Human Acellular Amniotic Membrane as Skin Substitute and Biological Scaffold: A Review of Its Preparation, Preclinical Research, and Clinical Application
Abstract
:1. Introduction
2. Materials and Methods
3. Preparation and Sterilization of HAAM
3.1. Preparation of HAAM
3.1.1. Chemical Methods
3.1.2. Biological Methods
3.1.3. Physical Methods
3.2. The Sterilization of HAAM
| Method | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Chemical method | |||
| surfactant | completely decellularized and degraded DNA | low efficiency; damages ECM and easily leaves chemical remains | [14] |
| acid/base solution | complete decellularization and high efficiency | damages ECM and growth factors | [28] |
| Hypotonic/hypertonic saline | gentle | low efficiency; not completely decellularized | [27,29] |
| chelating agent | gentle | not completely decellularized; combination application needed | [30] |
| Biological method | |||
| trypsin | completely decellularized, high efficiency and high biocompatibility | degraded ECM; high costs and influenced by temperature, environment, and pH | [34] |
| DNAase | enzymatic digestion of cell nucleus | low efficiency; not completely decellularized | [33] |
| lipase | hydrolysis lipids | low efficiency; not completely decellularized | [27] |
| Physical method | |||
| freeze–thaw | Less damage to ECM | Low efficiency; high requirement regarding temperature change rate | [36] |
| mechanical scraping | most commonly used auxiliary methods | not completely decellularized | [25] |
| nonthermal irreversible electroporation | preservation of ECM, no thermal damage, facilitating recellularization | reliance on host response; equipment complexity | [39] |
| High hydrostatic pressure | Non-Thermal Process, effective, No Chemical Residues | expensive, product variability, potential for uneven pressure distribution | [40] |
| Sterilization of HAAM | |||
| irradiation | complete sterilization | damages ECM structure | [42] |
| Ethylene oxide | complete sterilization and elimination of all microorganisms | flammable, explosive, and toxic | [44] |
| Peracetic acid | complete sterilization | interfere tissue structures | [43] |
| Supercritical carbon dioxide | complete sterilization, high biocompatibility, and environmental friendliness | accurate control of pressure rate | [45] |
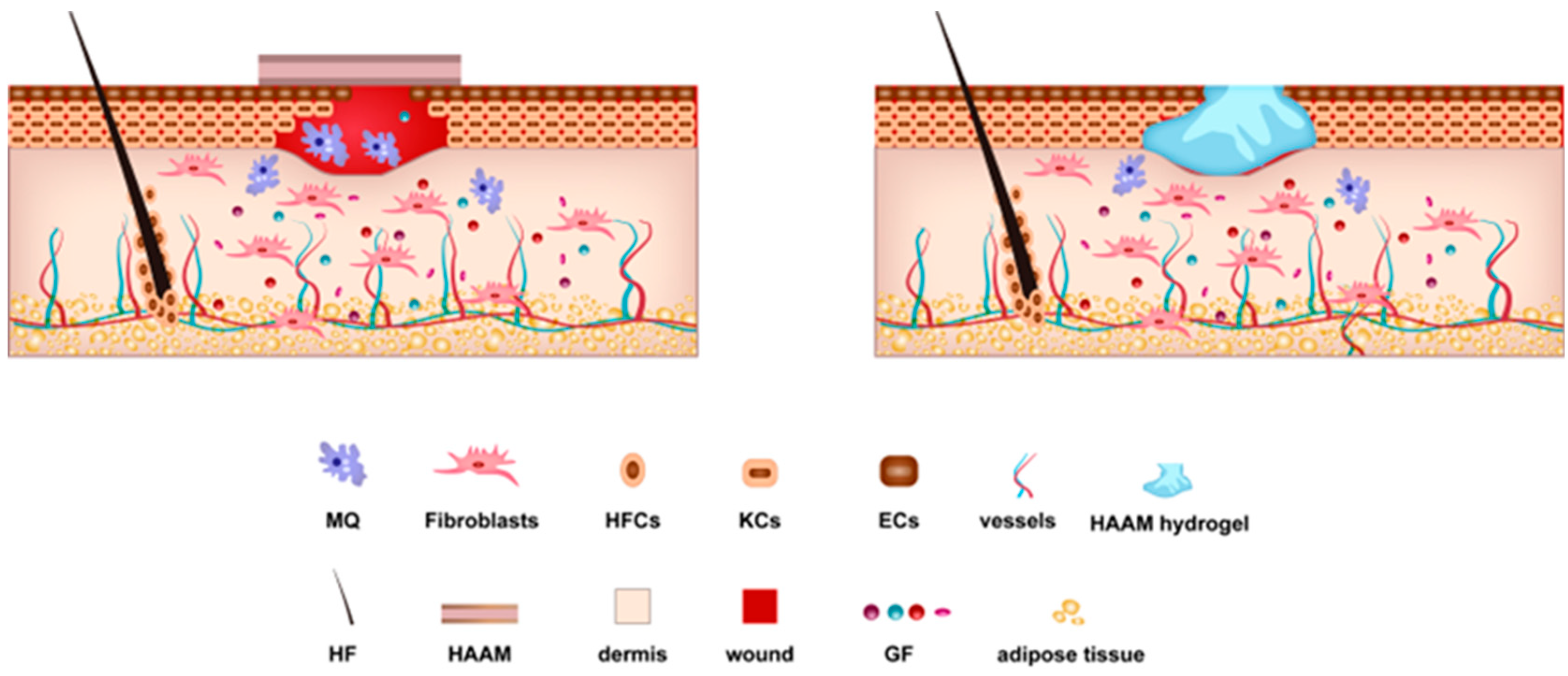
4. Mechanisms Underlying the Regenerative Ability of HAAM
4.1. The Role of Cells and Growth Factors in HAAM-Mediated Healing
4.2. HAAM as a Bioactive Scaffold and Cell Delivery System
4.3. Unique Material Properties of HAAM: Biocompatibility
5. Preclinical Research on HAAM
5.1. Independent Applications
5.2. Combined Applications
| Model | Number | Origin | Preparation Method | Sterilization Method | Group | Results | Reference |
|---|---|---|---|---|---|---|---|
| Full-thickness skin defects on the back of SD rats (2 month-old, male) | 25 | Healthy human placenta | 1% Triton X-100 for 4 h, lipase (2000 U/L) for 10 h, and DNAase (2000 U/L) for 4 h at 37 °C. | Decellularization under the sterile state | Two groups: HAAM and control | HAAM increased the expression level of VEGF and α-SMA and decreased TGF-β1 | [15] |
| In vitro experiment | N | Healthy human placenta | 0.5 M NaOH for 30s, 0.2% EDTA for 30 min, cell scraper | N | Two groups: HAAM and fresh AM | HAAM had an antibacterial effect on three standard strains of ATCC bacteria | [67] |
| Myocardial infarction in Wistar rats (2–3 months old, male) | 50 | Healthy human placenta | 0.01% SDS and 0.01% SD for 24 h at 37 °C | N | Three groups: HAAM, BMSC (bone-marrow mononuclear stem cells), control | HAAM has the potential for angiogenesis and cardiomyocyte regeneration | [69] |
| Iatrogenic defects in fetal membranes in a rabbit model. | 8 | Healthy human placenta | 0.5% sodium deoxycholate, 0.02% ethylenediamine tetraacetic acid, two protease inhibitor cocktail tablets for 1 h at 4 °C, cell scraper | N | One group: HAAM | HAAM could restore the integrity of the punctured fetal membrane | [70] |
| In vitro experiment | N | Healthy human placenta | 1.25% NaOCl for 5 min | 70% ethanol for 3 h | Two groups: 3D HAAM scaffold and 2D cell culture model | HAAM could serve as a 3D scaffold for in vitro cancer research | [73] |
| In vitro osteogenic differentiation experiment | N | Healthy human placenta | 0.1% EDTA for 2 h at 37 °C, cell scraper | N | One group: HAAM | HAAM promoted human APC (dental apical papilla cells) osteogenic differentiation | [71] |
