Chitosan-Coated SLN: A Potential System for Ocular Delivery of Metronidazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MTZ-Loaded SLNs
2.3. Preparation of CS-Coated MTZ-Loaded SLN
2.4. Characterization of CS-Coated SLN
2.4.1. Particle Size (PS) and Polydispersity Index (PDI)
2.4.2. Zeta Potential (ZP)
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. MTZ Loading (DL) and Encapsulation Efficiency (EE)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. pH and Osmolarity
2.4.7. Mucoadhesion
2.4.8. In Vitro Release of MTZ
3. Results
3.1. Preparation and Identification of a CS-Coated SLN Dispersion
3.2. Mucoadhesion
3.3. Characterisation of Optimized SLN
3.3.1. Particle Size
3.3.2. Zeta Potential
3.3.3. Transmission Electron Microscopy (TEM)
3.4. In Vitro Release of MTZ and Kinetic Modelling
3.5. CS-Coated SLN Stability Studies
3.5.1. Particle Size and Polydispersity Index
3.5.2. Zeta Potential
3.5.3. pH and Osmolarity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghanem, V.C.; Mehra, N.; Wong, S.; Mannis, M.J. The prevalence of ocular signs in acne rosacea: Comparing patients from ophthalmology and dermatology clinics. Cornea 2003, 22, 230–233. [Google Scholar] [CrossRef]
- Yaylali, V.; Ozyurt, C. Comparison of tear function tests and impression cytology with the ocular findings in acne rosacea. Eur. J. Ophthalmol. 2002, 12, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M. Ocular rosacea: Current concepts and therapy. Arch. Dermatol. 1997, 133, 89–90. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, L.S.; Mannis, M.J. Ocular rosacea. Ocul. Surf. 2005, 3, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Starr, P.A.J.; Macdonald, A. Oculocutaneous Aspects of Rosacea. J. R. Soc. Med. 1969, 62, 9–11. [Google Scholar] [CrossRef]
- Ramelet, A.A. Rosacea: A Reaction Pattern Associated With Ocular Lesions and Migraine? Arch. Dermatol. 1994, 130, 1448. [Google Scholar] [CrossRef]
- Vieira, A.C.C.; Höfling-Lima, A.L.; Mannis, M.J. Ocular rosacea—A review. Arq. Bras. Oftalmologia 2012, 75, 363–369. [Google Scholar] [CrossRef]
- Freeman, C.D.; Klutman, N.E.; Lamp, K.C. Metronidazole. A therapeutic review and update. Drugs 1997, 54, 679–708. [Google Scholar] [CrossRef]
- Metronidazole 0.75% Topical Gel—Uses, Side Effects, and More. Available online: https://www.webmd.com/drugs/2/drug-75148/metronidazole-topical/details (accessed on 18 January 2022).
- Esmaeili, F.; Hosseini-Nasr, M.; Rad-Malekshahi, M.; Samadi, N.; Atyabi, F.; Dinarvand, R. Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 161–167. [Google Scholar] [CrossRef]
- Luo, Y.F.; Chen, D.W.; Ren, L.X.; Zhao, X.L.; Qin, J. Solid lipid nanoparticles for enhancing vinpocetine’s oral bioavailability. J. Control. Release 2006, 114, 53–59. [Google Scholar] [CrossRef]
- Zhou, W.; Shuyu, X.; Luyan, Z.; Zhao, D.; Wang, Y.; Xiaofang, W. Preparation and evaluation of ofloxacin-loaded palmitic acid solid lipid nanoparticles. Int. J. Nanomed. 2011, 6, 547–555. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, X.; Xu, Q.; Su, J.; Li, X.; Zhou, W. Evaluation of the antibacterial activity of tilmicosin-SLN against Streptococcus agalactiae: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 4747–4755. [Google Scholar] [CrossRef]
- Lu, M.; Wang, Y.; Li, X.; Zhou, W. Surface Charge Modification and Antibacterial Study on Tilmicosin-Loaded Solid Lipid Nanoparticles against Avian. Ann. Pharmacol. Pharm. 2021, 6, 1198. [Google Scholar]
- Barnhorst, J.; Foster, J.A.; Chern, K.C.; Meisler, D.M. The efficacy of topical metronidazole in the treatment of ocular rosacea. Ophthalmology 1996, 103, 1880–1883. [Google Scholar] [CrossRef]
