Development of Novel Paclitaxel-Loaded ZIF-8 Metal-Organic Framework Nanoparticles Modified with Peptide Dimers and an Evaluation of Its Inhibitory Effect against Prostate Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines and Cell Culture
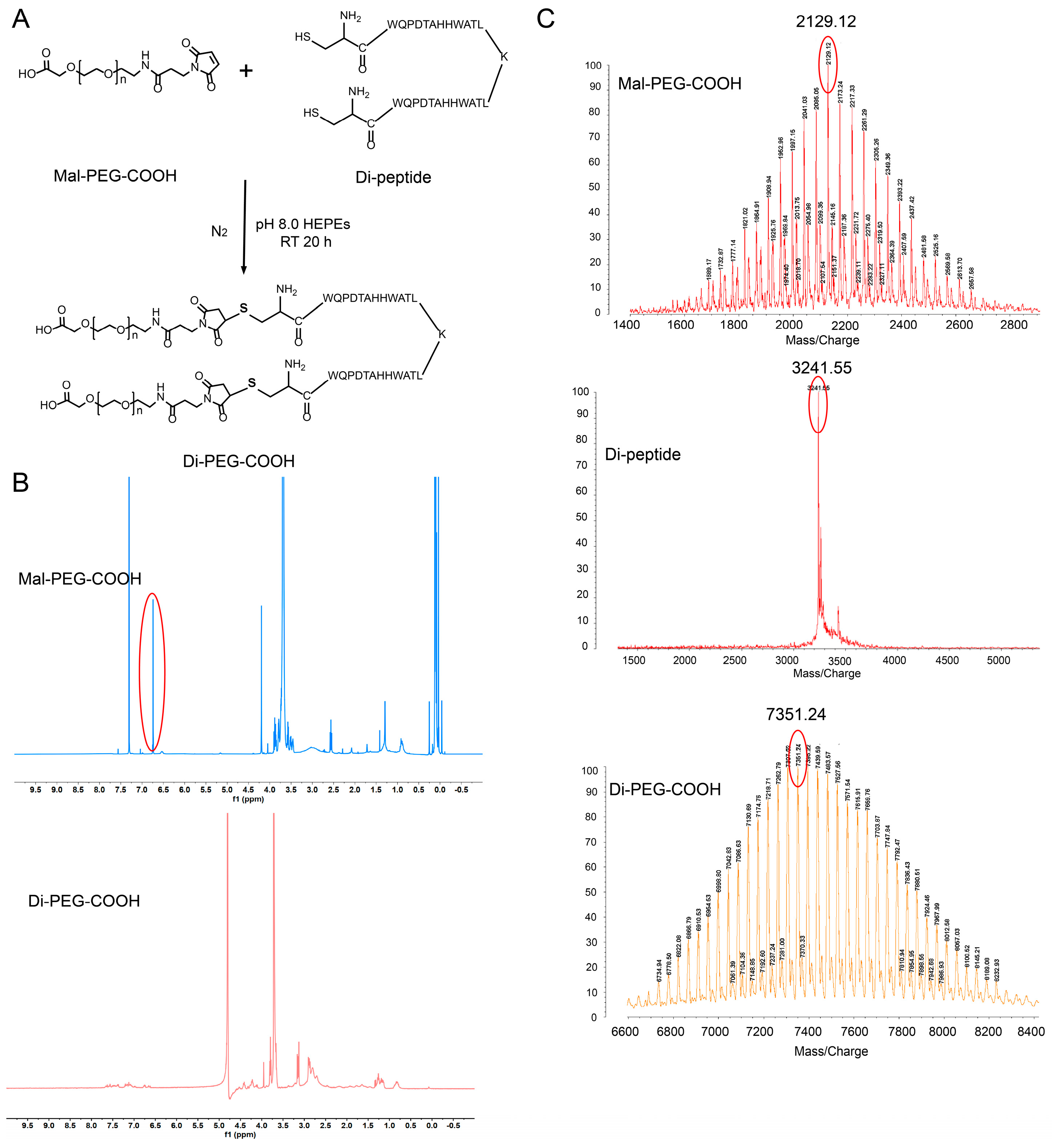
2.3. Synthesis and Characterization of Di-PEG-COOH
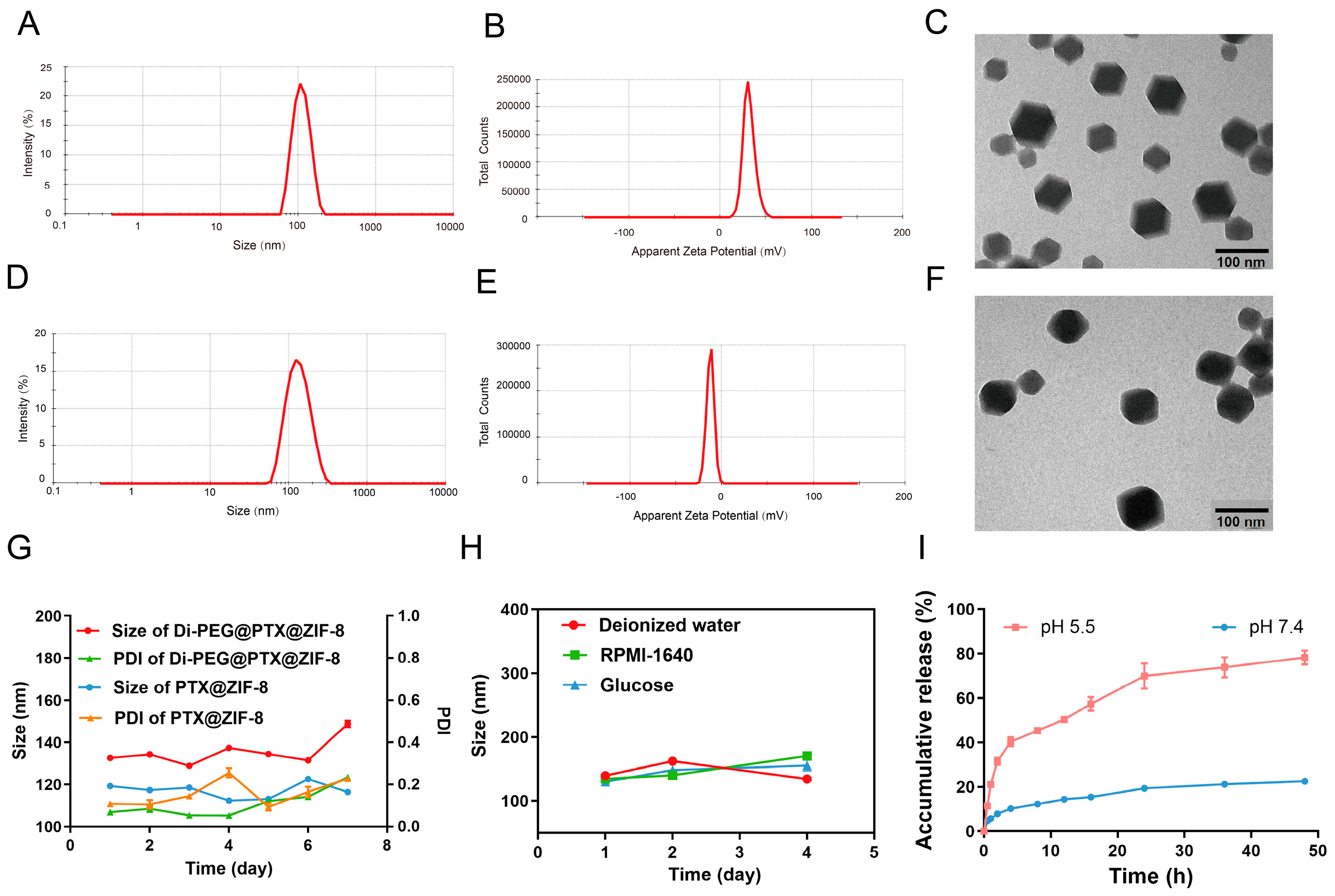
2.4. Preparation of PTX@ZIF-8 and Di-PEG@PTX@ZIF-8
2.5. Characterization of PTX@ZIF-8 and Di-PEG@PTX@ZIF-8
2.6. Stability Examination of Di-PEG@PTX@ZIF-8
2.7. In Vitro Release Assay
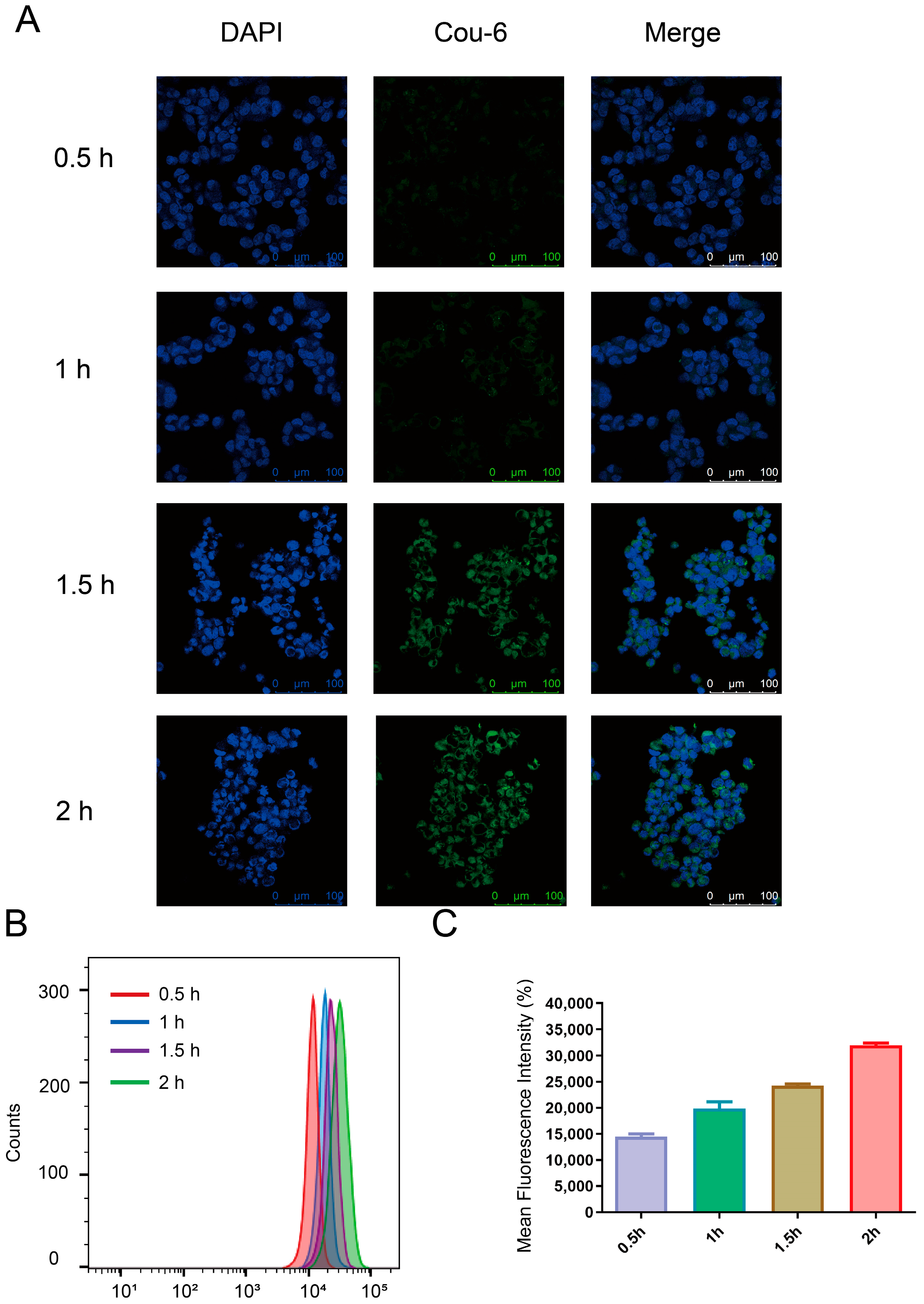
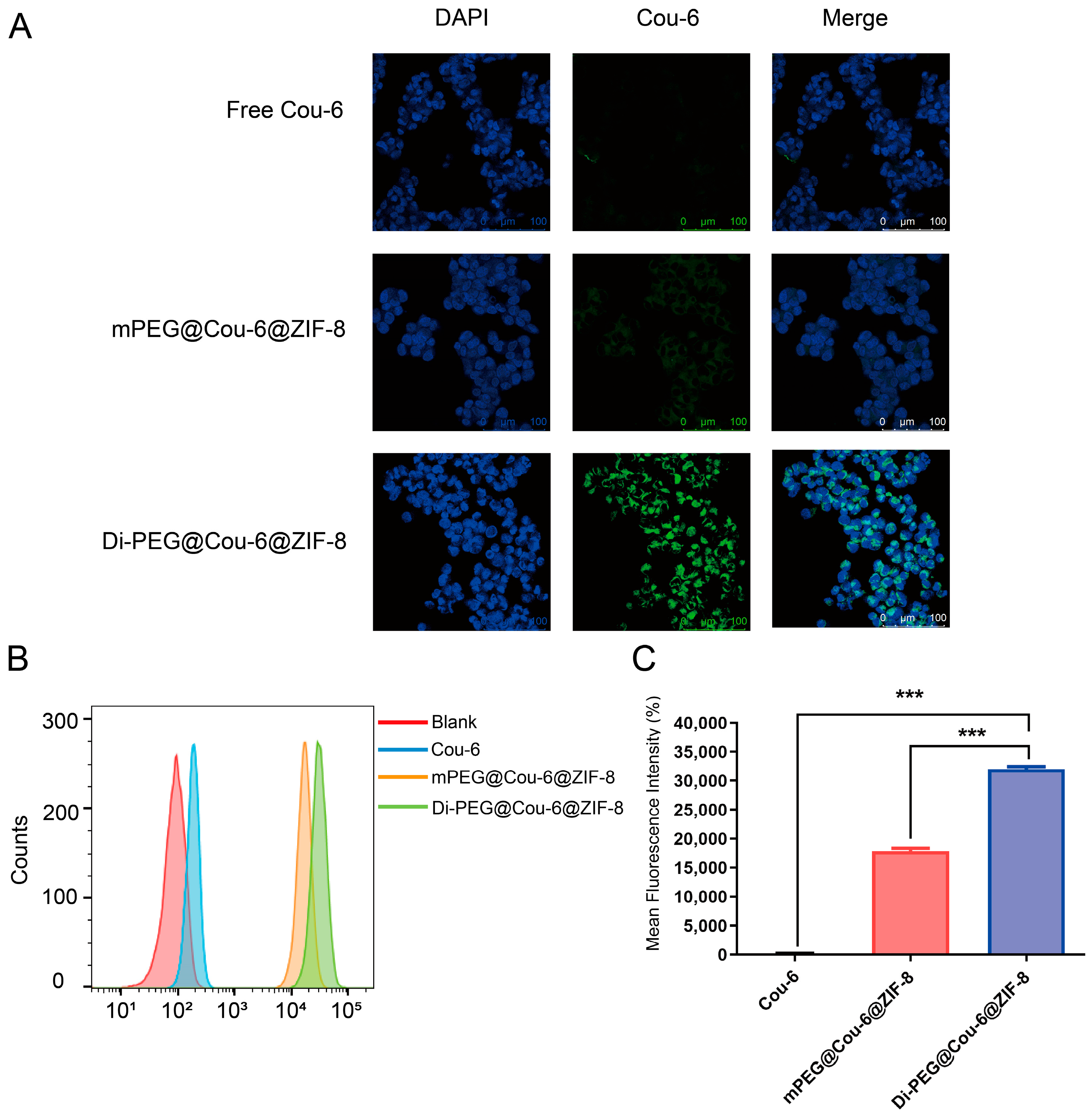
2.8. In Vitro Cellular Uptake Studies
2.9. Cell Viability Assay
2.10. Wound-Healing Assay
2.11. Cell Apoptosis Assay
2.12. Statistical Analysis
3. Results
3.1. Synthesis of Di-PEG-COOH
3.2. Characterization of PTX@ZIF-8 and Di-PEG@PTX@ZIF-8
3.3. In Vitro Drug Release
3.4. Cellular Uptake Study
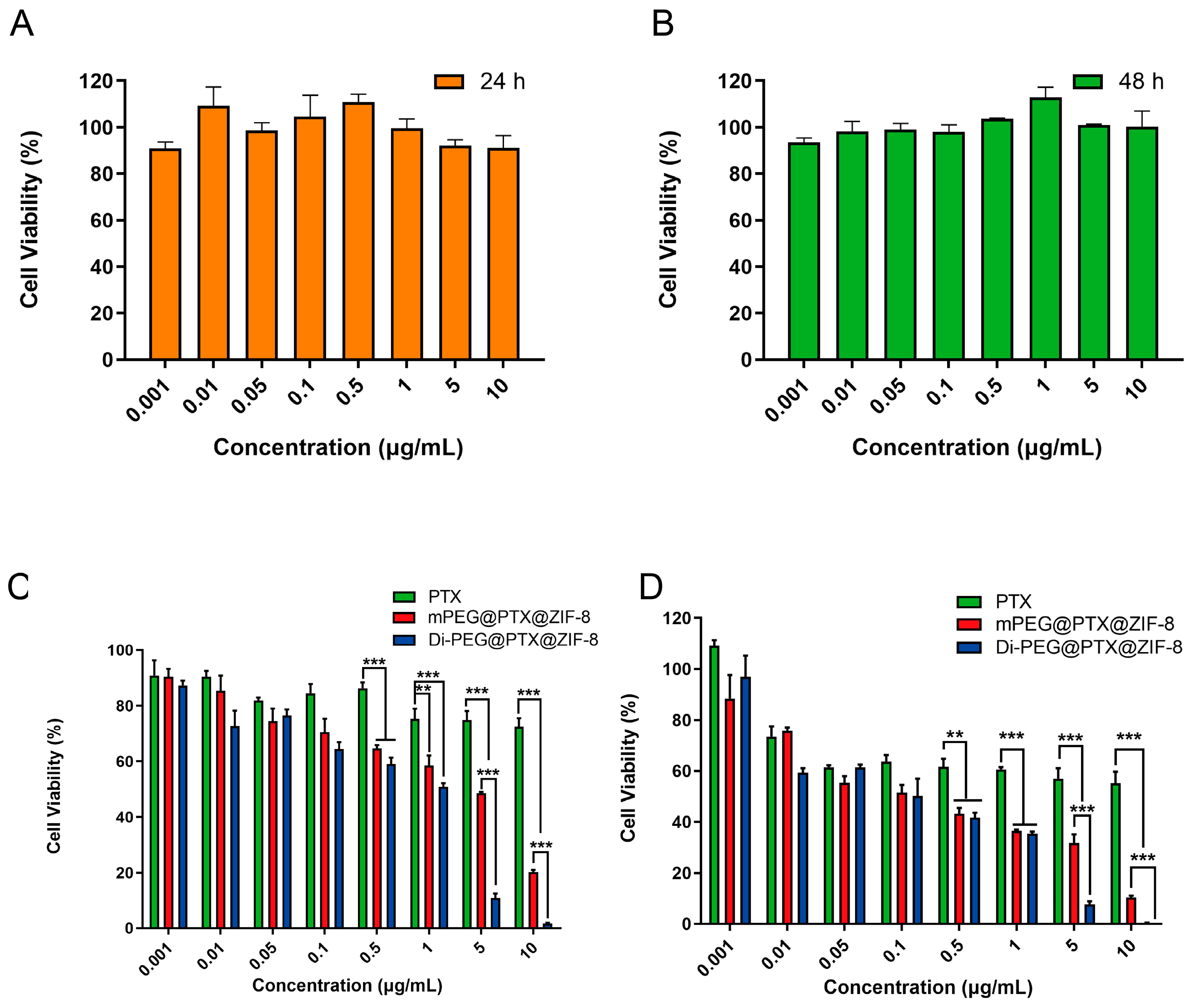
3.5. Cell Viability Assay
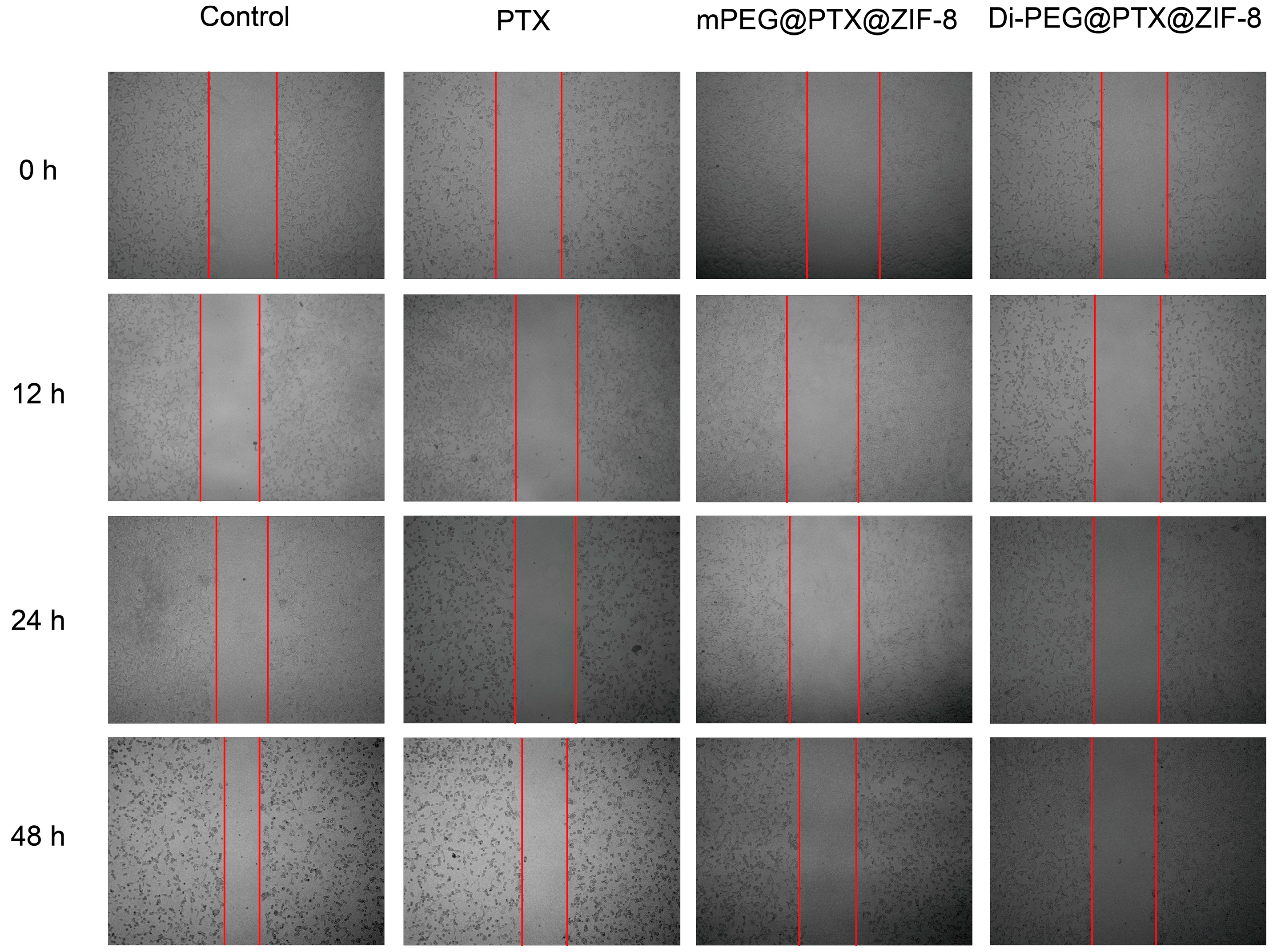
3.6. Wound-Healing Assay
3.7. Cell Apoptosis Study In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mandair, D.; Rossi, R.E.; Pericleous, M.; Whyand, T.; Caplin, M.E. Prostate cancer and the influence of dietary factors and supplements: A systematic review. Nutr. Metab. 