| In vitro experiment | N | Healthy human placenta | Tris-EDTA over night in a refrigerator, SDS over night at 25 °C, Pepsin for 24 h at room temperature | N | One group: HAAM hiPSCs-HLCs (Human-induced pluripotent stem cells-derived hepatocyte-like cells) | HAAM supported the differentiation of hiPSCs to HLCs | [72] |
| In vitro experiment | N | Healthy human placenta | 0.25% trypsin-EDTA for 20 min at 37 °C, cell scraper | N | One group: HAAM-e-CSF (Embryonic cerebrospinal fluid)-BM-MSCs (bone marrow derived mesenchymal stem cells) | HAAM could effectively improve BM-MSC cultivation and neural differentiation with e-CSF as a source of neurological factors | [78] |
| Second-degree burn injuries in balb/c mice (8–10 weeks old, male) | 30 | Healthy human placenta | acid peracetic | N | Two groups: HAAM and control | HAAM could promote the formation of vascularized granulation tissues and skin appendages while reducing the infiltration of inflammatory cells in the wound | [28] |
| Uterus of rabbits (1 year-old) | 2 | Healthy human placenta | 1% Triton X-100 for 1 d, 2000 U/L lipase for 10 h, and 2000 U/L DNAase for 3 h | N | Two groups: HAAM/PU (poly(ester urethane) and PP (Polypropylene mesh) | HAAM/PU showed anti-inflammatory, high biocompatibility, and non-adherent to surrounding organs compared to the control group | [27] |
| Tendon injury models in chickens | 30 | Healthy human placenta | 0.05% ethylenediaminetetraacetic acid at 37 °C for 2 h, cell scraper | ethylene oxide for 6 h | Two groups: HAAM and control | HAAM promoted the endogenous healing of the tendon and prevents exogenous adhesion | [54] |
| Burn wound dressing of mouse (6 month-old female) | 3 | Healthy human placenta | 2% sodium deoxycholeate (SD) (w/v) for 6 h; 2% SDS for 6 h | N | Three groups: HAAM synergistically activated PRP, silver nitrate gel, control | HAAM synergistically activated PRP-accelerated cell migration and skin regeneration compared to the other two groups | [17] |
| Abdominal defect in SD rats | 20 | Healthy human placenta | 1% TritonX-100 for 24 h; 0.25% trypsin and 0.02% EDTA for 4 h at 37 °C | γ-rays (30 kGy) | Two groups: electrospun HAAM and SIS mesh (small intestinal submucosa) | Electrospun HAAM showed superior bioactivity and reinforced mechanical support | [68] |
| Third-degree burn injuries in BALB/c mouse (male) | 75 | Healthy human placenta | N | N | Five groups: HAAM/ESF/AT-MSCs, HAAM/ESF, HAAM/AT-MSCs, HAAM, control | HAAM and other experimental groups showed accelerated wound healing, neo-vascularization, and early re-epithelialization compared to the control group (p < 0.005) | [74] |
| Excisional wound in Wistar rats (9–10 week-old, male) | 24 | Healthy human placenta | 0.05% Trypsin-EDTA for 30 min at 37 °C | N | Four groups: HAAM-PLMSCs (placenta-derived mesenchymal stem cells), HAAM-ADMSCs (adipose–derived mesenchymal stem cells), HAAM, control | HAAM and other experimental groups showed accelerated wound healing and regeneration of skin appendages compared to the control | [75] |
| Endometrial injury in SD rats (6–8 weeks old, female) | 48 | Healthy human placenta | 0.1% Triton X-100 for 36 h at 37 °C; 2.5% trypsin-EDTA for 4 h at 37 °C, cell scraper | N | Four groups: HAAM-UCMSCs (Umbilicalcord-derived mesenchymal stem cells), HAAM, normal, control | The HAAM-UCMSCs group could promote the proliferation of endometrial epithelial and stromal cells | [18] |
| In vitro experiment | N | Healthy human placenta | Three freeze–thaw; trypsin-EDTA overnight at 4 °C, cell scraper | N | One group: HAAM-PCL (Poly(ε-caprolactone)) | HAAM-PCL facilitated the myogenic differentiation of ADSCs | [76] |
| Tracheal defects in New Zealand rabbits | 30 | Healthy human placenta | 0.01% SDS and 0.01% SD for 24 h at 37 °C | N | Three groups: HAAM-hucMSCs (human umbilical cord mesenchymal stem cells), HAAM, control | HAAM facilitated hucMSCs differentiated into chondrocytes | [77] |
| In vitro experiment | N | Healthy human placenta | 10 mM Tris and 0.1% EDTA for 1 h; 0.5% SDS for 4 h at room temperature | N | One group: HAAM-human cardiac ECM hydrogel | HAAM-human cardiac ECM hydrogel could specifically support the culture and interaction of cardiac cells | [19] |
| In vitro experiment and in vivo experiment (full-thickness skin defects in New Zealand rabbits, male and female) | 72 | Healthy human placenta | N | N | One group (in vitro experiment): GelMA-dHAMMA composite hydrogel (methacrylated gelatin (GelMA), dHAM-methacrylic anhydride(dHAMMA)); Three groups: (vivo experiment): -GelMA-dHAMMA -GelMA -control | GelMA-dHAMMA could promote fibroblast proliferation and α-SMA expression in an in vitro experiment and promote wound healing in an in vivo experiment | [60] |
