Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles
Abstract
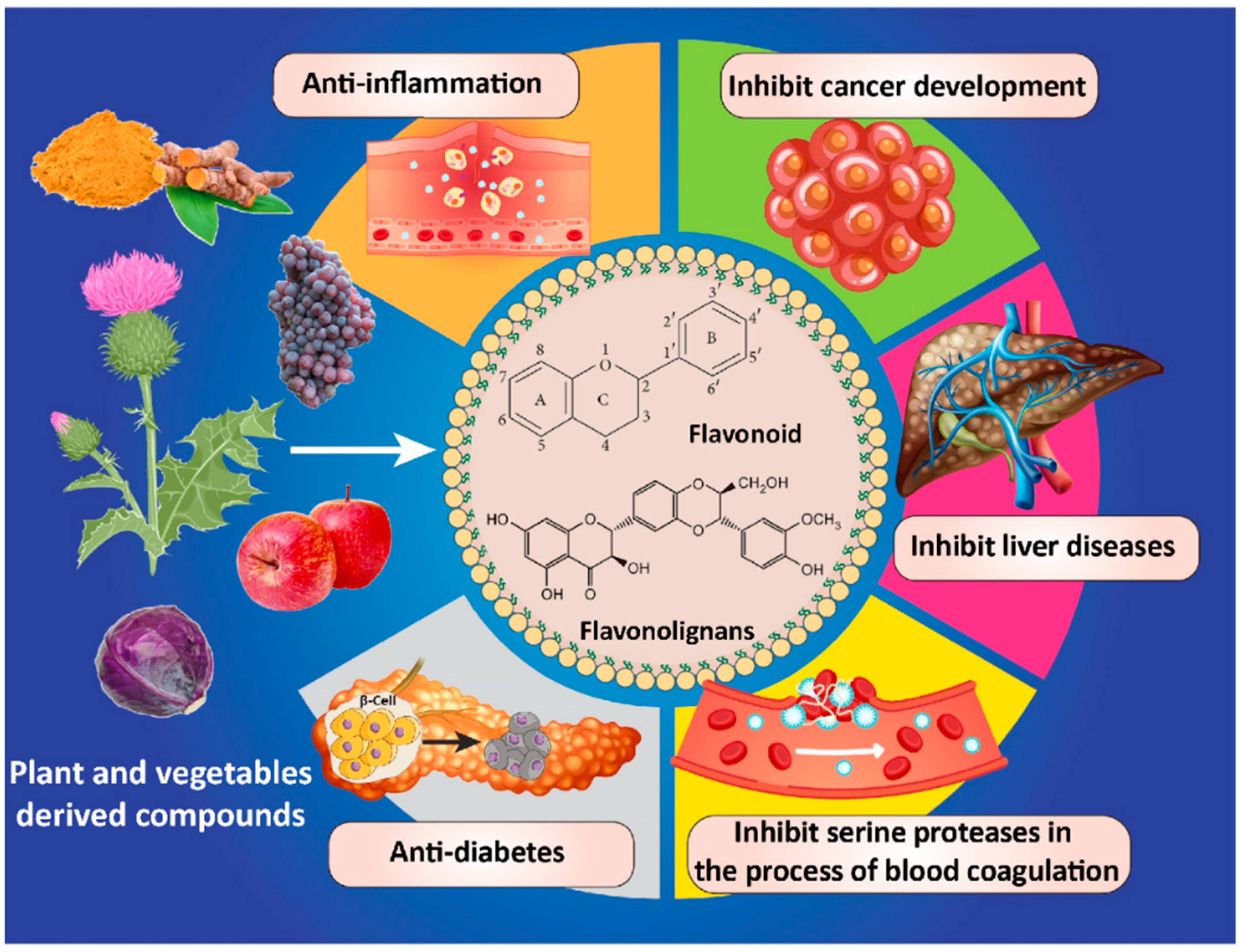
1. Introduction
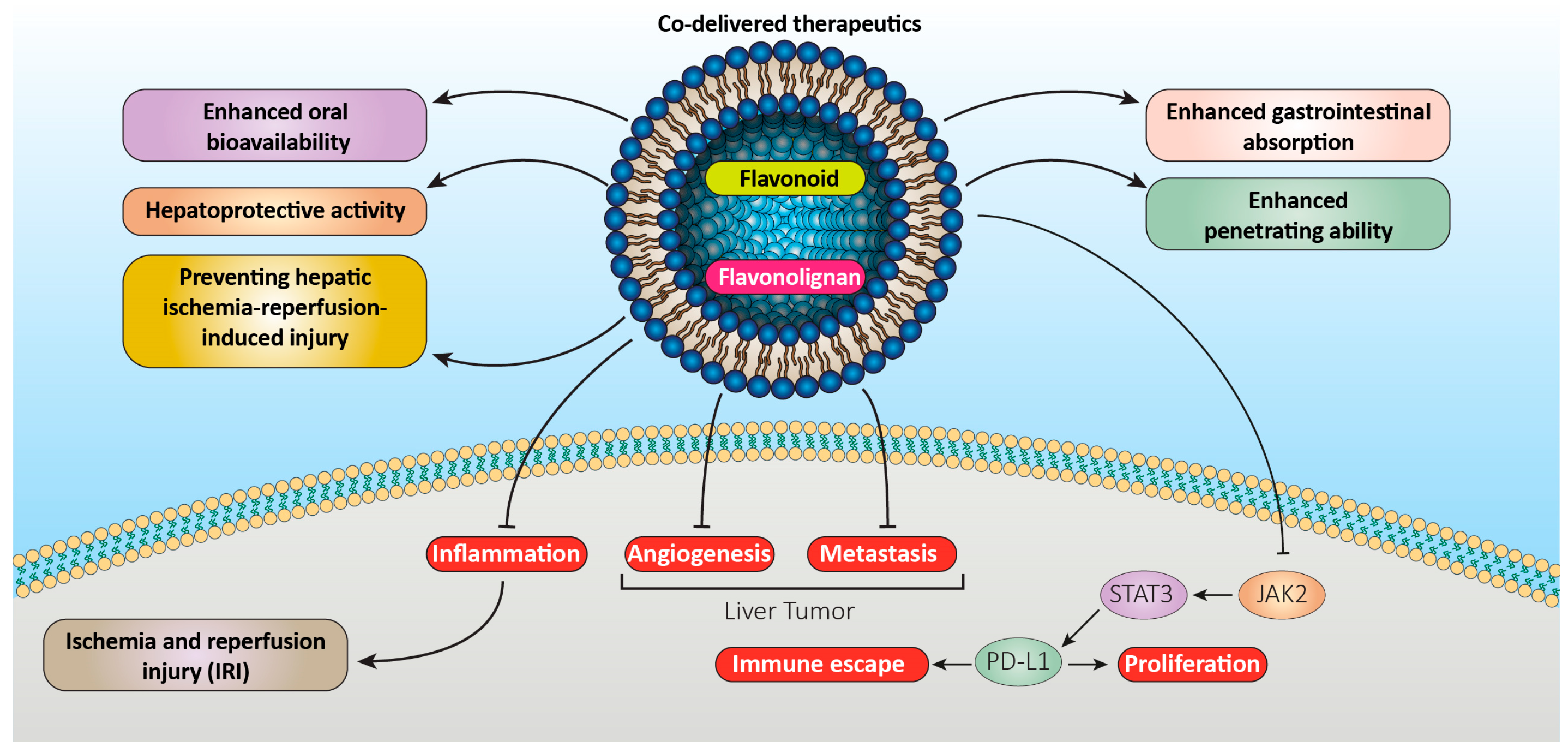
2. Lipid-Based Carrier Systems
2.1. Liposomes
2.1.1. Flavonoid Liposomes
2.1.2. Flavonolignan Liposomes
2.2. Micro- and Nanoemulsions
2.2.1. Flavonoid Nanoemulsions
2.2.2. Flavonolignan Nanoemulsions
2.3. Solid Lipid Nanoparticles
2.3.1. Flavonoid SLNs
2.3.2. Flavonolignan SLNs
3. Clinical Studies and Market Product
4. Limitation of Translation Research to Product and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Hu, K.; Di, L.; Wang, P.; Liu, Z.; Zhang, J.; Yue, P.; Song, W.; Zhang, J.; Chen, T.J. Traditional herbal medicine and nanomedicine: Converging disciplines to improve therapeutic efficacy and human health. Adv. Drug Deliv. Rev. 2021, 178, 113964. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Kaviyani, N.; Tavakol, S. Monoterpenes modulating autophagy: A review study. Basic Clin. Pharmacol. Toxicol. 2020, 126, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Afshar, E.G.; Farkhondeh, T.; Samarghandian, S. Potential therapeutic effects of curcumin mediated by JAK/STAT signaling pathway: A review. Phytother. Res. 2020, 34, 1745–1760. [Google Scholar] [CrossRef] [PubMed]
- Nagula, R.L.; Wairkar, S.J. Recent advances in topical delivery of flavonoids: A review. J. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Jasim, H.A.; Nahar, L.; Jasim, M.A.; Moore, S.A.; Ritchie, K.J.; Sarker, S.D. Chalcones: Synthetic chemistry follows where nature leads. Biomolecules 2021, 11, 1203. [Google Scholar] [CrossRef]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of lipid-based encapsulation on the bioaccessibility and bioavailability of phenolic compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef]
- Saito, A. Challenges and complexity of functionality evaluation of flavan-3-ol derivatives. Biosci. Biotechnol. Biochem. 2017, 81, 1055–1060. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Tavakol, S.; Ahmadi, Z.; Roomiani, S.; Mohammadinejad, R.; Samarghandian, S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother. Res. 2020, 34, 911–923. [Google Scholar] [CrossRef]
- Prasanna, P.; Upadhyay, A. Flavonoid-based nanomedicines in Alzheimer’s disease therapeutics: Promises made, a long way to go. ACS Pharmacol. Transl. Sci. 2021, 4, 74–95. [Google Scholar] [CrossRef]
- Velázquez-Lam, E.; Imperial, J.; Ponz, F. Polyphenol-functionalized plant viral-derived nanoparticles exhibit strong antimicrobial and antibiofilm formation activities. ACS Appl. Bio Mater. 2020, 3, 2040–2047. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, P.; Arya, S.K. Naringin: A potential natural product in the field of biomedical applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100068. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; pp. 200–211. [Google Scholar]
- Wang, X.; Yang, Z.; Su, F.; Li, J.; Boadi, E.O.; Chang, Y.-X.; Wang, H. Study on structure activity relationship of natural flavonoids against thrombin by molecular docking virtual screening combined with activity evaluation in vitro. Molecules 2020, 25, 422. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Izzo, A.A.; Milić, N.; Cicala, C.; Santini, A.; Capasso, R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother. Res. 2018, 32, 2202–2213. [Google Scholar] [CrossRef]
- Gorhe, A.A.; Kulkarni, A.; Pallavi, K.; Snehal, J. Silymarin Loaded Novel Drug Delivery for Oral and Topical Administration. J. Drug Deliv. Ther. 2020, 10, 262–270. [Google Scholar] [CrossRef]
- Csupor, D.; Csorba, A.; Hohmann, J. Recent advances in the analysis of flavonolignans of Silybum marianum. J. Pharm. Biomed. Anal. 2016, 130, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Elfaky, M.A.; Sirwi, A.; Ismail, S.H.; Awad, H.H.; Gad, S.S. Hepatoprotective effect of silver nanoparticles at two different particle sizes: Comparative study with and without silymarin. Curr. Issues Mol. Biol. 2022, 44, 2923–2938. [Google Scholar] [CrossRef]
- Islam, A.; Mishra, A.; Siddiqui, M.A.; Siddiquie, S.J. Recapitulation of Evidence of Phytochemical, Pharmacokinetic and Biomedical Application of Silybin. Drug Res. 2021, 71, 489–503. [Google Scholar] [CrossRef]
- Abrol, S.; Trehan, A.; Katare, O. Formulation, characterization, and in vitro evaluation of silymarin-loaded lipid microspheres. Drug Deliv. 2004, 11, 185–191. [Google Scholar] [CrossRef]
- El-Far, M.; Salah, N.; Essam, A.; Abd El-Azim, A.O.; El-Sherbiny, I.M. Silymarin nanoformulation as potential anticancer agent in experimental Ehrlich ascites carcinoma-bearing animals. Nanomedicine 2018, 13, 1858–1865. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Sayed, I.E.T.E.; El-Torgoman, A.M.A.; Kalam, A.; Wageh, S.; Kamel, M.A. Green Synthesis of Silymarin–Chitosan Nanoparticles as a New Nano Formulation with Enhanced Anti-Fibrotic Effects against Liver Fibrosis. Int. J. Mol. Sci. 2022, 23, 5420. [Google Scholar] [CrossRef]
- Yousefi, M.; Shadnoush, M.; Sohrabvandi, S.; Khorshidian, N.; Mortazavian, A.M. Encapsulation Systems for Delivery of Flavonoids: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13934–13951. [Google Scholar]
- Ding, Y.; Tong, Z.; Jin, L.; Ye, B.; Zhou, J.; Sun, Z.; Yang, H.; Hong, L.; Huang, F.; Wang, W. An NIR Discrete Metallacycle Constructed from Perylene Bisimide and Tetraphenylethylene Fluorophores for Imaging-Guided Cancer Radio-Chemotherapy. Adv. Mater. 2022, 34, 2106388. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Yang, J.; Shi, B.; Ye, B.; Wang, M.; Huang, F.; Stang, P.J. Polymeric nanoparticles integrated from discrete organoplatinum (II) metallacycle by stepwise post-assembly polymerization for synergistic cancer therapy. Chem. Mater. 2020, 32, 4564–4573. [Google Scholar] [CrossRef]
- Parveen, R.; Baboota, S.; Ali, J.; Ahuja, A.; Vasudev, S.S.; Ahmad, S. Oil based nanocarrier for improved oral delivery of silymarin: In vitro and in vivo studies. Int. J. Pharm. 2011, 413, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Y.; Du Hyeong Hwang, A.M.Y.; Kim, D.W.; Shin, Y.-J.; Bae, O.-N.; Kim, Y.-I.; Kim, J.O.; Yong, C.S.; Choi, H.-G. Silymarin-loaded solid nanoparticles provide excellent hepatic protection: Physicochemical characterization and in vivo evaluation. Int. J. Nanomed. 2013, 8, 3333. [Google Scholar]
