Electrosprayed Core (Cellulose Acetate)–Shell (Polyvinylpyrrolidone) Nanoparticles for Smart Acetaminophen Delivery
Abstract
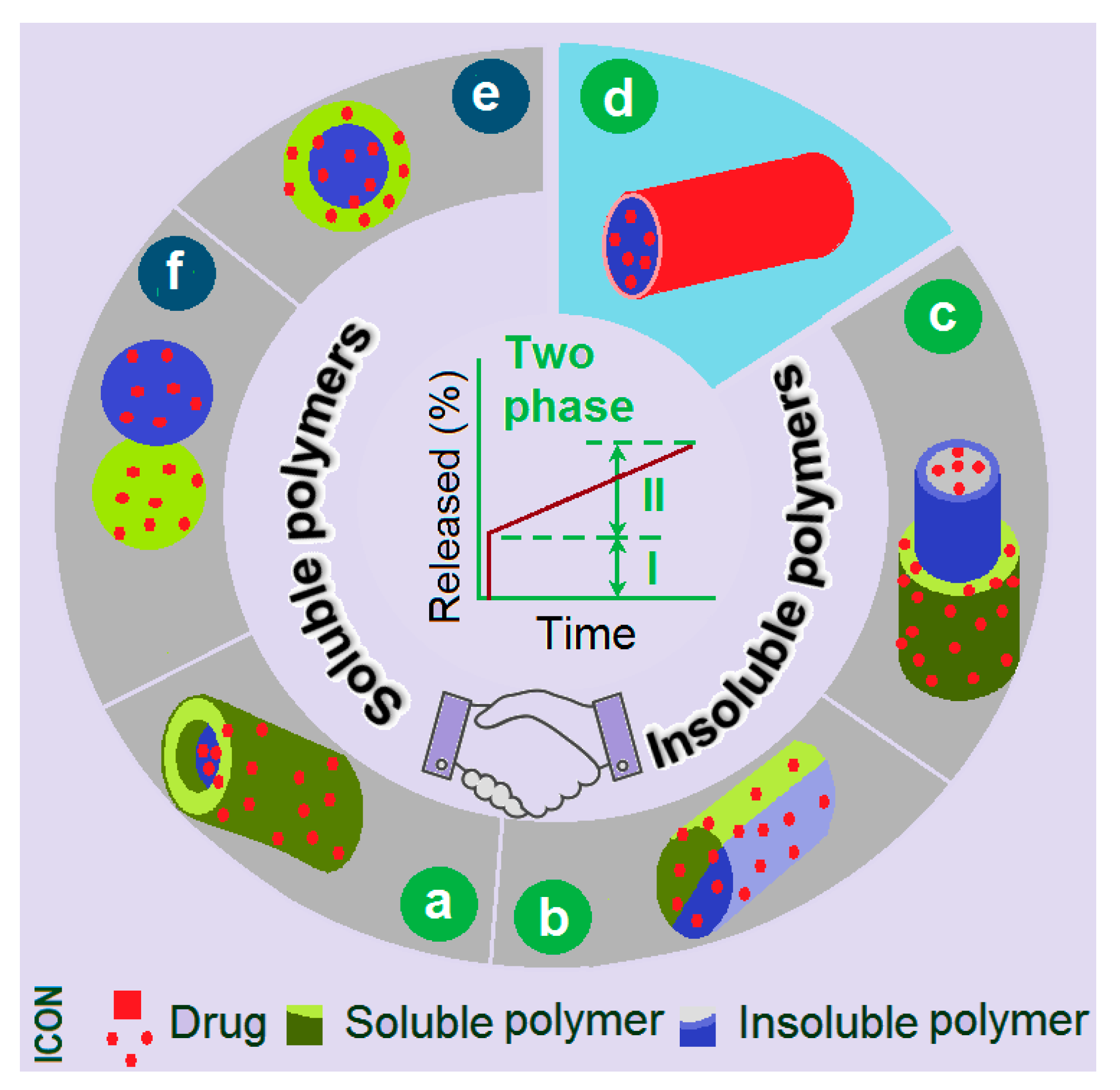
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
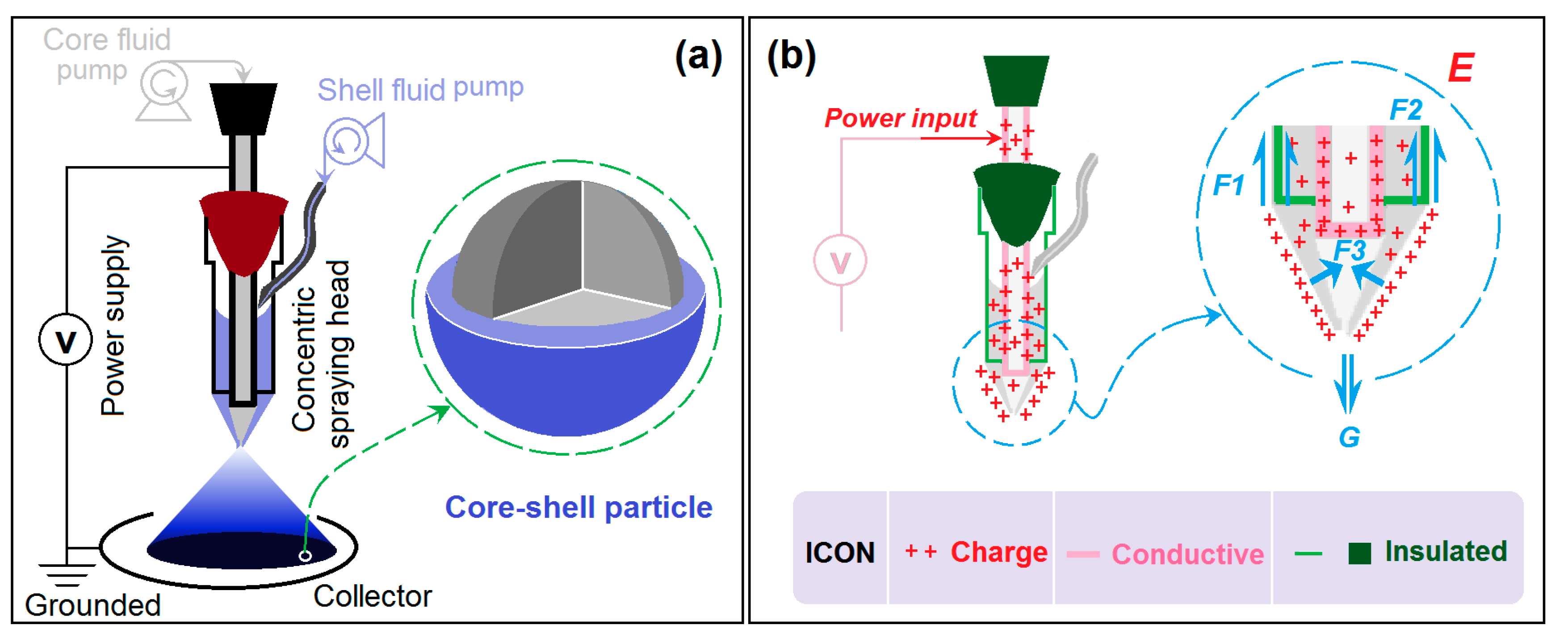
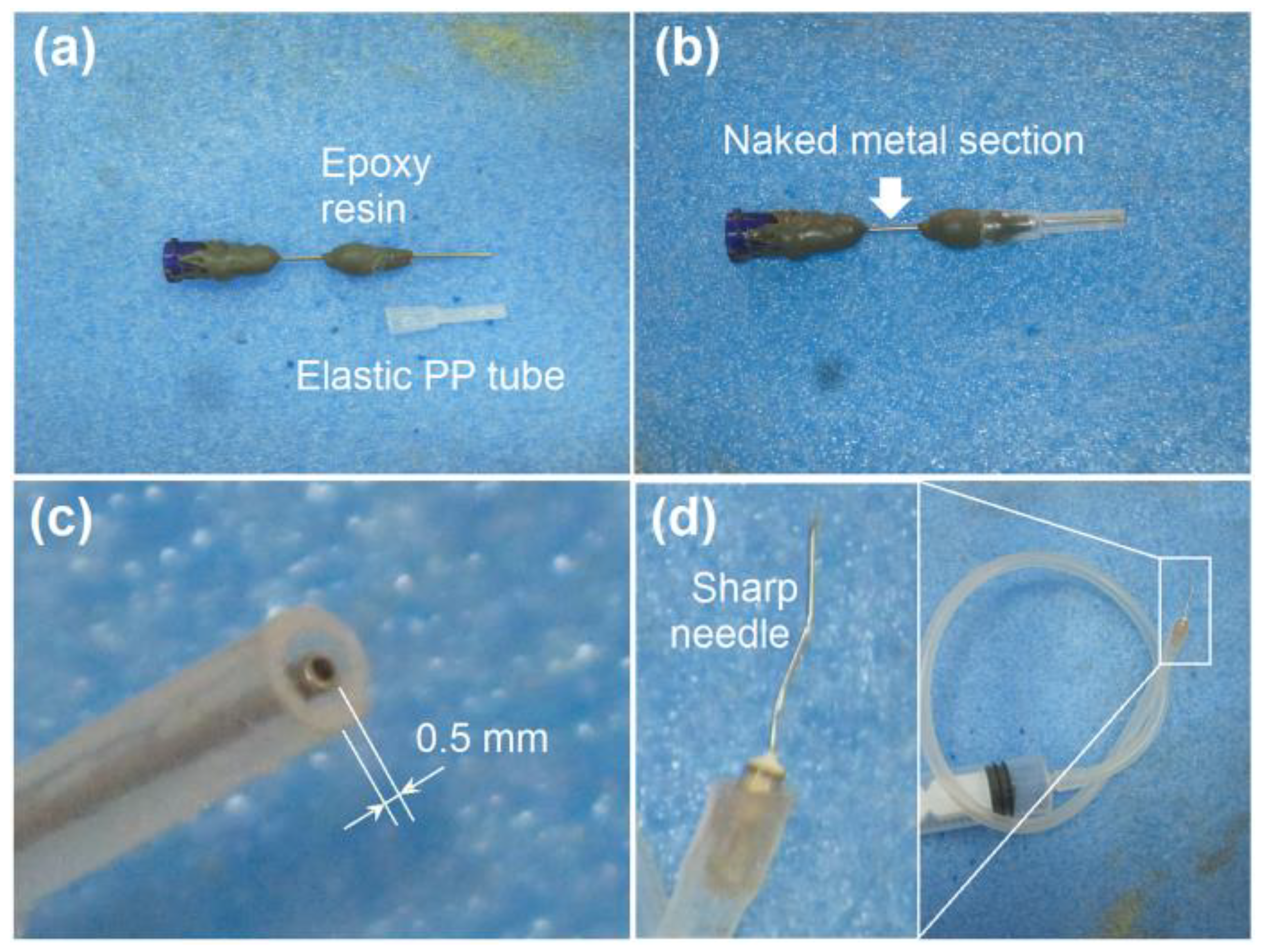
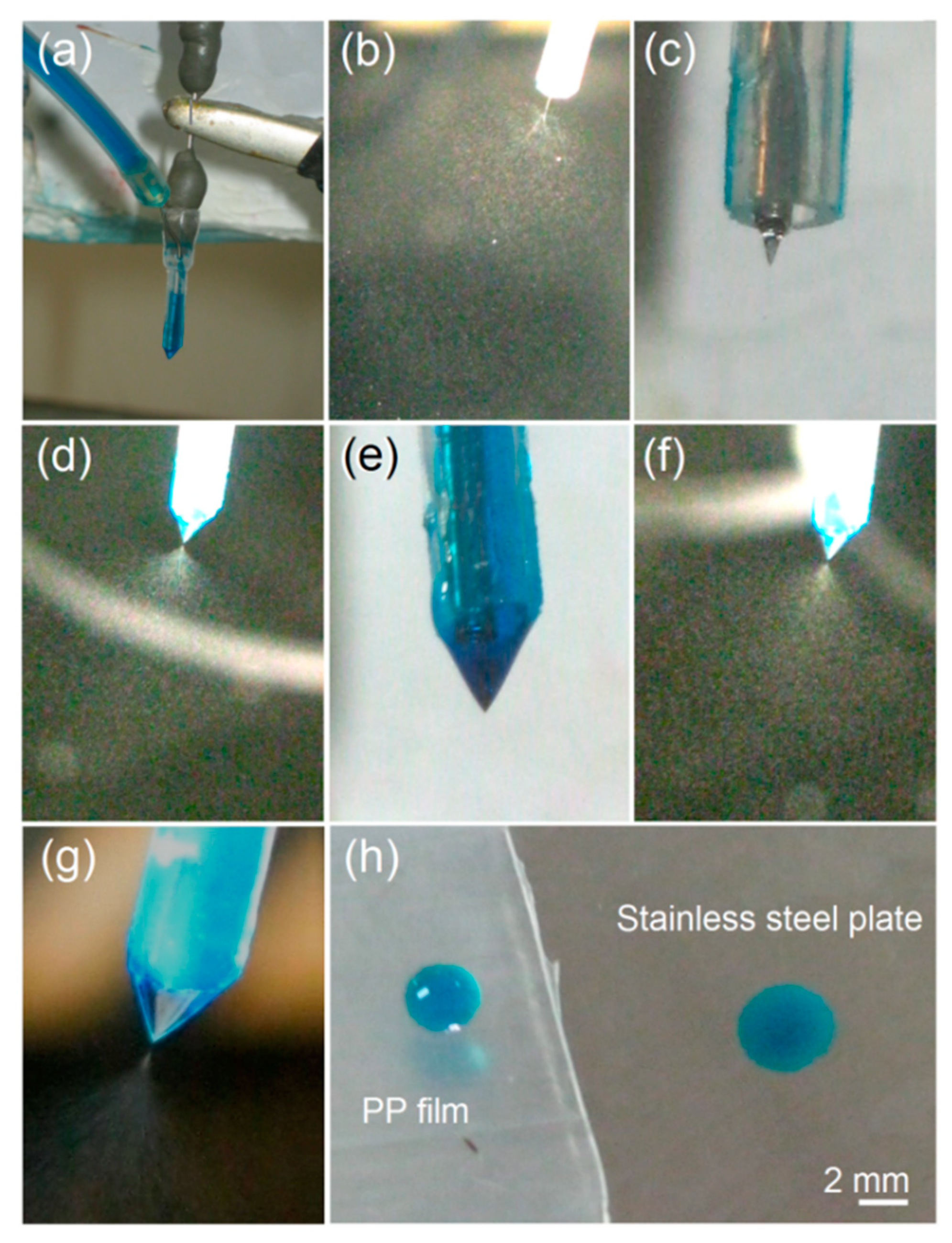
Modified Coaxial Electrospraying
2.3. Characterizations
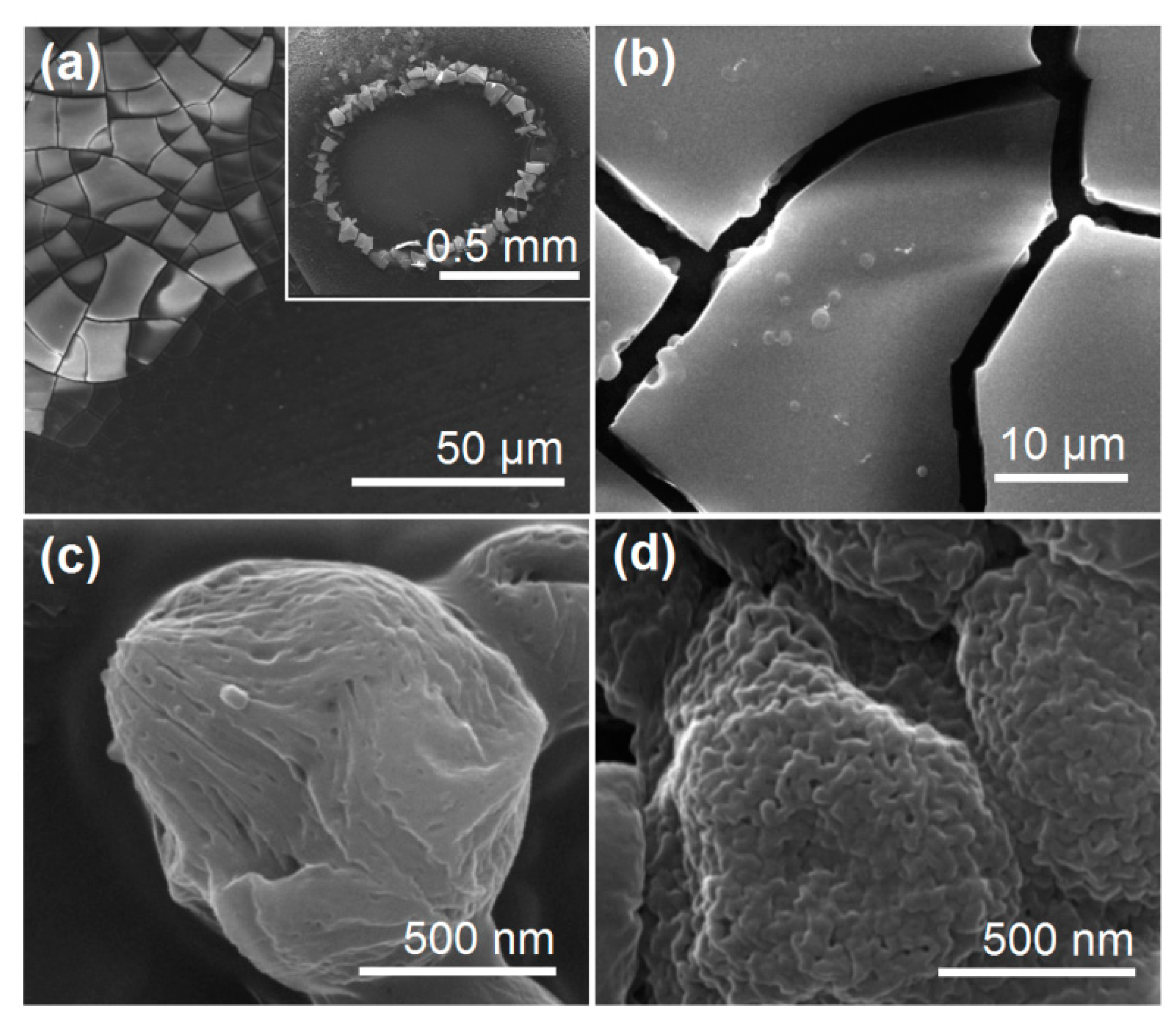
2.3.1. Morphology and Inner Structure
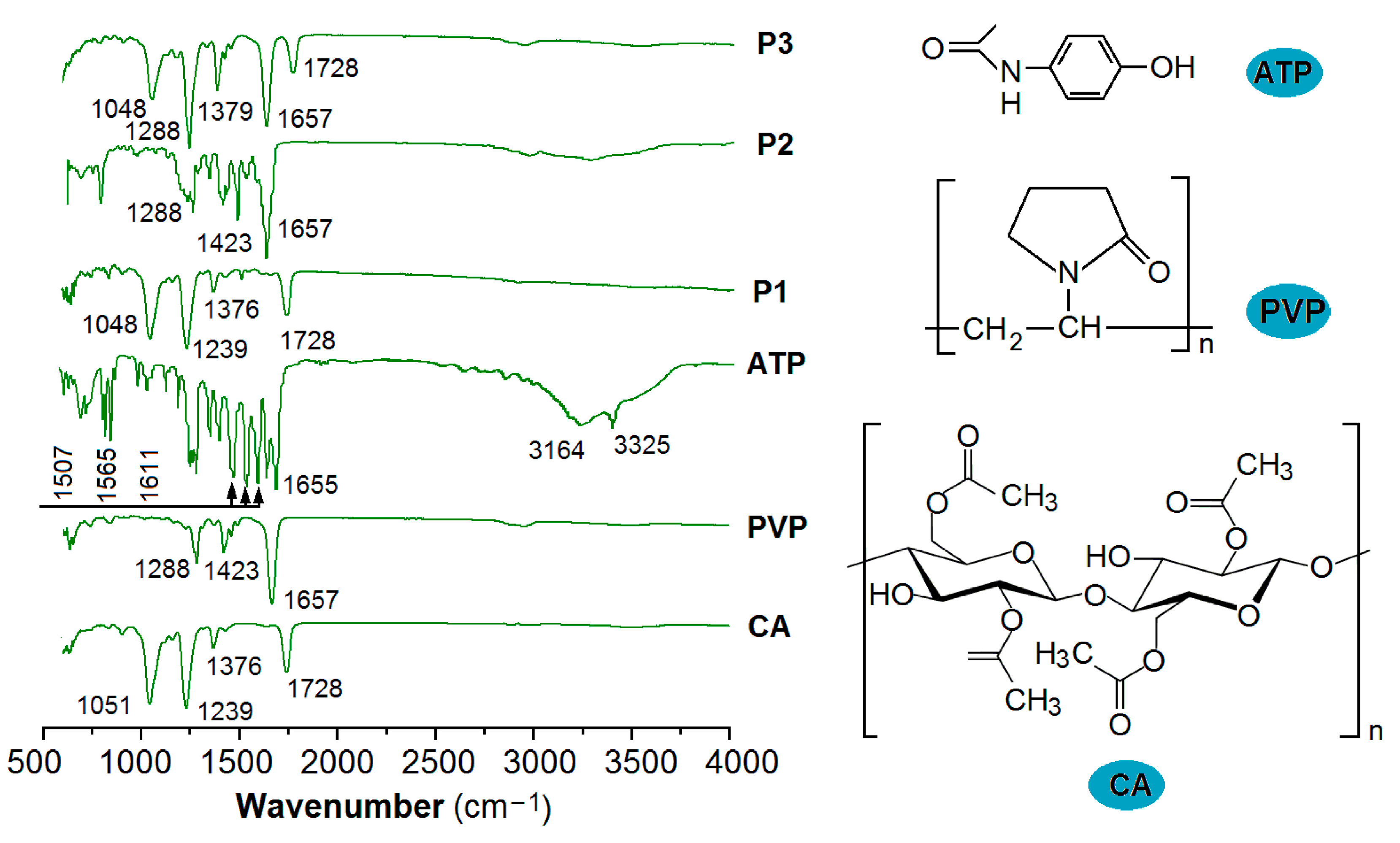
2.3.2. Physical State and Compatibility
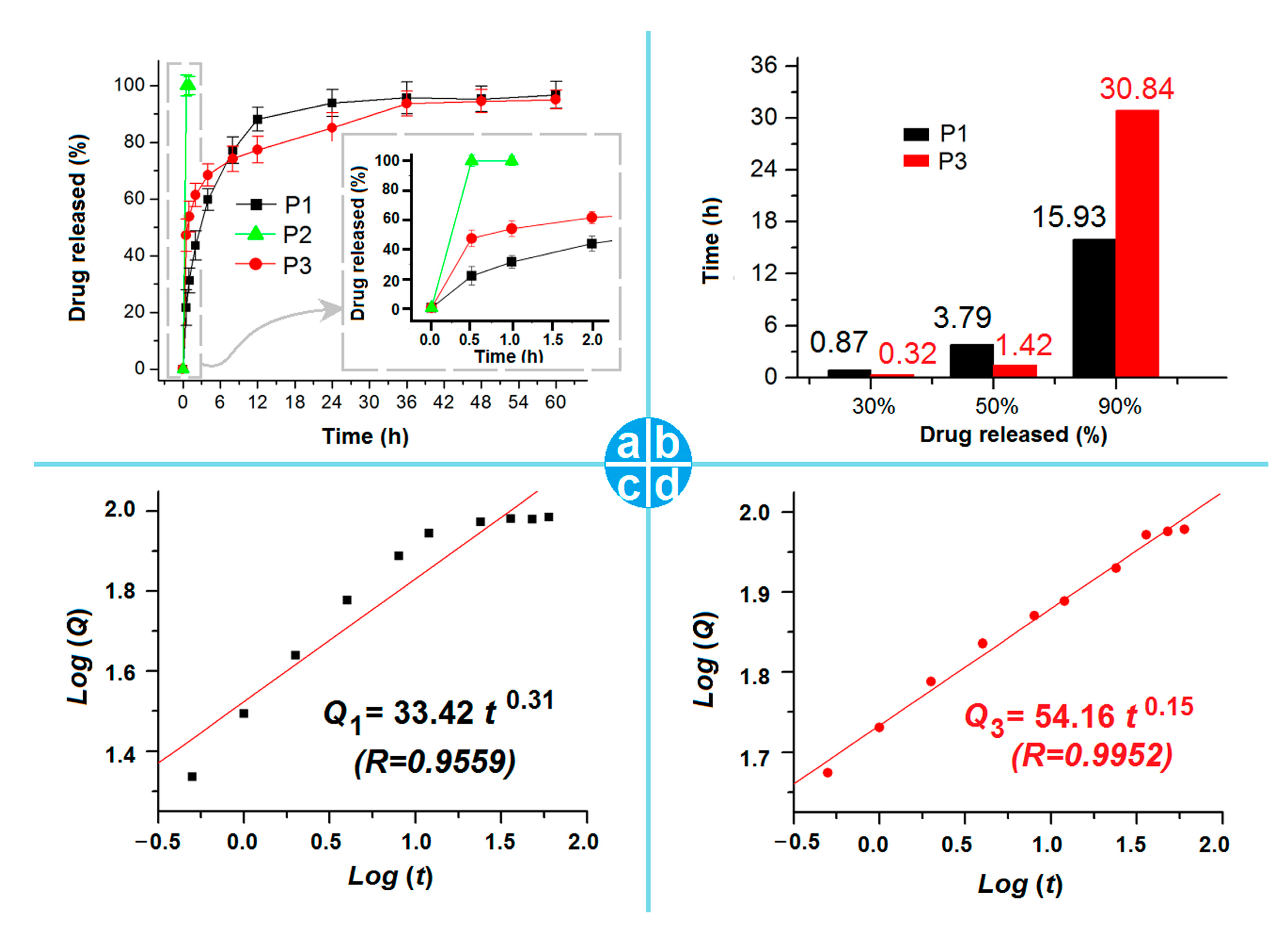
2.4. Drug Encapsulation Efficiency and the In Vitro Drug Release Profiles
3. Results and Discussion
3.1. The Modified Coaxial Electrospraying Process
3.2. The Morphologies and Inner Structures of the Electrosprayed Particles
3.3. The Physical State and Compatibility
3.4. The Drug EE% and In Vitro Release Profile
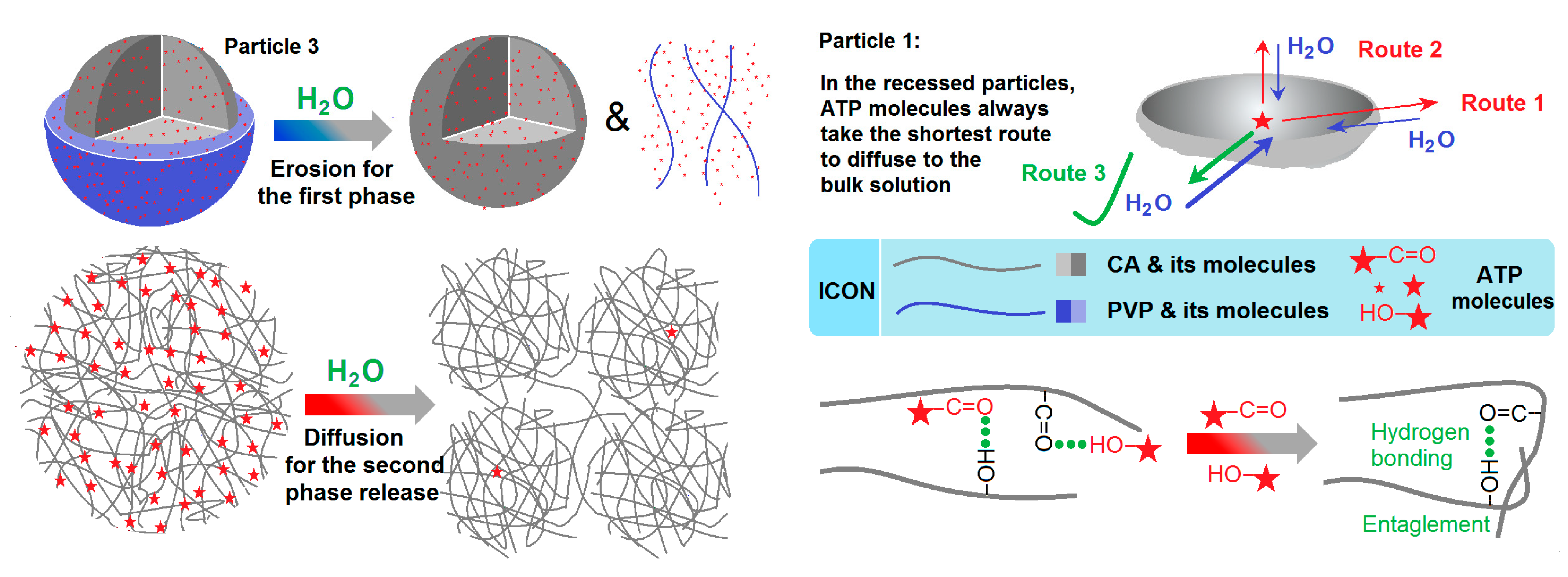
3.5. The Biphasic Release Mechanism from the CA-Based Hydrogel Particles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, W.; Li, T. Deepening the Understanding of the in Vivo and Cellular Fate of Nanocarriers. Adv. Drug Deliv. Rev. 2022, 189, 114529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Geng, C.; Shen, H.; Zhang, Q.; Miao, Y.; Wu, J.; Ouyang, R.; Zhou, S. Two Hawks with One Arrow: A Review on Bifunctional Scaffolds for Photothermal Therapy and Bone Regeneration. Nanomaterials 2023, 13, 551. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Liu, Z.; He, H.; Lu, Y.; Qi, J.; Wu, W. “Hook&Loop” Multivalent Interactions Based on Disk-Shaped Nanoparticles Strengthen Active Targeting. J. Control Release 2023, 354, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, L.; Chunta, S.; Wu, W.; Chen, Z.; Lu, Y. Enhanced Oral Bioavailability from Food Protein Nanoparticles: A Mini Review. J. Control Release 2023, 354, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ji, X.; Zhang, Y.; Liu, C.; Zhang, Z.; Lv, Y.; Dong, X.; He, H.; Qi, J.; Lu, Y.; et al. Near-Infrared Fluorophores with Absolute Aggregation-Caused Quenching and Negligible Fluorescence Re-Illumination for in Vivo Bioimaging of Nanocarriers. Aggregate 2022, 4, e277. [Google Scholar] [CrossRef]
- He, H.; Liu, C.; Ming, J.; Lv, Y.; Qi, J.; Lu, Y.; Dong, X.; Zhao, W.; Wu, W. Accurate and Sensitive Probing of Onset of Micellization Based on Absolute Aggregation-Caused Quenching Effect. Aggregate 2022, 3, e163. [Google Scholar] [CrossRef]
- Murugesan, R.; Raman, S. Recent Trends in Carbon Nanotubes Based Prostate Cancer Therapy: A Biomedical Hybrid for Diagnosis and Treatment. Curr. Drug Deliv. 2022, 19, 229–237. [Google Scholar] [CrossRef]
- Man, F.; Yang, Y.; He, H.; Qi, J.; Wu, W.; Lu, Y. Establishment of In Vitro Dissolution Based on Similarity with In Vivo Dissolution: A Case Study on Aripiprazole. Mol. Pharm. 2023, 20, 2579–2588. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, Q.; Li, Y.; Silva, D.Z.; Ruiz, M.E.L.; Ouyang, R.; Liu, B.; Miao, Y. Recent Development of Rhenium-Based Materials in the Application of Diagnosis and Tumor Therapy. Molecules 2023, 28, 2733. [Google Scholar] [CrossRef]