2014, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; O’Callaghan, C.; Ding, K.; Toren, P.; Dearnaley, D.; Higano, C.S.; Horwitz, E.; Malone, S.; Goldenberg, L.; Gospodarowicz, M.; et al. Nadir testosterone within first year of androgen-deprivation therapy (ADT) predicts for time to castration-resistant progression: A secondary analysis of the PR-7 trial of intermittent versus continuous ADT. J. Clin. Oncol. 2015, 33, 1151–1156. [Google Scholar] [CrossRef]
- Hashemi, M.; Zandieh, M.A.; Talebi, Y.; Rahmanian, P.; Shafiee, S.S.; Nejad, M.M.; Babaei, R.; Sadi, F.H.; Rajabi, R.; Abkenar, Z.O.; et al. Paclitaxel and docetaxel resistance in prostate cancer: Molecular mechanisms and possible therapeutic strategies. Biomed. Pharmacother. 2023, 160, 114392. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zieren, R.C.; Xue, W.; de Reijke, T.M.; Pienta, K.J. Metastatic prostate cancer remains incurable, why? Asian J. Urol. 2019, 6, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Barve, A.; Jin, W.; Cheng, K. Prostate cancer relevant antigens and enzymes for targeted drug delivery. J. Control. Release 2014, 187, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Anand, U.; Pandey, S.K.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Dey, A. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist. Updates 2021, 55, 100754. [Google Scholar] [CrossRef]
