Formulation and Evaluation of Prednisolone Sodium Metazoate-Loaded Mucoadhesive Quatsomal Gel for Local Treatment of Recurrent Aphthous Ulcers: Optimization, In Vitro, Ex Vivo, and In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
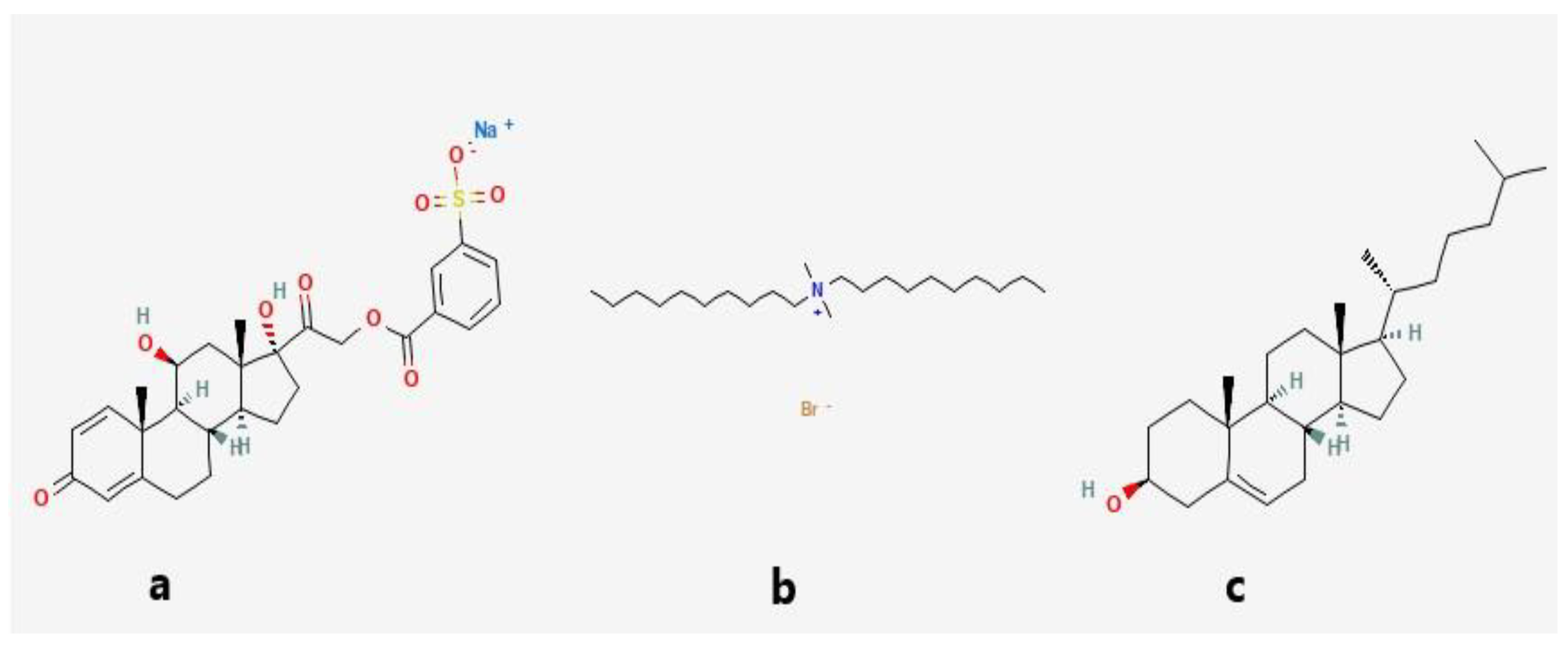
2.1. Materials
2.2. Preparation of Pred MS-Loaded Quatsomes
2.3. Characterization of Pred MS-Loaded Quatsomes
2.3.1. Determination of PS, PDI, and ZP of Quatsomes
2.3.2. Determination of EE%
2.3.3. In Vitro Drug Release Study
2.4. Optimization of Pred MS-Loaded Quatsome
2.5. Selecting the Optimized Pred MS-Loaded Quatsomes Formula
2.6. Morphology
2.7. Compatibility Evaluation of the Optimized Quatsomal Formula Using Fourier Transform Infrared (FTIR)
2.8. Stability Study of the Optimized Quatsomal Formula
2.9. Formulation of Pred MS Mucoadhesive Quatsomal Gel
2.10. Evaluation of Pred MS Mucoadhesive Quatsomal Gel
2.10.1. Evaluation of the Physical Properties
2.10.2. Determination of Gel pH
2.10.3. Determination of Drug Content
2.10.4. Evaluation of the Rheological Properties
2.10.5. Assessment of Gel Spreadability
2.10.6. In Vitro Drug Release Study
2.10.7. Ex Vivo Mucoadhesive Force (MF)
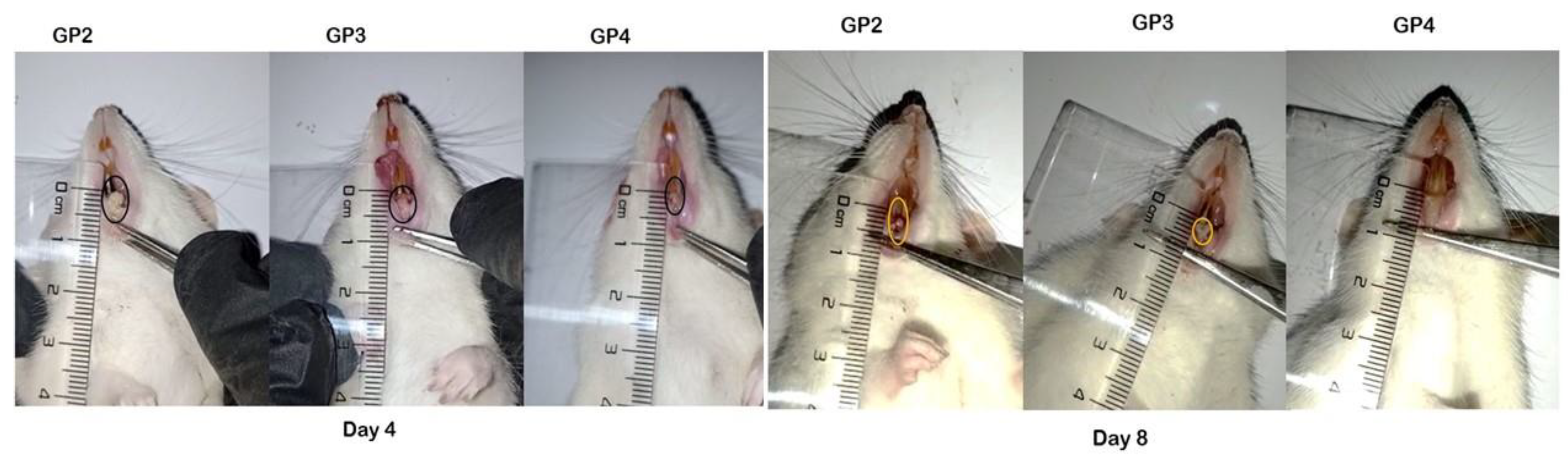
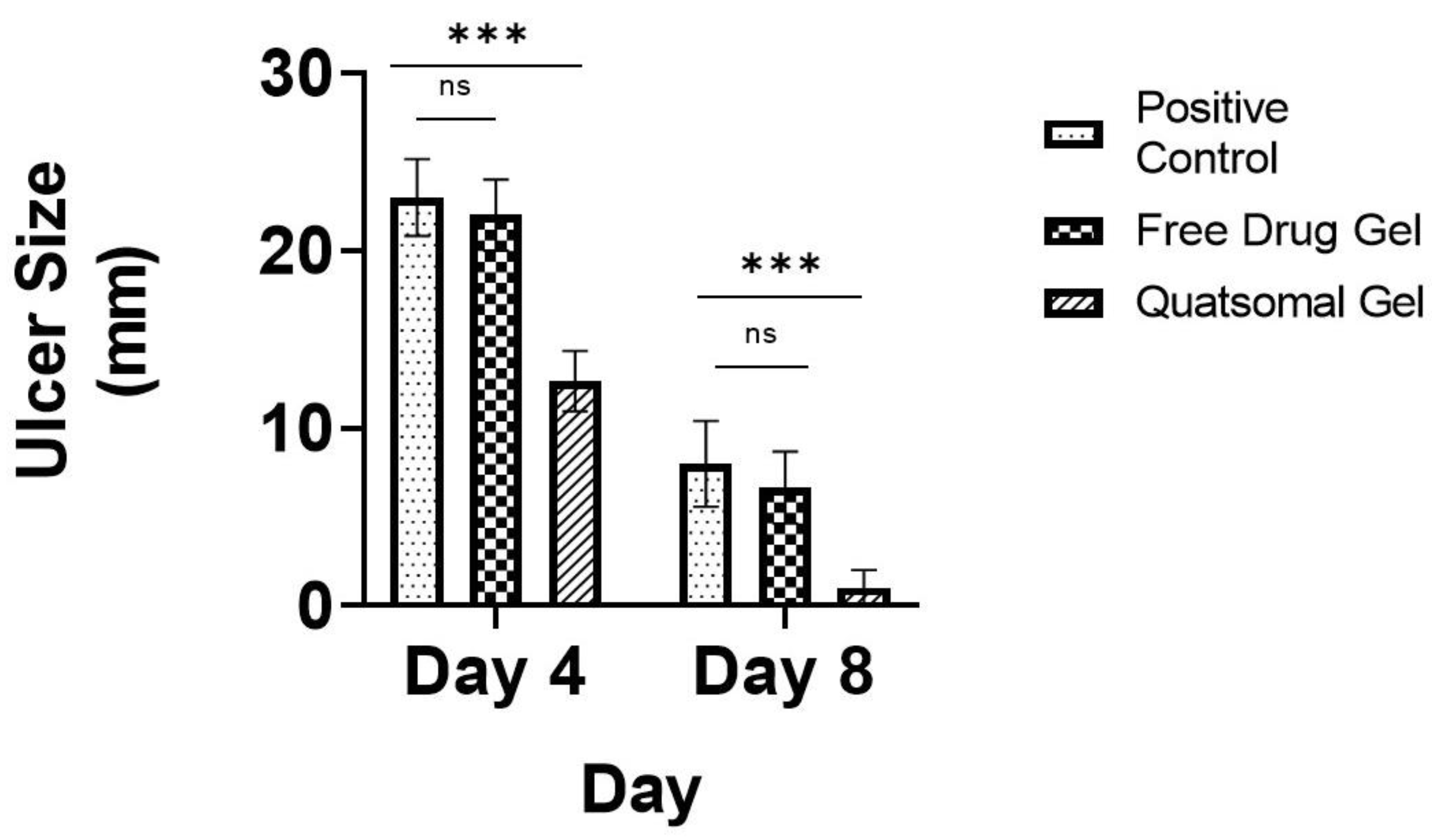
2.11. In Vivo Study
2.12. Statistical Analysis of Data
3. Results and Discussion
3.1. Factorial Design Optimization
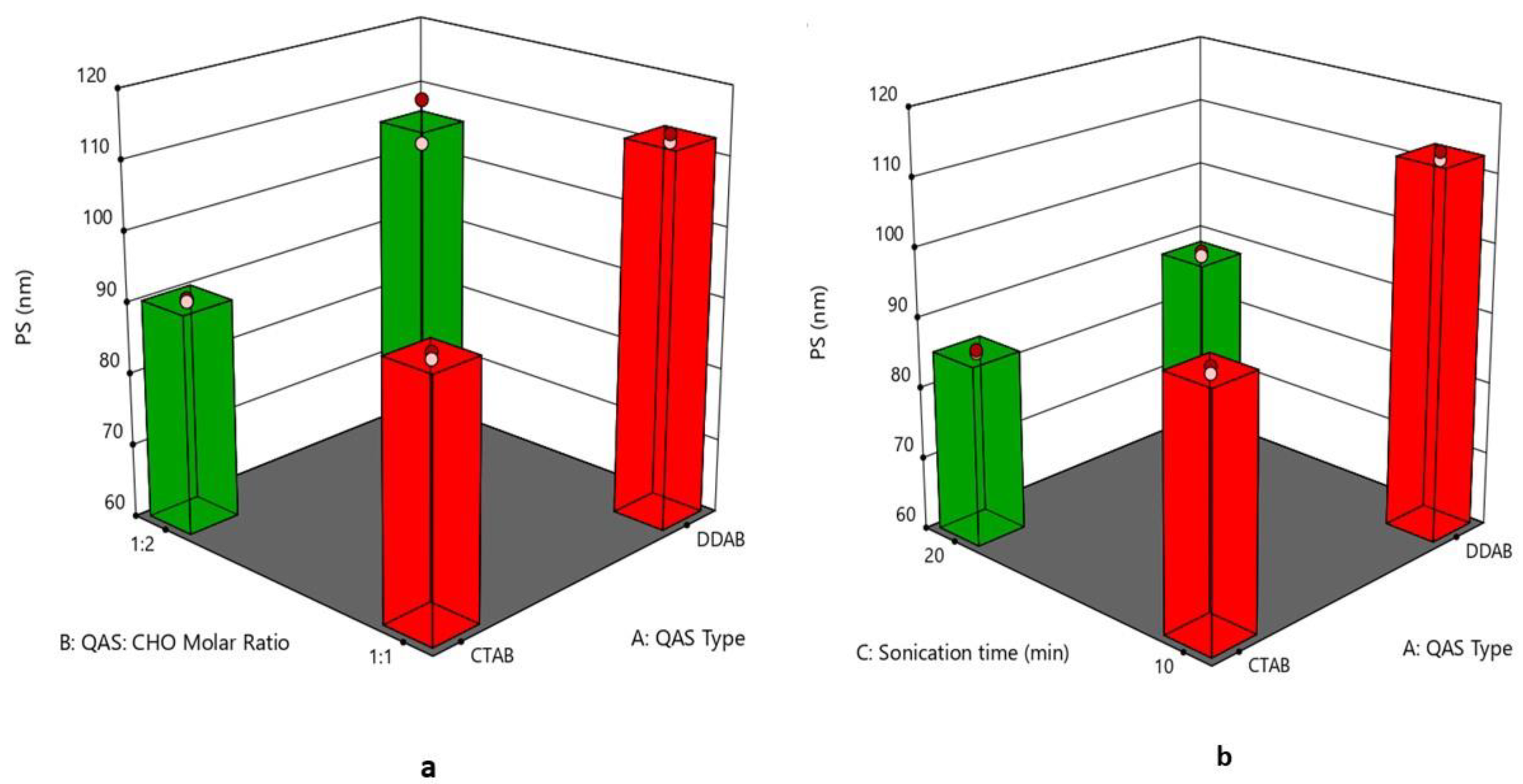
3.2. Effect of Formulation Variables on the Particle Size
3.3. Effect of Formulation Variables on Polydispersity Index
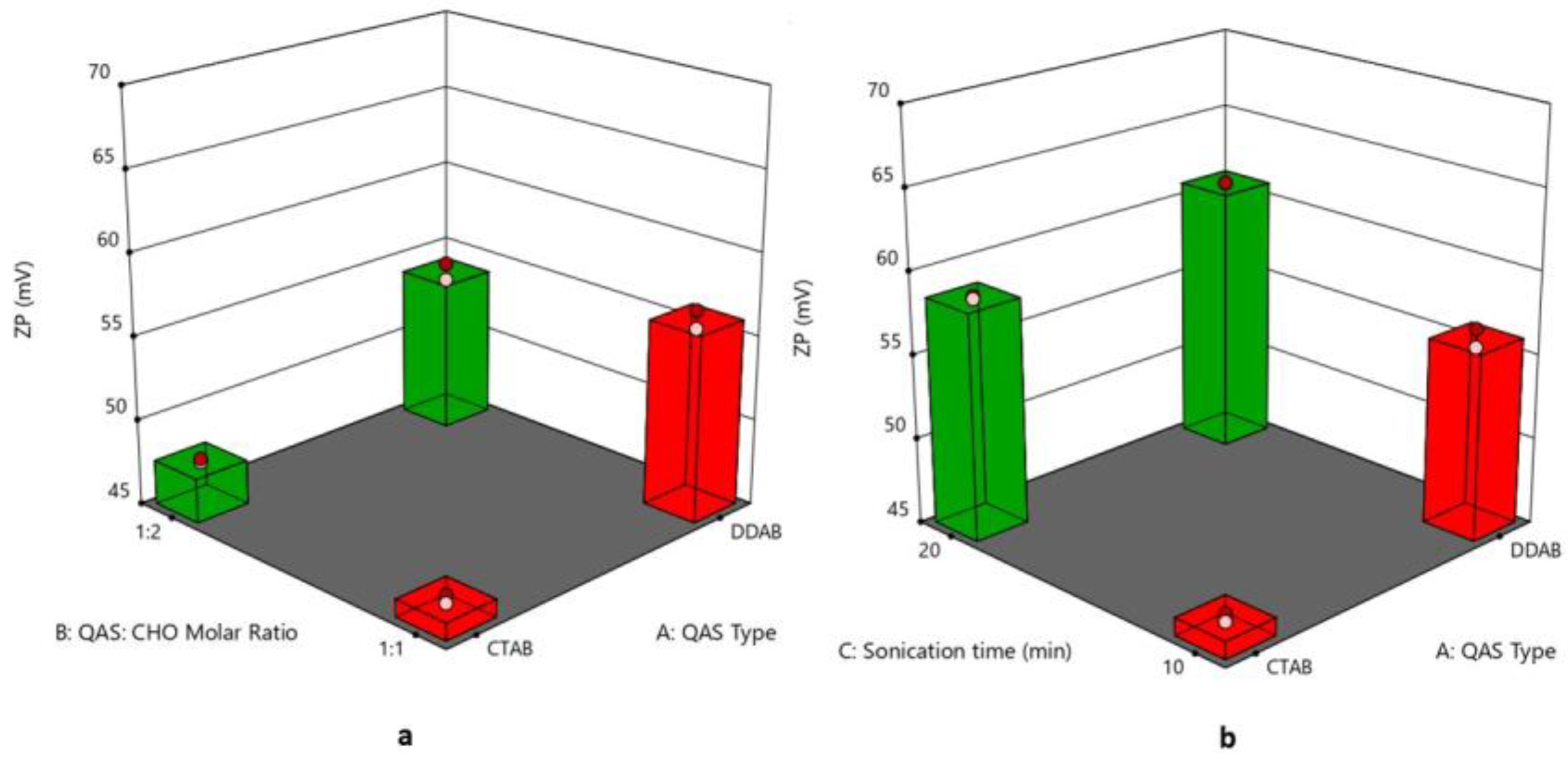
3.4. Effect of Formulation Variables on Zeta Potential
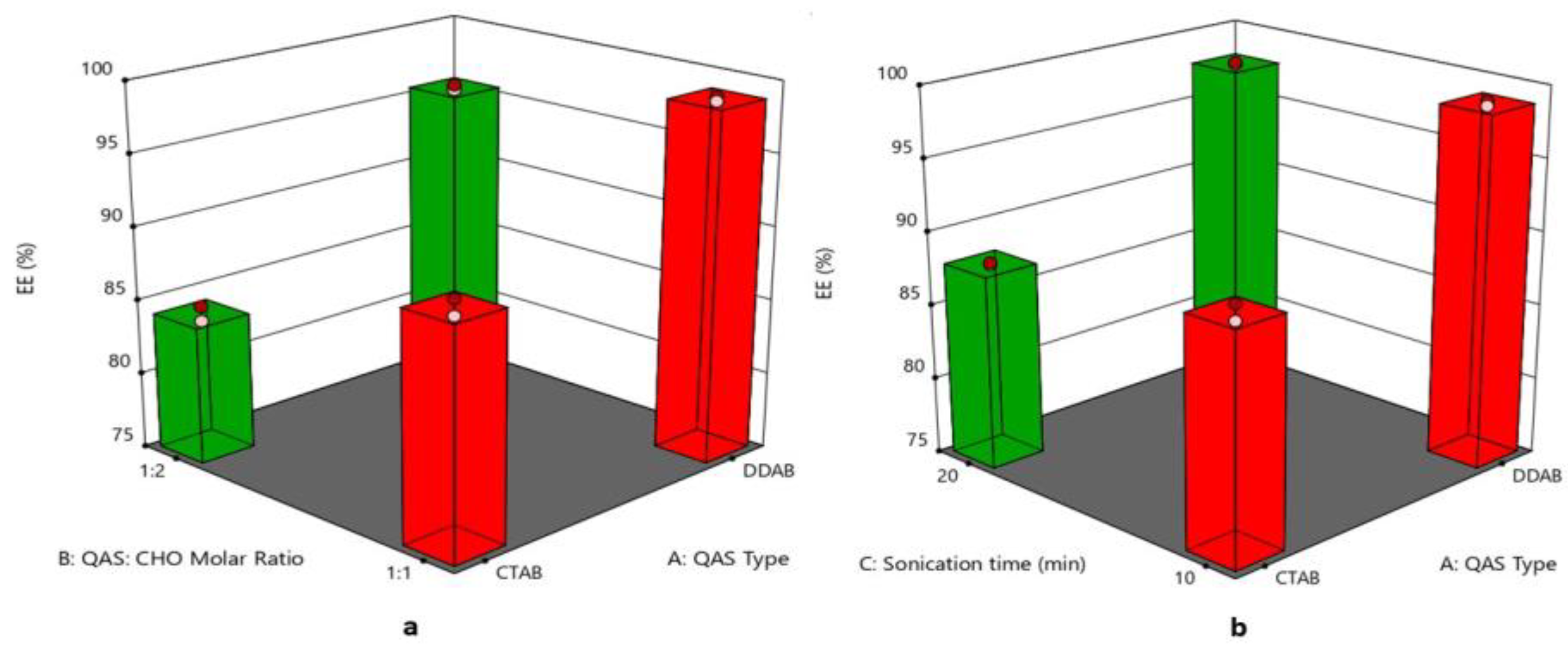
3.5. Effect of Formulation Variables on Entrapment Efficiency%
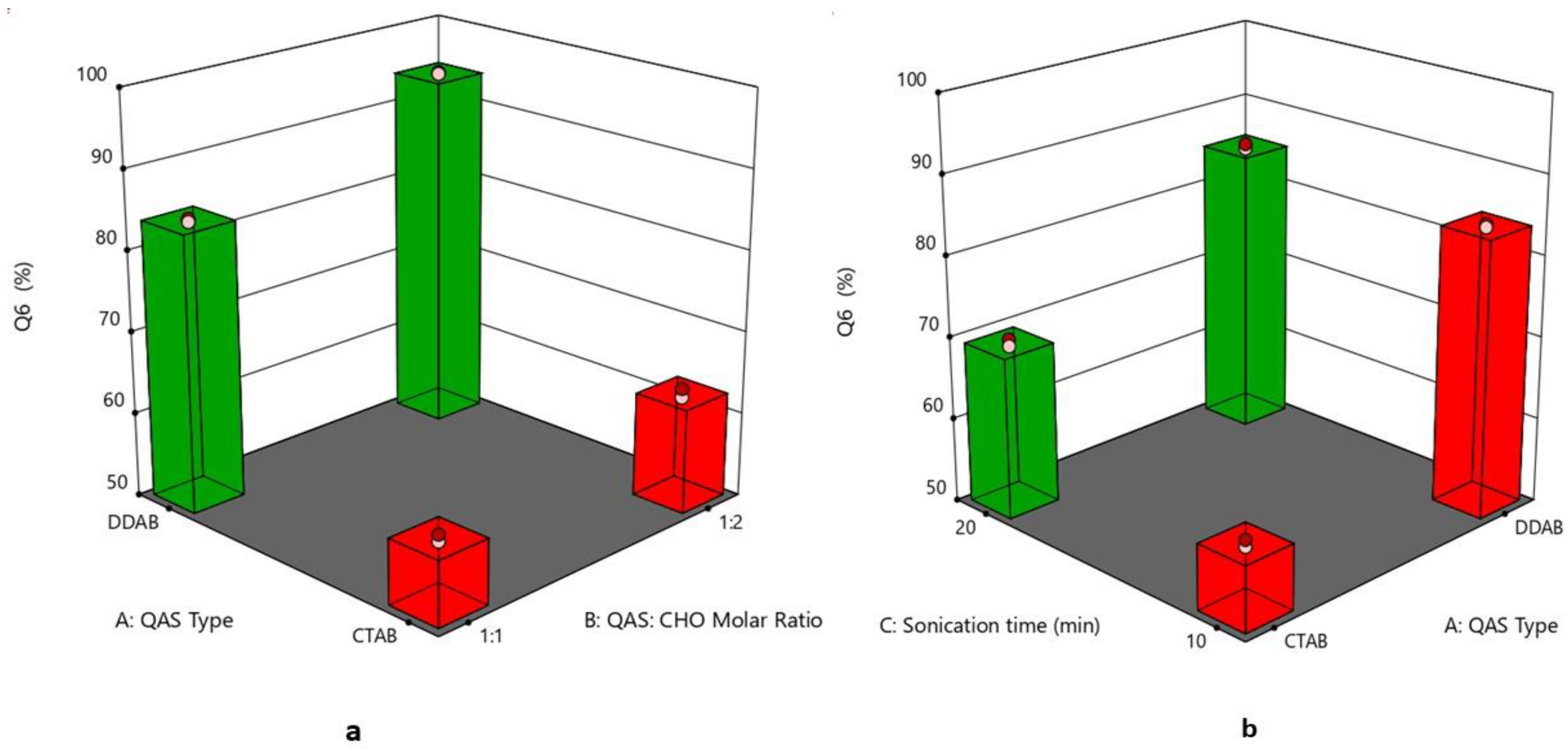
3.6. Effect of Formulation Variables on the In Vitro Drug Release
3.7. Selection of the Optimized Formula
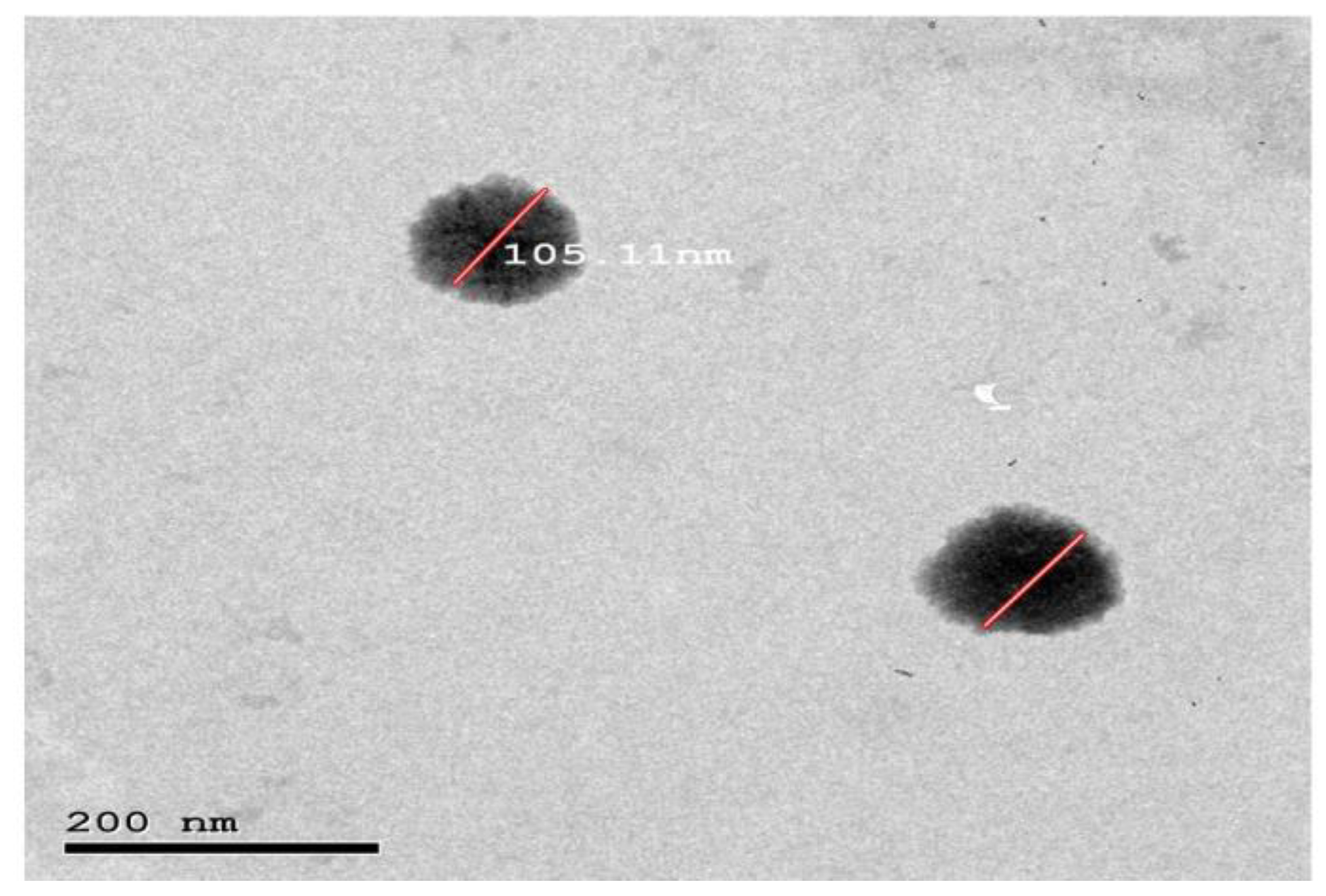
3.8. Morphology
3.9. Compatibility Assessment of the Optimized Formula Using Fourier Transform Infrared (FTIR)
3.10. Stability Study of the Optimized Quatsomal Formula
3.11. Evaluation of Pred MS Mucoadhesive Quatsomal Gel
3.11.1. Physical Appearance of the Gel
3.11.2. Determination of Gel pH
3.11.3. Determination of Drug Content
3.11.4. Evaluation of the Rheological Properties
3.11.5. Assessment of Gel Spreadability
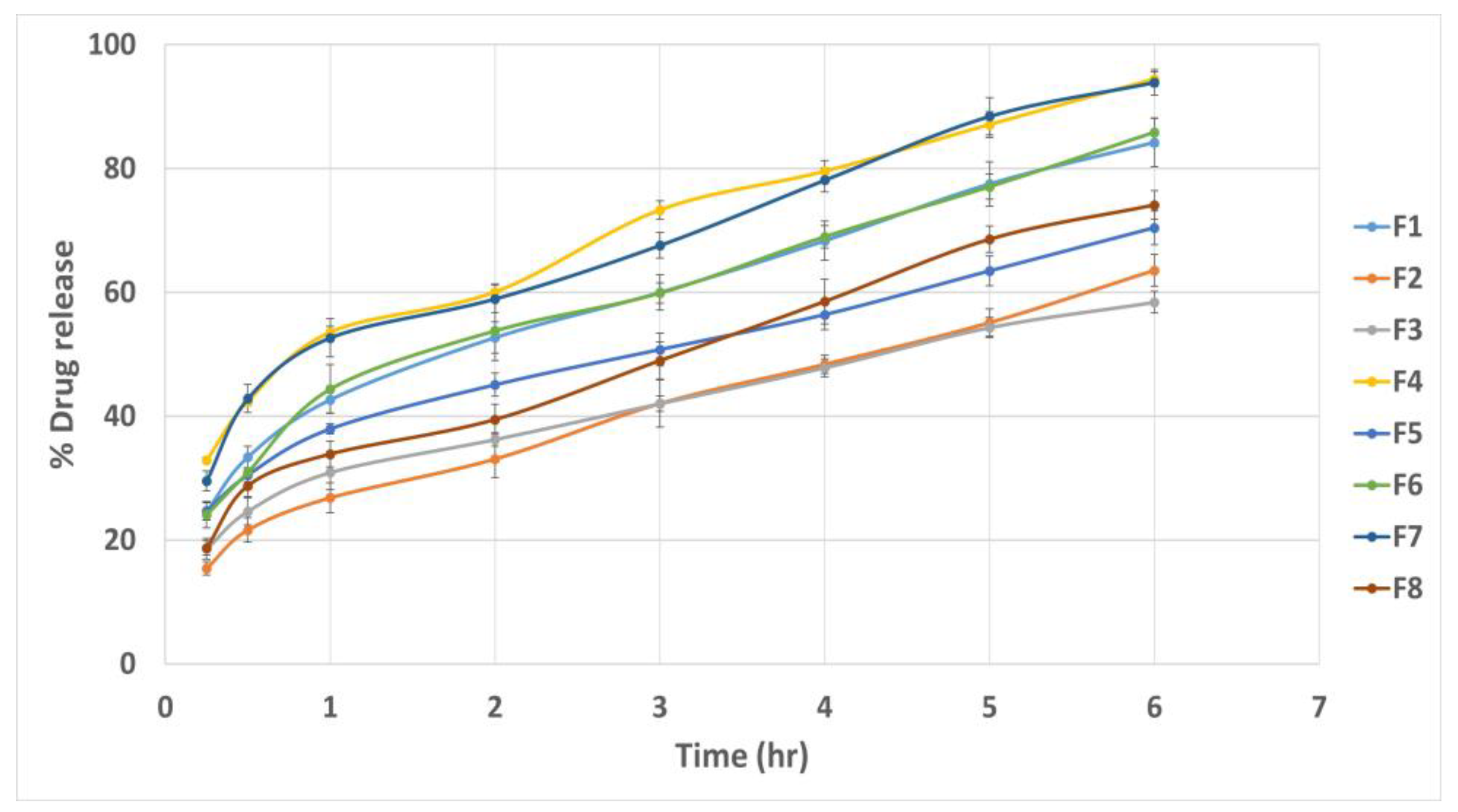
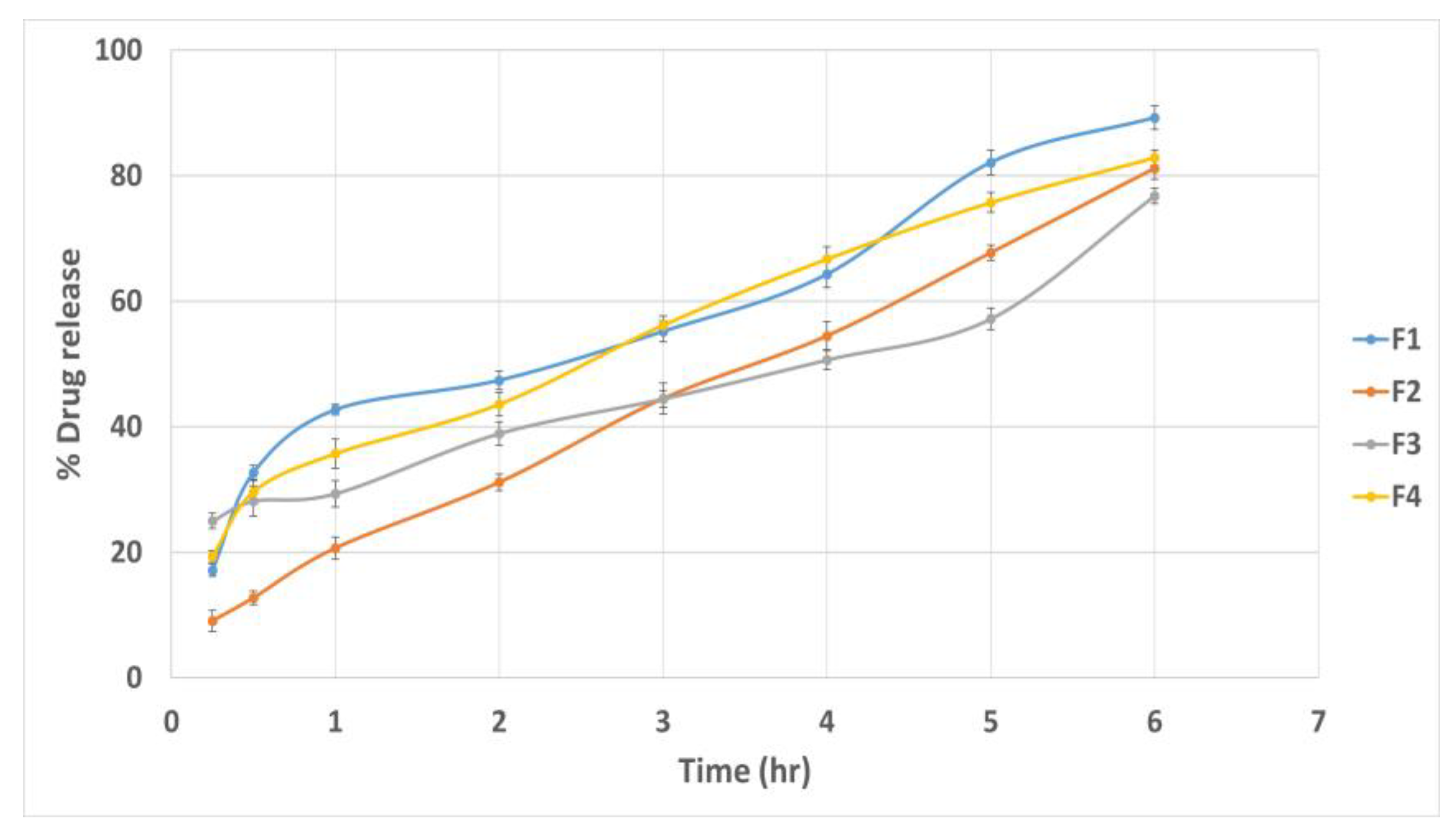
3.11.6. In Vitro Drug Release Study
3.11.7. Ex Vivo Muco-Adhesive Force
3.12. In Vivo Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibáñez-Mancera, N.G.; López-Callejas, R.; Toral-Rizo, V.H.; Rodríguez-Méndez, B.G.; Lara-Carrillo, E.; Peña-Eguiluz, R.; do Amaral, R.C.; Mercado-Cabrera, A.; Valencia-Alvarado, R. Healing of Recurrent Aphthous Stomatitis by Non-Thermal Plasma: Pilot Study. Biomedicines 2023, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.B.; Smith, G.P. Recurrent aphthous stomatitis: A comprehensive review and recommendations on therapeutic options. Dermatol. Ther. 2022, 35, e15500. [Google Scholar] [CrossRef]
