Cyclic RGD-Functionalized pH/ROS Dual-Responsive Nanoparticle for Targeted Breast Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Animals
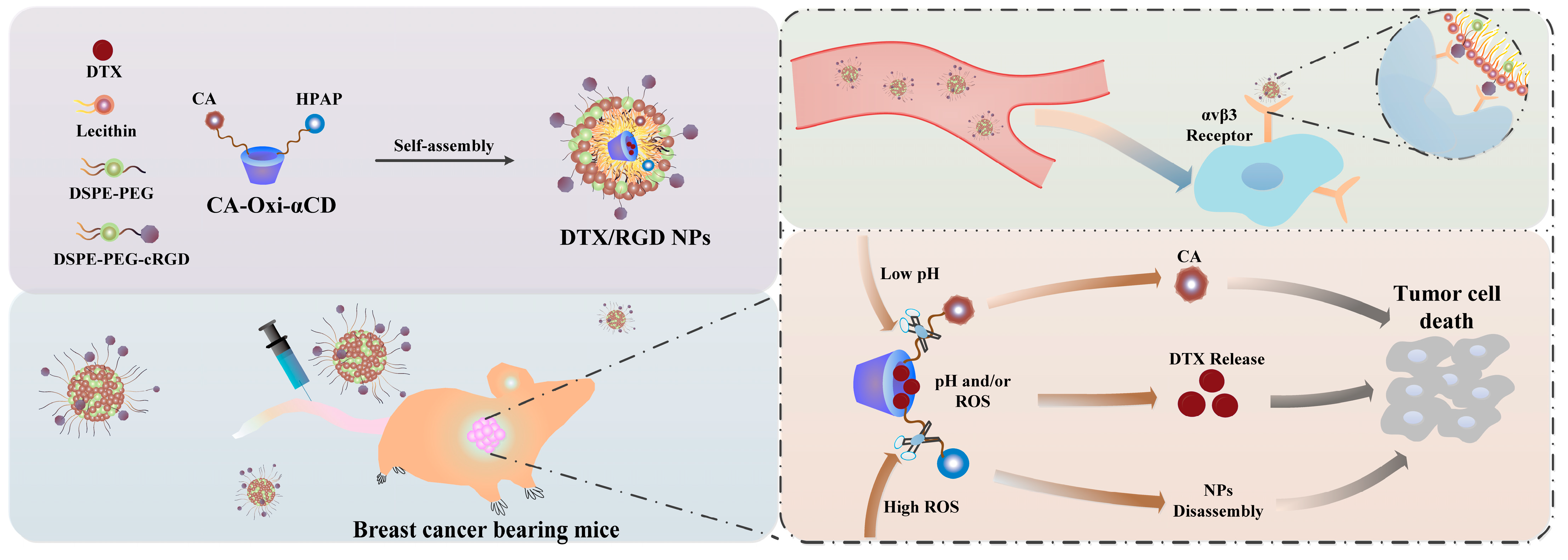
2.4. Fabrication and Characterization of DTX-Loaded NPs
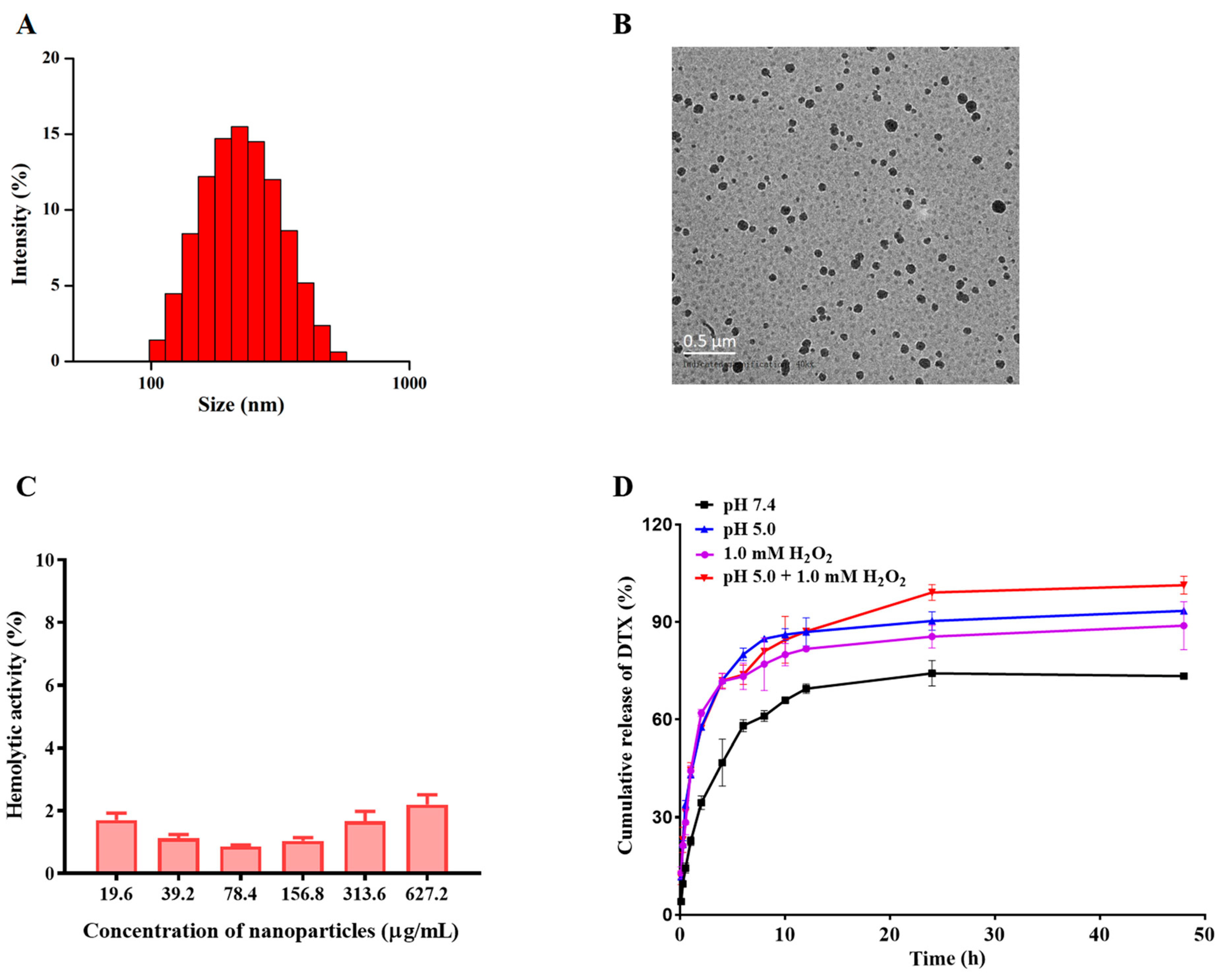
2.5. pH/ROS Responsiveness and Drug Release of NPs In Vitro
2.6. Hemolysis Assay
2.7. Intracellular H2O2 Detection
2.8. Intracellular Uptake
2.9. The Penetration in 3D Tumor Spheroids
2.10. Cytotoxicity Assays
2.11. Cell Cycle Assay
2.12. Cell Apoptosis Assay
2.13. In Vivo Biodistribution
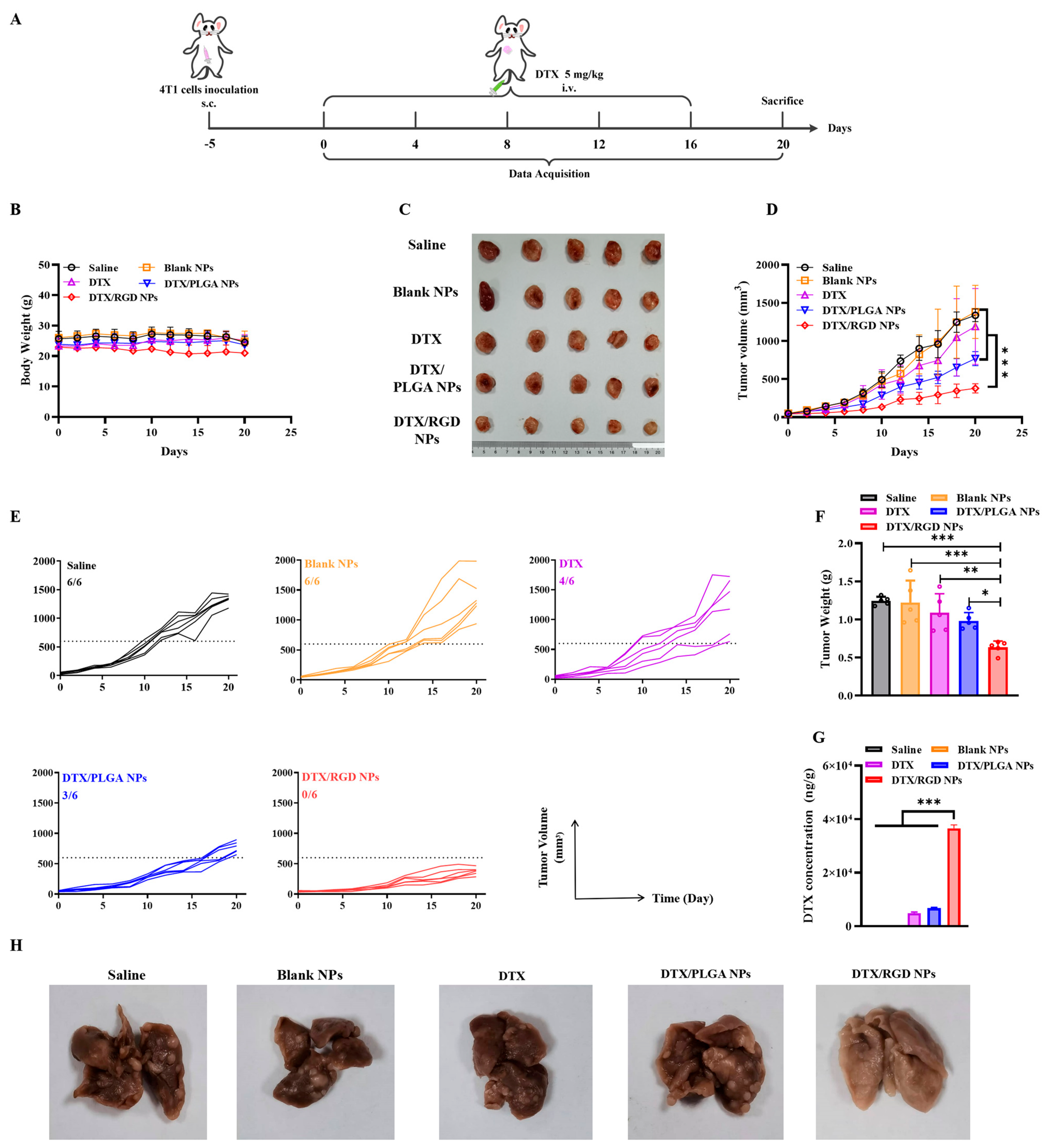
2.14. Antitumor Efficacy In Vivo
2.15. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Characterization of the NPs
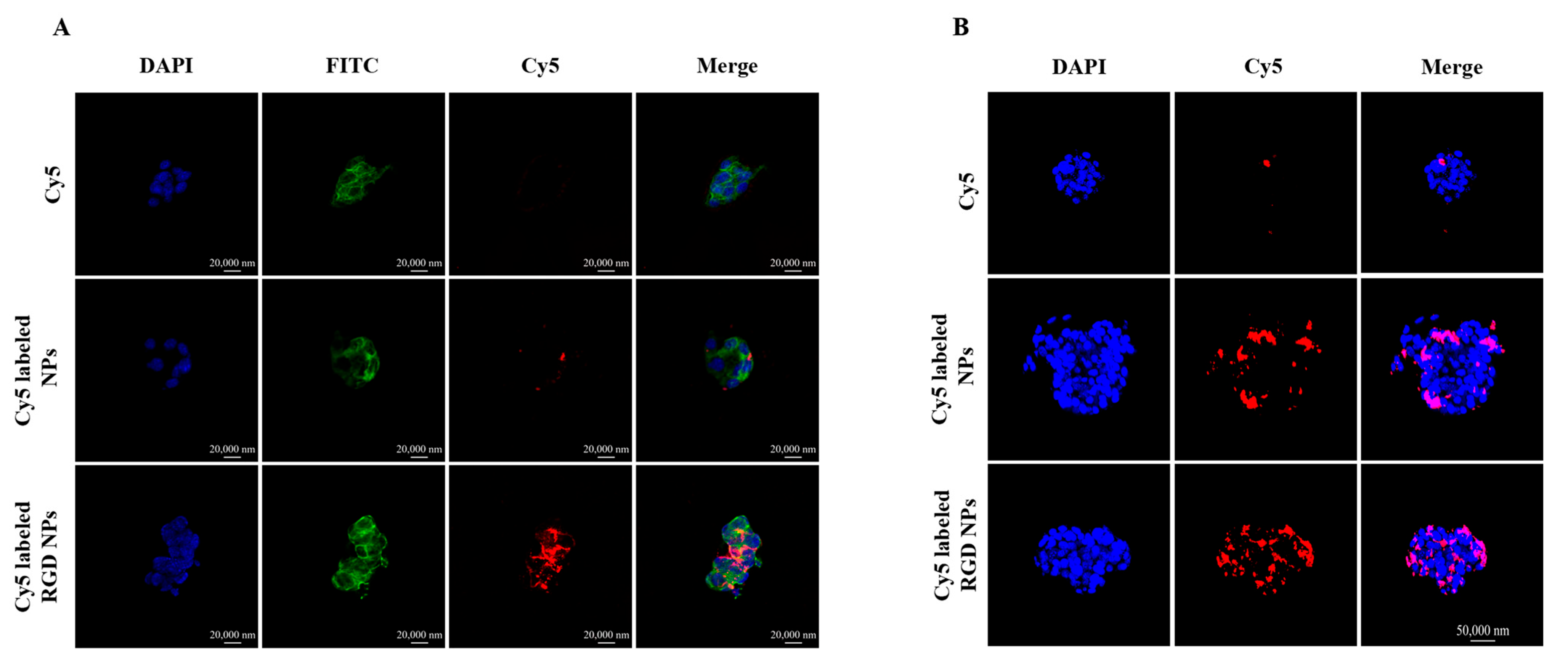
3.2. Cellular Uptake
3.3. Three-Dimensional (3D) Tumor Spheroid Penetration of the NPs
3.4. In Vitro Cytotoxicity of the NPs on Tumor Cells
3.5. Cell-Cycle Assay
3.6. Cell Apoptosis
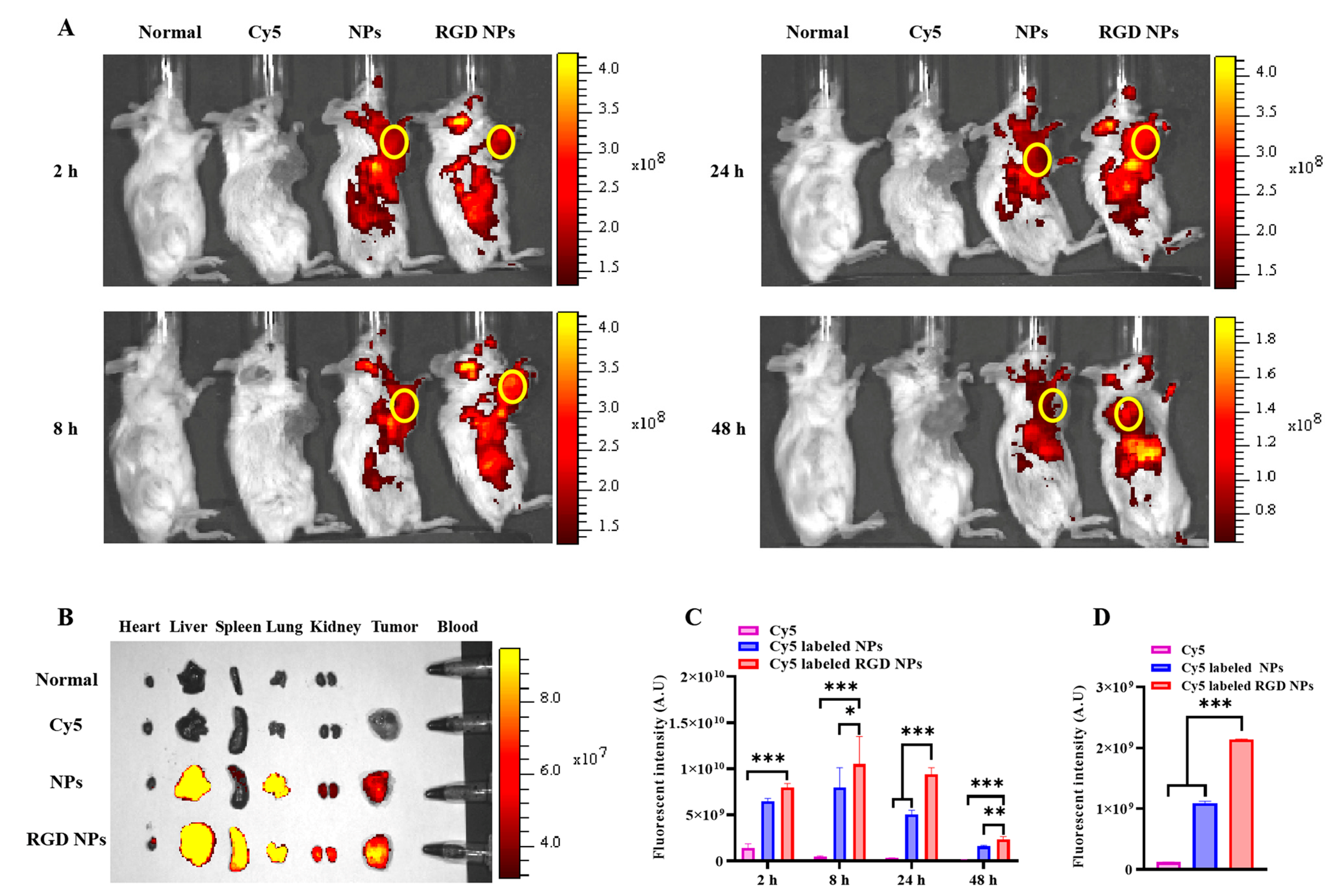
3.7. In Vivo Biodistribution
3.8. The Evaluation of Antitumor Effect of NPs In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davodabadi, F.; Sarhadi, M.; Arabpour, J.; Sargazi, S.; Rahdar, A.; Diez-Pascual, A.M. Breast cancer vaccines: New insights into immunomodulatory and nano-therapeutic approaches. J. Control. Release Off. J. Control. Release Soc. 2022, 349, 844–875. [Google Scholar] [CrossRef]
- Bahreyni, A.; Mohamud, Y.; Luo, H. Emerging nanomedicines for effective breast cancer immunotherapy. J. Nanobiotechnol. 2020, 18, 180. [Google Scholar] [CrossRef]
