Abstract
CRLX101 is a cyclodextrin-based nanopharmaceutical designed to improve the delivery and efficacy of the anti-cancer drug camptothecin. Cyclodextrins have unique properties that can enhance drug solubility, stability, and bioavailability, making them an attractive option for drug delivery. The use of cyclodextrin-based nanoparticles can potentially reduce toxicity and increase the therapeutic index compared to conventional chemotherapy. CRLX101 has shown promise in preclinical studies, demonstrating enhanced tumor targeting and prolonged drug release. This systematic review followed PRISMA guidelines, assessing the efficacy and toxicity of CRLX101 in cancer treatment using clinical trials. Studies from January 2010 to April 2023 were searched in PubMed, Scopus, Web of Science, and Cochrane Database of Systematic Reviews, using specific search terms. The risk of bias was assessed using ROBINS-I and Cochrane risk-of-bias tools. After screening 6018 articles, 9 were included in the final review. These studies, conducted between 2013 and 2022, focused on patients with advanced or metastatic cancer resistant to standard therapies. CRLX101 was often combined with other therapeutic agents, resulting in improvements such as increased progression-free survival and clinical benefit rates. Toxicity was generally manageable, with common adverse events including fatigue, nausea, and anemia.
1. Introduction
Cancer is the second leading cause of morbidity and mortality worldwide, affecting millions of people each year [1]. It is distinguished by its unusual and unchecked cell growth that can occur in any part of the body, presenting in over 100 distinct variations. The most prevalent types include lung, breast, prostate, colorectal, and gastric cancers [2]. The incidence of cancer fluctuates based on geographic location, demographic variables, and lifestyle habits [3]. This illness has a profound influence on public health due to its high occurrence rate, the costs associated with its treatment and healthcare, as well as the emotional strain it places on patients and their loved ones [4]. It also impinges on patients’ quality of life, with many suffering from debilitating symptoms and side effects from treatment, such as fatigue, pain, and impaired ability to carry out daily tasks [5]. Further, a social stigma often accompanies a cancer diagnosis, influencing the working, social, and emotional aspects of the lives of those affected [6].
Conventional cancer treatments include surgery, radiotherapy, and chemotherapy [7]. Surgery is used to remove solid tumors and can be curative if performed in early disease stages [7]. Radiotherapy employs ionizing radiation to destroy cancer cells and reduce tumors, often in combination with other treatments [8]. Chemotherapy uses drugs that target rapidly growing cells, including cancerous ones, but can also affect healthy cells, causing side effects [9]. Conversely, emerging cancer therapies, such as immunotherapy and targeted therapy, have gained ground in recent years [10]. Immunotherapy harnesses the patient’s immune system to fight cancer, using monoclonal antibodies, checkpoint inhibitors, and cellular therapies, such as chimeric antigen receptor T cells (CAR-T) [11]. Targeted therapy, on the other hand, employs drugs that specifically act on molecules or cellular pathways involved in cancer cell growth and survival, minimizing damage to healthy cells [12]. In this landscape of targeted therapy, cyclodextrins (CDs) have emerged as a promising tool in the formulation of anti-cancer drugs, thus making their history and specific properties crucial to understand.
CDs belong to a family of naturally derived cyclic oligosaccharides that are sourced from starch. They consist of six (known as α-), seven (referred to as β-), or eight (termed γ-cyclodextrins) glucose units. These units are interconnected through α(1→4) glycosidic bonds [13]. These molecules have a frustoconical structure with an internal hydrophobic cavity and an external hydrophilic surface, enabling them to form inclusion complexes with a wide variety of substances, enhancing solubility and stability [14]. Cyclodextrins have proven useful in pharmaceutical formulation, especially for improving bioavailability and drug delivery of poorly water-soluble drugs [15]. Motivated by their properties and the urgent need to overcome the limitations of conventional anticancer drugs such as toxicity and multidrug resistance, cyclodextrins have been employed in the development of new cancer treatments [16]. Several types of cyclodextrins, including α-, β-, and γ-cyclodextrin, as well as their hydroxypropyl and methylated derivatives, have been used in cancer drug formulation [17]. These cyclodextrins can form inclusion complexes with anticancer drugs, enhancing solubility, stability, and bioavailability, which may lead to increased efficacy and reduced toxicity [18].
CRLX101 serves as a prime example of the potential of cyclodextrin-based nanomedicines in cancer therapy, but it is not alone. Other formulations utilizing the versatility of cyclodextrins have also shown promising results in clinical settings. For instance, NK012, an SN-38-incorporating polymeric micelle, is formed by the self-assembly of a block copolymer composed of polyethylene glycol (PEG) and poly(glutamic acid), using the inherent properties of cyclodextrins [19]. The results from a phase II study indicated that NK012 is effective against advanced, recurrent, or metastatic colorectal cancer and well-tolerated [20].
Thus, the development and clinical investigation of CRLX101, along with formulations such as NK012, underscore the significant potential of cyclodextrin-based nanomedicines in cancer therapy. By enhancing drug delivery and therapeutic efficacy while mitigating toxicity, these groundbreaking treatments herald a promising future for more effective and personalized cancer therapeutic strategies.
CRLX101 is an innovative nanomedicine that was developed using a unique method called “self-assembly”, where a cyclodextrin-containing polymer, camptothecin (CPT), and a linker molecule spontaneously form a nanoparticle drug conjugate [21]. The drug CPT is covalently linked to the cyclodextrin-containing polymer through a cleavable linker, creating a drug-polymer conjugate. This conjugate then self-assembles into nanoparticles, encapsulating additional CPT within the nanoparticle core [22]. At present, CRLX101 is progressing through the final phases of clinical trials. Its potential effectiveness has been explored across a diverse range of cancer types, such as lung, kidney, and ovarian cancer [23].