- Agrawal, A.; Joshi, A.; Bhattacharya, S. Recent Excavation of Nanoethosomes in Current Drug Delivery. Curr. Drug Deliv. 2022, 20, 1–16. [Google Scholar] [CrossRef]
- Bobokalonov, J.; Muhidinov, Z.; Nasriddinov, A.; Jomnurodov, A.; Khojaeva, F.; Komilova, G.; Yusufi, S.; Liu, L. Evaluation of Extended-Release of Piroxicam-Loaded Pectin-Zein Hydrogel Microspheres: In Vitro, Ex Vivo, and In Vivo Studies. Curr. Drug Deliv. 2022, 19, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Spasova, M.; Stoyanova, N.; Nachev, N.; Ignatova, M.; Manolova, N.; Rashkov, I.; Georgieva, A.; Toshkova, R.; Markova, N. Innovative Fibrous Materials Loaded with 5-Nitro-8-hydroxyquinoline via Electrospinning/Electrospraying Demonstrate Antioxidant, Antimicrobial and Anticancer Activities. Antioxidants 2023, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Feng, C. Aniline Dimers Serving as Stable and Efficient Transfer Units for Intermolecular Charge-Carrier Transmission. iScience 2023, 26, 105762. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Chauhan, L.S. Effect of Surfactant Chain Length on Emulsification Dynamics of Self Emulsifying Formulation of Poorly Soluble Drug. Curr. Drug Deliv. 2022, 19, 874–888. [Google Scholar] [CrossRef] [PubMed]
- Strnad, S.; Zemljič, L.F. Cellulose–Chitosan Functional Biocomposites. Polymers 2023, 15, 425. [Google Scholar] [CrossRef]
- Cai, Y.; Qi, J.; Lu, Y.; He, H.; Wu, W. The in Vivo Fate of Polymeric Micelles. Adv. Drug Deliv. Rev. 2022, 188, 114463. [Google Scholar] [CrossRef]
- Fan, W.; Peng, H.; Yu, Z.; Wang, L.; He, H.; Ma, Y.; Qi, J.; Lu, Y.; Wu, W. The Long-Circulating Effect of Pegylated Nanoparticles Revisited via Simultaneous Monitoring of Both the Drug Payloads and Nanocarriers. Acta Pharm. Sin. B 2022, 12, 2479–2493. [Google Scholar] [CrossRef]
- Morais, D.C.; Fontes, M.L.; Oliveira, A.B.; Gabbai-Armelin, P.R.; Ferrisse, T.M.; De Oliveira, L.F.C.; Brighenti, F.L.; Barud, H.S.; De Sousa, F.B. Combining Polymer and Cyclodextrin Strategy for Drug Release of Sulfadiazine from Electrospun Fibers. Pharmaceutics 2023, 15, 1890. [Google Scholar] [CrossRef]
- Tan, P.K.; Kuppusamy, U.R.; Chua, K.H.; Arumugam, B. Emerging Strategies to Improve the Stability and Bioavailability of Insulin: An Update on Formulations and Delivery Approaches. Curr. Drug Deliv. 2022, 20, 1141–1162. [Google Scholar] [CrossRef]
- Phan, N.T.; Tran, Y.T.H.; Nguyen, L.T.; Hoang, Y.K.; Bui, C.K.; Nguyen, H.D.; Vu, G.T.T. Self Nanoelmusifying Drug Delivery System of Rosuvastatin: Bioavailability Evaluation and In Vitro–In Vivo Correlation. Curr. Drug Deliv. 2022, 20, 1–10. [Google Scholar] [CrossRef]
- Al-Nimry, S.S.; Khanfar, M.S. Enhancement of the Solubility of Asenapine Maleate Through the Preparation of Co-Crystals. Curr. Drug Deliv. 2022, 19, 788–800. [Google Scholar] [CrossRef]
- Gupta, N.; Yadav, V.; Patel, R. A Brief Review of the Essential Role of Nanovehicles for Improving the Therapeutic Efficacy of Pharmacological Agents against Tumours. Curr. Drug Deliv. 2022, 19, 301–316. [Google Scholar] [CrossRef]