- Britt, K.L.; Cuzick, J.; Phillips, K.A. Key steps for effective breast cancer prevention. Nat. Rev. Cancer 2020, 20, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.H.; Tan, A.M.; Shi, Y. New and Emerging Targeted Therapies for Advanced Breast Cancer. Int. J. Mol. Sci. 2022, 23, 2288. [Google Scholar] [CrossRef] [PubMed]
- Yao, V.J.; D’Angelo, S.; Butler, K.S.; Theron, C.; Smith, T.L.; Marchio, S.; Gelovani, J.G.; Sidman, R.L.; Dobroff, A.S.; Brinker, C.J.; et al. Ligand-targeted theranostic nanomedicines against cancer. J. Control. Release Off. J. Control. Release Soc. 2016, 240, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz, R.; Taciak, B.; Krol, M. Drug delivery systems improving chemical and physical properties of anticancer drugs currently investigated for treatment of solid tumors. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2017, 68, 165–174. [Google Scholar]
- de la Torre, P.; Perez-Lorenzo, M.J.; Alcazar-Garrido, A.; Flores, A.I. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715. [Google Scholar] [CrossRef]
- Seyyednia, E.; Oroojalian, F.; Baradaran, B.; Mojarrad, J.S.; Mokhtarzadeh, A.; Valizadeh, H. Nanoparticles modified with vasculature-homing peptides for targeted cancer therapy and angiogenesis imaging. J. Control. Release Off. J. Control. Release Soc. 2021, 338, 367–393. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Song, B.; Ma, Z.; Zhang, Y.; Sun, H.; Wei, X.; Bai, Y.; Lu, X.; Zhang, P.; et al. Polymeric PD-L1 blockade nanoparticles for cancer photothermal-immunotherapy. Biomaterials 2022, 280, 121312. [Google