| Full-thickness skin defects in New Zealand rabbits (6 months old) | 24 | Healthy rabbit placenta | 1% Triton X-100 for 12 h; 0.25% trypsin and 0.02% EDTA for 1 h, and RNaseA (0.02 mg/mL) and DNaseI(0.2 mg/mL) for 4 h at 37 °C | γ-rays | Four groups: AM (HAAM), PAM (polyacrylamide), AlgSr/PAM (Polyacrylamide-alginate gel), AlgSrIII/PAM-AM | AlgSrIII/PAM-AM could effectively promote the endothelialization process and repair blood vessels | [66] |
| Third-degree burn wound in New Zealand white rabbits (adult female/male) | 4 | Healthy human placenta | 1% Triton X-100 and 1% SDC at 4 °C for 48 h | plasma (H2O2) at 48 °C | Four groups: AMFIBHA (acellular amniotic membrane(AM), fibrin (FIB), hyaluronic acid (HA)); cpAM (cellular and plasma sterilized AM); pdAM (plasma sterilization dAM); control | AMFIBHA group could promote complete the epithelialization of the wound compared to the other groups and showed good stability | [58] |
6. Clinical Applications of HAAM
6.1. Independent Applications
6.2. Combined Applications
| Defects | Year | Preparation Method | Sterilization Method | Study Design | Number of Patients | Results | Follow-up (Months) | Trial Registration Number | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Split-thickness graft donor site | 2018 | EDTA (0.025%) for 1 h and cell scraper | γ-rays (25 kGy) | Three groups: Mepitel, Dried AM, HAAM | 20 | HAAM has no significant differences with Mepitel, including re-epithelization, pain sensation, scar formation, and infection rate. | 6 | IRCT201511118177N12 | [80] |
| Second-degree-burned skin | 2019 | acid peracetic | N | Two groups: HAAM and silver sulfadiazine gauze | 12 | HAAM showed accelerated wound healing compared to the control group (p < 0.001). | N | N | [28] |
| Venous lower-limb ulcers | 2013 | glutaraldehyde, 0.5% SDS for 24 h at 4 °C, 0.25% trypsin for 4 h at 37 °C | ethylene oxide | One group: HAAM | 4 | HAAM showed accelerated wound healing and generated less pain and lower medical costs. | 6 | N | [81] |
| Full-thickness defects in the lower third of the nose | 2012–2016 | SDS for 5 h; 0.25% trypsin for 6 h | γ-rays | Two groups: HAAM and Vaseline gauze | 180 | HAAM showed accelerated wound healing and fewer complications (p < 0.001). | 3 | ChiCTR1800017618 | [82] |
| Full- thickness auricular skin defects after benign tumor removal | 2016–2021 | N | N | Two groups: HAAM and Vaseline gauze | 36 | HAAM showed accelerated wound healing and fewer complications compared to the control group (p < 0.05). | 3 | N | [20] |
| Chronic diabetic foot ulcers | 2020 | 0.25% Trypsin- EDTA, mechanical isolation | N | One group: -HAAM-loaded WJ-MSCs and DFs | 5 | HAAM-loaded WJ-MSCs and DFs showed accelerated wound healing and no side effects or complications. | 1 | N | [84] |
| Chronic diabetic wounds | 2019 | N | N | One group: HAAM-loaded WJ-MSCs | 5 | HAAM-loaded WJ-MSCs showed accelerated wound healing (p < 0.002). | 1 | N | [85] |
| Ocular surface diseases | 2021 | EDTA (0.25% w/v) | γ-rays (25 kGy) | Two groups: PW-HAAM (processed wet HAAM) and PD-HAAM (processed dry HAAM) | N | HAAM repaired the conjunctival surface. | N | N | [16] |
7. Discussion
7.1. Potential of HAAM in the Treatment of Extensive and Deep Skin Damage
7.2. Challenges in HAAM Use and Future Directions
- Mechanism of Action Studies: More in-depth studies are needed to understand the role of HAAM’s ECM components, like collagen types III, IV, and V, proteoglycans, and glycoproteins, in cell adhesion, growth, and differentiation. Gaining a deeper understanding of how these components affect wound healing at the molecular level can lead to better application strategies;
- Customizing HAAM for Specific Applications: HAAM’s versatility as a surgical patch, tissue scaffold, and a cell delivery vehicle has been highlighted. However, different medical applications might have distinct requirements. Research can be directed towards customizing HAAM for specific uses, such as exploring its ability to deliver specific types of drugs, cells, or growth factors for targeted treatments;
- Mitigating Disease Transmission Risks: While the decellularization of the amniotic membrane reduces immunogenicity, potential risks of disease transmission remain. Studies to establish more robust sterilization processes or assess the risk profile for disease transmission would be beneficial;
- Integration with Other Therapies: As an ideal skin substitute and biological scaffold, HAAM’s compatibility with other therapeutic strategies, e.g., gene therapy, stem cell therapy, or nanoparticle delivery systems, could be further explored.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAAM | Human acellular amniotic membrane |
