Preparation and In Vitro Characterization of Microneedles Containing Inclusion Complexes Loaded with Progesterone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Progesterone Inclusion Complex
2.3. The Single-Factor Experiments
2.4. Central Composite Design (CCD)
2.5. Optimization and Validation of the Inclusion Process
2.6. Characterization of Progesterone Inclusion Complexes
2.6.1. Infrared Spectra of Progesterone Inclusion Complexes
2.6.2. Differential Scanning Calorimetry of the Progesterone Inclusion Complex
2.7. Preparation of Layered Microneedles of the Progesterone Inclusion Complex
2.8. Preparation of Microneedles with PVA as the Backing Layer Material
2.8.1. Selection of Needle Tip Materials
2.8.2. Selection of the Needle Tip Concentration
2.8.3. Screening of the PVA Layer Concentration
2.9. Preparation of Microneedles with HPC as the Backing Layer Material
2.9.1. Selection of Needle Tip Materials
2.9.2. Selection of the Needle Tip Concentration
2.9.3. Screening of the HPC Layer Concentration
2.10. Evaluation of the Appearance of the Progesterone Soluble Microneedles
2.11. Microneedle Mechanical Strength Evaluation of the Progesterone Inclusion Complex
2.12. Penetration Evaluation of Progesterone Inclusion Complex Microneedles
2.13. Microneedle In Vitro Release Evaluation of the Progesterone Inclusion Complex
2.14. In Vitro Transdermal Permeation Evaluation of Progesterone Inclusion Complex Microneedles
3. Results
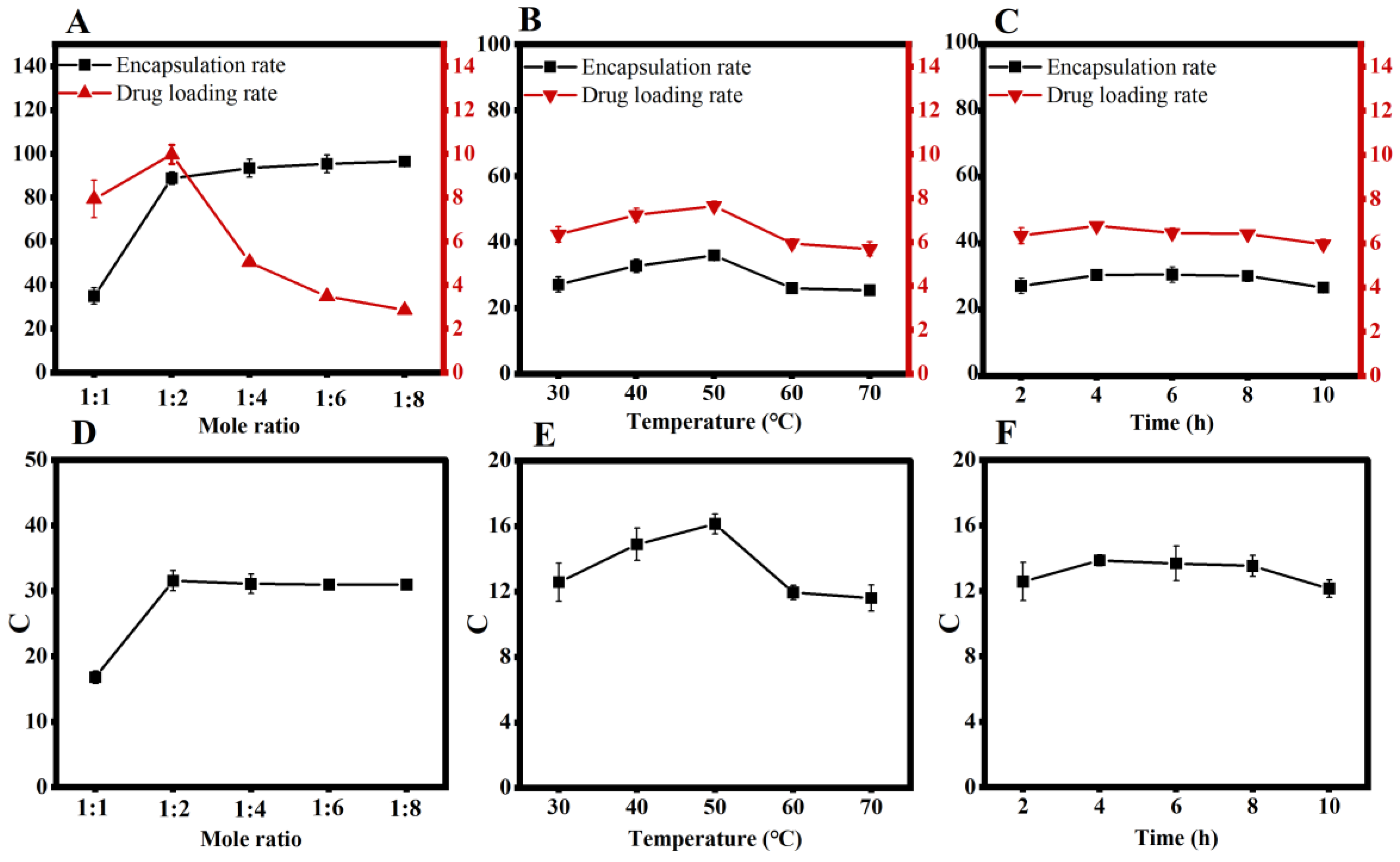
3.1. Single-Factor Experiments
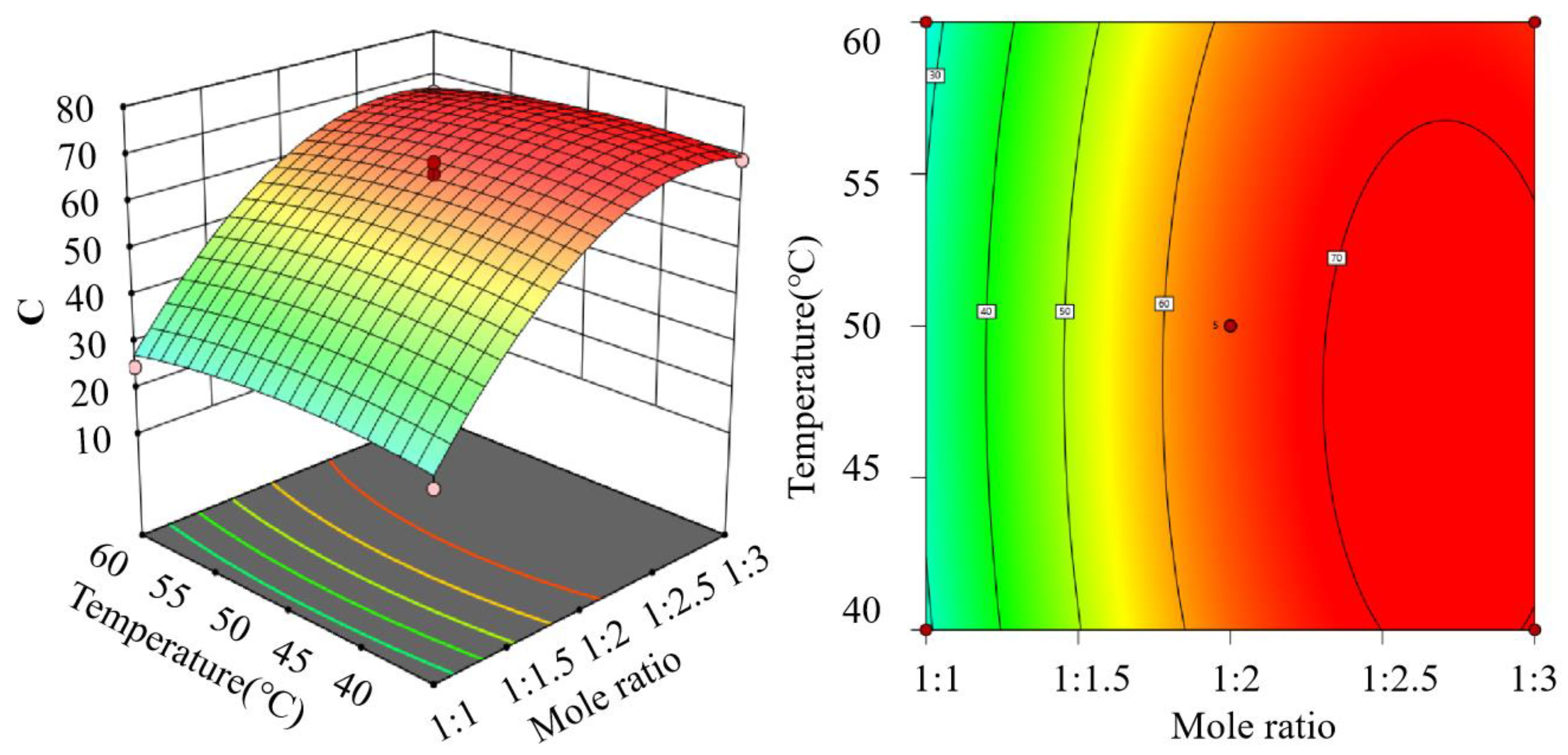
3.2. Central Composite Design
3.3. Optimization and Validation of the Inclusion Process