Scholar] [CrossRef]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010, 62, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, Y.; Guo, R.; Yao, X.; Sung, S.; Pang, Z.; Yang, W. Erythrocyte-cancer hybrid membrane-camouflaged melanin nanoparticles for enhancing photothermal therapy efficacy in tumors. Biomaterials 2019, 192, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Pu, K. Recent advances in dual- and multi-responsive nanomedicines for precision cancer therapy. Biomaterials 2022, 291, 121906. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Li, Y. Stimuli-Responsive Nanomedicines for Overcoming Cancer Multidrug Resistance. Theranostics 2018, 8, 1059–1074. [Google Scholar] [CrossRef]
- Antoniou, A.I.; Giofre, S.; Seneci, P.; Passarella, D.; Pellegrino, S. Stimulus-responsive liposomes for biomedical applications. Drug Discov. Today 2021, 26, 1794–1824. [Google Scholar] [CrossRef]
- Huu, V.A.; Luo, J.; Zhu, J.; Zhu, J.; Patel, S.; Boone, A.; Mahmoud, E.; McFearin, C.; Olejniczak, J.; de Gracia Lux, C.; et al. Light-responsive nanoparticle depot to control release of a small molecule angiogenesis inhibitor in the posterior segment of the eye. J. Control. Release Off. J. Control. Release Soc. 2015, 200, 71–77. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Wang, M.; Liao, Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J. Control. Release Off. J. Control. Release Soc. 2021, 335, 437–448. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Wang, Q.; Wang, X.; Yao, P.; Feng, W.; Yuan, Q.; Qi, X.; Chen, S.; Pu, W.; et al. Self-Illuminating Triggered Release of Therapeutics from Photocleavable Nanoprodrug for the Targeted Treatment of Breast Cancer. ACS Appl. Mater. Interfaces 2022, 14, 8766–8781. [Google Scholar] [CrossRef]