| SC-CO2 | Supercritical Carbon Dioxide |
| WHO | World Health Organization |
| STSG | split thickness skin grafting |
| AM | Amnion membrane |
| ECM | extracellular matrix |
| SDS | sodium dodecyl sulfate |
| HAM | Human Amniotic Membrane |
| EDTA | ethylenediaminetetraacetic acid |
| TGF-β | Transforming Growth Factor-beta |
| EGF | epidermal growth factor |
| VEGF | vascular endothelial growth factor |
| FGF | fibroblast growth factor |
| PRP | platelet rich plasma |
| MSCs | mesenchymal stem cells |
| NTIRE | nonthermal irreversible electroporation |
References
- Tiwari, N.; Kumar, D.; Priyadarshani, A.; Jain, G.K.; Mittal, G.; Kesharwani, P.; Aggarwal, G. Recent progress in polymeric biomaterials and their potential applications in skin regeneration and wound care management. J. Drug Deliv. Sci. Technol. 2023, 82, 104319. [Google Scholar] [CrossRef]
- Widjaja, W.; Tan, J.; Maitz, P.K.M. Efficacy of dermal substitute on deep dermal to full thickness burn injury: A systematic review. ANZ J. Surg. 2017, 87, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Goyer, B.; Larouche, D.; Kim, D.H.; Veillette, N.; Pruneau, V.; Bernier, V.; Auger, F.A.; Germain, L. Immune tolerance of tissue-engineered skin produced with allogeneic or xenogeneic fibroblasts and syngeneic keratinocytes grafted on mice. Acta Biomater. 2019, 90, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Climov, M.; Medeiros, E.; Farkash, E.A.; Qiao, J.; Rousseau, C.F.; Dong, S.; Zawadzka, A.; Racki, W.J.; Al-Musa, A.; Sachs, D.H.; et al. Bioengineered Self-assembled Skin as an Alternative to Skin Grafts. Plastic and reconstructive surgery. Glob. Open 2016, 4, e731. [Google Scholar]
- Fishman, J.A. Prevention of infection in xenotransplantation: Designated pathogen-free swine in the safety equation. Xenotransplantation 2020, 27, e12595. [Google Scholar] [CrossRef]
- Savoji, H.; Godau, B.; Hassani, M.S.; Akbari, M. Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front. Bioeng. Biotechnol. 2018, 6, 86. [Google Scholar] [CrossRef]
- Halim, A.S.; Khoo, T.L.; Yussof, S.J.M. Biologic and synthetic skin substitutes: An overview. Indian J. Plast. Surg. 2010, 43, S23–S28. [Google Scholar] [CrossRef]
- Loeffelbein, D.J.; Baumann, C.; Stoeckelhuber, M.; Hasler, R.; Mucke, T.; Steinstrasser, L.; Drecoll, E.; Wolff, K.D.; Kesting, M.R. Amniotic membrane as part of a skin substitute for full-thickness wounds: An experimental evaluation in a porcine model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1245–1256. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Jafari, S.M.S.; Kiasat, M.; Tavakkolian, A.R.; Imani, M.T.; Ayaz, M.; Tolide-ie, H.R. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns 2013, 39, 349–353. [Google Scholar] [CrossRef]
- Ditmars, F.S.; Lind, R.A.; Broderick, T.C.; Fagg, W.S. Safety and efficacy of acellular human amniotic fluid and membrane in the treatment of non-healing wounds in a patient with chronic venous insufficiency. SAGE Open Med. Case Rep. 2022, 10, 2050313x221100882. [Google Scholar] [CrossRef]
- Riau, A.K.; Beuerman, R.W.; Lim, L.S.; Mehta, J.S. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 2010, 31, 216–225. [Google Scholar] [CrossRef]
- Mohan, R.; Bajaj, A.; Gundappa, M. Human Amnion Membrane: Potential Applications in Oral and Periodontal Field. J. Int. Soc. Prev. Community Dent. 2017, 7, 15–21. [Google Scholar] [CrossRef]
- Filipas, D. Vaginal reconstruction/fistulae. Curr. Opin. Urol. 2001, 11, 267–270. [Google Scholar] [CrossRef]
- Wilshaw, S.P.; Kearney, J.; Fisher, J.; Ingham, E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng. Part A 2008, 14, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, W.; Ye, Q.; Bu, S.; Shen, Z.; Zhu, Y. The repairing of full-thickness skin deficiency and its biological mechanism using decellularized human amniotic membrane as the wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Krishnakumar, R.; Rekha, R.; Vaseeharan, B.; Saraswathi, K.; Raj, M.; Hanna, R.E.B.; Brennan, G.P.; Dayanithi, G.; Vijayakumar, S. Bio-Fabrication of Human Amniotic Membrane Zinc Oxide Nanoparticles and the Wet/Dry HAM Dressing Membrane for Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 695710. [Google Scholar] [CrossRef] [PubMed]
- Kshersagar, J.; Kshirsagar, R.; Desai, S.; Bohara, R.; Joshi, M. Decellularized amnion scaffold with activated PRP: A new paradigm dressing material for burn wound healing. Cell Tissue Bank. 2018, 19, 423–436. [Google Scholar] [CrossRef]