- Ma, Y.; He, H.; Xia, F.; Li, Y.; Lu, Y.; Chen, D.; Qi, J.; Lu, Y.; Zhang, W.; Wu, W. In vivo fate of lipid-silybin conjugate nanoparticles: Implications on enhanced oral bioavailability. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2643–2654. [Google Scholar] [CrossRef]
- Hanafy, N.A.; El-Kemary, M.A. Silymarin/curcumin loaded albumin nanoparticles coated by chitosan as muco-inhalable delivery system observing anti-inflammatory and anti COVID-19 characterizations in oleic acid triggered lung injury and in vitro COVID-19 experiment. Int. J. Biol. Macromol. 2022, 198, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.-J.; Choi, H.S.; Perumalsamy, H.; Shanmugam, R.; Thangavelu, L.; Balusamy, S.R.; Kim, Y.-J. Biosynthesis and cytotoxic effect of silymarin-functionalized selenium nanoparticles induced autophagy mediated cellular apoptosis via downregulation of PI3K/Akt/mTOR pathway in gastric cancer. Phytomedicine 2022, 99, 154014. [Google Scholar] [CrossRef]
- Cunha, C.; Daniel-da-Silva, A.L.; Oliveira, H. Drug Delivery Systems and Flavonoids: Current Knowledge in Melanoma Treatment and Future Perspectives. Micromachines 2022, 13, 1838. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Ranjbar, S.; Gupta, V.K.; Asif, M.; Pourseyedi, S.; Karimi, M.J.; Mohammadinejad, R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015, 207, 159–163. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Madamsetty, V.S.; Kumar, A.; Varzandeh, M.; Dehshahri, A.; Zarrabi, A.; Sharififar, F.; Mohammadi, M.; Fahimipour, A.; Ramakrishna, S. Electrospun nanocarriers for delivering natural products for cancer therapy. Trends Food Sci. Technol. 2021, 118, 887–904. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-derived nanostructures: Types and applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Madamsetty, V.S.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Schneible, J.D.; Fatemi, I.; Shirvani, A.; Zarrabi, A.; Azedi, F.; Dehshahri, A. Chitosan: A versatile bio-platform for breast cancer theranostics. J. Control. Release 2022, 341, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Dehshahri, A.; Sadeghpour, H.; Kazemi Oskuee, R.; Fadaei, M.; Sabahi, Z.; Alhashemi, S.H.; Mohazabieh, E. Interleukin-12 plasmid DNA delivery using l-thyroxine-conjugated polyethylenimine nanocarriers. J. Nanoparticle Res. 2014, 16, 2423. [Google Scholar] [CrossRef]
- Hajighahramani, N.; Eslami, M.; Negahdaripour, M.; Ghoshoon, M.B.; Dehshahri, A.; Erfani, N.; Heidari, R.; Gholami, A.; Nezafat, N.; Ghasemi, Y. Computational design of a chimeric epitope-based vaccine to protect against Staphylococcus aureus infections. Mol. Cell. Probes 2019, 46, 101414. [Google Scholar] [CrossRef] [PubMed]
- Dehshahri, A.; Ashrafizadeh, M.; Afshar, E.G.; Pardakhty, A.; Mandegary, A.; Mohammadinejad, R.; Sethi, G. Topoisomerase inhibitors: Pharmacology and emerging nanoscale delivery systems. Pharmacol. Res. 2020, 151, 104551. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.; Kalari, S.; Morowvat, M.H.; Sabahi, Z.; Dehshahri, A. Green synthesis of selenium nanoparticles by cyanobacterium Spirulina platensis (abdf2224): Cultivation condition quality controls. BioMed Res. Int. 2021, 2021, 6635297. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Dehshahri, A.; Madamsetty, V.S.; Zahmatkeshan, M.; Tavakol, S.; Makvandi, P.; Khorsandi, D.; Pardakhty, A.; Ashrafizadeh, M.; Afshar, E.G. In vivo gene delivery mediated by non-viral vectors for cancer therapy. J. Control. Release 2020, 325, 249–275. [Google Scholar] [CrossRef]
- Mahani, M.; Pourrahmani-Sarbanani, M.; Yoosefian, M.; Divsar, F.; Mousavi, S.M.; Nomani, A. Doxorubicin delivery to breast cancer cells with transferrin-targeted carbon quantum dots: An in vitro and in silico study. J. Drug Deliv. Sci. Technol. 2021, 62, 102342. [Google Scholar] [CrossRef]
- Chaudhary, S.; Garg, T.; Murthy, R.; Rath, G.; Goyal, A.K. Development, optimization and evaluation of long chain nanolipid carrier for hepatic delivery of silymarin through lymphatic transport pathway. Int. J. Pharm. 2015, 485, 108–121. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Mozafari, M. Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Lian, R.; Lu, Y.; Qi, J.; Tan, Y.; Niu, M.; Guan, P.; Hu, F.; Wu, W. Silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices: Physical characterization and enhanced oral bioavailability. Aaps Pharmscitech 2011, 12, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Sezer, C.V. An In Vitro Assessment of the Cytotoxic and Apoptotic Potency of Silymarin and Silymarin Loaded Solid Lipid Nanoparticles on Lung and Breast Cancer Cells. Pak. J. Zool. 2021, 53, 1407–1415. [Google Scholar] [CrossRef]
- Nazem, Z.; Firoozian, F.; Khodabandelou, S.; Mohammadi, M.; Mahboobian, M.M. Systematic Optimization of Solid Lipid Nanoparticles of Silybin for Improved Oral Drug Delivery by Box-Behnken Design: In Vitro and In Vivo Evaluations. J. Pharm. Innov. 2022, 1–13. [Google Scholar] [CrossRef]
- Hong, J.; Jeon, B.-J.; Myeong, J.; Kim, G.-Y.; Lee, J.; Kim, S. Emulsifying Solution Prepared by an AI-based Drug Delivery System (AIDDS™) Enhances Bioavailability of Silymarin. Food Suppl. Biomater. Health 2021, 1, e23. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Angelico, R. Formulation strategies for enhancing the bioavailability of silymarin: The state of the art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef]
- Javed, S.; Kohli, K.; Ali, M. Reassessing bioavailability of silymarin. Altern. Med. Rev. 2011, 16, 239. [Google Scholar]
- Kumar, N.; Rai, A.; Reddy, N.D.; Raj, P.V.; Jain, P.; Deshpande, P.; Mathew, G.; Kutty, N.G.; Udupa, N.; Rao, C.M. Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol. Rep. 2014, 66, 788–798. [Google Scholar] [CrossRef]
- Maheshwari, H.; Agarwal, R.; Patil, C.; Katare, O.P. Preparation and pharmacological evaluation of silibinin liposomes. Arzneimittelforschung 2003, 53, 420–427. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Nano-soldiers ameliorate silibinin delivery: A Review Study. Curr. Drug Deliv. 2020, 17, 15–22. [Google Scholar] [CrossRef]