- Han, Y.; Wang, L.; Li, Q.; Chen, H.; Ma, X. LncRNA NEAT1 is upregulated in recurrent aphthous stomatitis (RAS) and has predictive values. BMC Oral Health 2021, 21, 673. [Google Scholar] [CrossRef] [PubMed]
- Edgar, N.R.; Saleh, D.; Miller, R.A. Recurrent Aphthous Stomatitis: A Review. J. Clin. Aesthet. Dermatol. 2017, 10, 26–36, (From NLM). [Google Scholar] [PubMed]
- Sharma, D.; Sharma, A.; Garg, R. Preparation, Physicochemical Evaluation and Characterization of Mucoadhesive Buccal Gels Impregnated with Benzydamine Hydrochloride for the Effective Treatment of Aphthous Stomatitis: Effect of Different Grades of HPMC Polymer on In vitro and Ex vivo Performance. Drug Deliv. Lett. 2019, 9, 341–357. [Google Scholar]
- Chiang, C.-P.; Yu-Fong Chang, J.; Wang, Y.-P.; Wu, Y.-H.; Wu, Y.-C.; Sun, A. Recurrent aphthous stomatitis—Etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J. Formos. Med. Assoc. 2019, 118, 1279–1289. [Google Scholar] [CrossRef]
- Kavita, K.; Singh, R.; Singh, R.; Gonuguntla, S.; Luke, A.M.; Jois, H.S. Assessment of Efficacy of 5% Topical Amlexanox and 0.1% Topical Triamcinolone Acetonide in Management of Recurrent Aphthous Stomatitis. J. Pharm. Bioallied. Sci. 2020, 12 (Suppl. 1), S444–S447. [Google Scholar]
- Liu, H.; Tan, L.; Fu, G.; Chen, L.; Tan, H. Efficacy of Topical Intervention for Recurrent Aphthous Stomatitis: A Network Meta-Analysis. Medicina 2022, 58, 771. [Google Scholar] [CrossRef]
- Manfredini, M.; Guida, S.; Giovani, M.; Lippolis, N.; Spinas, E.; Farnetani, F.; Dattola, A.; Di Matteo, E.; Pellacani, G.; Giannetti, L. Recurrent Aphthous Stomatitis: Treatment and Management. Dermatol. Pract. Concept. 2021, 11, e2021099. [Google Scholar] [CrossRef]
- Daneshpazhooh, M.; Zhara Ghodsi, S.; Mahmoudi, H. Recurrent Aphthous Stomatitis. In Diseases of the Oral Mucosa: Study Guide and Review; Schmidt, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 125–133. [Google Scholar]
- Cheng, B.; Zeng, X.; Liu, S.; Zou, J.; Wang, Y. The efficacy of probiotics in management of recurrent aphthous stomatitis: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 21181. [Google Scholar] [CrossRef]
- D’haens, G. Systematic review: Second-generation vs. conventional corticosteroids for induction of remission in ulcerative colitis. Aliment. Pharmacol. Ther. 2016, 44, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Duan, W.; Tran, T.T.D. Recent developments of nanoparticle-delivered dosage forms for buccal delivery. Int. J. Pharm. 2019, 571, 118697. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Suharyani, I.; Fouad Abdelwahab Mohammed, A.; Muchtaridi, M.; Wathoni, N.; Abdassah, M. Evolution of Drug Delivery Systems for Recurrent Aphthous Stomatitis. Drug Des. Dev. Ther. 2021, 15, 4071–4089. [Google Scholar] [CrossRef] [PubMed]
- Şenel, S.; Özdoğan, A.I.; Akca, G. Current status and future of delivery systems for prevention and treatment of infections in the oral cavity. Drug Deliv. Transl. Res. 2021, 11, 1703–1734. [Google Scholar] [CrossRef]
