Piezodynamic Eradication of Both Gram-Positive and Gram-Negative Bacteria by Using a Nanoparticle Embedded Polymeric Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Synthesis of the Bismuth Ferrite NPs
2.4. Preparation of the PVDF-HFP@BFO Composite
2.5. Antibacterial Experiments
3. Results and Discussion
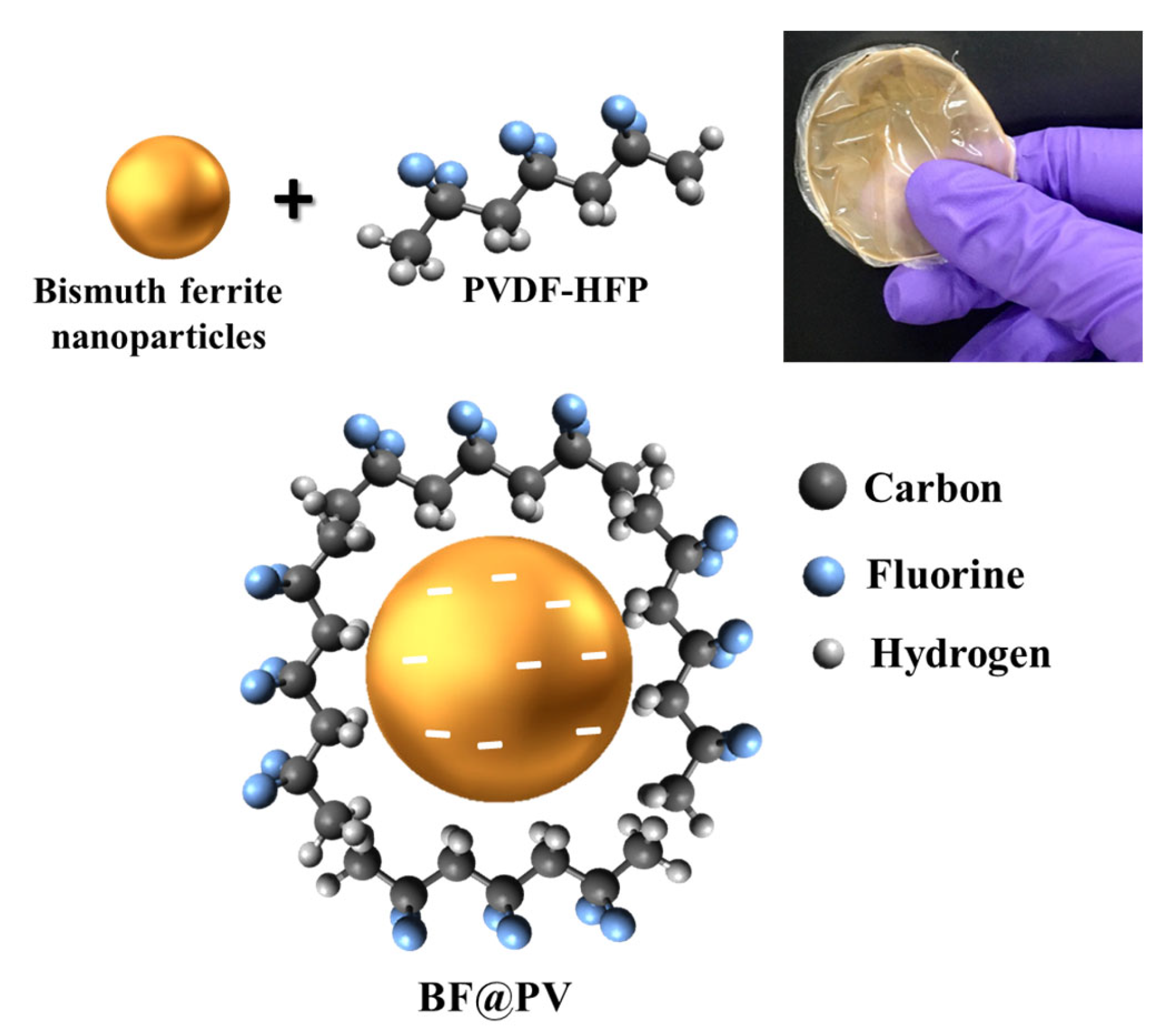
3.1. Design Rationale of the As-Prepared BF@PV Samples
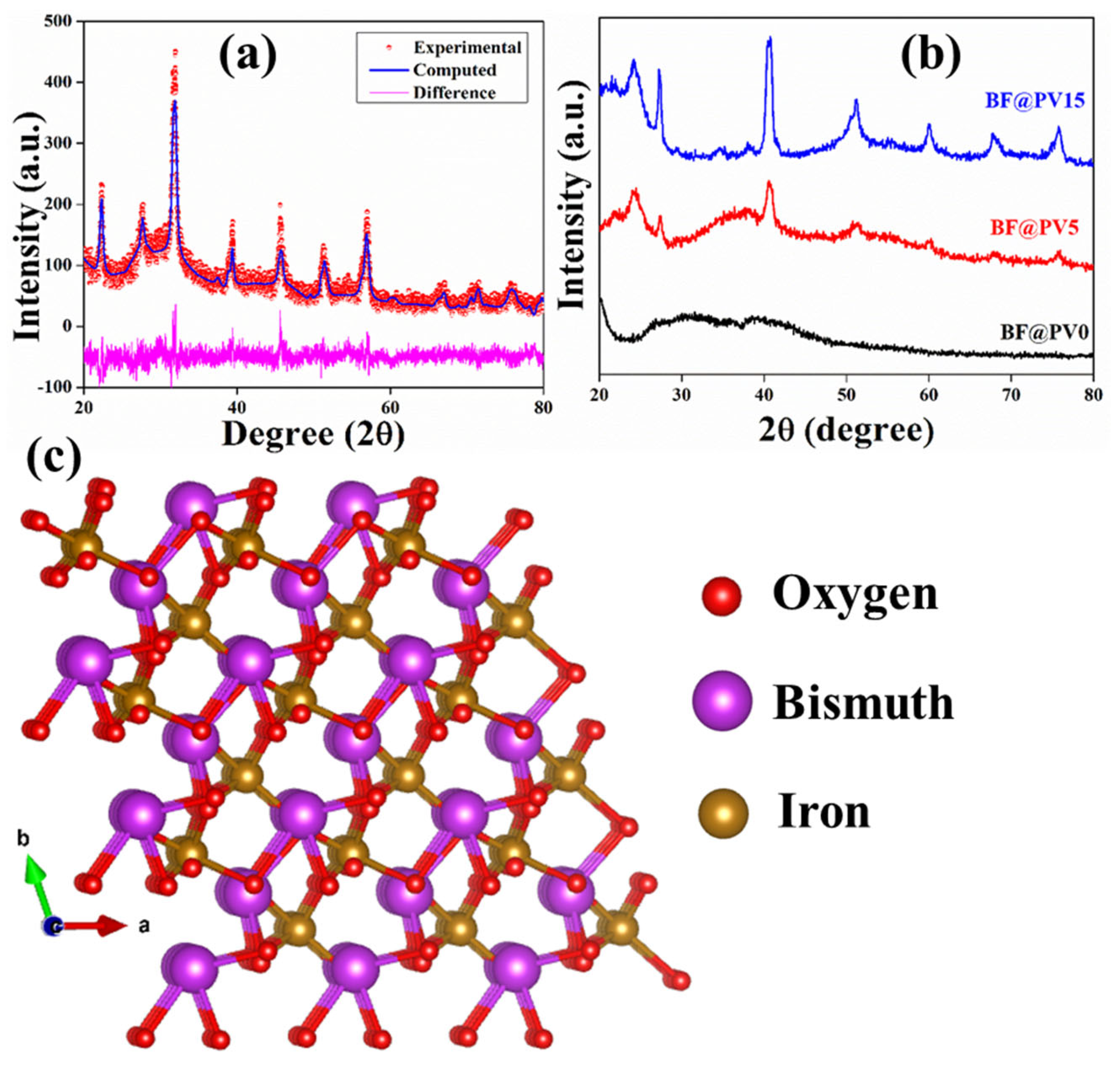
3.2. Structural Characteristics of the Synthesized BF@PV
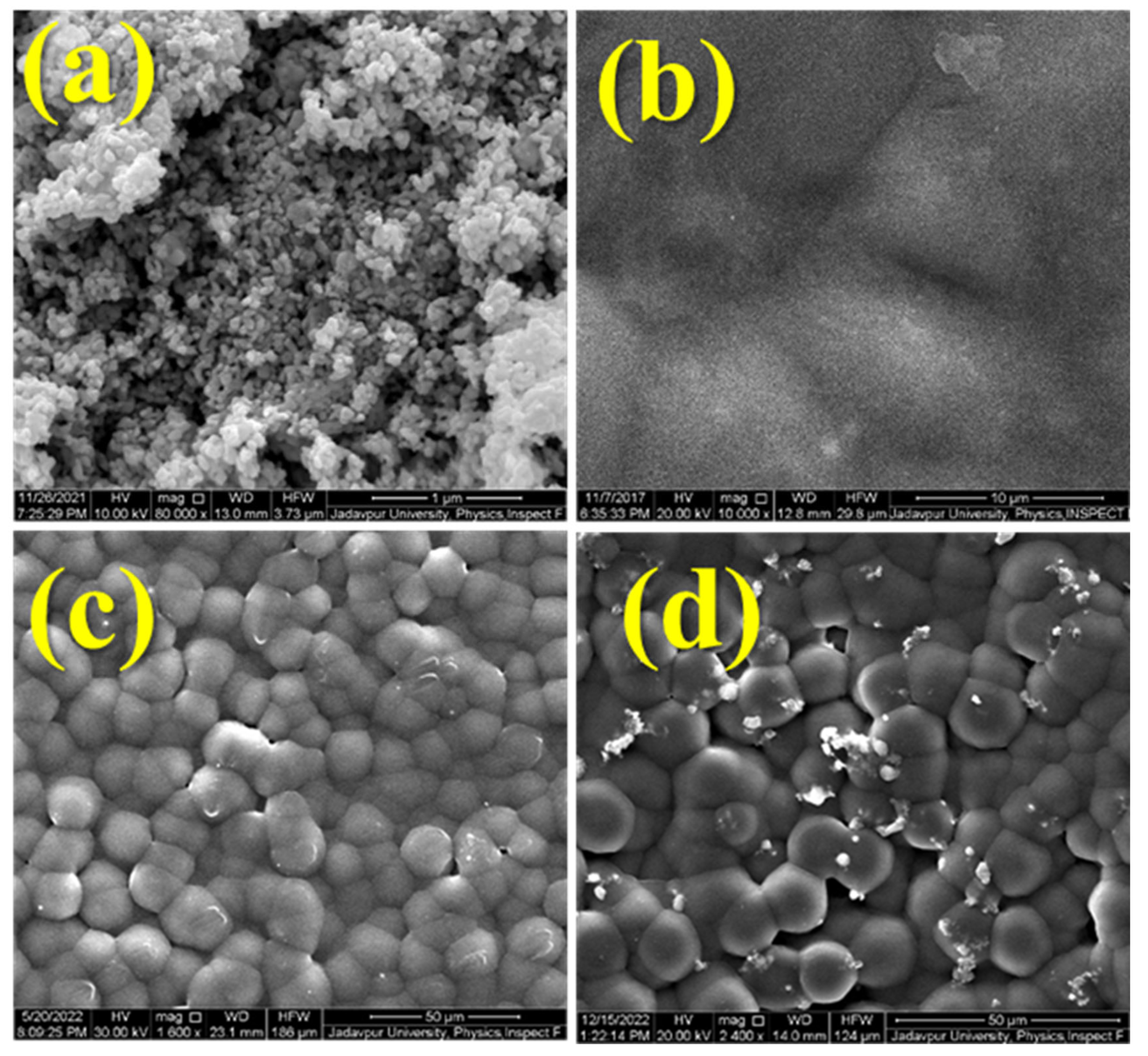
3.3. Morphological Features of the BF@PV Nanocomposites
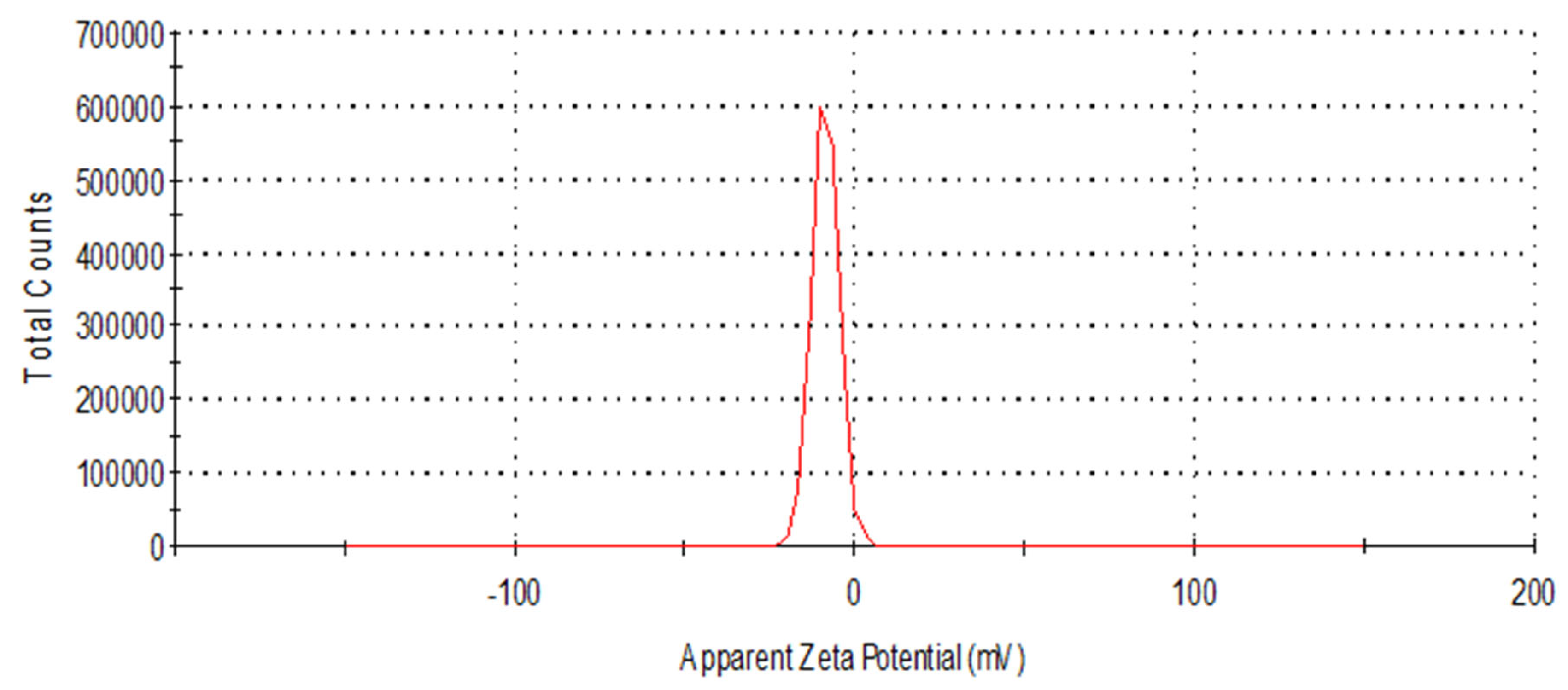
3.4. Estimation of the Degree of Polarization
3.5. Mechanism of the Piezo Response
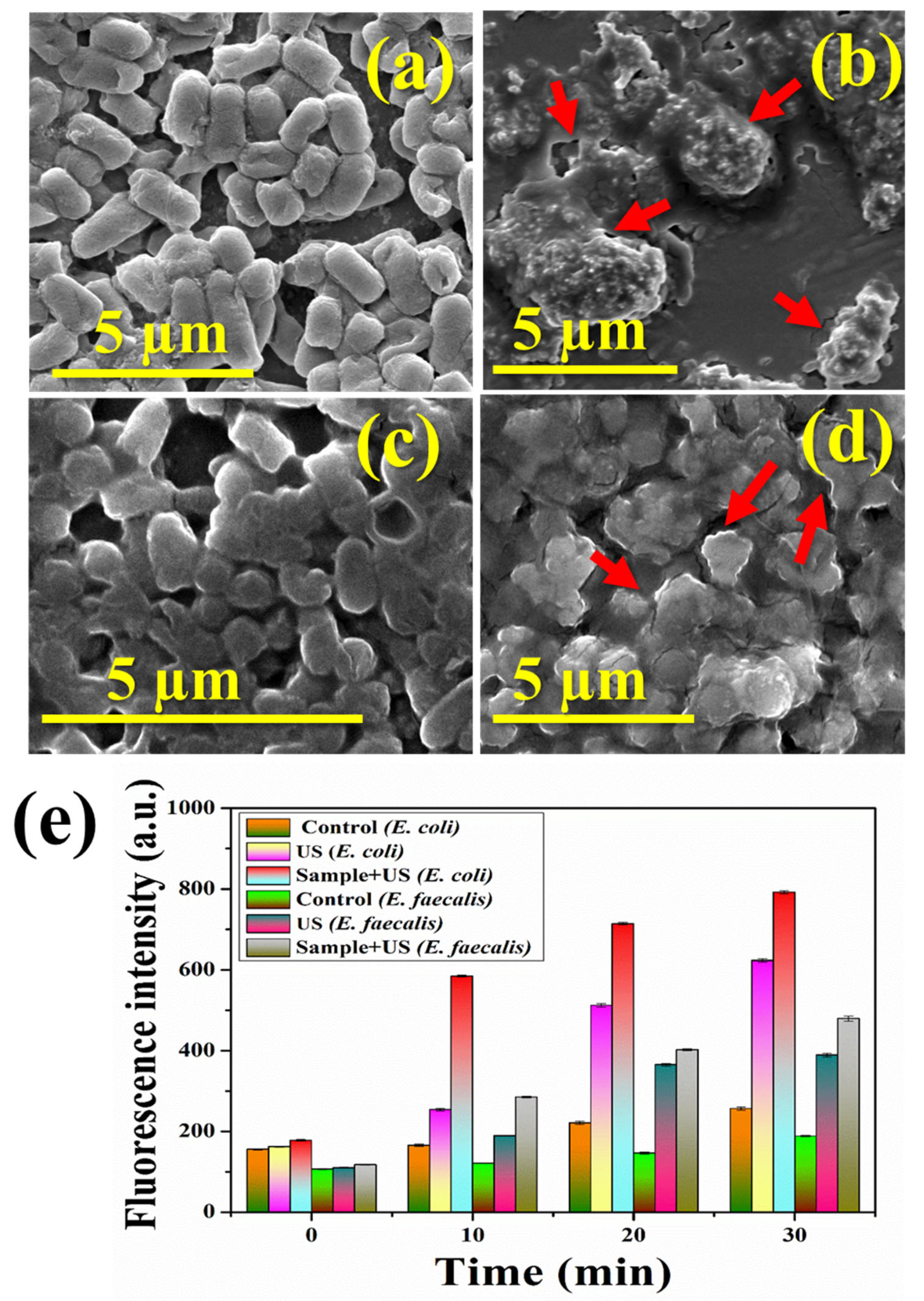
3.6. Piezodynamic Antibacterial Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bag, N.; Bardhan, S.; Roy, S.; Roy, J.; Mondal, D.; Das, S.; Guo, B. Nanoparticle Mediated Stimuli Responsive Antibacterial Therapy. Biomater. Sci. 2023, 11, 1994–2019. [Google Scholar] [CrossRef] [PubMed]
- Jajere, S.M. A Review of Salmonella Enterica with Particular Focus on the Pathogenicity and Virulence Factors, Host Specificity and Antimicrobial Resistance Including Multidrug Resistance. Vet. World 2019, 12, 504. [Google Scholar] [CrossRef]
- Tian, F.; Li, J.; Nazir, A.; Tong, Y. Bacteriophage—A Promising Alternative Measure for Bacterial Biofilm Control. Infect. Drug Resist. 2021, 14, 205. [Google Scholar] [CrossRef]
- Sieiro, C.; Areal-hermida, L.; Pichardo-gallardo, Á.; Almuiña-gonzález, R.; de Miguel, T.; Sánchez, S.; Sánchez-pérez, Á.; Villa, T.G. A Hundred Years of Bacteriophages: Can Phages Replace Antibiotics in Agriculture and Aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef] [PubMed]