- Iqbal, B.; Ali, J.; Baboota, S. Silymarin loaded nanostructured lipid carrier: From design and dermatokinetic study to mechanistic analysis of epidermal drug deposition enhancement. J. Mol. Liq. 2018, 255, 513–529. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, D.; Li, Z.; Duan, C.; Wang, Y.; Feng, F.; Wang, F.; Liu, Y.; Zhang, Q. Nanostructured lipid carriers for parenteral delivery of silybin: Biodistribution and pharmacokinetic studies. Colloids Surf. B Biointerfaces 2010, 80, 213–218. [Google Scholar] [CrossRef]
- Piazzini, V.; Lemmi, B.; D’Ambrosio, M.; Cinci, L.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Nanostructured lipid carriers as promising delivery systems for plant extracts: The case of silymarin. Appl. Sci. 2018, 8, 1163. [Google Scholar] [CrossRef]
- Ma, Y.; He, H.; Fan, W.; Li, Y.; Zhang, W.; Zhao, W.; Qi, J.; Lu, Y.; Dong, X.; Wu, W. In vivo fate of biomimetic mixed micelles as nanocarriers for bioavailability enhancement of lipid–drug conjugates. ACS Biomater. Sci. Eng. 2017, 3, 2399–2409. [Google Scholar] [CrossRef]
- Kumar, R. Lipid-based nanoparticles for drug-delivery systems. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–284. [Google Scholar]
- Rawat, M.; Singh, D.; Saraf, S.; Saraf, S. Nanocarriers: Promising vehicle for bioactive drugs. Biol. Pharm. Bull. 2006, 29, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Patel, B.; Thakkar, H. Lipid based nanocarriers: Promising drug delivery system for topical application. Eur. J. Lipid Sci. Technol. 2021, 123, 2000264. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef] [PubMed]
- Salawi, A. Self-emulsifying drug delivery systems: A novel approach to deliver drugs. Drug Deliv. 2022, 29, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Nayak, P.; Yadav, N.; Singh, M.; Tambuwala, M.M.; Aljabali, A.A. Orally administered self-emulsifying drug delivery system in disease management: Advancement and patents. Expert Opin. Drug Deliv. 2021, 18, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the scale-up methods for the preparation of solid lipid nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 2021, 335, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Izza, N.m.; Watanabe, N.; Okamoto, Y.; Suga, K.; Wibisono, Y.; Kajimura, N.; Mitsuoka, K.; Umakoshi, H. Dependence of the Core–Shell Structure on the Lipid Composition of Nanostructured Lipid Carriers: Implications for Drug Carrier Design. ACS Appl. Nano Mater. 2022, 5, 9958–9969. [Google Scholar] [CrossRef]
- Wang, J.; Gong, J.; Wei, Z. Strategies for liposome drug delivery systems to improve tumor treatment efficacy. AAPS PharmSciTech 2022, 23, 27. [Google Scholar] [CrossRef]
- Sharma, V.K.; Agrawal, M.K. A historical perspective of liposomes-a bio nanomaterial. Mater. Today Proc. 2021, 45, 2963–2966. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Li, Z.; Loh, X.J. Small molecule therapeutic-loaded liposomes as therapeutic carriers: From development to clinical applications. RSC Adv. 2016, 6, 70592–70615. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Suita, Y.; Miriyala, S.; Dean, J.; Tapinos, N.; Shen, J. Advances in lipid-based nanoparticles for cancer chemoimmunotherapy. Pharmaceutics 2021, 13, 520. [Google Scholar] [CrossRef]
- Elmowafy, M.; Viitala, T.; Ibrahim, H.M.; Abu-Elyazid, S.K.; Samy, A.; Kassem, A.; Yliperttula, M. Silymarin loaded liposomes for hepatic targeting: In vitro evaluation and HepG2 drug uptake. Eur. J. Pharm. Sci. 2013, 50, 161–171. [Google Scholar] [CrossRef]
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Evaluation of hybrid liposomes-encapsulated silymarin regarding physical stability and in vivo performance. Int. J. Pharm. 2006, 319, 121–129. [Google Scholar] [CrossRef]
- El-Samaligy, M.; Afifi, N.; Mahmoud, E. Increasing bioavailability of silymarin using a buccal liposomal delivery system: Preparation and experimental design investigation. Int. J. Pharm. 2006, 308, 140–148. [Google Scholar] [CrossRef]
- Chu, C.; Tong, S.-S.; Xu, Y.; Wang, L.; Fu, M.; Ge, Y.-R.; Yu, J.-N.; Xu, X.-M. Proliposomes for oral delivery of dehydrosilymarin: Preparation and evaluation in vitro and in vivo. Acta Pharmacol. Sin. 2011, 32, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Furneri, P.M.; Petronio, G.P.; Fuochi, V.; Cupri, S.; Pignatello, R. Nanosized devices as antibiotics and antifungals delivery: Past, news, and outlook. In Nanostructures for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 697–748. [Google Scholar]
- Khorasani, S.; Danaei, M.; Mozafari, M. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Mozafari, R.M. Nanoliposomes: From Fundamentals to Recent Developments; Trafford Publishing Co. Ltd.: Oxford, UK, 2005. [Google Scholar]
- Szoka, F., Jr.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef]
- Liao, A.-M.; Cai, B.; Huang, J.-H.; Hui, M.; Lee, K.-K.; Lee, K.Y.; Chun, C. Synthesis, anticancer activity and potential application of diosgenin modified cancer chemotherapeutic agent cytarabine. Food Chem. Toxicol. 2021, 148, 111920. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-processing techniques for the improvement of liposome stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M.; Varma, R.S.; Hamidi, M.; Mozafari, M.R. Conventional and novel methods for the preparation of micro and nanoliposomes. Micro Nano Bio Asp. 2022, 1, 18–29. [Google Scholar]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Jing, D.; Wu, W.; Chen, X.; Xiao, H.; Zhang, Z.; Chen, F.; Zhang, Z.; Liu, J.; Shao, Z.; Pu, F. Quercetin encapsulated in Folic Acid-Modified Liposomes is therapeutic against osteosarcoma by non-Covalent binding to the JH2 Domain of JAK2 via the JAK2-STAT3-PDL1. Pharmacol. Res. 2022, 182, 106287. [Google Scholar] [CrossRef]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef]
- Ferreira-Silva, M.; Faria-Silva, C.; Carvalheiro, M.C.; Simões, S.; Marinho, H.S.; Marcelino, P.; Campos, M.C.; Metselaar, J.M.; Fernandes, E.; Baptista, P.V. Quercetin Liposomal Nanoformulation for Ischemia and Reperfusion Injury Treatment. Pharmaceutics 2022, 14, 104. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Gao, Y.; Liu, S.; Li, K.; Wang, S.; Gao, L.; Shi, M.; Liu, Z.; Han, Z. Effect of a drug delivery system made of quercetin formulated into PEGylation liposomes on cervical carcinoma in vitro and in vivo. J. Nanomater. 2021, 2021, 9389934. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, R.; Huang, H. Anti-Allergic Effects of Quercetin and Quercetin Liposomes in RBL-2H3 Cells. Endocr. Metab. Immune Disord. Drug Targets 2022, 23, 692–701. [Google Scholar]
