Development and Evaluation of Phytosomes Containing Callistemon citrinus Leaf Extract: A Preclinical Approach for the Treatment of Obesity in a Rodent Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Callistemon citrinus Leaf Extract
2.2. Phytosome Preparation
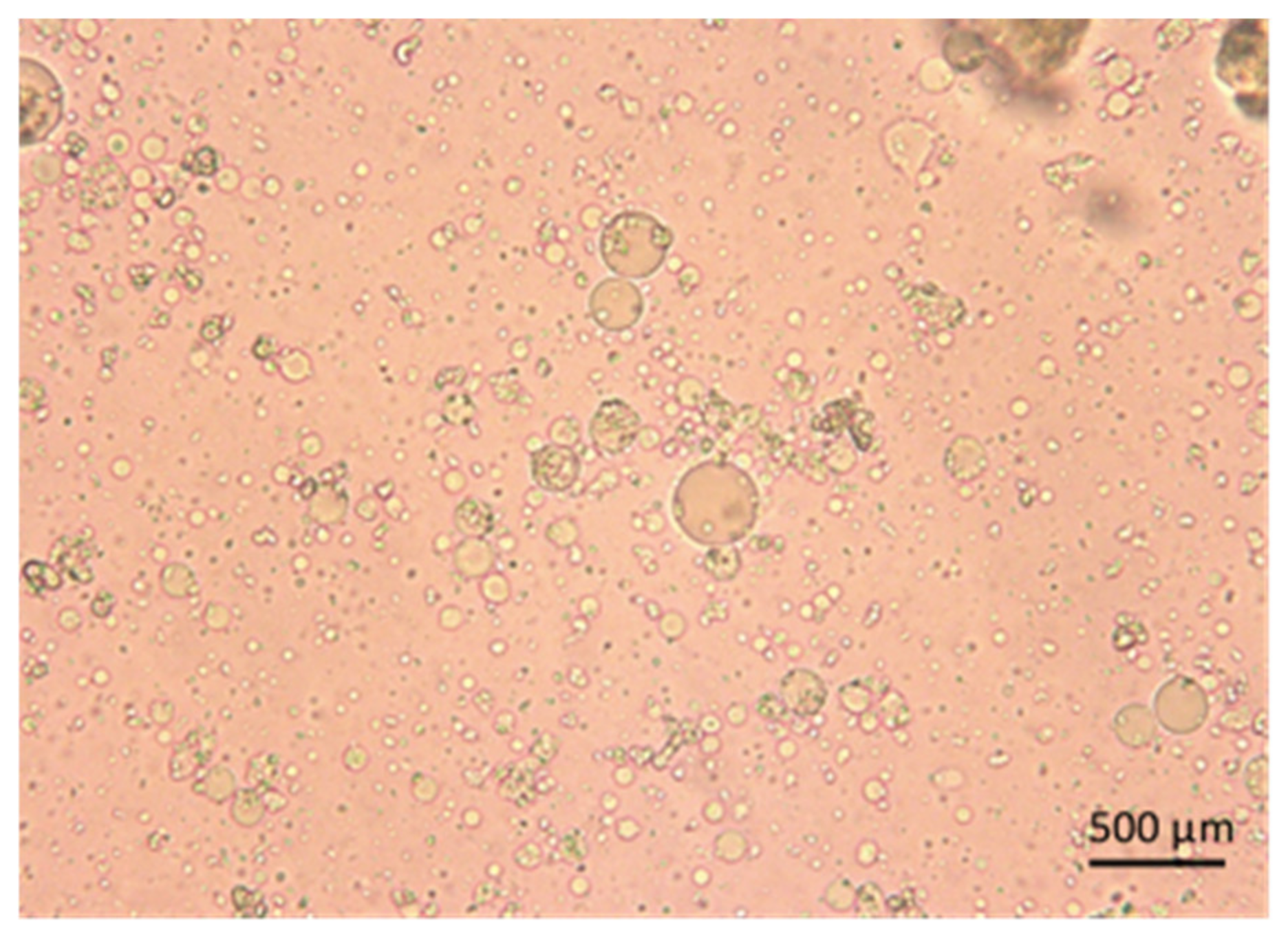
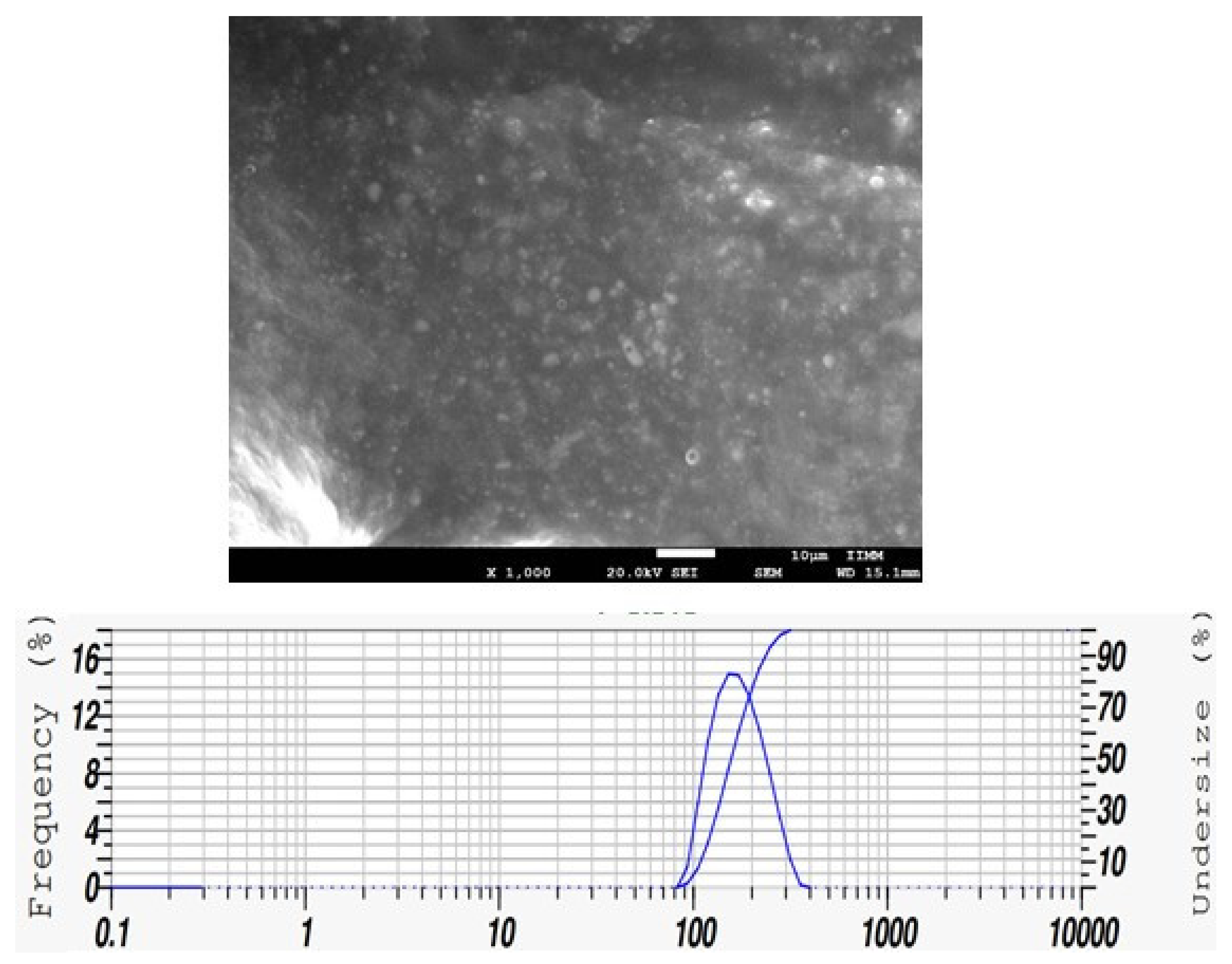
2.2.1. Lyophilization and Scanning Electron Microscopy (SEM)
2.2.2. Particle Size
2.2.3. Stability Study
2.2.4. Study of Vesicular Entrapment/Encapsulation and Solubility
2.3. In Vitro Antioxidant Activity
2.3.1. DPPH Radical Assay
2.3.2. ABTS Radical Scavenging Assay
2.3.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.3.4. Determination of Total Phenolic Content
2.3.5. Total Flavonoid Content
2.3.6. Total Terpenoid Content
2.4. GC-MS Determination
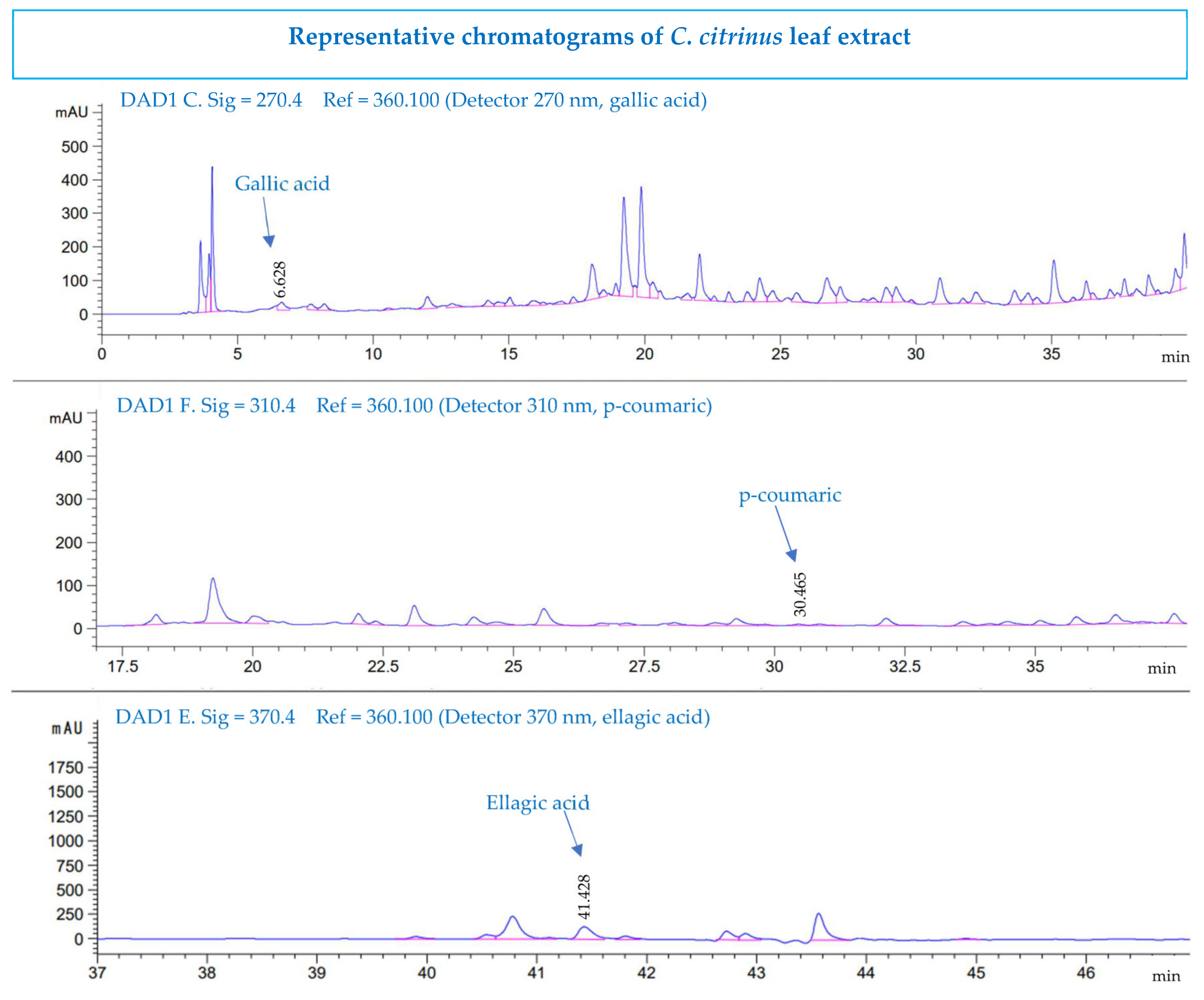
2.5. HPLC Determination
2.6. Anti-Obesity Evaluation of Phytosomes
2.6.1. In Vivo Study
Animals
2.6.2. Obesity Induction
2.6.3. Measurement of Morphometric and Biochemical Parameters
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphology and Particle-Size Analysis
3.2. Study of Vesicular Entrapment/Encapsulation
3.3. Study of Stability and Solubility
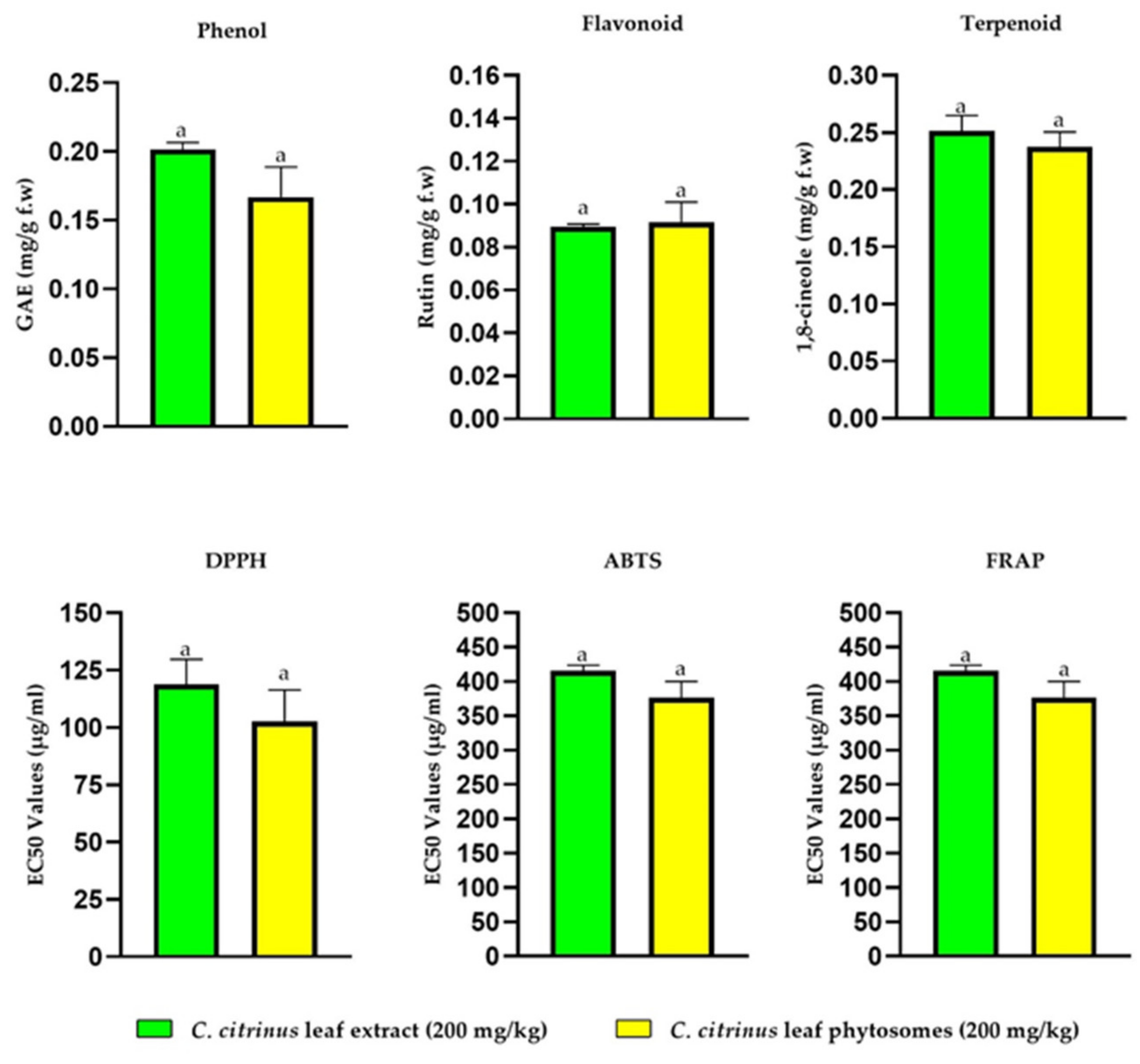
3.4. In Vitro Antioxidant Activity of Callistemon citrinus Phytosomes
3.5. Gas Chromatography and Mass Spectrometry Analysis
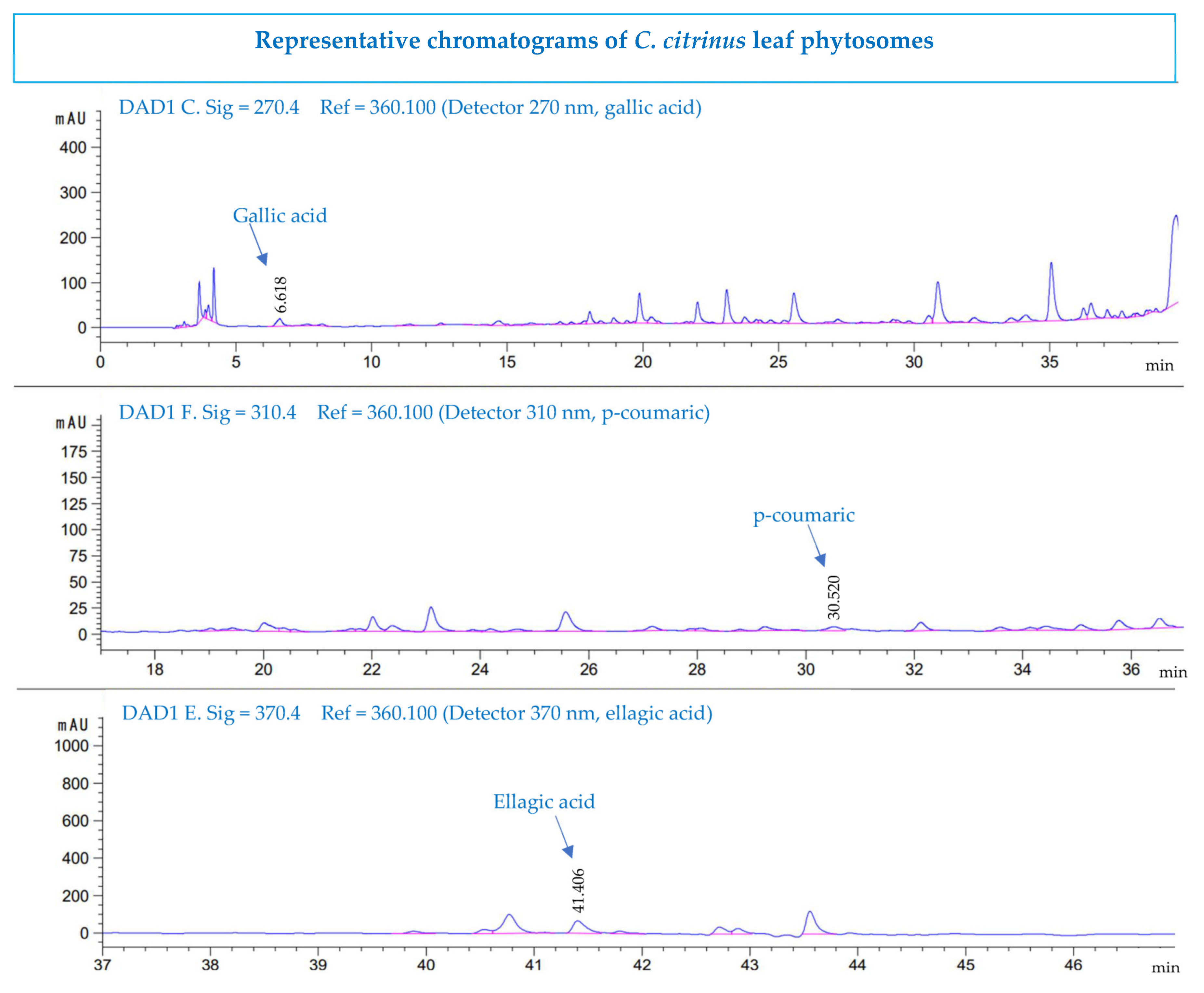
3.6. High-Performance Liquid Chromatography Analysis
3.7. Effect of Phytosomes on Morphometric and Biochemical Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandhiya, V.; Ubaidulla, U. A review on herbal drug loaded into pharmaceutical carrier techniques and its evaluation process. Future J. Pharm. Sci. 2020, 6, 51. [Google Scholar] [CrossRef]
