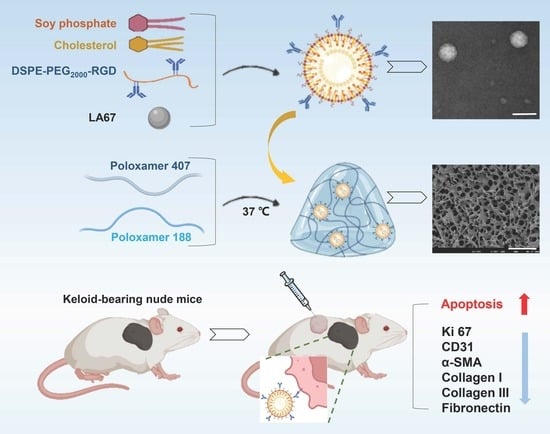
LA67 Liposome-Loaded Thermo-Sensitive Hydrogel with Active Targeting for Efficient Treatment of Keloid via Peritumoral Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Characterization of DSPE-PEG2000-RGD
2.3. Preparation of RGD-Modified LNP Loaded with LA67 (LA67-RL)
2.4. Characterization of LA67-RL
2.5. Preparation and Evaluation of Thermo-Sensitive Hydrogel
2.5.1. Preparation of Thermo-Sensitive Hydrogel
2.5.2. Determination of Gelation Time
2.5.3. Box–Behnken Design (BBD)
2.5.4. Preparation of LA67-RL-Gel
2.5.5. Surface Morphology of Hydrogels
2.5.6. Rheological Properties
2.6. Storage Stability
2.7. In Vitro Release
2.8. Cell Research
2.8.1. Cells Viability Assay
2.8.2. Cellular Uptake
2.8.3. Uptake Mechanism Study
2.8.4. Cell Apoptosis Assay
2.8.5. Cell Migration
2.9. In Vivo Studies
2.9.1. Establishment of Keloid Model
2.9.2. In Vivo Retention Studies
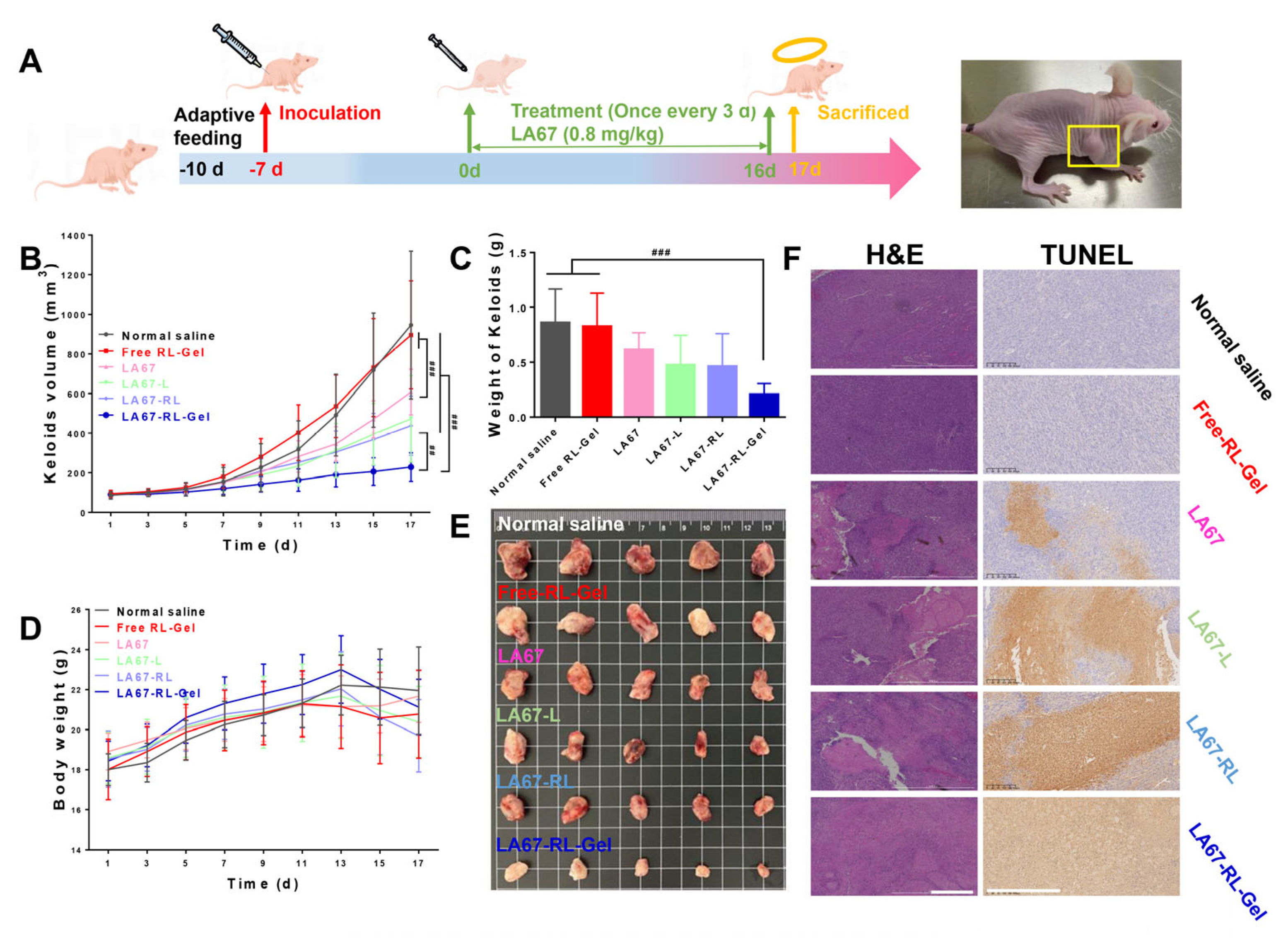
2.9.3. In Vivo Anti-Keloid Efficacy
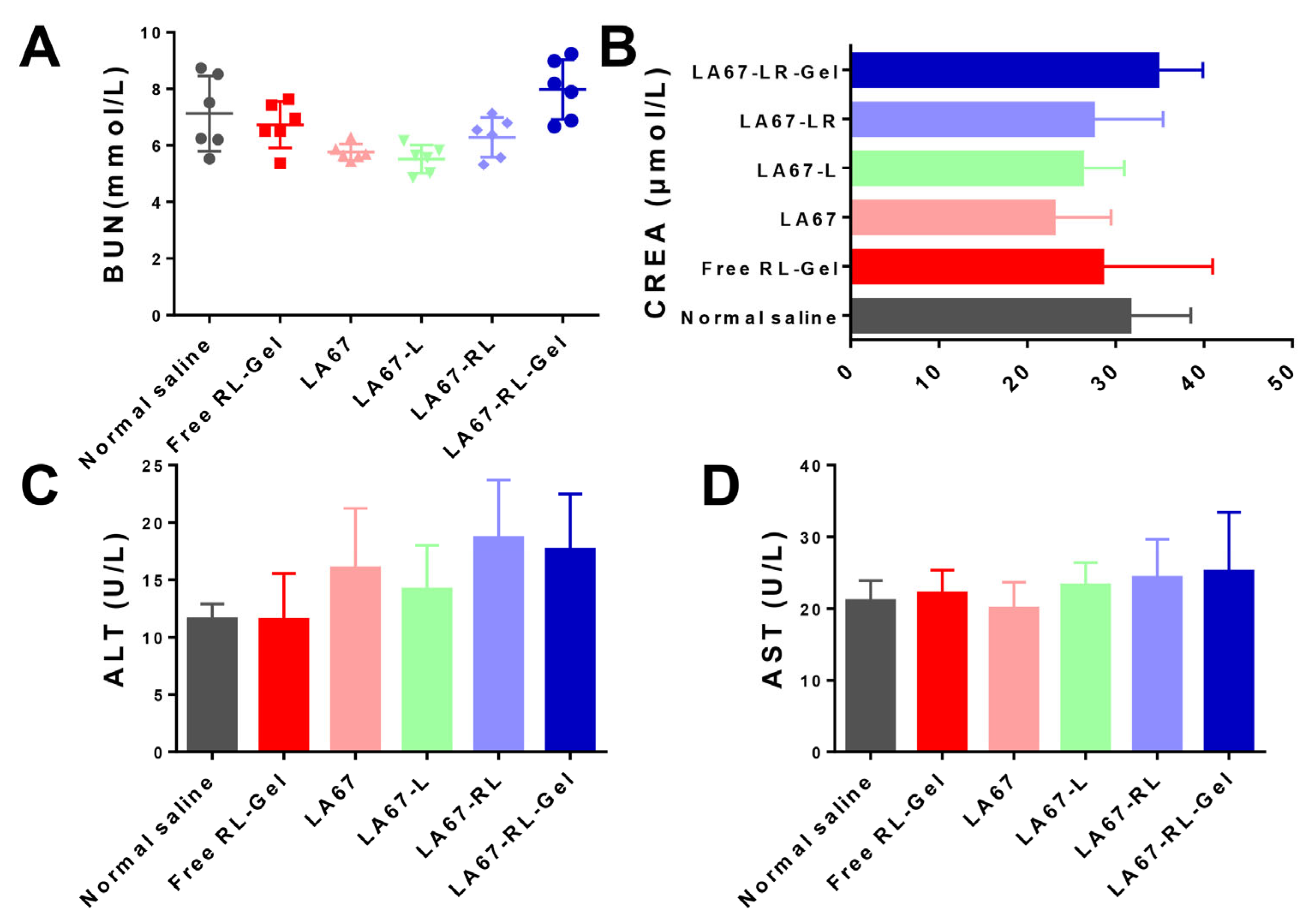
2.9.4. In Vivo Safety
2.10. Statistical Analysis
3. Results
3.1. Characterization of DSPE-PEG2000-RGD
3.2. Characterization of LA67-RL
3.2.1. Particle Size and Zeta Potential
3.2.2. Encapsulation Efficiency (EE)
3.3. Preparation and Characterization of Hydrogel
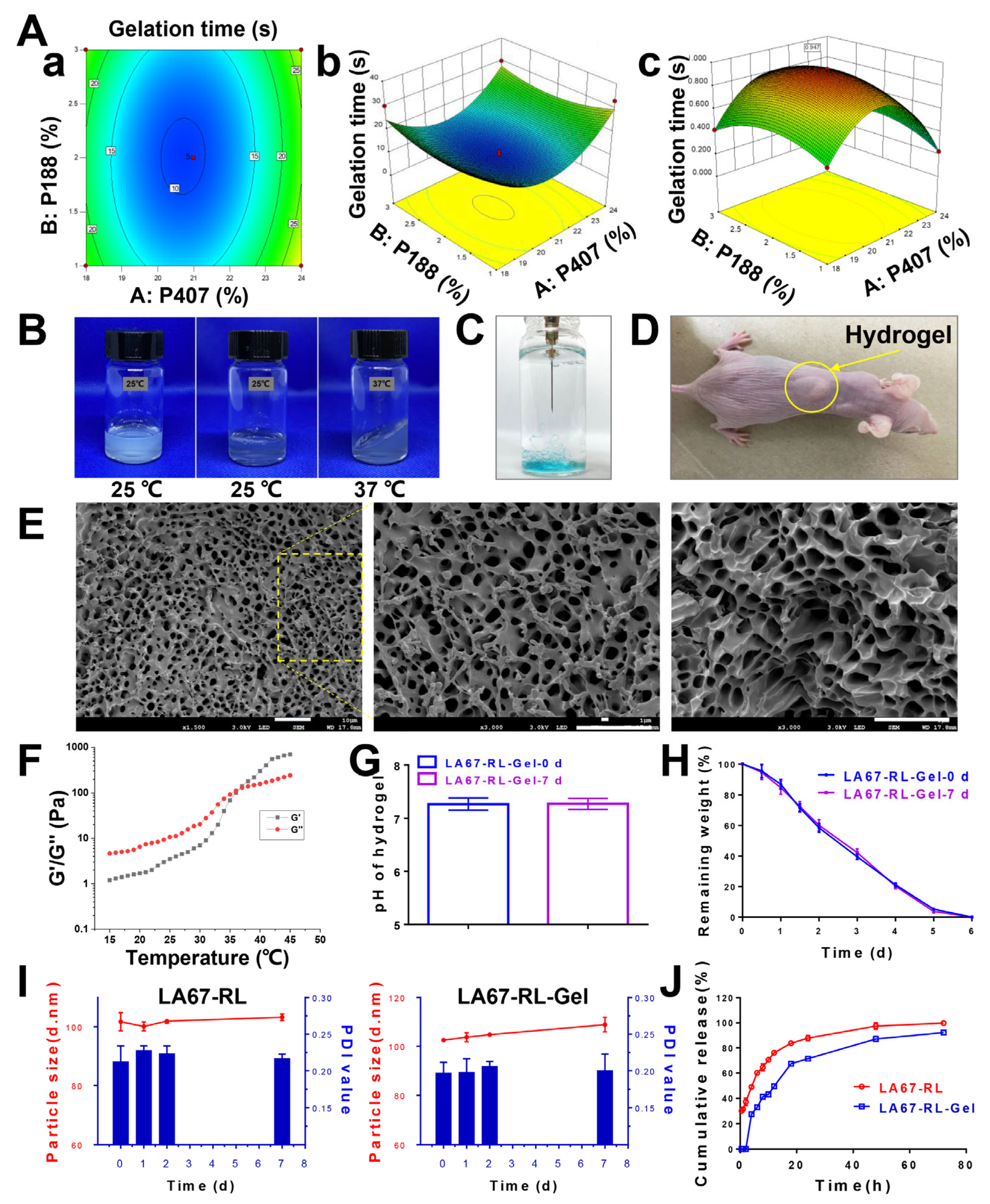
3.3.1. Box–Behnken Design (BBD)
3.3.2. Feasibility Check
3.3.3. Properties of Thermo-Sensitive Hydrogel
3.4. Stability
3.5. In Vitro Release
3.6. Cell Researches
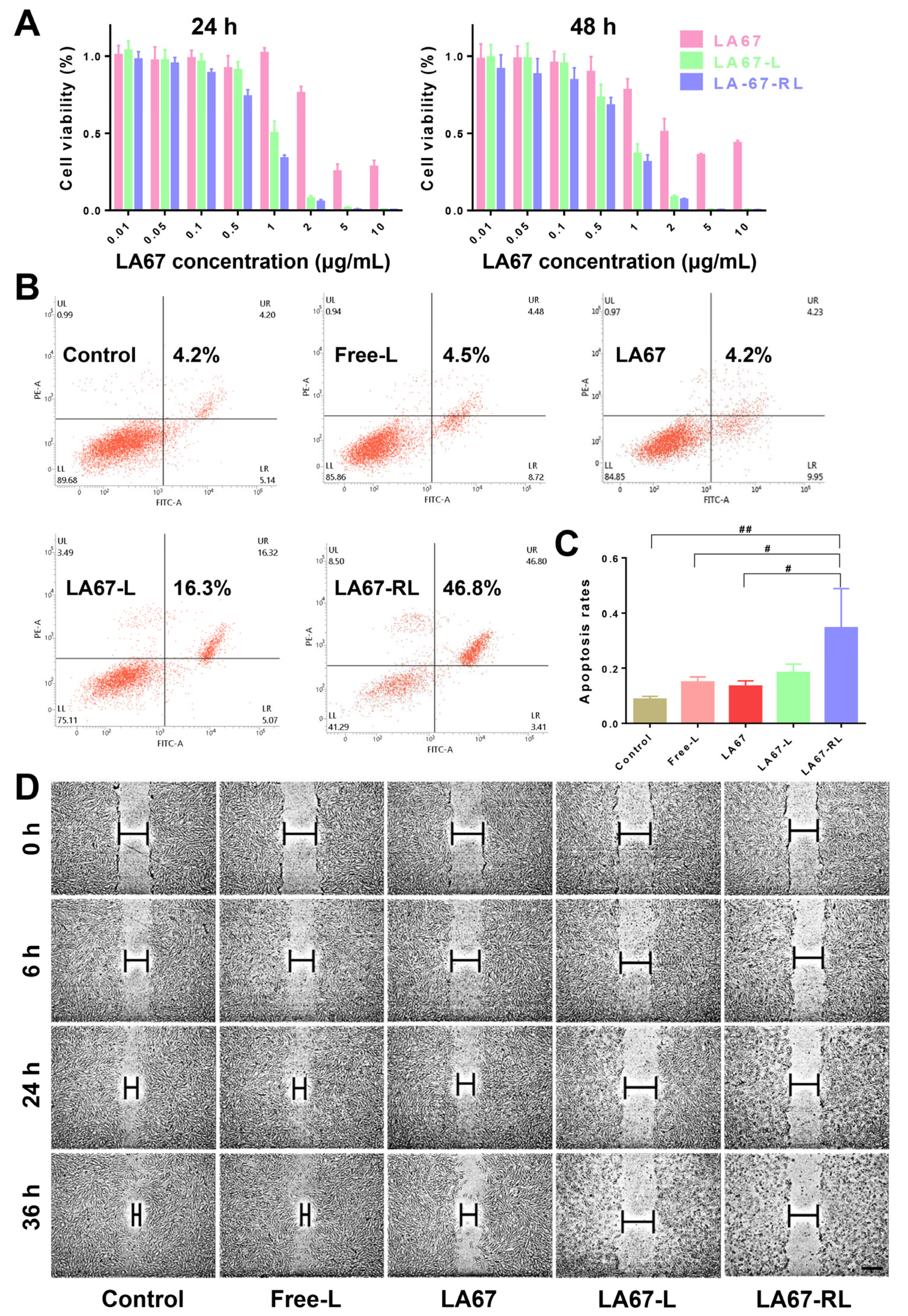
3.6.1. Cytotoxicity
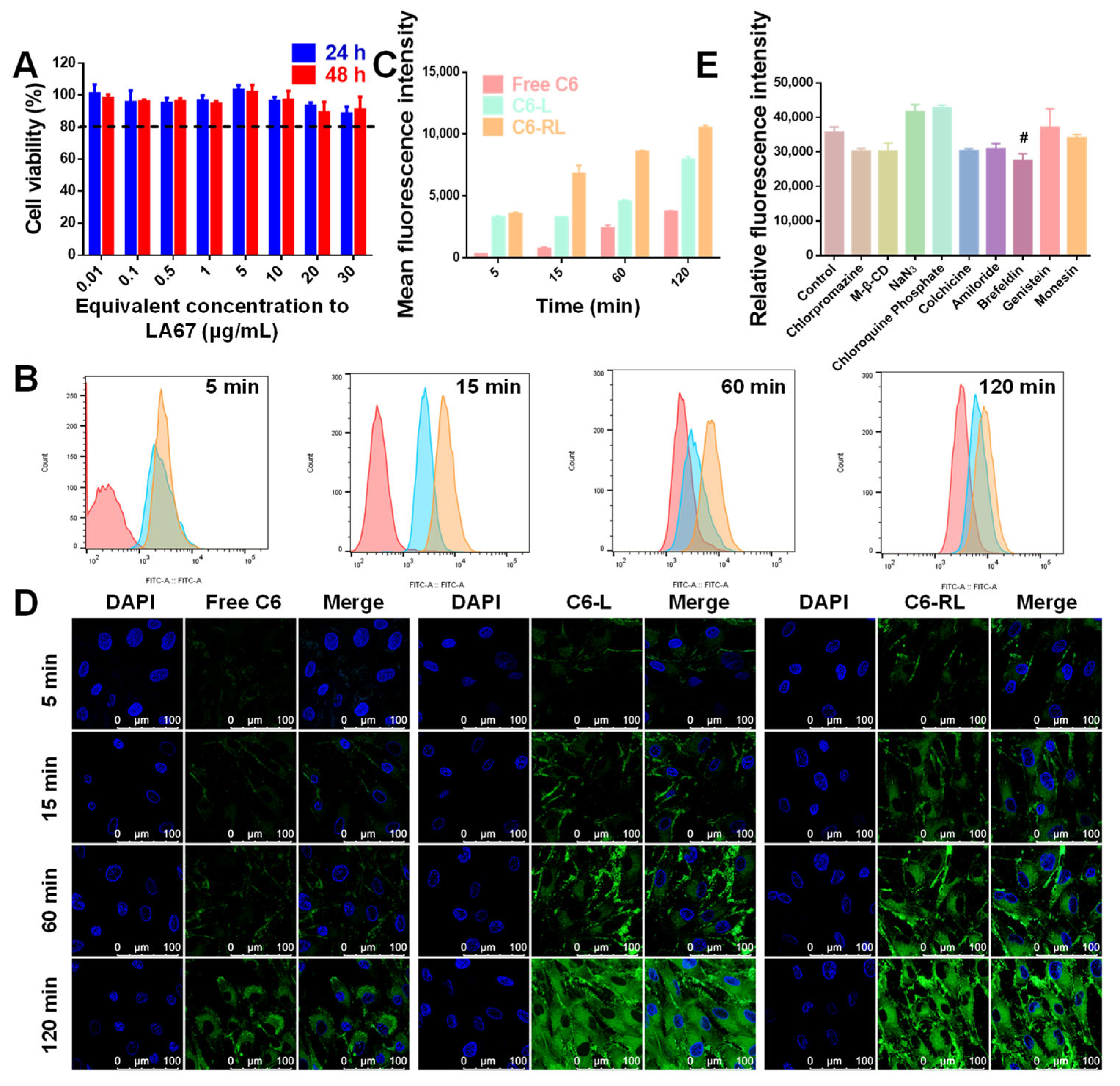
3.6.2. Cellular Uptake
3.6.3. Uptake Mechanism
3.6.4. Cell Viability
3.6.5. Cell Apoptosis
3.6.6. Cell Migration