- Bora, N.S.; Mazumder, B.; Pathak, M.P.; Joshi, K.; Chattopadhyay, P. Nanotechnology in Preventive and Emergency Healthcare. In Nanotechnology; CRC Press: Boca Raton, FL, USA, 2019; pp. 221–272. [Google Scholar] [CrossRef]
- Sabino, C.P.; Wainwright, M.; Ribeiro, M.S.; Sellera, F.P.; dos Anjos, C.; Baptista, M.D.S.; Lincopan, N. Global Priority Multidrug-Resistant Pathogens Do Not Resist Photodynamic Therapy. J. Photochem. Photobiol. B 2020, 208, 111893. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, D.; Hu, W.; Li, X.; Xia, Q.; Wen, T.; Jia, H. Nosocomial Infection of COVID-19: A New Challenge for Healthcare Professionals (Review). Int. J. Mol. Med. 2021, 47, 43. [Google Scholar] [CrossRef]
- Miller, K.P.; Wang, L.; Benicewicz, B.C.; Decho, A.W. Inorganic Nanoparticles Engineered to Attack Bacteria. Chem. Soc. Rev. 2015, 44, 7787–7807. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.H.; Hussain, I.; Rotello, V.M. Combatting Antibiotic-Resistant Bacteria Using Nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ali, A.; Liu, Y.; Xie, H.; Ullah, S.; Roy, S.; Song, Z.; Guo, B.; Xu, J. Advances in Image-Guided Drug Delivery for Antibacterial Therapy. Adv. Drug Deliv. Rev. 2023, 192, 114634. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Roy, S.; Bardhan, S.; Roy, J.; Kanungo, I.; Basu, R.; Das, S. Recent Advances in Piezocatalytic Polymer Nanocomposites for Wastewater Remediation. Dalton Trans. 2022, 51, 451–462. [Google Scholar] [CrossRef]