- Waszczykowska, A.; Żyro, D.; Jurowski, P.; Ochocki, J. Effect of treatment with silver(I) complex of metronidazole on ocular rosacea: Design and formulation of new silver drug with potent antimicrobial activity. J. Trace Elem. Med. Biol. 2020, 61, 126531. [Google Scholar] [CrossRef]
- Claros-Chacaltana, F.D.Y.; Aldrovani, M.; Kobashigawa, K.K.; Padua, I.R.M.; Valdetaro, G.P.; de Barros Sobrinho, A.A.F.; Abreu, T.G.M.; Laus, J.L. Effect of metronidazole ophthalmic solution on corneal neovascularization in a rat model. Int. Ophthalmol. 2019, 39, 1123–1135. [Google Scholar] [CrossRef]
- Kayser, O.; Lemke, A.; Hernandez-Trejo, N. The Impact of Nanobiotechnology on the Development of New Drug Delivery Systems. Curr. Pharm. Biotechnol. 2016, 6, 3–5. [Google Scholar] [CrossRef]
- Popa, L.; Ghica, M.V.; Dinu-Pîrvu, C.E.; Irimia, T. Chitosan: A Good Candidate for Sustained Release Ocular Drug Delivery Systems. Chitin-Chitosan Myriad Funct. Sci. Technol. 2018, 14, 283–311. [Google Scholar] [CrossRef]
- Andersen, T.; Bleher, S.; Flaten, G.E.; Tho, I.; Mattsson, S.; Škalko-Basnet, N. Chitosan in mucoadhesive drug delivery: Focus on local vaginal therapy. Mar. Drugs 2015, 13, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Fischak, C.; Klaus, R.; Werkmeister, R.M.; Hohenadl, C.; Prinz, M.; Schmetterer, L.; Garhöfer, G. Effect of Topically Administered Chitosan-N-acetylcysteine on Corneal Wound Healing in a Rabbit Model. J. Ophthalmol. 2017, 2017, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Cinci, L.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Solid Lipid Nanoparticles and Chitosan-coated Solid Lipid Nanoparticles as Promising Tool for Silybin Delivery: Formulation, Characterization, and in vitro evaluation. Curr. Drug Deliv. 2019, 16, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Makoni, P.A.; Khamanga, S.M.; Walker, R.B. Muco-adhesive clarithromycin-loaded nanostructured lipid carriers for ocular delivery: Formulation, characterization, cytotoxicity and stability. J. Drug Deliv. Sci. Technol. 2021, 61, 102171. [Google Scholar] [CrossRef]
- Pham, C.V.; Van, M.C.; Thi, H.P.; Thanh, C.Đ.; Ngoc, B.T.; Van, B.N.; Le Thien, G.; Van, B.N.; Nguyen, C.N. Development of ibuprofen-loaded solid lipid nanoparticle-based hydrogels for enhanced in vitro dermal permeation and in vivo topical anti-inflammatory activity. J. Drug Deliv. Sci. Technol. 2020, 57, 101758. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Nguyen, T.T.T.; Nguyen, H.T.; Tran, T.H. Nanostructured lipid carriers to enhance transdermal delivery and efficacy of diclofenac. Drug Deliv. Transl. Res. 2017, 7, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Badawi, N.M.; Hteaima, M.H.; El-Say, K.M.; Aattia, D.A.; Ael-Nabarawi, M.A.; Elmazar, M.M. Pomegranate extract-loaded solid lipid nanoparticles: Design, optimization, and in vitro cytotoxicity study. Int. J. Nanomed. 2018, 13, 1313–1326. [Google Scholar] [CrossRef]
- Naguib, Y.W.; Rodriguez, B.L.; Li, X.; Hursting, S.D.; Williams, R.O.; Cui, Z. Solid Lipid Nanoparticle Formulations of Docetaxel Prepared with High Melting Point Triglycerides: In Vitro and in Vivo Evaluation. Mol. Pharm. 2014, 11, 1239–1249. [Google Scholar] [CrossRef]
- Chaturvedi, S.P.; Kumar, V. Production techniques of lipid nanoparticles: A review. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 525–541. [Google Scholar]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M.; Panciani, P.P.; Ugazio, E.; Sapino, S.; Peira, E.; Chirio, D. Techniques for the Preparation of Solid Lipid Nano and Microparticles. Appl. Nanotechnol. Drug Deliv. 2014, 1, 51–75. [Google Scholar]
- Pharmaceuticals Encapsulated in Lipid Nanoparticles with Ultrasonics. Available online: https://www.hielscher.com/pharmaceuticals-encapsulated-in-lipid-nanoparticles-with-ultrasonics.htm (accessed on 16 February 2022).