3.4. Characterization of Progesterone Inclusion Complexes
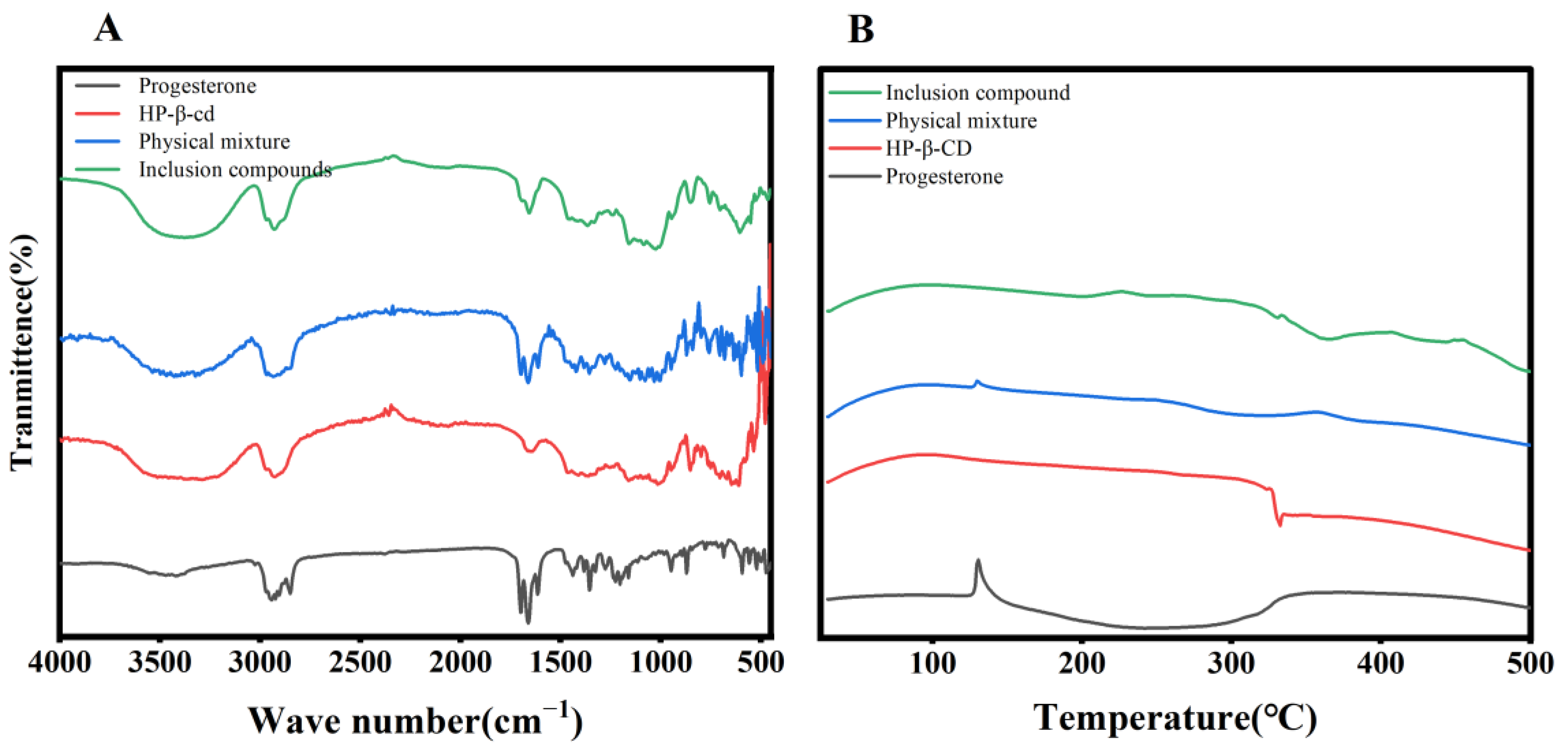
3.4.1. Infrared Spectra of Progesterone Inclusion Complexes
3.4.2. Differential Thermal Analysis of Progesterone Inclusion Compounds
3.5. Progesterone Dissolving Microneedle Loading Measurement
3.6. Preparation of Microneedles with PVA as the Backing Layer Material
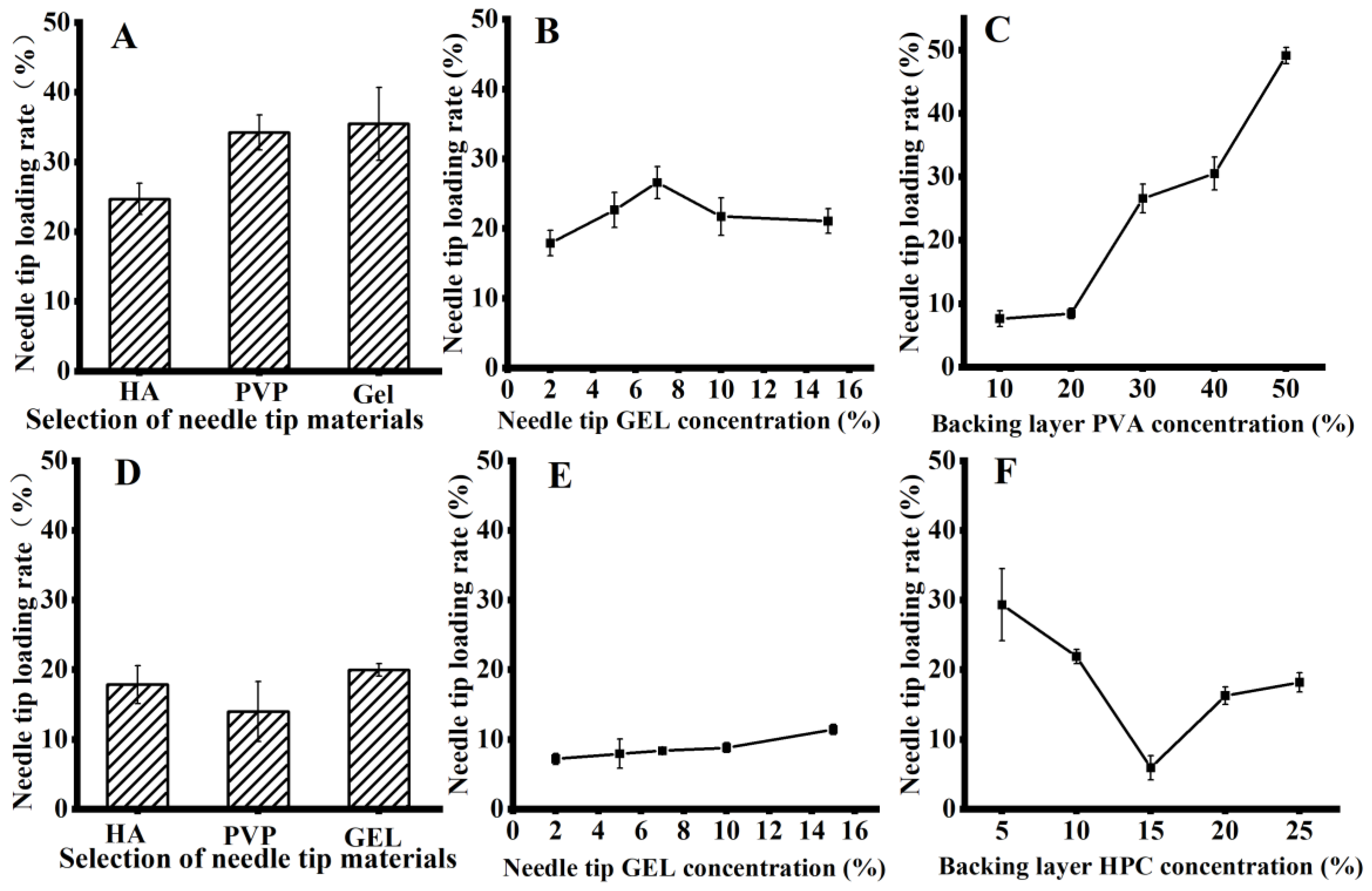
3.6.1. Screening of the Tip Material
3.6.2. Screening of the Needle Tip Concentration
3.6.3. Screening of the PVA Layer Concentration
3.7. Preparation of Microneedles with HPC as Backing Layer Material
3.7.1. Screening of the Needle Tip Materials
3.7.2. Needle Tip Concentration Screening
3.7.3. Screening of the HPC Layer Concentration
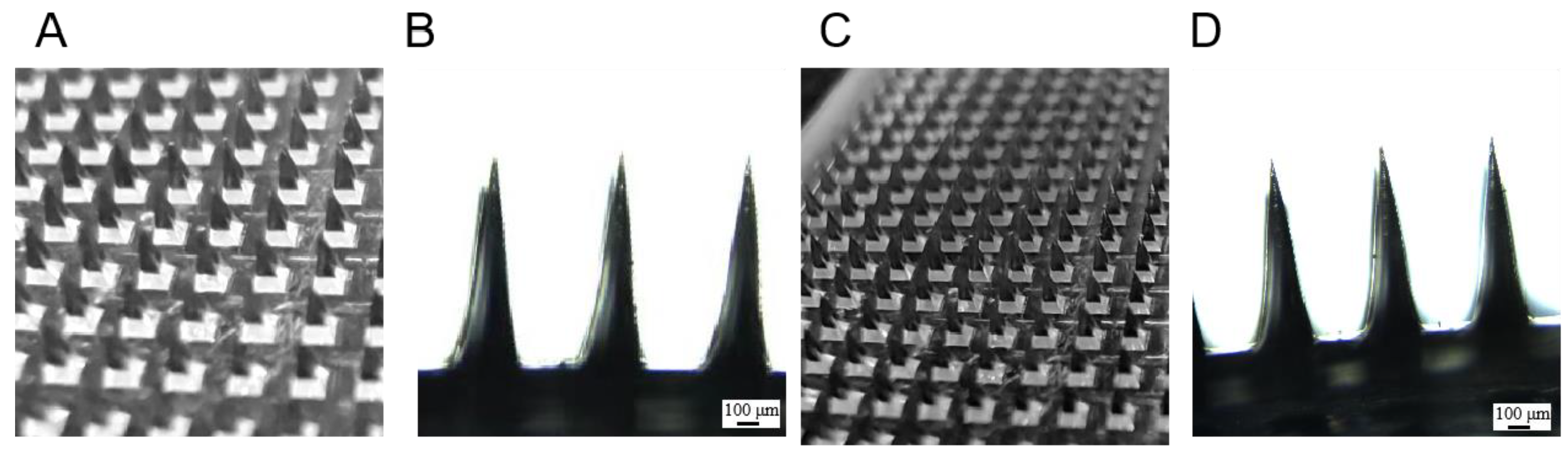
3.8. Evaluation of the Appearance of the Progesterone Soluble Microneedles
3.9. Mechanical Strength Evaluation of Progesterone Microneedles
3.10. Microneedle Penetration Assessment of the Progesterone Inclusion Compound
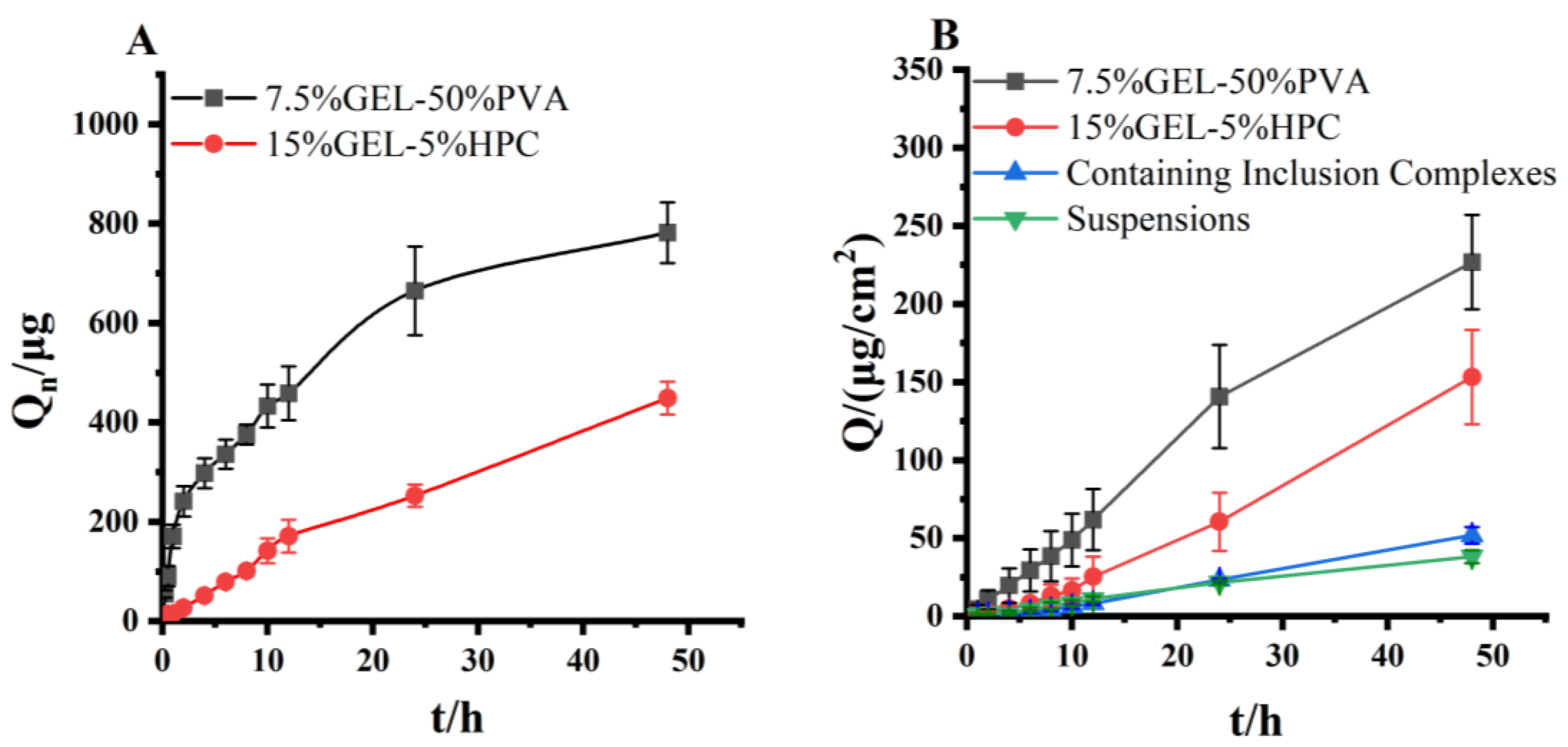
3.11. Evaluation of In Vitro Release of Progesterone Inclusion Compound Microneedles
3.12. Evaluation of In Vitro Transdermal Permeation Evaluation of Progesterone Inclusion Compound Microneedles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filicori, M. Clinical roles and applications of progesterone in reproductive medicine: An overview. Acta Obstet Gynecol Scand. 2015, 94 (Suppl. S161), 3–7. [Google Scholar] [CrossRef] [PubMed]
