Imidazoles as Serotonin Receptor Modulators for Treatment of Depression: Structural Insights and Structure–Activity Relationship Studies
Abstract
1. Introduction
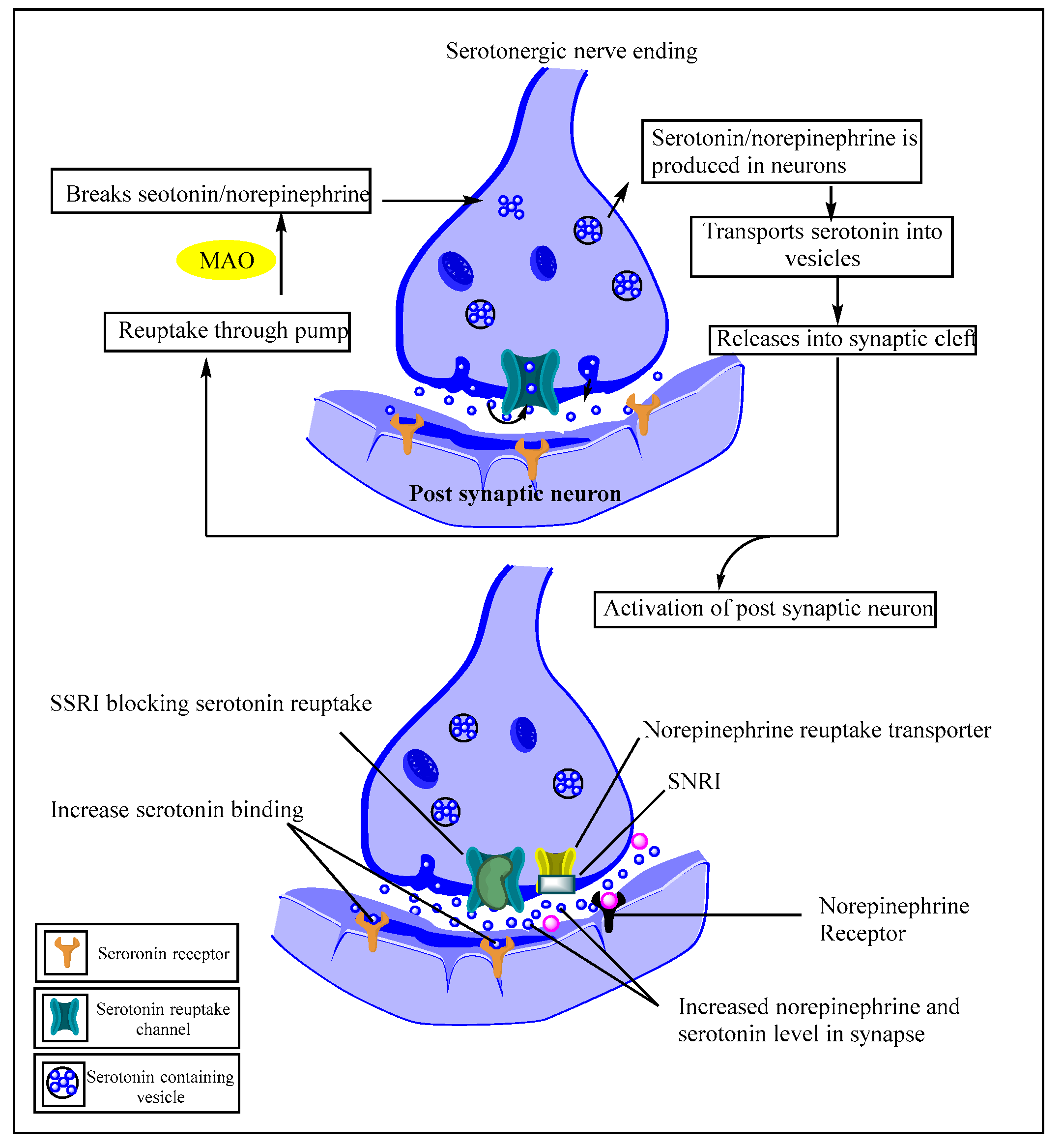
Etiology of Depression, Structural and Mechanistic Insights
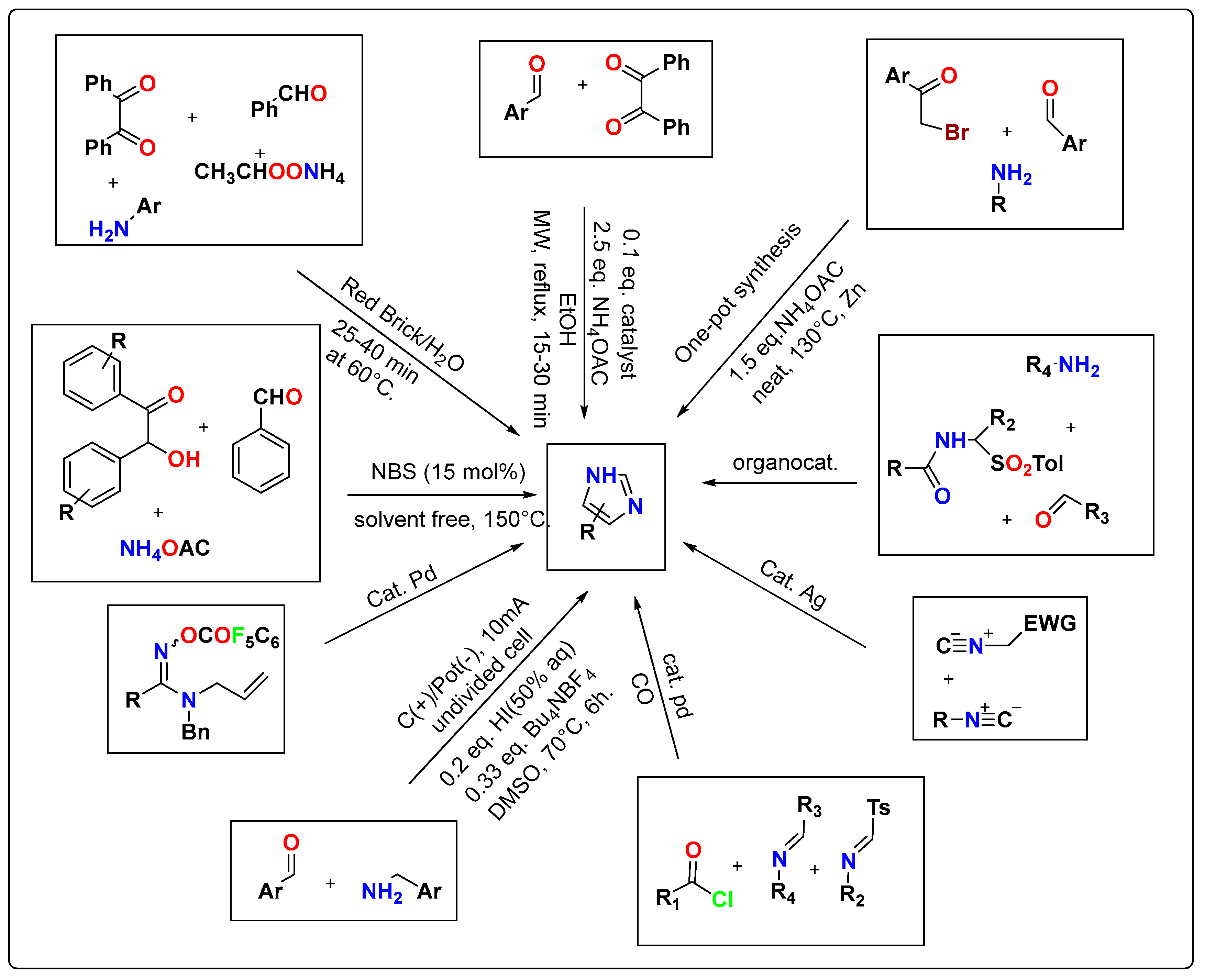
2. Modern Synthetic Methods for Substituted Imidazole Derivatives
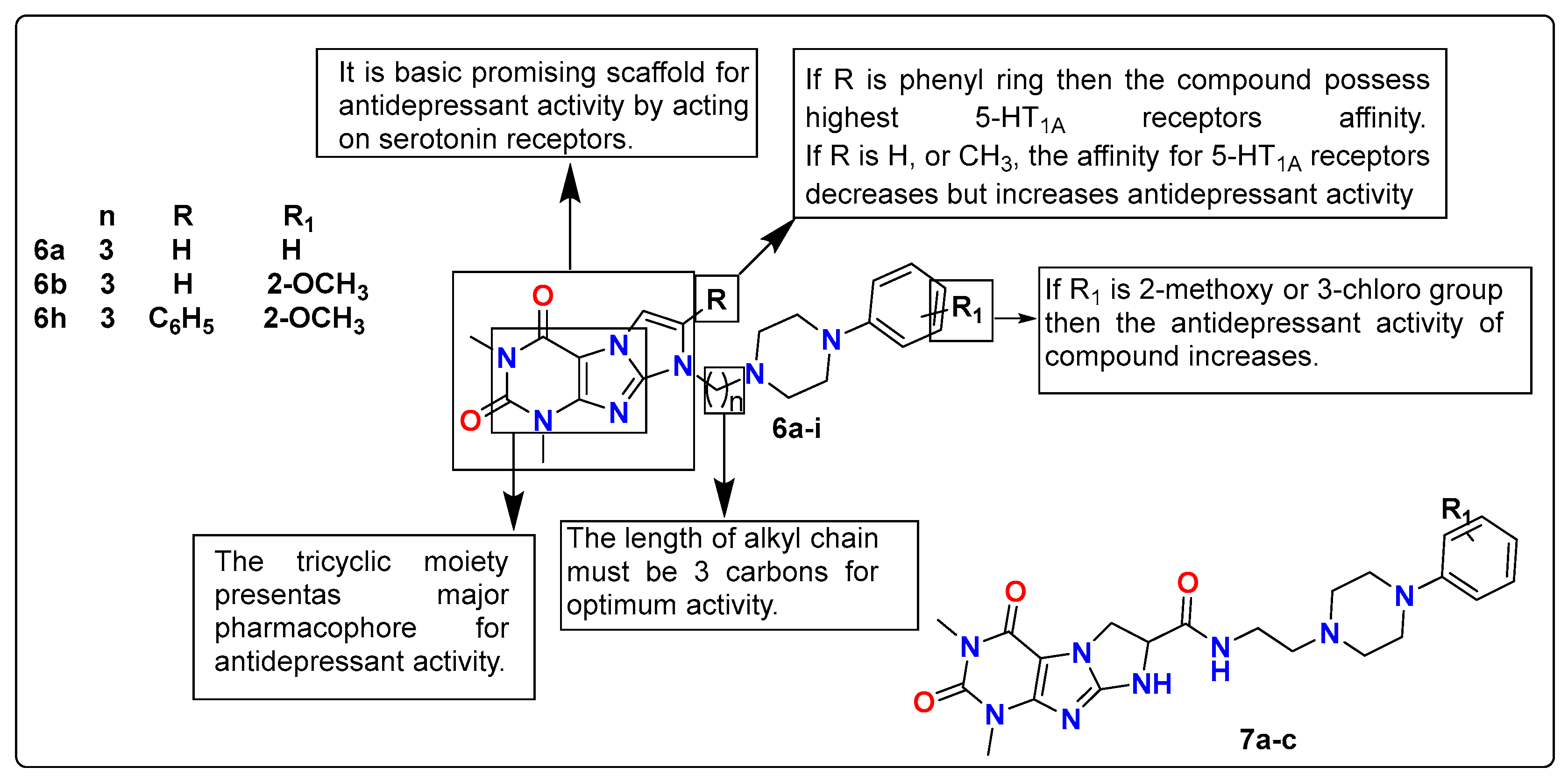
3. SAR of Various Imidazole Derivatives
4. Other Imidazole-Based Serotonin-Modulating Agents
5. Imidazole-Based Drugs under Clinical Trials against Depression
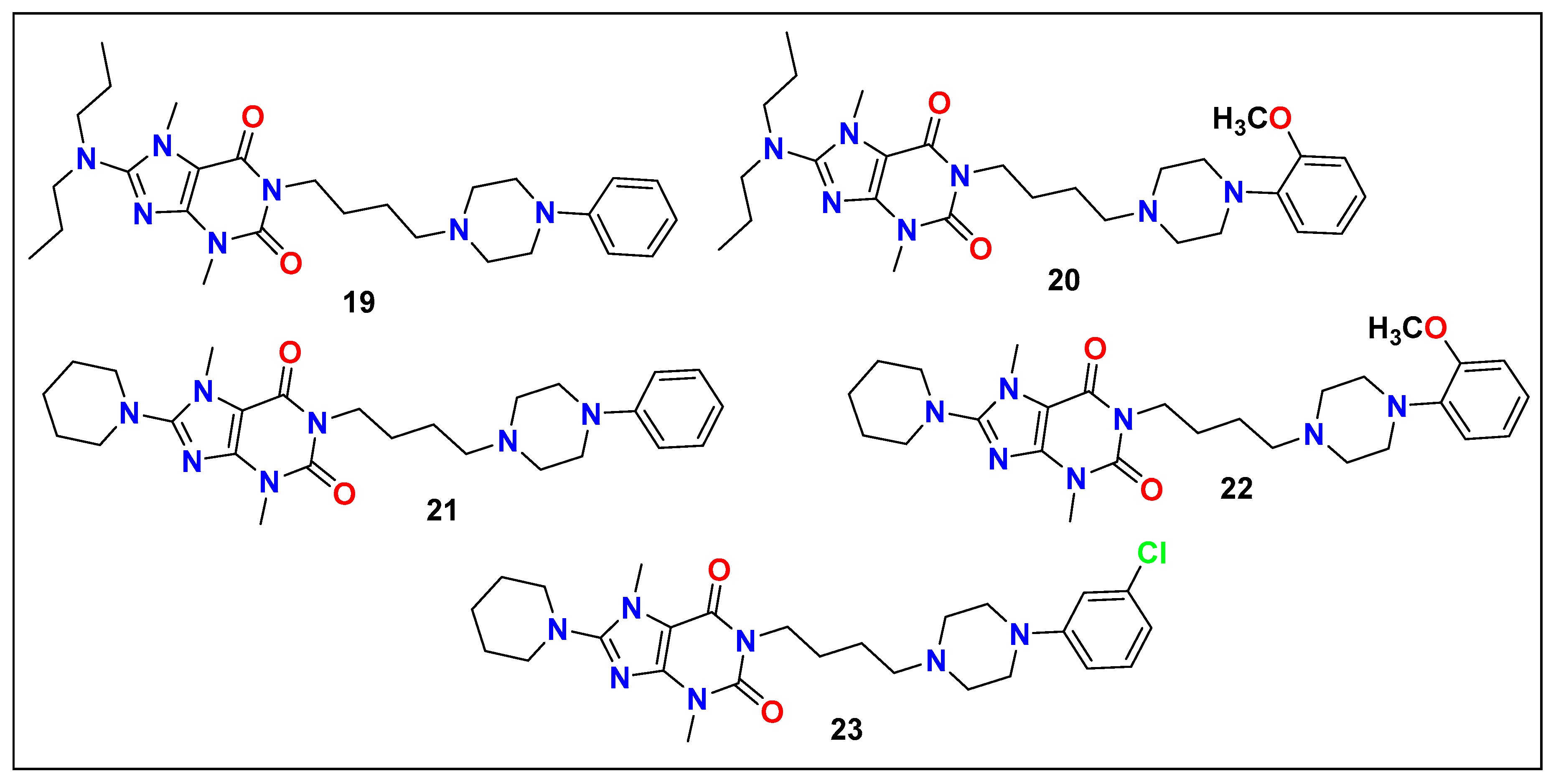
6. Recently Granted Patents on Imidazole-Based Compounds
7. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| α1R | Alpha-1 Receptors |
| σ2R | Sigma-2 Receptors |
| ADMET | Absorption, Distribution, Metabolism, Excretion, Toxicity |
| BBB | Blood Brain Barrier |
| BBP | Blood Brain Permeability |
| CDK | Cyclin-dependent Kinase |
| CNS | Central Nervous System |
| D receptor | Dopamine Receptor |
| EAAT3 | Excitatory amino acid transporter 3 |
| FST | Force swim test |
| hERG | human Ether-à-go-go-Related Gene |
| HLM | Human Liver Microsomes |
| hSerT | Human Serotonin Transporter |
| IC50 | Half Maximal Inhibitory Concentration |
| I.P. | Indian Pharmacopoeia |
| IL-17 | Interleukin-17 |
| Ki | Inhibition constant |
| MAO | Mono Amine Oxidase |
| MDCK | Madin-Darby Canine Kidney Cells |
| MDR | Multidrug Resistance Gene |
| MFST | Modified Force Swim Test |
| MEKC | Micellar Electrokinetic Chromatography |
| PD | Parkinson’s disease |
| Pgp | P-glycoprotein |
| PK | Pharmacokinetic |
| pKa | Acid Dissociation Constant |
| PNS | Peripheral Nervous System |
| PTZ | Pentylenetetrazole |
| SAR | Structure–Activity Relationship |
| SERT | Serotonin reuptake transporter |
| SSRI | Selective Serotonin Reuptake Inhibitors |
| TGF | Transforming growth factor |
| TNF | Tumor necrosis factor |
| TST | Tail Suspension Test |
References
- Whitaker-Azmitia, P.M. The Discovery of Serotonin and its Role in Neuroscience. Neuropsychopharmacology 1999, 21, 2–8. [Google Scholar] [CrossRef]
- Sjoerdsma, A.; Palfreyman, M.G. History of serotonin and serotonin disorders. Ann. New York Acad. Sci. 1990, 600, 1–7. [Google Scholar] [CrossRef]
- Andrews, A.M. Celebrating serotonin. ACS Chem. Neurosci. 2012, 3, 644–645. [Google Scholar] [CrossRef][Green Version]
- Maclean, J.A.; Schoenwaelder, S.M. Serotonin in platelets. In Serotonin; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–119. [Google Scholar]
- Jonnakuty, C.; Gragnoli, C. What do we know about serotonin? J. Cell. Physiol. 2008, 217, 301–306. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Kroeze, W.K.; Kristiansen, K.; Roth, B.L. Molecular biology of serotonin receptors-structure and function at the molecular level. Curr. Top. Med. Chem. 2002, 2, 507–528. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G. Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022. [Google Scholar] [CrossRef]
- Kendrick, T.; Collinson, S. Antidepressants and the serotonin hypothesis of depression. BMJ 2022, 378, o1993. [Google Scholar] [CrossRef]
- Bhutani, P.; Joshi, G.; Raja, N.; Bachhav, N.; Rajanna, P.K.; Bhutani, H.; Paul, A.T.; Kumar, R. U.S. FDA Approved Drugs from 2015–June 2020: A Perspective. J. Med. Chem. 2021, 64, 2339–2381. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, L.; Nandakumar, K.S.; Cheng, K. Imidazole Scaffold Based Compounds in the Development of Therapeutic Drugs. Curr. Top. Med. Chem. 2021, 21, 2514–2528. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.M.; Damu, G.L.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Hossain, M.; Nanda, A.K. A review on heterocyclic: Synthesis and their application in medicinal chemistry of imidazole moiety. Science 2018, 6, 83–94. [Google Scholar] [CrossRef]
- Siwach, A.; Verma, P.K. Synthesis and therapeutic potential of imidazole containing compounds. BMC Chem. 2021, 15, 1–69. [Google Scholar] [CrossRef]
- Demchenko, S.; Lesyk, R.; Yadlovskyi, O.; Zuegg, J.; Elliott, A.G.; Drapak, I.; Fedchenkova, Y.; Suvorova, Z.; Demchenko, A. Synthesis, antibacterial and antifungal activity of new 3-aryl-5H-pyrrolo [1, 2-a] imidazole and 5H-imidazo [1, 2-a] azepine quaternary salts. Molecules 2021, 26, 4253. [Google Scholar] [CrossRef]
- Chahal, S.; Rani, P.; Kiran; Sindhu, J.; Joshi, G.; Ganesan, A.; Kalyaanamoorthy, S.; Mayank; Kumar, P.; Singh, R. Design and Development of COX-II Inhibitors: Current Scenario and Future Perspective. ACS Omega 2023, 8, 17446–17498. [Google Scholar] [CrossRef]
- Ali, I.; Lone, M.N.; Aboul-Enein, H.Y. Imidazoles as potential anticancer agents. MedChemComm 2017, 8, 1742–1773. [Google Scholar] [CrossRef]
- Dhameliya, T.M.; Patel, K.I.; Tiwari, R.; Vagolu, S.K.; Panda, D.; Sriram, D.; Chakraborti, A.K. Design, synthesis, and biological evaluation of benzo [d] imidazole-2-carboxamides as new anti-TB agents. Bioorganic Chem. 2021, 107, 104538. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdel-Maksoud, M.S.; Hassan, R.M.; Mersal, K.I.; Ammar, U.M.; Se-In, C.; He-Soo, H.; Kim, H.-K.; Lee, A.; Lee, K.-T. Design, synthesis and anti-inflammatory activity of imidazol-5-yl pyridine derivatives as p38α/MAPK14 inhibitor. Bioorganic Med. Chem. 2021, 31, 115969. [Google Scholar] [CrossRef]
- Eliewi, A.; Al-Garawi, Z.; Al-Kazzaz, F.; Atia, A. Multi target-directed imidazole derivatives for neurodegenerative diseases. J. Phys. Conf. Ser. 2021, 1853, 012066. [Google Scholar] [CrossRef]
- Dhingra, A.K.; Chopra, B.; Jain, A.; Chaudhary, J. Imidazole: Multi-targeted therapeutic leads for the management of Alzheimer’s disease. Mini Rev. Med. Chem. 2022, 22, 1352–1373. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Gupta, S.M.; Dwivedi, S.; Kumar, D.; Shaikh, M.F.; Negi, A. Preclinical Models for Alzheimer’s Disease: Past, Present, and Future Approaches. ACS Omega 2022, 7, 47504–47517. [Google Scholar] [CrossRef]
- Georgiou, N.; Gkalpinos, V.K.; Katsakos, S.D.; Vassiliou, S.; Tzakos, A.G.; Mavromoustakos, T. Rational Design and Synthesis of AT1R Antagonists. Molecules 2021, 26, 2927. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.Y.; El-Sebaey, S.A.; El Deeb, M.A.; Elzoghbi, M.S. Potential antiviral and anticancer effect of imidazoles and bridgehead imidazoles generated by HPV-Induced cervical carcinomas via reactivating the P53/pRb pathway and inhibition of CA IX. J. Mol. Struct. 2021, 1230, 129865. [Google Scholar] [CrossRef]