- Patel, A. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef]
- Völker-Dieben, H.J.; Kok-Van Alphen, C.C.; Hollander, J.; Kruit, P.J.; Batenburg, K. The palliative treatment of the dry eye. Doc. Ophthalmol. 1987, 67, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Shinde, U.A.; Parmar, S.J.; Easwaran, S. Metronidazole-loaded nanostructured lipid carriers to improve skin deposition and retention in the treatment of rosacea. Drug Dev. Ind. Pharm. 2019, 45, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Hwang, I.C.; Park, J.W.; Park, H.J. Photoprotection for deltamethrin using chitosan-coated beeswax solid lipid nanoparticles. Pest Manag. Sci. 2012, 68, 1062–1068. [Google Scholar] [CrossRef]
- Polaczyk, A.L.; Amburgey, J.E.; Alansari, A.; Poler, J.C.; Propato, M.; Hill, V.R. Calculation and uncertainty of zeta potentials of microorganisms in a 1:1 electrolyte with a conductivity similar to surface water. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124097. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current-time relationship in electroosmotic flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Sikhondze, S.S. The Development, Formulation and Characterization of an Optimized Metronidazole Loaded Solid Lipid Nanoparticle Formulation for Ocular Drug Delivery; Rhodes University: Makhanda, South Africa, 2022. [Google Scholar]
- Üstündaǧ-Okur, N.; Gökçe, E.H.; Bozbiyik, D.I.; Eǧrilmez, S.; Özer, Ö.; Ertan, G. Preparation and in vitro-in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur. J. Pharm. Sci. 2014, 63, 204–215. [Google Scholar] [CrossRef]