- Ghosh, S.; Bardhan, S.; Mondal, D.; Sarkar, D.; Roy, J.; Roy, S.; Basu, R.; Das, S. Natural Hematite-Based Self-Poled Piezo-Responsive Membrane for Harvesting Energy from Water Flow and Catalytic Removal of Organic Dye. Ceram. Int. 2023, 49, 14710–14718. [Google Scholar] [CrossRef]
- Sarkar, D.; Das, N.; Saikh, M.M.; Biswas, P.; Roy, S.; Paul, S.; Hoque, N.A.; Basu, R.; Das, S. High β-Crystallinity Comprising Nitrogenous Carbon Dot/PVDF Nanocomposite Decorated Self-Powered and Flexible Piezoelectric Nanogenerator for Harvesting Human Movement Mediated Energy and Sensing Weights. Ceram. Int. 2023, 49, 5466–5478. [Google Scholar] [CrossRef]
- Bagchi, B.; Hoque, N.A.; Janowicz, N.; Das, S.; Tiwari, M.K. Re-Usable Self-Poled Piezoelectric/Piezocatalytic Films with Exceptional Energy Harvesting and Water Remediation Capability. Nano Energy 2020, 78, 105339. [Google Scholar] [CrossRef] [PubMed]
- Masimukku, S.; Hu, Y.C.; Lin, Z.H.; Chan, S.W.; Chou, T.M.; Wu, J.M. High Efficient Degradation of Dye Molecules by PDMS Embedded Abundant Single-Layer Tungsten Disulfide and Their Antibacterial Performance. Nano Energy 2018, 46, 338–346. [Google Scholar] [CrossRef]
- Mondal, D.; Bardhan, S.; Das, N.; Roy, J.; Ghosh, S.; Maity, A.; Roy, S.; Basu, R.; Das, S. Natural Clay-Based Reusable Piezo-Responsive Membrane for Water Droplet Mediated Energy Harvesting, Degradation of Organic Dye and Pathogenic Bacteria. Nano Energy 2022, 104, 107893. [Google Scholar] [CrossRef]
- Kumar, S.; Haikerwal, A.; Saxena, S.K. Epidemiology of Water-Associated Infectious Diseases. In Water-Associated Infectious Diseases; Springer: Singapore, 2019; pp. 19–25. [Google Scholar] [CrossRef]