- Modh, P.G.; Patel, L.J. Synthesis, Drug Likeness and In-vitro Screening of Some Novel Quinazolinone Derivatives for Anti-Obesity Activity. J. Pharm. Res. Int. 2021, 33, 81–92. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Eseola, A.O.; Plass, W.; Kato, K.; Otuechere, C.A.; Awakan, O.J.; Atolani, O.; Otohinoyi, D.A.; Elebiyo, T.C.; Evbuomwan, I.O. The anti-parasite action of imidazole derivatives likely involves oxidative stress but not HIF-1α signaling. Chem.-Biol. Interact. 2021, 349, 109676. [Google Scholar] [CrossRef]
- Negi, A.; Alex, J.M.; Amrutkar, S.M.; Baviskar, A.T.; Joshi, G.; Singh, S.; Banerjee, U.C.; Kumar, R. Imine/amide–imidazole conjugates derived from 5-amino-4-cyano-N1-substituted benzyl imidazole: Microwave-assisted synthesis and anticancer activity via selective topoisomerase-II-α inhibition. Bioorganic Med. Chem. 2015, 23, 5654–5661. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.A.; Stewart, M.L.; Gavathiotis, E.; Tepper, J.L.; Bruekner, S.R.; Koss, B.; Opferman, J.T.; Walensky, L.D. A competitive stapled peptide screen identifies a selective small molecule that overcomes MCL-1-dependent leukemia cell survival. Chem. Biol. 2012, 19, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Abou Samra, A.; Robert, A.; Gov, C.; Favre, L.; Eloy, L.; Jacquet, E.; Bignon, J.; Wiels, J.; Desrat, S.; Roussi, F. Dual inhibitors of the pro-survival proteins Bcl-2 and Mcl-1 derived from natural compound meiogynin A. Eur. J. Med. Chem. 2018, 148, 26–38. [Google Scholar] [CrossRef]
- Litaudon, M.; Bousserouel, H.; Awang, K.; Nosjean, O.; Martin, M.-T.; Dau, M.E.T.H.; Hadi, H.A.; Boutin, J.A.; Sevenet, T.; Gueritte, F. A dimeric sesquiterpenoid from a Malaysian Meiogyne as a new inhibitor of Bcl-xL/BakBH3 domain peptide interaction. J. Nat. Prod. 2009, 72, 480–483. [Google Scholar] [CrossRef]
- Negi, A.; Murphy, P.V. Natural products as Mcl-1 inhibitors: A comparative study of experimental and computational modelling data. Chemistry 2022, 4, 983–1009. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Wang, L.; Han, L.; Hou, X.; Fu, H.; Fang, H. Design, synthesis and preliminary bioactivity studies of imidazolidine-2, 4-dione derivatives as Bcl-2 inhibitors. Bioorganic Med. Chem. 2015, 23, 7359–7365. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Murphy, P.V. Development of Mcl-1 inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 210, 113038. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Ramarao, P.; Kumar, R. Recent advancements in small molecule inhibitors of insulin–like growth factor-1 receptor (IGF-1R) tyrosine kinase as anticancer agents. Mini Rev. Med. Chem. 2013, 13, 653–681. [Google Scholar] [CrossRef] [PubMed]
- Peled, N.; Wynes, M.W.; Ikeda, N.; Ohira, T.; Yoshida, K.; Qian, J.; Ilouze, M.; Brenner, R.; Kato, Y.; Mascaux, C. Insulin-like growth factor-1 receptor (IGF-1R) as a biomarker for resistance to the tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. Cell. Oncol. 2013, 36, 277–288. [Google Scholar] [CrossRef]
- Osmaniye, D.; Levent, S.; Sağlık, B.N.; Karaduman, A.B.; Özkay, Y.; Kaplancıklı, Z.A. Novel imidazole derivatives as potential aromatase and monoamine oxidase-B inhibitors against breast cancer. New J. Chem. 2022, 46, 7442–7451. [Google Scholar] [CrossRef]
- Çetiner, G.; Çevik, U.A.; Celik, I.; Bostancı, H.E.; Özkay, Y.; Kaplancıklı, Z.A. New imidazole derivatives as aromatase inhibitor: Design, synthesis, biological activity, molecular docking, and computational ADME-Tox studies. J. Mol. Struct. 2023, 1278, 134920. [Google Scholar] [CrossRef]
- Makar, S.; Saha, T.; Swetha, R.; Gutti, G.; Kumar, A.; Singh, S.K. Rational approaches of drug design for the development of selective estrogen receptor modulators (SERMs), implicated in breast cancer. Bioorganic Chem. 2020, 94, 103380. [Google Scholar] [CrossRef]
- Xie, B.; Xu, B.; Xin, L.; Wei, Y.; Guo, X.; Dong, C. Discovery of estrogen receptor α targeting caged hypoxia-responsive PROTACs with an inherent bicyclic skeleton for breast cancer treatment. Bioorganic Chem. 2023, 137, 106590. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K.; Voisin-Chiret, A.S. Estrogen Receptor-α Targeting: PROTACs, SNIPERs, Peptide-PROTACs, Antibody Conjugated PROTACs and SNIPERs. Pharmaceutics 2022, 14, 2523. [Google Scholar] [CrossRef]
- Li, S.-R.; Tan, Y.-M.; Zhang, L.; Zhou, C.-H. Comprehensive insights into medicinal research on imidazole-based supramolecular complexes. Pharmaceutics 2023, 15, 1348. [Google Scholar] [CrossRef]
- Ralhan, R.; Kaur, J. Alkylating agents and cancer therapy. Expert Opin. Ther. Pat. 2007, 17, 1061–1075. [Google Scholar] [CrossRef]
- Frezza, M.; Hindo, S.; Chen, D.; Davenport, A.; Schmitt, S.; Tomco, D.; Ping Dou, Q. Novel metals and metal complexes as platforms for cancer therapy. Curr. Pharm. Des. 2010, 16, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Zhang, Y.-Y.; Yan, C.-Y.; Wan, K.; Gan, L.-L.; Shi, Y. Recent researches in metal supramolecular complexes as anticancer agents. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2010, 10, 371–395. [Google Scholar] [CrossRef]
- Liu, P.; Jia, J.; Zhao, Y.; Wang, K.-Z. Recent advances on dark and light-activated cytotoxity of imidazole-containing ruthenium complexes. Mini Rev. Med. Chem. 2016, 16, 272–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, W.; Du, J.; Shen, P.; Yang, C. A Boron 2-(2′-pyridyl) Imidazole Fluorescence Probe for Bovine Serum Albumin: Discrimination over Other Proteins and Identification of Its Denaturation. Photochem. Photobiol. 2017, 93, 1414–1422. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Zhang, S.-Q.; Cui, M.-C.; Gao, L.-H.; Zhao, H.; Wang, K.-Z. pH-sensitive near-IR emitting dinuclear ruthenium complex for recognition, two-photon luminescent imaging, and subcellular localization of cancer cells. ACS Appl. Bio Mater. 2020, 3, 5420–5427. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, C.; Wang, Y.; Chen, Y.; Ding, X.; Yu, B. Luminescent detection of the lipopolysaccharide endotoxin and rapid discrimination of bacterial pathogens using cationic platinum (ii) complexes. RSC Adv. 2017, 7, 32632–32636. [Google Scholar] [CrossRef]
- Okda, H.E.; El Sayed, S.; Ferreira, R.C.; Costa, S.P.; Raposo, M.M.; Martinez-Manez, R.; Sancenon, F. 4-(4, 5-Diphenyl-1H-imidazole-2-yl)-N, N-dimethylaniline-Cu (II) complex, a highly selective probe for glutathione sensing in water-acetonitrile mixtures. Dye. Pigment. 2018, 159, 45–48. [Google Scholar] [CrossRef]