CRLX101 presents a significant edge over conventional CPT formulations due to its capacity to extend the duration of cancer cell exposure to the active pharmaceutical ingredient. Thanks to its nanoparticle construction, CRLX101 enables a gradual, sustained discharge of CPT, thereby augmenting its anti-cancer impacts and elevating the therapeutic index [24]. Moreover, by focusing on the tumor microenvironment rather than specific cancer cells, CRLX101 may circumvent prevalent drug resistance mechanisms, a substantial constraint of many traditional anti-cancer medications [25]. The selective accumulation of CRLX101 within tumors, coupled with the sustained drug release, could help lessen systemic exposure and toxicity often associated with free CPT [26].
Nevertheless, CRLX101 is not without its limitations. Despite the unique formulation enhancing drug delivery and curbing systemic toxicity, patients can still experience side effects such as fatigue, diarrhea, and neutropenia. Additionally, it is not uncommon for some tumors to exhibit resistance to CRLX101, whether inherent or acquired [27]. In instances of acquired resistance, prolonged usage of CRLX101 may induce modifications in tumor cells, reducing their susceptibility to the drug. Similarly, in the case of inherent resistance, some tumors may demonstrate initial resistance to CRLX101 attributable to intertumoral genetic variations [28].
Beyond CRLX101, cyclodextrins have been utilized in devising other drug delivery systems for cancer therapies, encompassing nanoparticles, liposomes, and micelles [17]. Drug delivery systems based on cyclodextrin, in combination with an array of therapeutic agents, including doxorubicin, paclitaxel, and cisplatin, have shown enhanced therapeutic efficacy and diminished side effects [18,29]. Leveraging cyclodextrins in the formulation of anti-cancer drugs and controlled-release systems presents an encouraging strategy to bolster the effectiveness and safety of oncological treatments.
Thus, this systematic review of clinical trials using CRLX101 for cancer treatment aspires to assess and compile the existing evidence concerning the efficacy and safety of CRLX101. The goal is to offer a more in-depth understanding of its therapeutic potential, pinpoint areas for refinement and optimization, and contribute meaningful insights for future investigations and the creation of personalized oncological treatments using cyclodextrins as a therapeutic platform.
2. Materials and Methods
This comprehensive review was carried out in adherence to the guidelines set forth by the Preferred Reporting Items for Systematic Review and Meta-Analyses [30]. The scope of the clinical trials included in this research endeavor was to evaluate the potency of CRLX101 in cancer management and gauge its potential toxic effects. Our systematic review and meta-analysis have been duly registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42023424511.
2.1. Eligibility Criteria
The following inclusion criteria were established: (a) participants—cancer patients undergoing treatment; (b) outcomes—measures of potential cancer improvement and toxicity of CRLX101; and (c) clinical trials. Searching was restricted to articles in English or Spanish language published in peer-reviewed journals.
The exclusion criteria were (a) studies comparing CRLX101 with other therapeutic agents or combinations without evaluating the impact on cancer; (b) review articles, case reports, and cross-sectional or longitudinal studies; (c) studies involving patients without cancer or not measuring the effect of therapy on cancer; and (d) duplicated studies.
2.2. Information Sources and Search Strategy
The investigators A.M.G.-M. and D.V.-M. executed a comprehensive search across various databases, namely PubMed, Scopus, Web of Science, and the Cochrane Database of Systematic Reviews. This was carried out within a specific timeframe, from January 2010 to April 2023. The decision to include studies in our review was based on factors such as participants involved, outcomes observed, and criteria for inclusion and exclusion. We employed a myriad of search terminologies to identify relevant information. These included terms related to the study’s focus: (a) “cyclodextrins”, “CRLX101”, “NLG207”, “beta-cyclodextrin”, “gamma-cyclodextrin”, “alpha-cyclodextrin”, “hydroxypropyl-beta-cyclodextrin”, “methyl-beta-cyclodextrin”, “sulfobutyl ether-beta-cyclodextrin”; (b) “cancer”, “neoplasms”, “tumor”, “carcinoma”, “malignancy”, “oncology”; (c) “drug delivery”, “drug administration”, “chemotherapy”, “targeted therapy”, “nanoparticles”, “nanomedicine”, and “drug carriers”. To ensure thoroughness, the search terminologies were customized to match each individual database and were run through the specific search parameters provided by these databases.
2.3. Selection Process
The identification of appropriate studies was followed by the utilization of Mendeley (Version for Windows 10; Elsevier, Amsterdam, The Netherlands) to remove duplicates. This process was independently executed by two investigators (A.M.G.-M. and D.V.-M.), who reviewed each title and abstract to determine potential articles requiring full-text examination. To address any disagreements, a third researcher, A.S.-M., was involved.
2.4. Data Items and Quality Assessment
In this research, the task of extracting specific variables such as patient improvements post CRLX101 intravenous treatment, toxicity levels, average age, type of clinical trial, patient’s health status, trial phase, and dosage was assigned to one researcher (D.V.-M.). A separate researcher (A.M.G.-M.) was responsible for confirming the accuracy of this data. Any disagreements between these two researchers were resolved by bringing in a third researcher (A.S.-M.) for further review.
For assessing the potential risk of bias within the studies, we utilized two different tools: the ROBINS-I tool (Risk of Bias in Non-randomized Studies—of Interventions) [31] for non-randomized studies, and the Cochrane risk-of-bias tool (RoB 2.0) [32] for randomized clinical trials. Each of these tools serves a specific purpose; ROBINS-I examines the presence of bias through seven categories, while RoB 2.0 evaluates bias across five different categories. This bias risk assessment for the included studies was performed independently by two researchers, D.V.-M. and A.M.G.-M.