- Labarta, E.; Mariani, G.; Paolelli, S.; Rodriguez-Varela, C.; Vidal, C.; Giles, J.; Bellver, J.; Cruz, F.; Marzal, A.; Celada, P.; et al. Impact of low serum progesterone levels on the day of embryo transfer on pregnancy outcome: A prospective cohort study in artificial cycles with vaginal progesterone. Hum Reprod. 2021, 36, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yan, Y.; Shen, X.; Sun, H.; Yan, G.; Kong, N.; Jiang, Y. Prolonging the time of progesterone supplementation to improve the pregnancy outcomes of single day 6 blastocyst transfer in frozen-thawed cycles: Study protocol for a randomized controlled trial. Trials 2022, 23, 1024. [Google Scholar] [CrossRef] [PubMed]
- Csapo, A.I.; Knobil, E.; van der Molen, H.J.; Wiest, W.G. Peripheral plasma progesterone levels during human pregnancy and labor. Am. J. Obstet. Gynecol. 1971, 110, 630–632. [Google Scholar] [CrossRef]
- Piette, P. The history of natural progesterone, the never-ending story. Climacteric 2018, 21, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Prior, J.C. Progesterone for the prevention and treatment of osteoporosis in women. Climacteric 2018, 21, 366–374. [Google Scholar] [CrossRef]
- Coomarasamy, A.; Devall, A.J.; Brosens, J.J.; Quenby, S.; Stephenson, M.D.; Sierra, S.; Christiansen, O.B.; Small, R.; Brewin, J.; Roberts, T.E.; et al. Micronized vaginal progesterone to prevent miscarriage: A critical evaluation of randomized evidence. Am. J. Obstet. Gynecol. 2020, 223, 167–176. [Google Scholar] [CrossRef]
- Griesinger, G.; Blockeel, C.; Sukhikh, G.T.; Patki, A.; Dhorepatil, B.; Yang, D.Z.; Chen, Z.J.; Kahler, E.; Pexman-Fieth, C.; Tournaye, H. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: A randomized clinical trial. Hum. Reprod. 2018, 33, 2212–2221. [Google Scholar] [CrossRef]