- Liu, L.; Bi, Y.; Zhou, M.; Chen, X.; He, X.; Zhang, Y.; Sun, T.; Ruan, C.; Chen, Q.; Wang, H.; et al. Biomimetic Human Serum Albumin Nanoparticle for Efficiently Targeting Therapy to Metastatic Breast Cancers. ACS Appl. Mater. Interfaces 2017, 9, 7424–7435. [Google Scholar] [CrossRef]
- Alves, R.C.; Fernandes, R.P.; Eloy, J.O.; Salgado, H.R.N.; Chorilli, M. Characteristics, Properties and Analytical Methods of Paclitaxel: A Review. Crit. Rev. Anal. Chem. 2018, 48, 110–118. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Mei, T.; Liu, Y.; Wu, K.; Le, W.; Hu, Y. Nanoscale MOFs in nanomedicine applications: From drug delivery to therapeutic agents. J. Mater. Chem. B 2023, 11, 3273–3294. [Google Scholar] [CrossRef]
- He, S.; Wu, L.; Li, X.; Sun, H.; Xiong, T.; Liu, J.; Huang, C.; Xu, H.; Sun, H.; Chen, W.; et al. Metal-organic frameworks for advanced drug delivery. Acta Pharm. Sin. B 2021, 11, 2362–2395. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, H.; Yu, F.; Zhang, J.; Li, Y.; Bao, W.; Wang, Z.; Lu, K.; Yu, J.; Bai, G.; et al. Enhanced oxygen reduction reaction performance of Co@N–C derived from metal-organic frameworks ZIF-67 via a continuous microchannel reactor. Chin. Chem. Lett. 2023, 34, 107128. [Google Scholar] [CrossRef]
