Abstract
Opioid utilization for pain management is prevalent among cancer patients. There is significant evidence describing the many effects of opioids on cancer development. Despite the pivotal role of metabolic reprogramming in facilitating cancer growth and metastasis, the specific impact of opioids on crucial oncogenic metabolic pathways remains inadequately investigated. This review provides an understanding of the current research on opioid-mediated changes to cellular metabolic pathways crucial for oncogenesis, including glycolysis, the tricarboxylic acid cycle, glutaminolysis, and oxidative phosphorylation (OXPHOS). The existing literature suggests that opioids affect energy production pathways via increasing intracellular glucose levels, increasing the production of lactic acid, and reducing ATP levels through impediment of OXPHOS. Opioids modulate pathways involved in redox balance which may allow cancer cells to overcome ROS-mediated apoptotic signaling. The majority of studies have been conducted in healthy tissue with a predominant focus on neuronal cells. To comprehensively understand the impact of opioids on metabolic pathways critical to cancer progression, research must extend beyond healthy tissue and encompass patient-derived cancer tissue, allowing for a better understanding in the context of the metabolic reprogramming already undergone by cancer cells. The current literature is limited by a lack of direct experimentation exploring opioid-induced changes to cancer metabolism as they relate to tumor growth and patient outcome.
1. Introduction
Opioids are widely used for pain management within the cancer patient population, for surgical and non-surgical pain treatment. Pain experienced by patients decreases quality of life and may also impact disease prognosis. Recent studies have reported that pain and subsequent opioid use were significant predictors of clinical outcomes in cancer patients [1,2,3]. Approximately 30–50% of patients undergoing antineoplastic treatment and 75–90% of patients with advanced disease experienced severe pain necessitating opioid therapy [4]. Opioids are administered through various routes including oral, intravenous, transdermal, and subcutaneous. These medications act primarily on the central nervous system by binding to opioid receptors in the brain and spinal cord involved in pain perception [5,6,7]. Binding of opioids to their cognate Class A G-protein coupled receptors in the central and peripheral nervous system results in receptor internalization and activation of multiple cellular signaling cascades [8]. This signal transduction eventually leads to the formation of descending inhibitory impulses through inhibition of GABA interneurons, ultimately decreasing nociceptive transmission and providing pain relief [7]. Despite their primary use for analgesia, administration of opioids can affect other organ systems through off-target and on-target receptor binding, including but not limited to gastrointestinal, respiratory, cardiovascular, neurological, and renal systems [9]. Newer opiate agonists, like oliceridine, have been developed to maintain analgesic efficacy but produce fewer undesirable side-effects [10,11].
There are three general types of opioids: natural, semi-synthetic, and synthetic. Morphine, a natural opiate derived from opium, is the most commonly used opioid for cancer pain [5,12,13]. Given the frequency of use during cancer treatment, tolerance to one opioid may require increasing doses or the need for a combination of drugs for effective pain relief. A comprehensive list of opioids, their classification, and their affinity for opioid receptors is in Table 1, accompanied by their chemical structures in Figure 1 [14]. Given the ubiquitous need for opioids in cancer pain management, their potential effects on tumor treatment and progression and patient prognosis has been studied [15,16,17,18,19]. A majority of this work has focused on morphine and concluded that the opioid has differential effects based on tumor classification. Despite the recognition that metabolism is a significant driving factor for tumor potentiation and growth, studies looking at the effect of opioids generally fail to address the possible impact of these drugs on cancer metabolism [20]. Opioid-induced changes to cellular metabolism are widely studied in the context of dependence, tolerance, and withdrawal. Research in this field is predominantly limited to changes observed in otherwise normal brains, urine, blood plasma and liver tissues. Given the role of metabolism in oncogenesis and the derangements induced by individual cancer subtypes, further changes to cancer-related metabolic pathways induced by analgesic use are of interest, especially for CNS-based tumors as this is the main site of action for opiates [21].
In this review, we provide an overview of changes to key cancer-specific metabolic pathways induced by opioids. Opioid-induced metabolic changes may have the potential to exacerbate or ameliorate certain pathways already affected by cancer metabolic reprogramming. Understanding these changes will better inform decisions regarding opioid prescription and usage for cancer patients, as well as the potential effects on patient outcomes [10]. While the outlined studies demonstrate a thorough examination of cellular metabolic changes, a significant gap in knowledge persists in opioids’ direct impact on cancer cell metabolism as it relates to disease pathogenicity and patient outcome. This review underscores opioid-induced alterations in crucial metabolic pathways pertinent to cancer. Given the heterogeneity of cancer, meticulous exploration of each tumor type, and possibly patient-specific investigation, is imperative. Such understanding is key to ascertain the therapeutic value or detriment of specific opioids in pain management, guided by metabolic nuances, for optimal patient outcome.

Table 1.
Common opioids use in cancer pain management and their opioid receptor specificity.
Table 1.
Common opioids use in cancer pain management and their opioid receptor specificity.
| Opioid Name | Classification | Opioid Receptor Specificity | Ref. |
|---|---|---|---|
| Morphine | Natural | MOR > DOR > KOR | [22,23] |
| Codeine | Natural | MOR | [24] |
| Oxycodone | Semi-synthetic | MOR > KOR > DOR | [25,26] |
| Hydromorphone | Semi-synthetic | MOR > DOR > KOR | [27,28] |
| Buprenorphine | Semi-synthetic | MOR | [29] |
| Hydrocodone | Semi-synthetic | MOR > KOR > DOR | [30] |
| Methadone | Synthetic | MOR > KOR > DOR | [31,32] |
| Fentanyl | Synthetic | MOR > KOR | [33,34] |
| Tramadol | Synthetic | Weak (MOR, KOR, DOR) | [35,36] |
MOR—mu (μ) opioid receptor; DOR—delta (δ) opioid receptor; KOR—kappa (κ) opioid receptor.
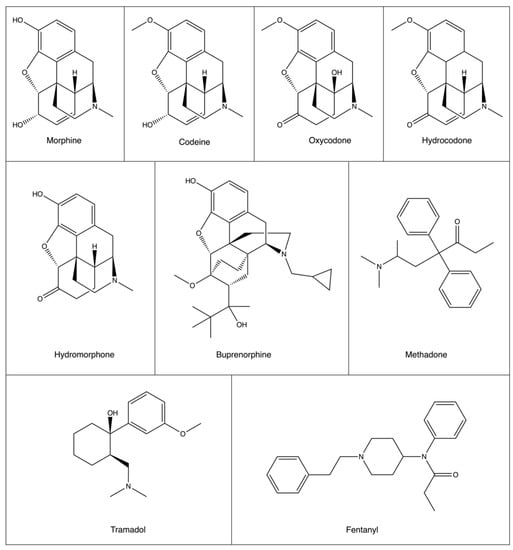
Figure 1.
Structures of common opioids used in cancer pain therapy. While there is structural similarity between synthetic and semi-synthetic opioids, synthetic opioids methadone, tramadol, and fentanyl are structurally very different.
2. Modulation of Cancer Growth by Opioids
Comprehensive cohort and retrospective studies conducted on patients with different types of cancer have shed light on the complex relationship between opioid use and cancer recurrence [37,38,39,40,41,42,43]. The investigations encompassed diverse cohorts, methodologies, and treatment modalities, revealing intriguing patterns and potential therapeutic implications. Because of their retrospective nature, numerous studies amalgamate the results around analgesia and anesthesia rendering it challenging to make truly distinct conclusions around the benefits or detriments of specific opioid use. For example, a clinical retrospective study of breast cancer patients who had either received a paravertebral nerve block with general anesthesia or general anesthesia with morphine found that anesthetic and analgesic technique had a significant impact on cancer recurrence. At 36 months, patients who received a paravertebral nerve block had a 94% recurrence-free and metastasis-free survival rate, compared to only 77% for those who received opioid analgesia [44]. Numerous retrospective studies focusing on non-small cell lung cancer patients and advanced prostate cancer demonstrated that higher dosages of postoperative morphine or generally higher opioid use was significantly correlated to higher cancer recurrence in a 5-year timeframe (hazard ratio (HR) 1.127), decreased progression-free survival, and decreased overall survival (HR 1.55) [38,39,42]. When comparing recurrence-free versus tumor recurrent groups, non-small cell lung cancer patients who had experienced tumor recurrence in a 5-year time period had received two times the total doses of postoperative opioid administration [42]. Dosage of pain management drugs were provided based on patient need. In lung adenocarcinoma patients, ketamine administration was protective for recurrence-specific survival (HR 0.44) but higher opioid use was associated with worse survival (HR 1.09). However, patients who exhibited genomic alterations in the oncogenic Wnt and Hippo pathways showed a positive correlation (~10–30% improvement) between increased opioid dose and recurrence-specific survival [37]. In a 12-year cohort study investigating over 200,000 patients with breast cancer, no strong association between opioid use or dosage and cancer recurrence was seen [41]. MOR antagonism, specifically in non-neuronal tissue, has also been explored as a possible method of attenuating cancer progression, focussing on mitigation of off-target consequences of opioid use. Methylnaltrexone, a peripheral MOR antagonist that cannot cross the blood—brain barrier was initially being investigated for its laxative properties in 229 patients with lung, breast, prostate, or pancreatic cancer experiencing opioid-induced constipation (NCT00401362, NCT00672477). However, when examining the pooled data from both randomized control trials, researchers also observed longer median overall survival by 76–118 days in patients receiving methylnaltrexone [40]. These findings challenge the notion of a universal relationship between opioids and cancer progression and emphasize the need for tumor-specific analyses to elucidate the cellular mechanisms involved.
The effect of opioids on tumor growth and proliferation has been studied using in vivo and in vitro models [15,45,46,47,48,49]. Numerous comprehensive reviews have highlighted that opioids, particularly morphine, have diverse effects on cancer phenotypes [15,17,50,51]. In in vitro models of breast cancer carcinoma and lung cancers, morphine has been shown to increase apoptosis and decrease cell proliferation through MOR/KOR activation and reduction in protein kinase C (pro-survival pathway) activity. Morphine was shown to inhibit the transcriptional regulator NFkβ and reduce expression of the pro-inflammatory cytokine TNF-α [52,53]. Both molecules have been associated with increased aggression of cancer phenotypes such as proliferation, metastasis, and immune evasion [54]. Morphine-induced reduction of NFkβ and TNF-α leads to inhibition of tumor cell growth in a variety of cancer cells [52,53]. Conversely, morphine has also been shown to upregulate VEGF and stimulate VEGF receptor transactivation as a pathway for increased cell proliferation [55,56,57]. Upregulation of VEGF has been connected to promotion of glycolysis and Warburg metabolism in pancreatic cancer [58]. Morphine-induced activation of Akt and mTORC1 pathways were shown to increase proliferation, cell migration, and invasion with indications that this may be through upregulated glucose metabolism, phosphorylation of sarcoma oncogene cellular homologue (Src), and augmentation of nutrient transports [55,59,60]. Fentanyl was shown to inhibit both tumor growth and invasion by downregulating β-catenin, a protein that has numerous roles as a signal transducer of the Wnt pathway and a modulator of lipid and carbohydrate metabolic enzymes [49,61].
The majority of studies examining the impact of opioids on cancer progression focus on phenotypic alterations, including proliferation, invasion, metastasis, and apoptosis. These studies delve into the translational and transcriptional mechanisms underlying these changes, often implying a potential involvement of metabolic processes [17,50]. The reprogramming of cellular metabolism is a fundamental characteristic of cancer and is a substantive mechanism through which opioids may influence the aforementioned cancer phenotypes [62,63].
3. Cancer Metabolism
Early discoveries indicated that cancer cells increase glucose consumption for energy synthesis, leading to an interest in downstream handling pathways of glycolysis and the tricarboxylic acid cycle (TCA), and anaplerotic pathways such as serine and glutamine metabolism [62]. While early concepts regarding cancer initiation suggested that heightened aerobic glycolysis could potentially serve as a distinguishing feature of cancer (involving reduced mitochondrial respiration and compensatory lactate fermentation), cancer metabolism is far more nuanced. Glycolytic upregulation is specific to certain cancer types. Significant mitochondrial ATP production via oxidative phosphorylation (OXPHOS) and the electron transport chain (ETC) is often needed for sufficient energy production for the hypergrowth of tumors [64]. A consequence of increased OXPHOS is the higher production of reactive oxygen species (ROS). Increased ROS can facilitate tumorigenesis through direct DNA damage and ROS-based signaling pathways but must be appropriately balanced with detoxification pathways such as the pentose phosphate pathway (PPP) and glutathione (GSH) cycle, in order to prevent tumor cell death [65]. Through the dysregulation of this non-exhaustive list of metabolic pathways, cancer cells promote growth, evade apoptosis, and initiate metastasis.
4. Glycolysis
4.1. Glycolysis in Cancer
A distinctive metabolic trait of many cancer cells is the increased uptake of glucose as a substrate for glycolysis. Despite being less efficient than OXPHOS, the Warburg effect, first described in 1920 by Otto Warburg, proposed that cancer cells preferentially upregulate glycolysis with lactate fermentation (potentially still in the presence of oxygen, hence the term aerobic glycolysis) to synthesize ATP [66]. This metabolic shift is initiated by several factors including the activation of oncogenes KRAS and BRAF, changes in the tumor microenvironment, and the inhibition of the tumor repressors NDRG2 and p53 [67,68,69,70]. Many cancer types overexpress GLUT (glucose transport) enzymes, particularly in hypoxic conditions, to accentuate cellular glucose uptake as a rate-limiting step of glycolysis [71,72]. Interestingly, glycolytic enzymes are ubiquitously upregulated in most cancers. In brain tumors, all glycolytic enzymes, including hexokinase-1 which is stringently allosterically regulated by glucose-6-phosphate (G6P), are overexpressed [73]. Phosphoglucose isomerase (PGI), phosphofructokinase (PFK), enolase-1 (ENO), and pyruvate kinase (PK) are the most frequently overexpressed glycolytic enzymes across various cancer types (Figure 2) [74]. Glycolytic upregulation provides a quick source of ATP and additionally provides a method to shuttle glycolytic intermediates into anabolic pathways to promote the synthesis of nucleotides, amino acids, and lipids for cell proliferation and survival [75,76,77]. For these reasons, modulation of glycolytic activity in cancer cells has been an ongoing point of investigation for treatment [78].
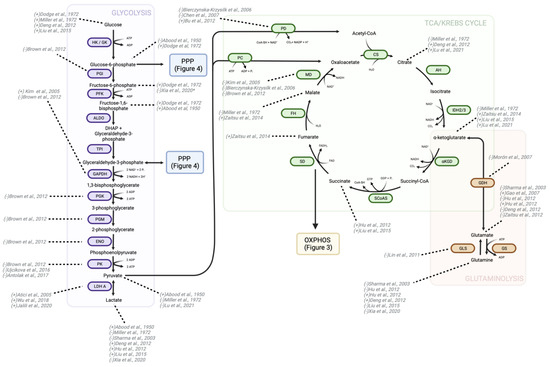
Figure 2.
Changes in metabolites and enzymes of glycolysis, TCA, and glutaminolysis due to morphine treatment. There is consensus among studies regarding increased glucose transport into the cell, upregulation of LDH-A, and decreased expression/activity of PK, but the remaining steps of glycolysis display variable patterns of upregulation and downregulation by morphine. Three separate studies have found that morphine decreases malate dehydrogenase expression; however, the change in other enzymes and metabolites of the TCA vary within the literature. Glutamine metabolism, or glutaminolysis, directly feeds into the TCA through α-ketoglutarate production. Morphine and other opioids affect glutaminolysis metabolites and enzymes, yet conflicting findings exist about whether they amplify or diminish this metabolic pathway. Directionality of change in metabolite or protein is indicated in the brackets preceding study authorship. “+” indicates an increase in either activity, expression, or level, while “-” indicates a decrease in the aforementioned parameters. HK—hexokinase; GK—glucokinase; PGI—phosphoglucose isomerase; PFK—phosphofructokinase; ALDO—aldolase; DHAP—dihydroxyacetone phosphate; TPI—triosephosphate isomerase; GAPDH—glyceraldehyde-3-phosphate dehydrogenase; PGK—phosphoglycerate kinase; PGM—phosphoglycerate mutase; ENO—enolase; PK—pyruvate kinase; LDH-A—lactate dehydrogenase A; PD—pyruvate dehydrogenase; PC—pyruvate carboxylase; CS—citrate synthase; AH—aconitase or aconitate hydratase; IDH—isocitrate dehydrogenase; a-KGD—α-ketoglutarate dehydrogenase; SCoAS—succinyl CoA synthetase; SD—succinate dehydrogenase; FH—fumarase or fumarate hydratase; MD—malate dehydrogenase; GLS—glutaminase; GS—glutamine synthase; GDH—glutamate dehydrogenase (also known as glutamic acid decarboxylase); OXPHOS—oxidative phosphorylation; PPP—pentose phosphate pathway [18,79,80,81,82,82,83,84,85,86,87,88,89,90,91,91,92,92,93,94,95,96,97,98,99,100].