- Elbhnsawi, N.A.; Elwakil, B.H.; Hassanin, A.H.; Shehata, N.; Elshewemi, S.S.; Hagar, M.; Olama, Z.A. Nano-Chitosan/Eucalyptus Oil/Cellulose Acetate Nanofibers: Manufacturing, Antibacterial and Wound Healing Activities. Membranes 2023, 13, 604. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Yang, H.; Yu, D.-G.; Lu, X. The Influence of the Ultrasonic Treatment of Working Fluids on Electrospun Amorphous Solid Dispersions. Front. Mol. Biosci. 2023, 10, 1184767. [Google Scholar] [CrossRef] [PubMed]
- Almoshari, Y. Osmotic Pump Drug Delivery Systems—A Comprehensive Review. Pharmaceuticals 2022, 15, 1430. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhou, X.; Xiao, B.; Duan, L.; Zhu, Z. Mucus-Penetrating Silk Fibroin-Based Nanotherapeutics for Efficient Treatment of Ulcerative Colitis. Biomolecules 2022, 12, 1263. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, X.; Cui, J.; Yu, F.; Liu, M.; Chen, Y.; Wu, J.; Sun, B.; Mo, X. Development of Biodegradable Polymeric Stents for the Treatment of Cardiovascular Diseases. Biomolecules 2022, 12, 1245. [Google Scholar] [CrossRef] [PubMed]
- Ajalli, N.; Pourmadadi, M.; Yazdian, F.; Abdouss, M.; Rashedi, H.; Rahdar, A. PVA Based Nanofiber Containing GO Modified with Cu Nanoparticles and Loaded Curcumin; High Antibacterial Activity with Acceleration Wound Healing. Curr. Drug Deliv. 2023, 20, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díaz, M.A.; Alvarado-Gómez, E.; Martínez-Pardo, M.E.; José Yacamán, M.; Flores-Santos, A.; Sánchez-Sánchez, R.; Martínez-Gutiérrez, F.; Bach, H. Development of Radiosterilized Porcine Skin Electrosprayed with Silver Nanoparticles Prevents Infections in Deep Burns. Int. J. Mol. Sci. 2022, 23, 13910. [Google Scholar] [CrossRef]
- Ejeta, F.; Gabriel, T.; Joseph, N.M.; Belete, A. Formulation, Optimization and In Vitro Evaluation of Fast Disintegrating Tablets of Salbutamol Sulphate Using a Combination of Superdisintegrantand Subliming Agent. Curr. Drug Deliv. 2022, 19, 129–141. [Google Scholar] [CrossRef]
- Köse, M.D.; Ungun, N.; Bayraktar, O. Eggshell Membrane Based Turmeric Extract Loaded Orally Disintegrating Films. Curr. Drug Deliv. 2022, 19, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Kolisnyk, O.; Vashchenko, O.; Ruban, O.; Fil, N.; Slipchenko, G. Assessing Compatibility of Excipients Selected for a Sustained Release Formulation of Bilberry Leaf Extract. Braz. J. Pharm. Sci. 2022, 58, e19753. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-layer Nanodepots for Sustained Release of Acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Rieber, J.; Meier-Bürgisser, G.; Miescher, I.; Weber, F.E.; Wolint, P.; Yao, Y.; Ongini, E.; Milionis, A.; Snedeker, J.G.; Calcagni, M.; et al. Bioactive and Elastic Emulsion Electrospun DegraPol Tubes Delivering IGF-1 for Tendon Rupture Repair. Int. J. Mol. Sci. 2023, 24, 10272. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, P.; Yu, D.-G.; Zhu, Y. Biphasic Drug Release from Electrospun Structures. Expert Opin. Drug Deliv. 2023, 20, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, X.; Liu, P.; Zhang, M.; Wang, C.; Zhang, B. Multifunctional Chitosan/Polycaprolactone Nanofiber Scaffolds with Varied Dual-Drug Release for Wound-Healing Applications. ACS Biomater. Sci. Eng. 2020, 6, 4666–4676. [Google Scholar] [CrossRef]