- Zhao, C.; Kong, X.; Shuang, S.; Wang, Y.; Dong, C. An anthraquinone-imidazole-based colorimetric and fluorescent sensor for the sequential detection of Ag+ and biothiols in living cells. Analyst 2020, 145, 3029–3037. [Google Scholar] [CrossRef]
- Tian, F.; Jiang, X.; Dou, X.; Wu, Q.; Wang, J.; Song, Y. Design and synthesis of novel adenine fluorescence probe based on Eu (III) complexes with dtpa-bis (guanine) ligand. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 194–200. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Sun, Q.; Shi, J.-W.; Xia, H.-Y.; Wang, J.-L.; Chen, H.-Y.; He, H.-F.; Shen, L.; Zhao, F.; Zhong, J. A novel triple substituted imidazole fluorescent sensor for Ag+ and its imaging in living cell and zebrafish. J. Photochem. Photobiol. A Chem. 2020, 389, 112244. [Google Scholar] [CrossRef]
- Suresh, S.; Bhuvanesh, N.; Raman, A.; Sugumar, P.; Padmanabhan, D.; Easwaramoorthi, S.; Ponnuswamy, M.N.; Kavitha, S.; Nandhakumar, R. Experimental and theoretical studies of imidazole based chemosensor for Palladium and their biological applications. J. Photochem. Photobiol. A Chem. 2019, 385, 112092. [Google Scholar] [CrossRef]
- Mehta, P.K.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Ratiometric detection of Cu+ in aqueous buffered solutions and in live cells using fluorescent peptidyl probe to mimic the binding site of the metalloprotein for Cu+. Sens. Actuators B Chem. 2018, 256, 393–401. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mahata, S.; Bandyopadhyay, A.; Mandal, B.B.; Manivannan, V. Application of 2, 4, 5-tris (2-pyridyl) imidazole as ‘turn-off’fluorescence sensor for Cu (II) and Hg (II) ions and in vitro cell imaging. Luminescence 2022, 37, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Cheng, F.; Liu, Z.; He, C.; Yang, Y.; Wang, K. Preparation, characterization, pH titration, and electrochemical properties of an anthracene-bridged binuclear ruthenium complex containing imidazole. J. Coord. Chem. 2019, 72, 2957–2967. [Google Scholar] [CrossRef]
- Niu, L.; Liu, J.; Gao, S.; Gao, J.; Zhou, Y.; Liu, S.; Ma, C.; Zhao, Y. Fluoride ions detection in aqueous media by unprecedented ring opening of fluorescein dye: A novel multimodal sensor for fluoride ions and its utilization in live cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122001. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, Y.; Shao, B.; Cheng, J.; Li, X. Recent advances of small-molecule fluorescent probes for detecting biological hydrogen sulfide. Front. Chem. Sci. Eng. 2022, 16, 34–63. [Google Scholar] [CrossRef]
- Strianese, M.; Brenna, S.; Ardizzoia, G.A.; Guarnieri, D.; Lamberti, M.; D’Auria, I.; Pellecchia, C. Imidazo-pyridine-based zinc (ii) complexes as fluorescent hydrogen sulfide probes. Dalton Trans. 2021, 50, 17075–17085. [Google Scholar] [CrossRef] [PubMed]
- Rabha, M.; Sen, B.; Sheet, S.K.; Aguan, K.; Khatua, S. Cyclometalated iridium (iii) complex of a 1, 2, 3-triazole-based ligand for highly selective sensing of pyrophosphate ion. Dalton Trans. 2022, 51, 11372–11380. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Liu, Q.; He, Y.; Zhao, K.; Wei, C.; Wojtas, L.; Shi, X.; Song, Z. Triazole-imidazole (TA-IM) derivatives as ultrafast fluorescent probes for selective Ag+ detection. Org. Biomol. Chem. 2018, 16, 7801–7805. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gao, G.; Cheng, J.; Chen, X.; Zhao, Y.; Ye, Y. Two new reversible naphthalimide-based fluorescent chemosensors for Hg2+. Luminescence 2016, 31, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, A.K.; Angamaly, S.A.; Pradeep, S.D.; Madhusoodhanan, D.T.; Manoharan, D.K.; Mohanan, P.V. A Novel Imidazole Bound Schiff Base as Highly Selective “Turn-on” Fluorescence Sensor for Zn2+ and Colorimetric Kit for Co2+. J. Fluoresc. 2022, 32, 189–202. [Google Scholar] [CrossRef]
- Pandith, A.; Uddin, N.; Choi, C.H.; Kim, H.-S. Highly selective imidazole-appended 9, 10-N, N′-diaminomethylanthracene fluorescent probe for switch-on Zn2+ detection and switch-off H2PO4− and CN− detection in 80% aqueous DMSO, and applications to sequential logic gate operations. Sens. Actuators B Chem. 2017, 247, 840–849. [Google Scholar] [CrossRef]
- Kırpık, H.; Kose, M.; Ballı, J.N. Tridentate benzimidazole ligand and its metal complexes: Synthesis, characterization, photo physical and sensor properties. Appl. Organomet. Chem. 2020, 34, e5992. [Google Scholar] [CrossRef]
- Mahnashi, M.H.; Mahmoud, A.M.; Alkahtani, S.A.; Ali, R.; El-Wekil, M.M. A novel imidazole derived colorimetric and fluorometric chemosensor for bifunctional detection of copper (II) and sulphide ions in environmental water samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117846. [Google Scholar] [CrossRef]
- Pan, J.; Yu, J.; Qiu, S.; Zhu, A.; Liu, Y.; Ban, X.; Li, W.; Yu, H.; Li, L. A novel dibenzimidazole-based fluorescent probe with high sensitivity and selectivity for copper ions. J. Photochem. Photobiol. A Chem. 2021, 406, 113018. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, S.; He, X.; Xu, X.; Zhang, G.; Yang, L.; Kong, L.; Yang, J. A novel tetraphenylethylene-functionalized arylimidazole AIEgen for detections of picric acid and Cu2+. Chem. Pap. 2021, 75, 6297–6306. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, X.; Mi, Z.; Zheng, R.; Fan, J.; Gu, Q.; Zhang, Y. A New Tetrasubstituted Imidazole Based Difunctional Probe for UV-spectrophotometric and Fluorometric Detecting of Fe 3+ Ion in Aqueous Solution. Chem. Res. Chin. Univ. 2019, 35, 200–208. [Google Scholar] [CrossRef]
- Daly, M.; Sutin, A.R.; Robinson, E. Depression reported by US adults in 2017–2018 and March and April 2020. J. Affect. Disord. 2021, 278, 131–135. [Google Scholar] [CrossRef]
- Sahu, B.; Bhatia, R.; Kaur, D.; Choudhary, D.; Rawat, R.; Sharma, S.; Kumar, B. Design, synthesis and biological evaluation of oxadiazole clubbed piperazine derivatives as potential antidepressant agents. Bioorganic Chem. 2023, 136, 106544. [Google Scholar] [CrossRef]
- Kumar, B.; Prakash Gupta, V.; Kumar, V. A perspective on monoamine oxidase enzyme as drug target: Challenges and opportunities. Curr. Drug Targets 2017, 18, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Mantha, A.K.; Kumar, V. Recent developments on the structure–activity relationship studies of MAO inhibitors and their role in different neurological disorders. RSC Adv. 2016, 6, 42660–42683. [Google Scholar] [CrossRef]