- Permana, A.D.; Utami, R.N.; Courtenay, A.J.; Manggau, M.A.; Donnelly, R.F.; Rahman, L. Phytosomal nanocarriers as platforms for improved delivery of natural antioxidant and photoprotective compounds in propolis: An approach for enhanced both dissolution behaviour in biorelevant media and skin retention profiles. J. Photochem. Photobiol. B Biol. 2020, 205, 111846. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant neutraceutical in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Warraich, U.M.; Hussain, F.; Kayani, H.U.R. Aging—Oxidative stress, antioxidant and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidant and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lin, W.C.; Yu, B.; Lee, T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals- A review. Asian-Australas. J. Anim. Sci. 2017, 30, 299–308. [Google Scholar]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Pinto, M.S. Bioavailability if bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2012, 75, 588–602. [Google Scholar] [CrossRef]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharma. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Quin, W.; Ketnawa, S.; Ogawa, Y. Effect of digestive enzyme and pH on variation of bioavailability of green tea during simulated in vitro gastrointestinal digestion. Food Sci. Hum. Wellness 2022, 11, 669–675. [Google Scholar] [CrossRef]
- Stevanovic, Z.D.; Sieniawska, E.; Glowniak, K.; Obradovoc, N.; Pajic-Lijakovic, I. Natural macromolecules as carriers for essential oils: From extraction to biomedical application. Front. Bioeng. Biotechnol. 2020, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Deepa, P.; Kim, S. A review of the chemical composition and biological activities of Callistemon lanceolatus (Sm.) Sweet. J. Appl. Pharm. Sci. 2021, 11, 65–73. [Google Scholar] [CrossRef]