- Li, H.; Chen, P.; Wang, M.; Wang, W.; Li, F.; Han, X.; Ren, J.; Duan, X. Liposome quercetin enhances the ablation effects of microwave ablation in treating the rabbit VX2 liver tumor model. Int. J. Hyperth. 2022, 39, 162–172. [Google Scholar] [CrossRef]
- Renault-Mahieux, M.; Vieillard, V.; Seguin, J.; Espeau, P.; Le, D.T.; Lai-Kuen, R.; Mignet, N.; Paul, M.; Andrieux, K. Co-Encapsulation of Fisetin and Cisplatin into Liposomes for Glioma Therapy: From Formulation to Cell Evaluation. Pharmaceutics 2021, 13, 970. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Hussain, A.; AlRajhi, M.; Alshehri, S.; Imam, S.S.; Qamar, W. Luteolin-loaded elastic liposomes for transdermal delivery to control breast cancer: In vitro and ex vivo evaluations. Pharmaceuticals 2021, 14, 1143. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, P.K.; Mutha, R.E.; Surana, S.J. Electrostatic deposition assisted preparation, characterization and evaluation of chrysin liposomes for breast cancer treatment. Drug Dev. Ind. Pharm. 2021, 47, 809–819. [Google Scholar] [CrossRef]
- Huang, R.; Zhao, Z.; Jiang, X.; Li, W.; Zhang, L.; Wang, B.; Tie, H. Liposomal chrysin attenuates hepatic ischaemia-reperfusion injury: Possible mechanism via inhibiting NLRP3 inflammasome. J. Pharm. Pharmacol. 2022, 74, 216–226. [Google Scholar] [CrossRef]
- Tian, J.-Y.; Chi, C.-L.; Bian, G.; Xing, D.; Guo, F.-J.; Wang, X.-Q. PSMA conjugated combinatorial liposomal formulation encapsulating genistein and plumbagin to induce apoptosis in prostate cancer cells. Colloids Surf. B Biointerfaces 2021, 203, 111723. [Google Scholar] [CrossRef]
- Ochi, M.M.; Amoabediny, G.; Rezayat, S.M.; Akbarzadeh, A.; Ebrahimi, B. In vitro co-delivery evaluation of novel pegylated nano-liposomal herbal drugs of silibinin and glycyrrhizic acid (nano-phytosome) to hepatocellular carcinoma cells. Cell J. (Yakhteh) 2016, 18, 135. [Google Scholar]
- Ripoli, M.; Angelico, R.; Sacco, P.; Ceglie, A.; Mangia, A. Phytoliposome-based silibinin delivery system as a promising strategy to prevent hepatitis C virus infection. J. Biomed. Nanotechnol. 2016, 12, 770–780. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, Y.; Zhang, Y.; Dang, B.; Liu, Y.; Feng, N. Enhanced oral bioavailability of silymarin using liposomes containing a bile salt: Preparation by supercritical fluid technology and evaluation in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6633. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Asfour, M.H.; Salama, A.A. Improved hepatoprotective activity of silymarin via encapsulation in the novel vesicular nanosystem bilosomes. Drug Dev. Ind. Pharm. 2017, 43, 2043–2054. [Google Scholar] [CrossRef]
- Mady, F.M.; Essa, H.; El-Ammawi, T.; Abdelkader, H.; Hussein, A.K. Formulation and clinical evaluation of silymarin pluronic-lecithin organogels for treatment of atopic dermatitis. Drug Des. Dev. Ther. 2016, 10, 1101. [Google Scholar]
- Xiao, Y.; Song, Y.; Chen, Z.; Ping, Q. Preparation of silymarin proliposomes and its pharmacokinetics in rats. Yao Xue Xue Bao Acta Pharm. Sin. 2005, 40, 758. [Google Scholar]
- Singh, I.R.; Pulikkal, A.K. Preparation, stability and biological activity of essential oil-based nano emulsions: A comprehensive review. OpenNano 2022, 8, 100066. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against β-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.B.U.; Cetin, M.; Orgul, D.; Taghizadehghalehjoughi, A.; Hacımuftuoglu, A.; Hekimoglu, S. Formulation and in vitro evaluation of topical nanoemulsion and nanoemulsion-based gels containing daidzein. J. Drug Deliv. Sci. Technol. 2019, 52, 189–203. [Google Scholar] [CrossRef]
- Hussein, J.; El-Naggar, M.E. Synthesis of an environmentally quercetin nanoemulsion to ameliorate diabetic-induced cardiotoxicity. Biocatal. Agric. Biotechnol. 2021, 33, 101983. [Google Scholar] [CrossRef]
- Mahadev, M.; Nandini, H.S.; Ramu, R.; Gowda, D.V.; Almarhoon, Z.M.; Al-Ghorbani, M.; Mabkhot, Y.N. Fabrication and evaluation of quercetin nanoemulsion: A delivery system with improved bioavailability and therapeutic efficacy in diabetes mellitus. Pharmaceuticals 2022, 15, 70. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Groo, A.-C.; Iacopetta, D.; Séguy, L.; Mariconda, A.; Puoci, F.; Saturnino, C.; Leroy, F.; Since, M.; Longo, P. A winning strategy to improve the anticancer properties of Cisplatin and Quercetin based on the nanoemulsions formulation. J. Drug Deliv. Sci. Technol. 2021, 66, 102907. [Google Scholar] [CrossRef]
- Son, H.-Y.; Lee, M.-S.; Chang, E.; Kim, S.-Y.; Kang, B.; Ko, H.; Kim, I.-H.; Zhong, Q.; Jo, Y.-H.; Kim, C.-T. Formulation and characterization of quercetin-loaded oil in water nanoemulsion and evaluation of hypocholesterolemic activity in rats. Nutrients 2019, 11, 244. [Google Scholar] [CrossRef]
- Ahmadi Oskooei, F.; Mehrzad, J.; Asoodeh, A.; Motavalizadehkakhky, A. Olive oil-based quercetin nanoemulsion (QuNE)’s interactions with human serum proteins (HSA and HTF) and its anticancer activity. J. Biomol. Struct. Dyn. 2021, 41, 778–791. [Google Scholar] [CrossRef]
- Marques, M.B.; Machado, A.P.; Santos, P.A.; Carrett-Dias, M.; Araújo, G.S.; da Silva Alves, B.; de Oliveira, B.S.; da Silva Júnior, F.M.; Dora, C.L.; Cañedo, A.D. Anti-MDR effects of quercetin and its Nanoemulsion in multidrug-resistant human Leukemia cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2021, 21, 1911–1920. [Google Scholar] [CrossRef]
- Magura, J.; Hassan, D.; Moodley, R.; Mackraj, I. Hesperidin-loaded nanoemulsions improve cytotoxicity, induce apoptosis, and downregulate miR-21 and miR-155 expression in MCF-7. J. Microencapsul. 2021, 38, 486–495. [Google Scholar] [CrossRef]
- Colombo, M.; Figueiró, F.; de Fraga Dias, A.; Teixeira, H.F.; Battastini, A.M.O.; Koester, L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018, 543, 214–223. [Google Scholar] [CrossRef]
- Singh, B.; Bandopadhyay, S.; Kapil, R.; Singh, R.; Katare, O.P. Self-emulsifying drug delivery systems (SEDDS): Formulation development, characterization, and applications. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 427–451. [Google Scholar] [CrossRef]