- Priyadarshini, R.; Raj, G.M. Histamine, Serotonin, Bradykinin, and the Ergot Alkaloids. In Introduction to Basics of Pharmacology and Toxicology: Volume 2: Essentials of Systemic Pharmacology: From Principles to Practice; Springer: Berlin/Heidelberg, Germany, 2021; Volume 2, p. 283. [Google Scholar]
- Paul, A.; Anandabaskar, N.; Mathaiyan, J.; Raj, G.M. Introduction to Basics of Pharmacology and Toxicology: Volume 2: Essentials of Systemic Pharmacology: From Principles to Practice; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Charvériat, M.; Guiard, B.P. Serotonergic neurons in the treatment of mood disorders: The dialogue with astrocytes. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 259, pp. 197–228. [Google Scholar]
- Park, J.; Jeong, W.; Yun, C.; Kim, H.; Oh, C.-M. Serotonergic Regulation of Hepatic Energy Metabolism. Endocrinol. Metab. 2021, 36, 1151. [Google Scholar] [CrossRef]
- Shah, P.A.; Park, C.J.; Shaughnessy, M.P.; Cowles, R.A. Serotonin as a mitogen in the gastrointestinal tract: Revisiting a familiar molecule in a new role. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1093–1104. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Serotonin—Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef]
- Zweckstetter, M.; Dityatev, A.; Ponimaskin, E. Structure of serotonin receptors: Molecular underpinning of receptor activation and modulation. Signal Transduct. Target. Ther. 2021, 6, 243. [Google Scholar] [CrossRef]
- Xu, P.; Huang, S.; Zhang, H.; Mao, C.; Zhou, X.E.; Cheng, X.; Simon, I.A.; Shen, D.-D.; Yen, H.-Y.; Robinson, C.V.; et al. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 2021, 592, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Healy, D. Serotonin and depression. BMJ 2015, 350, h1771. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Capitão, L.P.; Giles, S.L.; Cowen, P.J.; Stringaris, A.; Harmer, C.J. The knowns and unknowns of SSRI treatment in young people with depression and anxiety: Efficacy, predictors, and mechanisms of action. Lancet Psychiatry 2021, 8, 824–835. [Google Scholar] [CrossRef]
- Zoega, H.; Kieler, H.; Nørgaard, M.; Furu, K.; Valdimarsdottir, U.; Brandt, L.; Haglund, B. Use of SSRI and SNRI antidepressants during pregnancy: A population-based study from Denmark, Iceland, Norway and Sweden. PLoS ONE 2015, 10, e0144474. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.S.; Lee-Zimmerman, C.; Cartwright, S.; Ann Morrissette, D. Serotonergic drugs for depression and beyond. Curr. Drug Targets 2013, 14, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Xue, W.; Wang, P.; Yang, F.; Li, B.; Li, X.; Li, Y.; Yao, X.; Zhu, F. Exploring the inhibitory mechanism of approved selective norepinephrine reuptake inhibitors and reboxetine enantiomers by molecular dynamics study. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, D.M. Communication networks in the brain: Neurons, receptors, neurotransmitters, and alcohol. Alcohol Res. Health 2008, 31, 196–214. [Google Scholar]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- He, J.H.; Liu, R.P.; Peng, Y.M.; Guo, Q.; Zhu, L.B.; Lian, Y.Z.; Hu, B.L.; Fan, H.H.; Zhang, X.; Zhu, J.H. Differential and paradoxical roles of new-generation antidepressants in primary astrocytic inflammation. J. Neuroinflamm. 2021, 18, 47. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Singh, G.; Monga, V.; Kumar, B. Recent advancements in the development of heterocyclic anti-inflammatory agents. Eur. J. Med. Chem. 2020, 200, 112438. [Google Scholar] [CrossRef]
- Singh, K.; Bhatia, R.; Kumar, B.; Singh, G.; Monga, V. Design strategies, chemistry and therapeutic insights of multi-target directed ligands as antidepressant agents. Curr. Neuropharmacol. 2022, 20, 1329–1358. [Google Scholar] [CrossRef]
- Kerru, N.; Bhaskaruni, S.V.; Gummidi, L.; Maddila, S.N.; Maddila, S.; Jonnalagadda, S.B. Recent advances in heterogeneous catalysts for the synthesis of imidazole derivatives. Synth. Commun. 2019, 49, 2437–2459. [Google Scholar] [CrossRef]
- Naowarojna, N.; Cheng, R.; Lopez, J.; Wong, C.; Qiao, L.; Liu, P. Chemical modifications of proteins and their applications in metalloenzyme studies. Synth. Syst. Biotechnol. 2021, 6, 32–49. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, B.; Dwivedi, A.R.; Kumar, V. Regioselective alkylation of 1, 2, 4-triazole using ionic liquids under microwave conditions. Green Process. Synth. 2016, 5, 233–237. [Google Scholar] [CrossRef]
- Thenrajan, T.; Sankar, S.S.; Kundu, S.; Wilson, J. Bimetallic nickel iron zeolitic imidazolate fibers as biosensing platform for neurotransmitter serotonin. Colloid Polym. Sci. 2022, 300, 223–232. [Google Scholar] [CrossRef]
- Kamijo, S.; Yamamoto, Y. Recent progress in the catalytic synthesis of imidazoles. Chem. Asian J. 2007, 2, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, A.A.; Abu-Dief, A.M.; Abdelhamid, A.A. Hydrothermal preparation and characterization of ZnFe2O4 magnetic nanoparticles as an efficient heterogeneous catalyst for the synthesis of multi-substituted imidazoles and study of their anti-inflammatory activity. Appl. Organomet. Chem. 2018, 32, e3794. [Google Scholar] [CrossRef]
- Nejatianfar, M.; Akhlaghinia, B.; Jahanshahi, R. Cu (II) immobilized on guanidinated epibromohydrin-functionalized γ-Fe2O3@ TiO2 (γ-Fe2O3@ TiO2-EG-Cu (II)): A highly efficient magnetically separable heterogeneous nanocatalyst for one-pot synthesis of highly substituted imidazoles. Appl. Organomet. Chem. 2018, 32, e4095. [Google Scholar] [CrossRef]
- Eidi, E.; Kassaee, M.Z.; Nasresfahani, Z. Synthesis of 2, 4, 5-trisubstituted imidazoles over reusable CoFe2O4 nanoparticles: An efficient and green sonochemical process. Appl. Organomet. Chem. 2016, 30, 561–565. [Google Scholar] [CrossRef]
- Maleki, A.; Alrezvani, Z.; Maleki, S. Design, preparation and characterization of urea-functionalized Fe3O4/SiO2 magnetic nanocatalyst and application for the one-pot multicomponent synthesis of substituted imidazole derivatives. Catal. Commun. 2015, 69, 29–33. [Google Scholar] [CrossRef]
- Kazemi, M. Reusable nanomagnetic catalysts in synthesis of imidazole scaffolds. Synth. Commun. 2020, 50, 2095–2113. [Google Scholar] [CrossRef]
- Negi, A.; Mirallai, S.I.; Konda, S.; Murphy, P.V. An improved method for synthesis of non-symmetric triarylpyridines. Tetrahedron 2022, 121, 132930. [Google Scholar] [CrossRef]