- Wang, S.; Shi, C.; Cai, X.; Wang, Y.; Chen, X.; Han, H.; Shen, H. Human Acellular Amniotic Matrix with Previously Seeded Umbilical Cord Mesenchymal Stem Cells Restores Endometrial Function in a Rat Model of Injury. Mediat. Inflamm. 2021, 2021, 5573594. [Google Scholar] [CrossRef]
- Becker, M.; Maring, J.A.; Schneider, M.; Martin, A.X.H.; Seifert, M.; Klein, O.; Braun, T.; Falk, V.; Stamm, C. Towards a Novel Patch Material for Cardiac Applications: Tissue-Specific Extracellular Matrix Introduces Essential Key Features to Decellularized Amniotic Membrane. Int. J. Mol. Sci. 2018, 19, 1032. [Google Scholar] [CrossRef]
- Chen, Y.; Lyu, L.; Xue, S. Evaluation of human acellular amniotic membrane for promoting anterior auricle reconstruction. Exp. Dermatol. 2021, 31, 823–824. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Zhang, C.; Hu, J.; Chen, J.; Du, W.; Liu, F.; Ren, W.; Wang, J.; Quan, R. Feasibility of repairing full-thickness skin defects by iPSC-derived epithelial stem cells seeded on a human acellular amniotic membrane. Stem Cell Res. Ther. 2019, 10, 155. [Google Scholar] [CrossRef]
- Arrizabalaga, J.; Nollert, M.U. Human Amniotic Membrane: A Versatile Scaffold for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Chai, Y.; Yu, Y. Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res. Part A 2019, 107, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Sanluis-Verdes, A.; Vilar, M.Y.-P.; García-Barreiro, J.; García-Camba, M.; Ibáñez, J.; Doménech, N.; Rendal-Vázquez, M.E. Production of an acellular matrix from amniotic membrane for the synthesis of a human skin equivalent. Cell Tissue Bank. 2015, 16, 411–423. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Mozafari, M.; Salehi, M.; Seifalian, A.; Bandehpour, M.; Ghanbarian, H.; Urbanska, A.M.; Sameni, M.; Samadikuchaksaraei, A.; Seifalian, A.M. Development of a Cost-Effective and Simple Protocol for Decellularization and Preservation of Human Amniotic Membrane as a Soft Tissue Replacement and Delivery System for Bone Marrow Stromal Cells. Adv. Healthc. Mater. 2015, 4, 918–926. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Tahtinen, M.; Shearier, E.; Qian, Z.; Zhao, F. Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation. Tissue Eng. Part C Methods 2015, 21, 77–87. [Google Scholar] [CrossRef]
- Shi, P.; Gao, M.; Shen, Q.; Hou, L.; Zhu, Y.; Wang, J. Biocompatible surgical meshes based on decellularized human amniotic membrane. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Milan, P.B.; Amini, N.; Joghataei, M.T.; Ebrahimi, L.; Amoupour, M.; Sarveazad, A.; Kargozar, S.; Mozafari, M. Decellularized human amniotic membrane: From animal models to clinical trials. Methods 2020, 171, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.W.; Sellaro, T.L.; Badylak, S.F. Decellularization of tissues and organs. Biomaterials 2006, 27, 3675–3683. [Google Scholar] [CrossRef]
- Ashouri, S.; Hosseini, S.A.; Hoseini, S.J.; Tara, F.; Ebrahimzadeh-Bideskan, A.; Webster, T.J.; Kargozar, S. Decellularization of human amniotic membrane using detergent-free methods: Possibilities in tissue engineering. Tissue Cell 2022, 76, 101818. [Google Scholar] [CrossRef]
- Khosravimelal, S.; Momeni, M.; Gholipur, M.; Kundu, S.C.; Gholipourmalekabadi, M. Protocols for decellularization of human amniotic membrane. Methods Cell Biol. 2020, 157, 37–47. [Google Scholar]
- Guo, Q.; Lu, X.; Xue, Y.; Zheng, H.; Zhao, X.; Zhao, H. A new candidate substrate for cell-matrix adhesion study: The acellular human amniotic matrix. J. Biomed. Biotechnol. 2012, 2012, 306083. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, S.; Zhang, X.; Dai, P.; Gao, Y.; Nan, L.; Zhang, Y.; Zhao, F.; Zhou, L.; Xu, Z.; et al. Transplantation of Amniotic Scaffold-Seeded Mesenchymal Stem Cells and/or Endothelial Progenitor Cells from Bone Marrow to Efficiently Repair 3-cm Circumferential Urethral Defect in Model Dogs. Tissue Eng. Part A 2018, 24, 47–56. [Google Scholar] [CrossRef]
- Fenelon, M.; Maurel, D.B.; Siadous, R.; Gremare, A.; Delmond, S.; Durand, M.; Brun, S.; Catros, S.; Gindraux, F.; L’Heureux, N.; et al. Comparison of the impact of preservation methods on amniotic membrane properties for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109903. [Google Scholar] [CrossRef]
- Huang, G.; Ji, S.; Luo, P.; Liu, H.; Zhu, S.; Wang, G.; Zhou, P.; Xiao, S.; Xia, Z. Accelerated expansion of epidermal keratinocyte and improved dermal reconstruction achieved by engineered amniotic membrane. Cell Transplant. 2013, 22, 1831–1844. [Google Scholar] [CrossRef]
- Mahmoudi-Rad, M.; Abolhasani, E.; Moravvej, H.; Mahmoudi-Rad, N.; Mirdamadi, Y. Acellular amniotic membrane: An appropriate scaffold for fibroblast proliferation. Clin. Exp. Dermatol. 2013, 38, 646–651. [Google Scholar] [CrossRef]
