Manganese Sulfate Nanocomposites Fabricated by Hot-Melt Extrusion for Chemodynamic Therapy of Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production and Particle Characterization of MnSO4 Extrudate-Based Nanocomposites
2.2.1. Production of MnSO4 Formulations by HME
2.2.2. Particle Characterization of MnSO4 NPs
2.3. Solid-State Studies
2.3.1. Fourier-Transform Infrared (FT-IR) Spectroscopy Analysis
2.3.2. X-ray Diffractometry (XRD) Assay
2.3.3. X-ray Photoelectron Spectroscopy (XPS) Study
2.3.4. Field Emission Scanning Electron Microscopy (FE-SEM)/Energy Dispersive Spectrometry (EDS) Study
2.4. Catalytic Assays
2.4.1. 3,3′,5,5′- Tetramethylbenzidine (TMB) Assay
2.4.2. MB Assay
2.5. In Vitro Anticancer Activity Tests
2.5.1. Antiproliferation Assay
2.5.2. Cellular ROS Assay
2.5.3. Apoptosis Assay
2.6. Data Analysis
3. Results and Discussion
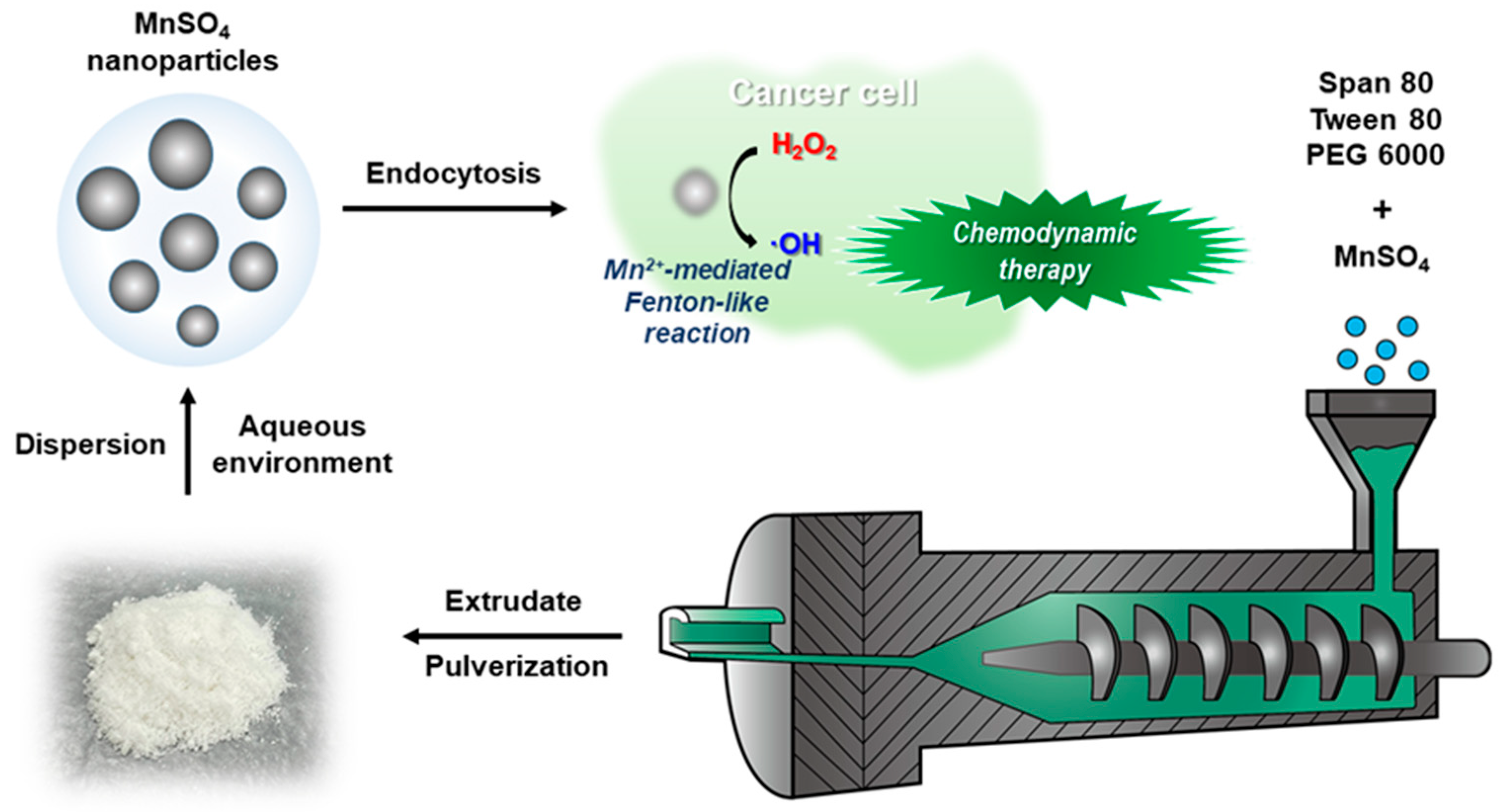
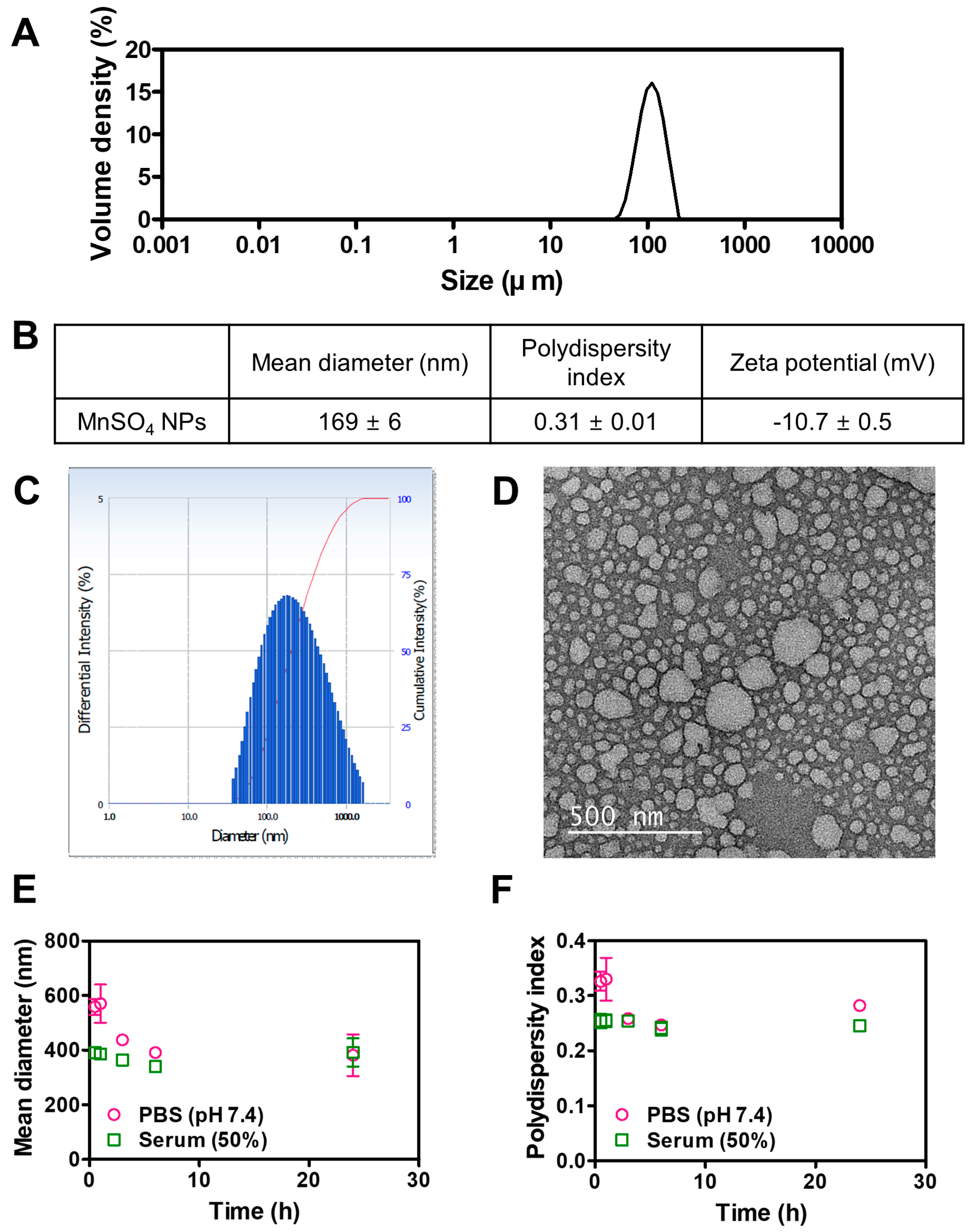
3.1. Fabrication and Particle Property Tests of MnSO4 NPs
3.2. Solid-State Features of MnSO4 NPs
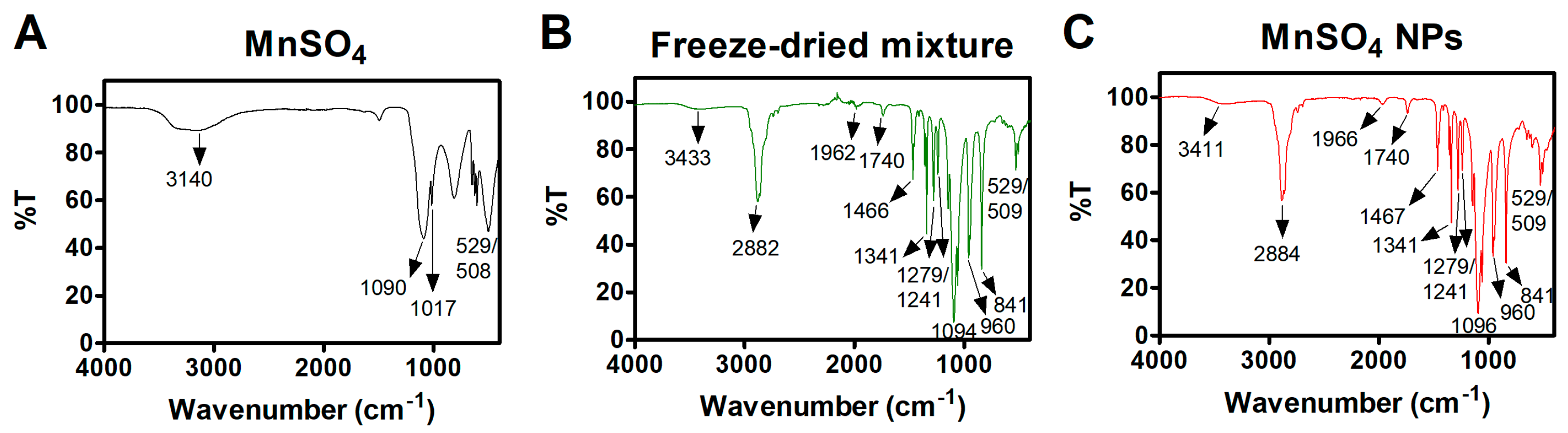

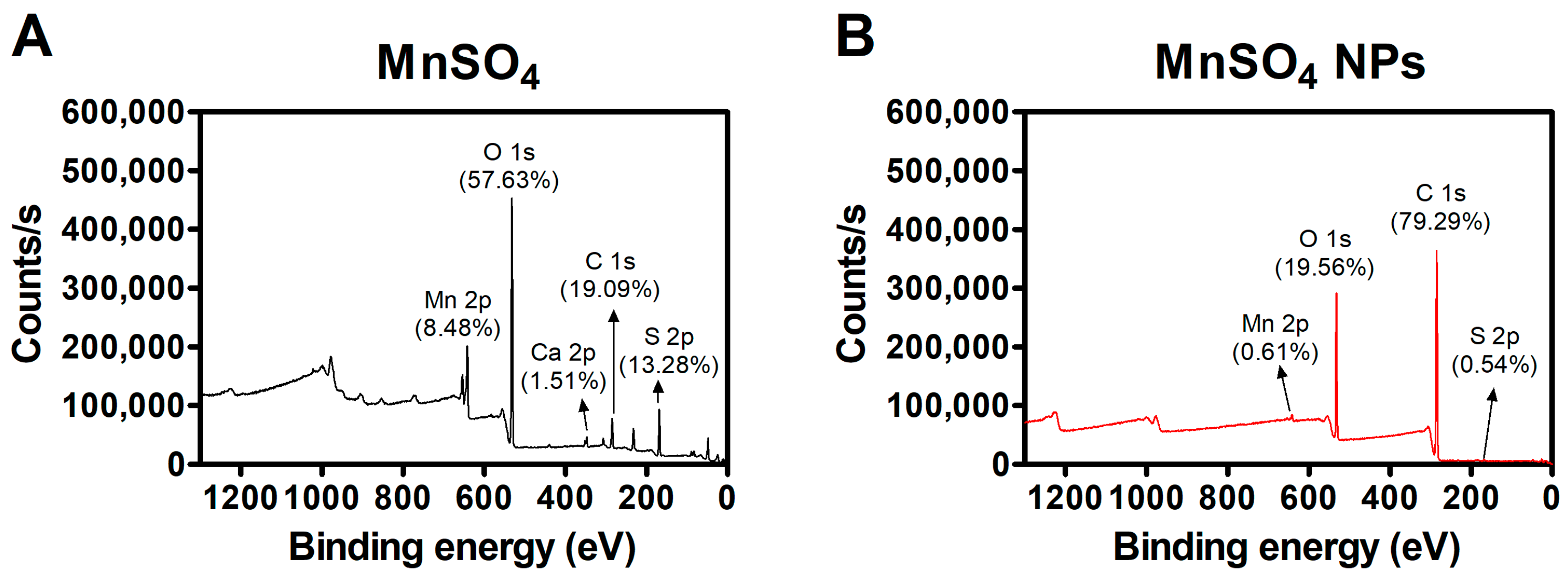
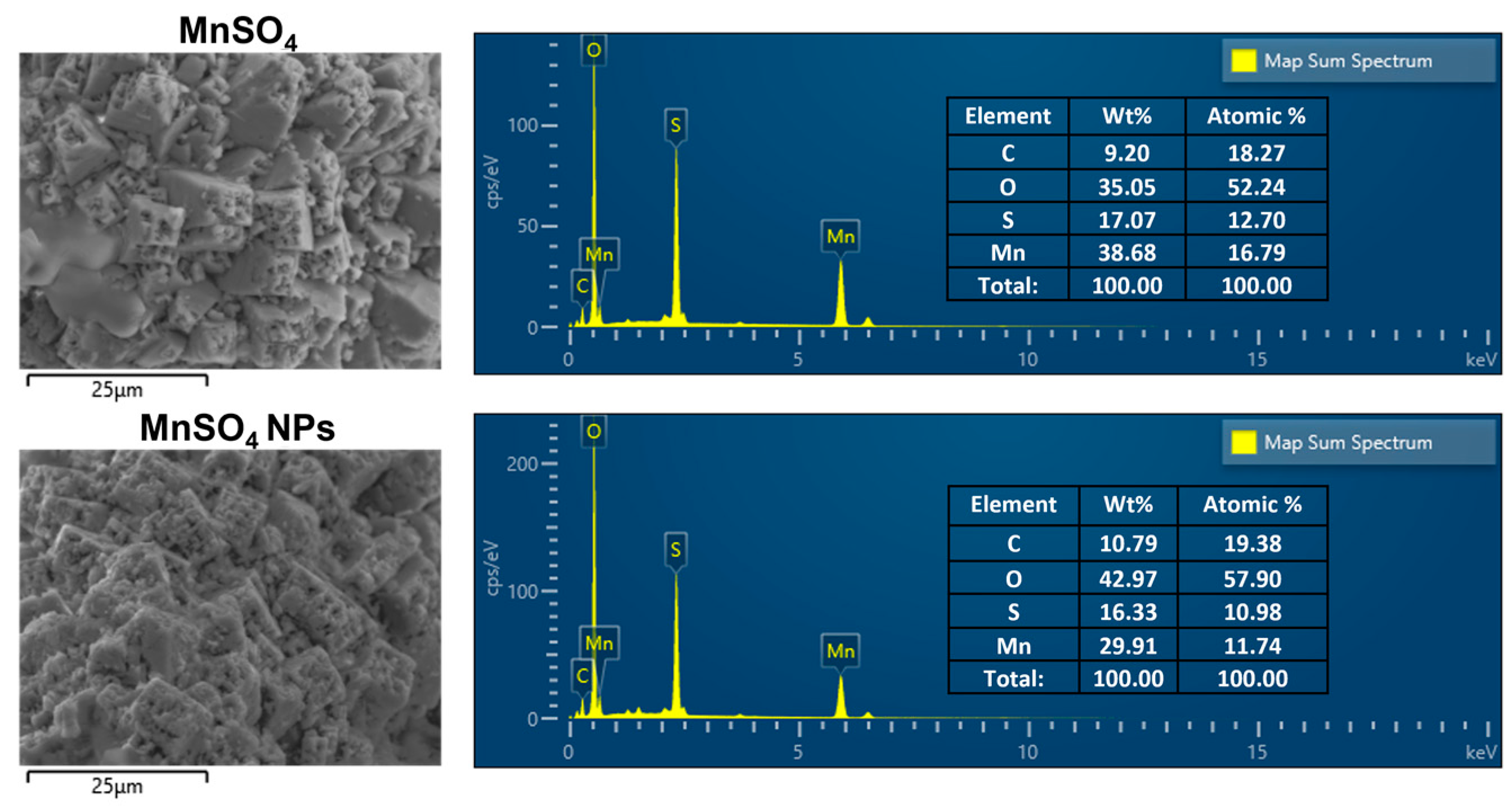
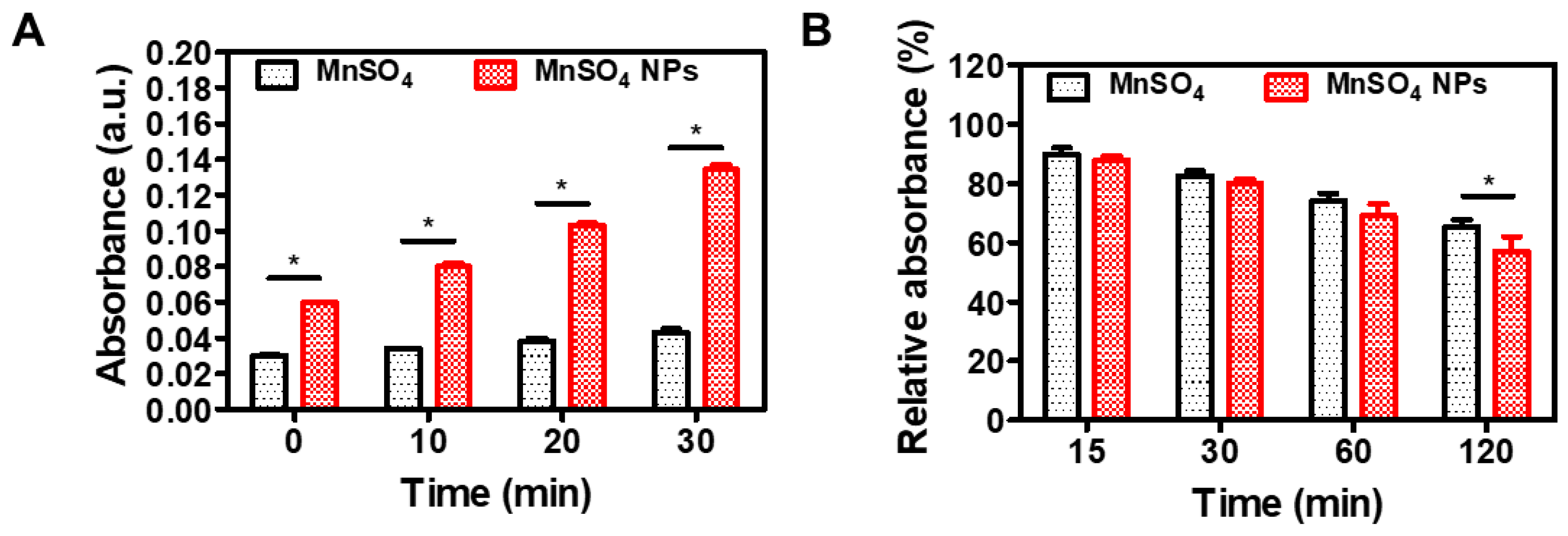
3.3. Catalytic Features
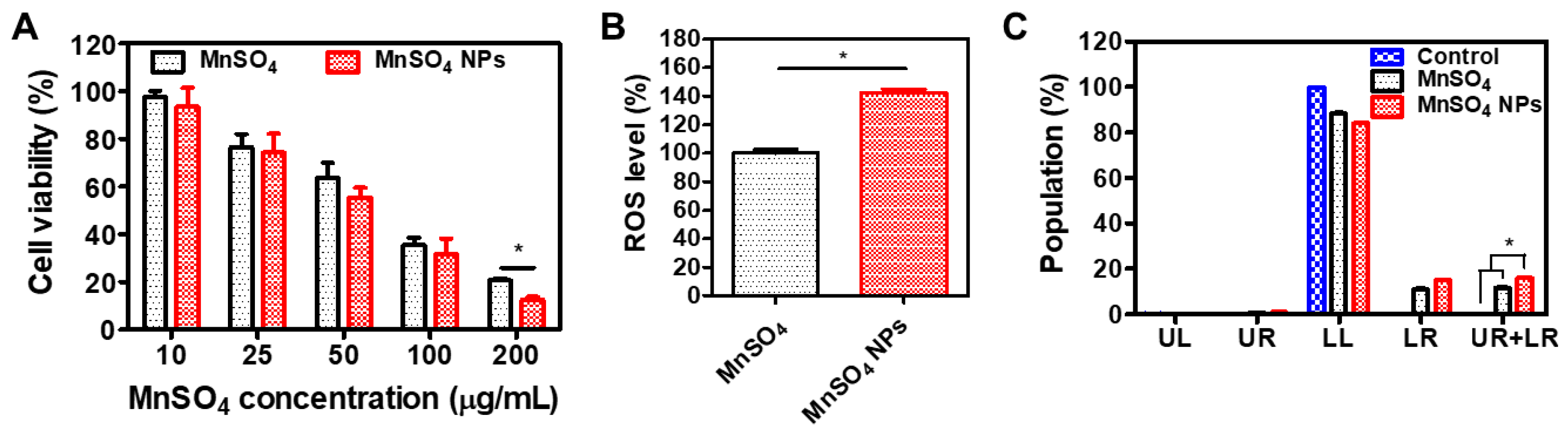