In more detail, ROBINS-I scrutinizes study bias through seven distinct domains: confounding factors, participant selection, intervention classification, deviations from planned interventions, handling of missing data, the way outcomes are measured, and the reporting of results. On the other hand, the RoB 2.0 tool focuses on five aspects of bias: bias stemming from the process of randomization, deviations from intended interventions, missing outcome data, the measurement methodology of the outcome, and selection in reporting results.
3. Results
3.1. Search Results
We conducted a systematic review of clinical trials to assess the effectiveness and improvements produced by CRLX101 in cancer patients as well as its toxicity. The search and selection strategy employed in the systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and is illustrated in Figure 1. A total of 6018 articles were initially identified from various databases: SCOPUS (n = 2927), Web of Science (n = 1789), PubMed (n = 1282), and Cochrane (n = 20). Among these, 2742 duplicates were removed, and the titles and abstracts of the remaining 3276 articles were reviewed. After this step, 20 articles were selected for further assessment. Upon full-text examination, 11 articles were excluded for various reasons: 4 being abstracts [33,34,35,36], 2 involving mice studies [23,24], 3 not measuring the effect on cancer [37,38,39], 1 being a duplicate [33], and 1 being a review [40]. This left nine articles for the final systematic review.
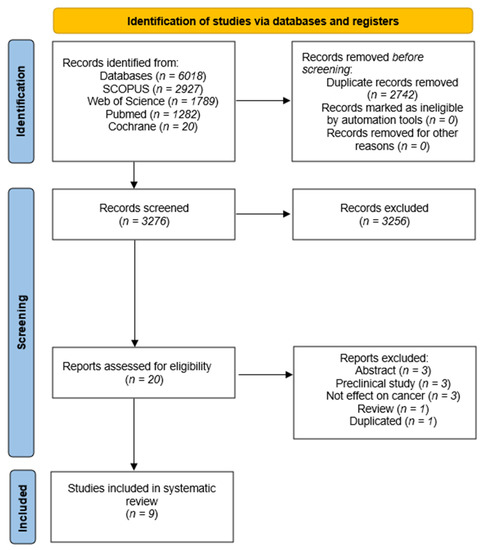
Figure 1.
Flow diagram of the systematic review.
3.2. Characteristics and Quality Assessment of Included Articles
The main characteristics of the nine included studies are summarized in Table 1. The articles were published between 2013 and 2022. A total of 323 participants (50.3% women) had a mean age ranging from 59 to 76 years. All studies focused on patients with advanced, metastatic, or inoperable cancer conditions resistant to standard therapies. Various therapies were employed in combination with CRLX101. All studies were conducted in the USA, except for Voss et al. [41], which was carried out in both the USA and Korea. The studies included in this systematic review share several common characteristics, such as investigating the therapeutic potential of CRLX101 in a range of cancer types at various stages, administering the drug in different dosing regimens, and assessing its impact on tumor progression, clinical outcomes, and associated toxicities (Table 1). Most of the studies were conducted in the context of phase I/II clinical trials or pilot studies. Specifically, Gaur et al. [26], Keefe et al. [42], and Weiss et al. [43] were phase I/IIa studies, while Duska et al. [44] and Sanoff et al. [45] were phase Ib/II studies. Krasner et al. [46], Schmidt et al. [47], and Voss et al. [41] were all phase II trials, and Chao et al. [48] conducted a single-center pilot trial.

Table 1.
Characteristics of the studies included (n = 9).
These studies focused on advanced or metastatic malignancies, encompassing gastric, ovarian, renal cell, and prostate cancers, among others. CRLX101 was administered at different dosages, and several studies shared similar dosing regimens. Chao et al. [48] and Voss et al. [41] used 15 mg/m2, Duska et al. [44] tested three dose levels (9, 12, and 15 mg/m2), and Gaur et al. [26] tested multiple dosages (6, 12, 15, and 18 mg/m2). Keefe et al. [42] utilized escalating doses of 12 and 15 mg/m2, while Krasner et al. [46] administered 15 mg/m2 every 21 or 28 days. Sanoff et al. [45] used two dosing phases of 15 mg/m2, Schmidt et al. [47] administered 12 mg/m2, and Weiss et al. [43] administered 15 mg/m2 bi-weekly.
On the other hand, in the studies, patients had a variety of conditions. Chao et al. [48] included patients resistant to at least one type of systemic treatment for advanced, inoperable, or metastasized stomach, gastroesophageal junction, or esophageal squamous cell or adenocarcinoma. Gaur et al. [26] studied patients with metastatic or unresectable solid tumor malignancies refractory to standard curative therapy. Duska et al. [44] focused on women with epithelial ovarian cancer (EOC). Keefe et al. [42] investigated patients with metastatic or locally advanced unresectable renal cell carcinoma who received a median of two prior therapies, including at least one prior vascular endothelial tyrosine kinase inhibitor therapy (VEGF-TKI). Krasner et al. [46] studied patients with recurrent epithelial ovarian, tubal, or primary peritoneal cancer who had up to three prior lines of treatment for Cohort A and up to two prior lines for Cohort B, excluding hormones and prior treatment with PARP inhibitors. Sanoff et al. [45] included patients with locally advanced rectal cancer. Schmidt et al. [47] investigated patients with metastatic castration-resistant prostate cancer who had a median of three prior systemic therapies. Voss et al. [41] examined patients with metastatic renal cell carcinoma (RCC) of any histologic subtype who had received two to three prior lines of molecularly targeted therapy, including at least one VEGF-inhibiting regimen, while Weiss et al. [43] studied patients with advanced solid tumor malignancies.
In these investigations, the drug CRLX101 was often combined with other therapeutic agents. For instance, bevacizumab was used in conjunction with CRLX101 by Duska et al. [44], Keefe et al. [42], Krasner et al. [46], and Voss et al. [41]. Furthermore, standard chemoradiotherapy was employed alongside CRLX101 in the study by Sanoff et al. [45]. Additionally, platinum and fluoropyrimidine therapy was used in combination with CRLX101 by Chao et al. [48].