- Zhuang, J.; Kuo, C.H.; Chou, L.Y.; Liu, D.Y.; Weerapana, E.; Tsung, C.K. Optimized metal-organic-framework nanospheres for drug delivery: Evaluation of small-molecule encapsulation. ACS Nano 2014, 8, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, G.; Liu, H.; Sang, C.; Liu, X.; Chen, T. Highly bioactive zeolitic imidazolate framework-8-capped nanotherapeutics for efficient reversal of reperfusion-induced injury in ischemic stroke. Sci. Adv. 2020, 6, eaay9751. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nystrom, A.M.; Zou, X. One-pot Synthesis of Metal-Organic Frameworks with Encapsulated Target Molecules and Their Applications for Controlled Drug Delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic Imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, J.; Wang, D.; Lv, J.; Hou, K.; Liu, Y.; Chen, C.; Tang, Z. Coordination-responsive drug release inside gold nanorod@metal-organic framework core–shell nanostructures for near-infrared-induced synergistic chemo-photothermal therapy. Nano Res. 2018, 11, 3294–3305. [Google Scholar] [CrossRef]
- Wrighton, N.C.; Balasubramanian, P.; Barbone, F.P.; Kashyap, A.K.; Farrell, F.X.; Jolliffe, L.K.; Barrett, R.W.; Dower, W.J. Increased potency of an erythropoietin peptide mimetic through covalent dimerization. Nat. Biotechnol. 1997, 15, 1261–1265. [Google Scholar] [CrossRef]