4.2. Opioid Modulation of Glycolysis
Metabolic studies on the effect of morphine in rodent brain samples have indicated an increase in intracellular glucose following both acute and chronic opioid treatment [79,80,81,101]. In murine colon cancer cells, morphine increased the expression of GLUT1, providing a mechanism for increased intracellular glucose levels and suggesting that morphine could induce changes to glucose import pathways or push for increased glycolytic ATP-generation over OXPHOS [18,102]. Aldolase C, the brain-specific isoform of fructose-1,6,-bisphosphatase responsible for the production of glycerol-3-phosphate and dihydroxyacetone during glycolysis, is increased in the mouse hippocampus following chronic morphine treatment at concentrations ranging from 20 to 50 mg/kg/day. This increase in protein expression is reversible with the administration of naloxone indicating an opioid receptor-specific pathway [103]. Acute morphine treatment at high doses of 50 mg/kg for fewer than 2 h in rats increased glycogen, fructose-1,6-bisphosphate (F-1,6-BP), pyruvic acid, lactic acid, ATP, and ADP, while decreasing G6P in the brain [80]. Within the same treatment time frame, lower morphine concentrations (15 mg/kg) increased G6P, and fructose-6-phosphate, but had no effect on F-1,6-BP. This observation may indicate delicate temporal regulation in the analysis and concentration-dependent variations. [79,82]. Changes to levels of G6P in the context of cancer may be of more relevance to the PPP, as fluctuations in abundance of this metabolite are often seen as a source of glucose-6-phosphate dehydrogenase (G6PDH) upregulation and will be discussed further in Section 9. Overexpression of G6PDH is negatively associated with tumor stage, depth of invasion, lymph node metastasis and ultimately survival rate [104]. In subcutaneous primary melanomas, glycolytic metabolite levels including both G6P and F6P were elevated in metastatic tumor samples compared to their non-metastatic counterparts. Elevation of these metabolites is suggested to support metastasis by upregulating the PPP serving as a mechanism to attenuate potential apoptotic signaling induced by high levels of ROS [105]. Thus, alterations to these metabolites brought on by morphine treatment may influence metastatic potential in cancer cells.
Many of the aforementioned glycolytic changes are mediated through the opioid receptor, as blocking the receptor with nalorphine treatment returned the metabolic phenotype back to control [79]. An extensive proteomic study investigating the immunosuppressive effects of chronic morphine treatment in African green monkeys showed reduced expression of multiple glycolytic enzymes. Consistent dosing with 5 mg/kg morphine over the 20-day treatment period reduced expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate mutase (PGM), ENO, and phosphoglycerate kinase (PGK) in varying tissue samples [83]. The activity and expression of PK following morphine exposure is contested in the literature. PK expression was elevated in neuronal tissue of rats and mice when treated for 1–5 days at concentrations of 5–15 mg/kg [84,106,107]. However, in investigations looking at non-primates and neuronal rat tissue treated with 5–50 mg/kg morphine for 10–20 days, PK expression decreased [83,85,86]. PK downregulation can be an indication of increased glycolytic flux and is a known mechanism for promoting cancer cell survival [108]. These studies may suggest that longer and higher concentrations of morphine exposure correspond to lower PK expression, while shorter and lower concentrations increase expression. While all metabolites and enzymes of the pathway are of interest for studying, changes to regulatory branches at HK, PFK, and PK are of particular importance as their modulation can significantly impact pathway flux [109].
Regulation of lactate dehydrogenase activity is important for the balance of pyruvate and lactate, and plays a role in tumorigenesis. Lactate dehydrogenase A (LDH-A) is a transcriptional target of the oncogene c-MYC and is involved in the upregulation of glycolysis in cancer cells that rely on Warburg metabolism [66,110]. Lactate dehydrogenase B (LDHB) more readily oxidizes lactate to pyruvate while the A subunit is involved with the reverse reaction as part of anerobic glycolysis. Both acute and chronic morphine administration (10–100 mg/kg) alter the expression level of components of the LDH complex, increasing LDHA but decreasing LDHB mRNA and protein expression; a change not observed with tramadol treatment [18,87,88,89,110]. The changes to LDH are thought to be a compensatory mechanism to help fulfil immediate energy demands. Along with changes at the enzymatic levels, metabolite and proton-NMR-based metabolomic analysis of rat brain and plasma following acute morphine treatment have shown an increase in lactic acid levels [81,90]. Lactic acid is produced when the energy demand of the brain exceeds the production capacity of OXPHOS leading to upregulation of glycolysis and glycolytic stress [111]. Regulation of lactic acid can significantly impact tumor growth and metabolic homeostasis. It is well-established that increased lactic acid levels act as fuel for oxidative metabolism in hypoxic tumor cells and have the potential to increase metastasis by approximately 10-fold [112,113,114]. Moreover, increased lactic acid in the tumor microenvironment allows for cancer cells to overcome immune surveillance by preventing natural killer cell activation and T-lymphocyte proliferation [115].
Expression of the components of pyruvate dehydrogenase, the connecting enzyme between glycolysis and the TCA by converting pyruvate to acetyl-CoA, are also reduced with chronic morphine treatment in mouse hippocampal tissue [87]. Changes in the expression levels of enzymes at the interface between aerobic and anerobic glycolysis, as well as the TCA, suggest morphine can alter the cell’s reliance on oxidative energy metabolism.
While some of the observed metabolic changes appear to be mediated through on-target effects (through the opioid receptor), others are independent of receptor presence or activation, indicating potential off-target binding. Fentanyl and tramadol, both of which also provide analgesia through MOR activation, do not increase lactic acid levels’ [91]. This suggests that the mechanism of morphine-induced increases in lactic acid may be independent of MOR activation. Because lactic acid levels in the tumor microenvironment can significantly impact patient prognosis, considerations should be made to choice of analgesic intervention, given that morphine can increase intercellular lactic acid levels where fentanyl treatment does not [116].
Five-week tramadol treatment in mice significantly decreases fructose-2,6-bisphospate and fructose-6-phosphate in the cerebellum [91]. Fructose-2,6-bisphosphate can modulate the activity of PFK, the second commitment step of glycolysis and a key regulatory point. A drop in levels of these two metabolites suggests an impedance of glycolysis by tramadol [117]. This finding may point to a shift in energy dependence to OXPHOS or suggest lower energy/ATP availability. Changes in metabolite levels in these studies were highly dependent on the concentration of drug used, illustrating dose-effect.
Noscapine, an opium alkaloid with limited analgesic properties, has recently displayed potent anti-tumor capabilities. A study in human renal cancer has suggested that noscapine treatment (concentration undisclosed) modulates the Warburg effect through activation of PI3K/mTOR signalling by lowering intercellular glucose uptake and both lactic acid and ATP production [118]. Despite not having analgesic use itself, noscapine can potentiate the analgesic effect of morphine three-fold when used in combination [119]. While noscapine has been investigated as an anti-tumor drug, further work is required to fully elucidate the mechanisms by which it increases apoptosis in cancer cells, and whether this holds true when combined with opioids for pain relief.
5. Creatine Metabolism
5.1. Creatine Metabolism in Cancer
Creatine kinase catalyzes the reversible reaction of phosphocreatine to creatine for ATP generation [120]. The enzyme can also act as an energy transport system to shuttle high-energy phosphate molecules to ATP utilization sites in the cytosol and mitochondria [121]. Creatine provides an important energy reserve predominantly in cells that are excitable and highly efficient—namely neurons and myocytes [122]. Historically, creatine treatment in cancer (artificially increasing creatine and phosphocreatine levels) has been associated with suppression of cell proliferation via modulation of energy buffering capacity and even increasing muscle mass in patients [123,124,125,126]. However, the role of creatine and its analogs in cancer are not straightforward. Studies in the last decade have called attention to creatine’s ability to induce cancer progression and initiate metastasis [127,128,129,130]. Increased creatine levels have been associated with poor survival in hepatocellular carcinoma, vulvar cancer, and breast cancer [131].
5.2. Opioid Modulation of Creatine Metabolism
Morphine can significantly alter phosphocreatine levels within acutely treated cells. Reports on the direction of change and the downstream effect on ATP production vary from study to study [79,80]. However, recent findings suggest that changes to creatine and phosphocreatine levels are dependent on specific brain structures. While chronic morphine treatment of 14 days increased creatine in the hippocampus and nucleus accumbens, it decreased in the prefrontal cortex and striatum [90]. In rhesus monkeys, chronic morphine treatment for 90 days at 10 mg/kg had the opposite effect, with increased creatine in the prefrontal cortex and a decrease in the hippocampus [92]. Increased creatine found in the brain of morphine-dependent rats could also point to a protective mechanism against oxidative stress [90,132,133]. Previous studies have suggested that elevated levels of creatine in the hippocampus are associated with improved memory and recall [134]. In a patient-derived xenograft mouse model of colon cancer, increased creatine abundance both through dietary intake and upregulation of enzymatic creatine production enhanced metastasis and reduced survival time [127]. This change is a result of downstream creatine-induced upregulation of snail and slug proteins which are well-established targets of Smad2/3 and promoters of tumor metastasis [135]. A morphine-induced creatine increase in sublocalized areas of brain tissue could significantly impact metastatic ability in the presence of tumors cells.
Morphine was shown to bind creatine kinase B at the micromolar level, inhibiting the enzyme’s ability to phosphorylate ADP and thus decreasing ATP production in rat brains [136]. Morphine treatment for only 2 days in mice decreased creatine kinase B expression in hippocampal tissue [84]. However, the overwhelming current data suggest that chronic morphine exposure reduces intercellular ATP levels [87,137,138]. High ATP production is the primary determinant of aggressive cancer phenotypes, and targeting the production of ATP has been successfully explored as cancer therapy [139,140]. Because morphine is able to reduce ATP production in cells, it may be a superior option to other opioids for cancers that are particularly sensitive to fluctuations in ATP levels [141].
6. Glutamine Metabolism
6.1. Glutamine Metabolism in Cancer
Glutamine is used as a major carbon source for cancer cells to feed into the TCA, acts as a nitrogen donor for the synthesis of purine and pyrimidines needed for DNA replication, and provides a precursor for GSH synthesis [142]. The metabolite is therefore integral for the production of energy and the maintenance of redox balance. Many tumors are so dependent on glutamine that they are categorized as “glutamine addicted” cancers, including lymphomas, glioblastomas, colorectal, and breast cancers [143]. These cancers heavily rely on glutamine for the synthesis of glutamate, α-ketoglutarate (αKG), and citrate [144]. Following entrance into the cell, glutamine is converted to glutamate through the activation of glutaminase (GLS) (Figure 2). Upregulation of GLS is associated with more advanced disease and poor prognosis of hepatocellular carcinoma, colorectal, breast, and pancreatic cancer [145,146,147,148]. These cancer types so heavily depend on glutamine such that disruption to its synthesis or levels is detrimental to tumor cell survival [144,149].
6.2. Opioid Modulation of Glutamine Metabolism
Glutamine can be synthesized by the enzyme glutamine synthetase (GS) through the combination of glutamate and ammonia. It can also be converted back into glutamate via GLSase as part of the glutamine–glutamate cycle, which is imperative for GABA production and neuron activation [150]. A study looking at changes to GS levels in rats following 72 h of consistent morphine treatment found that opioid exposure did not alter enzymatic activity, nor did it affect enzyme expression levels in brain tissue [107]. However, short-term treatment of under 1 h did decrease GLS protein expression [93]. NMR studies in the rat brain following chronic morphine treatment found a minor increase in both glutamate and glutamine levels in the locus coeruleus and periaqueductal gray [94]. Other studies have found glutamate to be increased in the hippocampus, nucleus accumbens, and striatum, but decreased in the prefrontal cortex of morphine dependent mice [90,95]. In rat plasma samples, both glutamine and 4-hydroxybutanoic acid, a precursor to glutamine, were decreased following morphine treatment (5–50 mg/kg), both acutely (within 24 h) and chronically at 5 days [81,96]. Using deuterium magnetic resonance spectroscopy (2H DMRS), the rate of cerebral glutamine consumption in morphine-treated mice was found to be approximately two-fold higher than control [151]. Moreover, a 90-day morphine addiction study performed in rhesus monkeys indicated that consistent exposure to 10 mg/kg morphine decreased both glutamate and glutamine by 7–10% in the hippocampus. Interestingly, the treatment resulted in a 7% increase in glutamine in the prefrontal cortex [92]. Morphine treatment induces differential regulation of GABA, glutamine, and glutamate in relation to brain structure location. Tramadol treatment in the cerebrum of mice resulted in increased γ-hydroxybutyric acid, a precursor to glutamine, while actual glutamine levels were found to decrease [91]. A study on neurotransmitter release rate in the hypothalamus found that rats treated with fentanyl for 75 min had slower glutamate and faster GABA release. However, no comment was made on total metabolite levels [152]. Rapidly proliferating cancer cells depend on glutamine uptake for anapleorosis into the TCA cycle to eventually produce ATP, NADH, and FADH2. As such, enzymes that regulate the glutaminolysis are attractive druggable targets [153]. Decreased glutamine uptake through inhibition of glutamine transporters has been shown to reduce cancer growth by preventing cell cycle progression through E2F transcription factors [154]. Similarly, depriving cancer cells of glutamine can enhance their sensitivity to apoptosis induced by TNF-α and heat shock [155]. Glutamine is also a key modulator of mTORC1 activation, a kinase which regulates cell growth and proliferation [156]. While morphine is reported to activate mTORC1 signaling independent of cellular metabolism, its ability to affect glutamine levels, metabolism, and uptake are likely systems by which it modulates cancer growth [60].
7. Tricarboxylic Acid (TCA) Cycle
7.1. The TCA Cycle in Cancer
The TCA cycle is a central hub for energy production, redox balance, and metabolite synthesis (Figure 2). Identification of mutations in isocitrate dehydrogenase, succinate dehydrogenase, fumarase, and malate dehydrogenase (MD) in a variety of cancer types has redirected attention to the role of the TCA in reprogramming tumor metabolism [157,158,159]. Increased levels of succinate were shown to enhance cell migratory and metastatic ability by activation of succinate receptor-1-mediated signaling [160]. αKG supplementation was shown to have anti-proliferative effects by attenuation of Wnt signaling and suppression of TGF-β [161,162,163]. Despite being initially overlooked as a dysregulated metabolic pathway in cancers, the TCA cycle has become a key target for cancer therapy [144,164,165,166].
7.2. Opioid Modulation of the TCA Cycle
The most extensive work regarding changes to TCA metabolites following opioid exposure were performed using blood and urine samples from rodents. Morphine has been reported to significantly impact numerous metabolites of the TCA cycle. In a study examining morphine relapse where exposure was increased over a 10-day period (3–9 mg/kg), followed by a 5-day period of withdrawal, then morphine relapse for up to 20 days, changes to 30 responsive metabolites in serum samples were noted. Upon initial exposure, mice had decreased levels of pyruvic acid and αKG, but no effective change was seen in citric acid. After withdrawal and relapse, a decrease in citric acid and increase in both αKG and pyruvic acid was observed [97]. Heroin was utilized as a comparative drug to morphine in these experiments. Heroin treatment for the same duration had dissimilar effects, despite being considered a prodrug that is metabolized into morphine in vivo, indicating potential off-targets for either (or both) drugs. Heroin resulted in an initial increase in citric acid, a decrease in αKG, and a decrease in pyruvic acid levels. Following withdrawal and relapse, almost all observed metabolites were decreased compared to the saline control group, except for pyruvic acid, which was found to be no different than control. Elevated pyruvic acid can inhibit cell growth through repression of histone gene expression and subsequent delay of S-phase entry [167]. Pyruvic acid increases expression of nicotinamide phosphoribosyltransferase, leading to increased NAD+ and downstream activation of histone deacetylase SIRT1 [168]. Maintaining low levels of pyruvic acid, through the upregulation of LDH-A to convert the metabolite into lactate, is one method by which cancers avoid apoptosis [169]. Changes in these metabolite levels as mice were put through withdrawal and re-exposure to both morphine and heroin indicate potential upregulation of compensatory pathways and underscore the impact of opioid exposure on TCA dysregulation and cancer-related pathways [97].