Moreover, the studies included in the analysis showed some common improvements across various clinical outcomes. Many studies reported increased progression-free survival (PFS), such as Duska et al. [44], Keefe et al. [42], Krasner et al. [46], and Weiss et al. [43]. Additionally, several studies observed clinical benefit rates (CBR) and overall response rates (ORR), as seen in Krasner et al. [46] and Sanoff et al. [45]. Some unique improvements were also noted, such as selective absorption of CRLX101 into gastric tumor tissue and reduced activity of potential drug targets such as carbonic anhydrase IX and HIF-1α in Chao et al. [48] and tumor downstaging in Sanoff et al. [45].
Regarding toxicity, most studies reported a manageable profile with common adverse events (AEs) such as fatigue, nausea, and anemia. Grade ≥ 3 AEs were observed in Keefe et al. [42], including non-infectious cystitis, fatigue, anemia, diarrhea, dizziness, and other individual events. In Krasner et al. [46], hypertension and qualitatively increased bladder toxicities were reported with the addition of bevacizumab, but no severe adverse events (SAEs) occurred. However, Schmidt et al. [47] found that CRLX101 was poorly tolerated in patients with metastatic castration-resistant prostate cancer, with intolerable toxicity attributed to non-infective cystitis.
The risk of bias assessment for the non-randomized studies was conducted using the ROBINS-I tool. The findings from the table indicate the predominant risk of bias for the included studies and highlight areas where biases may potentially influence the results. In this analysis, the predominant risk of bias among the studies was moderate. The domain with the highest risk was “Bias due to confounding”, which could be attributed to uncontrolled or residual confounding factors affecting the study outcomes. The domain with the lowest risk was “Bias in the classification of interventions” and “Bias due to deviations from intended interventions”, suggesting that these aspects of the studies were well handled (Figure 2). In addition to the non-randomized studies, the risk of bias for the randomized study by Voss et al. [41] was also assessed using the RoB 2.0 tool. The assessment indicated a minimal risk of bias across all categories: the process of randomization, divergence from planned interventions, missing outcome data, outcome measurement, and choice of the result reported (Figure 3).
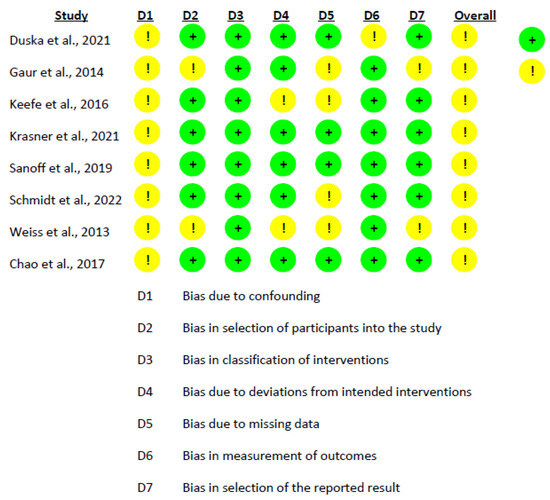
Figure 2.
Risk of Bias Assessment using ROBINS-I for the Included Intervention Studies [26,42,43,44,45,46,47,48].
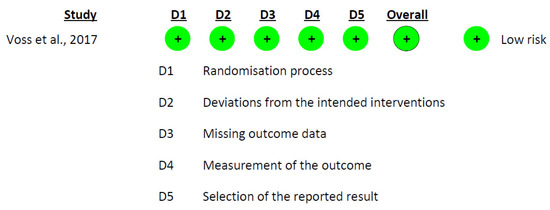
Figure 3.
Risk-of-Bias Assessment Using RoB 2.0 for the Included Intervention Studies [41].
4. Discussion
The current analysis aimed to examine the efficacy and safety of CRLX101, a novel nanoparticle drug conjugate, in the treatment of various advanced cancers. Evidence from multiple clinical trials, carried out in diverse patient demographics and under different conditions, have underlined the potential of CRLX101 as a possible therapeutic option for several types of cancer. These insights accentuate the need for more comprehensive studies on the application of CRLX101, either in tandem with other treatments or as a sole therapeutic option. In the following discussion, we will delve into several key aspects of the study outcomes, such as disparities in safety and efficacy, dosage and administration, along with possible rationales for the observed variations between different trials.
4.1. Efficacy and Safety of CRLX101 across Different Clinical Trials and Conditions
Considering the observed therapeutic improvements and related toxic side effects in various trials, it is evident that the effectiveness of CRLX101 might differ significantly based on the specific type of cancer, the applied treatment plan, and the patient demographic. For instance, a pilot study conducted by Chao et al. [48] in a single center observed that CRLX101 was selectively absorbed by gastric tumor tissue. This study, involving patients with advanced, inoperable, or metastasized stomach, gastroesophageal junction, or esophageal squamous cell or adenocarcinoma, reported a favorable toxicity profile. In contrast, a study by Schmidt et al. [47] found no notable improvements in patients suffering from metastatic castration-resistant prostate cancer, with the treatment showing low tolerability due to excessive toxicity.
To gain a better understanding of the varying degrees of efficacy and safety associated with CRLX101, it would be beneficial to juxtapose these results with other studies that involve cancer treatment and cyclodextrins. For example, a preclinical study by Hrkach et al. [49] explored the efficacy of a PSMA-targeted docetaxel nanoparticle conjugate in cancer models. While not CRLX101 per se, this approach shares similarities with the overall concept of nanoparticle-based chemotherapy delivery. The study found that the docetaxel nanoparticle conjugate exhibited a differentiated pharmacological profile and enhanced antitumor efficacy compared to free docetaxel in preclinical models. This highlights the potential benefits of combining nanomedicine-based therapeutics with traditional chemotherapy agents in the treatment of advanced cancers. On the other hand, new data from a large-scale genomic study on cyclodextrin-based therapies revealed significant patient-specific variability, hinting at the need for personalized dosing strategies [50].