3.7. In Vivo Studies
3.7.1. In Vivo Retention
3.7.2. Biodistribution
3.7.3. In Vivo Anti-Keloid Effect
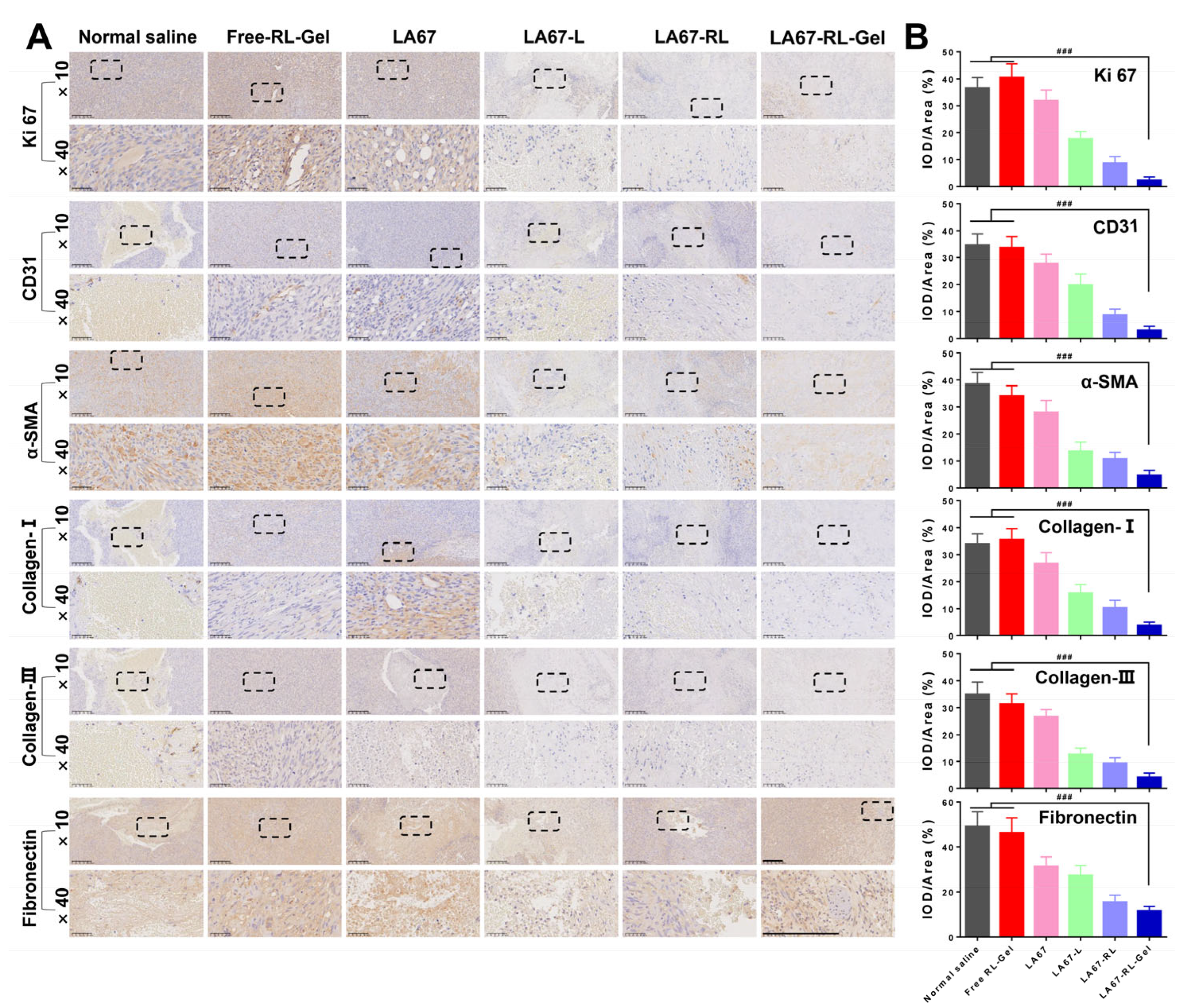
3.7.4. Anti-Keloid Mechanism
3.7.5. Biocompatibility and Tissue Cytotoxicity In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Direder, M.; Weiss, T.; Copic, D.; Vorstandlechner, V.; Laggner, M.; Pfisterer, K.; Mildner, C.S.; Klas, K.; Bormann, D.; Haslik, W.; et al. Schwann Cells Contribute to Keloid Formation. Matrix Biol. 2022, 108, 55–76. [Google Scholar] [CrossRef]
- Naik, P.P. Novel Targets and Therapies for Keloid. Clin. Exp. Dermatol. 2022, 47, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Kamalasanan, K. Drug Delivery to Optimize Angiogenesis Imbalance in Keloid: A Review. J. Control. Release 2021, 329, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yan, G.; Li, L.; Wang, D.; Wang, Y.; Jin, S.; Jin, Z.; Li, L.; Zhu, L. RUNX3 Mediates Keloid Fibroblast Proliferation through Deacetylation of EZH2 by SIRT1. BMC Mol. Cell Biol. 2022, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, F.; Wang, P.; Jin, X.; Liu, R. Natural-Product-Mediated Autophagy in the Treatment of Various Liver Diseases. Int. J. Mol. Sci. 2022, 23, 15109. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Qian, H.; Tao, W.; Zhang, Y.; Hu, C.; Mao, W.; Guo, Q. Natural Medicines of Targeted Rheumatoid Arthritis and Its Action Mechanism. Front. Immunol. 2022, 13, 945129. [Google Scholar] [CrossRef]
- Cai, J.; Yi, M.; Tan, Y.; Li, X.; Li, G.; Zeng, Z.; Xiong, W.; Xiang, B. Natural Product Triptolide Induces GSDME-Mediated Pyroptosis in Head and Neck Cancer through Suppressing Mitochondrial Hexokinase-II. J. Exp. Clin. Cancer Res. 2021, 40, 190. [Google Scholar] [CrossRef]