3.4. In Vitro Anticancer Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, H.; Tiwari, R.V.; Repka, M.A. 2016. Hot-melt extrusion: From theory to application in pharmaceutical formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.F.; Pinto, R.M.A.; Simões, S. Hot-melt extrusion in the pharmaceutical industry: Toward filing a new drug application. Drug Discov. Today 2019, 24, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- Tambe, S.; Jain, D.; Agarwal, Y.; Amin, P. Hot-melt extrusion: Highlighting recent advances in pharmaceutical applications. J. Drug Deliv. Sci. Technol. 2021, 63, 102452. [Google Scholar] [CrossRef]
- Almotairy, A.; Alyahya, M.; Althobaiti, A.; Almutairi, M.; Bandari, S.; Ashour, E.A.; Repka, M.A. Disulfiram 3D printed film produced via hot-melt extrusion techniques as a potential anticervical cancer candidate. Int. J. Pharm. 2023, 635, 122709. [Google Scholar] [CrossRef] [PubMed]
- Bagde, A.; Kouagou, E.; Singh, M. Formulation of topical flurbiprofen solid lipid nanoparticle gel formulation using hot melt extrusion technique. AAPS PharmSciTech 2022, 23, 257. [Google Scholar] [CrossRef]
- Patel, H.; Palekar, S.; Patel, A.; Patel, K. Ibrutinib amorphous solid dispersions with enhanced dissolution at colonic pH for the localized treatment of colorectal cancer. Int. J. Pharm. 2023, 641, 123056. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, G.; Wen, X.; Wu, L.; Deng, R.; Liang, Q.; Zhang, L.; Chen, H.; Feng, X.; He, J. Preparation, evaluation, and pharmacokinetics in beagle dogs of a taste-masked flunixin meglumine orally disintegrating tablet prepared using hot-melt extrusion technology and D-optimal mixture design. Eur. J. Pharm. Sci. 2022, 168, 106019. [Google Scholar] [CrossRef]