- Cheng, R.; Jiang, L.; Gao, H.; Liu, Z.; Makila, E.; Wang, S.; Saiding, Q.; Xiang, L.; Tang, X.; Shi, M.; et al. A pH-Responsive Cluster Metal-Organic Framework Nanoparticle for Enhanced Tumor Accumulation and Antitumor Effect. Adv. Mater. 2022, 34, e2203915. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release Off. J. Control. Release Soc. 2019, 308, 172–189. [Google Scholar] [CrossRef]
- Xu, Q.; Chu, C.C. Development of ROS-responsive amino acid-based poly(ester amide) nanoparticle for anticancer drug delivery. J. Biomed. Mater. Res. Part A 2021, 109, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Han, Y.; Tang, J.; Piao, Y.; Liu, X.; Zhou, Z.; Gao, J.; Rao, J.; Shen, Y. A Tumor-Specific Cascade Amplification Drug Release Nanoparticle for Overcoming Multidrug Resistance in Cancers. Adv. Mater. 2017, 29, 1702342. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yang, B.; Ye, H.; Zhang, X.; Song, H.; Wang, X.; Li, N.; Wei, L.; Wang, Y.; Zhang, H.; et al. Self-Strengthened Oxidation-Responsive Bioactivating Prodrug Nanosystem with Sequential and Synergistically Facilitated Drug Release for Treatment of Breast Cancer. ACS Appl. Mater. Interfaces 2019, 11, 18914–18922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, W.; Wang, Z.; Wu, X.; Zhang, H.; Fang, W.; Yan, R.; Jin, Y. Self-Supply Oxygen ROS Reactor via Fenton-like Reaction and Modulating Glutathione for Amplified Cancer Therapy Effect. Nanomaterials 2022, 12, 2509. [Google Scholar] [CrossRef]