One possible explanation for the differences in efficacy and safety may be the intrinsic tumor characteristics, such as genetic and molecular profiles, that could influence the response to treatment. For example, in the study by Weiss et al. [43], the median progression-free survival for patients treated at the maximum tolerated dose was 3.7 months, with stable disease observed in 28 patients (64%) and confirmed stable disease in 15 patients (34%). This suggests that the response to CRLX101 may depend on the molecular characteristics of the tumor, which could also impact the overall treatment outcomes. On the other hand, a phase I clinical trial conducted by Mita et al. [51] investigated the safety, tolerability, and pharmacokinetics of cyclodextrin-based nanoparticle-encapsulated paclitaxel (IT-101) in patients with advanced solid tumors. The study reported promising safety and preliminary antitumor activity, although the responses varied across different cancer types and patient populations. A study by Anselmo et al. [52] further showed how nanocarriers can alter the immunological response, indicating a complex interplay between the nanodrug and the patient’s immune system.
Considering these factors, a study by Liu et al. [53] highlighted the importance of understanding the tumor microenvironment and its potential impact on the efficacy of nanomedicine-based therapeutics. The authors suggested that factors such as tumor heterogeneity, stromal components, and immune cell infiltration could significantly affect the delivery and efficacy of nanoparticle-based therapies. This further emphasizes the need for more research to elucidate the complex relationship between tumor characteristics and the effectiveness of CRLX101. Furthermore, Jain et al. [54] emphasized how the tumor microenvironment could pose physical barriers to drug delivery, further complicating the scenario.
Another potential explanation for the differences in efficacy and safety could be the presence of other comorbidities or concurrent therapies in the patient population. For example, in the study by Sanoff et al. [45], patients with locally advanced rectal cancer received standard chemoradiotherapy and radiotherapy, along with concurrent administration of capecitabine, which could have influenced the overall response to CRLX101. Furthermore, the study by Gaur et al. [26] found that the lower toxicity observed in their patient cohort was not due to allele frequency differences in drug metabolism genes, suggesting that other factors, such as concurrent treatments or comorbidities, could be responsible for the observed differences in toxicity. For example, a comprehensive study by the Cancer Genome Atlas Research Network [55] highlighted the influence of patient’s genotypic variations and concurrent medications on the overall effectiveness and safety profile of oncological therapies.
In conclusion, the relatively small number of studies and diversity of patient populations currently available in the literature makes it a challenge to conclusively assess the efficacy of CRLX101 in different conditions.
4.2. Dosing and Administration of CRLX101
In optimizing the dose and administration of CRLX101, it is important to consider various aspects to maximize its therapeutic potential and minimize adverse effects. The varying degrees of efficacy and safety observed across different trials and conditions may be attributed to differences in dosing and administration as well as the intrinsic characteristics of the tumor and the patients receiving the treatment.
A general trend observed in CRLX101 clinical trials is that lower doses administered more frequently, such as 12 mg/m² every 2 weeks, tend to result in a more favorable toxicity profile [48]. Conversely, higher doses administered less frequently, such as 15 mg/m² every 3 weeks, have been associated with a higher incidence of intolerable toxicity [47]. This observation is consistent with the findings of Weiss et al. [43], who determined the maximum tolerated dose (MTD) of CRLX101 to be 12 mg/m² when administered every 3 weeks in their phase II study involving patients with advanced solid tumors. The efficacy of CRLX101 may also be influenced by its combination with other anticancer agents. In a study by Gaur et al. [26], the combination of CRLX101 with bevacizumab demonstrated promising response rates and a manageable safety profile in patients with metastatic renal cell carcinoma.
When comparing CRLX101 with the broader literature on nanoparticle-based drug delivery, it becomes apparent that the optimal dosing regimen for CRLX101 may depend on several factors, including the cancer type, treatment regimen, and patient population. For example, studies on other nanoparticle-based therapeutics, such as those utilizing liposomes or polymeric micelles, have also shown that lower doses administered more frequently tend to result in improved efficacy and reduced toxicity [56,57]. This finding is consistent across various cancer types and may be attributed to the unique properties of nanoparticle-based drug delivery systems.
One such property is the enhanced permeability and retention (EPR) effect, which allows nanoparticles to preferentially accumulate in tumor tissue due to the leaky vasculature and impaired lymphatic drainage often observed in tumors [58]. This effect may influence the optimal dosing regimen for CRLX101 and other cyclodextrin-based therapies, as higher drug concentrations in the tumor tissue could lead to increased efficacy and reduced systemic toxicity [59]. This observation is echoed in a review by Maeda et al. [60], where they discuss the impact of the EPR effect on nanoparticle drug delivery and how it might be leveraged to improve treatment outcomes.
Moreover, it is increasingly recognized that specific tumor characteristics, such as molecular and genetic profiles, could influence the response to treatment and, consequently, the optimal dosing regimen [61]. For instance, a study by Boehnke et al. [62] highlighted the role of tumor genetics in determining the efficacy of nanoparticle-based drugs. They found that tumors with certain genetic profiles were more likely to respond to nanoparticle-based therapies, suggesting that a personalized approach to treatment could improve outcomes for patients. As such, it becomes imperative to consider individual patient factors and tumor characteristics when determining the most appropriate dosing strategy for CRLX101.
4.3. Limitations and Future Directions
While the findings from the various clinical trials of CRLX101 offer valuable insights into the potential benefits of this cyclodextrin-based therapy for cancer treatment, several limitations must be acknowledged. These limitations should be considered when interpreting the results and planning future research.