- Zanchetta, B.; Chaud, M.; Santana, M. Self-emulsifying drug delivery systems (SEDDS) in pharmaceutical development. J. Adv. Chem. Eng. 2015, 5, 1000130. [Google Scholar]
- Li, X.; Yuan, Q.; Huang, Y.; Zhou, Y.; Liu, Y. Development of silymarin self-microemulsifying drug delivery system with enhanced oral bioavailability. Aaps Pharmscitech 2010, 11, 672–678. [Google Scholar] [CrossRef]
- Panapisal, V.; Charoensri, S.; Tantituvanont, A. Formulation of microemulsion systems for dermal delivery of silymarin. Aaps Pharmscitech 2012, 13, 389–399. [Google Scholar] [CrossRef]
- Wu, W.; Wang, Y.; Que, L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006, 63, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, H.; Xie, L.; Liu, K.; Zhang, X.; Li, X. Tea saponins as natural emulsifiers and cryoprotectants to prepare silymarin nanoemulsion. LWT 2022, 156, 113042. [Google Scholar] [CrossRef]
- Wei, Y.; Ye, X.; Shang, X.; Peng, X.; Bao, Q.; Liu, M.; Guo, M.; Li, F. Enhanced oral bioavailability of silybin by a supersaturatable self-emulsifying drug delivery system (S-SEDDS). Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 22–28. [Google Scholar] [CrossRef]
- Adhikari, M.; Arora, R. Nano-silymarin provides protection against γ-radiation-induced oxidative stress in cultured human embryonic kidney cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2015, 792, 1–11. [Google Scholar] [CrossRef]
- Calligaris, S.; Comuzzo, P.; Bot, F.; Lippe, G.; Zironi, R.; Anese, M.; Nicoli, M.C. Nanoemulsions as delivery systems of hydrophobic silybin from silymarin extract: Effect of oil type on silybin solubility, in vitro bioaccessibility and stability. LWT-Food Sci. Technol. 2015, 63, 77–84. [Google Scholar] [CrossRef]
- Nagi, A.; Iqbal, B.; Kumar, S.; Sharma, S.; Ali, J.; Baboota, S. Quality by design based silymarin nanoemulsion for enhancement of oral bioavailability. J. Drug Deliv. Sci. Technol. 2017, 40, 35–44. [Google Scholar] [CrossRef]
- Kumar, S.; Randhawa, J.K. High melting lipid based approach for drug delivery: Solid lipid nanoparticles. Mater. Sci. Eng. C 2013, 33, 1842–1852. [Google Scholar] [CrossRef]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; MaÈder, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.N.; Omidfar, K. Drug targeting using solid lipid nanoparticles. Chem. Phys. Lipids 2014, 181, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, M.; Qi, J.; Lu, Y.; Wu, W. Comparison of the oral bioavailability of silymarin-loaded lipid nanoparticles with their artificial lipolysate counterparts: Implications on the contribution of integral structure. Int. J. Pharm. 2015, 489, 195–202. [Google Scholar] [CrossRef]
- Xu, P.; Yin, Q.; Shen, J.; Chen, L.; Yu, H.; Zhang, Z.; Li, Y. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int. J. Pharm. 2013, 454, 21–30. [Google Scholar] [CrossRef]
- He, J.; Hou, S.; Lu, W.; Zhu, L.; Feng, J. Preparation, pharmacokinetics and body distribution of silymarin-loaded solid lipid nanoparticles after oral administration. J. Biomed. Nanotechnol. 2007, 3, 195–202. [Google Scholar] [CrossRef]
- Gill, B.; Singh, J.; Sharma, V.; Kumar, S.H. Emulsomes: An emerging vesicular drug delivery system. Asian J. Pharm. (AJP) 2014, 6, 133–142. [Google Scholar] [CrossRef]
- Khan, S.; Baboota, S.; Ali, J.; Khan, S.; Narang, R.S.; Narang, J.K. Nanostructured lipid carriers: An emerging platform for improving oral bioavailability of lipophilic drugs. Int. J. Pharm. Investig. 2015, 5, 182. [Google Scholar]
- Rishitha, N.; Muthuraman, A. Therapeutic evaluation of solid lipid nanoparticle of quercetin in pentylenetetrazole induced cognitive impairment of zebrafish. Life Sci. 2018, 199, 80–87. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Baskaran, R.; Jang, Y.S.; Oh, S.H.; Yoo, B.K. Quercetin-loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. Aaps Pharmscitech 2017, 18, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Banala, V.T.; Kushwaha, P.; Karvande, A.; Sharma, S.; Tripathi, A.K.; Verma, A.; Trivedi, R.; Mishra, P.R. Quercetin-loaded solid lipid nanoparticles improve osteoprotective activity in an ovariectomized rat model: A preventive strategy for post-menopausal osteoporosis. Rsc Adv. 2016, 6, 97613–97628. [Google Scholar] [CrossRef]
- Talarico, L.; Consumi, M.; Leone, G.; Tamasi, G.; Magnani, A. Solid lipid nanoparticles produced via a coacervation method as promising carriers for controlled release of quercetin. Molecules 2021, 26, 2694. [Google Scholar] [CrossRef]
- Azizi, M.; Li, Y.; Kaul, N.; Abbaspourrad, A. Study of the physicochemical properties of fish oil solid lipid nanoparticle in the presence of palmitic acid and quercetin. J. Agric. Food Chem. 2019, 67, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhu, L.; Wei, X.-K.; Shen, Q. Solid lipid nanoparticles of quercetin (a flavonoid) in recovery of motor function after spinal injuries. J. Biomater. Tissue Eng. 2015, 5, 509–513. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, H.; Shu, L.; Zhang, Y.; Okeke, C.; Zhang, L.; Li, J.; Li, N. Preparation and evaluation of Baicalin-loaded cationic solid lipid nanoparticles conjugated with OX26 for improved delivery across the BBB. Drug Dev. Ind. Pharm. 2015, 41, 353–361. [Google Scholar] [CrossRef]
- Komath, S.; Garg, A.; Wahajuddin, M. Development and evaluation of Chrysin-Phospholipid complex loaded solid lipid nanoparticles-storage stability and in vitro anti-cancer activity. J. Microencapsul. 2018, 35, 600–617. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schrader, K.; Schwarz, K. Encapsulation of (-)-epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2019, 244, 91–100. [Google Scholar] [CrossRef]
- Cengiz, M.; Kutlu, H.M.; Burukoglu, D.D.; Ayhancı, A. A comparative study on the therapeutic effects of silymarin and silymarin-loaded solid lipid nanoparticles on D-GaIN/TNF-α-induced liver damage in Balb/c mice. Food Chem. Toxicol. 2015, 77, 93–100. [Google Scholar] [CrossRef]
- Li, P.; Bukhari, S.N.A.; Khan, T.; Chitti, R.; Bevoor, D.B.; Hiremath, A.R.; SreeHarsha, N.; Singh, Y.; Gubbiyappa, K.S. Apigenin-loaded solid lipid nanoparticle attenuates diabetic nephropathy induced by streptozotocin nicotinamide through Nrf2/HO-1/NF-kB signalling pathway. Int. J. Nanomed. 2020, 15, 9115. [Google Scholar] [CrossRef]