- Almeida, H.; Lobão, P.; Frigerio, C.; Fonseca, J.; Silva, R.; Quaresma, P.; Lobo, J.M.S.; Amaral, M.H. Development of mucoadhesive and thermosensitive eyedrops to improve the ophthalmic bioavailability of ibuprofen. J. Drug Deliv. Sci. Technol. 2016, 35, 69–80. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sánchez, A.; Alonso, M.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Cunha, S.; Costa, C.P.; Loureiro, J.A.; Alves, J.; Peixoto, A.F.; Forbes, B.; Lobo, J.M.S.; Silva, A.C. Double optimization of rivastigmine-loaded nanostructured lipid carriers (NLC) for nose-to-brain delivery using the quality by design (QbD) approach: Formulation variables and instrumental parameters. Pharmaceutics 2020, 12, 599. [Google Scholar] [CrossRef]
- Smith, R.S.; Rudt, L.A. Ocular vascular and epithelial barriers to microperoxidase. Investig. Ophthalmol. 1975, 14, 556–560. [Google Scholar]
- Takeuchi, H.; Thongborisute, J.; Matsui, Y.; Sugihara, H.; Yamamoto, H.; Kawashima, Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Nikogeorgos, N.; Efler, P.; Kayitmazer, A.B.; Lee, S. “Bio-glues” to enhance slipperiness of mucins: Improved lubricity and wear resistance of porcine gastric mucin (PGM) layers assisted by mucoadhesion with chitosan. Soft Matter 2015, 11, 489–498. [Google Scholar] [CrossRef]
- Mendes, A.C.; Moreno, J.S.; Hanif, M.; Douglas, T.E.L.; Chen, M.; Chronakis, I.S. Morphological, mechanical and mucoadhesive properties of electrospun chitosan/phospholipid hybrid nanofibers. Int. J. Mol. Sci. 2018, 19, 2266. [Google Scholar] [CrossRef]
- Fefelova, N.A.; Nurkeeva, Z.S.; Mun, G.A.; Khutoryanskiy, V.V. Mucoadhesive interactions of amphiphilic cationic copolymers based on [2-(methacryloyloxy)ethyl]trimethylammonium chloride. Int. J. Pharm. 2007, 339, 25–32. [Google Scholar] [CrossRef]