- Aliabadi, N.; Jamalidoust, M.; Pouladfar, G.; Ziyaeyan, A.; Ziyaeyan, M. Antiviral Activity of Triptolide on Herpes Simplex Virus in Vitro. Immun. Inflamm. Dis. 2022, 10, e667. [Google Scholar] [CrossRef]
- Lai, K.; Li, Y.; Li, L.; Gong, Y.; Huang, C.; Zhang, Y.; Cheng, L.; Xu, F.; Zhao, H.; Li, C.; et al. Intravitreal Injection of Triptolide Attenuates Subretinal Fibrosis in Laser-Induced Murine Model. Phytomedicine 2021, 93, 153747. [Google Scholar] [CrossRef]
- Huang, R.; Guo, F.; Li, Y.; Liang, Y.; Li, G.; Fu, P.; Ma, L. Activation of AMPK by Triptolide Alleviates Nonalcoholic Fatty Liver Disease by Improving Hepatic Lipid Metabolism, Inflammation and Fibrosis. Phytomedicine 2021, 92, 153739. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, Q.; Wang, Y.; Zhang, H.; Liu, X.; Zhou, H.; Yang, T. The Molecular Pathogenesis of Triptolide-Induced Hepatotoxicity. Front. Pharmacol. 2022, 24, 979307. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Lai, F.; Fu, J.; Li, C.; Ma, J.; Chen, C.; Liu, K.; Zhang, T.; Chen, X.; Zhang, D. Novel Nitric Oxide-Releasing Derivatives of Triptolide as Antitumor and Anti-Inflammatory Agents: Design, Synthesis, Biological Evaluation, and Nitric Oxide Release Studies. Eur. J. Med. Chem. 2020, 190, 112079. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Li, M.; Deng, Y.; Lu, A.; Lu, J. Triptolide Delivery: Nanotechnology-Based Carrier Systems to Enhance Efficacy and Limit Toxicity. Pharmacol. Res. 2021, 165, 105377. [Google Scholar] [CrossRef]
- Peng, Z.H.; Jogdeo, C.M.; Li, J.; Xie, Y.; Wang, Y.; Sheinin, Y.M.; Kopeček, J.; Oupický, D. Tumor Microenvironment-Responsive Polymeric IRGD and Doxorubicin Conjugates Reduce Spontaneous Lung Metastasis in an Orthotopic Breast Cancer Model. Pharmaceutics 2022, 14, 1725. [Google Scholar] [CrossRef]