- Xu, X.-M.; Li, Q.; Zhu, Y.; Shen, S.; Shen, Z.; Yu, J.-N. Study on the preparation and bio-distribution of silybin lipid nanospheres. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2005, 30, 1912–1914. [Google Scholar]
- Sharma, T.; Singh, D.; Mahapatra, A.; Mohapatra, P.; Sahoo, S.; Sahoo, S.K. Advancements in clinical translation of flavonoid nanoparticles for cancer treatment. Opennano 2022, 8, 100074. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W.J.V. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Patel, R.J.; Saraf, S.; Saraf, S.J. Recent expansion of pharmaceutical nanotechnologies and targeting strategies in the field of phytopharmaceuticals for the delivery of herbal extracts and bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Elena, M.; Eleftheria, G.; Yiannis, S.; Lefteris, Z.C.; Michael, P.; Georgios, A.; Christos, P.C. Clinical trials of nanovesicles for drug delivery applications. In Applications of Nanovesicular Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 467–486. [Google Scholar]
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A translational perspective. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2023–2050. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Wang, G.; Yao, Z.; Dang, X.J. Pharmacokinetics and antitumor efficacy of DSPE-PEG2000 polymeric liposomes loaded with quercetin and temozolomide: Analysis of their effectiveness in enhancing the chemosensitization of drug-resistant glioma cells. Int. J. Mol. Med. 2016, 37, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sharma, V.; Bhushan, B.; Malviya, R.; Awasthi, R.; Kulkarni, G.T. Nanocarriers for protein and peptide delivery: Recent advances and progress. J. Res. Pharm. 2021, 25, 99–116. [Google Scholar]
- Mohanty, S.; Sahoo, A.K.; Konkimalla, V.B.; Pal, A.; Si, S.C.J.A.o. Naringin in combination with isothiocyanates as liposomal formulations potentiates the anti-inflammatory activity in different acute and chronic animal models of rheumatoid arthritis. ACS Omega 2020, 5, 28319–28332. [Google Scholar] [CrossRef]
- Halevas, E.G.; Avgoulas, D.I.; Katsipis, G.; Pantazaki, A.A.J. Flavonoid-liposomes formulations: Physico-chemical characteristics, biological activities and therapeutic applications. Eur. J. Med. Chem. Rep. 2022, 5, 100059. [Google Scholar] [CrossRef]
- Agarawal, K.; Anant Kulkarni, Y.; Wairkar, S. Nanoformulations of flavonoids for diabetes and microvascular diabetic complications. Drug Deliv. Transl. Res. 2023, 13, 18–36. [Google Scholar] [CrossRef]
| Phytochemical Type | Cargo | Carrier | Disease | In Vitro/In Vivo | Major Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Flavonoid | Quercetin | Folic-acid-modified liposome | Osteosarcoma (OS) | In Vitro | The results indicated a new quercetin-based anti-OS treatment via the JAK2-STAT3-PD-L1 signaling axis. | [82] |
| Flavonoid | Quercetin | PEGylated liposomes | Diabetes mellitus (DM) | In Vivo | Attenuating AGE expression, mitigating oxidative stress, and arresting DN development in STZ-induced DN rats. | [83] |
| Flavonoid | Quercetin | Liposomes | IRI | In Vitro/In Vivo | The results indicated that quercetin liposomes could be a useful instrument for resolving therapeutic bottlenecks in hepatic IRI. | [84] |
| Flavonoid | Quercetin | PEGylated liposomes | Cervical carcinoma | In Vitro/In Vivo | Based on the results, PEG-Que-NLs exhibited promising application prospects in treating malignant tumors due to their ability to target tumors, gradual release, and improved quercetin solubility. | [85] |
| Flavonoid | Quercetin | Liposomes | Allergic effects in RBL-2H3 cells | In Vitro | The results indicated that quercetin liposomes may reduce histamine calcium influx and hexosaminidase release significantly. | [86] |
| Flavonoid | Quercetin | Liposomes | Liver neoplasms | In Vivo | Suppressed excessive angiogenesis and residual tumor growth reduce metastasis and increase experimental lifespan among animals. | [87] |
| Flavonoid | Fisetin | PEGylated liposomes | GBM | In Vitro | The liposomal formulation contains both medications with a diameter of 173 8 nm (PDI = 0.12 0.01), as well as a drug loading of fisetin and cisplatin of 1.7 0.3 and 0.8 0.1%, respectively. In addition, the liposomal formulation contains DOPC/cholesterol/DODA-GLY-PEG2000 at a molar ratio of 75.3/20.8/3.9. | [88] |
| Flavonoid | Luteolin | Elastic liposomes | Breast cancer | In Vitro/Ex Vivo | The results indicated that Span 80 was considered as the best edge activator among the studied surfactants (Span 60 and Brij), which is related to negative glass transition temperature. | [89] |
| Flavonoid | Chrysin | Liposomes | Breast cancer | In Silico/In Vivo | Based on the current study, chitosan may shield and encase chrysin, resulting in increasing its cytotoxicity and bioavailability. | [90] |
| Flavonoid | Chrysin | Liposomes | HIR | In Vitro | Chrysin biocompatibility is enhanced by the liposomal drug delivery technology, and LC may lessen liver I/R toxicity and be a useful medication for treating and preventing HIR-induced injury. | [91] |
| Flavonoid | Genistein and plumbagin | PSMA-specific antibody conjugated liposomes | Prostate cancer cells | In Vitro | The created liposomal formulations exhibit long-term (several weeks) stability in PBS and water, and a hydrodynamic diameter of between 80 and 100 nm. | [92] |
| Flavonolignan | Silibinin | Liposomes | Liver neoplasms | In Vitro | The results indicated that cholesterol affects drug-entrapment and drug-leakage properties significantly. About 90% of the medication was loaded into such vesicles. | [50] |
| Flavonolignan | SLM | Liposomes | Improved the SLM bioavailability | In Vitro | This formulation which employs safe components and a buccal spray liposomal dose form can meet the SLM needs of children with liver conditions. | [72] |
| Flavonolignan | SLM | Liposomes | Cancer (pharmacokinetics evaluation) | In Vivo | SLM liposomes could encapsulate materials at a rate of over 90% with an average particle size of roughly 238.8 nm. Oral administration of SLM proliposomes leads to their high bioavailability. | [98] |