- Fournier, P.; Dumulon-Perreault, V.; Ait-Mohand, S.; Langlois, R.; Benard, F.; Lecomte, R.; Guerin, B. Comparative study of 64Cu/NOTA-[D-Tyr6,betaAla11,Thi13,Nle14]BBN(6-14) monomer and dimers for prostate cancer PET imaging. EJNMMI Res. 2012, 2, 8. [Google Scholar] [CrossRef]
- Kristensen, M.; Kucharz, K.; Felipe Alves Fernandes, E.; Stromgaard, K.; Schallburg Nielsen, M.; Cederberg Helms, H.C.; Bach, A.; Ulrikkaholm Tofte-Hansen, M.; Irene Aldana Garcia, B.; Lauritzen, M.; et al. Conjugation of Therapeutic PSD-95 Inhibitors to the Cell-Penetrating Peptide Tat Affects Blood-Brain Barrier Adherence, Uptake, and Permeation. Pharmaceutics 2020, 12, 661. [Google Scholar] [CrossRef]
- Aggarwal, S.; Singh, P.; Topaloglu, O.; Isaacs, J.T.; Denmeade, S.R. A dimeric peptide that binds selectively to prostate-specific membrane antigen and inhibits its enzymatic activity. Cancer Res. 2006, 66, 9171–9177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, H.; Zhao, H.; Qi, L.; Liu, Y.; Zhang, Z.; Liu, C.; Chen, L.; Jin, M.; Guan, Y.; et al. Development and Evaluation of a PSMA-Targeted Nanosystem Co-Packaging Docetaxel and Androgen Receptor siRNA for Castration-Resistant Prostate Cancer Treatment. Pharmaceutics 2022, 14, 964. [Google Scholar] [CrossRef]