- Kemker, I.; Feiner, R.C.; Müller, K.M.; Sewald, N. Size-Dependent Cellular Uptake of RGD Peptides. ChemBioChem 2020, 21, 496–499. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, X.; Zhou, B.; Wang, G.; Guan, X.; Xu, Y.; Zhang, J.; Hong, Z.; Cao, J.; Sun, X.; et al. Tumor Acidic Microenvironment-Induced Drug Release of RGD Peptide Nanoparticles for Cellular Uptake and Cancer Therapy. Colloids Surf. B Biointerfaces 2021, 202, 111673. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, S.; Dou, H.; Liu, Q.; Shu, G.; Lin, J.; Zhang, W.; Peng, G.; Zhong, Z.; Fu, H. Novel Glucosamine-Loaded Thermosensitive Hydrogels Based on Poloxamers for Osteoarthritis Therapy by Intra-Articular Injection. Mater. Sci. Eng. C 2021, 118, 111352. [Google Scholar] [CrossRef]
- Li, Y.; Liu, G.; Wang, M.; Zhang, Y.; You, S.; Zhang, J.; Guo, G.; Han, B.; Li, L.; Zhao, N. The Controlled Release and Prevention of Abdominal Adhesion of Tannic Acid and Mitomycin C-Loaded Thermosensitive Gel. Polymers 2023, 15, 975. [Google Scholar] [CrossRef]
- Hirun, N.; Kraisit, P.; Tantishaiyakul, V. Thermosensitive Polymer Blend Composed of Poloxamer 407, Poloxamer 188 and Polycarbophil for the Use as Mucoadhesive In Situ Gel. Polymers 2022, 14, 1836. [Google Scholar] [CrossRef]
- Tan, M.S.A.; Pandey, P.; Falconer, J.R.; Siskind, D.J.; Balmanno, A.; Parekh, H.S. Clozapine-Encapsulated Binary Mixed Micelles in Thermosensitive Sol—Gels for Intranasal Administration. Gels 2022, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Ottonelli, I.; Bighinati, A.; Adani, E.; Loll, F.; Caraffi, R.; Vandelli, M.A.; Boury, F.; Tosi, G.; Duskey, J.T.; Marigo, V.; et al. Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles. Pharmaceutics 2023, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Postolović, K.S.; Antonijević, M.D.; Ljujić, B.; Kovačević, M.M.; Janković, M.G.; Stanić, Z.D. PH-Responsive Hydrogel Beads Based on Alginate, κ-Carrageenan and Poloxamer for Enhanced Curcumin, Natural Bioactive Compound, Encapsulation and Controlled Release Efficiency. Molecules 2022, 27, 4045. [Google Scholar] [CrossRef] [PubMed]
- Shawky, S.; Makled, S.; Awaad, A.; Boraie, N. Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive in Situ Gel—A Novel Intravesical Therapy Tackling Bladder Cancer. Pharmaceutics 2022, 14, 2527. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Kaleem, M.; Dalhat, M.H. Thermosensitive Hydrogels Loaded with Resveratrol Nanoemulsion: Formulation Optimization by Central Composite Design and Evaluation in MCF-7 Human Breast Cancer Cell Lines. Gels 2022, 8, 450. [Google Scholar] [CrossRef]
- Su, R.; Li, P.; Zhang, Y.; Lv, Y.; Wen, F.; Su, W. Polydopamine/Tannic Acid/Chitosan/Poloxamer 407/188 Thermosensitive Hydrogel for Antibacterial and Wound Healing. Carbohydr. Polym. 2023, 302, 120349. [Google Scholar] [CrossRef]
- Gupta, I.; Adin, S.N.; Rashid, M.A.; Alhamhoom, Y.; Aqil, M.; Mujeeb, M. Linalool-Incorporated Synergistically Engineered Modified Liposomal Nanocarriers for Enhanced Transungual Delivery of Terbinafine against Onychomycosis. Materials 2023, 16, 4424. [Google Scholar] [CrossRef]
- Wang, S.; Meng, S.; Zhou, X.; Gao, Z.; Piao, M.G. PH-Responsive and Mucoadhesive Nanoparticles for Enhanced Oral Insulin Delivery: The Effect of Hyaluronic Acid with Different Molecular Weights. Pharmaceutics 2023, 15, 820. [Google Scholar] [CrossRef]