| Flavonolignan | SLM | Liposomes containing a bile salt | Chronic liver, cirrhosis and hepatocellular carcinoma (pharmacokinetics evaluation) | In Vitro/In Vivo | SM-Lip-SEDS indicated enhanced in vitro drug release compared to SM powder. | [95] |
| Flavonolignan | SLM | Bilosomes | Hepatoprotective | In Vitro/In Vivo | The results indicated the possible application of bilosomes for enhancing SM hepatoprotective effect when administered orally. | [96] |
| Flavonolignan | SLM | Glyceryl monooleate/poloxamer 407 liquid crystalline matrices | Improving the bioavailability of SLM | In Vitro/In Vivo | Pharmacokinetic tests revealed 3.5 times more SLM oral bioavailability compared to Legalon®, a commercial SLM formulation, and considerably higher peak concentrations with SLM GMO/P407 LCM. | [43] |
| Flavonolignan | SLM | Organogel with pluronic-lecithin (PLO) | Atopic dermatitis | In Vitro/Ex Vivo | A unique topical formulation of SLM which is loaded with PLO gel might be launched. | [97] |
| Phytochemical Type | Cargo | Carrier | Disease | In Vitro/In Vivo | Major Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Flavonoid | Naringenin | NEs | Alzheimer disease (AD) | In Vitro | The naringenin-loaded NE alleviated the direct neurotoxic consequences of Aβ on SH-SY5Y cells significantly. This process was once related to a downregulation of APP and BACE expression, indicating decreased amyloidogenesis. | [104] |
| Flavonoid | Daidzein | NEs and NEGs | Melanoma | In Vitro | All of the formulations benefitted from appropriate pH values for pores and skin complying with zero-order launch kinetics. The DZ launch fees acquired for DZ-NE and DZ-NEG equaled 2.701 ± 0.265 and 1.325 ± 0.117 μg/cm2/h, respectively. | [105] |
| Flavonoid | Quercetin | NEs | Heart failure and vascular disease (CVD) | In Vitro/In Vivo | Based on the results, quercetin and other polyphenolic substances are enhanced by therapeutic NE. | [106] |
| Flavonoid | Quercetin | NEs | Diabetes mellitus | In Vivo | The optimized quercetin NE exhibited appropriate stability for forty-five days. The Que-NE demonstrated most useful oral bioavailability unlike Que-PD. | [107] |
| Flavonoid | Quercetin and cisplatin | NEs | Human breast carcinoma | In Vitro | The new formulations multiplied the antitumor recreation of each of the molecules and the synergistic impact of the cisplatin/quercetin unlike the MDA-MB-231. | [108] |
| Flavonoid | Quercetin | NEs | High cholesterol | In Vitro/In Vivo | Based on the results, hepatic bile acid synthesis and fecal cholesterol excretion play at least a minimal role in quercetin preventive effects on lipid abnormalities. | [109] |
| Flavonoid | Quercetin | Olive oil NEs | Cancer | In Vitro | Quercetin-entrapped NEs formed stable complexes with HSA and HTF through improving hydrophilic–hydrophobic interactions compared to the non-entrapped quercetin. | [110] |
| Flavonoid | Quercetin and vincristine | NEs | Leukemia | In Silico/In Vitro | Direct inhibition of ABCB1 efflux activity by the unloaded NE demonstrated the structure–activity link. | [111] |
| Flavonoid | Hesperidin | NEs | Cancer | In Vitro | HPNEM reduced mir-21 and mir-155 expression, demonstrating its therapeutic potential for breast cancer. | [112] |
| Flavonoid | Kaempferol | Muco-adhesive NE | Glioma cell line | In Vitro/Ex Vivo/In Vivo | KPF from KPF-MNE displayed higher permeation across the mucosa in ex vivo diffusion studies. | [113] |
| Flavonolignan | SLM | NEs | Stabilization of SM-NE droplets | In Vitro | Based on the studies, tea saponin has the capacity to prepare SM-NE as natural emulsifiers and act as cryoprotectants to lessen SM-NE damage during freeze-drying. | [119] |
| Flavonolignan | SLM | MEs | Evaluated the drug delivery systems | In Vitro | The studies indicated prolonged release for MEs compared to SLM solution. | [117] |
| Flavonolignan | SLM | Improving the bioavailability of SLM | In Vitro | In vitro release of SLM from SMEDDS determined by a dialysis method and an ultrafiltration method showed first-order kinetics and was typical of sustained characteristics. | [118] | |
| Flavonolignan | SLM | MEs | Improving the bioavailability of SLM | In Vitro/In Vivo | SMEDDS is considered as an effective oral drug delivery system for non-water-soluble medications. The most useful system with the exceptional self-microemulsifying and solubilization potential contained 10% (w/w) of ethyl linoleate, 30% of Cremophor EL, and 60% of ethyl alcohol. | [116] |
| Flavonolignan | Silybin | MEs | Improving the bioavailability of SLM | In Vitro/In Vivo | This study indicates the effectiveness of hyper-saturable formulations as a delivery method for improving the oral bioavailability of drugs which are not regarded as highly soluble. | [120] |
| Flavonolignan | SLM | NEs | Hepatic diseases | In Vitro | The nanosized formulation allows enhancing bioavailability inside body. | [121] |
| Flavonolignan | SLM | NEs | A pharmacokinetic study | In Vitro/In Vivo | This study indicates that NE technology can enhance SLM biopharmaceutical capabilities. | [25] |
| Flavonolignan | SLM | NEs | Hepatic | In Vitro/In Vivo | The drug dissolved rapidly from the NPs, reaching almost 80% during 15 min, indicating three-fold higher dissolution than that of the commercial product. | [26] |
| Flavonolignan | SLM | NEs | Analyzing bio-accessibility and -stability | In Vitro | Castor oil deteriorated silybin with less stability than extra virgin olive or sunflower oil. | [122] |
| Flavonolignan | SLM | NEs | Pharmacokinetic study | In Vitro | Based on a pharmacokinetic study, SLM in NE exhibited a significantly (P 0.05) better oral bioavailability than that in oral solution, indicating that NE may be an appropriate oral delivery method for SLM. | [123] |
| Phytochemical Type | Cargo | Carrier | Disease | In Vitro/In Vivo | Major Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Flavonoid | Apigenin | SLNs | Diabetic nephropathy | In Vitro/In Vivo | The encapsulation efficiency of pigenin–SLNP equaled 78.90%. A medicine releases on average 71.52% of its dose every 24 h with a fast release of 34.28% every five hours. | [144] |
| Flavonoid | Quercetin | SLNs | Neurodegenerative disorders | In Vitro | Employing as a therapy for neurodegenerative illnesses like memory impairments. | [134] |