- Bertrand, J.; Dostálová, H.; Kryštof, V.; Jorda, R.; Delgado, T.; Castro-Alvarez, A.; Mella, J.; Cabezas, D.; Faúndez, M.; Espinosa-Bustos, C. Design, Synthesis, In Silico Studies and Inhibitory Activity towards Bcr-Abl, BTK and FLT3-ITD of New 2, 6, 9-Trisubstituted Purine Derivatives as Potential Agents for the Treatment of Leukaemia. Pharmaceutics 2022, 14, 1294. [Google Scholar] [CrossRef]
- Georgiou, M.; Lougiakis, N.; Tenta, R.; Gioti, K.; Baritaki, S.; Gkaralea, L.-E.; Deligianni, E.; Marakos, P.; Pouli, N.; Stellas, D. Discovery of New 1, 4, 6-Trisubstituted-1 H-pyrazolo [3, 4-b] pyridines with Anti-Tumor Efficacy in Mouse Model of Breast Cancer. Pharmaceutics 2023, 15, 787. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Naeem, A.; Xiao, S.; Hu, L.; Zhang, J.; Zheng, Q. Safety challenges and application strategies for the use of dendrimers in medicine. Pharmaceutics 2022, 14, 1292. [Google Scholar] [CrossRef] [PubMed]
- Skwarecki, A.S.; Nowak, M.G.; Milewska, M.J. Synthetic strategies in construction of organic low molecular-weight carrier-drug conjugates. Bioorganic Chem. 2020, 104, 104311. [Google Scholar] [CrossRef] [PubMed]
- Girish, Y.R.; Kumar, K.S.S.; Thimmaiah, K.N.; Rangappa, K.S.; Shashikanth, S. ZrO2-β-cyclodextrin catalyzed synthesis of 2, 4, 5-trisubstituted imidazoles and 1, 2-disubstituted benzimidazoles under solvent free conditions and evaluation of their antibacterial study. RSC Adv. 2015, 5, 75533–75546. [Google Scholar] [CrossRef]
- Bajpai, S.; Singh, S.; Srivastava, V. Nano zirconia catalysed one-pot synthesis of some novel substituted imidazoles under solvent-free conditions. RSC Adv. 2015, 5, 28163–28170. [Google Scholar] [CrossRef]
- Fang, S.; Yu, H.; Yang, X.; Li, J.; Shao, L. Nickel-Catalyzed Construction of 2, 4-Disubstituted Imidazoles via C–C Coupling and C− N Condensation Cascade Reactions. Adv. Synth. Catal. 2019, 361, 3312–3317. [Google Scholar] [CrossRef]
- Shi, S.; Xu, K.; Jiang, C.; Ding, Z. ZnCl2-Catalyzed [3+ 2] Cycloaddition of Benzimidates and 2 H-Azirines for the Synthesis of Imidazoles. J. Org. Chem. 2018, 83, 14791–14796. [Google Scholar] [CrossRef]
- Man, L.; Copley, R.C.; Handlon, A.L. Thermal and photochemical annulation of vinyl azides to 2-aminoimidazoles. Org. Biomol. Chem. 2019, 17, 6566–6569. [Google Scholar] [CrossRef]
- Harisha, M.B.; Dhanalakshmi, P.; Suresh, R.; Kumar, R.R.; Muthusubramanian, S.; Bhuvanesh, N. TMSOTf-Catalysed Synthesis of 2, 4, 5-Trisubstituted Imidazoles from Vinyl Azides and Nitriles. ChemistrySelect 2019, 4, 2954–2958. [Google Scholar] [CrossRef]
- Tian, Y.; Qin, M.; Yang, X.; Zhang, X.; Liu, Y.; Guo, X.; Chen, B. Acid-catalyzed synthesis of imidazole derivatives via N-phenylbenzimidamides and sulfoxonium ylides cyclization. Tetrahedron 2019, 75, 2817–2823. [Google Scholar] [CrossRef]
- Hogendorf, A.S.; Hogendorf, A.; Popiołek-Barczyk, K.; Ciechanowska, A.; Mika, J.; Satała, G.; Walczak, M.; Latacz, G.; Handzlik, J.; Kieć-Kononowicz, K. Fluorinated indole-imidazole conjugates: Selective orally bioavailable 5-HT7 receptor low-basicity agonists, potential neuropathic painkillers. Eur. J. Med. Chem. 2019, 170, 261–275. [Google Scholar] [CrossRef]
- Vanda, D.; Soural, M.; Canale, V.; Chaumont-Dubel, S.; Satała, G.; Kos, T.; Funk, P.; Fülöpová, V.; Lemrova, B.; Koczurkiewicz, P. Novel non-sulfonamide 5-HT6 receptor partial inverse agonist in a group of imidazo [4, 5-b] pyridines with cognition enhancing properties. Eur. J. Med. Chem. 2018, 144, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Zagórska, A.; Bucki, A.; Kołaczkowski, M.; Siwek, A.; Głuch-Lutwin, M.; Starowicz, G.; Kazek, G.; Partyka, A.; Wesołowska, A.; Słoczyńska, K. Synthesis and biological evaluation of 2-fluoro and 3-trifluoromethyl-phenyl-piperazinylalkyl derivatives of 1 H-imidazo [2, 1-f] purine-2, 4 (3 H, 8 H)-dione as potential antidepressant agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kumar, N.; Thakur, A.; Kumar, V.; Kumar, R.; Kumar, V. A Review on the Arylpiperazine Derivatives as Potential Therapeutics for the Treatment of Various Neurological Disorders. Curr. Drug Targets 2022, 23, 729–751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Sahu, B.; Pathania, S.; Singh, P.K.; Akhtar, M.J.; Kumar, B. Piperazine, a Key Substructure for Antidepressants: Its Role in Developments and Structure-Activity Relationships. ChemMedChem 2021, 16, 1878–1901. [Google Scholar] [CrossRef]
- Kumar, R.R.; Kumar, V.; Kaur, D.; Nandi, N.K.; Dwivedi, A.R.; Kumar, V.; Kumar, B. Investigation of indole-3-piperazinyl derivatives as potential antidepressants: Design, synthesis, in-vitro, in-vivo and in-silico analysis. ChemistrySelect 2021, 6, 11276–11284. [Google Scholar] [CrossRef]
- Zagórska, A.; Kołaczkowski, M.; Bucki, A.; Siwek, A.; Kazek, G.; Satała, G.; Bojarski, A.J.; Partyka, A.; Wesołowska, A.; Pawłowski, M. Structure–activity relationships and molecular studies of novel arylpiperazinylalkyl purine-2, 4-diones and purine-2, 4, 8-triones with antidepressant and anxiolytic-like activity. Eur. J. Med. Chem. 2015, 97, 142–154. [Google Scholar] [CrossRef]
- Zagórska, A.; Jurczyk, S.; Pawłowski, M.; Dybała, M.; Nowak, G.; Tatarczyńska, E.; Nikiforuk, A.; Chojnacka-Wójcik, E. Synthesis and preliminary pharmacological evaluation of imidazo [2, 1-f] purine-2, 4-dione derivatives. Eur. J. Med. Chem. 2009, 44, 4288–4296. [Google Scholar] [CrossRef]
- Tokgöz, G.; Demir Özkay, Ü.; Osmaniye, D.; Turan Yücel, N.; Can, Ö.D.; Kaplancıklı, Z.A. Synthesis of Novel Benzazole Derivatives and Evaluation of Their Antidepressant-Like Activities with Possible Underlying Mechanisms. Molecules 2018, 23, 2881. [Google Scholar] [CrossRef]
- Czopek, A.; Kołaczkowski, M.; Bucki, A.; Byrtus, H.; Pawłowski, M.; Siwek, A.; Bojarski, A.J.; Bednarski, M.; Wróbel, D.; Wesołowska, A. Novel mannich bases, 5-arylimidazolidine-2, 4-dione derivatives with dual 5-HT1A receptor and serotonin transporter affinity. Arch. Der Pharm. 2013, 346, 98–109. [Google Scholar] [CrossRef]
- Seo, H.J.; Park, E.-J.; Kim, M.J.; Kang, S.Y.; Lee, S.H.; Kim, H.J.; Lee, K.N.; Jung, M.E.; Lee, M.; Kim, M.-S. Design and synthesis of novel arylpiperazine derivatives containing the imidazole core targeting 5-HT2A receptor and 5-HT transporter. J. Med. Chem. 2011, 54, 6305–6318. [Google Scholar] [CrossRef] [PubMed]
- Ulak, G.; Mutlu, O.; Akar, F.Y.; Komsuoğlu, F.I.; Tanyeri, P.; Erden, B.F. Neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole augment the effects of antidepressants acting via serotonergic system in the forced swimming test in rats. Pharmacol. Biochem. Behav. 2008, 90, 563–568. [Google Scholar] [CrossRef]