- Hantoushzadeh, S.; Sheikh, M.; Shariat, M.; Mansouri, R.; Ghamari, A.; Golshahi, F. The effects of progesterone therapy in pregnancy: Vaginal and intramuscular administration. J. Matern. Fetal Neonatal Med. 2021, 34, 2033–2040. [Google Scholar] [CrossRef]
- Iwase, A.; Ando, H.; Toda, S.; Ishimatsu, S.; Harata, T.; Kurotsuchi, S.; Shimomura, Y.; Goto, M.; Kikkawa, F. Oral progestogen versus intramuscular progesterone for luteal support after assisted reproductive technology treatment: A prospective randomized study. Arch. Gynecol. Obstet. 2008, 277, 319–324. [Google Scholar] [CrossRef]
- Maher, M.A.; Abdelaziz, A.; Ellaithy, M.; Bazeed, M.F. Prevention of preterm birth: A randomized trial of vaginal compared with intramuscular progesterone. Acta Obstet. Gynecol. Scand. 2013, 92, 215–222. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, M.; Huang, D.; Sun, Z.; Ao, B.; Xu, F.; Zhang, F. Mechanism insight into the in situ reactions of repeated intramuscular progesterone injections. Basic Clin. Pharmacol. Toxicol. 2023, 132, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Ghazarossian, V.E.; Schantz, E.J.; Schnoes, H.K.; Strong, F.M. A biologically active acid hydrolysis product of saxitoxin. Biochem. Biophys. Res. Commun. 1976, 68, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2022, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Kovacik, A.; Kopecna, M.; Vavrova, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Swain, S. Transdermal Evaporation Drug Delivery System: Concept to Commercial Products. Adv. Pharm Bull. 2018, 8, 535–550. [Google Scholar] [CrossRef]