- Nagy, A.; Hollingsworth, J.A.; Hu, B.; Steinbrück, A.; Stark, P.C.; Rios Valdez, C.; Vuyisich, M.; Stewart, M.H.; Atha, D.H.; Nelson, B.C.; et al. Functionalization-Dependent Induction of Cellular Survival Pathways by CdSe Quantum Dots in Primary Normal Human Bronchial Epithelial Cells. ACS Nano 2013, 7, 8397–8411. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, H.; Shu, L.; Zhang, Y.; Okeke, C.; Zhang, L.; Li, J.; Li, N. Preparation and Evaluation of Baicalin-Loaded Cationic Solid Lipid Nanoparticles Conjugated with OX26 for Improved Delivery across the BBB. Drug Dev. Ind. Pharm. 2015, 41, 353–361. [Google Scholar] [CrossRef]
- Sheng, F.; Luo, Y.; Wu, D.; Yuan, J.; Zheng, G.; Zheng, Y.; Jin, Z. Poly Amino Acid Thermosensitive Hydrogel Loaded with ICG-001 for Inhibiting Keloid by down-Regulating the Wnt/β-Catenin Pathway. Mater. Des. 2022, 222, 111050. [Google Scholar] [CrossRef]
- Hou, W.; Liu, B.; Xu, H. Triptolide: Medicinal Chemistry, Chemical Biology and Clinical Progress. Eur. J. Med. Chem. 2019, 176, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Lv, Z.; Yang, Q.; Chang, R.; Wu, J. Research Progress on Stimulus-Responsive Polymer Nanocarriers for Cancer Treatment. Pharmaceutics 2023, 15, 1928. [Google Scholar] [CrossRef] [PubMed]
- Nik, M.E.; Jaafari, M.R.; Mashreghi, M.; Nikoofal-Sahlabadi, S.; Amin, M.; Sadeghnia, H.R.; Iranshahi, M.; Navashenaq, J.G.; Malaekeh-Nikouei, B. The Effect of RGD-Targeted and Non-Targeted Liposomal Galbanic Acid on the Therapeutic Efficacy of Pegylated Liposomal Doxorubicin: From Liposomal Preparation to in-Vivo Studies. Int. J. Pharm. 2021, 604, 120710. [Google Scholar] [CrossRef]
