Preclinical Application of CEST MRI to Detect Early and Regional Tumor Response to Local Brain Tumor Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Drug-Loaded Liposomal Hydrogel
2.2. Liposome Characterization, Drug Loading Determination
2.3. Hydrogel Rheology Studies
2.4. Drug Release Test
2.5. Cytotoxicity Test
2.6. MR Imaging
2.7. Animal Protocol
2.8. Histological Examination
3. Results
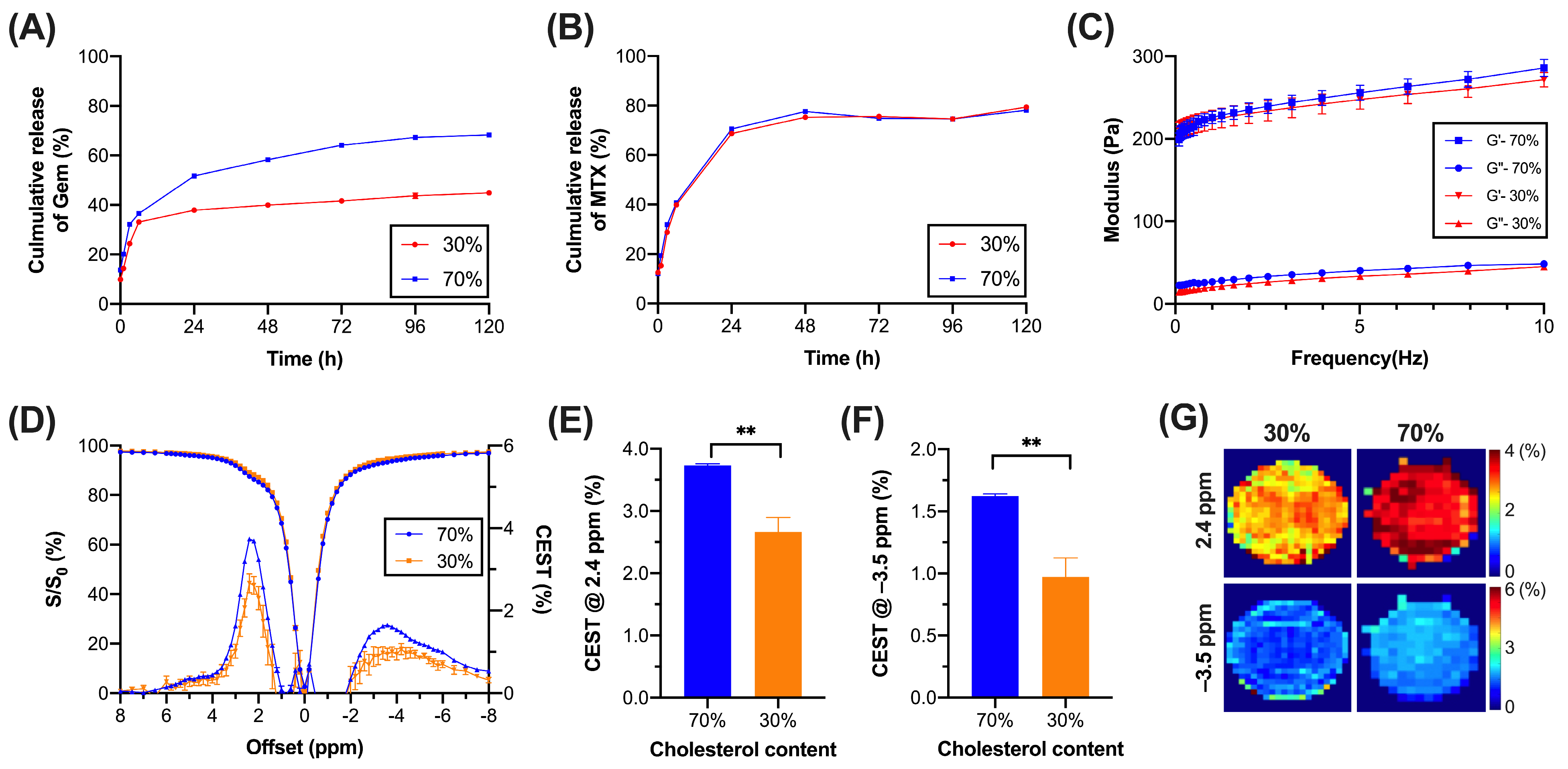
3.1. Liposomal Hydrogel with Sustainable Release and Low Rigidity
3.2. CEST Properties of Liposomal Hydrogel
3.3. Characterization of MGLH and LH
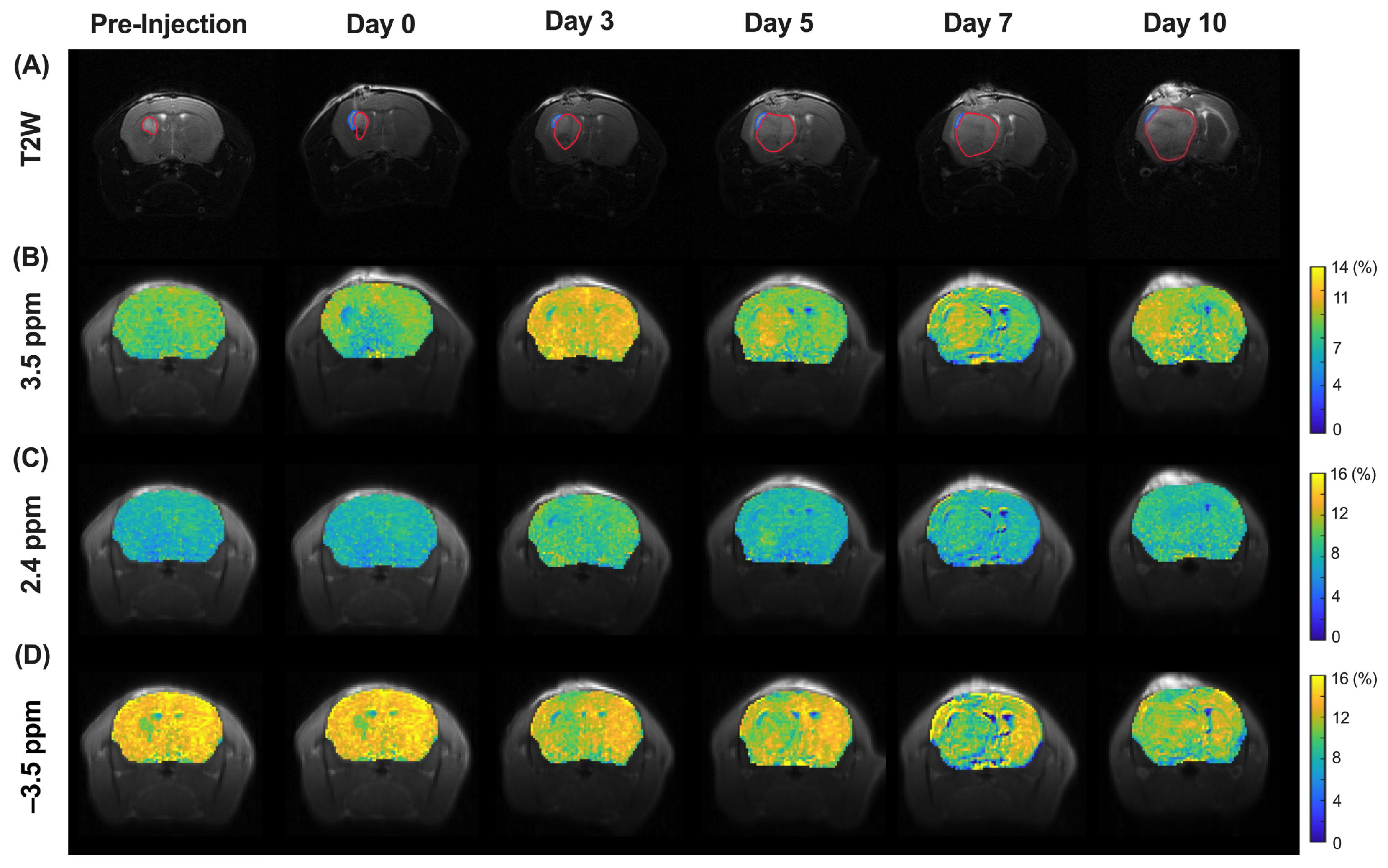
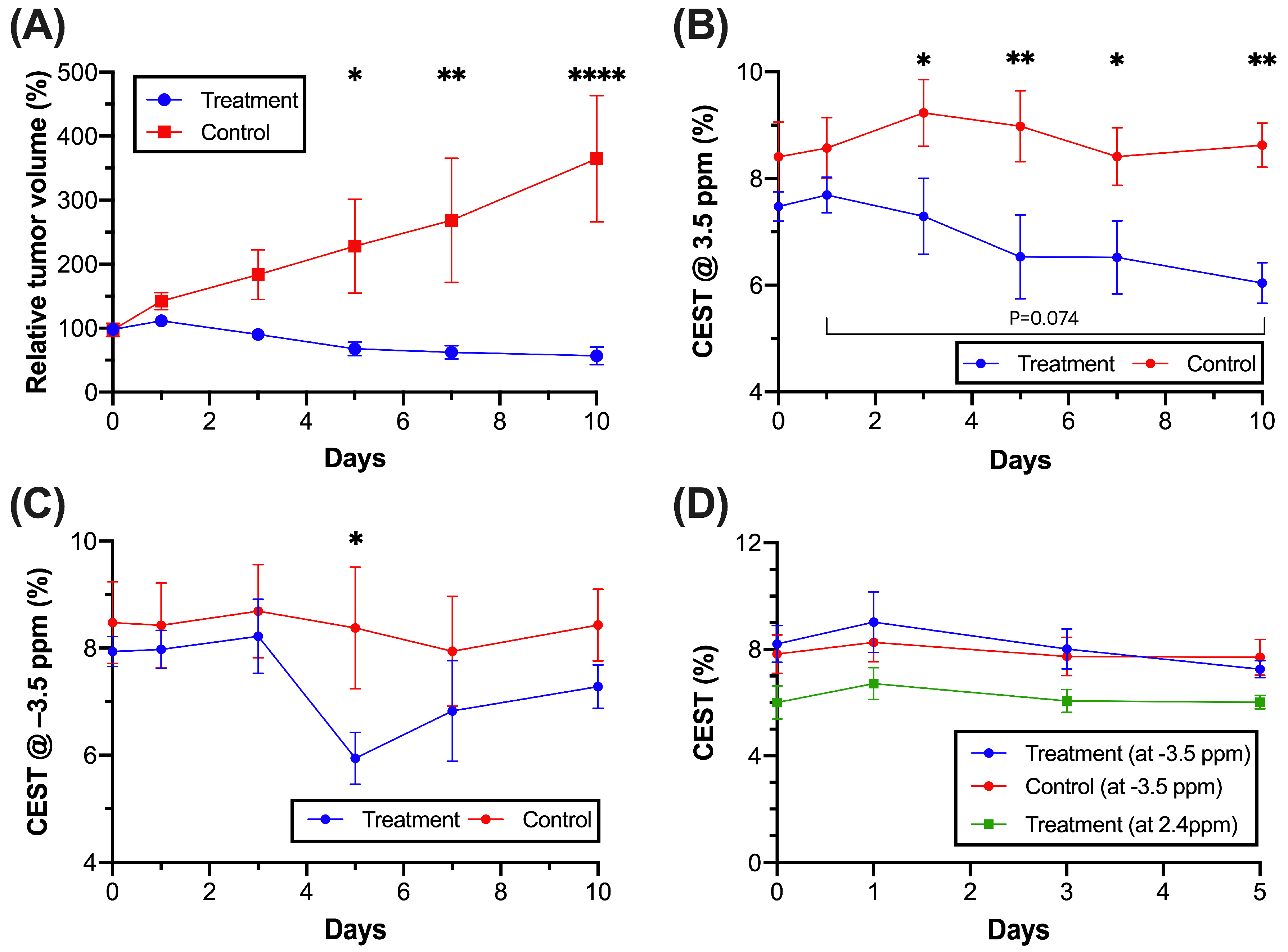
3.4. CEST MRI of Molecular and Morphological Change in Tumor Region during Treatment
3.5. CEST MRI of Liposome and Drug Release during Treatment
3.6. Regional Tumor Analysis Assessed by Histology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CEST | Chemical Exchange Saturation Transfer |
| MRI | Magnetic Resonance Imaging |
| APT | Amide Proton Transfer |
| rNOE | relayed Nuclear Overhauser Effect |
| MGLH | Methotrexate Gemcitabine encapsulated Liposomal Hydrogel |
| LH | Liposomal Hydrogel |
References
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neurooncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of care for treatment of recurrent glioblastoma—Are we there yet? Neuro-Oncology 2013, 15, 4–27. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncology 2013, 15 (Suppl. 2), ii1–ii56. [Google Scholar] [CrossRef]
- Westphal, M.; Ram, Z.; Riddle, V.; Hilt, D.; Bortey, E. Gliadel wafer in initial surgery for malignant glioma: Long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006, 148, 269–275; discussion 275. [Google Scholar] [CrossRef]