- Pulver; Shevtsov, A.; Leybovich, B.; Artyuhov, I.; Maleev, Y.; Peregudov, A. Production of organ extracellular matrix using a freeze-thaw cycle employing extracellular cryoprotectants. Cryoletters 2014, 35, 400–406. [Google Scholar]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Phillips, M.; Maor, E.; Rubinsky, B. Nonthermal irreversible electroporation for tissue decellularization. J. Biomech. Eng. 2010, 132, 091003. [Google Scholar] [CrossRef]
- Zemmyo, D.; Yamamoto, M.; Miyata, S. Efficient Decellularization by Application of Moderate High Hydrostatic Pressure with Supercooling Pretreatment. Micromachines 2021, 12, 1486. [Google Scholar] [CrossRef]
- Bujang-Safawi, E.; Halim, A.S.; Khoo, T.L.; Dorai, A.A. Dried irradiated human amniotic membrane as a biological dressing for facial burns—A 7-year case series. Burns 2010, 36, 876–882. [Google Scholar] [CrossRef]
- Singh, R.; Purohit, S.; Chacharkar, M.P.; Bhandari, P.S.; Bath, A.S. Microbiological safety and clinical efficacy of radiation sterilized amniotic membranes for treatment of second-degree burns. Burns 2007, 33, 505–510. [Google Scholar] [CrossRef] [PubMed]
- von Versen-Höynck, F.; Syring, C.; Bachmann, S.; Möller, D.E. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts—Light and scanning electron microscopic studies. Cell Tissue Bank. 2004, 5, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Xiao, C.; Miao, Y.; Wang, J.; Chen, R.; Fan, Z.; Hu, Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res. Ther. 2021, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.; Lin, Y.; Wu, S.; Lin, S.; Srinivasan, P.; Hsieh, D.; Huang, S.H. Supercritical Carbon Dioxide-decellularized Porcine Acellular Dermal Matrix combined with Autologous Adipose-derived Stem Cells: Its Role in Accelerated Diabetic Wound Healing. Int. J. Med. Sci. 2020, 17, 354–367. [Google Scholar] [CrossRef]
- Wehmeyer, J.L.; Natesan, S.; Christy, R.J. Development of a Sterile Amniotic Membrane Tissue Graft Using Supercritical Carbon Dioxide. Tissue Eng. Part C Methods 2015, 21, 649–659. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y. Preventive effects of transplantation of oral mucosal epithelial cells seeded on a decellularized amniotic membrane in a model of intrauterine adhesion. Int. J. Clin. Exp. Pathol. 2018, 11, 1510. [Google Scholar]
- Tang, K.; Wu, J.; Xiong, Z.; Ji, Y.; Sun, T.; Guo, X. Human acellular amniotic membrane: A potential osteoinductive biomaterial for bone regeneration. J. Biomater. Appl. 2018, 32, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Francisco, J.C.; Cunha, R.C.; Cardoso, M.A.; Simeoni, R.B.; Mogharbel, B.F.; Picharski, G.L.; Dziedzic, D.S.M.; Guarita-Souza, L.C.; Carvalho, K.A.T. Decellularized amniotic membrane scaffold as a pericardial substitute: An in vivo study. Transplant. Proc. 2016, 48, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Salah, R.A.; Mohamed, I.K.; El-Badri, N. Development of decellularized amniotic membrane as a bioscaffold for bone marrow-derived mesenchymal stem cells: Ultrastructural study. J. Mol. Histol. 2018, 49, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ma, Y.; Lu, X.; Li, W.; Xia, E.; Li, T.-C.; Zhang, H.; Huang, X. Transplantation of human adipose stem cells using acellular human amniotic membrane improves angiogenesis in injured endometrial tissue in a rat intrauterine adhesion model. Cell Transplant. 2020, 29, 0963689720952055. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nair, M.G. Macrophages in wound healing: Activation and plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–411. [Google Scholar]
- Sang, R.; Liu, Y.; Kong, L.; Qian, L.; Liu, C. Effect of Acellular Amnion with Increased TGF-β and bFGF Levels on the Biological Behavior of Tenocytes. Front. Bioeng. Biotechnol. 2020, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Laleh, M.; Tahernejad, M.; Bonakdar, S.; Asefnejad, A.; Golkar, M.; Kazemi-Lomedasht, F.; Habibi-Anbouhi, M. Positive effect of acellular amniotic membrane dressing with immobilized growth factors in skin wound healing. J. Biomed. Mater. Res. Part A 2023, 111, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Sreelatha, H.; Anil, A.; Arumugham, S.; Varkey, P.; Senan, M.; Krishnan, L.K. Human-Derived Scaffold Components and Stem Cells Creating Immunocompatible Dermal Tissue Ensuing Regulated Nonfibrotic Cellular Phenotypes. ACS Biomater. Sci. Eng. 2020, 6, 2740–2756. [Google Scholar] [CrossRef] [PubMed]