- Koo, J.S.; Lee, S.Y.; Nam, S.; Azad, M.O.K.; Kim, M.; Kim, K.; Chae, B.J.; Kang, W.S.; Cho, H.J. Preparation of cupric sulfate-based self-emulsifiable nanocomposites and their application to the photothermal therapy of colon adenocarcinoma. Biochem. Biophys. Res. Commun. 2018, 503, 2471–2477. [Google Scholar] [CrossRef]
- Koo, J.S.; Lee, S.Y.; Azad, M.O.K.; Kim, M.; Hwang, S.J.; Nam, S.; Kim, S.; Chae, B.J.; Kang, W.S.; Cho, H.J. Development of iron(II) sulfate nanoparticles produced by hot-melt extrusion and their therapeutic potentials for colon cancer. Int. J. Pharm. 2019, 558, 388–395. [Google Scholar] [CrossRef]
- Lee, S.Y.; Nam, S.; Choi, Y.; Kim, M.; Koo, J.S.; Chae, B.J.; Kang, W.S.; Cho, H.J. Fabrication and characterizations of hot-melt extruded nanocomposites based on zinc sulfate monohydrate and Soluplus. Appl. Sci. 2017, 7, 902. [Google Scholar] [CrossRef]
- Thapa, R.K.; Kim, J.O. Nanomedicine-based commercial formulations: Current developments and future prospects. J. Pharm. Investig. 2023, 53, 19–33. [Google Scholar] [CrossRef]
- López-Lázaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef]
- Lin, L.S.; Song, J.; Song, L.; Ke, K.; Liu, Y.; Zhou, Z.; Shen, Z.; Li, J.; Yang, Z.; Tang, W.; et al. Simultaneous Fenton-like ion delivery and glutathione depletion by MnO2-based nanoagent enhances chemodynamic therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 4902–4906. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhu, Y.; Xu, C.; Pan, C.; Shi, Y.; Zou, J.; Li, Y.; Hu, X.; Zhou, B.; Zhao, C.; et al. Biomimetic yolk-shell nanocatalysts for activatable dual-modal-image-guided triple-augmented chemodynamic therapy of cancer. ACS Nano 2022, 16, 19038–19052. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Su, L.; He, J.; Ruan, R.; Wang, J.; Wang, Z.; Xiao, P.; Liu, C.; Cao, Y.; Li, W.; et al. Dual-modal imaging and synergistic spinal tumor therapy enabled by hierarchical-structured nanofibers with cascade release and postoperative anti-adhesion. ACS Nano 2022, 16, 16880–16897. [Google Scholar] [CrossRef]
- Kim, M.J.; Hosseindoust, A.; Kim, K.Y.; Moturi, J.; Lee, J.H.; Kim, T.G.; Mun, J.Y.; Chae, B.J. Improving the bioavailability of manganese and meat quality of broilers by using hot-melt extrusion nano method. Brit. Poult. Sci. 2022, 63, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, S.Y.; Cho, E.J.; Jeong, D.I.; Kim, D.D.; Kim, H.C.; Cho, H.J. Gas generating microspheres for immediate release of Hsp90 inhibitor aiming at postembolization hypoxia in transarterial chemoembolization therapy of hepatocellular carcinoma. Int. J. Pharm. 2021, 607, 120988. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.I.; Kim, S.; Lee, S.Y.; Kim, H.J.; Lee, J.; Lee, K.; Cho, H.J. Iron sulfate-reinforced hydrogel reactors with glucose deprivation, serial reactive oxygen species generation, ferroptosis induction, and photothermal ablation for cancer therapy. Chem. Eng. J. 2022, 438, 135584. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.; Jeong, D.I.; Hwang, C.; Lee, J.; Lee, K.; Kim, H.J.; Cho, H.J. Ferrocene and glucose oxidase-installed multifunctional hydrogel reactors for local cancer therapy. J. Control. Release 2022, 349, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Kiio, T.M.; Park, S. Physical properties of nanoparticles do matter. J. Pharm. Investig. 2021, 51, 35–51. [Google Scholar] [CrossRef]
- Haque, S.T.; Karim, M.E.; Othman, I.; Chowdhury, E.H. Mitigating off-target distribution and enhancing cytotoxicity in breast cancer cells with alpha-ketoglutaric acid-modified Fe/Mg-CA nanoparticles. J. Pharm. Investig. 2022, 52, 367–386. [Google Scholar] [CrossRef]
- Zalba, S.; Ten Hagen, T.L.M.; Burgui, C.; Garrido, M.J. Stealth nanoparticles in oncology: Facing the PEG dilemma. J. Control. Release 2022, 351, 22–36. [Google Scholar] [CrossRef]
- Guo, X.; Xiao, H.S.; Wang, F.; Zhang, Y.H. Micro-Raman and FTIR spectroscopic observation on the phase transitions of MnSO4 droplets and ionic interactions between Mn2+ and SO42-. J. Phys. Chem. A 2010, 114, 6480–6486. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, X.; Shi, J.; Zhao, J.; Hua, Z.; Gao, J.; Ruan, M.; Yan, D. Templated synthesis of hierarchically porous manganese oxide with a crystalline nanorod framework and its high electrochemical performance. J. Mater. Chem. 2007, 17, 855–860. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Qian, D.; Li, Y.; Zhang, W. Single-crystal α-MnO2 nanorods: Synthesis and electrochemical properties. Nanotechnology 2007, 18, 115616. [Google Scholar] [CrossRef]
- Stella, C.; Soundararajan, N.; Ramachandran, K. Structural, optical, dielectric and magnetic properties of Mn1−xCoxO2 nanowires. Superlattices Microstruc. 2014, 71, 203–210. [Google Scholar] [CrossRef]
- Nosal, H.; Moser, K.; Warzała, M.; Holzer, A.; Stańczyk, D.; Sabura, E. Selected fatty acids esters as potential PHB-V bioplasticizers: Effect on mechanical properties of the polymer. J. Polym. Environ. 2021, 29, 38–53. [Google Scholar] [CrossRef]
- Nayl, A.A.; Ismail, I.M.; Aly, H.F. Recovery of pure MnSO4∙H2O by reductive leaching of manganese from pyrolusite ore by sulfuric acid and hydrogen peroxide. Int. J. Miner. Process. 2011, 100, 116–123. [Google Scholar] [CrossRef]
- Bhatt, S.; Pulpytel, J.; Mirshahi, M.; Arefi-Khonsari, F. Catalyst-free plasma-assisted copolymerization of poly(ε-caprolactone)-poly(ethylene glycol) for biomedical applications. ACS Macro Lett. 2012, 1, 764–767. [Google Scholar] [CrossRef]
- Mondal, H.; Karmakar, M.; Ghosh, N.N.; Maiti, D.K.; Chattopadhyay, P.K.; Singha, N.R. One-pot synthesis of sodium alginate-grafted-terpolymer hydrogel for As(III) and V(V) removal: In situ anchored comonomer and DFT studies on structures. J. Environ. Manag. 2021, 294, 112932. [Google Scholar] [CrossRef]