- Zhou, J.; Dai, Y.; Fu, J.; Yan, C.; Yu, D.-G.; Yi, T. Dual-Step Controlled Release of Berberine Hydrochloride from the Trans-Scale Hybrids of Nanofibers and Microparticles. Biomolecules 2023, 13, 1011. [Google Scholar] [CrossRef]
- Kanak, N.A.; Shahruzzaman, M.; Islam, M.S.; Takafuji, M.; Rahman, M.M.; Kabir, S.F. Fabrication of Electrospun PLA-nHAp Nanocomposite for Sustained Drug Release in Dental and Orthopedic Applications. Materials 2023, 16, 3691. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, L.; Yu, C.; Chen, J.; Ye, X.; Zhang, F.; Linhardt, R.J.; Chen, S.; Pan, H. One-Pot Self-Assembly of Core-Shell Nanoparticles within Fibers by Coaxial Electrospinning for Intestine-Targeted Delivery of Curcumin. Foods 2023, 12, 1623. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Gong, W.; Wang, B.; Yu, D.-G.; Zhu, Y. Integrating Chinese Herbs and Western Medicine for New Wound Dressings through Handheld Electrospinning. Biomedicines 2023, 11, 2146. [Google Scholar] [CrossRef]
- Yu, D.-G.; Xu, L. Impact Evaluations of Articles in Current Drug Delivery Based on Web of Science. Curr. Drug Deliv. 2023, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, D.-G.; Liu, Y.; Liu, Y.-N. Progress of Electrospun Nanofibrous Carriers for Modifications to Drug Release Profiles. J. Funct. Biomater. 2022, 13, 289. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, W.; Zhou, J.; Yu, D.-G.; Liu, H. The Applications of Ferulic-Acid-Loaded Fibrous Films for Fruit Preservation. Polymers 2022, 14, 4947. [Google Scholar] [CrossRef] [PubMed]
- Partheniadis, I.; Stathakis, G.; Tsalavouti, D.; Heinämäki, J.; Nikolakakis, I. Essential Oil—Loaded Nanofibers for Pharmaceutical and Biomedical Applications: A Systematic Mini-Review. Pharmaceutics 2022, 14, 1799. [Google Scholar] [CrossRef] [PubMed]
- Râpă, M.; Gaidau, C.; Mititelu-Tartau, L.; Berechet, M.-D.; Berbecaru, A.C.; Rosca, I.; Chiriac, A.P.; Matei, E.; Predescu, A.-M.; Predescu, C. Bioactive Collagen Hydrolysate-Chitosan/Essential Oil Electrospun Nanofibers Designed for Medical Wound Dressings. Pharmaceutics 2021, 13, 1939. [Google Scholar] [CrossRef]
- Zhu, M.; Yu, J.; Li, Z.; Ding, B. Self-Healing Fibrous Membranes. Angew. Chem. Int. Ed. 2022, 61, e202208949. [Google Scholar] [CrossRef]
- Du, Y.; Yang, Z.; Kang, S.; Yu, D.-G.; Chen, X.; Shao, J. A Sequential Electrospinning of a Coaxial and Blending Process for Creating Double-Layer Hybrid Films to Sense Glucose. Sensors 2023, 23, 3685. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Y.; Bai, Y.; Shi, H.; Zhou, W.; Chen, Y.; Liu, Y.; Yu, D.-G. Recent Combinations of Electrospinning with Photocatalytic Technology for Treating Polluted Water. Catalysts 2023, 13, 758. [Google Scholar] [CrossRef]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef]