- Abouzid, A.; Moustafa, A.Y.; Allcock, N.; Najlah, M.; Elhissi, A.; Stanley, C.W.; Ahmed, W.; Seville, P.; Crean, S.; Forbes, R.T.; et al. Amlexanox-loaded nanoliposomes showing enhanced anti-inflammatory activity in cultured macrophages: A potential formulation for treatment of oral aphthous stomatitis. J. Drug Deliv. Sci. Technol. 2023, 79, 104052. [Google Scholar] [CrossRef]
- Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Almatroudi, A.; Rahmani, A.H. Recent strategies towards the surface modification of liposomes: An innovative approach for different clinical applications. 3 Biotech 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-Coating Effect on the Characteristics of Liposomes: A Focus on Bioactive Compounds and Essential Oils: A Review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Vargas-Nadal, G.; Muñoz-Úbeda, M.; Álamo, P.; Mitjans, M.; Céspedes, V.; Köber, M.; González-Mira, E.; Ferrer-Tasies, L.; Vinardell, M.P.; Mangues, R.; et al. MKC-Quatsomes: A stable nanovesicle platform for bio-imaging and drug-delivery applications. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102136. [Google Scholar] [CrossRef]
- Morla-Folch, J.; Vargas-Nadal, G.; Zhao, T.; Sissa, C.; Ardizzone, A.; Kurhuzenkau, S.; Köber, M.; Uddin, M.; Painelli, A.; Veciana, J.; et al. Dye-Loaded Quatsomes Exhibiting FRET as Nanoprobes for Bioimaging. ACS Appl. Mater. Interfaces 2020, 12, 20253–20262. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Tasies, L.; Santana, H.; Cabrera-Puig, I.; González-Mira, E.; Ballell-Hosa, L.; Castellar-Álvarez, C.; Córdoba, A.; Merlo-Mas, J.; Gerónimo, H.; Chinea, G.; et al. Recombinant Human Epidermal Growth Factor/Quatsome Nanoconjugates: A Robust Topical Delivery System for Complex Wound Healing. Adv. Ther. 2021, 4, 2000260. [Google Scholar] [CrossRef]
- Hassan, D.H.; Abdelmonem, R.; Abdellatif, M.M. Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study. Pharmaceutics 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Qiu, F.; Sloat, B.R. Lecithin-based cationic nanoparticles as a potential DNA delivery system. Int. J. Pharm. 2006, 313, 206–213. [Google Scholar] [CrossRef]
- Zheng, W.; Hao, Y.; Wang, D.; Huang, H.; Guo, F.; Sun, Z.; Shen, P.; Sui, K.; Yuan, C.; Zhou, Q. Preparation of triamcinolone acetonide-loaded chitosan/fucoidan hydrogel and its potential application as an oral mucosa patch. Carbohydr. Polym. 2021, 272, 118493. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bao, Q.; Shen, J.; Lalla, R.V.; Burgess, D.J. Mucoadhesive in situ forming gel for oral mucositis pain control. Int. J. Pharm. 2020, 580, 119238. [Google Scholar] [CrossRef]
- Dong, D.; Thomas, N.; Ramezanpour, M.; Psaltis, A.J.; Huang, S.; Zhao, Y.; Thierry, B.; Wormald, P.-J.; Prestidge, C.A.; Vreugde, S. Inhibition of Staphylococcus aureus and Pseudomonas aeruginosa biofilms by quatsomes in low concentrations. Exp. Biol. Med. 2020, 245, 34–41. [Google Scholar] [CrossRef]