First, the number of clinical trials and patient populations studied for CRLX101 is relatively small. This limits the generalizability of the results and makes it difficult to draw definitive conclusions about the effectiveness and safety of CRLX101 across different cancer types and patient populations. More extensive clinical trials with larger and more diverse patient populations will be required to further validate the efficacy and safety of CRLX101. Second, the studies included in the analysis had varying designs, methodologies, and endpoints, which may have contributed to the observed heterogeneity in the results. Future studies should aim for a more standardized approach in terms of trial design and outcome assessment to enable more accurate comparisons between studies and a more comprehensive understanding of the treatment effects. Third, many of the trials were early-phase studies, which primarily focused on safety, tolerability, and pharmacokinetics rather than clinical efficacy. To establish the therapeutic benefits of CRLX101, more advanced trials (e.g., phase II or III) with larger patient cohorts and well-defined clinical endpoints are necessary. Lastly, the underlying mechanisms through which CRLX101 exerts its antitumor effects are not yet fully understood. A deeper understanding of the molecular and cellular mechanisms that drive the efficacy and safety of CRLX101 could help optimize the treatment strategy and potentially improve patient outcomes. Further preclinical and clinical research is needed to elucidate these mechanisms and identify potential biomarkers that could predict treatment response and aid in patient selection.
5. Conclusions
In this systematic review, we analyzed the potential of CRLX101, a cyclodextrin-based nanopharmaceutical, as a therapeutic agent for various cancer types. Our findings suggest that the unique properties of cyclodextrin-based nanoparticles might provide certain advantages over conventional chemotherapy, possibly enhancing the therapeutic index and reducing systemic toxicity. However, the efficacy and safety of CRLX101 appear to be influenced by several factors, including cancer type, treatment regimen, patient population, and dosing strategy. While these preliminary findings are encouraging, further research is necessary to fully understand the clinical implications of CRLX101 in the treatment of cancer.
Author Contributions
Conceptualization, D.V.-M. and A.M.G.-M.; methodology, A.M.G.-M. and D.V.-M.; software, A.M.G.-M.; validation, P.H.-S.; investigation, A.S.-M.; resources, C.L.-A., A.S.-M. and R.G.-L.; writing—original draft preparation, A.S.-M., D.V.-M. and R.G.-L.; writing—review and editing, C.L.-A., A.S.-M., P.H.-S. and A.M.G.-M.; visualization, P.H.-S.; supervision, R.G.-L.; project administration, C.L.-A.; funding acquisition, C.L.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. Diet, Activity and Cancer; WCRF Int.: London, UK, 2018. [Google Scholar]
- de Souza, J.A.; Yap, B.J.; Wroblewski, K.; Blinder, V.; Araújo, F.S.; Hlubocky, F.J.; Nicholas, L.H.; O’Connor, J.M.; Brockstein, B.; Ratain, M.J.; et al. Measuring Financial Toxicity as a Clinically Relevant Patient-Reported Outcome: The Validation of the COmprehensive Score for Financial Toxicity (COST). Cancer 2017, 123, 476–484. [Google Scholar] [CrossRef]
- Quinten, C.; Coens, C.; Mauer, M.; Comte, S.; Sprangers, M.A.G.; Cleeland, C.; Osoba, D.; Bjordal, K.; Bottomley, A. EORTC Clinical Groups Baseline Quality of Life as a Prognostic Indicator of Survival: A Meta-Analysis of Individual Patient Data from EORTC Clinical Trials. Lancet Oncol. 2009, 10, 865–871. [Google Scholar] [CrossRef]
- Cataldo, J.K.; Slaughter, R.; Jahan, T.M.; Pongquan, V.L.; Hwang, W.J. Measuring Stigma in People with Lung Cancer: Psychometric Testing of the Cataldo Lung Cancer Stigma Scale. Oncol. Nurs. Forum 2011, 38, E46–E54. [Google Scholar] [CrossRef]
- DeVita, V.T.; Lawrence, T.S.; Rosenberg, S.A. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; ISBN 978-0-7817-7207-5. [Google Scholar]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The Role of Radiotherapy in Cancer Treatment: Estimating Optimal Utilization from a Review of Evidence-Based Clinical Guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Longley, D.B.; Johnston, P.G. Molecular Mechanisms of Drug Resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer Immunotherapy Using Checkpoint Blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations I: Structure and Physicochemical Properties, Formation of Complexes, and Types of Complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Mura, P. Analytical Techniques for Characterization of Cyclodextrin Complexes in the Solid State: A Review. J. Pharm. Biomed. Anal. 2015, 113, 226–238. [Google Scholar] [CrossRef]
- Swaminathan, S.; Cavalli, R.; Trotta, F. Cyclodextrin-Based Nanosponges: A Versatile Platform for Cancer Nanotherapeutics Development. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 579–601. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Erdoğar, N.; Hıncal, A.A.; Bilensoy, E. Amphiphilic Cyclodextrin Nanoparticles. Int. J. Pharm. 2017, 531, 457–469. [Google Scholar] [CrossRef]