- Guler, E.; Nur Hazar-Yavuz, A.; Tatar, E.; Morid Haidari, M.; Sinemcan Ozcan, G.; Duruksu, G.; Graça, M.P.F.; Kalaskar, D.M.; Gunduz, O.; Emin Cam, M. Oral Empagliflozin-Loaded Tri-layer Core-Sheath Fibers Fabricated Using Tri-axial Electrospinning: Enhanced In Vitro and in Vivo Antidiabetic Performance. Int. J. Pharm. 2023, 635, 122716. [Google Scholar] [CrossRef]
- Zhao, T.; Zheng, Y.; Zhang, X.; Teng, D.; Xu, Y.; Zeng, Y. Design of Helical Groove/Hollow Nanofibers via Tri-Fluid Electrospinning. Mater. Des. 2021, 205, 109705. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Yang, F.; Ye, C.; Xu, X.; Wang, S.; Zhang, L.; Zou, D. Novel PVA-Based Microspheres Co-Loaded with Photothermal Transforming Agent and Chemotherapeutic for Colorectal Cancer Treatment. Pharmaceutics 2021, 13, 984. [Google Scholar] [CrossRef]
- Du, Y.; Yu, D.-G.; Yi, T. Electrospun Nanofibers as Chemosensors for Detecting Environmental Pollutants: A Review. Chemosensors 2023, 11, 208. [Google Scholar] [CrossRef]
- Rimann, M.; Jüngel, A.; Mousavi, S.; Moeschlin, N.; Calcagni, M.; Wuertz-Kozak, K.; Brunner, F.; Dudli, S.; Distler, O.; Adlhart, C. Acrylonitrile and Pullulan Based Nanofiber Mats as Easily Accessible Scaffolds for 3D Skin Cell Models Containing Primary Cells. Cells 2022, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Ren, Z.; Chen, Y.; Huang, J.; Lei, Z.; Qian, X.; Lai, Y.; Zhang, S. A Quadruple Biomimetic Hydrophilic/Hydrophobic Janus Composite Material Integrating Cu(OH)2 Micro-Needles and Embedded Bead-On-String Nanofiber Membrane for Efficient Fog Harvesting. Chem. Eng. J. 2023, 455, 140863. [Google Scholar] [CrossRef]
- Gentile, P.; McColgan-Bannon, K.; Gianone, N.C.; Sefat, F.; Dalgarno, K.; Ferreira, A.M. Biosynthetic PCL-graft-Collagen Bulk Material for Tissue Engineering Applications. Materials 2017, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Sun, C.; Lin, H.; Li, G.; Lian, Z.; Song, R.; Zhuang, S.; Zhang, D. Electrospun Bi-Decorated BixBiyOz/TiO2 Flexible Carbon Nanofibers and Their Applications on Degradating of Organic Pollutants under Solar Radiation. J. Mater. Sci. Technol. 2023, 150, 114–123. [Google Scholar] [CrossRef]
- Qian, C.; Liu, Y.; Chen, S.; Zhang, C.; Chen, X.; Liu, Y.; Liu, P. Electrospun Core–Sheath PCL Nanofibers Loaded with nHA and Simvastatin and Their Potential Bone Regeneration Applications. Front. Bioeng. Biotechnol. 2023, 11, 1205252. [Google Scholar] [CrossRef] [PubMed]
- Brimo, N.; Serdaroglu, D.C.; Uysal, B. Comparing Antibiotic Pastes with Electrospun Nanofibers as Modern Drug Delivery Systems for Regenerative Endodontics. Curr. Drug Deliv. 2022, 19, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun Polycaprolactone (PCL) Degradation: An In Vitro and In Vivo Study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhong, M.; Song, N.; Wang, C.; Lu, X. General Synthesis of Pt and Ni Co-Doped Porous Carbon Nanofibers to Boost HER Performance in Both Acidic and Alkaline Solutions. Chin. Chem. Lett. 2023, 34, 107359. [Google Scholar] [CrossRef]