In an investigation performed on rat urine and serum samples, chronic morphine treatment (2–10 mg/kg/day for 5–14 days) increased metabolites including αKG, succinate, malic acid, and fumarate [81,96]. Interestingly, when rats were treated with both morphine and naloxone, many of the effected TCA metabolites remained elevated compared to control but reduced compared to the opioid-only treatment group, suggesting that metabolic changes brought on by opioid treatment may not entirely depend on opioid receptor activation [81]. αKG is also central to tumor growth dynamics as it is both a metabolite of the TCA, a key player in glutaminolysis, and acts as an epigenetic regulator through its role as co-factor for ten-eleven translocation enzymes and JmjC domain-containing histone demethylases [170]. In general, increased αKG induces anti-cancer effects [171]. The metabolite was shown to induce apoptosis through the ROS-PI3K/Akt/mTOR pathway, increase antineoplastic treatment efficacy through epigenetic upregulation of PD-L1, and reduce metastatic potential by suppression of TGF-β and VEGF production [162,172,173].
In brain tissue samples from morphine-dependant rats, changes to TCA metabolites were dependant on substructure localization. Succinate was increased in the nucleus accumbens but decreased in the striatum, while αKG was drastically decreased in only the hippocampus. Almost all observed changes to TCA metabolites disappeared with methadone co-treatment. It appears that chronic and consistent morphine treatment increases αKG in serum but decreases the compound in neuronal tissue. The combination of increased lactic acid, mentioned previously, and decreased succinate brought on by morphine treatment suggests a mitochondrial functional deficiency and decreased TCA cycle activity leading to decreased ATP levels with opiate exposure [90]. Proteomic changes across numerous studies in neuronal tissue following morphine treatment indicated changes to only one TCA enzyme, MD. The expression of this enzyme was reduced in rat brain tissue and monkey cerebral spinal fluid [83,98,99]. MD is often overexpressed in cancers and is associated with poor prognosis [174,175]. Cytosolic MD can act as a source of NAD required by cancer cells to maintain high levels of glycolysis [100,176]. Reduction in both cytosolic and mitochondrial MD expression as a result of morphine treatment may offer a potential strategy to mitigate the overexpression and enzyme hyperactivity observed in highly proliferative tumors.
A sweeping, definitive assertion regarding the consistent up- or downregulation of TCA cycle activity induced by opioids, specifically morphine, cannot be made. The complex and context-dependent nature of opioid-induced modulation of TCA activity necessitates careful consideration of multiple factors such as treatment protocols (including dosing amounts, frequency, and length), tissue types, anatomical substructure, and genetic heterogeneity. However, it can be concluded that opioid treatment leads to significant fluctuations of metabolites within the TCA cycle. These findings serve to emphasize the fundamental interconnectivity that characterizes cellular metabolism, whereby the TCA cycle acts as a central hub integrating diverse metabolic pathways highly influential in cancer cell growth and survival. Consequently, the intricate network of metabolic interactions becomes disrupted upon opioid exposure, leading to pronounced alterations in energy utilization and biosynthesis that may also occur in the context of tumor metabolism [177].
8. Oxidative Phosphorylation (OXPHOS)
8.1. OXPHOS in Cancer
Mitochondria are dynamic organelles consistently undergoing fusion and fission throughout the cell cycle. These organelles exist as either branched tubular networks, or fragmented granules depending on the cellular state, energy demand, and oxidative stress regulation [178]. Numerous studies have highlighted the imbalance of increased fission or decreased fusion in cancers leading to fragmented mitochondrial networks [179]. Enhanced mitochondrial fission has been shown to push cells into mitosis and increase replication [180]. Moreover, prevention of mitochondrial fragmentation through upregulation of the fusion protein Mfn1 or downregulation of the fission protein Drp1 significantly reduces metastatic abilities in certain cancers [181]. Cells with fragmented mitochondria have decreased OXPHOS capabilities and more heavily rely on glycolytic energy production [182,183]. The ETC is the most efficient metabolic pathway for the generation of ATP per unit of carbon source [184]. The pathway consists of five complexes, the first four of which generate a proton gradient across the inner mitochondrial membrane and thus produce the mitochondrial membrane potential (Figure 3). The fifth complex, ATP synthase, produces ATP while pumping protons back into the mitochondrial matrix [185]. Due to leaking electrons during this transport, superoxide and hydrogen peroxide formation occur at complex I and complex III [186,187]. The increase in ROS can promote the development and progression of tumors but must be balanced appropriately with detoxifying metabolic pathways to prevent ROS-activated apoptotic signaling [65,188].
Downregulation of mitochondrial metabolic enzymes, particularly those involved in OXPHOS, show a significant negative correlation with endothelial-to-mesenchymal transition (EMT) across over 20 types of cancers [189]. Metastatic cancers exhibit a profound upregulation of EMT in stark contrast to their parental tissue, underscoring the pivotal role of this molecular process in the acquisition of aggressive phenotypes [189,190]. An in-depth investigation into patient survival reveals an association between dysregulated EMT and the impairment of ETC enzyme expression wherein OXPHOS was discovered to be the most affect pathway among cohorts classified as low vs. high survival rates. ETC enzymes were found to be downregulated in 60% of cancer types. In samples with high induction of EMT and metastatic potential, OXPHOS was the most significantly downregulated metabolic pathway. The most frequently downregulated gene among low survival patients are subunits of complex I and IV [189]. The association between poor clinical outcome and downregulation of OXPHOS enzymes is attributed to the correlated increase in EMT. Targeting complexes I–IV of the ETC was shown to successfully reduce tumor growth and induce apoptosis [191,192,193]. Overall regulation of OXPHOS unsurprisingly varies in different cancer types and can even be heterogenous within tumors [194,195,196].
8.2. Opioid Modulation of OXPHOS
A study examining mitochondrial morphology following opioid treatment found that morphine had a minimal effect, while fentanyl and methadone significantly altered organelle morphology. Following four hours of treatment to murine neurons, methadone and fentanyl at concentrations between 25 and 100 μM caused a decrease in total mitochondrial area and branching [197]. A reduction in mitochondrial branching can be indicative of impaired ATP synthesis, disruption in ROS balance, and impaired calcium regulation [198]. As previously mentioned, mitochondrial structure and function are integrally related. Increased fragmentation of mitochondria, such as that induced by fentanyl treatment, can lead to higher levels of cancer cell proliferation and metastasis [180,181]. No significant changes to complex I or III activity in mouse brain tissue occurred after 14 days of treatment with morphine at high concentrations (10–40 mg/kg) [86], but the expression levels of complex I and III were decreased in the amygdala of acutely treated mice [93]. A reduction in complex I expression was found in the hippocampus of rats following chronic morphine treatment [87]. Morphine stimulates nitric oxide (NO) release from the mitochondria in many cell types [199,200,201]. NO can interact with and inhibit complex IV of the ETC, impeding cellular respiration [202]. Morphine treatment of glioma cells resulted in a 30% decrease in the mitochondrial membrane potential via activation of NO-release pathways. The observed effect was believed to be a result of complex IV inhibition but interestingly was unique to morphine. Fentanyl and methadone do not substantially increase NO release, again indicating the off-target effects of morphine [203]. Mitochondrial functional analysis (oxygen consumption) in isolated rat liver mitochondria after incubation with morphine, cocaine, and a combination of the two, indicated that morphine treatment decreases complex I activity by about 20%. No significant changes to complex I or II activity were observed in neuronal mitochondria in this study. All three drug groups did result in decreased ATP synthase activity, however, no changes to mitochondrial membrane potential were observed [204]. In rhesus monkeys treated with 3–15 mg/kg of morphine for 7–20 days, ATP synthase expression was decreased two-fold [83,205]. A similar conclusion was drawn from morphine exposure in rats which identified a two-fold decrease in ATP synthase activity following an acute treatment period of three days [98,106]. This supports similar findings of decrease in ATP metabolite levels following morphine treatment [87,138]. Investigation of complex II–IV expression in a variety of murine and primate models predominantly indicate no change following morphine treatment; however, studies demonstrate either a decrease in activity or expression of both complex I and ATP synthase [83,85,87,93,98,106,204,205,206,207]. As previously mentioned, outside of glycolysis, ATP synthase is a large source of ATP for cancer cells. In clear cell renal cell carcinoma, glioblastomas, and HER2+ breast tumors, components of ATP synthase are often overexpressed [208,209,210]. Several drugs targeting the activity of ATP synthase, both synthetically designed (e.g., bedaquiline) and naturally occurring (e.g., curcumin; NCT03769766; NCT02439385) are currently under preclinical or early stage clinical trial investigation for amelioration of current antineoplastic agents [141,211,212]. Reduction in ATP levels and ATP synthase activity as a result of opioid treatment may synergistically work with antineoplastic agents much like the aforementioned drugs undergoing clinical trials.
In human hepatoma cells, in vitro fentanyl treatment did not result in significant changes to cellular respiration, the activity of any components of the ETC, or an increase in intracellular ROS levels. Despite decreased ATP levels, fentanyl did not impact ATP synthase activity [213]. Interestingly in non-cancerous rat brain samples, fentanyl did impact mitochondrial bioenergetics. Moderate concentrations of fentanyl were able to inhibit complexes II to IV of the ETC, while higher concentrations inhibited ATP synthase by over 50% [214]. Similarly, 30-day tramadol treatment in mice resulted in significant inhibition of complex I, III, and IV in isolated mitochondria from brain samples [215].
The majority of oxidative metabolism analysis in the context of opioids is studied in brain tissue samples since mitochondrial glucose oxidation is the main energy source in neurons and the brain is the primary clinical opioid effect site [216]. While drug concentration and treatment model vary from study to study, the literature supports the idea that opioids reduce OXPHOS capacity, particularly in neuronal tissue. Numerous studies have recently indicated that inhibition of OXPHOS can be a strategy to overcome chemotherapeutic resistance [217,218,219]. Other OXPHOS inhibitors, such as IACS-010759, have been shown to strongly inhibit proliferation [220]. Although not all cancers heavily rely on OXPHOS for energy production, the concomitant administration of opioids alongside antineoplastic interventions may confer additional advantages for those cancer subtypes exhibiting energy dependence.
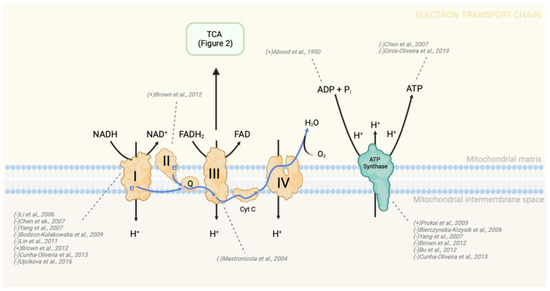
Figure 3.
Changes in OXPHOS enzyme activity due to morphine treatment. Morphine treatment consistently leads to a decrease in the activity of complex I and ATP synthase. Consequently, a reduction in intercellular ATP levels is expected following morphine treatment. To indicate the directionality of change in metabolite or protein levels, brackets preceding the study authorship are used. In this context, a “+” symbol denotes an increase in activity, expression, or level, while a “-” symbol indicates a decrease in the aforementioned parameters. NADH—nicotinamide adenine dinucleotide (reduced form), FADH2—flavin adenine dinucleotide (reduced form); Complex I—NADH dehydrogenase; Q—coenzyme Q (also known as ubiquinone); Complex II—succinate dehydrogenase; Complex III—cytochrome bc1 complex; Cyt c—cytochrome c; Complex IV—cytochrome c oxidase [80,83,85,87,93,98,106,137,203,204,205,206,207].
9. Metabolic Mitigation of Oxidative Stress
9.1. Metabolic Mitigation of Oxidative Stress in Cancer
With elevated levels of metabolism and cellular proliferation, cancer cells have higher quantities of ROS compared to non-cancer cells [221,222]. While increased ROS can act as a pro-oncogenic signaling pathway, they can also trigger apoptosis, autophagy, and other forms of cell death [223,224]. As such, many cancers upregulate compensatory pathways such as the PPP, MD-1, malic enzyme-1, isocitrate dehydrogenase isoenzymes, as well as serine metabolism, to increase NADPH production to be used for ROS detoxification by the GSH cycle (Figure 4) [225]. The PPP branches from glycolysis at the node of G6P formation to generate phosphopentoses and ribonucleotides for the production of nucleic acids. It is also a large supplier of NADPH, required for reduction of GSH and scavenging of ROS, showing involvement in both redox balance and the biosynthesis of macromolecules [226].The pathway has an oxidative and non-oxidative phase that can be preferentially upregulated allosterically via the pathway’s own products depending on the demand of the cell. Each G6P molecule made during glycolysis can be funneled into the oxidative phase of the PPP for the formation of two NADPH molecules and one phosphorylated pentose ring. The non-oxidative phase of the pathway can then produce glyceraldehyde-3-phosphate and fructose-6-phosphate, which may be fed back into glycolysis. NADPH produced from the PPP can be used in combination with acetyl-coA by fatty acid synthases for fatty acid production [227,228]. More importantly, in the context of cancer, NADPH is used by the GSH cycle for detoxification of peroxide radicals. In many cancers, the high consumption of NADPH for ROS mitigation results in upregulation of expression or activation of G6PDH, the first PPP enzyme that produces NADPH and also the rate limiting step of the pathway [229]. Similarly, artificial downregulation or mutation of G6PDH increases cellular oxidative stress, ROS accumulation, and leads to increased apoptosis [230,231]. Glutathione reductase (GR) drives the NADPH-dependent reduction of glutathione disulfide (GSSG) to GSH. In turn, GSH can then be oxidized back into GSSG by members of the glutathione peroxidase (GPx) family as they convert organic hydroperoxides into corresponding alcohol or H2O2 into water [232]. Elevated levels of GSH have been found in a variety of tumors including ovarian, breast, and lung cancers and has also been suggested as necessary for proliferation and tumor initiation [233,234,235]. In other tumor types, such as brain and gastrointestinal tumors, GSH reduction is observed [235]. This heterogeneity is predominantly attributed to the varying levels of GSH regulation by the healthy tissue [235]. Though some studies present a relationship between high GSH levels and poor patient outcome, others do not support such findings [236,237,238,239].
9.2. Opioid Modulation of Oxidative Stress Mitigation Pathways
G6PDH is the first enzyme and rate-limiting step of the PPP [240]. Enzyme kinetic investigations on G6PDH isolated from healthy human erythrocytes indicated that morphine is a weak non-competitive inhibitor of G6PDH with a Ki of 25.93 ± 6.48 mM and IC50 of 43.58 mM. The study found that vancomycin, a glycopeptide antibiotic, and omeprazole, a proton pump inhibitor, were also non-competitive inhibitors of G6PDH. The effect of ketamine and remifentanil were also partially investigated and despite showing some inhibitory effects, the IC50 values of these anesthetic agents were sufficiently higher than morphine, indicating less inhibition of G6PDH [241]. Contrary to changes in activity level, expression of G6PDH does not appear to change following chronic morphine treatment (10 mg/kg for 5 days in isolated rat amygdala) [93]. As previously mentioned, acute morphine treatment at high doses of 50 mg/kg for fewer than 2 h in rats decreased G6P in the brain while lower concentrations (15 mg/kg) increased G6P [80]. Unfortunately, there are insufficient data on the effect of morphine or other opioids on direct changes to NADPH, the product of G6PDH. While the PPP is a major source of NADPH, other enzymes, such as isocitrate dehydrogenase-1 (IDH1) and MD, can also produce this molecule [242]. G6PDH upregulation is highly observed in a variety of cancers including breast carcinoma, glioma, and lung adenocarcinoma. This upregulation is strongly correlated with poor patient prognosis through perpetuation of aggressive cancer phenotypes such as increased growth, invasion, migration, and chemotherapy resistance [230,243,244,245]. It is therefore not surprising that inhibitors of G6PDH and transcriptional regulators are being investigated as therapeutic options. Polydatin, a glucoside of resveratrol and inhibitor of G6PDH, was shown to impede cell proliferation and block transition into S-phase [246]. In cancers that are highly sensitive to G6PDH activity, enzymatic inhibition by opioids could have a beneficial effect similar to that of polydatin.
Anesthetic drugs, midazolam and propofol, were found to significantly upregulate numerous PPP metabolites in cerebral spinal fluid of subarachnoid hemorrhage patients. Samples taken up to 72 h post sedation indicated that midazolam led to increased 6-phosphogluconate, ribulose-5-phosphate, erythrose-4-phosphate, and glyceraldehye-3-phosphate, while propofol increased ribose-5-phosphate, erythrose-4-phosphate, glyceraldehyde-3-phosphate, and fructose-6-phosphate. Conversely, dexmedetomidine slightly inhibited the PPP, resulting in decreased NADP and sedoheptulose-7-phosphate [247]. Given the neuroprotective and antioxidant properties associated with both propofol and midazolam in the brain, the increase in PPP activity is likely not in response to ROS accumulation or upregulation of pathways to counteract oxidative damage brought on by anesthetic treatment [248,249,250]. Instead, upregulation of the PPP is thought to occur to promote biosynthesis given that purine and pyrimidine metabolism were both also upregulated in cerebral spinal fluid samples. The observed phenomenon was thought by the authors to represent a source of “rapid sedative induced DNA synthesis”.