- Terreni, E.; Chetoni, P.; Tampucci, S.; Burgalassi, S.; Al-Kinani, A.A.; Alany, R.G.; Monti, D. Assembling surfactants-mucoadhesive polymer nanomicelles (ASMP-nano) for ocular delivery of cyclosporine-A. Pharmaceutics 2020, 12, 253. [Google Scholar] [CrossRef]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Lamprecht, A. Standardized in vitro drug release test for colloidal drug carriers using modified USP dissolution apparatus I. Drug Dev. Ind. Pharm. 2011, 37, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Tomlinson, A.; Khanal, S.; Ramaesh, K.; Diaper, C.; McFadyen, A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4309–4315. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Mishra, S.; Mohanty, B. Effect of formulation factors on in vitro transcorneal permeation of voriconazole from aqueous drops. J. Adv. Pharm. Technol. Res. 2013, 4, 210. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G. Ocular ciprofloxacin hydrochloride mucoadhesive chitosan-coated liposomes. Pharm. Dev. Technol. 2011, 16, 44–56. [Google Scholar] [CrossRef]
- Particle Size Distribution Functions. Available online: https://www.eng.uc.edu/~beaucag/Classes/Nanopowders/ParticleSizeDistribuhtml/ParticleSizeDistribu.htm (accessed on 30 January 2022).
- Kulkarni, V.S. Handbook of Non-Invasive Drug Delivery Systems: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780815520252. [Google Scholar]
- Dingler, A.; Blum, R.P.; Niehus, H.; Müller, R.H.; Gohla, S. Solid lipid nanoparticles (SLNTM/LipopearlsTM)—A pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products. J. Microencapsul. 1999, 16, 751–767. [Google Scholar] [CrossRef]
- WaKasongo, K. An Investigation into the Feasability of Incorporating Didanosine into Innovative Solid Lipid Nanocarriers; Rhodes University: Makhanda, South Africa, 2010. [Google Scholar]
- Teeranachaideekul, V.; Souto, E.B.; Junyaprasert, V.B.; Müller, R.H. Cetyl palmitate-based NLC for topical delivery of Coenzyme Q10—Development, physicochemical characterization and in vitro release studies. Eur. J. Pharm. Biopharm. 2007, 67, 141–148. [Google Scholar] [CrossRef]