- Yu, D.-G.; Huang, C. Electrospun Biomolecule-Based Drug Delivery Systems. Biomolecules 2023, 13, 1152. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Wang, L.; Li, Q.; Ma, B.; He, C.; Guo, X.; Nie, J.; Ma, G. A Review: Current Status and Emerging Developments on Natural Polymer-Based Electrospun Fibers. Macromol. Rapid Commun. 2022, 43, 2200456. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, R.G. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Miklaszewski, A.; Cielecka-Piontek, J. Improving Solubility and Permeability of Hesperidin through Electrospun Orange-Peel-Extract-Loaded Nanofibers. Int. J. Mol. Sci. 2023, 24, 7963. [Google Scholar] [CrossRef]
- Schiødt, F.V.; Rochling, F.A.; Casey, D.L.; Lee, W.M. Acetaminophen Toxicity in An Urban County Hospital. N. Engl. J. Med. 1997, 337, 1112–1118. [Google Scholar] [CrossRef]
- Wang, L.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Multi-Compartment Centrifugal Electrospinning Based Composite Fibers. Chem. Eng. J. 2017, 330, 541–549. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Zhang, C.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Designer Fibers from 2D to 3D-Simultaneous and Controlled Engineering of Morphology, Shape and Size. Chem. Eng. J. 2018, 334, 89–98. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Tabakoglu, S.; Kolbuk, D.; Sajkiewicz, P. Multifluid Electrospinning for Multi-drug Delivery Systems: Pros and Cons, Challenges, and Future Directions. Biomater. Sci. 2022, 11, 37–61. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Jiang, L. Bio-Mimic Multichannel Microtubes by A Facile Method. J. Am. Chem. Soc. 2007, 129, 764–765. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Hauzerova, S.; Novotny, V.; Hedvicakova, V.; Jencova, V.; Kostakova, E.K.; Schindler, M.; Lukas, D. AC Electrospinning: Impact of High Voltage and Solvent on the Electrospinnability and Productivity of Polycaprolactone Electrospun Nanofibrous Scaffolds. Mater. Today Chem. 2022, 26, 101025. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, H.; Lv, F.; Wang, J.; Zhao, S.; Li, Y.; Xue, X.; Liu, Y.; Wei, G.; Lu, W. Sequential Release of Paclitaxel and Imatinib from Core–Shell Microparticles Prepared by Coaxial Electrospray for Vaginal Therapy of Cervical Cancer. Int. J. Mol. Sci. 2021, 22, 8760. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Li, Y.; Tan, Y.; Liu, Y.; Pan, W.; Tan, G. Stimuli-Responsive Electrospun Nanofibers for Drug Delivery, Cancer Therapy, Wound Dressing, and Tissue Engineering. J. Nanobiotechnol. 2023, 21, 237. [Google Scholar] [CrossRef]
- Chen, J.-M.; Liu, K.-C.; Yeh, W.-L.; Chen, J.-C.; Liu, S.-J. Sustained Release of Levobupivacaine, Lidocaine, and Acemetacin from Electrosprayed Microparticles: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2020, 21, 1093. [Google Scholar] [CrossRef]