- López-Mejía, A.; Ortega-Pérez, L.G.; Godínez-Hernández, D.; Nateras-Marin, B.; Meléndez-Herrera, E.; Rios-Chavez, P. Chemopreventive effect of Callistemon citrinus (Curtis) Skeels against colon cancer induced by 1, 2-dimethylhydrazine in rats. J. Cancer Res. Clin. Oncol. 2019, 145, 1417–1426. [Google Scholar] [CrossRef]
- Ortega-Pérez, L.G.; Piñón-Simental, J.S.; Magaña-Rodríguez, O.R.; López-Mejía, A.; Ayala-Ruiz, L.A.; García-Calderón, A.J.; Godínez-Hernández, D.; Rios-Chavez, P. Evaluation of the toxicology, anti-lipase, and antioxidant effects of Callistemon citrinus in rats fed with a high fat-fructose diet. Pharm. Biol. 2022, 60, 1384–1393. [Google Scholar] [CrossRef]
- Petronilho, S.; Rocha, S.M.; Ramírez-Chávez, E.; Molina-Torres, J.; Rios-Chavez, P. Assessment of the terpenic profile of Callistemon citrinus (Curtis) Skeels from Mexico. Ind. Crops Prod. 2013, 46, 369–379. [Google Scholar] [CrossRef]
- Ayala-Ruiz, L.A.; Ortega-Pérez, L.G.; Piñón-Simental, J.S.; Magaña-Rodríguez, O.R.; Meléndez-Herrera, E.; Rios-Chavez, P. Role of the major terpenes of Callistemon citrinus against the oxidative stress during a hypercaloric diet in rats. Biomed. Pharmacother. 2022, 153, 113505. [Google Scholar] [CrossRef]
- Khanh, P.N.; Duc, H.V.; Huong, T.T.; Ha, V.T.; Van, D.T.; Son, N.T.; Kim, Y.H.; Viet, D.Q.; Cuong, N.M. Phenolic compounds from Callistemon citrinus leaves and stem. J. Sci. Technol. 2016, 54, 190–197. [Google Scholar] [CrossRef][Green Version]
- Ravichandran, S.; Paluri, V.; Kumar, G.; Loganathan, K.; Venkata, R.K. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2016, 11, 445–458. [Google Scholar] [CrossRef]