- Sherwin, E.; Gigliucci, V.; Harkin, A. Regional specific modulation of neuronal activation associated with nitric oxide synthase inhibitors in an animal model of antidepressant activity. Behav. Brain Res. 2017, 316, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Lauro, F.V.; Francisco, D.C.; Ricardo, G.; Heidari, A.; Maria, L.R.; Virginia, M.A.M.; Magdalena, A.R.; Tomas, L.G. Design and synthesis of two Strychnidin-oxiran-naphthalenol derivatives and their theoretical evaluation as noradrenaline and serotonin reuptake inhibitors. Vietnam J. Chem. 2022, 60, 245–256. [Google Scholar]
- Czopek, A.; Partyka, A.; Bucki, A.; Pawłowski, M.; Kołaczkowski, M.; Siwek, A.; Głuch-Lutwin, M.; Koczurkiewicz, P.; Pękala, E.; Jaromin, A. Impact of N-Alkylamino Substituents on Serotonin Receptor (5-HTR) Affinity and Phosphodiesterase 10A (PDE10A) Inhibition of Isoindole-1, 3-dione Derivatives. Molecules 2020, 25, 3868. [Google Scholar] [CrossRef]
- Żmudzki, P.; Satała, G.; Chłoń-Rzepa, G.; Bojarski, A.J.; Kazek, G.; Siwek, A.; Gryboś, A.; Głuch-Lutwin, M.; Wesołowska, A.; Pawłowski, M. Structure–5-HT/D2 Receptor Affinity Relationship in a New Group of 1-Arylpiperazynylalkyl Derivatives of 8-Dialkylamino-3, 7-dimethyl-1H-purine-2, 6 (3H, 7H)-dione. Arch. Der Pharm. 2016, 349, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Hogendorf, A.S.; Hogendorf, A.; Kurczab, R.; Kalinowska-Tłuścik, J.; Popik, P.; Nikiforuk, A.; Krawczyk, M.; Satała, G.; Lenda, T.; Knutelska, J. 2-Aminoimidazole-based antagonists of the 5-HT6 receptor–A new concept in aminergic GPCR ligand design. Eur. J. Med. Chem. 2019, 179, 1–15. [Google Scholar] [CrossRef]
- Bromidge, S.M.; Arban, R.; Bertani, B.; Bison, S.; Borriello, M.; Cavanni, P.; Dal Forno, G.; Di-Fabio, R.; Donati, D.; Fontana, S. Design and Synthesis of Novel Tricyclic Benzoxazines as Potent 5-HT1A/B/D Receptor Antagonists Leading to the Discovery of 6-{2-[4-(2-methyl-5-quinolinyl)-1-piperazinyl] ethyl}-4 H-imidazo [5, 1-c][1, 4] benzoxazine-3-carboxamide (GSK588045). J. Med. Chem. 2010, 53, 5827–5843. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Sanacora, G. A new generation of antidepressants: An update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 2019, 24, 606–615. [Google Scholar] [CrossRef]
- El-Haggar, S.M.; Eissa, M.A.; Mostafa, T.M.; El-Attar, K.S.; Abdallah, M.S. The phosphodiesterase inhibitor pentoxifylline as a novel adjunct to antidepressants in major depressive disorder patients: A proof-of-concept, randomized, double-blind, placebo-controlled trial. Psychother. Psychosom. 2018, 87, 331–339. [Google Scholar] [CrossRef]
- Bah, T.M.; Kaloustian, S.; Rousseau, G.; Godbout, R. Pretreatment with pentoxifylline has antidepressant-like effects in a rat model of acute myocardial infarction. Behav. Pharmacol. 2011, 22, 779–784. [Google Scholar] [CrossRef]
- Yasrebi, S.-O.; Momtazmanesh, S.; Moghaddam, H.S.; Shahmansouri, N.; Mehrpooya, M.; Arbabi, M.; Ghazizadeh-Hashemi, F.; Akhondzadeh, S. Pentoxifylline for treatment of major depression after percutaneous coronary intervention or coronary artery bypass grafting: A randomized, double-blind, placebo-controlled trial. J. Psychosom. Res. 2021, 150, 110635. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.H.; Izadi, A.; Hajiesmaeili, M.R.; Ghanizadeh, A.; Dastjerdi, G.; Hosseini, H.A.; Ghiamat, M.M.; Abbasi, H.R. Effect of etomidate versus thiopental on major depressive disorder in electroconvulsive therapy, a randomized double-blind controlled clinical trial. J. ECT 2012, 28, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.; Vadakkadath Meethal, S.; Bowen, R.L.; Atwood, C.S. Leuprolide acetate: A drug of diverse clinical applications. Expert Opin. Investig. Drugs 2007, 16, 1851–1863. [Google Scholar] [CrossRef]
- Lindemann, L.; Porter, R.H.; Scharf, S.H.; Kuennecke, B.; Bruns, A.; von Kienlin, M.; Harrison, A.C.; Paehler, A.; Funk, C.; Gloge, A. Pharmacology of basimglurant (RO4917523, RG7090), a unique metabotropic glutamate receptor 5 negative allosteric modulator in clinical development for depression. J. Pharmacol. Exp. Ther. 2015, 353, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Stedenfeld, K.A.; Clinton, S.M.; Kerman, I.A.; Akil, H.; Watson, S.J.; Sved, A.F. Candesartan reverses depression-like behavior in a rodent model of depression. FASEB J. 2010, 24, 1052.3. [Google Scholar] [CrossRef]
- Luo, C.; Fan, H.; Li, S.; Zou, Y. Therapeutic of Candesartan and Music Therapy in Diabetic Retinopathy with Depression in Rats. Evid. -Based Complement. Altern. Med. 2021, 2021, 5570356. [Google Scholar] [CrossRef]
- Rizvi, S.J.; Kennedy, S.H. Emerging drugs for major depressive disorder: An update. Expert Opin. Emerg. Drugs 2012, 17, 285–294. [Google Scholar] [CrossRef][Green Version]
- Ar, M.; Ozbalak, M.; Tuzuner, N.; Bekoz, H.; Ozer, O.; Ugurlu, K.; Tabak, F.; Ferhanoglu, B. Severe bone marrow failure due to valganciclovir overdose after renal transplantation from cadaveric donors: Four consecutive cases. Transplant. Proc. 2009, 41, 1648–1653. [Google Scholar] [CrossRef]
- Treggiari-Venzi, M.; Borgeat, A.; Fuchs-Buder, T.; Gachoud, J.-P.; Suter, P.M. Overnight sedation with midazolam or propofol in the ICU: Effects on sleep quality, anxiety and depression. Intensive Care Med. 1996, 22, 1186–1190. [Google Scholar] [CrossRef]
- Grunebaum, M.F.; Galfalvy, H.C.; Choo, T.-H.; Keilp, J.G.; Moitra, V.K.; Parris, M.S.; Marver, J.E.; Burke, A.K.; Milak, M.S.; Sublette, M.E. Ketamine for rapid reduction of suicidal thoughts in major depression: A midazolam-controlled randomized clinical trial. Am. J. Psychiatry 2018, 175, 327–335. [Google Scholar] [CrossRef]
- Grunebaum, M.F.; Ellis, S.P.; Keilp, J.G.; Moitra, V.K.; Cooper, T.B.; Marver, J.E.; Burke, A.K.; Milak, M.S.; Sublette, M.E.; Oquendo, M.A. Ketamine versus midazolam in bipolar depression with suicidal thoughts: A pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017, 19, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.; Bin, L.; Ray, M.D.; Michael, S.J.; Deyi, Z. Imidazole Carboxamides. Indian Patent 271995, 18 March 2016. [Google Scholar]
- Lee, J.; Seo, H.J.; Kang, S.Y.; Park, E.-J.; Kim, M.J.; Lee, S.H.; Kim, J.Y.; Kim, J.; Jung, M.E.; Kim, H.J.; et al. Arylpiperazine-Containing Imidazole 4-carboxamide Derivatives and Pharmaceutical Composition Comprising Same. U.S. Patent US 8,835,436, 16 September 2014. [Google Scholar]