Much like an increase in G6PDH expression and activity, upregulation of the PPP, in particular for the purpose of biosynthesis, supports the biomaterial demand required of cancer cells to maintain high levels of proliferation. Despite being beneficial for its neuroprotective properties, propofol has also been shown to transcriptionally activate Nrf2 pathways involved in enhanced expression of ROS detoxification enzymes, and thus increase proliferation and invasion of gallbladder and breast cancer cells [251,252]. Propofol-induced upregulation of the PPP is likely a metabolic pathway through which the anesthetic agent may promote metastatic phenotypes.
A predominant utility for PPP-produced NADPH is for the mitigation of ROS through antioxidant pathways, most notably GSH metabolism [253]. Morphine treatment decreases GSH metabolite levels in murine models and human samples [254,255,256,257]. Following a single intraperitoneal dose of morphine at 3, 6, or 12 mg/kg in adult mice, GSH levels were significantly reduced in brain samples [257]. In a different study investigating the attenuation of morphine tolerance in mice by inhibition of nitric oxide synthase (NOS), subcutaneous morphine injections of 5 mg/kg twice a day resulted in a significant decrease in GSH and GSH peroxidase activity in brain samples. Changes to both the metabolite level and enzyme activity were first detected at 3 days but were sustained for the full 7-day experiment [255]. A similar drop in GSH and GSH peroxidase and an increase in ROS was observed in primary hippocampus cultures prepared from newborn mice following a 24-h incubation with 1 μM morphine. However, pre-treatment with L-NAME, a NOS inhibitor, increased GSH concentrations and reduced ROS levels relative to morphine-only treatment, suggesting that inhibition of the NOS system plays a key role in the oxidative stress response of the hippocampus [256]. Along with the ability for NADPH to attenuate ROS via GSH metabolism, it can also act as a substrate for NOX enzymes, also known as NADPH-oxidases [258]. This is of particular importance in the context of both cancer and opioid metabolism given that morphine has been shown to activate NOX enzymes which consume NADPH for superoxide production [259,260,261]. According to the existing literature, morphine induces a global reduction in PPP activity, accompanied by an elevation in NADPH-consuming enzymes, thereby compromising cancer cells’ ability to counteract ROS and reducing the availability of nucleotides necessary for DNA synthesis and proliferation. Conversely, other anesthetic agents appear to upregulate the PPP. However, the majority of investigations into PPP upregulation by anesthetic agents are primarily conducted within the framework of intraoperative neuroprotection as opposed to directly investigating changes in cancer cells.
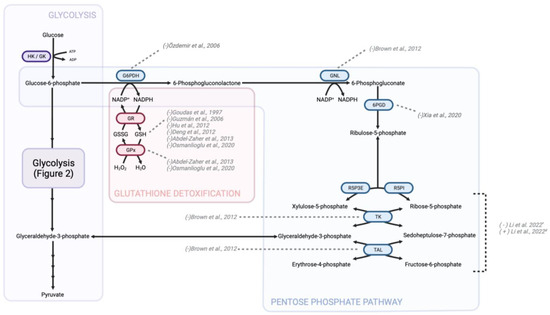
Figure 4.
Changes to GSH detoxification and the PPP secondary to opioid treatment. GSH detoxification is a vital cellular process involved in the neutralization of reactive oxygen compounds and maintenance of redox balance. Opioids were found to impact this pathway by influencing GSH levels and the activity of GSH-related enzymes, such as GPx and glutathione-S-transferase. Studies suggest that chronic opioid use can lead to a decrease in GSH levels and impair the efficiency of GSH detoxification, potentially contributing to oxidative stress and cellular damage. To indicate the directionality of change in metabolite or protein levels, brackets preceding the study authorship are used. The accompanying figure illustrates the alterations induced by morphine treatment, with the exception of those marked with a “#” signifying changes attributable to midazolam and propofol, or a “*” signifying changes resulting from dexmedetomidine administration. HK—hexokinase; GK—glucokinase; G6PDH—glucose-6-phosphate dehydrogenase; GSH—glutathione (reduced form); GSSG—glutathione disulfide (oxidized form); GPx—glutathione peroxidase; GR—glutathione reductase; NADPH—nicotinamide adenine dinucleotide phosphate (reduced form); NADP+—nicotinamide adenine dinucleotide phosphate (oxidized form); GNL—gluconolactonase; 6PGD—6-Phosphogluconate dehydrogenase; R5PI—ribose-5-phosphate isomerase; R5P3E—ribose-5-phosphate-3-epimerase; TK—transketolase; TAL—transaldolase [83,90,91,92,241,247,254,255,256,257].
10. Conclusions
Opioid use for pain management is essential and ubiquitous for patients with cancer. While effective for reducing nociception, opioids, regardless of class, have off-target interactions which lead to predominantly unwanted side effects [262]. Retrospective and cohort studies suggest that opioid use is predominantly associated with cancer recurrence, though this observation is nuanced and dependent on tissue specificity and genomic alteration [43,263]. Unfortunately, investigations into the impact of opioids on tumorigenesis with data specific to metabolic changes are not widely available [19]. In this review, we have focused on metabolic changes brought on by opioid exposure in a variety of tissue sources. Research in this field is limited by the diversity in sample origin, treatment model, and choice of opioid [137]. Observations from pre-clinical in vivo models treated with morphine specifically focusing on biomarkers of addiction and withdrawal dominate the data pool, while limited research exists on the effects of other opioids like fentanyl, methadone, oxycodone, and tramadol. More surprisingly, no reliable data can be found on other commonly used opioids in relation to cellular metabolism, such as hydromorphone or buprenorphine. Given the fundamental importance of metabolic reprogramming in cancer cells, any changes to key metabolic pathways brought on by external factors, such as pain management options, must be considered to tailor treatment and improve patient prognosis through precision medicine. Cancer patients are often treated with opioids for long durations and receive a cocktail of medications for disease management [5,14]. The profound impact of cellular metabolism reprogramming on cancer growth and chemotherapeutic resistance is widely recognized. The amassed data presented above unmistakably demonstrate that opioids exert influence on cellular metabolism and redox equilibrium. However, better comprehending the mechanisms, extents, and shifts in metabolic pathways within distinct cancer types is pivotal. Such understanding could profoundly influence decisions regarding pain management strategies, potentially affecting disease progression and patient outcomes. Apart from fundamental similarities in proliferation, cancer is a heterogenous disease with hundreds of further classifications by which tumors can be differentiated [62]. This includes genetic mutations, epigenetic signatures, and distinct changes in metabolism—as discussed above [264,265,266,267].
Advocacy for precision medicine among the cancer patient population has increased [268]. Given the outlined opioid-induced changes to cellular metabolism and the diversity in tumor metabolic reprograming, it is likely that the choice of pain management can play a larger role in disease treatment and response than previously appreciated. Patient heterogeneity must be leveraged by data-driven methodology to inform treatment decisions such that the correct patient receives the appropriately tailored treatment based on their individual characteristics [269]. A nuanced understanding of these complexities is essential for unraveling the broader impact of opioids on cellular metabolism and may contribute to the development of targeted therapeutic interventions.
Author Contributions
Conceptualization, D.T. and J.T.M.; methodology, D.T. and J.T.M.; validation, D.T. and J.T.M.; formal analysis, D.T.; investigation, D.T.; resources, D.T. and J.T.M.; data curation, D.T.; writing—original draft preparation, D.T.; writing—review and editing, D.T. and J.T.M.; visualization, D.T.; supervision, J.T.M.; project administration, J.T.M.; funding acquisition, J.T.M. All authors have read and agreed to the published version of the manuscript.
Funding
SickKids Foundation through the Curtis Joseph and Harold Groves Chair in Anesthesia and Pain Medicine (J.T.M.) and a Garron Family Cancer Center Pitblado Discovery Grant (J.T.M.), and the Department of Anesthesiology and Pain Medicine, University of Toronto through a Merit Award (J.T.M.).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Zheng, J.; He, J.; Wang, W.; Zhou, H.; Cai, S.; Zhu, L.; Qian, X.; Wang, J.; Lu, Z.; Huang, C. The Impact of Pain and Opioids Use on Survival in Cancer Patients: Results from a Population-Based Cohort Study and a Meta-Analysis. Medicine 2020, 99, e19306. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Garrett-Mayer, E.S.; Yang, Y.-C.O.; De Wit, R.; Tannock, I.F.; Eisenberger, M. A Contemporary Prognostic Nomogram for Men with Hormone-Refractory Metastatic Prostate Cancer: A TAX327 Study Analysis. Clin. Cancer Res. 2007, 13, 6396–6403. [Google Scholar] [CrossRef]
- Roviello, G.; Gallicchio, R.; Bozza, G.; Rodriquenz, M.G.; Aieta, M.; Storto, G. Pain Predicts Overall Survival in Men with Metastatic Castration-Resistant Prostate Cancer Treated with Radium-223. OncoTargets Ther. 2019, 12, 9. [Google Scholar] [CrossRef]
- Van Den Beuken-van Everdingen, M.H.J.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; Van Kleef, M.; Patijn, J. High Prevalence of Pain in Patients with Cancer in a Large Population-Based Study in The Netherlands. Pain 2007, 132, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Bruera, E.; Paice, J.A. Cancer Pain Management: Safe and Effective Use of Opioids. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e593–e599. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.; Bruera, E. Balancing Opioid Analgesia with the Risk of Nonmedical Opioid Use in Patients with Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 213–226. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Gupta, M. Physiology, Opioid Receptor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Beckett, A.H.; Casy, A.F. Synthetic Analgesics: Stereochemical Considerations. J. Pharm. Pharmacol. 1954, 6, 986–1001. [Google Scholar] [CrossRef]
- de Boer, H.D.; Detriche, O.; Forget, P. Opioid-Related Side Effects: Postoperative Ileus, Urinary Retention, Nausea and Vomiting, and Shivering. A Review of the Literature. Best Pract. Res. Clin. Anaesthesiol. 2017, 31, 499–504. [Google Scholar] [CrossRef]
- Paton, K.F.; Atigari, D.V.; Kaska, S.; Prisinzano, T.; Kivell, B.M. Strategies for Developing κ Opioid Receptor Agonists for the Treatment of Pain with Fewer Side Effects. J. Pharmacol. Exp. Ther. 2020, 375, 332–348. [Google Scholar] [CrossRef]
- Singla, N.K.; Skobieranda, F.; Soergel, D.G.; Salamea, M.; Burt, D.A.; Demitrack, M.A.; Viscusi, E.R. APOLLO-2: A Randomized, Placebo and Active-Controlled Phase III Study Investigating Oliceridine (TRV 130), a G Protein–Biased Ligand at the μ-Opioid Receptor, for Management of Moderate to Severe Acute Pain Following Abdominoplasty. Pain Pract. 2019, 19, 715–731. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Wee, B.; Moore, R.A. Oral Morphine for Cancer Pain. Cochrane Database Syst. Rev. 2016, 4, CD003868. [Google Scholar] [CrossRef] [PubMed]
- Hanks, G.W.; de Conno, F.; Cherny, N.; Hanna, M.; Kalso, E.; McQuay, H.J.; Mercadante, S.; Meynadier, J.; Poulain, P.; Ripamonti, C.; et al. Morphine and Alternative Opioids in Cancer Pain: The EAPC Recommendations. Br. J. Cancer 2001, 84, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Cancer Pain (PDQ®). Patient Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. [Google Scholar]
- Afsharimani, B.; Cabot, P.; Parat, M.-O. Morphine and Tumor Growth and Metastasis. Cancer Metastasis Rev. 2011, 30, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Rea, D.; Palma, G.; Luciano, A.; Cuomo, A.; Arra, C.; Izzo, F. Morphine Promotes Tumor Angiogenesis and Increases Breast Cancer Progression. BioMed Res. Int. 2015, 2015, e161508. [Google Scholar] [CrossRef] [PubMed]
- Gach, K.; Wyrębska, A.; Fichna, J.; Janecka, A. The Role of Morphine in Regulation of Cancer Cell Growth. Naunyn. Schmiedebergs Arch. Pharmacol. 2011, 384, 221. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, X.; Wang, J.; Sun, P.; Weng, M.; Chen, W.; Sun, Z.; Zhu, M.; Miao, C. Nalmefene Attenuates Malignant Potential in Colorectal Cancer Cell via Inhibition of Opioid Receptor. Acta Biochim. Biophys. Sin. 2018, 50, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.F.; Gorur, A.; Cata, J.P. Opioids and Cancer Prognosis: A Summary of the Clinical Evidence. Neurosci. Lett. 2021, 746, 135661. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Kim, S.S.; Lee, J. Cancer Cell Metabolism: Implications for Therapeutic Targets. Exp. Mol. Med. 2013, 45, e45. [Google Scholar] [CrossRef]
- Richards, C. Actions of General Anaesthetics on Synaptic Transmission in the CNS. Br. J. Anaesth. 1983, 55, 201–207. [Google Scholar] [CrossRef]
- Corbett, A.; Paterson, S.; Kosterlitz, H. Selectivity of Ligands for Opioid Receptors. In Opioids; Springer: Berlin/Heidelberg, Germany, 1993; pp. 645–679. [Google Scholar] [CrossRef]
- Chen, J.-C.; Smith, E.R.; Cahill, M.; Cohen, R.; Fishman, J.B. The Opioid Receptor Binding of Dezocine, Morphine, Fentanyl, Butorphanol and Nalbuphine. Life Sci. 1993, 52, 389–396. [Google Scholar] [CrossRef]
- Raynor, K.; Kong, H.; Chen, Y.; Yasuda, K.; Yu, L.; Bell, G.I.; Reisine, T. Pharmacological Characterization of the Cloned Kappa-, Delta-, and Mu-Opioid Receptors. Mol. Pharmacol. 1994, 45, 330–334. [Google Scholar] [PubMed]
- Davis, M.P.; Varga, J.; Dickerson, D.; Walsh, D.; LeGrand, S.B.; Lagman, R. Normal-Release and Controlled-Release Oxycodone: Pharmacokinetics, Pharmacodynamics, and Controversy. Support. Care Cancer 2003, 11, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.K.; Ross, F.B.; Lotfipour, S.; Saini, K.S.; Edwards, S.R.; Smith, M.T. Oxycodone and Morphine Have Distinctly Different Pharmacological Profiles: Radioligand Binding and Behavioural Studies in Two Rat Models of Neuropathic Pain. Pain 2007, 132, 289–300. [Google Scholar] [CrossRef]