- Paosen, S.; Saising, J.; Wira Septama, A.; Piyawan Voravuthikunchai, S. Green synthesis of silver nanoparticles using plants from Myrtaceae family and characterization of their antibacterial activity. Mater. Lett. 2017, 209, 201–209. [Google Scholar] [CrossRef]
- Rotimi, L.; Ojemaye, M.O.; Okoh, O.O.; Okoh, A.I. Silver nanoparticles mediated by Callistemon citrinus extracts and their antimalaria, antitrypanosomal and antibacterial efficacy. J. Mol. Liq. 2019, 273, 615–625. [Google Scholar]
- Rotimi, L.; Ojemaye, M.O.; Okoh, O.O.; Sadimennko, A.; Okoh, A.I. Synthesis, characterization, antimalarial, antitrypanocidal and antimicrobialproperties of gold nanoparticle. Green Chem. Lett. Rev. 2019, 12, 61–68. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ahmad, I.S.; Fouly, H.; Abbas, F.I.; Hasan, A.; Kushad, M. Poly (lactic-co-glycolic acid) nanoparticles loaded with Callistemon citrinus phenolics exhibited anticancer properties against three breast cancer cell lines. J. Food Qual. 2019, 2019, 2638481. [Google Scholar] [CrossRef]
- Gharibvand, Z.S.; Behbahani, B.A.; Noshad, M.; Jooyandeh, H. Green synthesis of silver nanoparticles using Callistemon citrinus leaf extract and evaluation of its antibacterial activity. Iran. Food Sci. Technol. Res. J. 2022, 18, 151–163. [Google Scholar]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.S.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Adiki, S.K.; Sangeetha, S.; Kamireddy, S.; Katakam, P.; Obilineni, I. Phytosomes: A novel phytoconstituent delivery approach to improve the efficacy of obesity treatment. Curr. Nutr. Food Sci. 2023, 19, 229–237. [Google Scholar] [CrossRef]
- Chakkhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. Lancet 2023, 58, 1–29. [Google Scholar] [CrossRef]
- Tham, K.W.; Lim, A.Y.L.; Baur, L.A. The global agenda on obesity: What does this mean for Singapore. Singapore Med. J. 2023, 64, 182–187. [Google Scholar] [CrossRef]
- Lee, P.C.; Lim, C.H.; Asokkumar, R.; Chua, M.W.J. Current treatment landscape for obesity in Singapore. Singapore Med. J. 2023, 64, 172–181. [Google Scholar] [CrossRef]
- Gehan, F.; Raoof, A. Anti-obesity potential of natural products. Egypt. J. Chem. 2022, 65, 329–358. [Google Scholar]
- López-Mejía, A.; Ortega-Pérez, L.G.; Magaña-Rodríguez, O.R.; Ayala-Ruiz, L.A.; Piñon-Simental, J.S.; Godínez-Hernández, D.; Rios-Chavez, P. Protective effect of Callistemon citrinus on oxidative stress in rats with 1,2-dimethylhydrazine-induced colon cancer. Biomed. Pharmacother. 2021, 142, 112070. [Google Scholar] [CrossRef] [PubMed]
- Baradaran, S.; Hajizadeh Moghaddam, A.; Khanjani Jelodar, S.; Moradi-Kor, N. Protective effects of curcumin and its nano-phytosome on carrageenan-induced inflammation in mice model: Behavioral and biochemical responses. J. Inflamm. Res. 2020, 13, 45–51. [Google Scholar] [CrossRef]
- Alvarez Cortes, O. Elaboración de una Biopelícula Comestible Incorporando Nanocápsulas de Aceite Esencial y Extractos Polifenólicos de Aloysia citriodora con Actividad Antiinflamatoria Evaluado en un Modelo Biológico. Master’s Thesis, Facultad de Químico Farmacobiología, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Mexico, 2020. [Google Scholar]
- Horiba. Colloidal Silica as a Particle Size and Charge Reference Material. 2009. Available online: https://www.horiba.com/fileadmin/uploads/Scientific/Documents/PSA/TN158.pdf (accessed on 22 January 2023).