- Moniz, T.; Costa Lima, S.A.; Reis, S. Marine polymeric microneedles for transdermal drug delivery. Carbohydr. Polym. 2021, 266, 118098. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Vitorino, C.; Sousa, J.; Pais, A. Overcoming the skin permeation barrier: Challenges and opportunities. Curr. Pharm. Des. 2015, 21, 2698–2712. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, X.; Yi, X.; Guo, X.; Li, L.; Hao, Y.; Wang, W. Lidocaine-Loaded Hyaluronic Acid Adhesive Microneedle Patch for Oral Mucosal Topical Anesthesia. Pharmaceutics 2022, 14, 686. [Google Scholar] [CrossRef]
- Xu, J.; Xu, D.; Xuan, X.; He, H. Advances of Microneedles in Biomedical Applications. Molecules 2021, 26, 5912. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Ex Vivo Transdermal Delivery of Nicotinamide Mononucleotide Using Polyvinyl Alcohol Microneedles. Polymers 2023, 15, 2031. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’Souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef]
- Richter-Johnson, J.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Therapeutic applications and pharmacoeconomics of microneedle technology. Expert Rev. Pharmacoecon. Outcomes Res. 2018, 18, 359–369. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated microneedles for drug delivery to the eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef]
- Fabbrocini, G.; De Vita, V.; Fardella, N.; Pastore, F.; Annunziata, M.C.; Mauriello, M.C.; Monfrecola, A.; Cameli, N. Skin needling to enhance depigmenting serum penetration in the treatment of melasma. Plast. Surg. Int. 2011, 2011, 158241. [Google Scholar] [CrossRef]