- Gholipourmalekabadi, M.; Sameni, M.; Radenkovic, D.; Mozafari, M.; Mossahebi-Mohammadi, M.; Seifalian, A. Decellularized human amniotic membrane: How viable is it as a delivery system for human adipose tissue-derived stromal cells? Cell Prolif. 2016, 49, 115–121. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, C.; Qian, C.; Xiao, W.; Zhu, H.; Guo, J.; Meng, Z.; Cui, W.; Ge, Z. Photo-crosslinkable amniotic membrane hydrogel for skin defect healing. Acta Biomater. 2021, 125, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Soncini, M.; Evangelista, M.; Schmidt, D. Amniotic membrane and amniotic fluid-derived cells: Potential tools for regenerative medicine? Regen. Med. 2009, 4, 275–291. [Google Scholar] [CrossRef]
- Moravvej, H.; Memariani, H.; Memariani, M.; Kabir-Salmani, M.; Shoae-Hassani, A.; Abdollahimajd, F. Evaluation of fibroblast viability seeded on acellular human amniotic membrane. BioMed Res. Int. 2021, 2021, 5597758. [Google Scholar] [CrossRef]
- Francisco, J.C.; Uemura, L.; Simeoni, R.B.; da Cunha, R.C.; Mogharbel, B.F.; Simeoni, P.R.B.; Naves, G.; Napimoga, M.H.; Noronha, L.; Carvalho, K.A.T.; et al. Acellular human amniotic membrane scaffold with 15d-PGJ2 nanoparticles in postinfarct rat model. Tissue Eng. Part A 2020, 26, 1128–1137. [Google Scholar] [CrossRef]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef]
- Bhawna; Gujjar, S.; Venkataprasanna, K.S.; Tiwari, S.; Sharma, J.C.; Sharma, P.; Pujani, M.; Pandey, A.K.; Abnave, P.; Kalyanasundaram, D.; et al. Stabilized human amniotic membrane for enhanced sustainability and biocompatibility. Process Biochem. 2023, 129, 67–75. [Google Scholar] [CrossRef]
- Lei, X.; Wu, Y.; Peng, X.; Zhao, Y.; Zhou, X.; Yu, X. Research on alginate-polyacrylamide enhanced amnion hydrogel, a potential vascular substitute material. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111145. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Bandehpour, M.; Mozafari, M.; Hashemi, A.; Ghanbarian, H.; Sameni, M.; Salimi, M.; Gholami, M.; Samadikuchaksaraei, A. Decellularized human amniotic membrane: More is needed for an efficient dressing for protection of burns against antibiotic-resistant bacteria isolated from burn patients. Burns 2015, 41, 1488–1497. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Zhu, T.; Tang, R. Evaluation of a Biocomposite Mesh Modified with Decellularized Human Amniotic Membrane for Intraperitoneal Onlay Mesh Repair. ACS Omega 2020, 5, 3550–3562. [Google Scholar] [CrossRef]
- Blume, G.G.; Machado-Junior, P.A.B.; Simeoni, R.B.; Bertinato, G.P.; Tonial, M.S.; Nagashima, S.; Pinho, R.A.; de Noronha, L.; Olandoski, M.; de Carvalho, K.A.T.; et al. Bone-Marrow Stem Cells and Acellular Human Amniotic Membrane in a Rat Model of Heart Failure. Life 2021, 11, 958. [Google Scholar] [CrossRef]
- Mallik, A.S.; Fichter, M.A.; Rieder, S.; Bilic, G.; Stergioula, S.; Henke, J.; Schneider, K.T.; Kurmanavicius, J.; Biemer, E.; Zimmermann, R.; et al. Fetoscopic closure of punctured fetal membranes with acellular human amnion plugs in a rabbit model. Obstet. Gynecol. 2007, 110, 1121–1129. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chung, M.C.; Yao, C.C.J.; Huang, C.H.; Chang, H.H.; Jeng, J.H.; Young, T.H. The effects of acellular amniotic membrane matrix on osteogenic differentiation and ERK1/2 signaling in human dental apical papilla cells. Biomaterials 2012, 33, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.F.; Soleimanifar, F.; Enderami, S.E.; Nasiri, N.; Nejati, F.; Mousavi, S.A.; Soleimani, M.; Kiani, J.; Ghoraeian, P.; Kehtari, M. Decellularized amniotic membrane Scaffolds improve differentiation of iPSCs to functional hepatocyte-like cells. J. Cell. Biochem. 2020, 121, 1169–1181. [Google Scholar] [CrossRef]
- Ganjibakhsh, M.; Mehraein, F.; Koruji, M.; Aflatoonian, R.; Farzaneh, P. Three-dimensional decellularized amnion membrane scaffold as a novel tool for cancer research; cell behavior, drug resistance and cancer stem cell content. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Gholipourmalekabadi, M.; Seifalian, A.M.; Urbanska, A.M.; Omrani, M.D.; Hardy, J.G.; Madjd, Z.; Hashemi, S.M.; Ghanbarian, H.; Milan, P.B.; Mozafari, M.; et al. 3D Protein-Based Bilayer Artificial Skin for the Guided Scarless Healing of Third-Degree Burn Wounds in Vivo. Biomacromolecules 2018, 19, 2409–2422. [Google Scholar] [CrossRef]
- Aghayan, H.R.; Hosseini, M.S.; Gholami, M.; Mohamadi-Jahani, F.; Tayanloo-Beik, A.; Alavi-Moghadam, S.; Payab, M.; Goodarzi, P.; Abdollahi, M.; Larijani, B.; et al. Mesenchymal stem cells’ seeded amniotic membrane as a tissue-engineered dressing for wound healing. Drug Deliv. Transl. Res. 2022, 12, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, A.; Bayati, V.; Rashno, M.; Orazizadeh, M. Aligned Poly(epsilon-caprolactone) Nanofibers Superimposed on Decellularized Human Amniotic Membrane Promoted Myogenic Differentiation of Adipose Derived Stem Cells. Cell J. 2021, 23, 603–611. [Google Scholar]