- Brem, H.; Piantadosi, S.; Burger, P.C.; Walker, M.; Selker, R.; Vick, N.A.; Black, K.; Sisti, M.; Brem, S.; Mohr, G.; et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet 1995, 345, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Tabet, A.; Jensen, M.P.; Parkins, C.C.; Patil, P.G.; Watts, C.; Scherman, O.A. Designing Next-Generation Local Drug Delivery Vehicles for Glioblastoma Adjuvant Chemotherapy: Lessons from the Clinic. Adv. Healthc. Mater. 2019, 8, 1801391. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.B.; Saltzman, W.M. Pharmacokinetics of the carmustine implant. Clin. Pharmacokinet. 2002, 41, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; To, A.K.W.; Lai, J.H.C.; Xiao, P.; Wu, E.X.; Xu, J.; Chan, K.W.Y. CEST MRI detectable liposomal hydrogels for multiparametric monitoring in the brain at 3T. Theranostics 2020, 10, 2215–2228. [Google Scholar] [CrossRef]
- Pathak, A.; Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. USA 2012, 109, 10334–10339. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Tabet, A.; Mommer, S.; Vigil, J.A.; Hallou, C.; Bulstrode, H.; Scherman, O.A. Mechanical Characterization of Human Brain Tissue and Soft Dynamic Gels Exhibiting Electromechanical Neuro-Mimicry. Adv. Healthc. Mater. 2019, 8, e1900068. [Google Scholar] [CrossRef]
- Budday, S.; Nay, R.; de Rooij, R.; Steinmann, P.; Wyrobek, T.; Ovaert, T.C.; Kuhl, E. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 2015, 46, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, T.A.; de Juan Pardo, E.M.; Kumar, S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009, 69, 4167–4174. [Google Scholar] [CrossRef] [PubMed]
- Polacheck, W.J.; Zervantonakis, I.K.; Kamm, R.D. Tumor cell migration in complex microenvironments. Cell. Mol. Life Sci. 2013, 70, 1335–1356. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Z.; Park, S.-W.; Lai, J.H.C.; Chan, K.W.Y. Molecular Imaging of Brain Tumors and Drug Delivery Using CEST MRI: Promises and Challenges. Pharmaceutics 2022, 14, 451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lal, B.; Wilson, D.A.; Laterra, J.; van Zijl, P.C. Amide proton transfer (APT) contrast for imaging of brain tumors. Magn. Reson. Med. 2003, 50, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Msayib, Y.; Harston, G.W.J.; Tee, Y.K.; Sheerin, F.; Blockley, N.P.; Okell, T.W.; Jezzard, P.; Kennedy, J.; Chappell, M.A. Quantitative CEST imaging of amide proton transfer in acute ischaemic stroke. Neuroimage Clin. 2019, 23, 101833. [Google Scholar] [CrossRef]