- Watcharasukarn, M.; Kaparaju, P.; Steyer, J.P.; Krogfelt, K.A.; Angelidaki, I. Screening Escherichia coli, Enterococcus faecalis, and Clostridium perfringens as Indicator Organisms in Evaluating Pathogen-Reducing Capacity in Biogas Plants. Microb. Ecol. 2009, 58, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fotadar, U.; Zaveloff, P.; Terracio, L. Growth of Escherichia coli at Elevated Temperatures. J. Basic Microbiol. 2005, 45, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.; Ramirez-Arcos, S. A Comparative Study of McFarland Turbidity Standards and the Densimat Photometer to Determine Bacterial Cell Density. Curr. Microbiol. 2015, 70, 907–909. [Google Scholar] [CrossRef]
- Roy, J.; Mukhopadhyay, L.; Bardhan, S.; Mondal, D.; Ghosh, S.; Chakraborty, S.; Bag, N.; Roy, S.; Basu, R.; Das, S. Piezo-Responsive Bismuth Ferrite Nanoparticle-Mediated Catalytic Degradation of Rhodamine B and Pathogenic E. coli in Aqueous Medium and Its Extraction Using External Magnetic Stimulation after Successful Treatment. Dalton Trans. 2022, 51, 16926–16936. [Google Scholar] [CrossRef]
- Thakur, P.; Kool, A.; Bagchi, B.; Hoque, N.A.; Das, S.; Nandy, P. Improvement of Electroactive β Phase Nucleation and Dielectric Properties of WO3·H2O Nanoparticle Loaded Poly(Vinylidene Fluoride) Thin Films. RSC Adv. 2015, 5, 62819–62827. [Google Scholar] [CrossRef]
- Dong, C.; Fu, Y.; Zang, W.; He, H.; Xing, L.; Xue, X. Self-Powering/Self-Cleaning Electronic-Skin Basing on PVDF/TiO2 Nanofibers for Actively Detecting Body Motion and Degrading Organic Pollutants. Appl. Surf. Sci. 2017, 416, 424–431. [Google Scholar] [CrossRef]