- Jain, P.K.; Kharya, M.; Gajbhiye, A. Pharmacological evaluation of mangiferin herbosomes for antioxidant and hepatoprotection potential against ethanol induced hepatic damage. Drug Dev. Ind. Pharm. 2013, 39, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Khurana, N.; Pounikar, Y.; Gajbhiye, A.; Kharya, M.D. Enhancement of absorption and hepatoprotective potential through soya-phosphatidylcholine-andrographolide vesicular system. J. Liposome Res. 2013, 23, 110–118. [Google Scholar] [CrossRef]
- Karamac, M.; Kosiñska, A.; Pegg, R.B. Comparison of radical-scavenging activities for selected phenolic acids. Pol. J. Food Nutr. Sci. 2005, 14, 165–170. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; de Brito, E.S.; Perez-Jimenez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Comp. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Chumpolsri, W.; Suttiarporn, P.; Wongpornchai, S. The chemical composition and antioxidant activities of basil from Thailand using retention indices and comprehensive two-dimensional gas chromatography. J. Serb. Chem. Soc. 2010, 75, 1503–1513. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Chang, C.L.; Lin, C.S. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid.-Based Complement. Altern. Med. 2012, 2012, 125247. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, J.A.S. Phytosome: An emerging trend in herbal drug treatment. J. Genet. Genom. 2011, 3, 109–114. [Google Scholar]
- Xie, J.; Li, Y.; Song, L.; Pan, Z.; Ye, S.; Hou, Z. Design of a novel curcumin-soybean phosphatidylcholine complex-based targeted drug delivery system. Drug Deliv. 2017, 24, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, S.M. Optimization and characterization of Cuscuta reflexa extract loaded phytosomes by the box-behnken design to improve the oral bioavailability. J. Oleo Sci. 2022, 71, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as innovative delivery systems for phytochemicals: A comprehensive review of literate. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef] [PubMed]
- Surendra, T.; Patel, D.K.; Baro, L.; Nair, S.K. A review on phytosomes, their characterization, advancement & potential for transdermal application. J. Drug Deliv. Ther. 2013, 3, 147–152. [Google Scholar]
- Boligon, A.A.; Machadi, M.M.; Athayde, M.L. Technical evaluation of antioxidant activity. Med. Chem. 2014, 4, 517–522. [Google Scholar] [CrossRef]
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Ahmed, M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem. Cent. J. 2012, 6, 12. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Saonere, J.A.; Channawar, M.A.; Kochar, N.I.; Mohale, D.; Chandewar, A.V. Preparation and evaluation of phytophospholipid complex of phenolic fractions of G. glabra for antioxidant and antimicrobial activity. Acta Sci. Pharm. Sci. 2023, 7, 37–44. [Google Scholar]
- Palachai, N.; Wattanathorn, J.; Muchimapura, S.; Thukham-Mee, W. Antimetabolic syndrome effect of phytosome containing the combined extracts of mulberry and ginger in an animal model of metabolic syndrome. Oxidative Med. Cell. Longev. 2019, 2019, 5972572. [Google Scholar] [CrossRef] [PubMed]
- Tung, B.H.; Hai, N.T.; Son, P.K. Hepatoprotective effect of phytosome curcumin against paracetamol-induced liver toxicity in mice. Braz. J. Pharm. Sci. 2017, 53, e16136. [Google Scholar] [CrossRef]
- Deleanu, M.; Toma, L.; Sanda, G.M.; Barbălată, T.; Niculescu, L.S.; Sima, A.V.; Deleanu, C.; Săcărescy, L.; Suciu, A.; Alexandru, G.; et al. Formulation of phytosomes with extracts of ginger rhizomes and rosehips with improved bioavailability, antioxidant and anti-inflammatory effects in vivo. Pharmaceutics 2023, 15, 1066. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural monoterpenes: Much more than only a scent. Chem. Biodivers 2019, 16, e1900434. [Google Scholar] [CrossRef]
- Silva, J.C.; Barbosa, L.C.A.; Demuner, J.A.; Montanari, R.M.; Pinheiro, A.L.; Dias, I.; Andrade, N.J. Chemical composition and bacterial activities from the essential oils of Myrtaceae species planted in Brazil. Química Nova 2010, 33, 104–108. [Google Scholar] [CrossRef]