- Chen, L.; van Zijl, P.C.M.; Wei, Z.; Lu, H.; Duan, W.; Wong, P.C.; Li, T.; Xu, J. Early detection of Alzheimer’s disease using creatine chemical exchange saturation transfer magnetic resonance imaging. Neuroimage 2021, 236, 118071. [Google Scholar] [CrossRef]
- Huang, J.; van Zijl, P.C.M.; Han, X.; Dong, C.M.; Cheng, G.W.Y.; Tse, K.H.; Knutsson, L.; Chen, L.; Lai, J.H.C.; Wu, E.X.; et al. Altered d-glucose in brain parenchyma and cerebrospinal fluid of early Alzheimer’s disease detected by dynamic glucose-enhanced MRI. Sci. Adv. 2020, 6, eaba3884. [Google Scholar] [CrossRef]
- Huang, J.; Xu, J.; Lai, J.H.C.; Chen, Z.; Lee, C.Y.; Mak, H.K.F.; Chan, K.H.; Chan, K.W.Y. Relayed nuclear Overhauser effect weighted (rNOEw) imaging identifies multiple sclerosis. Neuroimage Clin. 2021, 32, 102867. [Google Scholar] [CrossRef] [PubMed]
- van Zijl, P.C.; Yadav, N.N. Chemical exchange saturation transfer (CEST): What is in a name and what isn’t? Magn. Reson. Med. 2011, 65, 927–948. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Xu, J.; Yadav, N.N.; Chan, K.W.; Luo, L.; McMahon, M.T.; Vogelstein, B.; van Zijl, P.C.; Zhou, S.; et al. CEST theranostics: Label-free MR imaging of anticancer drugs. Oncotarget 2016, 7, 6369–6378. [Google Scholar] [CrossRef]
- Jones, C.K.; Schlosser, M.J.; van Zijl, P.C.; Pomper, M.G.; Golay, X.; Zhou, J. Amide proton transfer imaging of human brain tumors at 3T. Magn. Reson. Med. 2006, 56, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.K.; Huang, A.; Xu, J.; Edden, R.A.; Schär, M.; Hua, J.; Oskolkov, N.; Zacà, D.; Zhou, J.; McMahon, M.T.; et al. Nuclear Overhauser enhancement (NOE) imaging in the human brain at 7T. Neuroimage 2013, 77, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Goerke, S.; Soehngen, Y.; Deshmane, A.; Zaiss, M.; Breitling, J.; Boyd, P.S.; Herz, K.; Zimmermann, F.; Klika, K.D.; Schlemmer, H.P.; et al. Relaxation-compensated APT and rNOE CEST-MRI of human brain tumors at 3 T. Magn. Reson. Med. 2019, 82, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Payen, J.F.; Wilson, D.A.; Traystman, R.J.; van Zijl, P.C. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat. Med. 2003, 9, 1085–1090. [Google Scholar] [CrossRef]
- Zhou, J.; Zaiss, M.; Knutsson, L.; Sun, P.Z.; Ahn, S.S.; Aime, S.; Bachert, P.; Blakeley, J.O.; Cai, K.; Chappell, M.A.; et al. Review and consensus recommendations on clinical APT-weighted imaging approaches at 3T: Application to brain tumors. Magn. Reson. Med. 2022, 88, 546–574. [Google Scholar] [CrossRef]
- Zu, Z.; Lin, E.C.; Louie, E.A.; Xu, J.; Li, H.; Xie, J.; Lankford, C.L.; Chekmenev, E.Y.; Swanson, S.D.; Does, M.D.; et al. Relayed nuclear Overhauser enhancement sensitivity to membrane Cho phospholipids. Magn. Reson. Med. 2020, 84, 1961–1976. [Google Scholar] [CrossRef]
- Huang, J.; Han, X.; Chen, L.; Xu, X.; Xu, J.; Chan, K.W.Y. Relayed nuclear Overhauser enhancement imaging with magnetization transfer contrast suppression at 3T. Magn. Reson. Med. 2021, 85, 254–267. [Google Scholar] [CrossRef]
- Han, X.; Lai, J.H.C.; Huang, J.; Park, S.W.; Liu, Y.; Chan, K.W.Y. Imaging Self-Healing Hydrogels and Chemotherapeutics Using CEST MRI at 3 T. ACS Appl. Bio Mater. 2021, 4, 5605–5616. [Google Scholar] [CrossRef] [PubMed]
- Sagiyama, K.; Mashimo, T.; Togao, O.; Vemireddy, V.; Hatanpaa, K.J.; Maher, E.A.; Mickey, B.E.; Pan, E.; Sherry, A.D.; Bachoo, R.M.; et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc. Natl. Acad. Sci. USA 2014, 111, 4542–4547. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.K.; Polders, D.; Hua, J.; Zhu, H.; Hoogduin, H.J.; Zhou, J.; Luijten, P.; van Zijl, P.C. In vivo three-dimensional whole-brain pulsed steady-state chemical exchange saturation transfer at 7 T. Magn. Reson. Med. 2012, 67, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, M.; Schmitt, B.; Bachert, P. Quantitative separation of CEST effect from magnetization transfer and spillover effects by Lorentzian-line-fit analysis of z-spectra. J. Magn. Reson. 2011, 211, 149–155. [Google Scholar] [CrossRef]