- Tu, Y.; Xiao, X.; Dong, Y.; Li, J.; Liu, Y.; Zong, Q.; Yuan, Y. Cinnamaldehyde-based poly(thioacetal): A ROS-awakened self-amplifying degradable polymer for enhanced cancer immunotherapy. Biomaterials 2022, 289, 121795. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, S.S.; Li, C.X.; Xu, L.; Cheng, H.; Zhang, X.Z. An Adenosine Triphosphate-Responsive Autocatalytic Fenton Nanoparticle for Tumor Ablation with Self-Supplied H2O2 and Acceleration of Fe(III)/Fe(II) Conversion. Nano Lett. 2018, 18, 7609–7618. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Miyanaga, S.; Ninomiya, I.; Tsukada, T.; Okamoto, K.; Harada, S.; Nakanuma, S.; Sakai, S.; Makino, I.; Kinoshita, J.; Hayashi, H.; et al. Concentration-dependent radiosensitizing effect of docetaxel in esophageal squamous cell carcinoma cells. Int. J. Oncol. 2016, 48, 517–524. [Google Scholar] [CrossRef]
- Xu, P.; Meng, Q.; Sun, H.; Yin, Q.; Yu, H.; Zhang, Z.; Cao, M.; Zhang, Y.; Li, Y. Shrapnel nanoparticles loading docetaxel inhibit metastasis and growth of breast cancer. Biomaterials 2015, 64, 10–20. [Google Scholar] [CrossRef]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef]
- Chen, H.; Niu, G.; Wu, H.; Chen, X. Clinical Application of Radiolabeled RGD Peptides for PET Imaging of Integrin αvβ3. Theranostics 2016, 6, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, L.; Wang, X.; Zhang, X.; Liu, M.; Zeng, W. Integrin (αvβ3) Targeted RGD Peptide Based Probe for Cancer