- Simeoni, P.R.B.; Simeoni, R.B.; Junior, P.A.B.M.; de Almeida, M.B.; Dziedzic, D.S.M.; da Rosa, N.N.; Stricker, P.E.F.; Miggiolaro, A.F.R.D.S.; Naves, G.; Neto, N.B.; et al. Tracheal Repair with Human Umbilical Cord Mesenchymal Stem Cells Differentiated in Chondrocytes Grown on an Acellular Amniotic Membrane: A Pre-Clinical Approach. Life 2021, 11, 879. [Google Scholar] [CrossRef]
- Dorazehi, F.; Nabiuni, M.; Jalali, H. Potential Use of Amniotic Membrane—Derived Scaffold for Cerebrospinal Fluid Applications. Int. J. Mol. Cell Med. 2018, 7, 91–101. [Google Scholar] [PubMed]
- Deus, I.; Santos, S.; Custódio, C.; Mano, J.F. Designing highly customizable human based platforms for cell culture using proteins from the amniotic membrane. Mater. Sci. Eng. C 2021, 134, 112574. [Google Scholar] [CrossRef]
- Nouri, M.; Ebrahimi, M.; Bagheri, T.; Fatemi, M.J.; Najafbeygi, A.; Araghi, S.; Molaee, M. Healing Effects of Dried and Acellular Human Amniotic Membrane and Mepitelas for Coverage of Skin Graft Donor Areas; A Randomized Clinical Trial. Bull. Emerg. Trauma 2018, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, X.; Yuan, D.; Zhao, J. Human acellular amniotic membrane is adopted to treat venous ulcers. Exp. Ther. Med. 2018, 16, 1285–1289. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.L.; Liu, K.; Parolini, O.; Wang, Y.; Deng, L.; Huang, Y.C. Human acellular amniotic membrane implantation for lower third nasal reconstruction: A promising therapy to promote wound healing. Burn. Trauma 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Kakabadze, A.; Mardaleishvili, K.; Loladze, G.; Karalashvili, L.; Chutkerashvili, G.; Chakhunashvili, D.; Kakabadze, Z. Reconstruction of mandibular defects with autogenous bone and decellularized bovine bone grafts with freeze-dried bone marrow stem cell paracrine factors. Oncol. Lett. 2017, 13, 1811–1818. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mohammadi, A.A.; Moshirabadi, K.; Zardosht, M. Effect of dermal fibroblasts and mesenchymal stem cells seeded on an amniotic membrane scaffold in skin regeneration: A case series. J. Cosmet. Dermatol. 2021, 20, 4040–4047. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.S.; Mohammadi, A.A.; Kabiri, H.; Hashempoor, M.R.; Mahmoodi, M.; Amini, M.; Mehrabani, D. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. J. Cosmet. Dermatol. 2019, 18, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Greaves, N.S.; Iqbal, S.A.; Hodgkinson, T.; Morris, J.; Benatar, B.; Alonso-Rasgado, T.; Baguneid, M.; Bayat, A. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen. 2015, 23, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.; Aslan, B.; Hosseinian, P.; Aydin, H.M. Supercritical Carbon Dioxide-Assisted Decellularization of Aorta and Cornea. Tissue Eng. Part C Methods 2017, 23, 540–547. [Google Scholar] [CrossRef]
- Amensag, S.; McFetridge, P.S. Tuning scaffold mechanics by laminating native extracellular matrix membranes and effects on early cellular remodeling. J. Biomed. Mater. Res. Part A 2014, 102, 1325–1333. [Google Scholar] [CrossRef]
- Bankoti, K.; Rameshbabu, A.; Datta, S.; Roy, M.; Goswami, P.; Roy, S.; Das, A.; Ghosh, S.; Dhara, S. Carbon nanodot decorated acellular dermal matrix hydrogel augments chronic wound closure. J. Mater. Chem. B 2020, 8, 9277–9294. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; An, S.; Deng, C.; Xiao, S. Human Acellular Amniotic Membrane as Skin Substitute and Biological Scaffold: A Review of Its Preparation, Preclinical Research, and Clinical Application. Pharmaceutics 2023, 15, 2249. https://doi.org/10.3390/pharmaceutics15092249
Li Y, An S, Deng C, Xiao S. Human Acellular Amniotic Membrane as Skin Substitute and Biological Scaffold: A Review of Its Preparation, Preclinical Research, and Clinical Application. Pharmaceutics. 2023; 15(9):2249. https://doi.org/10.3390/pharmaceutics15092249
Chicago/Turabian StyleLi, Yanqi, Siyu An, Chengliang Deng, and Shune Xiao. 2023. "Human Acellular Amniotic Membrane as Skin Substitute and Biological Scaffold: A Review of Its Preparation, Preclinical Research, and Clinical Application" Pharmaceutics 15, no. 9: 2249. https://doi.org/10.3390/pharmaceutics15092249
APA StyleLi, Y., An, S., Deng, C., & Xiao, S. (2023). Human Acellular Amniotic Membrane as Skin Substitute and Biological Scaffold: A Review of Its Preparation, Preclinical Research, and Clinical Application. Pharmaceutics, 15(9), 2249. https://doi.org/10.3390/pharmaceutics15092249