- Lin, D.J.; Chang, C.L.; Lee, C.K.; Cheng, L.P. Preparation and Characterization of Microporous PVDF/PMMA Composite Membranes by Phase Inversion in Water/DMSO Solutions. Eur. Polym. J. 2006, 42, 2407–2418. [Google Scholar] [CrossRef]
- Roy, S.; Maity, A.; Mandal, P.; Chanda, D.K.; Pal, K.; Bardhan, S.; Das, S. Effects of Various Morphologies on the Optical and Electrical Properties of Boehmite Nanostructures. CrystEngComm 2018, 20, 6338–6350. [Google Scholar] [CrossRef]
- Bormashenko, Y.; Pogreb, R.; Stanevsky, O.; Bormashenko, E. Vibrational Spectrum of PVDF and Its Interpretation. Polym. Test 2004, 23, 791–796. [Google Scholar] [CrossRef]
- Salimi, A.; Yousefi, A.A. Conformational Changes and Phase Transformation Mechanisms in PVDF Solution-Cast Films. J. Polym. Sci. B Polym. Phys. 2004, 42, 3487–3495. [Google Scholar] [CrossRef]
- Barrau, S.; Ferri, A.; Da Costa, A.; Defebvin, J.; Leroy, S.; Desfeux, R.; Lefebvre, J.M. Nanoscale Investigations of α- And γ-Crystal Phases in PVDF-Based Nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 13092–13099. [Google Scholar] [CrossRef]
- He, Z.; Rault, F.; Lewandowski, M.; Mohsenzadeh, E.; Salaün, F. Electrospun PVDF Nanofibers for Piezoelectric Applications: A Review of the Influence of Electrospinning Parameters on the β Phase and Crystallinity Enhancement. Polymers 2021, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Hoque, N.A.; Thakur, P.; Biswas, P.; Saikh, M.M.; Roy, S.; Bagchi, B.; Das, S.; Ray, P.P. Biowaste Crab Shell-Extracted Chitin Nanofiber-Based Superior Piezoelectric Nanogenerator. J. Mater. Chem. A Mater. 2018, 6, 13848–13858. [Google Scholar] [CrossRef]