- Cai, D.; Gao, W.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. Hydrophobic penetrating peptide PFVYLI-modified stealth liposomes for doxorubicin delivery in breast cancer therapy. Biomaterials 2014, 35, 2283–2294. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Y.; Wang, Y.; Zhang, M.; Luo, Y.; Tang, J.; Wang, Z.; Wang, D.; Hao, L.; Wang, Z. Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. Int. J. Nanomed. 2017, 12, 5313–5330. [Google Scholar] [CrossRef]
- Yang, H.W.; Hua, M.Y.; Liu, H.L.; Tsai, R.Y.; Chuang, C.K.; Chu, P.C.; Wu, P.Y.; Chang, Y.H.; Chuang, H.C.; Yu, K.J.; et al. Cooperative dual-activity targeted nanomedicine for specific and effective prostate cancer therapy. ACS Nano 2012, 6, 1795–1805. [Google Scholar] [CrossRef]
- To, P.K.; Do, M.H.; Cho, J.H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int. J. Mol. Sci. 2020, 21, 2991. [Google Scholar] [CrossRef]
- Xue, Y.N.; Yu, B.B.; Liu, Y.N.; Guo, R.; Li, J.L.; Zhang, L.C.; Su, J.; Sun, L.K.; Li, Y. Zinc promotes prostate cancer cell chemosensitivity to paclitaxel by inhibiting epithelial-mesenchymal transition and inducing apoptosis. Prostate 2019, 79, 647–656. [Google Scholar] [CrossRef]