- Battista, S.; Köber, M.; Bellio, P.; Celenza, G.; Galantini, L.; Vargas-Nadal, G.; Fagnani, L.; Veciana, J.; Ventosa, N.; Giansanti, L. Quatsomes Formulated with l-Prolinol-Derived Surfactants as Antibacterial Nanocarriers of (+)-Usnic Acid with Antioxidant Activity. ACS Appl. Nano Mater. 2022, 5, 6140–6148. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Ahmed, S.M.; El-Nabarawi, M.A.; Teaima, M. Oral Bioavailability Enhancement of Vancomycin Hydrochloride with Cationic Nanocarrier (Leciplex): Optimization, In Vitro, Ex Vivo, and In Vivo Studies. Sci. Pharm. 2023, 91, 1. [Google Scholar] [CrossRef]
- Fouad, S.A.; Teaima, M.H.; Gebril, M.I.; Abd Allah, F.I.; El-Nabarawi, M.A.; Elhabal, S.F. Formulation of novel niosomal repaglinide chewable tablets using coprocessed excipients: In vitro characterization, optimization and enhanced hypoglycemic activity in rats. Drug Deliv. 2023, 30, 2181747. [Google Scholar] [CrossRef]
- Sindi, A.M.; Alharbi, W.S.; Alkhalidi, H.M.; Alghaith, A.F.; Hosny, K.M. Development and optimization of Clotrimazole-Rosehip oil nanoethosomal-gel for oral thrush and gingivitis. J. Drug Deliv. Sci. Technol. 2021, 63, 102482. [Google Scholar] [CrossRef]
- Thomas, N.; Dong, D.; Richter, K.; Ramezanpour, M.; Vreugde, S.; Thierry, B.; Wormald, P.-J.; Prestidge, C.A. Quatsomes for the treatment of Staphylococcus aureus biofilm. J. Mater. Chem. B 2015, 3, 2770–2777. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; Eltabeeb, M.A.; El-Nabarawi, M.A.; Abdellatif, M.M. Utilization of propranolol hydrochloride mucoadhesive invasomes as a locally acting contraceptive: In-vitro, ex-vivo, and in-vivo evaluation. Drug Deliv. 2022, 29, 2549–2560. [Google Scholar] [CrossRef]
- Kuo, A.-T.; Tu, C.-L.; Yang, Y.-M.; Chang, C.-H. Enhanced physical stability of mixed ion pair amphiphile/double-chained cationic surfactant vesicles in the presence of cholesterol. J. Oleo Sci. 2018, 67, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; Ghaffar, S.A.; Hager, R.; Elzohairy, N.A.; Khalifa, M.M.; Mohie, P.M.; Gad, R.A.; Omar, N.N.; Elkomy, M.H.; Khasawneh, M.A.; et al. Development of thermosensitive hydrogel of Amphotericin-B and Lactoferrin combination-loaded PLGA-PEG-PEI nanoparticles for potential eradication of ocular fungal infections: In-vitro, ex-vivo and in-vivo studies. Int. J. Pharm. X 2023, 5, 100174. [Google Scholar] [CrossRef]
- Lyapunov, A.N.; Bezuglaya, E.P.; Lyapunov, N.A.; Kirilyuk, I.A. Studies of Carbomer Gels Using Rotational Viscometry and Spin Probes. Pharm. Chem. J. 2015, 49, 639–644. [Google Scholar] [CrossRef]
- Shukla, R.; Tiwari, G.; Tiwari, R.; Rai, A.K. Formulation and evaluation of the topical ethosomal gel of melatonin to prevent UV radiation. J. Cosmet. Dermatol. 2020, 19, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, D.C.; Senthilnathan, R.D.; Maanvizhi, S.; Madhavan, Y.; Sankarapandian, S.; Ramshankar, V.; Kalachaveedu, M. Preparation and Characterization of Silymarin Gel: A Novel Topical Mucoadhesive Formulation for Potential Applicability in Oral Pathologies. Gels 2023, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Reddy Hv, R.; Bhattacharyya, S. In vitro evaluation of mucoadhesive in situ nanogel of celecoxib for buccal delivery. Ann. Pharm. Françaises 2021, 79, 418–430. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Rahman, M.A.; Warsi, M.H.; Yusuf, M.; Alam, P. Development of Nanogel Loaded with Lidocaine for Wound-Healing: Illustration of Improved Drug Deposition and Skin Safety Analysis. Gels 2022, 8, 466. [Google Scholar] [CrossRef]
- Syed, M.A.; Hanif, S.; Ain, N.u.; Syed, H.K.; Zahoor, A.F.; Khan, I.U.; Abualsunun, W.A.; Jali, A.M.; Qahl, S.H.; Sultan, M.H.; et al. Assessment of Binary Agarose–Carbopol Buccal Gels for Mucoadhesive Drug Delivery: Ex Vivo and In Vivo Characterization. Molecules 2022, 27, 7004. [Google Scholar] [CrossRef]
- Hitomi, S.; Nodai, T.; Kokabu, S.; Shikayama, T.; Sago-Ito, M.; Nakatomi, C.; Terawaki, K.; Omiya, Y.; Shinoda, M.; Ono, K. Hepcidin expression in the trigeminal ganglion and the oral mucosa in an oral ulcerative mucositis rat model. PLoS ONE 2023, 18, e0284617. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Khalil, I.A.; Elakkad, Y.E.; Eliwa, H.A.; Samir, T.M.; Al-Mokaddem, A.K. Formulation and Characterization of Sertaconazole Nitrate Mucoadhesive Liposomes for Vaginal Candidiasis. Int. J. Nanomed. 2020, 15, 4079–4090. [Google Scholar] [CrossRef] [PubMed]
- Date, A.A.; Srivastava, D.; Nagarsenker, M.S.; Mulherkar, R.; Panicker, L.; Aswal, V.; Hassan, P.A.; Steiniger, F.; Thamm, J.; Fahr, A. Lecithin-based novel cationic nanocarriers (LeciPlex) I: Fabrication, characterization and evaluation. Nanomedicine 2011, 6, 1309–1325. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.; Omolo, C.A.; Fasiku, V.O.; Elrashedy, A.A.; Mocktar, C.; Nkambule, B.; Soliman, M.E.S.; Govender, T. Formulation of pH-Responsive Quatsomes from Quaternary Bicephalic Surfactants and Cholesterol for Enhanced Delivery of Vancomycin against Methicillin Resistant Staphylococcus aureus. Pharmaceutics 2020, 12, 1093. [Google Scholar] [CrossRef]
- Ansari, M.D.; khan, I.; Solanki, P.; Pandit, J.; Jahan, R.N.; Aqil, M.; Sultana, Y. Fabrication and optimization of raloxifene loaded spanlastics vesicle for transdermal delivery. J. Drug Deliv. Sci. Technol. 2022, 68, 103102. [Google Scholar] [CrossRef]
- Rossetti, M.; Stella, L.; Morlà-Folch, J.; Bobone, S.; Boloix, A.; Baranda, L.; Moscone, D.; Roldán, M.; Veciana, J.; Segura, M.F.; et al. Engineering DNA-Grafted Quatsomes as Stable Nucleic Acid-Responsive Fluorescent Nanovesicles. Adv. Funct. Mater. 2021, 31, 2103511. [Google Scholar] [CrossRef]
- Qu, Y.; Wu, Z.; Liu, Y.; Lin, J.; Zhang, L.; Luo, X. Impact of double-chain surfactant stabilizer on the free active surface sites of gold nanoparticles. Mol. Catal. 2021, 501, 111377. [Google Scholar] [CrossRef]
- Kuo, A.-T.; Tu, C.-L.; Yang, Y.-M.; Chang, C.-H. Enhanced physical stability of positively charged catanionic vesicles: Role of cholesterol-adjusted molecular packing. J. Taiwan Inst. Chem. Eng. 2018, 92, 29–35. [Google Scholar] [CrossRef]