- Bardhan, S.; Pal, K.; Roy, S.; Das, S.; Chakraborty, A.; Karmakar, P.; Basu, R.; Das, S. Nanoparticle Size-Dependent Antibacterial Activities in Natural Minerals. J. Nanosci. Nanotechnol. 2019, 19, 7112–7122. [Google Scholar] [CrossRef]
- Ali, A.; Chen, L.; Nasir, M.S.; Wu, C.; Guo, B.; Yang, Y. Piezocatalytic Removal of Water Bacteria and Organic Compounds: A Review. Environ. Chem. Lett. 2022, 1, 1–18. [Google Scholar] [CrossRef]
- Ince, N.H. Ultrasound-Assisted Advanced Oxidation Processes for Water Decontamination. Ultrason. Sonochem. 2018, 40, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Masekela, D.; Hintsho-Mbita, N.C.; Sam, S.; Yusuf, T.L.; Mabuba, N. Application of BaTiO3-Based Catalysts for Piezocatalytic, Photocatalytic and Piezo-Photocatalytic Degradation of Organic Pollutants and Bacterial Disinfection in Wastewater: A Comprehensive Review. Arab. J. Chem. 2023, 16, 104473. [Google Scholar] [CrossRef]
- Palomino, A.; Gewurz, D.; DeVine, L.; Zajmi, U.; Moralez, J.; Abu-Rumman, F.; Smith, R.P.; Lopatkin, A.J. Metabolic Genes on Conjugative Plasmids Are Highly Prevalent in Escherichia Coli and Can Protect against Antibiotic Treatment. ISME J. 2022, 17, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Bai, L.; Liu, K.; Shi, Y.; Minakata, D.; Huang, C.H.; Spinney, R.; Seth, R.; Dionysiou, D.D.; Wei, Z.; et al. Elucidating Sulfate Radical-Mediated Disinfection Profiles and Mechanisms of Escherichia coli and Enterococcus faecalis in Municipal Wastewater. Water Res. 2020, 173, 115552. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, M.; Vaish, R. Flexible Ag@LiNbO3/PVDF Composite Film for Piezocatalytic Dye/Pharmaceutical Degradation and Bacterial Disinfection. ACS Appl Mater Interfaces 2021, 13, 22914–22925. [Google Scholar] [CrossRef]