- Ceccarelli, S.M.; Jagasia, R.; Jakob-roetne, R.; Wichmann, J. Benzimidazoles as CNS Active Agents. U.S. Patent US 20,150,203,472A, 23 July 2015. [Google Scholar]
- Schwartz, J.-C.; Lecomte, J.-M. Combination Product Comprising an Antagonist or Inverse Agonist of Histamine Receptor H3 and an Antipsychotic and Antidepressant agent, and Use Thereof for the Preparation of a Medicament That Prevents the Adverse Effects of Psychotropic Drugs. U.S. Patent US8,106,041B2, 31 January 2012. [Google Scholar]
- Thurkauf, A.; Horvath, R.F.; Yuan, J.; Peterson, J.M. Certain 4-Aminomethyl-2-substituted Imidazole Derivatives and 2-Aminomethyl-4-substituted Imidazole Derivatives; New Classes of Dopamine Receptor Subtype Specific Ligands. U.S. Patent US6,797,824B2, 28 September 2004. [Google Scholar]
- Borroto-Escuela, D.O.; Li, X.; Tarakanov, A.O.; Savelli, D.; Narváez, M.; Shumilov, K.; Andrade-Talavera, Y.; Jimenez-Beristain, A.; Pomierny, B.; Díaz-Cabiale, Z. Existence of brain 5-HT1A–5-HT2A isoreceptor complexes with antagonistic allosteric receptor–receptor interactions regulating 5-HT1A receptor recognition. ACS Omega 2017, 2, 4779–4789. [Google Scholar] [CrossRef] [PubMed]
- Bricker, B.A.; Voshavar, C.; Onyameh, E.K.; Gonela, U.M.; Lin, X.; Swanson, T.L.; Kozell, L.B.; Schmachtenberg, J.L.; Bloom, S.H.; Janowsky, A.J. Enantiomeric Separation, Absolute Configuration by X-ray Crystallographic Analysis, and Functional Evaluation of Enantiomers of the Dual Ligand, SYA0340 at 5-HT1A and 5-HT7A Receptors. ACS Omega 2023, 8, 21736–21744. [Google Scholar] [CrossRef]
- Ostrowska, K.; Leśniak, A.; Karczyńska, U.; Jeleniewicz, P.; Głuch-Lutwin, M.; Mordyl, B.; Siwek, A.; Trzaskowski, B.; Sacharczuk, M.; Bujalska-Zadrożny, M. 6-Acetyl-5-hydroxy-4, 7-dimethylcoumarin derivatives: Design, synthesis, modeling studies, 5-HT1A, 5-HT2A and D2 receptors affinity. Bioorganic Chem. 2020, 100, 103912. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Reidy, M.; O’Reilly, C.; Jarikote, D.V.; Negi, A.; Samali, A.; Szegezdi, E.; Murphy, P.V. Decorated macrocycles via ring-closing double-reductive amination. Identification of an apoptosis inducer of leukemic cells that at least partially antagonizes a 5-HT2 receptor. Org. Lett. 2015, 17, 1672–1675. [Google Scholar] [CrossRef]
- Negi, A.; Reilly, C.O.; Jarikote, D.V.; Zhou, J.; Murphy, P.V. Multi-targeting protein-protein interaction inhibitors: Evolution of macrocyclic ligands with embedded carbohydrates (MECs) to improve selectivity. Eur. J. Med. Chem. 2019, 176, 292–309. [Google Scholar] [CrossRef]
- Foley, C.A.; Al-Issa, Y.A.; Hiller, K.P.; Mulcahy, S.P. Synthesis and Structure–Activity Relationships of 1-Aryl-β-carbolines as Affinity Probes for the 5-Hydroxytryptamine Receptor. ACS Omega 2019, 4, 9807–9812. [Google Scholar] [CrossRef]
- Singh, D.; Singh, P.; Srivastava, P.; Kakkar, D.; Pathak, M.; Tiwari, A.K. Development and Challenges in the discovery of 5-HT1A and 5-HT7 receptor ligands. Bioorganic Chem. 2023, 131, 106254. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rakshit, J.; Bandyopadhyay, J.; Basu, S. Multi-target inhibition ability of neohesperidin dictates its neuroprotective activity: Implication in Alzheimer’s disease therapeutics. Int. J. Biol. Macromol. 2021, 176, 315–324. [Google Scholar] [CrossRef]
- Tzschentke, T.M. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998, 56, 613–672. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Gabr, M.T. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2019, 14, 437. [Google Scholar]
- Jenwitheesuk, E.; Horst, J.A.; Rivas, K.L.; Van Voorhis, W.C.; Samudrala, R. Novel paradigms for drug discovery: Computational multitarget screening. Trends Pharmacol. Sci. 2008, 29, 62–71. [Google Scholar] [CrossRef]
- Negi, A.; Zhou, J.; Sweeney, S.; Murphy, P.V. Ligand design for somatostatin receptor isoforms 4 and 5. Eur. J. Med. Chem. 2019, 163, 148–159. [Google Scholar] [CrossRef]
- Dantas, D.; Ribeiro, A.I.; Carvalho, F.; Gil-Martins, E.; Silva, R.; Remião, F.; Zille, A.; Cerqueira, F.; Pinto, E.; Dias, A.M. Red-shifted and pH-responsive imidazole-based azo dyes with potent antimicrobial activity. Chem. Commun. 2023, 59, 2791–2794. [Google Scholar] [CrossRef] [PubMed]
- Slassi, S.; Aarjane, M.; El-Ghayoury, A.; Allain, M.; Amine, A. Synthesis, crystal structure, photoisomerization, and DFT studies of novel azo compounds based on imidazole. J. Phys. Org. Chem. 2023, 36, e4486. [Google Scholar] [CrossRef]
- Negi, A.; Kieffer, C.; Voisin-Chiret, A.S. Azobenzene photoswitches in proteolysis targeting chimeras: Photochemical control strategies and therapeutic benefits. ChemistrySelect 2022, 7, e202200981. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K.; Voisin-Chiret, A.S. Light-Activating PROTACs in Cancer: Chemical Design, Challenges, and Applications. Appl. Sci. 2022, 12, 9674. [Google Scholar] [CrossRef]
- Narasimhan, B.; Sharma, D.; Kumar, P. Biological importance of imidazole nucleus in the new millennium. Med. Chem. Res. 2011, 20, 1119–1140. [Google Scholar] [CrossRef]
- Negi, A.; Voisin-Chiret, A.S. Strategies to reduce the on-target platelet toxicity of Bcl-xL inhibitors: PROTACs, SNIPERs and prodrug-based approaches. ChemBioChem 2022, 23, e202100689. [Google Scholar] [CrossRef]
- Singh, P.K.; Negi, A.; Gupta, P.K.; Chauhan, M.; Kumar, R. Toxicophore exploration as a screening technology for drug design and discovery: Techniques, scope and limitations. Arch. Toxicol. 2016, 90, 1785–1802. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Y.; Li, S.; Zhang, P.; Yao, Q. Synthesis and modification of ZIF-8 and its application in drug delivery and tumor therapy. RSC Adv. 2020, 10, 37600–37620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gong, L.; Wu, H.; Liu, C.; Liu, Y.; Xiao, C.; Liu, C.; Chen, L.; Jin, M.; Gao, Z. Development of Novel Paclitaxel-Loaded ZIF-8 Metal-Organic Framework Nanoparticles Modified with Peptide Dimers and an Evaluation of Its Inhibitory Effect against Prostate Cancer Cells. Pharmaceutics 2023, 15, 1874. [Google Scholar] [CrossRef] [PubMed]
- Asadi, V.; Marandi, A.; Kardanpour, R.; Tangestaninejad, S.; Moghadam, M.; Mirkhani, V.; Mohammadpoor-Baltork, I.; Mirzaei, R. Carbonic Anhydrase-Embedded ZIF-8 Electrospun PVA Fibers as an Excellent Biocatalyst Candidate. ACS Omega 2023, 8, 17809–17818. [Google Scholar] [CrossRef]