| Flavonoid | Quercetin | SLNs | Carcinoma (Caco-2 cell line) | In Vitro | Enhancing Caco-2 cells’ absorption of quercetin by SLN and more quickly than pure quercetin; quercetin from SLN dispersion was released. | [135] |
| Flavonoid | Quercetin | SLNs | Post-menopausal osteoporosis | In Vitro/In Vivo | A notable distinction in the release pattern of QSLNs and quercetin. A continuous release was observed in the case of the QSLNs and 45 6.33% of the medication was released by the end of 12 h unlike a release of less than 10% for pure quercetin. | [136] |
| Flavonoid | Quercetin | SLNs. Stearic acid as a core lipid and arabic gum as a stabilizer | Improving the bioavailability of SLM | In Vitro | The system displayed a regulated antioxidant impact compared to free quercetin, indicating that the encapsulated nutraceutical retains a significant amount of its antioxidant activity (around 81% of that of free quercetin). | [137] |
| Flavonoid | Quercetin | SLNs stabilized by whey protein isolate | Studying the physicochemical properties | In Vitro | Quercetin improved fish oil oxidative stability at low palmitic acid concentrations significantly. | [138] |
| Flavonoid | Quercetin | SLNs | Spinal injuries | In Vitro/In Vivo | Quercetin only displayed a 62.20 1.06% drug release at the end of the 12-h dissolving research. However, its lipid SLNs showed an 84.99 1.42% drug release. The bioavailability enhanced significantly with the SLNs. | [139] |
| Flavonoid | Baicalin | PEGylated cationic SLNs conjugated with OX26 antibody | Brain disorders | In Vitro | OX26-PEG-CSLN is among possible delivery strategies for treating brain diseases throughout the BBB. | [140] |
| Flavonoid | Chrysin–phospholipid | SLNs | Cancer | In Vitro | Ch-PC-SLNs demonstrated zero-order release kinetics and a better encapsulation efficiency than Ch-SLNs. | [141] |
| Flavonoid | (─)-epigallocatechin-3-gallate (EGCG) | SLNs | For food applications | In Vitro | This technique is considered as efficient for incorporating EGCG on a wide scale into food items. | [142] |
| Flavonolignan | Silibinin and d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) | SLNs | Breast cancer | In Vitro/In Vivo | The optimized SLNs exhibited an appropriate serum stability with around 45 nm in size entering MDA-MB-231 breast cancer cells effectively. | [130] |
| Flavonolignan | SLM | Lipid NPs | Studying the drug delivery system | In Vitro | Improved solubility, alternative routes including improved absorption and lymphatic transport, or perhaps both can increase bioavailability. | [129] |
| Flavonolignan | SLM | SLNs | Study of the drug delivery system | In Vitro | Based on the studies, cold-SM-SLNs can increase SM oral bioavailability, indicating a potential use for SM oral drug delivery system to the liver. | [131] |
| Flavonolignan | SLM | SLNs | Diverse liver and gallbladder disorders | In Vitro/In Vivo | SM-loaded SLN might be a beneficial approach for distributing poorly water-soluble Sm beyond offering favorable hepatic protection. | [143] |
| Flavonolignan | SLM | NLCs | Studying the drug delivery system | In Vitro | Possibility of controlled drug release and targeting, lack of carrier biotoxicity and biodegradation, and absence of manufacturing scale-up difficulties in the food and pharmaceutical sectors. | [54] |
| Flavonolignan | Silybin | Mixed micelles | Studying the drug delivery system | In Vivo | Based on the studies, bioavailability increased by 7–9 times compared to a fast-release silybin solid dispersion formulation. | [55] |
| Flavonolignan | Silybin | NLCs | Biodistribution and pharmacokinetic studies | In Vitro | The results indicate that silybin continuous release and targeting through NLC is a possibility. | [53] |
| Flavonolignan | SLM | NLCs | Analyzing epidermal drug deposition enhancement | In Vitro | The results indicate the capability of NLC gel to deliver SLM topically. | [52] |
| Flavonolignan | Silybin | Lipid nanospheres | Study of pharmacokinetics | In Vitro/In Vivo | Unlike SLM, SLM proliposome is regarded as a stable and easy-to-make formulation which supports SLM gastrointestinal absorption. | [145] |
| Product Name | Drug | Nanostructure | Application | Clinical Trials |
|---|---|---|---|---|
| Gallic acid phytosome® | Gallic acid | Phytosome | Antimicrobial and anti-apoptotic activities | NM 1 |
| Ginkgoselect® phytosome® | Ginkgoselect | Phytosome | Antioxidant activity and hepatoprotective feature | NM |
| Silybin phytosome® | Silybin | Phytosome | Hepatoprotective and antioxidant abilities | NM |
| Ginseng phytosome® | Ginseng | Phytosome | Nutraceutical compound and immunomodulator agent | NM |
| Green tea phytosome® | Green tea | Phytosome | Antioxidant and anticancer ability | NM |
| Grape seed phytosome® | Grape seed | Phytosome | Antioxidant and cardioprotective ability | NM |
| Hawthorn phytosome® | Hawthorn | Phytosome | Cardioprotective agent | NM |
| Quercetin phytosome® | Quercetin | Phytosome | Antioxidant and anticancer ability | NM |
| Naringenin phytosomes® | Naringenin | Phytosomes | Antioxidant ability | NM |
| Dasatinib + quercetin phytosome | Quercetin | Phytosomes | Antioxidant | 2 (NCT04313634) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. https://doi.org/10.3390/pharmaceutics15071944
Ranjbar S, Emamjomeh A, Sharifi F, Zarepour A, Aghaabbasi K, Dehshahri A, Sepahvand AM, Zarrabi A, Beyzaei H, Zahedi MM, et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics. 2023; 15(7):1944. https://doi.org/10.3390/pharmaceutics15071944
Chicago/Turabian StyleRanjbar, Shahla, Abbasali Emamjomeh, Fatemeh Sharifi, Atefeh Zarepour, Kian Aghaabbasi, Ali Dehshahri, Azadeh Mohammadi Sepahvand, Ali Zarrabi, Hamid Beyzaei, Mohammad Mehdi Zahedi, and et al. 2023. "Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles" Pharmaceutics 15, no. 7: 1944. https://doi.org/10.3390/pharmaceutics15071944
APA StyleRanjbar, S., Emamjomeh, A., Sharifi, F., Zarepour, A., Aghaabbasi, K., Dehshahri, A., Sepahvand, A. M., Zarrabi, A., Beyzaei, H., Zahedi, M. M., & Mohammadinejad, R. (2023). Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics, 15(7), 1944. https://doi.org/10.3390/pharmaceutics15071944