- Radulovic, N.; Lazarevic, J.; Ristic, N.; Palic, R. Chemotaxonomic significance of the volatiles in the genus Stachys (Lamiaceae): Essential oil composition of four Balkan Stachys species. Biochem. Syst. Ecol. 2007, 35, 196–208. [Google Scholar] [CrossRef]
- Muselli, A.; Rossi, P.-G.; Desjobert, J.-M.; Bernardini, A.-F.; Berti, L.; Costa, J. Chemical composition and antibacterial activity of Otanthus maritimus (L.) Hoffmanns. & Link essential oils from Corsica. Flavour Fragr. J. 2007, 22, 217–223. [Google Scholar]
- Al-Omar, M.S. Phytochemical analysis, antioxidant, and anti-microbial activities of Suaeda vermiculata n-hexane extract in comparison to the plant’s hydrodistilled volatile oil. Pharmacogn. J. 2021, 13, 853–859. [Google Scholar] [CrossRef]
- Boti, J.B.; Koukoua, G.; N’Guessan, T.Y.; Casanova, J. Chemical variability of Conyza sumatrensis and Microglossa pyrifolia from Côte d’Ivoire. Flavour Fragr. J. 2007, 22, 27–31. [Google Scholar] [CrossRef]
- Babushok, V.-I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Zhao, C.X.; Li, X.N.; Liang, Y.Z.; Fang, H.Z.; Huang, L.F.; Guo, F.Q. Comparative analysis of chemical components of essential oils from different samples of Rhododendron with the help of chemometrics methods. Chemom. Intell. Lab. Syst. 2006, 82, 218–222. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902. [Google Scholar] [CrossRef]
- Das, S.R.; Kundu, C.N. Promising opportunities and potential risk of nanoparticle on the society. IET Nanobiotechnol. 2020, 14, 253–260. [Google Scholar] [CrossRef] [PubMed]


| Temperature | 25 °C |
| Particle | LUDOX (1.45–0.000i) |
| Dispersion medium | Water |
| Cell | Plastic |
| Distribution type | Monodisperse narrow |
| Parameter | Abs |
|---|---|
| Tdrug | 0.189 ± 0.01 |
| Udrug | 0.045 ± 0.07 |
| EE | 80.49 ± 0.07% |
| Temperature | ||
|---|---|---|
| Days | 20 ± 2 °C | 4 ± 1 °C |
| 1 | 193.62 ± 27.33 a | 285.07 ± 14.04 ab |
| 3 | 218.06 ± 59.55 a | 412.80 ± 248.22 abc |
| 5 | 256.50 ± 29.00 a | 454.23 ± 175.28 abc |
| 10 | 279.64 ± 61.21 a | 570.70 ± 132.73 bc |
| 106 | 283.82 ± 51.87 ab | 623.23 ± 142.18 c |
| Solvent | C. citrinus Extract (200 mg/kg) | C. citrinus Extract (200 mg/kg) + Tween 80 | C. citrinus Phytosomes (200 mg/kg) | Soybean Liposomes + Tween 80 | Soybean Liposomes-Tween 80 |
|---|---|---|---|---|---|
| Distilled water | Partially | Partially | Soluble | Soluble | Micellar shape |
| Methanol | Soluble | Soluble | Partially | Unsolvable | Soluble |
| Dichloromethane | Soluble | Partially | Soluble | Soluble | Soluble |
| Chloroform | Soluble | Soluble | Soluble | Soluble | Partially |
| Hexane | Partially | Soluble | Soluble | Soluble | Soluble |
| RT | RIlit | RIcalc | Ref. RIlit | Match Factor | Prob (%) | Compounds | Extract | Phytosomes |
|---|---|---|---|---|---|---|---|---|
| 7.47 | 1041 | 1059 | Silva et al. [57] | 972 | 93.8% | 1,8-Cineole | 0.613 ± 0.05 | 0.224 ± 0.04 |
| 10.91 | 1143 | 1131 | Radulovic et al. [58] | 950 | 67.2% | L-Pinocarveol | 0.097 ± 0.007 | 0.030 ± 0.005 |
| 11.65 | 1140 | 1114 | Muselli et al. [59] | 883 | 68.6% | Pinocarvone | 0.016 ± 0.003 | nd |
| 11.76 | 1170 | 1166 | Al-Omar [60] | 923 | 63.9% | Borneol | 0.0081 ± 0.001 | nd |
| 12.54 | 1172 | 1143 | Boti et al. [61] | 952 | 74.5% | α-Terpineol | 0.0894 ± 0.04 | 0.0233 ± 0.003 |
| 23.04 | 1567 | 1530 | Babushok et al. [62] | 929 | 55.5% | Globulol | 0.011 ± 0.002 | 0.0012 ± 0.001 |
| 33.78 | 2099 | 2045 | Babushok et al. [62] | 894 | 81.2% | Phytol | 0.1714 ± 0.03 | 0.0637 ± 0.01 |
| 45.04 | 2847 | 2914 | Zhao et al. [63] | 963 | 50.4% | Squalene | 0.1041 ± 0.01 | 0.0044 ± 0.001 |
| 53.66 | - | 2886 | - | 886 | 59.9% | Unknown 1 | 0.0957 ± 0.02 | 0.0364 ± 0.006 |
| 54.94 | - | 2848 | - | 941 | 86.5% | Unknow 2 | 0.8505 ± 0.05 | 0.2187 ± 0.03 |
| Compounds | Extract (µg/mL) | Phytosomes (µg/mL) |
|---|---|---|
| gallic acid | 6.94 ± 0.06 | 5.93 ± 0.0 |