- Matsumura, Y. Preclinical and Clinical Studies of NK012, an SN-38-Incorporating Polymeric Micelles, Which Is Designed Based on EPR Effect. Adv. Drug Deliv. Rev. 2011, 63, 184–192. [Google Scholar] [CrossRef]
- Hamaguchi, T.; Tsuji, A.; Yamaguchi, K.; Takeda, K.; Uetake, H.; Esaki, T.; Amagai, K.; Sakai, D.; Baba, H.; Kimura, M.; et al. A Phase II Study of NK012, a Polymeric Micelle Formulation of SN-38, in Unresectable, Metastatic or Recurrent Colorectal Cancer Patients. Cancer Chemother. Pharmacol. 2018, 82, 1021–1029. [Google Scholar] [CrossRef]
- Heidel, J.D.; Schluep, T. Cyclodextrin-Containing Polymers: Versatile Platforms of Drug Delivery Materials. J. Drug Deliv. 2012, 2012, 262731. [Google Scholar] [CrossRef]
- Ghitman, J.; Voicu, S.I. Controlled Drug Delivery Mediated by Cyclodextrin-Based Supramolecular Self-Assembled Carriers: From Design to Clinical Performances. Carbohydr. Polym. Technol. Appl. 2023, 5, 100266. [Google Scholar] [CrossRef]
- Eliasof, S.; Lazarus, D.; Peters, C.G.; Case, R.I.; Cole, R.O.; Hwang, J.; Schluep, T.; Chao, J.; Lin, J.; Yen, Y.; et al. Correlating Preclinical Animal Studies and Human Clinical Trials of a Multifunctional, Polymeric Nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 15127–15132. [Google Scholar] [CrossRef]
- Young, C.; Schluep, T.; Hwang, J.; Eliasof, S. CRLX101 (Formerly IT-101)-A Novel Nanopharmaceutical of Camptothecin in Clinical Development. Curr. Bioact. Compd. 2011, 7, 8–14. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Gaur, S.; Wang, Y.; Kretzner, L.; Chen, L.; Yen, T.; Wu, X.; Yuan, Y.-C.; Davis, M.; Yen, Y. Pharmacodynamic and Pharmacogenomic Study of the Nanoparticle Conjugate of Camptothecin CRLX101 for the Treatment of Cancer. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1477–1486. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Chacko, A.-M.; Hood, E.D.; Zern, B.J.; Muzykantov, V.R. Targeted Nanocarriers for Imaging and Therapy of Vascular Inflammation. Curr. Opin. Colloid Interface Sci. 2011, 16, 215–227. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.; Bossuyt, P. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews|The BMJ. 2021. Available online: https://www.bmj.com/content/372/bmj.n71 (accessed on 18 October 2022).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Alarcón, M.; Ojeda, R.; Huaricancha, I.; Hilario, K. Análisis Crítico de Ensayos Clínicos Aleatorizados: Riesgo de Sesgo. 2015. Available online: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1019-43552015000400008 (accessed on 18 October 2022).
- Voss, M.H.; Coates, A.; Garmey, E.G.; Haas, N.B.; Hutson, T.; Keefe, S.M.; Motzer, R.; Piscitelli, A.; Vogelzang, N.J.; Figlin, R.A. Randomized Phase 2 Study to Assess the Safety and Efficacy of CRLX101 in Combination with Bevacizumab in Patients (Pts.) with Metastatic Renal Cell Carcinoma (RCC) versus Standard of Care (SOC). J. Clin. Oncol. 2015, 33, TPS4579. [Google Scholar] [CrossRef]
- Pham, E.; Lee, C.; Xu, P.; Man, S.; Yin, M.; Peters, C.; Lazarus, D.; Eliasof, S.; Kerbel, R. Abstract 4124: Potent Anti-Tumor and Metastatic Breast Cancer Efficacy of Bevacizumab with CRLX101, an Investigational Chemotherapy Nanoparticle-Drug Conjugate That Secondarily Suppresses HIF-1α. Cancer Res. 2015, 75, 4124. [Google Scholar] [CrossRef]
- Wang, A.; Sanoff, H.K.; McRee, A.J.; O’Neil, B.H.; Calvo, B.F.; Hennessy, M.G.; Murphy, C.; Tynan, M.T.; Blackstock, A.W.; Garmey, E.G.; et al. Phase IB/II Study of Neoadjuvant Chemoradiotherapy with CRLX101 and Capecitabine for Locally Advanced Rectal Cancer. J. Clin. Oncol. 2015, 33, TPS3629. [Google Scholar] [CrossRef]
- Krasner, C.N.; Schilder, R.J.; Brady, W.E.; Duska, L.R.; O’Malley, D.; Disilvestro, P.A.; Li, J.; Downing, W.; Senderowicz, A. A Phase 1b Study of the Nanoparticle-Drug Conjugate (NDC) CRLX101 in Combination with Weekly Paclitaxel in Patients (Pts) with Platinum-Resistant Ovarian Cancer (OC). Ann. Oncol. 2016, 27, vi298. [Google Scholar] [CrossRef]
- Clark, A.J.; Wiley, D.T.; Zuckerman, J.E.; Webster, P.; Chao, J.; Lin, J.; Yen, Y.; Davis, M.E. CRLX101 Nanoparticles Localize in Human Tumors and Not in Adjacent, Nonneoplastic Tissue after Intravenous Dosing. Proc. Natl. Acad. Sci. USA 2016, 113, 3850–3854. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Peer, C.J.; Huitema, A.D.R.; Williams, M.D.; Wroblewski, S.; Schellens, J.H.M.; Madan, R.A.; Figg, W.D. Measurement of NLG207 (Formerly CRLX101) Nanoparticle-Bound and Released Camptothecin in Human Plasma. J. Pharm. Biomed. Anal. 2020, 181, 113073. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Huitema, A.D.R.; Dorlo, T.P.C.; Peer, C.J.; Cordes, L.M.; Sciuto, L.; Wroblewski, S.; Pommier, Y.; Madan, R.A.; Thomas, A.; et al. Population Pharmacokinetic Analysis of Nanoparticle-Bound and Free Camptothecin after Administration of NLG207 in Adults with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2020, 86, 475–486. [Google Scholar] [CrossRef]