- Slovin, S.F. Targeting novel antigens for prostate cancer treatment: Focus on prostate-specific membrane antigen. Expert Opin. Ther. Targets 2005, 9, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Nielsen, M.; Borre, M. Diagnostic and Therapeutic Strategies for Prostate Cancer. Semin. Nucl. Med. 2016, 46, 484–490. [Google Scholar] [CrossRef]
- Adashek, J.J.; Reed, J.P.; Tandon, A.; Freedland, S.J.; Posadas, E.; Bhowmick, N.; Chung, L.W.; Freeman, M.; Figlin, R.A.; Gong, J. Combination Androgen Receptor Inhibition and Docetaxel in Metastatic Castration-sensitive Prostate Cancer: The Next Step in First-line Treatment? Clin. Genitourin. Cancer 2020, 18, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Gong, H.; Zhou, J.; Zhang, Q.; Gao, W.; Fang, R.H.; Zhang, L. Targeted gene silencing in vivo by platelet membrane-coated metal-organic framework nanoparticles. Sci. Adv. 2020, 6, eaaz6108. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |

| Time | Control | PTX | mPEG@PTX@ZIF-8 | Di-PEG@PTX@ZIF-8 |
|---|---|---|---|---|
| 0 h | 0 | 0 | 0 | 0 |
| 12 h | 13.10% | 6.00% | 1.61% | 0.36% |
| 24 h | 24.15% | 8.19% | 4.53% | 1.79 |
| 48 h | 48.45% | 31.48 | 21.63% | 3.21% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Gong, L.; Wu, H.; Liu, C.; Liu, Y.; Xiao, C.; Liu, C.; Chen, L.; Jin, M.; Gao, Z.; et al. Development of Novel Paclitaxel-Loaded ZIF-8 Metal-Organic Framework Nanoparticles Modified with Peptide Dimers and an Evaluation of Its Inhibitory Effect against Prostate Cancer Cells. Pharmaceutics 2023, 15, 1874. https://doi.org/10.3390/pharmaceutics15071874