- Demel, R.A.; De Kruyff, B. The function of sterols in membranes. Biochim. Biophys. Acta Biomembr. 1976, 457, 109–132. [Google Scholar] [CrossRef]
- Mohammed, A.; Weston, N.; Coombes, A.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shi, A.; Wang, Q.; Zhang, G. Multivesicular Liposomes for the Sustained Release of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Peanuts: Design, Characterization, and In Vitro Evaluation. Molecules 2019, 24, 1746. [Google Scholar] [CrossRef]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: A marriage of convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Johannessen, M.; Gravningen, K.; Puolakkainen, M.; Acharya, G.; Basnet, P.; Škalko-Basnet, N. Liposomes-In-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection. Pharmaceutics 2020, 12, 1203. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, P.; Hu, M.; Huang, W.; Zhu, X.; Yan, D. Self-Delivery Nanoparticles of Amphiphilic Methotrexate-Gemcitabine Prodrug for Synergistic Combination Chemotherapy via Effect of Deoxyribonucleotide Pools. Bioconjug. Chem. 2016, 27, 2722–2733. [Google Scholar] [CrossRef]
- Forte, A.E.; Gentleman, S.M.; Dini, D. On the characterization of the heterogeneous mechanical response of human brain tissue. Biomech. Model. Mechanobiol. 2017, 16, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Parkins, C.C.; McAbee, J.H.; Ruff, L.; Wendler, A.; Mair, R.; Gilbertson, R.J.; Watts, C.; Scherman, O.A. Mechanically matching the rheological properties of brain tissue for drug-delivery in human glioblastoma models. Biomaterials 2021, 276, 120919. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.; Halicek, M.; Fabelo, H.; Camacho, R.; Plaza, M.L.; Godtliebsen, F.; Callicó, G.M.; Fei, B. Hyperspectral Imaging for the Detection of Glioblastoma Tumor Cells in H&E Slides Using Convolutional Neural Networks. Sensors 2020, 20, 1911. [Google Scholar] [PubMed]
