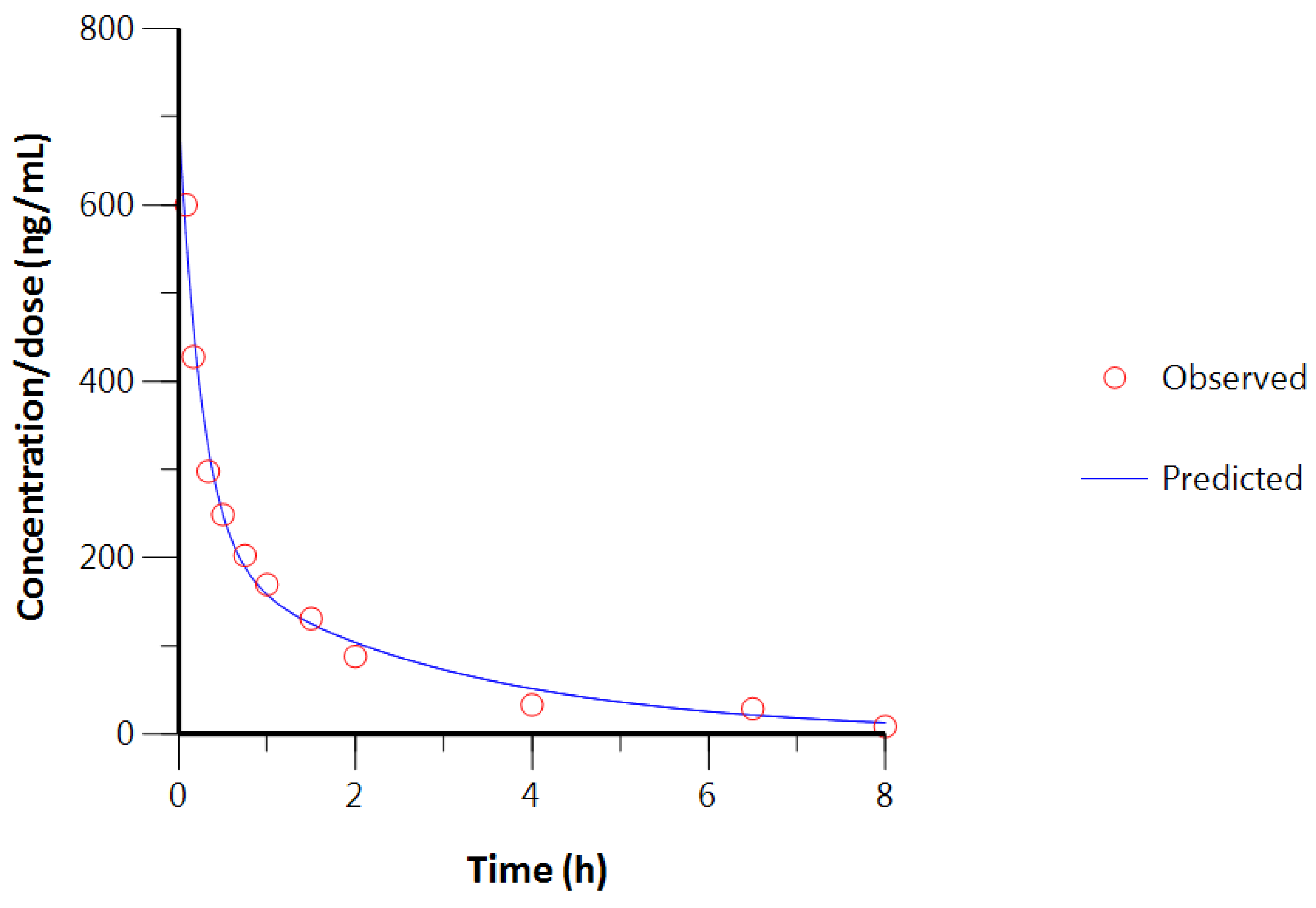
3.2. Intravenous Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mlost, J.; Bryk, M.; Starowicz, K. Cannabidiol for pain treatment: Focus on pharmacology and mechanism of action. Int. J. Mol. Sci. 2020, 21, 8870. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Babalonis, S.; Walsh, S.H. Cannabidiol: Pharmacology and Therapeutic Targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, A.E.; Fadus, M.C.; Gruber, S.A.; Gray, K.M.; Wilens, T.E.; Squeglia, L.M. A scoping review of the use of cannabidiol in psychiatric disorders. Psychiatry Res. 2022, 308, 114347. [Google Scholar] [CrossRef] [PubMed]
- Franco, V.; Perucca, E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 2019, 79, 1435–1454. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Bellman, Z.D.; Yates, A.S.; England, T.J.; O’Sullivan, S.E. A systematic review of cannabidiol dosing in clinical populations. Br. J. Clin. Pharmacol. 2019, 85, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A systematic review on the pharmacokinetics of cannabidiol in humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Wakshlag, J.J.; Schwark, W.S.; Deabold, K.A.; Talsma, B.N.; Cital, S.; Lyubimov, A.; Iqbal, A.; Zakharov, A. Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract. Front. Vet. Sci. 2020, 7, 505. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Williams, M.R.; Holbrook, T.C.; Maxwell, L.; Croft, C.H.; Ientile, M.M.; Cliburn, K. Pharmacokinetic Evaluation of a Cannabidiol Supplement in Horses. J. Equine Vet. Sci. 2022, 110, 103842. [Google Scholar] [CrossRef]
- Xu, C.; Chang, T.; Du, Y.; Yu, C.; Tan, X.; Li, X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ. Toxicol. Pharmacol. 2019, 70, 103202. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.E.; Knych, H.K.; Adams, A.A. Pharmacokinetics of cannabidiol in a randomized crossover trial in senior horses. Am. J. Vet. Res. 2022, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hložek, T.; Uttl, L.; Kadeřábek, L.; Balíková, M.; Lhotková, E.; Horsley, R.R.; Nováková, P.; Šíchová, K.; Štefková, K.; Tylš, F.; et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol. 2017, 27, 1223–1237. [Google Scholar] [CrossRef] [PubMed]
- Izgelov, D.; Davidson, E.; Barasch, D.; Regev, A.; Domb, A.J.; Hoffman, A. Pharmacokinetic investigation of synthetic cannabidiol oral formulations in healthy volunteers. Eur. J. Pharm. Biopharm. 2020, 154, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Jennotte, O.; Koch, N.; Lechanteur, A.; Evrard, B. Development of amorphous solid dispersions of cannabidiol: Influence of the carrier, the hot-melt extrusion parameters and the use of a crystallization inhibitor. J. Drug Deliv. Sci. Technol. 2022, 71, 103372. [Google Scholar] [CrossRef]