- Nayak, D.; Tawale, R.M.; Aranjani, J.M.; Tippavajhala, V.K. Formulation, Optimization and Evaluation of Novel Ultra-deformable Vesicular Drug Delivery System for an Anti-fungal Drug. AAPS PharmSciTech 2020, 21, 140. [Google Scholar] [CrossRef] [PubMed]
- Refai, H.; Osman, D.A.; Khalifa, M.K.; Elsalhy, S. Trifluoperazine-loaded leciplexes as potential nasal delivery systems for treatment of depression. Azhar Int. J. Pharm. Med. Sci. 2022, 2, 70–82. [Google Scholar] [CrossRef]
- Cano-Sarabia, M.; Angelova, A.; Ventosa, N.; Lesieur, S.; Veciana, J. Cholesterol induced CTAB micelle-to-vesicle phase transitions. J. Colloid Interface Sci. 2010, 350, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, F.; Kharaziha, M.; Emadi, R. Inspiring biomimetic system based on red blood cell membrane vesicles for effective curcumin loading and release. Int. J. Pharm. 2022, 613, 121419. [Google Scholar] [CrossRef] [PubMed]
- Albash, R.; Abdellatif, M.M.; Hassan, M.; Badawi, N.M. Tailoring Terpesomes and Leciplex for the Effective Ocular Conveyance of Moxifloxacin Hydrochloride (Comparative Assessment): In-vitro, Ex-vivo, and In-vivo Evaluation. Int. J. Nanomed. 2021, 16, 5247–5263. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Tasies, L.; Moreno-Calvo, E.; Cano-Sarabia, M.; Aguilella-Arzo, M.; Angelova, A.; Lesieur, S.; Ricart, S.; Faraudo, J.; Ventosa, N.; Veciana, J. Quatsomes: Vesicles Formed by Self-Assembly of Sterols and Quaternary Ammonium Surfactants. Langmuir 2013, 29, 6519–6528. [Google Scholar] [CrossRef]
- Khan, M.I.; Madni, A.; Hirvonen, J.; Peltonen, L. Ultrasonic Processing Technique as a Green Preparation Approach for Diacerein-Loaded Niosomes. AAPS PharmSciTech 2017, 18, 1554–1563. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary; National Center for Biotechnology Information: Bethesda, MD, USA, 2023. [Google Scholar]
- Köber, M.; Illa-Tuset, S.; Ferrer-Tasies, L.; Moreno-Calvo, E.; Tatkiewicz, W.I.; Grimaldi, N.; Piña, D.; Pérez, A.P.; Lloveras, V.; Vidal-Gancedo, J.; et al. Stable nanovesicles formed by intrinsically planar bilayers. J. Colloid Interface Sci. 2023, 631, 202–211. [Google Scholar] [CrossRef]
- Elmowafy, E.; Cespi, M.; Bonacucina, G.; Soliman, M.E. In situ composite ion-triggered gellan gum gel incorporating amino methacrylate copolymer microparticles: A therapeutic modality for buccal applicability. Pharm. Dev. Technol. 2019, 24, 1258–1271. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Eid, A.M.; Dawaba, A.M.; Ibrahim, A.G.; Fouda, A.; Hassan, S.M. Synthesis and characterization of new functionalized chitosan and its antimicrobial and in-vitro release behavior from topical gel. Int. J. Biol. Macromol. 2022, 207, 242–253. [Google Scholar] [CrossRef]
- Cid, Y.P.; Pedrazzi, V.; de Sousa, V.P.; Pierre, M.B.R. In Vitro Characterization of Chitosan Gels for Buccal Delivery of Celecoxib: Influence of a Penetration Enhancer. AAPS PharmSciTech 2012, 13, 101–111. [Google Scholar] [CrossRef]