- Qi, Z.; Yan, Z.; Tan, G.; Jia, T.; Geng, Y.; Shao, H.; Kundu, S.C.; Lu, S. Silk Fibroin Microneedles for Transdermal Drug Delivery: Where Do We Stand and How Far Can We Proceed? Pharmaceutics 2023, 15, 355. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.C.; Rizal, B.; Guanes, G.; Baek, S.K.; Park, J.H.; Betz, A.R.; Choi, S.O. Fabrication of Circular Obelisk-Type Multilayer Microneedles Using Micro-Milling and Spray Deposition. Front. Bioeng. Biotechnol. 2018, 6, 54. [Google Scholar] [CrossRef]
- Damiri, F.; Kommineni, N.; Ebhodaghe, S.O.; Bulusu, R.; Jyothi, V.; Sayed, A.A.; Awaji, A.A.; Germoush, M.O.; Al-Malky, H.S.; Nasrullah, M.Z.; et al. Microneedle-Based Natural Polysaccharide for Drug Delivery Systems (DDS): Progress and Challenges. Pharmaceuticals 2022, 15, 190. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Alzahrani, T.A.; Hussain, A.; Altamimi, M.A. Formulation and Evaluation of Supramolecular Food-Grade Piperine HP beta CD and TPGS Complex: Dissolution, Physicochemical Characterization, Molecular Docking, In Vitro Antioxidant Activity, and Antimicrobial Assessment. Molecules 2020, 25, 4716. [Google Scholar] [CrossRef] [PubMed]
- Sharifpour, E.; Arabkhani, P.; Sadegh, F.; Mousavizadeh, A.; Asfaram, A. In-situ hydrothermal synthesis of CNT decorated by nano ZnS/CuO for simultaneous removal of acid food dyes from binary water samples. Sci. Rep. 2022, 12, 12381. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Quan, G.; Lin, S.; Peng, T.; Wang, Q.; Ran, H.; Chen, H.; Zhang, Q.; Wang, L.; Pan, X.; et al. Novel dissolving microneedles for enhanced transdermal delivery of levonorgestrel: In vitro and in vivo characterization. Int. J. Pharm. 2017, 534, 378–386. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, B.; Ruiz, E.F.; Zhang, F. Advances in the polymeric delivery of nucleic acid vaccines. Theranostics 2022, 12, 4081–4109. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, J.; Liu, Z.; Zhou, Q.; Wang, X.; Zhao, F.; Zhang, Y.; Wang, J.; Liu, M.; Du, R. Promising Strategies for Transdermal Delivery of Arthritis Drugs: Microneedle Systems. Pharmaceutics 2022, 14, 1736. [Google Scholar] [CrossRef]
- Göktürk, S.; Çalışkan, E.; Talman, R.Y.; Var, U. A study on solubilization of poorly soluble drugs by cyclodextrins and micelles: Complexation and binding characteristics of sulfamethoxazole and trimethoprim. Sci. World J. 2012, 2012, 718791. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Yun, C.H.; Han, S.H. Induction of Dendritic Cell Maturation and Activation by a Potential Adjuvant, 2-Hydroxypropyl-β-Cyclodextrin. Front. Immunol. 2016, 7, 435. [Google Scholar] [CrossRef] [PubMed]

| Std. | Run | Mole Ratio (A) | Temperature (B) (°C) | C |
|---|---|---|---|---|
| 13 | 1 | 1:2 | 50 | 66.08 |
| 2 | 2 | 1:3 | 40 | 68.95 |
| 9 | 3 | 1:2 | 50 | 68.48 |
| 3 | 4 | 1:1 | 60 | 24.64 |
| 8 | 5 | 1:2 | 64.14 | 59.54 |
| 11 | 6 | 1:2 | 50 | 63.50 |
| 5 | 7 | 1:0.58 | 50 | 11.78 |
| 1 | 8 | 1:1 | 40 | 26.36 |
| 6 | 9 | 1:3.4 | 50 | 65.74 |
| 7 | 10 | 1:2 | 35.86 | 62.65 |
| 10 | 11 | 1:2 | 50 | 62.65 |
| 12 | 12 | 1:2 | 50 | 64.65 |
| 4 | 13 | 1:3 | 60 | 65.97 |
| Source | Sum of Squares | F-Value | p-Value | |
|---|---|---|---|---|
| Model | 4594.34 | 123.30 | <0.0001 | significant |
| A | 3209.25 | 430.65 | <0.0001 | significant |
| B | 10.35 | 1.39 | 0.2772 | |
| AB | 0.3969 | 0.0533 | 0.8241 | |
| A2 | 1366.96 | 183.43 | <0.0001 | significant |
| B2 | 56.52 | 7.58 | 0.0283 | |
| Residual | 52.16 | |||
| Lack of Fit | 31.02 | 1.96 | 0.2626 | Not significant |