- Garrity, M.M.; Burgart, L.J.; Riehle, D.L.; Hill, E.M.; Sebo, T.J.; Witzig, T. Identifying and Quantifying Apoptosis: Navigating Technical Pitfalls. Mod. Pathol. 2003, 16, 389–394. [Google Scholar] [CrossRef]
- Ellingson, B.M.; Wen, P.Y.; van den Bent, M.J.; Cloughesy, T.F. Pros and cons of current brain tumor imaging. Neuro-Oncology 2014, 16 (Suppl. 7), vii2–vii11. [Google Scholar] [CrossRef]
- de Sousa Cavalcante, L.; Monteiro, G. Gemcitabine: Metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur. J. Pharmacol. 2014, 741, 8–16. [Google Scholar] [CrossRef]
- Spurlock, C.F., 3rd; Aune, Z.T.; Tossberg, J.T.; Collins, P.L.; Aune, J.P.; Huston, J.W., 3rd; Crooke, P.S.; Olsen, N.J.; Aune, T.M. Increased sensitivity to apoptosis induced by methotrexate is mediated by JNK. Arthritis Rheum. 2011, 63, 2606–2616. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-W.; Lai, J.H.C.; Han, X.; Leung, V.W.M.; Xiao, P.; Huang, J.; Chan, K.W.Y. Preclinical Application of CEST MRI to Detect Early and Regional Tumor Response to Local Brain Tumor Treatment. Pharmaceutics 2024, 16, 101. https://doi.org/10.3390/pharmaceutics16010101
Park S-W, Lai JHC, Han X, Leung VWM, Xiao P, Huang J, Chan KWY. Preclinical Application of CEST MRI to Detect Early and Regional Tumor Response to Local Brain Tumor Treatment. Pharmaceutics. 2024; 16(1):101. https://doi.org/10.3390/pharmaceutics16010101
Chicago/Turabian StylePark, Se-Weon, Joseph H. C. Lai, Xiongqi Han, Vivian W. M. Leung, Peng Xiao, Jianpan Huang, and Kannie W. Y. Chan. 2024. "Preclinical Application of CEST MRI to Detect Early and Regional Tumor Response to Local Brain Tumor Treatment" Pharmaceutics 16, no. 1: 101. https://doi.org/10.3390/pharmaceutics16010101
APA StylePark, S.-W., Lai, J. H. C., Han, X., Leung, V. W. M., Xiao, P., Huang, J., & Chan, K. W. Y. (2024). Preclinical Application of CEST MRI to Detect Early and Regional Tumor Response to Local Brain Tumor Treatment. Pharmaceutics, 16(1), 101. https://doi.org/10.3390/pharmaceutics16010101