- Sulistiawati; Saka Dwipayanti, K.; Azhar, M.; Rahman, L.; Pakki, E.; Himawan, A.; Permana, A.D. Enhanced skin localization of metronidazole using solid lipid microparticles incorporated into polymeric hydrogels for potential improved of rosacea treatment: An ex vivo proof of concept investigation. Int. J. Pharm. 2022, 628, 122327. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abdelgawad, W.Y.; Gad, M.K.; Mohamed, M.I. A novel approach for the treatment of oral ulcerative lesion using mucoadhesive proniosome gel. J. Drug Deliv. Sci. Technol. 2021, 63, 102460. [Google Scholar] [CrossRef]
- Khan, B.A.; Ali, A.; Hosny, K.M.; Halwani, A.A.; Almehmady, A.M.; Iqbal, M.; Alharbi, W.S.; Abualsunun, W.A.; Bakhaidar, R.B.; Murshid, S.S.A.; et al. Carbopol emulgel loaded with ebastine for urticaria: Development, characterization, in vitro and in vivo evaluation. Drug Deliv. 2022, 29, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Asane, G.S.; Nirmal, S.A.; Rasal, K.B.; Naik, A.A.; Mahadik, M.S.; Rao, Y.M. Polymers for Mucoadhesive Drug Delivery System: A Current Status. Drug Dev. Ind. Pharm. 2008, 34, 1246–1266. [Google Scholar] [CrossRef]
- Ossama, M.; Lamie, C.; Tarek, M.; Wagdy, H.A.; Attia, D.A.; Elmazar, M.M. Management of recurrent aphthous ulcers exploiting polymer-based Muco-adhesive sponges: In-Vitro and in-vivo evaluation. Drug Deliv. 2021, 28, 87–99. [Google Scholar] [CrossRef]
- Cevher, E.; Taha, M.A.M.; Orlu, M.; Araman, A. Evaluation of Mechanical and Mucoadhesive Properties of Clomiphene Citrate Gel Formulations Containing Carbomers and Their Thiolated Derivatives. Drug Deliv. 2008, 15, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.; Tuğcu-Demiröz, F.; Vural, İ.; Çelebi, N. Development and characterization of gels and liposomes containing ovalbumin for nasal delivery. J. Drug Deliv. Sci. Technol. 2018, 44, 108–117. [Google Scholar] [CrossRef]




| Independent Variables | Levels | |
|---|---|---|
| Low | High | |
| X1: QAS type | DDAB | CTAB |
| X2: QAS: CHO molar ratio | 1:1 | 1:2 |
| X3: Sonication time (min) | 10 | 20 |
| Responses (dependent variables) | Constraints | |
| Y1: PS (nm) | Minimize | |
| Y2: PDI | Minimize | |
| Y3: ZP (mV) | Maximize (absolute value) | |
| Y4: EE (%) | Maximize | |
| Y5: Q6% | Maximize | |
| Formulation Code | QAS Type | QAS: CHO Molar Ratio | Sonication Time (min) | PS (nm) (Y1) | PDI (Y2) | ZP (mV) (Y3) | EE % (Y4) | Q6 (%) (Y5) |
|---|---|---|---|---|---|---|---|---|
| F1 | DDAB | 1:1 | 10 | 113.28 ± 0.79 | 0.324 ± 0.001 | 56.9 ± 0.42 | 98.60 ± 1.22 | 84.21 ± 2.70 |
| F2 | CTAB | 1:2 | 10 | 91.35 ± 0.29 | 0.278 ± 0.002 | 48.6 ± 0.63 | 84.89 ± 1.71 | 63.54 ± 2.45 |
| F3 | CTAB | 1:1 | 10 | 95.93 ± 0.67 | 0.328 ± 0.004 | 45.15 ± 0.19 | 90.81 ± 0.74 | 58.39 ± 1.75 |
| F4 | DDAB | 1:2 | 10 | 100.50 ± 0.74 | 0.234 ± 0.003 | 53.4 ± 0.14 | 95.90 ± 1.35 | 93.89 ± 1.19 |
| F5 | CTAB | 1:1 | 20 | 86.10 ± 0.26 | 0.269 ± 0.007 | 58.1 ± 0.35 | 88.23 ± 1.52 | 70.41 ± 2.94 |