- Xu, W.; Li, H.; Zheng, Y.; Lei, W.; Wang, Z.; Cheng, Y.; Qi, R.; Peng, H.; Lin, H.; Yue, F.; et al. Atomic insights into Ti Doping on the stability enhancement of truncated octahedron LiMn2O4 nanoparticles. Nanomaterials 2021, 11, 508. [Google Scholar] [CrossRef] [PubMed]
- Mondal, H.; Karmakar, M.; Dutta, A.; Mahapatra, M.; Deb, M.; Mitra, M.; Roy, J.S.D.; Roy, C.; Chattopadhyay, P.K.; Singha, N.R. Tetrapolymer network hydrogels via gum ghatti-grafted and N−H/C−H-activated allocation of monomers for composition-dependent superadsorption of metal ions. ACS Omega 2018, 3, 10692–10708. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Jin, M.; Yang, K.; Chen, B.; Xiong, M.; Li, X.; Cao, G. Fenton/Fenton-like metal-based nanomaterials combine with oxidase for synergistic tumor therapy. J. Nanobiotechnol. 2021, 19, 325. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, X.; He, Y.; Peng, Y.; Pan, J.; Niu, X.; Zhao, H.; Lan, M. Colorimetric evaluation of the hydroxyl radical scavenging ability of antioxidants using carbon-confined CoOx as a highly active peroxidase mimic. Mikrochim. Acta 2019, 186, 354. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on methylene blue: Its properties, uses, toxicity and photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Satoh, A.Y.; Trosko, J.E.; Masten, S.J. Methylene blue dye test for rapid qualitative detection of hydroxyl radicals formed in a Fenton’s reaction aqueous solution. Environ. Sci. Technol. 