- Levana, O.; Hong, S.; Kim, S.H.; Jeong, J.H.; Hur, S.S.; Lee, J.W.; Kwon, K.-S.; Hwang, Y. A Novel Strategy for Creating an Antibacterial Surface Using a Highly Efficient Electrospray-Based Method for Silica Deposition. Int. J. Mol. Sci. 2022, 23, 513. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, C. Chiral Fiber Supramolecular Hydrogels for Tissue Engineering. WIREs Nanomed. Nanobiotechnol. 2023, 15, 1847. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Cristiano, M.C.; Paolino, D.; Celesti, C.; Iannazzo, D.; Pistarà, V.; Iraci, N.; Ventura, C.A. Bicalutamide Anticancer Activity Enhancement by Formulation of Soluble Inclusion Complexes with Cyclodextrins. Biomolecules 2022, 12, 1716. [Google Scholar] [CrossRef]
- Permyakov, E.A. α-Lactalbumin, Amazing Calcium-Binding Protein. Biomolecules 2020, 10, 1210. [Google Scholar] [CrossRef]
- Ye, P.; Wei, S.; Luo, C.; Wang, Q.; Li, A.; Wei, F. Long-Term Effect against Methicillin-Resistant Staphylococcus aureus of Emodin Released from Coaxial Electrospinning Nanofiber Membranes with a Biphasic Profile. Biomolecules 2020, 10, 362. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Vas, A.; Árvai, G.; Fenyvesi, É.; Jicsinszky, L.; Budai, I.; Bényei, A.; Regdon, G., Jr.; Rusznyák, Á.; Vasvári, G.; et al. Investigation of the Drug Carrier Properties of Insoluble Cyclodextrin Polymer Microspheres. Biomolecules 2022, 12, 931. [Google Scholar] [CrossRef]
- Liu, Z.; Lansley, A.B.; Duong, T.N.; Smart, J.D.; Pannala, A.S. Increasing Cellular Uptake and Permeation of Curcumin Using a Novel Polymer-Surfactant Formulation. Biomolecules 2022, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Darie-Ion, L.; Jayathirtha, M.; Hitruc, G.E.; Zaharia, M.-M.; Gradinaru, R.V.; Darie, C.C.; Pui, A.; Petre, B.A. A Proteomic Approach to Identify Zein Proteins upon Eco-Friendly Ultrasound-Based Extraction. Biomolecules 2021, 11, 1838. [Google Scholar] [CrossRef] [PubMed]
- La Hoz, R.; Diban, N.; Berciano, M.T.; San Emeterio, C.; Urtiaga, A.; Lafarga, M.; Rodríguez-Rey, J.C.; Tapia, O. Coaxial Synthesis of PEI-Based Nanocarriers of Encapsulated RNA-Therapeutics to Specifically Target Muscle Cells. Biomolecules 2022, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Hama, R.; Ulziibayar, A.; Reinhardt, J.W.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Developments in Biopolymer-Based Hydrogels for Tissue Engineering Applications. Biomolecules 2023, 13, 280. [Google Scholar] [CrossRef]
- Esquivel, S.V.; Bhatt, H.N.; Diwan, R.; Habib, A.; Lee, W.-Y.; Khatun, Z.; Nurunnabi, M. β-Glucan and Fatty Acid Based Mucoadhesive Carrier for Gastrointestinal Tract Specific Local and Sustained Drug Delivery. Biomolecules 2023, 13, 768. [Google Scholar] [CrossRef]
- Khadka, B.; Lee, B.; Kim, K.-T. Drug Delivery Systems for Personal Healthcare by Smart Wearable Patch System. Biomolecules 2023, 13, 929. [Google Scholar] [CrossRef]
- Peppas, N. Analysis of Fickian and Non-Fickian Drug Release from Polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Zhu, L.F.; Zheng, Y.; Fan, J.; Yao, Y.; Ahmad, Z.; Chang, M.W. A Novel Core-Shell Nanofiber Drug Delivery System Intended for the Synergistic Treatment of Melanoma. Eur. J. Pharm. Sci. 2019, 137, 105002. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef]