- Filizola, M.; Villar, H.O.; Loew, G.H. Molecular Determinants of Non-Specific Recognition of δ, μ, and κ Opioid Receptors. Bioorg. Med. Chem. 2001, 9, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.; Strain, E.C.; Abreu, M.E.; Bigelow, G.E. Enadoline, a Selective Kappa Opioid Agonist: Comparison with Butorphanol and Hydromorphone in Humans. Psychopharmacology 2001, 157, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Khroyan, T.V.; Wu, J.; Polgar, W.E.; Cami-Kobeci, G.; Fotaki, N.; Husbands, S.M.; Toll, L. BU 08073 a Buprenorphine Analogue with Partial Agonist Activity at μ-Receptors in Vitro but Long-Lasting Opioid Antagonist Activity in Vivo in Mice. Br. J. Pharmacol. 2015, 172, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Codd, E.E.; Shank, R.P.; Schupsky, J.J.; Raffa, R.B. Serotonin and Norepinephrine Uptake Inhibiting Activity of Centrally Acting Analgesics: Structural Determinants and Role in Antinociception. J. Pharmacol. Exp. Ther. 1995, 274, 1263–1270. [Google Scholar]
- Neil, A. Affinities of Some Common Opioid Analgesics towards Four Binding Sites in Mouse Brain. Naunyn. Schmiedebergs Arch. Pharmacol. 1984, 328, 24–29. [Google Scholar] [CrossRef]
- Kristensen, K. The Mu1, Mu2, Delta, Kappa Opioid Receptor Binding Profiles of Methadone Stereoisomers and Morphine. Life Sci. 1995, 56, PL45–PL50. [Google Scholar] [CrossRef]
- Maguire, P.; Tsai, N.; Kamal, J.; Cometta-Morini, C.; Upton, C.; Loew, G. Pharmacological Profiles of Fentanyl Analogs at μ, δ and κ Opiate Receptors. Eur. J. Pharmacol. 1992, 213, 219–225. [Google Scholar] [CrossRef]
- Negus, S.S.; Schrode, K.; Stevenson, G.W. Mu/Kappa Opioid Interactions in Rhesus Monkeys: Implications for Analgesia and Abuse Liability. Exp. Clin. Psychopharmacol. 2008, 16, 386. [Google Scholar] [CrossRef]
- Barakat, A. Revisiting Tramadol: A Multi-Modal Agent for Pain Management. CNS Drugs 2019, 33, 481–501. [Google Scholar] [CrossRef]
- Hennies, H.; Friderichs, E.; Schneider, J. Receptor Binding, Analgesic and Antitussive Potency of Tramadol and Other Selected Opioids. Arzneimittelforschung 1988, 38, 877–880. [Google Scholar]
- Connolly, J.G.; Tan, K.S.; Mastrogiacomo, B.; Dycoco, J.; Caso, R.; Jones, G.D.; McCormick, P.J.; Sanchez-Vega, F.; Irie, T.; Scarpa, J.R.; et al. Intraoperative Opioid Exposure, Tumour Genomic Alterations, and Survival Differences in People with Lung Adenocarcinoma. Br. J. Anaesth. 2021, 127, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Cata, J.P.; Keerty, V.; Keerty, D.; Feng, L.; Norman, P.H.; Gottumukkala, V.; Mehran, J.R.; Engle, M. A Retrospective Analysis of the Effect of Intraoperative Opioid Dose on Cancer Recurrence after Non—Small Cell Lung Cancer Resection. Cancer Med. 2014, 3, 900–908. [Google Scholar] [CrossRef]
- Zylla, D.; Gourley, B.L.; Vang, D.; Jackson, S.; Boatman, S.; Lindgren, B.; Kuskowski, M.A.; Le, C.; Gupta, K.; Gupta, P. Opioid Requirement, Opioid Receptor Expression, and Clinical Outcomes in Patients with Advanced Prostate Cancer: Opioids and Prostate Cancer Outcomes. Cancer 2013, 119, 4103–4110. [Google Scholar] [CrossRef]
- Janku, F.; Johnson, L.K.; Karp, D.D.; Atkins, J.T.; Singleton, P.A.; Moss, J. Treatment with Methylnaltrexone Is Associated with Increased Survival in Patients with Advanced Cancer. Ann. Oncol. 2016, 27, 2032–2038. [Google Scholar] [CrossRef]
- Cronin-Fenton, D.P.; Heide-Jørgensen, U.; Ahern, T.P.; Lash, T.L.; Christiansen, P.M.; Ejlertsen, B.; Sjøgren, P.; Kehlet, H.; Sørensen, H.T. Opioids and Breast Cancer Recurrence: A Danish Population-Based Cohort Study: Opioids and Breast Cancer Recurrence. Cancer 2015, 121, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Maher, D.P.; Wong, W.; White, P.F.; McKenna, R.; Rosner, H.; Shamloo, B.; Louy, C.; Wender, R.; Yumul, R.; Zhang, V. Association of Increased Postoperative Opioid Administration with Non-Small-Cell Lung Cancer Recurrence: A Retrospective Analysis. Br. J. Anaesth. 2014, 113, i88–i94. [Google Scholar] [CrossRef] [PubMed]
- Wall, T.; Sherwin, A.; Ma, D.; Buggy, D.J. Influence of Perioperative Anaesthetic and Analgesic Interventions on Oncological Outcomes: A Narrative Review. Br. J. Anaesth. 2019, 123, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Exadaktylos, A.K.; Buggy, D.J.; Moriarty, D.C.; Mascha, E.; Sessler, D.I. Can Anesthetic Technique for Primary Breast Cancer Surgery Affect Recurrence or Metastasis? Anesthesiology 2006, 105, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Celik, F.; Duran, T. Effects of Fentanyl on Pancreatic Cancer Cell Proliferation and Cancer Stem Cell Differentiation. Cell. Mol. Biol. 2019, 65, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Lennon, F.E.; Moss, J.; Singleton, P.A.; Riou, B. The μ-Opioid Receptor in Cancer Progression: Is There a Direct Effect? Anesthesiology 2012, 116, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Kawaraguchi, Y.; Sugimoto, H.; Furuya, H.; Kawaguchi, M. Effects of Morphine and Fentanyl on 5-Fluorouracil Sensitivity in Human Colon Cancer HCT116 Cells. J. Anesth. 2014, 28, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Novy, D.M.; Nelson, D.V.; Koyyalagunta, D.; Cata, J.P.; Gupta, P.; Gupta, K. Pain, Opioid Therapy, and Survival: A Needed Discussion. Pain 2020, 161, 496–501. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Chen, M.-L.; Zhou, S.-L. Fentanyl Inhibits Proliferation and Invasion of Colorectal Cancer via β-Catenin. Int. J. Clin. Exp. Pathol. 2015, 8, 227–235. [Google Scholar]
- Tuerxun, H.; Cui, J. The Dual Effect of Morphine on Tumor Development. Clin. Transl. Oncol. 2019, 21, 695–701. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Palma, G.; Arra, C. The Role of Morphine in Animal Models of Human Cancer: Does Morphine Promote or Inhibit the Tumor Growth? BioMed Res. Int. 2013, 2013, 258141. [Google Scholar] [CrossRef]
- Sueoka, E.; Sueoka, N.; Kai, Y.; Okabe, S.; Suganuma, M.; Kanematsu, K.; Yamamoto, T.; Fujiki, H. Anticancer Activity of Morphine and Its Synthetic Derivative, KT-90, Mediated through Apoptosis and Inhibition of NF-κB Activation. Biochem. Biophys. Res. Commun. 1998, 252, 566–570. [Google Scholar] [CrossRef]
- Iglesias, M. μ-Opioid Receptor Activation Prevents Apoptosis Following Serum Withdrawal in Differentiated SH-SY5Y Cells and Cortical Neurons via Phosphatidylinositol 3-Kinase. Neuropharmacology 2003, 44, 482–492. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, B.P. TNF-α/NF-κB/Snail Pathway in Cancer Cell Migration and Invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef]
- Chen, C.; Farooqui, M.; Gupta, K. Morphine Stimulates Vascular Endothelial Growth Factor-like Signaling in Mouse Retinal Endothelial Cells. Curr. Neurovasc. Res. 2006, 3, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Poonawala, T.; Levay-Young, B.K.; Hebbel, R.P.; Gupta, K. Opioids Heal Ischemic Wounds in the Rat. Wound Repair Regen. 2005, 13, 165–174. [Google Scholar] [CrossRef]
- Singleton, P.; Lingen, M.; Fekete, M.; Garcia, J.; Moss, J. Methylnaltrexone Inhibits Opiate and VEGF-Induced Angiogenesis: Role of Receptor Transactivation. Microvasc. Res. 2006, 72, 3–11. [Google Scholar] [CrossRef]
- Shi, S.; Xu, J.; Zhang, B.; Ji, S.; Xu, W.; Liu, J.; Jin, K.; Liang, D.; Liang, C.; Liu, L.; et al. VEGF Promotes Glycolysis in Pancreatic Cancer via HIF1α Up-Regulation. Curr. Mol. Med. 2016, 16, 394–403. [Google Scholar] [CrossRef]
- Gupta, K.; Kshirsagar, S.; Chang, L.; Schwartz, R.; Law, P.-Y.; Yee, D.; Hebbel, R.P. Morphine Stimulates Angiogenesis by Activating Proangiogenic and Survival-Promoting Signaling and Promotes Breast Tumor Growth. Cancer Res. 2002, 62, 4491–4498. [Google Scholar]
- Liu, X.; Yang, J.; Yang, C.; Huang, X.; Han, M.; Kang, F.; Li, J. Morphine Promotes the Malignant Biological Behavior of Non-Small Cell Lung Cancer Cells through the MOR/Src/mTOR Pathway. Cancer Cell Int. 2021, 21, 622. [Google Scholar] [CrossRef] [PubMed]
- Vergara, D.; Stanca, E.; Guerra, F.; Priore, P.; Gaballo, A.; Franck, J.; Simeone, P.; Trerotola, M.; De Domenico, S.; Fournier, I.; et al. β-Catenin Knockdown Affects Mitochondrial Biogenesis and Lipid Metabolism in Breast Cancer Cells. Front. Physiol. 2017, 8, 544. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Chandel, N.S. Targeting Mitochondria Metabolism for Cancer Therapy. Nat. Chem. Biol. 2015, 11, 9–15. [Google Scholar] [CrossRef]
- Harris, I.S.; DeNicola, G.M. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Schwartzenberg-Bar-Yoseph, F.; Armoni, M.; Karnieli, E. The Tumor Suppressor P53 Down-Regulates Glucose Transporters GLUT1 and GLUT4 Gene Expression. Cancer Res. 2004, 64, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef] [PubMed]
- McGrail, K.; Granado-Martínez, P.; Esteve-Puig, R.; García-Ortega, S.; Ding, Y.; Sánchez-Redondo, S.; Ferrer, B.; Hernandez-Losa, J.; Canals, F.; Manzano, A.; et al. BRAF Activation by Metabolic Stress Promotes Glycolysis Sensitizing NRASQ61-Mutated Melanomas to Targeted Therapy. Nat. Commun. 2022, 13, 7113. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, J.; Sun, X.; Guo, Y.; Chu, D.; Wei, L.; Li, X.; Yang, G.; Liu, X.; Yao, L.; et al. Tumor Suppressor NDRG2 Inhibits Glycolysis and Glutaminolysis in Colorectal Cancer Cells by Repressing C-Myc Expression. Oncotarget 2015, 6, 26161–26176. [Google Scholar] [CrossRef]
- Kunkel, M.; Reichert, T.E.; Benz, P.; Lehr, H.-A.; Jeong, J.-H.; Wieand, S.; Bartenstein, P.; Wagner, W.; Whiteside, T.L. Overexpression of Glut-1 and Increased Glucose Metabolism in Tumors Are Associated with a Poor Prognosis in Patients with Oral Squamous Cell Carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 1015–1024. [Google Scholar] [CrossRef]
- Brown, R.S.; Wahl, R.L. Overexpression of Glut-1 Glucose Transporter in Human Breast Cancer an Immunohistochemical Study. Cancer 1993, 72, 2979–2985. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Rolleston, F.S.; Taylor, K. Factors Affecting the Glucose 6-Phosphate Inhibition of Hexokinase from Cerebral Cortex Tissue of the Guinea Pig. Biochem. J. 1968, 106, 193–201. [Google Scholar] [CrossRef]
- Altenberg, B.; Greulich, K.O. Genes of Glycolysis Are Ubiquitously Overexpressed in 24 Cancer Classes. Genomics 2004, 84, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhao, F.; Thompson, C.B. The Molecular Determinants of de Novo Nucleotide Biosynthesis in Cancer Cells. Curr. Opin. Genet. Dev. 2009, 19, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Zheng, J. Energy Metabolism of Cancer: Glycolysis versus Oxidative Phosphorylation (Review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.-F.H. Tumor Glycolysis as a Target for Cancer Therapy: Progress and Prospects. Mol. Cancer 2013, 12, 152. [Google Scholar] [CrossRef]
- Dodge, P.W.; Takemori, A.E. Effects of Morphine, Nalorphine and Pentobarbital Alone and in Combination on Cerebral Glycolytic Substrates and Cofactors of Rats in Vivo. Biochem. Pharmacol. 1972, 21, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Abood, L.G.; Kun, E.; Geiling, E.M.K. Phosphorylated Intermediates of Chronically and Acutely Morphinized Rats. J. Pharmacol. Exp. Ther. 1950, 98, 373. [Google Scholar]
- Liu, R.; Cheng, J.; Yang, J.; Ding, X.; Yang, S.; Dong, F.; Guo, N.; Liu, S. GC-MS-Based Plasma Metabolomic Investigations of Morphine Dependent Rats at Different States of Euphoria, Tolerance and Naloxone-Precipitated Withdrawal. Metab. Brain Dis. 2015, 30, 767–776. [Google Scholar] [CrossRef]
- Miller, A.L.; Hawkins, R.A.; Harris, R.L.; Veech, R.L. The Effects of Acute and Chronic Morphine Treatment and of Morphine Withdrawal on Rat Brain in Vivo. Biochem. J. 1972, 129, 463–469. [Google Scholar] [CrossRef][Green Version]
- Brown, J.N.; Ortiz, G.M.; Angel, T.E.; Jacobs, J.M.; Gritsenko, M.; Chan, E.Y.; Purdy, D.E.; Murnane, R.D.; Larsen, K.; Palermo, R.E.; et al. Morphine Produces Immunosuppressive Effects in Nonhuman Primates at the Proteomic and Cellular Levels. Mol. Cell. Proteom. 2012, 11, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Morón, J.A.; Abul-Husn, N.S.; Rozenfeld, R.; Dolios, G.; Wang, R.; Devi, L.A. Morphine Administration Alters the Profile of Hippocampal Postsynaptic Density-Associated Proteins. Mol. Cell. Proteom. 2007, 6, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Ujcikova, H.; Vosahlikova, M.; Roubalova, L.; Svoboda, P. Proteomic Analysis of Protein Composition of Rat Forebrain Cortex Exposed to Morphine for 10 Days; Comparison with Animals Exposed to Morphine and Subsequently Nurtured for 20 Days in the Absence of This Drug. J. Proteom. 2016, 145, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Antolak, A.; Bodzoń-Kułakowska, A.; Cetnarska, E.; Pietruszka, M.; Marszałek-Grabska, M.; Kotlińska, J.; Suder, P. Proteomic Data in Morphine Addiction Versus Real Protein Activity: Metabolic Enzymes. J. Cell. Biochem. 2017, 118, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-L.; Lu, G.; Gong, Y.-X.; Zhao, L.-C.; Chen, J.; Chi, Z.-Q.; Yang, Y.-M.; Chen, Z.; Li, Q.; Liu, J.-G. Expression Changes of Hippocampal Energy Metabolism Enzymes Contribute to Behavioural Abnormalities during Chronic Morphine Treatment. Cell Res. 2007, 17, 689–700. [Google Scholar] [CrossRef]
- Atici, S.; Cinel, I.; Cinel, L.; Doruk, N.; Eskandari, G.; Oral, U. Liver and Kidney Toxicity in Chronic Use of Opioids: An Experimental Long Term Treatment Model. J. Biosci. 2005, 30, 245–252. [Google Scholar] [CrossRef]
- Jalili, C.; Rashidi, I.; Roshankhah, S.; Jalili, F.; Salahshoor, M.R. Protective Effect of Genistein on the Morphine-Induced Kidney Disorders in Male Mice. Electron. J. Gen. Med. 2020, 17, em213. [Google Scholar] [CrossRef][Green Version]
- Hu, Z.; Deng, Y.; Hu, C.; Deng, P.; Bu, Q.; Yan, G.; Zhou, J.; Shao, X.; Zhao, J.; Li, Y.; et al. 1H NMR-Based Metabonomic Analysis of Brain in Rats of Morphine Dependence and Withdrawal Intervention. Behav. Brain Res. 2012, 231, 11–19. [Google Scholar] [CrossRef]