- Bhasarkar, J.; Bal, D. Kinetic investigation of a controlled drug delivery system based on alginate scaffold with embedded voids. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018817462. [Google Scholar] [CrossRef]
- Nabi-Meibodi, M.; Vatanara, A.; Najafabadi, A.R.; Rouini, M.R.; Ramezani, V.; Gilani, K.; Etemadzadeh, S.M.H.; Azadmanesh, K. The effective encapsulation of a hydrophobic lipid-insoluble drug in solid lipid nanoparticles using a modified double emulsion solvent evaporation method. Colloids Surf. B Biointerfaces 2013, 112, 408–414. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chung, J.F. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2011, 83, 299–306. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Chen, H.H. Entrapment and release of saquinavir using novel cationic solid lipid nanoparticles. Int. J. Pharm. 2009, 365, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Remya, P.N.; Damodharan, N. Formulation, development and characterisation of nimodipine loaded solid lipid nanoparticles. Int. J. Appl. Pharm. 2020, 12, 265–271. [Google Scholar] [CrossRef]
- Zimmermann, E.; Müller, R.; Mäder, K. Influence of different parameters on reconstitution of lyophilized SLN. Int. J. Pharm. 2000, 196, 211–213. [Google Scholar] [CrossRef]
- Havanoor, S.M.; Manjunath, K.; Bhagawati, S.T.; Veerapur, V.P. Isradipine Loaded Solid Lipid Nanoparticles for Better Treatment. Int. J. Biopharm. 2014, 3, 218–224. [Google Scholar]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yan, H.; Jiang, X.; Xu, H.; Tsao, R.; Zhang, L. Preparation of 9 Z-β-Carotene and 9 Z-β-Carotene High-Loaded Nanostructured Lipid Carriers: Characterization and Storage Stability. J. Agric. Food Chem. 2020, 68, 13844–13853. [Google Scholar] [CrossRef]