Zhao H, Gong L, Wu H, Liu C, Liu Y, Xiao C, Liu C, Chen L, Jin M, Gao Z, et al. Development of Novel Paclitaxel-Loaded ZIF-8 Metal-Organic Framework Nanoparticles Modified with Peptide Dimers and an Evaluation of Its Inhibitory Effect against Prostate Cancer Cells. Pharmaceutics. 2023; 15(7):1874. https://doi.org/10.3390/pharmaceutics15071874
Chicago/Turabian StyleZhao, Heming, Liming Gong, Hao Wu, Chao Liu, Yanhong Liu, Congcong Xiao, Chenfei Liu, Liqing Chen, Mingji Jin, Zhonggao Gao, and et al. 2023. "Development of Novel Paclitaxel-Loaded ZIF-8 Metal-Organic Framework Nanoparticles Modified with Peptide Dimers and an Evaluation of Its Inhibitory Effect against Prostate Cancer Cells" Pharmaceutics 15, no. 7: 1874. https://doi.org/10.3390/pharmaceutics15071874
APA StyleZhao, H., Gong, L., Wu, H., Liu, C., Liu, Y., Xiao, C., Liu, C., Chen, L., Jin, M., Gao, Z., Guan, Y., & Huang, W. (2023). Development of Novel Paclitaxel-Loaded ZIF-8 Metal-Organic Framework Nanoparticles Modified with Peptide Dimers and an Evaluation of Its Inhibitory Effect against Prostate Cancer Cells. Pharmaceutics, 15(7), 1874. https://doi.org/10.3390/pharmaceutics15071874