- Xia, W.; Liu, G.; Shao, Z.; Xu, E.; Yuan, H.; Liu, J.; Gao, L. Toxicology of Tramadol Following Chronic Exposure Based on Metabolomics of the Cerebrum in Mice. Sci. Rep. 2020, 10, 11130. [Google Scholar] [CrossRef]
- Deng, Y.; Bu, Q.; Hu, Z.; Deng, P.; Yan, G.; Duan, J.; Hu, C.; Zhou, J.; Shao, X.; Zhao, J.; et al. 1H-Nuclear Magnetic Resonance-Based Metabonomic Analysis of Brain in Rhesus Monkeys with Morphine Treatment and Withdrawal Intervention. J. Neurosci. Res. 2012, 90, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Q.; Cheng, Y.; Ji, J.; Yu, L.-C. Changes of Protein Expression Profiles in the Amygdala during the Process of Morphine-Induced Conditioned Place Preference in Rats. Behav. Brain Res. 2011, 221, 197–206. [Google Scholar] [CrossRef]
- Sharma, S.K.; Yashpal, K.; Fundytus, M.E.; Sauriol, F.; Henry, J.L.; Coderre, T.J. Alterations in Brain Metabolism Induced by Chronic Morphine Treatment: NMR Studies in Rat CNS. Neurochem. Res. 2003, 28, 1369–1373. [Google Scholar] [CrossRef]
- Gao, H.; Xiang, Y.; Sun, N.; Zhu, H.; Wang, Y.; Liu, M.; Ma, Y.; Lei, H. Metabolic Changes in Rat Prefrontal Cortex and Hippocampus Induced by Chronic Morphine Treatment Studied Ex Vivo by High Resolution 1H NMR Spectroscopy. Neurochem. Int. 2007, 50, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Zaitsu, K.; Miyawaki, I.; Bando, K.; Horie, H.; Shima, N.; Katagi, M.; Tatsuno, M.; Bamba, T.; Sato, T.; Ishii, A.; et al. Metabolic Profiling of Urine and Blood Plasma in Rat Models of Drug Addiction on the Basis of Morphine, Methamphetamine, and Cocaine-Induced Conditioned Place Preference. Anal. Bioanal. Chem. 2014, 406, 1339–1354. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, R.; Sheng, W.; Feng, L.; Xu, P.; Wang, Y.; Xie, Y.; Xu, H.; Wang, G.; Aa, J. Identification of Morphine and Heroin-Treatment in Mice Using Metabonomics. Metabolites 2021, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Bierczynska-Krzysik, A.; Pradeep John, J.; Silberring, J.; Kotlinska, J.; Dylag, T.; Cabatic, M.; Lubec, G. Proteomic Analysis of Rat Cerebral Cortex, Hippocampus and Striatum after Exposure to Morphine. Int. J. Mol. Med. 2006, 18, 775–784. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Chudapongse, N.; Lee, S.-M.; Levin, M.C.; Oh, J.-T.; Park, H.-J.; Ho, I.K. Proteomic Analysis of Phosphotyrosyl Proteins in Morphine-Dependent Rat Brains. Mol. Brain Res. 2005, 133, 58–70. [Google Scholar] [CrossRef]
- Mansouri, S.; Shahriari, A.; Kalantar, H.; Moini Zanjani, T.; Haghi Karamallah, M. Role of Malate Dehydrogenase in Facilitating Lactate Dehydrogenase to Support the Glycolysis Pathway in Tumors. Biomed. Rep. 2017, 6, 463–467. [Google Scholar] [CrossRef]
- Dedrick, D.F.; Scherer, Y.D.; Biebuyck, J.F. Use of a Rapid Brain-Sampling Technique in a Physiologic Preparation: Effects of Morphine, Ketamine, and Halothane on Tissue Energy Intermediates. Anesthesiology 1975, 42, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Yousif, S.; Saubaméa, B.; Cisternino, S.; Marie-Claire, C.; Dauchy, S.; Scherrmann, J.-M.; Declèves, X. Effect of Chronic Exposure to Morphine on the Rat Blood-Brain Barrier: Focus on the P-Glycoprotein. J. Neurochem. 2008, 107, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-Y.; Pu, X.-P. Chronic Morphine Administration Induces Over-Expression of Aldolase C with Reduction of CREB Phosphorylation in the Mouse Hippocampus. Eur. J. Pharmacol. 2009, 609, 51–57. [Google Scholar] [CrossRef]
- Song, J.; Sun, H.; Zhang, S.; Shan, C. The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance. Life 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Aurora, A.B.; Khivansara, V.; Leach, A.; Gill, J.G.; Martin-Sandoval, M.; Yang, C.; Kasitinon, S.Y.; Bezwada, D.; Tasdogan, A.; Gu, W.; et al. Loss of Glucose 6-Phosphate Dehydrogenase Function Increases Oxidative Stress and Glutaminolysis in Metastasizing Melanoma Cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2120617119. [Google Scholar] [CrossRef]
- Yang, L.; Sun, Z.S.; Zhu, Y. Proteomic Analysis of Rat Prefrontal Cortex in Three Phases of Morphine-Induced Conditioned Place Preference. J. Proteome Res. 2007, 6, 2239–2247. [Google Scholar] [CrossRef]
- Bodzon-Kulakowska, A.; Suder, P.; Drabik, A.; Kotlinska, J.H.; Silberring, J. Constant Activity of Glutamine Synthetase after Morphine Administration versus Proteomic Results. Anal. Bioanal. Chem. 2010, 398, 2939–2942. [Google Scholar] [CrossRef][Green Version]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of Pyruvate Kinase M2 by Reactive Oxygen Species Contributes to Cellular Antioxidant Responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef]
- Scrutton, M.C.; Utter, M.F. The Regulation of Glycolysis and Gluconeogenesis in Animal Tissues. Annu. Rev. Biochem. 1968, 37, 249–302. [Google Scholar] [CrossRef]
- Shim, H.; Dolde, C.; Lewis, B.C.; Wu, C.S.; Dang, G.; Jungmann, R.A.; Dalla-Favera, R.; Dang, C.V. C-Myc Transactivation of LDH-A: Implications for Tumor Metabolism and Growth. Proc. Natl. Acad. Sci. USA 1997, 94, 6658–6663. [Google Scholar] [CrossRef]
- Lü, L.; Li, J.; Yew, D.T.; Rudd, J.A.; Mak, Y.T. Oxidative Stress on the Astrocytes in Culture Derived from a Senescence Accelerated Mouse Strain. Neurochem. Int. 2008, 52, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Tsirigos, A.; Whitaker-Menezes, D.; Pavlides, S.; Pestell, R.G.; Chiavarina, B.; Frank, P.G.; Flomenberg, N.; Howell, A.; Martinez-Outschoorn, U.E.; et al. Ketones and Lactate “Fuel” Tumor Growth and Metastasis: Evidence That Epithelial Cancer Cells Use Oxidative Mitochondrial Metabolism. Cell Cycle Georget. Tex 2010, 9, 3506–3514. [Google Scholar] [CrossRef]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting Lactate-Fueled Respiration Selectively Kills Hypoxic Tumor Cells in Mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef]
- Dhup, S.; Kumar Dadhich, R.; Ettore Porporato, P.; Sonveaux, P. Multiple Biological Activities of Lactic Acid in Cancer: Influences on Tumor Growth, Angiogenesis and Metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Fischer, K.; Hoffmann, P.; Voelkl, S.; Meidenbauer, N.; Ammer, J.; Edinger, M.; Gottfried, E.; Schwarz, S.; Rothe, G.; Hoves, S.; et al. Inhibitory Effect of Tumor Cell-Derived Lactic Acid on Human T Cells. Blood 2007, 109, 3812–3819. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Oikonomou, K.G.; Gibilaro, E.; Apergis, G. Elevated Lactic Acid Is a Negative Prognostic Factor in Metastatic Lung Cancer. Cancer Biomark. 2015, 15, 725–734. [Google Scholar] [CrossRef]
- Ros, S.; Schulze, A. Balancing Glycolytic Flux: The Role of 6-Phosphofructo-2-Kinase/Fructose 2,6-Bisphosphatases in Cancer Metabolism. Cancer Metab. 2013, 1, 8. [Google Scholar] [CrossRef]
- Tian, X.; Liu, M.; Huang, X.; Zhu, Q.; Liu, W.; Chen, W.; Zou, Y.; Cai, Y.; Huang, S.; Chen, A.; et al. Noscapine Induces Apoptosis in Human Colon Cancer Cells by Regulating Mitochondrial Damage and Warburg Effect via PTEN/PI3K/mTOR Signaling Pathway. OncoTargets Ther. 2020, 13, 5419–5428. [Google Scholar] [CrossRef]
- Rida, P.C.G.; LiVecche, D.; Ogden, A.; Zhou, J.; Aneja, R. The Noscapine Chronicle: A Pharmaco-Historic Biography of the Opiate Alkaloid Family and Its Clinical Applications: THE NOSCAPINE CHRONICLE. Med. Res. Rev. 2015, 35, 1072–1096. [Google Scholar] [CrossRef] [PubMed]
- Cain, D.F.; Infante, A.A.; Davies, R.E. Chemistry of Muscle Contraction. Adenosine Triphosphate and Phosphorylcreatine as Energy Supplies for Single Contractions of Working Muscle. Nature 1962, 196, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Sweeney, H.L.; Kushmerick, M.J. A Simple Analysis of the “Phosphocreatine Shuttle”. Am. J. Physiol.-Cell Physiol. 1984, 246, C365–C377. [Google Scholar] [CrossRef]
- Miller, E.E.; Evans, A.E.; Cohn, M. Inhibition of Rate of Tumor Growth by Creatine and Cyclocreatine. Proc. Natl. Acad. Sci. USA 1993, 90, 3304–3308. [Google Scholar] [CrossRef]
- Kristensen, C.A.; Askenasy, N.; Jain, R.K.; Koretsky, A.P. Creatine and Cyclocreatine Treatment of Human Colon Adenocarcinoma Xenografts: 31P and 1H Magnetic Resonance Spectroscopic Studies. Br. J. Cancer 1999, 79, 278–285. [Google Scholar] [CrossRef][Green Version]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition Interventions to Treat Low Muscle Mass in Cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Fairman, C.M.; Kendall, K.L.; Hart, N.H.; Taaffe, D.R.; Galvao, D.A.; Newton, R.U. The Potential Therapeutic Effects of Creatine Supplementation on Body Composition and Muscle Function in Cancer. Crit. Rev. Oncol. Hematol. 2019, 133, 46–57. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.; Yan, H.; Wang, W.; Wu, Z.; Zhang, F.; Zhang, Q.; Shi, G.; Du, J.; Cai, H.; et al. Creatine Promotes Cancer Metastasis through Activation of Smad2/3. Cell Metab. 2021, 33, 1111–1123. [Google Scholar] [CrossRef]
- Maguire, O.A.; Ackerman, S.E.; Szwed, S.K.; Maganti, A.V.; Marchildon, F.; Huang, X.; Kramer, D.J.; Rosas-Villegas, A.; Gelfer, R.G.; Turner, L.E.; et al. Creatine-Mediated Crosstalk between Adipocytes and Cancer Cells Regulates Obesity-Driven Breast Cancer. Cell Metab. 2021, 33, 499–512. [Google Scholar] [CrossRef]
- Papalazarou, V.; Zhang, T.; Paul, N.R.; Juin, A.; Cantini, M.; Maddocks, O.D.; Salmeron-Sanchez, M.; Machesky, L.M. The Creatine–Phosphagen System Is Mechanoresponsive in Pancreatic Adenocarcinoma and Fuels Invasion and Metastasis. Nat. Metab. 2020, 2, 62–80. [Google Scholar] [CrossRef]
- Loo, J.M.; Scherl, A.; Nguyen, A.; Man, F.Y.; Weinberg, E.; Zeng, Z.; Saltz, L.; Paty, P.B.; Tavazoie, S.F. Extracellular Metabolic Energetics Can Promote Cancer Progression. Cell 2015, 160, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bu, P. The Two Sides of Creatine in Cancer. Trends Cell Biol. 2022, 32, 380–390. [Google Scholar] [CrossRef]
- Berti, S.L.; Nasi, G.M.; Garcia, C.; de Castro, F.L.; Nunes, M.L.; Rojas, D.B.; Moraes, T.B.; Dutra-Filho, C.S.; Wannmacher, C.M.D. Pyruvate and Creatine Prevent Oxidative Stress and Behavioral Alterations Caused by Phenylalanine Administration into Hippocampus of Rats. Metab. Brain Dis. 2012, 27, 79–89. [Google Scholar] [CrossRef]
- Guidi, C.; Potenza, L.; Sestili, P.; Martinelli, C.; Guescini, M.; Stocchi, L.; Zeppa, S.; Polidori, E.; Annibalini, G.; Stocchi, V. Differential Effect of Creatine on Oxidatively-Injured Mitochondrial and Nuclear DNA. Biochim. Biophys. Acta BBA-Gen. Subj. 2008, 1780, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Knox, D.; Perrine, S.A.; George, S.A.; Galloway, M.P.; Liberzon, I. Single Prolonged Stress Decreases Glutamate, Glutamine, and Creatine Concentrations in the Rat Medial Prefrontal Cortex. Neurosci. Lett. 2010, 480, 16–20. [Google Scholar] [CrossRef]
- Cai, J.; Xia, L.; Li, J.; Ni, S.; Song, H.; Wu, X. Tumor-Associated Macrophages Derived TGF-β-Induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells through Smad2,3-4/Snail Signaling Pathway. Cancer Res. Treat. 2019, 51, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Weinsanto, I.; Mouheiche, J.; Laux-Biehlmann, A.; Delalande, F.; Marquette, A.; Chavant, V.; Gabel, F.; Cianferani, S.; Charlet, A.; Parat, M.-O.; et al. Morphine Binds Creatine Kinase B and Inhibits Its Activity. Front. Cell. Neurosci. 2018, 12, 464. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolism and Metabolomics of Opiates: A Long Way of Forensic Implications to Unravel. J. Forensic Leg. Med. 2019, 61, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Sebők, V.; Sultana, N.; Swann, J.R.; Bailey, A. Metabolic Phenotyping of Opioid and Psychostimulant Addiction: A Novel Approach for Biomarker Discovery and Biochemical Understanding of the Disorder. Br. J. Pharmacol. 2022, 179, 1578–1606. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, M.; Ózsvári, B.; Sotgia, F.; Lisanti, M.P. High ATP Production Fuels Cancer Drug Resistance and Metastasis: Implications for Mitochondrial ATP Depletion Therapy. Front. Oncol. 2021, 11, 740720. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Ma, F.; Qian, H. Defueling the Cancer: ATP Synthase as an Emerging Target in Cancer Therapy. Mol. Ther.-Oncolytics 2021, 23, 82–95. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The Diverse Functions of Glutamine in Metabolism, Cell Biology and Cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Wise, D.R.; Thompson, C.B. Glutamine Addiction: A New Therapeutic Target in Cancer. Trends Biochem. Sci. 2010, 35, 427–433. [Google Scholar] [CrossRef]
- Seltzer, M.J.; Bennett, B.D.; Joshi, A.D.; Gao, P.; Thomas, A.G.; Ferraris, D.V.; Tsukamoto, T.; Rojas, C.J.; Slusher, B.S.; Rabinowitz, J.D.; et al. Inhibition of Glutaminase Preferentially Slows Growth of Glioma Cells with Mutant IDH1. Cancer Res. 2010, 70, 8981–8987. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, Q.; Ma, H.; Lv, Q.; Zhang, T. Expression of Glutaminase Is Upregulated in Colorectal Cancer and of Clinical Significance. Int. J. Clin. Exp. Pathol. 2014, 7, 1093–1100. [Google Scholar]
- Yu, D.; Shi, X.; Meng, G.; Chen, J.; Yan, C.; Jiang, Y.; Wei, J.; Ding, Y. Kidney-Type Glutaminase (GLS1) Is a Biomarker for Pathologic Diagnosis and Prognosis of Hepatocellular Carcinoma. Oncotarget 2015, 6, 7619. [Google Scholar] [CrossRef] [PubMed]
- Qie, S.; Chu, C.; Li, W.; Wang, C.; Sang, N. ErbB2 Activation Upregulates Glutaminase 1 Expression Which Promotes Breast Cancer Cell Proliferation. J. Cell. Biochem. 2014, 115, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Udupa, S.; Nguyen, S.; Hoang, G.; Nguyen, T.; Quinones, A.; Pham, K.; Asaka, R.; Nguyen, K.; Zhang, C.; Elgogary, A. Upregulation of the Glutaminase II Pathway Contributes to Glutamate Production upon Glutaminase 1 Inhibition in Pancreatic Cancer. Proteomics 2019, 19, 1800451. [Google Scholar] [CrossRef]
- Chiodi, I.; Picco, G.; Martino, C.; Mondello, C. Cellular Response to Glutamine and/or Glucose Deprivation in In vitro Transformed Human Fibroblasts. Oncol. Rep. 2019, 41, 3555–3564. [Google Scholar] [CrossRef]