Optical Imaging. Curr. Protein Pept. Sci. 2016, 17, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Perrins, R.D.; McCarthy, L.A.; Robinson, A.; Spry, K.L.; Cognet, V.; Ferreira, A.; Porter, J.; Garciotaa, C.E.; Rodriguez, M.A.; Lopez, D.; et al. Targeting Ultrasmall Gold Nanoparticles with cRGD Peptide Increases the Uptake and Efficacy of Cytotoxic Payload. Nanomaterials 2022, 12, 4013. [Google Scholar] [CrossRef] [PubMed]
- Shan, L. (131)I-Labeled arginine-arginine-leucine (RRL)-containing cyclic peptide (YCGGRRLGGC) for imaging prostate carcinoma. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Yang, X.; Hong, H.; Grailer, J.J.; Rowland, I.J.; Javadi, A.; Hurley, S.A.; Xiao, Y.; Yang, Y.; Zhang, Y.; Nickles, R.J.; et al. cRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials 2011, 32, 4151–4160. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, L.; Wang, J.; Luo, J.; Tang, J.; Zhong, L.; Xiao, Q.; Niu, W.; Li, J.; Zhu, J.; et al. cRGD-functionalized nanoparticles for combination therapy of anti-endothelium dependent vessels and anti-vasculogenic mimicry to inhibit the proliferation of ovarian cancer. Acta Biomater. 2019, 94, 495–504. [Google Scholar] [CrossRef]
- Capello, A.; Krenning, E.P.; Bernard, B.F.; Breeman, W.A.; Erion, J.L.; de Jong, M. Anticancer activity of targeted proapoptotic peptides. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2006, 47, 122–129. [Google Scholar]