- Pornputtapitak, W.; Pantakitcharoenkul, J.; Teeranachaideekul, V.; Sinthiptharakoon, K.; Sapcharoenkun, C.; Meemuk, B. Effect of oil content on physiochemical characteristics of γ-oryzanol-loaded nanostructured lipid carriers. J. Oleo Sci. 2019, 68, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.E.; Benoit, J.P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Effect of light and temperature on zeta potential and physical stability in solid lipid nanoparticle (SLN®) dispersions. Int. J. Pharm. 1998, 168, 221–229. [Google Scholar] [CrossRef]
- Zulkifli, Z.A.; Razak, K.A.; Rahman, W.N.W.A. The effect of reaction temperature on the particle size of bismuth oxide nanoparticles synthesized via hydrothermal method. In AIP Conference Proceedings; AIP Publishing LLC: College Park, MD, USA, 2018; Volume 1958. [Google Scholar]
- Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf. B Biointerfaces 2010, 81, 412–421. [Google Scholar] [CrossRef]
- Makoni, P.A.; Kasongo, K.W.; Walker, R.B. Short term stability testing of efavirenz-loaded solid lipid nanoparticle (SLN) and nanostructured lipid carrier (NLC) dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophthalmic drug dosage forms: Characterisation and research methods. Sci. World J. 2014, 2014, 861904. [Google Scholar] [CrossRef]
- Silva, A.C.; González-Mira, E.; García, M.L.; Egea, M.A.; Fonseca, J.; Silva, R.; Santos, D.; Souto, E.B.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B Biointerfaces 2011, 86, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Makoni, P.A. Lipid Nanocarriers: A Novel Approach to Delivering Ophthalmic Clarithromycin; Rhodes University: Makhanda, South Africa, 2020. [Google Scholar]