- Koch, N.; Jennotte, O.; Gasparrini, Y.; Vandenbroucke, F.; Lechanteur, A.; Evrard, B. Cannabidiol aqueous solubility enhancement: Comparison of three amorphous formulations strategies using different type of polymers. Int. J. Pharm. 2020, 589, 119812. [Google Scholar] [CrossRef]
- Cherniakov, I.; Izgelov, D.; Barasch, D.; Davidson, E.; Domb, A.J.; Hoffman, A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: Pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J. Control. Release 2017, 266, 1–7. [Google Scholar] [CrossRef]
- De Prá, M.A.A.; Vardanega, R.; Loss, C.G. Lipid-based formulations to increase cannabidiol bioavailability: In vitro digestion tests, pre-clinical assessment and clinical trial. Int. J. Pharm. 2021, 609, 121159. [Google Scholar] [CrossRef]
- Jennotte, O.; Koch, N.; Lechanteur, A.; Rosoux, F.; Emmerechts, C.; Beeckman, E.; Evrard, B. Feasibility study of the use of a homemade direct powder extrusion printer to manufacture printed tablets with an immediate release of a BCS II molecule. Int. J. Pharm. 2023, 646, 123506. [Google Scholar] [CrossRef]
- Koch, N.; Jennotte, O.; Toussaint, C.; Lechanteur, A.; Evrard, B. Production challenges of tablets containing lipid excipients: Case study using cannabidiol as drug model. Int. J. Pharm. 2023, 633, 122639. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, D.Y.; Kang, J.H.; Jeong, J.W.; Kim, J.H.; Kim, H.W.; Oh, D.H.; Kim, J.M.; Rhim, S.J.; Kim, G.D.; et al. Alternative experimental approaches to reduce animal use in biomedical studies. J. Drug Deliv. Sci. Technol. 2022, 68, 103131. [Google Scholar] [CrossRef]
- Pestieau, A.; Lebrun, S.; Cahay, B.; Brouwers, A.; Streel, B.; Cardot, J.M.; Evrard, B. Evaluation of different in vitro dissolution tests based on level A in vitro–in vivo correlations for fenofibrate self-emulsifying lipid-based formulations. Eur. J. Pharm. Biopharm. 2017, 112, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Kraft, K.; Rusch, L.; Fein, M.; Leone-Bay, A. Improved Bioavailability with Dry Powder Cannabidiol Inhalation: A Phase 1 Clinical Study. J. Pharm. Sci. 2021, 110, 3946–3952. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Kevin, R.C.; Vogel, R.; Doohan, P.; Berger, M.; Amminger, G.P.; McGregor, I.S. A validated method for the simultaneous quantification of cannabidiol, Δ9-tetrahydrocannabinol, and their metabolites in human plasma and application to plasma samples from an oral cannabidiol open-label trial. Drug Test. Anal. 2021, 13, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Malaca, S.; Gottardi, M.; Pigliasco, F.; Barco, S.; Cafaro, A.; Amadori, E.; Riva, A.; Marcenaro, M.; Striano, P.; Cangemi, G.; et al. UHPLC-MS/MS analysis of cannabidiol and its metabolites in serum of patients with resistant epilepsy treated with CBD formulations. Pharmaceuticals 2021, 14, 630. [Google Scholar] [CrossRef] [PubMed]
- Lo Faro, A.F.; Tini, A.; Gottardi, J.; Pichini, S.; Carlier, J.; Giorgetti, R.; Busardò, F.P. UHPLC-MS-MS Determination of THC, CBD and Their Metabolites in Whole Blood of Light Cannabis Smokers. J. Anal. Toxicol. 2023, 46, e256–e261. [Google Scholar] [CrossRef]
- Nicolaou, A.G.; Christodoulou, M.C.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Analysis of cannabinoids in conventional and alternative biological matrices by liquid chromatography: Applications and challenges. J. Chromatogr. A 2021, 1651, 462277. [Google Scholar] [CrossRef]
- González, O.; Alonso, R.M. Validation of bioanalytical chromatographic methods for the quantification of drugs in biological fluids. Handb. Anal. Sep. 2020, 7, 115–134. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R. Bioanalytical method validation: An updated review. Pharm. Methods 2010, 1, 25–38. [Google Scholar] [CrossRef]
- Moein, M.M.; El Beqqali, A.; Abdel-Rehim, M. Bioanalytical method development and validation: Critical concepts and strategies. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Hahirwa, I.; Charlier, C.; Karangwa, C.; Denooz, R. Validation of an analytical method for the determination in serum of psychotropic drugs by High-Performance Liquid Chromatography with Diode Array Detection. Rwanda J. 2015, 2, 13–23. [Google Scholar] [CrossRef]
- Pirard, C.; Charlier, C. Simple and fast method for the measurement of legacy and novel brominated flame retardants in human serum. Chemosphere 2018, 211, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Pichini, S.; Mannocchi, G.; Gottardi, M.; Pérez-Acevedo, A.P.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Farré, M.; Pacifici, R.; Busardò, F.P. Fast and sensitive UHPLC-MS/MS analysis of cannabinoids and their acid precursors in pharmaceutical preparations of medical cannabis and their metabolites in conventional and non-conventional biological matrices of treated individual. Talanta 2020, 209, 120537. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Medina, A.; Serrano-Rodríguez, J.M.; Díez de Castro, E.; García-Valverde, M.T.; Saitua, A.; Becero, M.; Muñoz, A.; Ferreiro-Vera, C.; Sánchez de Medina, V. Pharmacokinetics and oral bioavailability of cannabidiol in horses after intravenous and oral administration with oil and micellar formulations. Equine Vet. J. 2023, 55, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Langos, M.; Brenneisen, R. Human Pharmacokinetics and Adverse Effects of Pulmonary and Intravenous THC-CBD Formulations. Med. Cannabis Cannabinoids 2018, 1, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cherniakov, I.; Izgelov, D.; Domb, A.J.; Hoffman, A. The effect of Pro NanoLipospheres (PNL) formulation containing natural absorption enhancers on the oral bioavailability of delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) in a rat model. Eur. J. Pharm. Sci. 2017, 109, 21–30. [Google Scholar] [CrossRef]
- Ferreira, L.; Campos, J.; Veiga, F.; Cardoso, C.; Paiva-Santos, A.C. Cyclodextrin-based delivery systems in parenteral formulations: A critical update review. Eur. J. Pharm. Biopharm. 2022, 178, 35–52. [Google Scholar] [CrossRef]
- Jones, D.S.; Dressman, J.B.; Loftsson, T.; Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A. Pharmacokinetics of cyclodextrins and drugs after oral and parenteral administration of drug/cyclodextrin complexes. J. Pharm. Pharmacol. 2016, 68, 544–555. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Bethanis, K.; Koniari, E.; Christoforides, E.; Nikola, O.; Andreou, A.; Mantzou, A.; Chrousos, G.P.; Kanaka-Gantenbein, C.; Lambrou, G.I. Biophysical Studies and In Vitro Effects of Tumor Cell Lines of Cannabidiol and Its Cyclodextrin Inclusion Complexes. Pharmaceutics 2022, 14, 706. [Google Scholar] [CrossRef]
- Piel, G.; Evrard, B.; Van Hees, T.; Delattre, L. Comparison of the IV pharmacokinetics in sheep of miconazole-cyclodextrin solutions and a micellar solution. Int. J. Pharm. 1999, 180, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Salles, É.L.; Khodadadi, H.; Jarrahi, A.; Ahluwalia, M.; Paffaro, V.A.; Costigliola, V.; Yu, J.C.; Hess, D.C.; Dhandapani, K.M.; Baban, B. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J. Cell. Mol. Med. 2020, 24, 12869–12872. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, A.R.; Greogory, L.K. Handbook of Basic Pharmacokinetics: Including Clinical Applictions; American Pharmacists Association: Washington, DC, USA, 2009. [Google Scholar]
- Linderkamp, O.; Betke, K.; Güntner, M.; Jap, G.H.; Riegel, K.P.; Walser, K. Blood volume in newborn piglets: Effects of time of natural cord rupture, intra-uterine growth retardation, asphyxia, and prostaglandin-induced prematurity. Pediatr. Res. 1981, 15, 53–57. [Google Scholar] [CrossRef] [PubMed]