- Abdelhakim, H.E.; Coupe, A.; Tuleu, C.; Edirisinghe, M.; Craig, D.Q.M. Utilising Co-Axial Electrospinning as a Taste-Masking Technology for Paediatric Drug Delivery. Pharmaceutics 2021, 13, 1665. [Google Scholar] [CrossRef] [PubMed]
- Alshaya, H.A.; Alfahad, A.J.; Alsulaihem, F.M.; Aodah, A.H.; Alshehri, A.A.; Almughem, F.A.; Alfassam, H.A.; Aldossary, A.M.; Halwani, A.A.; Bukhary, H.A.; et al. Fast-Dissolving Nifedipine and Atorvastatin Calcium Electrospun Nanofibers as a Potential Buccal Delivery System. Pharmaceutics 2022, 14, 358. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhao, H.; Liu, H.; Zhao, P.; Yu, D.-G. Electrosprayed Stearic-Acid-Coated Ethylcellulose Microparticles for an Improved Sustained Release of Anticancer Drug. Gels 2023, 9, 700. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, Z.; Falconer, J.; Whittaker, A.K.; Popat, A.; Smith, M.T.; Kumeria, T.; Han, F.Y. Sustained Release Ketamine-Loaded Porous Silicon-PLGA Microparticles Prepared by an Optimized Supercritical CO2 Process. Drug Deliv. Transl. Res. 2022, 12, 676–694. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Controlled-Release Antihistamines Using Supercritical Antisolvent Process. J. Supercrit. Fluids 2021, 171, 105201. [Google Scholar] [CrossRef]
- Jordan, F.; Naylor, A.; Kelly, C.A.; Howdle, S.M.; Lewis, A.; Illum, L. Sustained Release HGH Microsphere Formulation Produced by a Novel Supercritical Fluid Technology: In Vivo Studies. J. Control. Release 2010, 141, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Zhou, J. How Can Electrospinning Further Service Well for Pharmaceutical Researches? J. Pharm. Sci. 2023. [Google Scholar] [CrossRef]


| No. | Electro- Spraying | Applied Voltage (kV) | Fluid Flow Rate (mL/h) | Theoretical Drug Content (%) | Products | |
|---|---|---|---|---|---|---|
| Core 1 | Shell 2 | |||||
| P1 | Single-fluid | 23 | 1.0 | -- | 28.6% | Monolithic particles |
| P2 | Single-fluid | 16 | -- | 1.0 | 28.6% | Monolithic particles |
| P3 | Coaxial | 18 | 0.6 | 0.4 | 28.6% | Core–shell nanoparticles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; He, H.; Du, Y.; Zhang, S.; Yu, D.-G.; Liu, P. Electrosprayed Core (Cellulose Acetate)–Shell (Polyvinylpyrrolidone) Nanoparticles for Smart Acetaminophen Delivery. Pharmaceutics 2023, 15, 2314. https://doi.org/10.3390/pharmaceutics15092314
Xu L, He H, Du Y, Zhang S, Yu D-G, Liu P. Electrosprayed Core (Cellulose Acetate)–Shell (Polyvinylpyrrolidone) Nanoparticles for Smart Acetaminophen Delivery. Pharmaceutics. 2023; 15(9):2314. https://doi.org/10.3390/pharmaceutics15092314
Chicago/Turabian StyleXu, Lin, Hua He, Yutong Du, Shengwei Zhang, Deng-Guang Yu, and Ping Liu. 2023. "Electrosprayed Core (Cellulose Acetate)–Shell (Polyvinylpyrrolidone) Nanoparticles for Smart Acetaminophen Delivery" Pharmaceutics 15, no. 9: 2314. https://doi.org/10.3390/pharmaceutics15092314
APA StyleXu, L., He, H., Du, Y., Zhang, S., Yu, D.-G., & Liu, P. (2023). Electrosprayed Core (Cellulose Acetate)–Shell (Polyvinylpyrrolidone) Nanoparticles for Smart Acetaminophen Delivery. Pharmaceutics, 15(9), 2314. https://doi.org/10.3390/pharmaceutics15092314