| F6 | DDAB | 1:1 | 20 | 89.30 ± 0.45 | 0.291 ± 0.001 | 61.2 ± 0.43 | 97.74 ± 0.83 | 85.84 ± 2.24 |
| F7 | DDAB | 1:2 | 20 | 69.47 ± 0.41 | 0.207 ± 0.004 | 68.1 ± 0.54 | 94.56 ± 1.16 | 94.42 ± 2.15 |
| F8 | CTAB | 1:2 | 20 | 75.21 ± 0.34 | 0.213 ± 0.009 | 61.3 ± 0.28 | 79.62 ± 1.44 | 74.11 ± 2.27 |
| Source | PS (nm) | PDI | ZP (mV) | EE (%) | Q6 (%) |
|---|---|---|---|---|---|
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| X1 = A = QAS type | <0.0001 | 0.0011 | 0.0004 | <0.0001 | <0.0001 |
| X2 = B = QAS: CHO | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| X3 = C = Sonication time | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Adequate precision | 36.4477 | 55.8875 | 86.7139 | 69.0594 | 103.0599 |
| R2 | 0.9924 | 0.9976 | 0.9991 | 0.9981 | 0.9992 |
| Adjusted R2 | 0.9857 | 0.9955 | 0.9983 | 0.9964 | 0.9986 |
| Predicted R2 | 0.9695 | 0.9904 | 0.9964 | 0.9924 | 0.9970 |
| Significant factors | X1, X2, X3 | X1, X2, X3 | X1, X2, X3 | X1, X2, X3 | X1, X2, X3 |
| Gel Formulae | Polymers Type | Polymers Percent (%) | pH | Drug Content (%) | Spreadability (g·cm/s) | MF (dyne/cm2) | Viscosity cp | Q6 (%) |
|---|---|---|---|---|---|---|---|---|
| G1 | Noveon | 1% | 6.9 ± 0.1 | 96.8 ± 0.41 | 6.67 ± 0.78 | 9417.6 ± 82.09 | 4286 ± 41.51 | 90.66 ± 0.49 |
| G2 | Noveon | 3% | 6.7 ± 0.14 | 97.3 ± 0.92 | 5.5 ± 0.75 | 10398.6 ± 90.54 | 7938 ± 45.76 | 85.53 ± 0.58 |
| G3 | Chitosan | 3% | 5.8 ± 0.22 | 94.9 ± 0.45 | 7.5 ± 0.65 | 7259.4 ± 118.25 | 9843 ± 59.75 | 88.31 ± 0.46 |
| G4 | Chitosan | 1% | 5.8 ± 0.13 | 93.2 ± 0.61 | 12.91 ± 0.59 | 5787.9 ± 105.76 | 277.3 ± 38.21 | 92.24 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassem, A.; Refai, H.; El-Nabarawi, M.A.; Abdellatif, M.M. Formulation and Evaluation of Prednisolone Sodium Metazoate-Loaded Mucoadhesive Quatsomal Gel for Local Treatment of Recurrent Aphthous Ulcers: Optimization, In Vitro, Ex Vivo, and In Vivo Studies. Pharmaceutics 2023, 15, 1947. https://doi.org/10.3390/pharmaceutics15071947
Kassem A, Refai H, El-Nabarawi MA, Abdellatif MM. Formulation and Evaluation of Prednisolone Sodium Metazoate-Loaded Mucoadhesive Quatsomal Gel for Local Treatment of Recurrent Aphthous Ulcers: Optimization, In Vitro, Ex Vivo, and In Vivo Studies. Pharmaceutics. 2023; 15(7):1947. https://doi.org/10.3390/pharmaceutics15071947
Chicago/Turabian StyleKassem, Ashraf, Hanan Refai, Mohamed A. El-Nabarawi, and Menna M. Abdellatif. 2023. "Formulation and Evaluation of Prednisolone Sodium Metazoate-Loaded Mucoadhesive Quatsomal Gel for Local Treatment of Recurrent Aphthous Ulcers: Optimization, In Vitro, Ex Vivo, and In Vivo Studies" Pharmaceutics 15, no. 7: 1947. https://doi.org/10.3390/pharmaceutics15071947
APA StyleKassem, A., Refai, H., El-Nabarawi, M. A., & Abdellatif, M. M. (2023). Formulation and Evaluation of Prednisolone Sodium Metazoate-Loaded Mucoadhesive Quatsomal Gel for Local Treatment of Recurrent Aphthous Ulcers: Optimization, In Vitro, Ex Vivo, and In Vivo Studies. Pharmaceutics, 15(7), 1947. https://doi.org/10.3390/pharmaceutics15071947