- Ali, A.; Zhao, J.; Yao, R.; Ahmed, S.; Wang, L.; Guo, B.; Rao, W.F.; Yang, Y. Stimulated Piezotronical Decontamination Using Cu2MgSnS4 Modified BaTiO3. Mater. Today Energy 2021, 21, 100717. [Google Scholar] [CrossRef]
- Lei, J.; Wang, C.; Feng, X.; Ma, L.; Liu, X.; Luo, Y.; Tan, L.; Wu, S.; Yang, C. Sulfur-Regulated Defect Engineering for Enhanced Ultrasonic Piezocatalytic Therapy of Bacteria-Infected Bone Defects. Chem. Eng. J. 2022, 435, 134624. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, M.; Kumar, A.; Powar, S.; Vaish, R. Rapid Bacterial Disinfection Using Low Frequency Piezocatalysis Effect. J. Ind. Eng. Chem. 2019, 77, 355–364. [Google Scholar] [CrossRef]
- Thakur, D.; Sharma, M.; Balakrishnan, V.; Vaish, R. Reusable Piezocatalytic Water Disinfection Activity of CVD-Grown Few-Layer WS2 on Sapphire Substrate. Environ. Sci. Nano 2022, 9, 805–814. [Google Scholar] [CrossRef]




| Parameters | Values |
|---|---|
| a (Å) | 5.589 |
| b (Å) | 5.575 |
| c (Å) | 5.659 |
| alpha (°) | 60.334 |
| beta (°) | 119.928 |
| gamma (°) | 119.768 |
| V (Å^3) | 124.639455 |
| size (Å) | 330.581 |
| strain | 3.7689 × 10−5 |
| Rp (%) | 10.258 |
| Rwp (%) | 13.488 |
| Material | Frequency and Power of Ultrasound | Bacterial Type | Time of Ablation | Degradation Efficacy | Year | Reference |
|---|---|---|---|---|---|---|
| Ag@LiNbO3/PVDF | 40 kHz, 70 W | S. aureus | 180 | 96.6 | 2021 | [37] |
| Ag@LiNbO3/PVDF | 40 kHz, 70 W | E. coli | 180 | 99 | 2021 | [37] |
| Cu2MgSnS4/BaTiO3 | 40 kHz | E. coli and S. aureus | 180 | >90 | 2021 | [38] |
| BTO | - | S. aureus | - | 97.12 | 2022 | [39] |