- Xia, Y.; Hong, Y.; Geng, R.; Li, X.; Qu, A.; Zhou, Z.; Zhang, Z. Amine-functionalized ZIF-8 as a fluorescent probe for breath volatile organic compound biomarker detection of lung cancer patients. ACS Omega 2020, 5, 3478–3486. [Google Scholar] [CrossRef]
- Costa, B.A.; Abuçafy, M.P.; Barbosa, T.W.L.; da Silva, B.L.; Fulindi, R.B.; Isquibola, G.; da Costa, P.I.; Chiavacci, L.A. ZnO@ ZIF-8 nanoparticles as nanocarrier of ciprofloxacin for antimicrobial activity. Pharmaceutics 2023, 15, 259. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.; Kesari, K.K. Chitosan nanoparticle encapsulation of antibacterial essential oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef]
- Mujtaba, M.; Negi, A.; King, A.W.; Zare, M.; Kuncova-Kallio, J. Surface modifications of nanocellulose for drug delivery applications; a critical review. Curr. Opin. Biomed. Eng. 2023, 100475. [Google Scholar] [CrossRef]
- Ramos, V.C.; Reyes, C.B.G.; García, G.M.; Quesada, M.I.S.; Barrero, F.J.M.-C.; Rábago, J.J.S.; Polo, M.S. ZIF-8 and Its Magnetic Functionalization as Vehicle for the Transport and Release of Ciprofloxacin. Pharmaceutics 2022, 14, 2546. [Google Scholar] [CrossRef]
- Gao, Z.; Mansor, M.H.; Winder, N.; Demiral, S.; Maclnnes, J.; Zhao, X.; Muthana, M. Microfluidic-Assisted ZIF-Silk-Polydopamine Nanoparticles as Promising Drug Carriers for Breast Cancer Therapy. Pharmaceutics 2023, 15, 1811. [Google Scholar] [CrossRef]
- Franco, A.; Negi, A.; Luque, R.; Carrillo-Carrión, C. Selectivity Control in the Oxidative Ring-Opening of Dimethylfuran Mediated by Zeolitic-Imidazolate Framework-8 Nanoparticles. ACS Sustain. Chem. Eng. 2021, 9, 8090–8096. [Google Scholar] [CrossRef]
- Tolomeu, H.V.; Fraga, C.A.M. Imidazole: Synthesis, Functionalization and Physicochemical Properties of a Privileged Structure in Medicinal Chemistry. Molecules 2023, 28, 838. [Google Scholar] [CrossRef]
- Zheng, X.; Ma, Z.; Zhang, D. Synthesis of imidazole-based medicinal molecules utilizing the van leusen imidazole synthesis. Pharmaceuticals 2020, 13, 37. [Google Scholar] [CrossRef]
- Andrei, G.Ş.; Andrei, B.F.; Roxana, P.R. Imidazole derivatives and their antibacterial activity—A mini-review. Mini Rev. Med. Chem. 2021, 21, 1380–1392. [Google Scholar] [CrossRef]
- Parwani, D.; Bhattacharya, S.; Rathore, A.; Mallick, C.; Asati, V.; Agarwal, S.; Rajoriya, V.; Das, R.; Kashaw, S.K. Current insights into the chemistry and antitubercular potential of benzimidazole and imidazole derivatives. Mini Rev. Med. Chem. 2021, 21, 643–657. [Google Scholar] [CrossRef]












| S. No. | Compound | Structure | Clinical Trial Status | Clinical Trial Number |
|---|---|---|---|---|
| 1 | EVT101 |  | Phase 2 Terminated | NCT01128452 |
| 2 | Pentoxifylline | 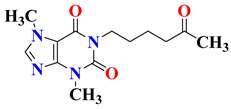 | Phases 1 and 2 Completed | NCT05324735 |
| Early Phase 1 Completed | NCT04417049 | |||
| Not Applicable Completed | NCT03554447 | |||
| Phase 1 Completed | NCT05271084 | |||
| 3 | Etomidate | 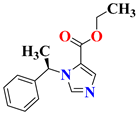 | Not Applicable Completed | NCT02667353 |
| Phase 4 Unknown | NCT02924090 | |||
| 4 | Leuprolide Acetate | Not Applicable Completed | NCT01762943 | |
| Phase 2 Completed | NCT04051320 | |||
| Phase 4 Completed | NCT01116401 | |||
| 5 | RO4917523 | 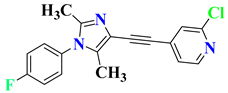 | Phase 2 Completed | NCT00809562 |
| Phase 2 Completed | NCT01437657 | |||
| 6 | Candesartan | 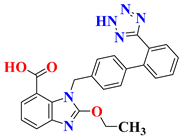 | Early Phase 1 Withdrawn | NCT04430959 |
| Phase 4 Terminated | NCT01794455 | |||
| 7 | Cimicoxib |  | Phase 2 Completed | NCT00510822 |
| 8 | Valganciclovir |  | Phases 1 and 2 Recruited | NCT04724447 |
| 9 | Midazolam |  | Phase 1 Terminated | NCT04082858 |
| Phase 4 Recruiting | NCT05026203 | |||
| Phase 2 Completed | NCT02360280 | |||
| Phase 2 Recruiting | NCT05383313 | |||
| Not yet recruiting | NCT05528718 | |||
| Phase 4 Completed | NCT01700829 | |||
| Phase 3 Active, not recruiting | NCT03889756 | |||
| Phase 4 Recruiting | NCT04220125 | |||
| Phase 3 Recruiting | NCT04939649 |
| Patentee Name/Inventors | Title | Patent No. | Year | Ref. |
|---|---|---|---|---|
| Albert K, Bin L, Ray MD, Michael SJ, and Deyi Z | Imidazole carboxamides | Indian patent No. 271995 | 2016 | [147] |
| Lee J, Seo HJ, Kang SY, Park EJ, Kim MJ, Lee SH, Kim JY, Kim J, Jung ME, Kim HJ, and Kim MS | Arylpiperazine-containing imidazole 4-carboxamide derivatives and a pharmaceutical composition comprising the same | US 8,835,436 | 2014 | [148] |
| Ceccarelli SM, Jagasia R, Jakob-roetne R, and Wichmann J | Benzimidazoles as CNS active agents. | US 20,150,203,472A | 2014 | [149] |
| Schwartz JC and Lecomte JM | Combination product comprising an antagonist or inverse agonist of histamine receptor H3 and an antipsychotic and antidepressant agent, and use thereof for the preparation of a medicament that prevents the adverse effects of psychotropic drugs. | US 8,106,041 | 2012 | [150] |
| Thurkauf A, Horvath RF, Yuan J, and Peterson JM | Certain 4-aminomethyl-2-substituted imidazole derivatives and 2-aminomethyl-4-substituted imidazole derivatives; new classes of dopamine receptor subtype-specific ligands. | US 6,797,824 | 2004 | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goel, K.K.; Thapliyal, S.; Kharb, R.; Joshi, G.; Negi, A.; Kumar, B. Imidazoles as Serotonin Receptor Modulators for Treatment of Depression: Structural Insights and Structure–Activity Relationship Studies. Pharmaceutics 2023, 15, 2208. https://doi.org/10.3390/pharmaceutics15092208
Goel KK, Thapliyal S, Kharb R, Joshi G, Negi A, Kumar B. Imidazoles as Serotonin Receptor Modulators for Treatment of Depression: Structural Insights and Structure–Activity Relationship Studies. Pharmaceutics. 2023; 15(9):2208. https://doi.org/10.3390/pharmaceutics15092208
Chicago/Turabian StyleGoel, Kapil Kumar, Somesh Thapliyal, Rajeev Kharb, Gaurav Joshi, Arvind Negi, and Bhupinder Kumar. 2023. "Imidazoles as Serotonin Receptor Modulators for Treatment of Depression: Structural Insights and Structure–Activity Relationship Studies" Pharmaceutics 15, no. 9: 2208. https://doi.org/10.3390/pharmaceutics15092208
APA StyleGoel, K. K., Thapliyal, S., Kharb, R., Joshi, G., Negi, A., & Kumar, B. (2023). Imidazoles as Serotonin Receptor Modulators for Treatment of Depression: Structural Insights and Structure–Activity Relationship Studies. Pharmaceutics, 15(9), 2208. https://doi.org/10.3390/pharmaceutics15092208