- Gu, Y.; Du, Y.; Jiang, L.; Tang, X.; Li, A.; Zhao, Y.; Lang, Y.; Liu, X.; Liu, J. αvβ3 integrin-specific exosomes engineered with cyclopeptide for targeted delivery of triptolide against malignant melanoma. J. Nanobiotechnol. 2022, 20, 384. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, Z.; Li, M.; Qu, Q.; Ma, X.; Yu, S.H.; Zhao, Y. A preloaded amorphous calcium carbonate/doxorubicin@silica nanoreactor for pH-responsive delivery of an anticancer drug. Angew. Chem. 2015, 54, 919–922. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Sun, W.; Zhou, Z.; Huang, Y. Dual Stimuli-Responsive Hybrid Polymeric Nanoparticles Self-Assembled from POSS-Based Starlike Copolymer-Drug Conjugates for Efficient Intracellular Delivery of Hydrophobic Drugs. ACS Appl. Mater. Interfaces 2016, 8, 13251–13261. [Google Scholar] [CrossRef]
- Deng, L.; Feng, Z.; Deng, H.; Jiang, Y.; Song, K.; Shi, Y.; Liu, S.; Zhang, J.; Bai, S.; Qin, Z.; et al. Rational Design of Nanoparticles to Overcome Poor Tumor Penetration and Hypoxia-Induced Chemotherapy Resistance: Combination of Optimizing Size and Self-Inducing High Level of Reactive Oxygen Species. ACS Appl. Mater. Interfaces 2019, 11, 31743–31754. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Feng, W.; Yuan, Q.; Qi, X.; Chen, S.; Yao, P.; Dai, Q.; Xia, P.; Zhang, D.; et al. Folic acid-modified ROS-responsive nanoparticles encapsulating luteolin for targeted breast cancer treatment. Drug Deliv. 2021, 28, 1695–1708. [Google Scholar] [CrossRef] [PubMed]