- Walls, A.B.; Waagepetersen, H.S.; Bak, L.K.; Schousboe, A.; Sonnewald, U. The Glutamine–Glutamate/GABA Cycle: Function, Regional Differences in Glutamate and GABA Production and Effects of Interference with GABA Metabolism. Neurochem. Res. 2015, 40, 402–409. [Google Scholar] [CrossRef]
- Lu, M.; Zhu, X.-H.; Zhang, Y.; Mateescu, G.; Chen, W. Quantitative Assessment of Brain Glucose Metabolic Rates Using in Vivo Deuterium Magnetic Resonance Spectroscopy. J. Cereb. Blood Flow Metab. 2017, 37, 3518–3530. [Google Scholar] [CrossRef]
- Pourzitaki, C.; Tsaousi, G.; Papazisis, G.; Kyrgidis, A.; Zacharis, C.; Kritis, A.; Malliou, F.; Kouvelas, D. Fentanyl and Naloxone Effects on Glutamate and GABA Release Rates from Anterior Hypothalamus in Freely Moving Rats. Eur. J. Pharmacol. 2018, 834, 169–175. [Google Scholar] [CrossRef]
- Halama, A.; Suhre, K. Advancing Cancer Treatment by Targeting Glutamine Metabolism-A Roadmap. Cancers 2022, 14, 553. [Google Scholar] [CrossRef]
- Wang, Q.; Hardie, R.; Hoy, A.J.; Van Geldermalsen, M.; Gao, D.; Fazli, L.; Sadowski, M.C.; Balaban, S.; Schreuder, M.; Nagarajah, R.; et al. Targeting ASCT2—Mediated Glutamine Uptake Blocks Prostate Cancer Growth and Tumour Development. J. Pathol. 2015, 236, 278–289. [Google Scholar] [CrossRef]
- Ko, Y.G.; Kim, E.Y.; Kim, T.; Park, H.; Park, H.S.; Choi, E.J.; Kim, S. Glutamine-Dependent Antiapoptotic Interaction of Human Glutaminyl-tRNA Synthetase with Apoptosis Signal-Regulating Kinase 1. J. Biol. Chem. 2001, 276, 6030–6036. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Park, K.-G. Targeting Glutamine Metabolism for Cancer Treatment. Biomol. Ther. 2018, 26, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, I.P.M.; Alam, N.A.; Rowan, A.J.; Barclay, E.; Jaeger, E.E.M.; Kelsell, D.; Leigh, I.; Gorman, P.; Lamlum, H.; Rahman, S.; et al. Germline Mutations in FH Predispose to Dominantly Inherited Uterine Fibroids, Skin Leiomyomata and Papillary Renal Cell Cancer. Nat. Genet. 2002, 30, 406–410. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-Associated IDH1 Mutations Produce 2-Hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, G.; Tennant, D.A. Isocitrate Dehydrogenase (IDH), Succinate Dehydrogenase (SDH), Fumarate Hydratase (FH): Three Players for One Phenotype in Cancer? Biochem. Soc. Trans. 2016, 44, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227. [Google Scholar] [CrossRef]
- Rzeski, W.; Walczak, K.; Juszczak, M.; Langner, E.; PoŻarowski, P.; Kandefer-Szerszeń, M.; Pierzynowski, S.G. Alpha-Ketoglutarate (AKG) Inhibits Proliferation of Colon Adenocarcinoma Cells in Normoxic Conditions. Scand. J. Gastroenterol. 2012, 47, 565–571. [Google Scholar] [CrossRef]
- Kaławaj, K.; Sławińska-Brych, A.; Mizerska-Kowalska, M.; Żurek, A.; Bojarska-Junak, A.; Kandefer-Szerszeń, M.; Zdzisińska, B. Alpha Ketoglutarate Exerts In Vitro Anti-Osteosarcoma Effects through Inhibition of Cell Proliferation, Induction of Apoptosis via the JNK and Caspase 9-Dependent Mechanism, and Suppression of TGF-β and VEGF Production and Metastatic Potential of Cells. Int. J. Mol. Sci. 2020, 21, 9406. [Google Scholar] [CrossRef]
- Tran, T.Q.; Hanse, E.A.; Habowski, A.N.; Li, H.; Ishak Gabra, M.B.; Yang, Y.; Lowman, X.H.; Ooi, A.M.; Liao, S.Y.; Edwards, R.A.; et al. α-Ketoglutarate Attenuates Wnt Signaling and Drives Differentiation in Colorectal Cancer. Nat. Cancer 2020, 1, 345–358. [Google Scholar] [CrossRef]
- Sainero-Alcolado, L.; Liaño-Pons, J.; Ruiz-Pérez, M.V.; Arsenian-Henriksson, M. Targeting Mitochondrial Metabolism for Precision Medicine in Cancer. Cell Death Differ. 2022, 29, 1304–1317. [Google Scholar] [CrossRef]
- Anderson, N.M.; Mucka, P.; Kern, J.G.; Feng, H. The Emerging Role and Targetability of the TCA Cycle in Cancer Metabolism. Protein Cell 2018, 9, 216–237. [Google Scholar] [CrossRef]
- Lycan, T.W.; Pardee, T.S.; Petty, W.J.; Bonomi, M.; Alistar, A.; Lamar, Z.S.; Isom, S.; Chan, M.D.; Miller, A.A.; Ruiz, J. A Phase II Clinical Trial of CPI-613 in Patients with Relapsed or Refractory Small Cell Lung Carcinoma. PLoS ONE 2016, 11, e0164244. [Google Scholar] [CrossRef]
- Nelson, D.M.; Ye, X.; Hall, C.; Santos, H.; Ma, T.; Kao, G.D.; Yen, T.J.; Harper, J.W.; Adams, P.D. Coupling of DNA Synthesis and Histone Synthesis in S Phase Independent of Cyclin/Cdk2 Activity. Mol. Cell. Biol. 2002, 22, 7459–7472. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wu, Y.; Zhai, Y.; Hu, B.; Ma, W.; Yang, W.; Yu, Q.; Chen, Z.; Workman, J.L.; Yu, X.; et al. Exogenous Pyruvate Represses Histone Gene Expression and Inhibits Cancer Cell Proliferation via the NAMPT–NAD+–SIRT1 Pathway. Nucleic Acids Res. 2019, 47, 11132–11150. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, Y.; Tang, D.; Dorfman, R.G.; Xu, L.; Zhou, Q.; Zhou, L.; Wang, Y.; Li, Y.; Yin, Y.; et al. Low Levels of Pyruvate Induced by a Positive Feedback Loop Protects Cholangiocarcinoma Cells from Apoptosis. Cell Commun. Signal. 2019, 17, 23. [Google Scholar] [CrossRef]
- TeSlaa, T.; Chaikovsky, A.C.; Lipchina, I.; Escobar, S.L.; Hochedlinger, K.; Huang, J.; Graeber, T.G.; Braas, D.; Teitell, M.A. α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell Metab. 2016, 24, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Abla, H.; Sollazzo, M.; Gasparre, G.; Iommarini, L.; Porcelli, A.M. The Multifaceted Contribution of α-Ketoglutarate to Tumor Progression: An Opportunity to Exploit? Semin. Cell Dev. Biol. 2020, 98, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhang, J.; Yan, M.; Chen, L.; Wu, J.; Tao, Q.; Yan, B.; Chen, X.; Peng, C. Supplementation with α-Ketoglutarate Improved the Efficacy of Anti-PD1 Melanoma Treatment through Epigenetic Modulation of PD-L1. Cell Death Dis. 2023, 14, 170. [Google Scholar] [CrossRef]
- Wu, F.; Xie, X.; Li, G.; Bao, D.; Li, H.; Wu, G.; Lai, Y.; Xing, Y.; Ouyang, P.; Chen, G.; et al. AKG Induces Cell Apoptosis by Inducing Reactive Oxygen Species—Mediated Endoplasmic Reticulum Stress and by Suppressing PI3K/AKT/mTOR—Mediated Autophagy in Renal Cell Carcinoma. Environ. Toxicol. 2023, 38, 17–27. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, P.; Chen, Z.; Yue, D.; Liu, C.; Li, C.; Chen, C.; Zhang, H.; Liu, H.; Zhang, Z.; et al. Urinary Malate Dehydrogenase 2 Is a New Biomarker for Early Detection of Non-small-cell Lung Cancer. Cancer Sci. 2021, 112, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-P.; Zhou, W.; Wang, J.; Huang, X.; Zuo, Y.; Wang, T.-S.; Gao, X.; Xu, Y.-Y.; Zou, S.-W.; Liu, Y.-B.; et al. Arginine Methylation of MDH1 by CARM1 Inhibits Glutamine Metabolism and Suppresses Pancreatic Cancer. Mol. Cell 2016, 64, 673–687. [Google Scholar] [CrossRef]
- Hanse, E.A.; Ruan, C.; Kachman, M.; Wang, D.; Lowman, X.H.; Kelekar, A. Cytosolic Malate Dehydrogenase Activity Helps Support Glycolysis in Actively Proliferating Cells and Cancer. Oncogene 2017, 36, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Ghanbari, R.; Pathmasiri, W.; McRitchie, S.; Poustchi, H.; Shayanrad, A.; Roshandel, G.; Etemadi, A.; Pollock, J.D.; Malekzadeh, R.; et al. Untargeted Metabolomics: Biochemical Perturbations in Golestan Cohort Study Opium Users Inform Intervention Strategies. Front. Nutr. 2020, 7, 584585. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Metabolic Regulation of Mitochondrial Dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef]
- Senft, D.; Ronai, Z.A. Regulators of Mitochondrial Dynamics in Cancer. Curr. Opin. Cell Biol. 2016, 39, 43–52. [Google Scholar] [CrossRef]
- Rehman, J.; Zhang, H.J.; Toth, P.T.; Zhang, Y.; Marsboom, G.; Hong, Z.; Salgia, R.; Husain, A.N.; Wietholt, C.; Archer, S.L. Inhibition of Mitochondrial Fission Prevents Cell Cycle Progression in Lung Cancer. FASEB J. 2012, 26, 2175–2186. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Yu, M.; Xie, Y.; Huang, Y.; Wolff, D.W.; Abel, P.W.; Tu, Y. Mitochondrial Dynamics Regulates Migration and Invasion of Breast Cancer Cells. Oncogene 2013, 32, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, D.C. Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell Metab. 2017, 26, 39–48. [Google Scholar] [CrossRef]
- Zhou, Y.; Long, D.; Zhao, Y.; Li, S.; Liang, Y.; Wan, L.; Zhang, J.; Xue, F.; Feng, L. Oxidative Stress-Mediated Mitochondrial Fission Promotes Hepatic Stellate Cell Activation via Stimulating Oxidative Phosphorylation. Cell Death Dis. 2022, 13, 689. [Google Scholar] [CrossRef]
- Mitchell, P. Possible Molecular Mechanisms of the Protonmotive Function of Cytochrome Systems. J. Theor. Biol. 1976, 62, 327–367. [Google Scholar] [CrossRef]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; King, M.S.; Pryde, K.R. The Production of Reactive Oxygen Species by Complex I. Biochem. Soc. Trans. 2008, 36, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Tahara, E.B.; Navarete, F.D.T.; Kowaltowski, A.J. Tissue-, Substrate-, and Site-Specific Characteristics of Mitochondrial Reactive Oxygen Species Generation. Free Radic. Biol. Med. 2009, 46, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Indran, I.R.; Hande, M.P.; Pervaiz, S. hTERT Overexpression Alleviates Intracellular ROS Production, Improves Mitochondrial Function, and Inhibits ROS-Mediated Apoptosis in Cancer CellshTERT, Mitochondria, and ROS. Cancer Res. 2011, 71, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Gaude, E.; Frezza, C. Tissue-Specific and Convergent Metabolic Transformation of Cancer Correlates with Metastatic Potential and Patient Survival. Nat. Commun. 2016, 7, 13041. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Yang, J. Epithelial–Mesenchymal Plasticity in Carcinoma Metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef]
- Rohlena, J.; Dong, L.; Neuzil, J. Targeting the Mitochondrial Electron Transport Chain Complexes for the Induction of Apoptosis and Cancer Treatment. Curr. Pharm. Biotechnol. 2013, 14, 377–389. [Google Scholar] [CrossRef]
- Li, B.; Yang, L. Creatine in T Cell Antitumor Immunity and Cancer Immunotherapy. Nutrients 2021, 13, 1633. [Google Scholar] [CrossRef]
- Luna Yolba, R.; Visentin, V.; Hervé, C.; Chiche, J.; Ricci, J.; Méneyrol, J.; Paillasse, M.R.; Alet, N. EVT-701 Is a Novel Selective and Safe Mitochondrial Complex 1 Inhibitor with Potent Anti-tumor Activity in Models of Solid Cancers. Pharmacol. Res. Perspect. 2021, 9, e00854. [Google Scholar] [CrossRef]
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.; Sánchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene Ablation-Resistant Pancreatic Cancer Cells Depend on Mitochondrial Function. Nature 2014, 514, 628–632. [Google Scholar] [CrossRef]
- Hernández-Reséndiz, I.; Gallardo-Pérez, J.C.; López-Macay, A.; Robledo-Cadena, D.X.; García-Villa, E.; Gariglio, P.; Saavedra, E.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S. Mutant p53R248Q Downregulates Oxidative Phosphorylation and Upregulates Glycolysis under Normoxia and Hypoxia in Human Cervix Cancer Cells. J. Cell. Physiol. 2019, 234, 5524–5536. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.M.; McKenna, W.G.; Kunz-Schughart, L.A.; Higgins, G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018, 24, 2482–2490. [Google Scholar] [CrossRef]
- Nylander, E.; Zelleroth, S.; Nyberg, F.; Grönbladh, A.; Hallberg, M. The Effects of Morphine, Methadone, and Fentanyl on Mitochondria: A Live Cell Imaging Study. Brain Res. Bull. 2021, 171, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A Mitochondrial Love-Hate Triangle. Am. J. Physiol.-Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef] [PubMed]
- Stefano, G.B. Autoimmunovascular Regulation: Morphine and Ancondamide and Ancondamide Stimulated Nitric Oxide Release. J. Neuroimmunol. 1998, 83, 70–76. [Google Scholar] [CrossRef]
- Machelska, H.; Ziólkowska, B.; Mika, J.; Przewlocka, B.; Przewlocki, R. Chronic Morphine Increases Biosynthesis of Nitric Oxide Synthase in the Rat Spinal Cord. Neuroreport 1997, 8, 2743–2747. [Google Scholar] [CrossRef]
- Hsiao, P.-N.; Chang, M.-C.; Cheng, W.-F.; Chen, C.-A.; Lin, H.-W.; Hsieh, C.-Y.; Sun, W.-Z. Morphine Induces Apoptosis of Human Endothelial Cells through Nitric Oxide and Reactive Oxygen Species Pathways. Toxicology 2009, 256, 83–91. [Google Scholar] [CrossRef]
- Brown, G.C. Regulation of Mitochondrial Respiration by Nitric Oxide Inhibition of Cytochrome c Oxidase. Biochim. Biophys. Acta BBA-Bioenerg. 2001, 1504, 46–57. [Google Scholar] [CrossRef]
- Mastronicola, D.; Arcuri, E.; Arese, M.; Bacchi, A.; Mercadante, S.; Cardelli, P.; Citro, G.; Sarti, P. Morphine but Not Fentanyl and Methadone Affects Mitochondrial Membrane Potential by Inducing Nitric Oxide Release in Glioma Cells. Cell. Mol. Life Sci. CMLS 2004, 61, 2991–2997. [Google Scholar] [CrossRef]
- Cunha-Oliveira, T.; Silva, L.; Silva, A.M.; Moreno, A.J.; Oliveira, C.R.; Santos, M.S. Acute Effects of Cocaine, Morphine and Their Combination on Bioenergetic Function and Susceptibility to Oxidative Stress of Rat Liver Mitochondria. Life Sci. 2013, 92, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Yang, Y.; Yan, G.; Hu, Z.; Hu, C.; Duan, J.; Lv, L.; Zhou, J.; Zhao, J.; Shao, X.; et al. Proteomic Analysis of the Nucleus Accumbens in Rhesus Monkeys of Morphine Dependence and Withdrawal Intervention. J. Proteom. 2012, 75, 1330–1342. [Google Scholar] [CrossRef]
- Bodzon-Kulakowska, A.; Suder, P.; Mak, P.; Bierczynska-Krzysik, A.; Lubec, G.; Walczak, B.; Kotlinska, J.; Silberring, J. Proteomic Analysis of Striatal Neuronal Cell Cultures after Morphine Administration. J. Sep. Sci. 2009, 32, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Li, K.W.; Jimenez, C.R.; van der Schors, R.C.; Hornshaw, M.P.; Schoffelmeer, A.N.M.; Smit, A.B. Intermittent Administration of Morphine Alters Protein Expression in Rat Nucleus Accumbens. Proteomics 2006, 6, 2003–2008. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, L.; Qian, K.; Wang, G.; Lu, M.; Qian, G.; Cao, X.; Jiang, W.; Xiao, Y.; Wang, X. A Novel Correlation between ATP5A1 Gene Expression and Progression of Human Clear Cell Renal Cell Carcinoma Identified by Co-Expression Analysis. Oncol. Rep. 2018, 39, 525–536. [Google Scholar] [CrossRef]