- Cossu, J.; Thoreau, F.; Boturyn, D. Multimeric RGD-Based Strategies for Selective Drug Delivery to Tumor Tissues. Pharmaceutics 2023, 15, 525. [Google Scholar] [CrossRef]
- Beba, L.; Baloglu, E.; Suer, K.; Guler, E.; Burgaz, E.V. Development and Optimization of In-Situ Gels for Vaginal Delivery of Metronidazole and Curcumin via Box-Behnken Design: In Vitro Characterization and Anti-Trichomonas Activity. J. Drug Deliv. Sci. Technol. 2023, 86, 104739. [Google Scholar] [CrossRef]
- Budden, T.; Gaudy-Marqueste, C.; Porter, A.; Kay, E.; Gurung, S.; Earnshaw, C.H.; Roeck, K.; Craig, S.; Traves, V.; Krutmann, J.; et al. Ultraviolet Light-Induced Collagen Degradation Inhibits Melanoma Invasion. Nat. Commun. 2021, 12, 2742. [Google Scholar] [CrossRef]



| Independent Variables | Range | ||
|---|---|---|---|
| Minimum | Maximum | ||
| X1 | P407 (%) | 18 | 24 |
| X2 | P188 (%) | 1 | 3 |
| Responses | Target | ||
| Y | Gelation time (s) | Minimum | |
| Independent Variables | Response Variable | ||
|---|---|---|---|
| Run | X1 (%) | X2 (%) | Y (S) |
| 1 | 25.3 | 2.0 | 36.0 ± 3.2 |
| 2 | 21.0 | 2.0 | 11.0 ± 2.1 |
| 3 | 21.0 | 2.0 | 9.1 ± 1.2 |
| 4 | 16.8 | 2.0 | 23.9 ± 2.1 |
| 5 | 21.0 | 3.4 | 14.1 ± 2.3 |
| 6 | 24.0 | 3.0 | 29.9 ± 3.3 |
| 7 | 21.0 | 2.0 | 8.1 ± 1.1 |
| 8 | 24.0 | 1.0 | 32.9 ± 4.3 |
| 9 | 21.0 | 2.0 | 10.3 ± 2.0 |
| 10 | 18.0 | 3.0 | 29.8 ± 3.4 |
| 11 | 21.0 | 2.0 | 10.2 ± 1.0 |
| 12 | 18.0 | 1.0 | 30.3 ± 3.2 |
| 13 | 21.0 | 0.6 | 13.0 ± 2.1 |
| Source | Sum of Squares | df | Mean Squares | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1177.7 | 5 | 235.5 | 8.5 | 0.0069 significant |