| Number | Loading Rate | Encapsulation Rate | Actual C | Mean C | Predict the C | Deviation (%) |
|---|---|---|---|---|---|---|
| 1 | 9.78 | 95.35 | 69.68 | |||
| 2 | 9.36 | 93.44 | 68.22 | 68.31 | 67.90 | 0.61 |
| 3 | 9.50 | 91.69 | 67.03 |
| Type | The Dosage of Needle Tip (μg) | Needapload per Unit Area (μg/cm2) | Whole Microneedle Drug Loading Volume (mg) |
|---|---|---|---|
| 7.5% GEL-50% PVA | 526.11 ± 32.37 | 233.82 ± 14.39 | 1.07 ± 0.01 |
| 15% GEL-5% HPC | 402.86 ± 18.61 | 179.05 ± 8.27 | 1.41 ± 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Wang, Z.; Aikelamu, K.; Bai, J.; Shen, Q.; Gao, X.; Wang, M. Preparation and In Vitro Characterization of Microneedles Containing Inclusion Complexes Loaded with Progesterone. Pharmaceutics 2023, 15, 1765. https://doi.org/10.3390/pharmaceutics15061765
He H, Wang Z, Aikelamu K, Bai J, Shen Q, Gao X, Wang M. Preparation and In Vitro Characterization of Microneedles Containing Inclusion Complexes Loaded with Progesterone. Pharmaceutics. 2023; 15(6):1765. https://doi.org/10.3390/pharmaceutics15061765
Chicago/Turabian StyleHe, Hongji, Zhaozhi Wang, Kadireya Aikelamu, Jingya Bai, Qi Shen, Xiaoli Gao, and Mei Wang. 2023. "Preparation and In Vitro Characterization of Microneedles Containing Inclusion Complexes Loaded with Progesterone" Pharmaceutics 15, no. 6: 1765. https://doi.org/10.3390/pharmaceutics15061765
APA StyleHe, H., Wang, Z., Aikelamu, K., Bai, J., Shen, Q., Gao, X., & Wang, M. (2023). Preparation and In Vitro Characterization of Microneedles Containing Inclusion Complexes Loaded with Progesterone. Pharmaceutics, 15(6), 1765. https://doi.org/10.3390/pharmaceutics15061765