- Papaioannou, L.; Avgoustakis, K. Responsive Nanomedicines Enhanced by or Enhancing Physical Modalities to Treat Solid Cancer Tumors: Preclinical and Clinical Evidence of Safety and Efficacy. Adv. Drug Deliv. Rev. 2022, 181, 114075. [Google Scholar] [CrossRef]
- Voss, M.H.; Hussain, A.; Vogelzang, N.; Lee, J.L.; Keam, B.; Rha, S.Y.; Vaishampayan, U.; Harris, W.B.; Richey, S.; Randall, J.M.; et al. A Randomized Phase II Trial of CRLX101 in Combination with Bevacizumab versus Standard of Care in Patients with Advanced Renal Cell Carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2754–2760. [Google Scholar] [CrossRef]
- Keefe, S.M.; Hoffman-Censits, J.; Cohen, R.B.; Mamtani, R.; Heitjan, D.; Eliasof, S.; Nixon, A.; Turnbull, B.; Garmey, E.G.; Gunnarsson, O.; et al. Efficacy of the Nanoparticle-Drug Conjugate CRLX101 in Combination with Bevacizumab in Metastatic Renal Cell Carcinoma: Results of an Investigator-Initiated Phase I-IIa Clinical Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 1579–1585. [Google Scholar] [CrossRef]
- Weiss, G.J.; Chao, J.; Neidhart, J.D.; Ramanathan, R.K.; Bassett, D.; Neidhart, J.A.; Choi, C.H.J.; Chow, W.; Chung, V.; Forman, S.J.; et al. First-in-Human Phase 1/2a Trial of CRLX101, a Cyclodextrin-Containing Polymer-Camptothecin Nanopharmaceutical in Patients with Advanced Solid Tumor Malignancies. Investig. New Drugs 2013, 31, 986–1000. [Google Scholar] [CrossRef]
- Duska, L.R.; Krasner, C.N.; O’Malley, D.M.; Hays, J.L.; Modesitt, S.C.; Mathews, C.A.; Moore, K.N.; Thaker, P.H.; Miller, A.; Purdy, C.; et al. A Phase Ib/II and Pharmacokinetic Study of EP0057 (Formerly CRLX101) in Combination with Weekly Paclitaxel in Patients with Recurrent or Persistent Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. Gynecol. Oncol. 2021, 160, 688–695. [Google Scholar] [CrossRef]
- Sanoff, H.K.; Moon, D.H.; Moore, D.T.; Boles, J.; Bui, C.; Blackstock, W.; O’Neil, B.H.; Subramaniam, S.; McRee, A.J.; Carlson, C.; et al. Phase I/II Trial of Nano-Camptothecin CRLX101 with Capecitabine and Radiotherapy as Neoadjuvant Treatment for Locally Advanced Rectal Cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 189–195. [Google Scholar] [CrossRef]
- Krasner, C.N.; Campos, S.M.; Young, C.L.; Chadda, K.R.; Lee, H.; Birrer, M.J.; Horowitz, N.S.; Konstantinopoulos, P.A.; D’Ascanio, A.M.; Matulonis, U.A.; et al. Sequential Phase II Clinical Trials Evaluating CRLX101 as Monotherapy and in Combination with Bevacizumab in Recurrent Ovarian Cancer. Gynecol. Oncol. 2021, 162, 661–666. [Google Scholar] [CrossRef]
- Schmidt, K.T.; Karzai, F.; Bilusic, M.; Cordes, L.M.; Chau, C.H.; Peer, C.J.; Wroblewski, S.; Huitema, A.D.R.; Schellens, J.H.M.; Gulley, J.L.; et al. A Single-Arm Phase II Study Combining NLG207, a Nanoparticle Camptothecin, with Enzalutamide in Advanced Metastatic Castration-Resistant Prostate Cancer Post-Enzalutamide. Oncologist 2022, 27, 718-e694. [Google Scholar] [CrossRef]
- Chao, J.; Lin, J.; Frankel, P.; Clark, A.J.; Wiley, D.T.; Garmey, E.; Fakih, M.; Lim, D.; Chung, V.; Luevanos, E.; et al. Pilot Trial of CRLX101 in Patients with Advanced, Chemotherapy-Refractory Gastroesophageal Cancer. J. Gastrointest. Oncol. 2017, 8, 962–969. [Google Scholar] [CrossRef]
- Hrkach, J.; Von Hoff, D.; Mukkaram Ali, M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical Development and Clinical Translation of a PSMA-Targeted Docetaxel Nanoparticle with a Differentiated Pharmacological Profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Mita, M.M.; Spear, M.A.; Yee, L.K.; Mita, A.C.; Heath, E.I.; Papadopoulos, K.P.; Federico, K.C.; Reich, S.D.; Romero, O.; Malburg, L.; et al. Phase 1 First-in-Human Trial of the Vascular Disrupting Agent Plinabulin(NPI-2358) in Patients with Solid Tumors or Lymphomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 5892–5899. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Liu, J.; Liao, S.; Diop-Frimpong, B.; Chen, W.; Goel, S.; Naxerova, K.; Ancukiewicz, M.; Boucher, Y.; Jain, R.K.; Xu, L. TGF-β Blockade Improves the Distribution and Efficacy of Therapeutics in Breast Carcinoma by Normalizing the Tumor Stroma. Proc. Natl. Acad. Sci. USA 2012, 109, 16618–16623. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional, Stimuli-Sensitive Nanoparticulate Systems for Drug Delivery. Nat. Rev. Drug Discov. 2014, 13, 813–827. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the Enhanced Permeability and Retention Effect for Tumor Targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Prasad, V.; Fojo, T.; Brada, M. Precision Oncology: Origins, Optimism, and Potential. Lancet Oncol. 2016, 17, e81–e86. [Google Scholar] [CrossRef]
- Boehnke, N.; Straehla, J.P.; Safford, H.C.; Kocak, M.; Rees, M.G.; Ronan, M.; Rosenberg, D.; Adelmann, C.H.; Chivukula, R.R.; Nabar, N.; et al. Massively Parallel Pooled Screening Reveals Genomic Determinants of Nanoparticle Delivery. Science 2022, 377, eabm5551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).