| Time (min) | Mobile Phase A (Ammonium Buffer, pH 10) | Mobile Phase B (Methanol) | Mode |
|---|---|---|---|
| 0.0–1.0 | 70% | 30% | Isocratic |
| 1.0–4.5 | 3% | 97% | Gradient |
| 4.5–5.0 | 3% | 97% | Isocratic |
| 5.0–5.05 | 70% | 30% | Gradient |
| Analytes | Precursor Ion (m/z) | Product Ion (m/z) | Cone Voltage (V) | CE (eV) | RT (min) |
|---|---|---|---|---|---|
| CBD | 315.1 | 193.1 (Quantification) 259.1 (Identification) | 25 25 | 20 20 | 4.20 |
| CBD-d3 | 318.2 | 196.1 | 20 | 20 | 4.19 |
| 7-COOH-CBD | 345.2 | 193.1 (Quantification) 299.2 (Identification) | 40 40 | 28 16 | 3.10 |
| 7-COOH-CBD-d3 | 348.2 | 302.3 | 34 | 24 | 3.09 |
| Piglet | Weight (kg) | Volume Injected (mL) | Injection Route |
|---|---|---|---|
| P1 | 9.45 | 2.36 | Cranial vena cava |
| P2 | 9.04 | 2.26 | Auricular catheter |
| P3 | 8.85 | 2.21 | Auricular catheter |
| Concentration Level (ng/mL) | Intra-Day Relative Bias (%) | |||
|---|---|---|---|---|
| CBD | 7-COOH-CBD | Series | CBD | 7-COOH-CBD |
| 0.5 | 5 | 1 | 0.5 | 3.0 |
| 0.5 | 5 | 2 | −3.2 | 2.1 |
| 0.5 | 5 | 3 | −7.4 | 2.6 |
| 1.5 | 15 | 1 | −6.9 | −6.4 |
| 1.5 | 15 | 2 | −9.0 | −2.7 |
| 1.5 | 15 | 3 | 3.6 | −4.5 |
| 15 | 150 | 1 | 1.3 | −0.8 |
| 15 | 150 | 2 | −1.5 | −1.4 |
| 15 | 150 | 3 | 4.9 | −1.1 |
| 50 | 500 | 1 | 0.2 | −3.5 |
| 50 | 500 | 2 | 0.2 | −0.4 |
| 50 | 500 | 3 | 1.2 | −2.0 |
| Concentration Level (ng/mL) | Inter-Day Relative Bias (%) | |||
| CBD | 7-COOH-CBD | CBD | 7-COOH-CBD | |
| 0.5 | 5 | −3.4 | 2.4 | |
| 1.5 | 1.5 | −4.1 | −3.0 | |
| 15 | 15 | 1.6 | 0.8 | |
| 50 | 500 | 0.5 | −2.8 | |
| Concentration Level (ng/mL) | Repeatability (RSD%) | Intermediate Precision (RSD%) | |||
|---|---|---|---|---|---|
| CBD | 7-COOH-CBD | CBD | 7-COOH-CBD | CBD | 7-COOH-CBD |
| 0.5 | 5 | 5.1 | 3.0 | 6.0 | 3.0 |
| 1.5 | 15 | 5.0 | 2.3 | 8.1 | 3.9 |
| 15 | 150 | 2.8 | 2.6 | 4.1 | 4.0 |
| 50 | 500 | 2.7 | 2.4 | 2.7 | 3.0 |
| Replicate | CBD (20 ng/mL) | 7-COOH-CBD (200 ng/mL) |
|---|---|---|
| 1 | −9.8 | −4.7 |
| 2 | −10.2 | −5.1 |
| 3 | −7.1 | −5 |
| 4 | −9.3 | −1.6 |
| 5 | −11.2 | −5.2 |
| Mean (±SD) | −9.5 ± 1.5 | −4.3 ± 1.5 |
| IV-Bolus Model | Two Comp. | Two Comp. | Three Comp. | Three Comp. |
|---|---|---|---|---|
| Number of Animals | n = 3 | n = 2 | n = 3 | n = 2 |
| PK Parameters | Values (±SD) | |||
| A | 542.53 ± 116.45 | 500.89 ± 129.30 | 640.14 ± 43.16 | 638.52 ± 60.91 |
| α | 3.71 ± 2.26 | 2.75 ± 2.18 | 12.28 ± 7.90 | |
| B | 168.95 ± 95.92 | 133.91 ± 105.05 | 330.39 ± 41.34 | |
| β | 0.34 ± 0.07 | 0.33 ± 0.10 | 0.70 ± 0.21 | 0.80 ± 0.19 |
| C | - | - | 10.56 ± 6.33 | 17.71 ± 15.74 |
| γ | - | - | 316.02 ± 38.40 | 0.09 ± 0.12 |
| Statistics | ||||