- Morarasu, S.; Morarasu, B.C.; Ghiarasim, R.; Coroaba, A.; Tiron, C.; Iliescu, R.; Dimofte, G.M. Targeted Cancer Therapy via pH-Functionalized Nanoparticles: A Scoping Review of Methods and Outcomes. Gels 2022, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Ghasemi, S.; Akbarian, M.; Hoseini-Ghahfarokhi, M.; Moghoofei, M.; Doroudian, M. Physically stimulus-responsive nanoparticles for therapy and diagnosis. Front. Chem. 2022, 10, 952675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, X.; Shen, Q.; Xing, D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun. 2019, 10, 1704. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, P.; Wang, X.; Wang, Q.; Dai, Q.; Peng, Y.; Yuan, Q.; Mou, N.; Lv, S.; Weng, B.; Wang, Y.; et al. Cyclic RGD-Functionalized pH/ROS Dual-Responsive Nanoparticle for Targeted Breast Cancer Therapy. Pharmaceutics 2023, 15, 1827. https://doi.org/10.3390/pharmaceutics15071827
Yao P, Wang X, Wang Q, Dai Q, Peng Y, Yuan Q, Mou N, Lv S, Weng B, Wang Y, et al. Cyclic RGD-Functionalized pH/ROS Dual-Responsive Nanoparticle for Targeted Breast Cancer Therapy. Pharmaceutics. 2023; 15(7):1827. https://doi.org/10.3390/pharmaceutics15071827
Chicago/Turabian StyleYao, Pu, Xiaowen Wang, Qianmei Wang, Qing Dai, Yu Peng, Qian Yuan, Nan Mou, Shan Lv, Bangbi Weng, Yu Wang, and et al. 2023. "Cyclic RGD-Functionalized pH/ROS Dual-Responsive Nanoparticle for Targeted Breast Cancer Therapy" Pharmaceutics 15, no. 7: 1827. https://doi.org/10.3390/pharmaceutics15071827
APA StyleYao, P., Wang, X., Wang, Q., Dai, Q., Peng, Y., Yuan, Q., Mou, N., Lv, S., Weng, B., Wang, Y., & Sun, F. (2023). Cyclic RGD-Functionalized pH/ROS Dual-Responsive Nanoparticle for Targeted Breast Cancer Therapy. Pharmaceutics, 15(7), 1827. https://doi.org/10.3390/pharmaceutics15071827