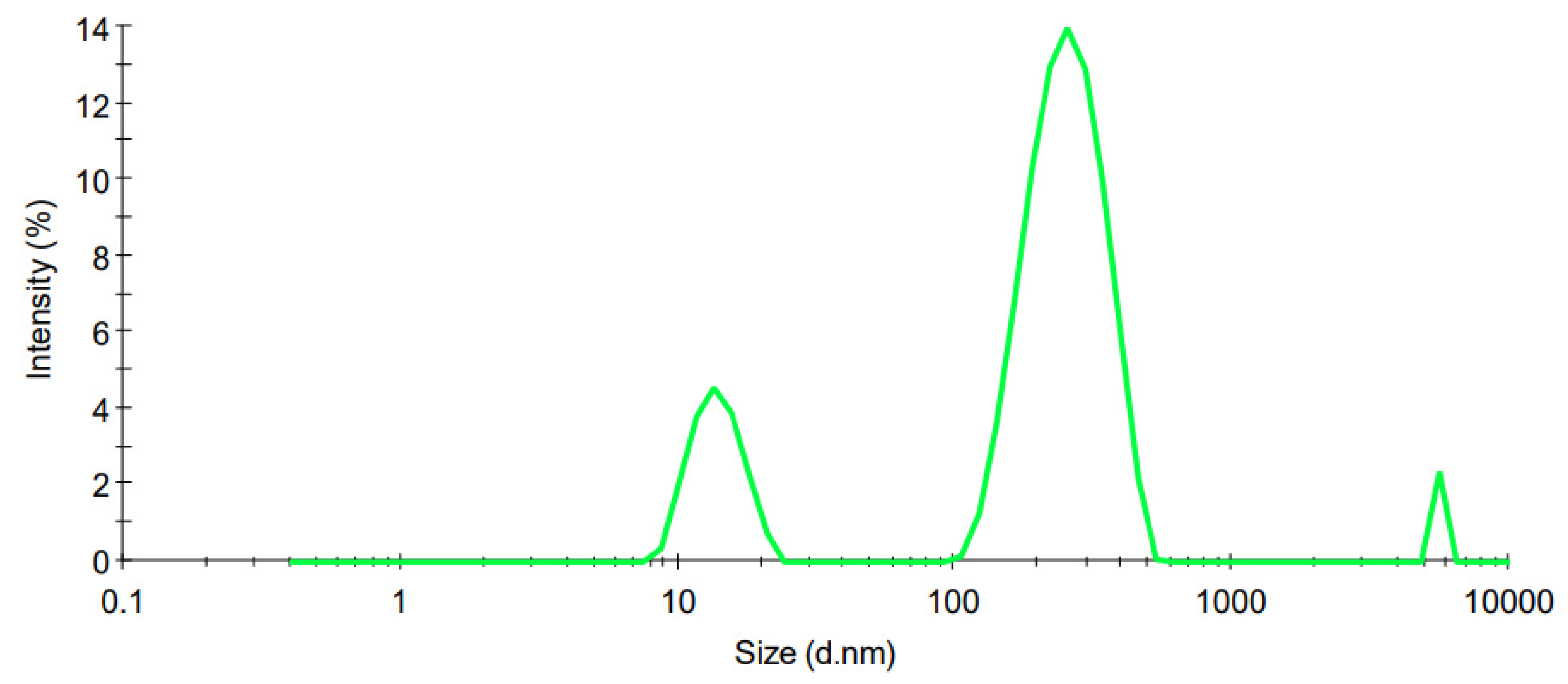




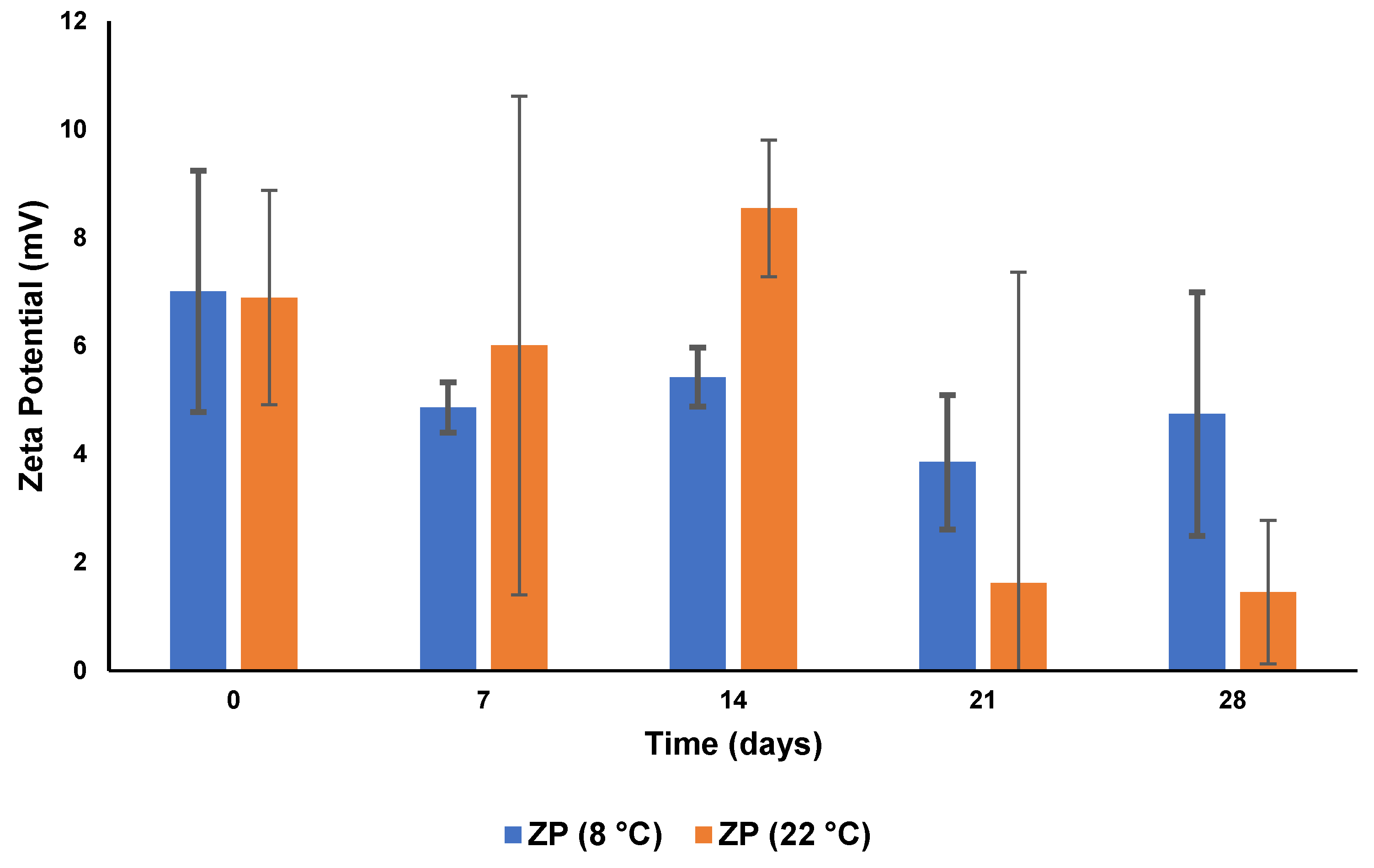
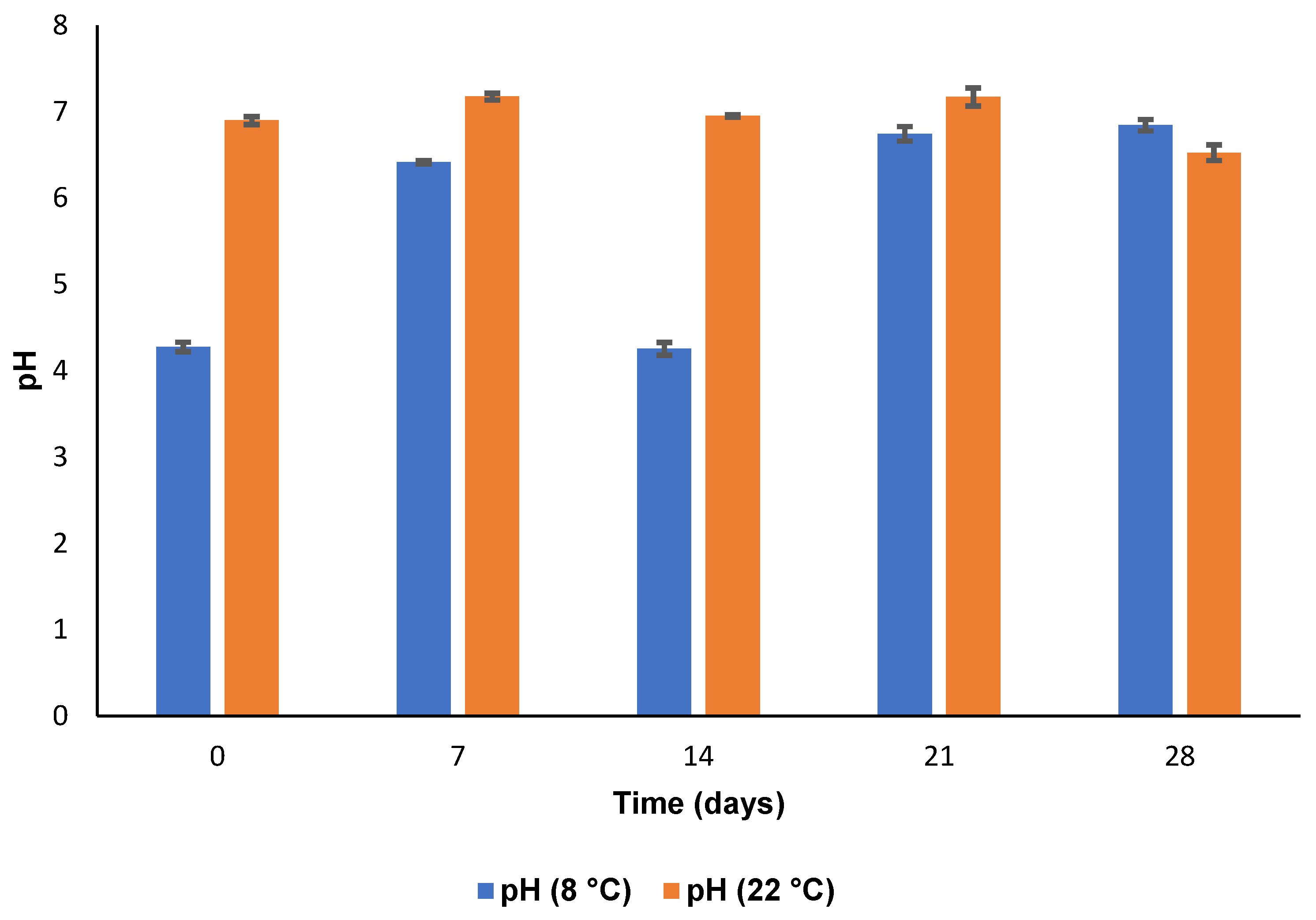

| CS Concentration in SLN Dispersion (% w/v) | PS (nm) | ZP (mV) | PDI | EE (%) | DL (%) | pH |
|---|---|---|---|---|---|---|
| 0.01 | 453.33 ± 74.86 | 6.67 ± 0.40 | 0.43 ± 0.04 | 99.91 | 4.99 | 6.72 ± 0.03 |
| 0.03 | 274.27 ± 141.31 | 7.39 ± 2.98 | 0.37 ± 0.10 | 99.91 | 4.99 | 4.81 ± 0.02 |
| 0.05 | 247.69 ± 223.69 | 4.9867 ± 2.25 | 0.37 ± 0.16 | 99.90 | 4.99 | 4.40 ± 0.01 |
| Model and Equation | Code Evaluation Criteria | ||
|---|---|---|---|
| R2adj | AIC | MSC | |
| Zero-order (A) | 0.3892 | 71.5384 | −0.2779 |
| First order (B) | 0.9924 | 36.449 | 4.1083 |
| Higuchi (C) | 0.8789 | 58.5922 | 1.3404 |
| Korsmeyer–Peppas (D) | 0.9164 | 56.3928 | 1.6153 |
| Hixson–Crowell (E) | 0.9701 | 47.3949 | 2.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikhondze, S.S.; Makoni, P.A.; Walker, R.B.; Khamanga, S.M.M. Chitosan-Coated SLN: A Potential System for Ocular Delivery of Metronidazole. Pharmaceutics 2023, 15, 1855. https://doi.org/10.3390/pharmaceutics15071855
Sikhondze SS, Makoni PA, Walker RB, Khamanga SMM. Chitosan-Coated SLN: A Potential System for Ocular Delivery of Metronidazole. Pharmaceutics. 2023; 15(7):1855. https://doi.org/10.3390/pharmaceutics15071855
Chicago/Turabian StyleSikhondze, Simise S., Pedzisai A. Makoni, Roderick B. Walker, and Sandile M. M. Khamanga. 2023. "Chitosan-Coated SLN: A Potential System for Ocular Delivery of Metronidazole" Pharmaceutics 15, no. 7: 1855. https://doi.org/10.3390/pharmaceutics15071855
APA StyleSikhondze, S. S., Makoni, P. A., Walker, R. B., & Khamanga, S. M. M. (2023). Chitosan-Coated SLN: A Potential System for Ocular Delivery of Metronidazole. Pharmaceutics, 15(7), 1855. https://doi.org/10.3390/pharmaceutics15071855