- Speransky, S.; Serafini, P.; Caroli, J.; Bicciato, S.; Lippman, M.; Bishopric, N. A Novel RNA Aptamer Identifies Plasma Membrane ATP Synthase Beta Subunit as an Early Marker and Therapeutic Target in Aggressive Cancer. Breast Cancer Res. Treat. 2019, 176, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, J.Y. ATP5A1 and ATP5B Are Highly Expressed in Glioblastoma Tumor Cells and Endothelial Cells of Microvascular Proliferation. J. Neurooncol. 2016, 126, 405–413. [Google Scholar] [CrossRef]
- Bianchi, G.; Ravera, S.; Traverso, C.; Amaro, A.; Piaggio, F.; Emionite, L.; Bachetti, T.; Pfeffer, U.; Raffaghello, L. Curcumin Induces a Fatal Energetic Impairment in Tumor Cells in Vitro and in Vivo by Inhibiting ATP-Synthase Activity. Carcinogenesis 2018, 39, 1141–1150. [Google Scholar] [CrossRef]
- Fiorillo, M.; Scatena, C.; Naccarato, A.G.; Sotgia, F.; Lisanti, M.P. Bedaquiline, an FDA-Approved Drug, Inhibits Mitochondrial ATP Production and Metastasis in Vivo, by Targeting the Gamma Subunit (ATP5F1C) of the ATP Synthase. Cell Death Differ. 2021, 28, 2797–2817. [Google Scholar] [CrossRef]
- Djafarzadeh, S.; Vuda, M.; Jeger, V.; Takala, J.; Jakob, S.M. The Effects of Fentanyl on Hepatic Mitochondrial Function. Anesth. Analg. 2016, 123, 311–325. [Google Scholar] [CrossRef]
- Vilela, S.M.F. Are Fentanyl and Remifentanil Safe Opioids for Rat Brain Mitochondrial Bioenergetics? Mitochondrion 2009, 9, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.M.; Ghaffar, H.M.A.; El Husseiny, R.M. Effects of Tramadol, Clonazepam, and Their Combination on Brain Mitochondrial Complexes. Toxicol. Ind. Health 2015, 31, 1325–1333. [Google Scholar] [CrossRef]
- Chih, C.-P.; Roberts, E.L. Energy Substrates for Neurons during Neural Activity: A Critical Review of the Astrocyte-Neuron Lactate Shuttle Hypothesis. J. Cereb. Blood Flow Metab. 2003, 23, 1263–1281. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, F.; Lim, J.-H.; Chim, H.; Bhalla, K.; Girnun, G.; Pierce, K.; Clish, C.B.; Granter, S.R.; Widlund, H.R.; Spiegelman, B.M.; et al. PGC1α Expression Defines a Subset of Human Melanoma Tumors with Increased Mitochondrial Capacity and Resistance to Oxidative Stress. Cancer Cell 2013, 23, 287–301. [Google Scholar] [CrossRef]
- Farge, T.; Saland, E.; De Toni, F.; Aroua, N.; Hosseini, M.; Perry, R.; Bosc, C.; Sugita, M.; Stuani, L.; Fraisse, M.; et al. Chemotherapy-Resistant Human Acute Myeloid Leukemia Cells Are Not Enriched for Leukemic Stem Cells but Require Oxidative Metabolism. Cancer Discov. 2017, 7, 716–735. [Google Scholar] [CrossRef]
- Lee, K.; Giltnane, J.M.; Balko, J.M.; Schwarz, L.J.; Guerrero-Zotano, A.L.; Hutchinson, K.E.; Nixon, M.J.; Estrada, M.V.; Sánchez, V.; Sanders, M.E.; et al. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017, 26, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Rodon Ahnert, J.; Piha-Paul, S.A.; Fu, S.; Janku, F.; Karp, D.D.; Naing, A.; Ileana Dumbrava, E.E.; Pant, S.; Subbiah, V.; et al. Phase I Trial of IACS-010759 (IACS), a Potent, Selective Inhibitor of Complex I of the Mitochondrial Electron Transport Chain, in Patients (Pts) with Advanced Solid Tumors. J. Clin. Oncol. 2019, 37, 3014. [Google Scholar] [CrossRef]
- Soga, T. Cancer Metabolism: Key Players in Metabolic Reprogramming. Cancer Sci. 2013, 104, 275–281. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer Metabolism: Looking Forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Radisky, D.C.; Levy, D.D.; Littlepage, L.E.; Liu, H.; Nelson, C.M.; Fata, J.E.; Leake, D.; Godden, E.L.; Albertson, D.G.; Angela Nieto, M.; et al. Rac1b and Reactive Oxygen Species Mediate MMP-3-Induced EMT and Genomic Instability. Nature 2005, 436, 123–127. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The Role of ROS in Tumour Development and Progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Horecker, B.L. The Pentose Phosphate Pathway. J. Biol. Chem. 2002, 277, 47965–47971. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S.J.; Porter, J.W.; Gibson, D.M. Studies on the Mechanism of Fatty Acid Synthesis: I. Preparation and Purification of an Enzyme System for Reconstruction of Fatty Acid Synthesis. Biochim. Biophys. Acta 1957, 24, 453–461. [Google Scholar] [CrossRef]
- Volpe, J.; Vagelos, P.R. Saturated Fatty Acid Biosynthesis and Its Regulation. Annu. Rev. Biochem. 1973, 42, 21–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Wu, Y.-H.; Yen, W.-C.; Liu, H.-Y.; Hwang, T.-L.; Stern, A.; Chiu, D.T.-Y. The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells 2019, 8, 1055. [Google Scholar] [CrossRef]
- Mele, L.; la Noce, M.; Paino, F.; Regad, T.; Wagner, S.; Liccardo, D.; Papaccio, G.; Lombardi, A.; Caraglia, M.; Tirino, V.; et al. Glucose-6-Phosphate Dehydrogenase Blockade Potentiates Tyrosine Kinase Inhibitor Effect on Breast Cancer Cells through Autophagy Perturbation. J. Exp. Clin. Cancer Res. 2019, 38, 160. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Z.; Zhu, Z.; Chen, A.; Fu, G.; Wang, Y.; Pan, H.; Jin, B. Modulation of G6PD Affects Bladder Cancer via ROS Accumulation and the AKT Pathway in Vitro. Int. J. Oncol. 2018, 53, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, K.; Rajakumar, S.; Sarkar, M.N.; Nachiappan, V. Glutathione Peroxidase3 of Saccharomyces Cerevisiae Protects Phospholipids during Cadmium-Induced Oxidative Stress. Antonie Van Leeuwenhoek 2011, 99, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.L.; Saha, A.; Liu, J.; Tadi, S.; Tiziani, S.; Yan, W.; Triplett, K.; Lamb, C.; Alters, S.E.; Rowlinson, S.; et al. Systemic Depletion of L-Cyst (e) Ine with Cyst (e) Inase Increases Reactive Oxygen Species and Suppresses Tumor Growth. Nat. Med. 2017, 23, 120–127. [Google Scholar] [CrossRef]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione Levels in Human Tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Barranco, S.C.; Perry, R.R.; Durm, M.E.; Quraishi, M.; Werner, A.L.; Gregorcyk, S.G.; Kolm, P. Relationship between Colorectal Cancer Glutathione Levels and Patient Survival. Dis. Colon Rectum 2000, 43, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.; Pilch, H.; Schmidt, M.; Dahlenburg, H.; Sagemüller, J.; Schiffer, I.; Oesch, F.; Knapstein, P.; Kaina, B.; Tanner, B. Metallothionein Expression in Ovarian Cancer in Relation to Histopathological Parameters and Molecular Markers of Prognosis. Int. J. Cancer 2001, 95, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Kigawa, J.; Minagawa, Y.; Kanamori, Y.; Itamochi, H.; Cheng, X.; Okada, M.; Oishi, T.; Terakawa, N. Glutathione Concentration May Be a Useful Predictor of Response to Second-Line Chemotherapy in Patients with Ovarian Cancer. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1998, 82, 697–702. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive Molecular Characterization of Clear Cell Renal Cell Carcinoma. Nature 2013, 499, 43–49. [Google Scholar] [CrossRef]
- Kornber, A.; Horecker, B.L.; Smyrniotis, P. Glucose-6-Phosphate Dehydrogenase-6-Phosphogluconic Dehydrogenase. Methods Enzymol. 1955, 1, 323–327. [Google Scholar]
- Özdemir, H.; Çiftçi, M. In Vitro Effects of Some Drugs on Human Red Blood Cell Glucose-6-Phosphate Dehydrogenase Enzyme Activity. J. Enzyme Inhib. Med. Chem. 2006, 21, 75–80. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, J.; Xu, Z.; Peng, B.; Huang, Q.; Arnold, E.; Ding, J. Structures of Human Cytosolic NADP-Dependent Isocitrate Dehydrogenase Reveal a Novel Self-Regulatory Mechanism of Activity. J. Biol. Chem. 2004, 279, 33946–33957. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhang, X.; Fan, R.; Gu, H.; Shi, Y.; Liu, H. Glucose-6-Phosphate Dehydrogenase Expression Is Correlated with Poor Clinical Prognosis in Esophageal Squamous Cell Carcinoma. Eur. J. Surg. Oncol. EJSO 2015, 41, 1293–1299. [Google Scholar] [CrossRef]
- Ju, H.-Q.; Lu, Y.-X.; Wu, Q.-N.; Liu, J.; Zeng, Z.-L.; Mo, H.-Y.; Chen, Y.; Tian, T.; Wang, Y.; Kang, T.-B.; et al. Disrupting G6PD-Mediated Redox Homeostasis Enhances Chemosensitivity in Colorectal Cancer. Oncogene 2017, 36, 6282–6292. [Google Scholar] [CrossRef]
- Li, R.; Wang, W.; Yang, Y.; Gu, C. Exploring the Role of Glucose-6-phosphate Dehydrogenase in Cancer (Review). Oncol. Rep. 2020, 44, 2325–2336. [Google Scholar] [CrossRef]
- Mele, L.; Paino, F.; Papaccio, F.; Regad, T.; Boocock, D.; Stiuso, P.; Lombardi, A.; Liccardo, D.; Aquino, G.; Barbieri, A.; et al. A New Inhibitor of Glucose-6-Phosphate Dehydrogenase Blocks Pentose Phosphate Pathway and Suppresses Malignant Proliferation and Metastasis in Vivo. Cell Death Dis. 2018, 9, 572. [Google Scholar] [CrossRef]
- Li, Y.-C.; Wang, R.; A, J.-Y.; Sun, R.-B.; Na, S.-J.; Liu, T.; Ding, X.-S.; Ge, W.-H. Cerebrospinal Fluid Metabolic Profiling Reveals Divergent Modulation of Pentose Phosphate Pathway by Midazolam, Propofol and Dexmedetomidine in Patients with Subarachnoid Hemorrhage: A Cohort Study. BMC Anesthesiol. 2022, 22, 34. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Zhao, J.; Li, L.; Wang, Y.; Zhang, Y.; Chen, Y.; Liu, W.; Gao, L. Midazolam Attenuates Autophagy and Apoptosis Caused by Ketamine by Decreasing Reactive Oxygen Species in the Hippocampus of Fetal Rats. Neuroscience 2018, 388, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, E.; Demirbilek, S.; But, A.K.; Saricicek, V.; Gulec, M.; Akyol, O.; Ersoy, M.O. Antioxidant Properties of Propofol and Erythropoietin after Closed Head Injury in Rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Harman, F.; Hasturk, A.E.; Yaman, M.; Arca, T.; Kilinc, K.; Sargon, M.F.; Kaptanoglu, E. Neuroprotective Effects of Propofol, Thiopental, Etomidate, and Midazolam in Fetal Rat Brain in Ischemia-Reperfusion Model. Childs Nerv. Syst. 2012, 28, 1055–1062. [Google Scholar] [CrossRef]
- Meng, C.; Song, L.; Wang, J.; Li, D.; Liu, Y.; Cui, X. Propofol Induces Proliferation Partially via Downregulation of P53 Protein and Promotes Migration via Activation of the Nrf2 Pathway in Human Breast Cancer Cell Line MDA-MB-231. Oncol. Rep. 2017, 37, 841–848. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, N.; Zhou, S.; Ye, W.; Jing, G.; Zhang, M. Propofol Induces Proliferation and Invasion of Gallbladder Cancer Cells through Activation of Nrf2. J. Exp. Clin. Cancer Res. 2012, 31, 66. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.-Q.; Lin, J.-F.; Tian, T.; Xie, D.; Xu, R.-H. NADPH Homeostasis in Cancer: Functions, Mechanisms and Therapeutic Implications. Signal Transduct. Target. Ther. 2020, 5, 231. [Google Scholar] [CrossRef] [PubMed]
- Goudas, L.C.; Langlade, A.; Serrie, A.; Matson, W.; Milbury, P.; Thurel, C.; Sandouk, P.; Carr, D.B. Acute Decreases in Cerebrospinal Fluid Glutathione Levels after Intracerebroventricular Morphine for Cancer Pain. Anesth. Analg. 1997, 89, 1209–1215. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Mostafa, M.G.; Farghly, H.M.; Hamdy, M.M.; Omran, G.A.; Al-Shaibani, N.K.M. Inhibition of Brain Oxidative Stress and Inducible Nitric Oxide Synthase Expression by Thymoquinone Attenuates the Development of Morphine Tolerance and Dependence in Mice. Eur. J. Pharmacol. 2013, 702, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Osmanlıoğlu, H.Ö.; Yıldırım, M.K.; Akyuva, Y.; Yıldızhan, K.; Nazıroğlu, M. Morphine Induces Apoptosis, Inflammation, and Mitochondrial Oxidative Stress via Activation of TRPM2 Channel and Nitric Oxide Signaling Pathways in the Hippocampus. Mol. Neurobiol. 2020, 57, 3376–3389. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, D.C.; Vázquez, I.E.; Brizuela, N.O.; Alvarez, R.G.; Mejía, G.B.; García, E.H.; Santamaría, D.; de Apreza, M.L.R.; Olguín, H.J. Assessment of Oxidative Damage Induced by Acute Doses of Morphine Sulfate in Postnatal and Adult Rat Brain. Neurochem. Res. 2006, 31, 549–554. [Google Scholar] [CrossRef]
- Koike, H.; Sasaki, H.; Kobori, T.; Zenno, S.; Saigo, K.; Murphy, M.E.; Adman, E.T.; Tanokura, M. 1.8 A Crystal Structure of the Major NAD(P)H:FMN Oxidoreductase of a Bioluminescent Bacterium, Vibrio Fischeri: Overall Structure, Cofactor and Substrate-Analog Binding, and Comparison with Related Flavoproteins. J. Mol. Biol. 1998, 280, 259–273. [Google Scholar] [CrossRef]
- Ju, H.-Q.; Ying, H.; Tian, T.; Ling, J.; Fu, J.; Lu, Y.; Wu, M.; Yang, L.; Achreja, A.; Chen, G.; et al. Mutant Kras-and P16-Regulated NOX4 Activation Overcomes Metabolic Checkpoints in Development of Pancreatic Ductal Adenocarcinoma. Nat. Commun. 2017, 8, 14437. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.; Esposito, E.; Bryant, L.; Cuzzocrea, S.; Salvemini, D. NADPH-Oxidase 2 Activation Promotes Opioid-Induced Antinociceptive Tolerance in Mice. Neuroscience 2013, 241, 1–9. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef]
- Ryungsa, K. Anesthetic Technique and Cancer Recurrence in Oncologic Surgery: Unraveling the Puzzle. Cancer Metastasis Rev. 2017, 36, 159–177. [Google Scholar] [CrossRef]
- Hausser, J.; Szekely, P.; Bar, N.; Zimmer, A.; Sheftel, H.; Caldas, C.; Alon, U. Tumor Diversity and the Trade-off between Universal Cancer Tasks. Nat. Commun. 2019, 10, 5423. [Google Scholar] [CrossRef]
- Kim, J.; DeBerardinis, R.J. Mechanisms and Implications of Metabolic Heterogeneity in Cancer. Cell Metab. 2019, 30, 434–446. [Google Scholar] [CrossRef]
- Mroz, E.A.; Rocco, J.W. The Challenges of Tumor Genetic Diversity. Cancer 2017, 123, 917–927. [Google Scholar] [CrossRef]
- Prokai, L.; Zharikova, A.D.; Stevens, S.M. Effect of Chronic Morphine Exposure on the Synaptic Plasma-Membrane Subproteome of Rats: A Quantitative Protein Profiling Study Based on Isotope-Coded Affinity Tags and Liquid Chromatography/Mass Spectrometry. J. Mass Spectrom. 2005, 40, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.A.; Letai, A.; Fisher, D.E.; Flaherty, K.T. Precision Medicine for Cancer with Next-Generation Functional Diagnostics. Nat. Rev. Cancer 2015, 15, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Kosorok, M.R.; Laber, E.B. Precision Medicine. Annu. Rev. Stat. Its Appl. 2019, 6, 263–286. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).