2007, 41, 2881–2887. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, D.I.; Kim, S.; Koo, J.S.; Lee, S.Y.; Kim, M.; Kim, K.Y.; Azad, M.O.K.; Karmakar, M.; Chu, S.; Chae, B.-J.; et al. Manganese Sulfate Nanocomposites Fabricated by Hot-Melt Extrusion for Chemodynamic Therapy of Colorectal Cancer. Pharmaceutics 2023, 15, 1831. https://doi.org/10.3390/pharmaceutics15071831
Jeong DI, Kim S, Koo JS, Lee SY, Kim M, Kim KY, Azad MOK, Karmakar M, Chu S, Chae B-J, et al. Manganese Sulfate Nanocomposites Fabricated by Hot-Melt Extrusion for Chemodynamic Therapy of Colorectal Cancer. Pharmaceutics. 2023; 15(7):1831. https://doi.org/10.3390/pharmaceutics15071831
Chicago/Turabian StyleJeong, Da In, Sungyun Kim, Ja Seong Koo, Song Yi Lee, Minju Kim, Kwang Yeol Kim, Md Obyedul Kalam Azad, Mrinmoy Karmakar, Seongnam Chu, Byung-Jo Chae, and et al. 2023. "Manganese Sulfate Nanocomposites Fabricated by Hot-Melt Extrusion for Chemodynamic Therapy of Colorectal Cancer" Pharmaceutics 15, no. 7: 1831. https://doi.org/10.3390/pharmaceutics15071831
APA StyleJeong, D. I., Kim, S., Koo, J. S., Lee, S. Y., Kim, M., Kim, K. Y., Azad, M. O. K., Karmakar, M., Chu, S., Chae, B.-J., Kang, W.-S., & Cho, H.-J. (2023). Manganese Sulfate Nanocomposites Fabricated by Hot-Melt Extrusion for Chemodynamic Therapy of Colorectal Cancer. Pharmaceutics, 15(7), 1831. https://doi.org/10.3390/pharmaceutics15071831