| BaTiO3 | 8 Hz | E. coli | 30 | 99 | 2019 | [40] |
| WS2 on sapphire substrate | 40 kHz | E. coli | 120 | 99 | 2022 | [41] |
| MWCNT-kaolinite-PVDF | 33 kHz, 50 W | E. coli | 45 | 99 | 2022 | [16] |
| BFO | 15 kHz, 250 W | E. coli | 60 | 94 | 2022 | [21] |
| BF@PV5 | 10 kHz, 20 W | E. coli | 30 | 99.7 | This work | This work |
| BF@PV5 | 10 kHz, 20 W | E. faecalis | 30 | 99.9 | This work | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Roy, S.; Wang, J.; Lu, X.; Li, S.; Yang, H.; Cheng, M.; Guo, B.; Xu, Y. Piezodynamic Eradication of Both Gram-Positive and Gram-Negative Bacteria by Using a Nanoparticle Embedded Polymeric Membrane. Pharmaceutics 2023, 15, 2155. https://doi.org/10.3390/pharmaceutics15082155
Chen C, Roy S, Wang J, Lu X, Li S, Yang H, Cheng M, Guo B, Xu Y. Piezodynamic Eradication of Both Gram-Positive and Gram-Negative Bacteria by Using a Nanoparticle Embedded Polymeric Membrane. Pharmaceutics. 2023; 15(8):2155. https://doi.org/10.3390/pharmaceutics15082155
Chicago/Turabian StyleChen, Chan, Shubham Roy, Jingjing Wang, Xiafen Lu, Siyi Li, Hao Yang, Minggang Cheng, Bing Guo, and Yuzhong Xu. 2023. "Piezodynamic Eradication of Both Gram-Positive and Gram-Negative Bacteria by Using a Nanoparticle Embedded Polymeric Membrane" Pharmaceutics 15, no. 8: 2155. https://doi.org/10.3390/pharmaceutics15082155
APA StyleChen, C., Roy, S., Wang, J., Lu, X., Li, S., Yang, H., Cheng, M., Guo, B., & Xu, Y. (2023). Piezodynamic Eradication of Both Gram-Positive and Gram-Negative Bacteria by Using a Nanoparticle Embedded Polymeric Membrane. Pharmaceutics, 15(8), 2155. https://doi.org/10.3390/pharmaceutics15082155