| R2 | 0.988 ± 0.010 | 0.983 ± 0.10 | 0.997 ± 0.002 | 0.996 ± 0.002 |
| AIC | −13.66 ± 5.21 | −12.22 ± 6.47 | −22.11 ± 8.22 | −21.94 ± 11.62 |
| Two-Compartment | Three-Compartment | |
|---|---|---|
| Weighted residual sum of square (WRSS) | 0.13 ± 0.07 | 0.04 ± 0.03 |
| Degrees of freedom (DF) | 6 | 4 |
FTable for α = 0.05 = 6.16 | (1) | |
| PK Parameters | Value (±SD) |
|---|---|
| V1 (mL) | 1512.43 ± 538.41 |
| Vss (mL) | 3260.35 ± 286.66 |
| k1-0 (h−1) | 1.04 ± 0.20 |
| k1-2 (h−1) | 1.80 ± 1.43 |
| k2-1 (h−1) | 1.20 ± 0.74 |
| Depth (k10/k12) | 0.58 |
| Capacity (k12/k21) | 1.50 |
| Cl (mL·h−1) | 1514.5 ± 261.16 |
| fc | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koch, N.; Jennotte, O.; Lechanteur, A.; Deville, M.; Charlier, C.; Cardot, J.-M.; Chiap, P.; Evrard, B. An Intravenous Pharmacokinetic Study of Cannabidiol Solutions in Piglets through the Application of a Validated Ultra-High-Pressure Liquid Chromatography Coupled to Tandem Mass Spectrometry Method for the Simultaneous Quantification of CBD and Its Carboxylated Metabolite in Plasma. Pharmaceutics 2024, 16, 140. https://doi.org/10.3390/pharmaceutics16010140
Koch N, Jennotte O, Lechanteur A, Deville M, Charlier C, Cardot J-M, Chiap P, Evrard B. An Intravenous Pharmacokinetic Study of Cannabidiol Solutions in Piglets through the Application of a Validated Ultra-High-Pressure Liquid Chromatography Coupled to Tandem Mass Spectrometry Method for the Simultaneous Quantification of CBD and Its Carboxylated Metabolite in Plasma. Pharmaceutics. 2024; 16(1):140. https://doi.org/10.3390/pharmaceutics16010140
Chicago/Turabian StyleKoch, Nathan, Olivier Jennotte, Anna Lechanteur, Marine Deville, Corinne Charlier, Jean-Michel Cardot, Patrice Chiap, and Brigitte Evrard. 2024. "An Intravenous Pharmacokinetic Study of Cannabidiol Solutions in Piglets through the Application of a Validated Ultra-High-Pressure Liquid Chromatography Coupled to Tandem Mass Spectrometry Method for the Simultaneous Quantification of CBD and Its Carboxylated Metabolite in Plasma" Pharmaceutics 16, no. 1: 140. https://doi.org/10.3390/pharmaceutics16010140
APA StyleKoch, N., Jennotte, O., Lechanteur, A., Deville, M., Charlier, C., Cardot, J.-M., Chiap, P., & Evrard, B. (2024). An Intravenous Pharmacokinetic Study of Cannabidiol Solutions in Piglets through the Application of a Validated Ultra-High-Pressure Liquid Chromatography Coupled to Tandem Mass Spectrometry Method for the Simultaneous Quantification of CBD and Its Carboxylated Metabolite in Plasma. Pharmaceutics, 16(1), 140. https://doi.org/10.3390/pharmaceutics16010140