| 4-hydroxybenzoic acid | nd | nd |
| chlorogenic acid | nd | nd |
| caffeic acid | nd | nd |
| Vanillic acid | nd | nd |
| Syringic acid | nd | nd |
| p-coumaric acid | 0.47 ± 0.05 | 0.65 ± 0.07 |
| ferulic acid | nd | nd |
| synaptic acid | nd | nd |
| ellagic acid | 74.3 ± 1.3 | 67.3 ± 1.4 |
| t-cinnamic acid | nd | nd |
| quercetin | nd | nd |
| rutin | nd | nd |
| Measurements | Control | Control + Vehicle | Control + C. citrinus Extract (200 mg/kg) | Hypercaloric-fat Diet (HFD) | HFD + Orlistat (5 mg/kg) | HFD + C. citrinus Extract (250 mg/kg) | HFD + Phytosomes (50 mg/kg) | HFD + Phytosomes (100 mg/kg) | HFD + Phytosomes (200 mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Morphometric parameters | |||||||||
| Abdominal circumference (cm) | 20.50 ± 0.45 a | 20.50 ± 45 a | 21.00 ± 0.45 a | 25.50 ± 0.45 b | 22.25 ± 0.45 a | 20.33 ± 1.36 a | 21.0 ± 0.52 a | 21.20 ± 20 a | 21.50 ± 0.45 a |
| Nose-to-anus length (cm) | 25.25 ± 0.60 a | 24.37 ± 0.60 a | 24.60 ± 0.54 a | 24.41 ± 0.91 a | 23.66 ± 0.91 a | 24.41 ± 0.91 a | 23.80 ± 0.54 a | 24.12 ± 0.60 a | 23.50 ± 0.60 a |
| Nose-to-tail length (cm) | 46.40 ± 0.42 a | 46.40 ± 0.42 a | 46.87 ± 0.47 a | 45.66 ± 0.38 a | 45.71 ± 0.47 a | 44.66 ± 1.63 a | 45.87 ± 0.47 a | 45.75 ± 0.47 a | 45.12 ± 0.47 a |
| Markers of obesity | |||||||||
| BMI (kg/m2) | 0.67 ± 0.03 b | 0.72 ± 0.03 ab | 0.70 ± 0.03 ab | 0.88 ± 0.04 a | 0.72 ± 0.04 ab | 0.69 ± 0.09 b | 0.66 ± 0.04 b | 0.68 ± 0.04 b | 0.76 ± 0.04 ab |
| Adiposity index | 2.78 ± 0.55 c | 2.77 ± 0.55 c | 2.52 ± 0.62 c | 9.43 ± 0.62 a | 5.56 ± 0.71 bc | 6.18 ± 0.39 b | 5.98 ± 0.71 b | 4.82 ± 0.71 bc | 4.02 ± 0.62 bc |
| Lee index | 0.30 ± 0.01 | 0.30 ± 0.02 | 0.30 ± 0.01 | 0.33 ± 0.01 a | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.31 ± 0.01 |
| Biochemical parameters | |||||||||
| Triacylglycerol (mg/dL) | 90.66 ± 11.64 c | 103.66 ± 11.64 c | 109.66 ± 11.64 c | 202.66 ± 11.64 a | 90.66 ± 11.64 c | 136.33 ± 66.96 b | 103.50 ± 11.64 c | 105.33 ± 11.64 c | 118.66 ± 11.64 b |
| Blood glucose (mg/dL) | 93.99 ± 8.24 a | 101.37 ± 8.24 a | 95.59 ± 8.24 a | 111.11 ± 8.24 b | 100.24 ± 8.24 a | 97.00 ± 4.24 a | 104.18 ± 8.24 a | 92.24 ± 8.24 a | 96.65 ± 8.24 a |
| Total cholesterol (mg/dL) | 161.33 ± 2.69 a | 160.33 ± 2.69 a | 162.00 ± 2.69 a | 159.66 ± 2.69 a | 156.66 ± 2.69 a | 162.00 ± 2.69 a | 155.66 ± 2.69 a | 161.00 ± 2.69 a | 159.30 ± 2.69 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Pérez, L.G.; Ayala-Ruiz, L.A.; Magaña-Rodríguez, O.R.; Piñón-Simental, J.S.; Aguilera-Méndez, A.; Godínez-Hernández, D.; Rios-Chavez, P. Development and Evaluation of Phytosomes Containing Callistemon citrinus Leaf Extract: A Preclinical Approach for the Treatment of Obesity in a Rodent Model. Pharmaceutics 2023, 15, 2178. https://doi.org/10.3390/pharmaceutics15092178
Ortega-Pérez LG, Ayala-Ruiz LA, Magaña-Rodríguez OR, Piñón-Simental JS, Aguilera-Méndez A, Godínez-Hernández D, Rios-Chavez P. Development and Evaluation of Phytosomes Containing Callistemon citrinus Leaf Extract: A Preclinical Approach for the Treatment of Obesity in a Rodent Model. Pharmaceutics. 2023; 15(9):2178. https://doi.org/10.3390/pharmaceutics15092178
Chicago/Turabian StyleOrtega-Pérez, Luis Gerardo, Luis Alberto Ayala-Ruiz, Oliver Rafid Magaña-Rodríguez, Jonathan Saúl Piñón-Simental, Asdrubal Aguilera-Méndez, Daniel Godínez-Hernández, and Patricia Rios-Chavez. 2023. "Development and Evaluation of Phytosomes Containing Callistemon citrinus Leaf Extract: A Preclinical Approach for the Treatment of Obesity in a Rodent Model" Pharmaceutics 15, no. 9: 2178. https://doi.org/10.3390/pharmaceutics15092178
APA StyleOrtega-Pérez, L. G., Ayala-Ruiz, L. A., Magaña-Rodríguez, O. R., Piñón-Simental, J. S., Aguilera-Méndez, A., Godínez-Hernández, D., & Rios-Chavez, P. (2023). Development and Evaluation of Phytosomes Containing Callistemon citrinus Leaf Extract: A Preclinical Approach for the Treatment of Obesity in a Rodent Model. Pharmaceutics, 15(9), 2178. https://doi.org/10.3390/pharmaceutics15092178