| A-P407 | 49.9 | 1 | 49.9 | 1.8 | 0.2218 |
| B-P188 | 0.3 | 1 | 0.3 | 0 | 0.9182 |
| X1X2 | 2.3 | 1 | 2.3 | 0.1 | 0.7840 |
| X1 | 1078.3 | 1 | 1078.3 | 38.9 | 0.0004 |
| X2 | 122.7 | 1 | 122.7 | 4.4 | 0.0734 |
| Residual | 194.0 | 7 | 27.7 | ||
| Lack of Fit | 188.8 | 3 | 62.9 | 48.4 | 0.0013 |
| Pure Error | 5.2 | 4 | 1.3 | ||
| Cor Total | 1371.7 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, H.; Wang, S.; Li, C.; Zeng, B.; Wu, H.; Liu, C.; Chen, L.; Jin, M.; Huang, W.; Zang, Y.; et al. LA67 Liposome-Loaded Thermo-Sensitive Hydrogel with Active Targeting for Efficient Treatment of Keloid via Peritumoral Injection. Pharmaceutics 2023, 15, 2157. https://doi.org/10.3390/pharmaceutics15082157
Wan H, Wang S, Li C, Zeng B, Wu H, Liu C, Chen L, Jin M, Huang W, Zang Y, et al. LA67 Liposome-Loaded Thermo-Sensitive Hydrogel with Active Targeting for Efficient Treatment of Keloid via Peritumoral Injection. Pharmaceutics. 2023; 15(8):2157. https://doi.org/10.3390/pharmaceutics15082157
Chicago/Turabian StyleWan, Hongshuang, Shuangqing Wang, Chuying Li, Bowen Zeng, Hao Wu, Chao Liu, Liqing Chen, Mingji Jin, Wei Huang, Yingda Zang, and et al. 2023. "LA67 Liposome-Loaded Thermo-Sensitive Hydrogel with Active Targeting for Efficient Treatment of Keloid via Peritumoral Injection" Pharmaceutics 15, no. 8: 2157. https://doi.org/10.3390/pharmaceutics15082157
APA StyleWan, H., Wang, S., Li, C., Zeng, B., Wu, H., Liu, C., Chen, L., Jin, M., Huang, W., Zang, Y., Zhang, D., Gao, Z., & Jin, Z. (2023). LA67 Liposome-Loaded Thermo-Sensitive Hydrogel with Active Targeting for Efficient Treatment of Keloid via Peritumoral Injection. Pharmaceutics, 15(8), 2157. https://doi.org/10.3390/pharmaceutics15082157