Combination Therapy as a Promising Way to Fight Oral Cancer
Abstract
1. Introduction
2. Current Therapeutic Options for Oral Cancer Treatment
| Therapies | Advantages | Disadvantages | References |
|---|---|---|---|
| Surgery |
|
| [29,30] |
| Radiotherapy |
|
| [30,31,32] |
| Chemotherapy |
|
| [30,33] |
3. New Combinatorial Approaches for Oral Cancer Treatment
3.1. DNA Damage Response Inhibition-Based Combination Therapies
| Combination Therapies | Study Design/Treatment Type | Cancer Type and Stage | Main Reported Outcome | References |
|---|---|---|---|---|
| PLA nanoparticles loaded with Cisplatin–chloroquine | Preclinical trials | N/A | Inhibited OSCC proliferation through oxidative stress and apoptosis. | [39,40] |
| Transfersomes loaded with 5-FU and COX-2 inhibitors | Preclinical trial | N/A | Exerted synergistic effects, increasing drug delivery efficiency. | [44] |
| Cisplatin plus Gemcitabine and Rituximab | Phase 1 clinical trial Patients previously treated | - R/M HNSCC | The treatment was considered safe, but no clinical benefit could be ascertained. | [89] |
| Cisplatin with a PARP inhibitor | Preclinical trial | N/A | Exerted synergistic effects in vitro and potentiated in vivo tumor growth suppression. | [47] |
| Combination of a PARP inhibitor with Cisplatin and Paclitaxel | Phase 1 clinical trial 1st line (IC) | LA-HNSCC IVA-B | Displayed low toxicity and was well tolerated. | [48] |
| PARP inhibitors combined with Curcumin | Preclinical trials | N/A | Enhanced anti-angiogenic activity and increased cell death. | [50,51] |
| ATO combined with Cisplatin | Preclinical trial | N/A | Exerted synergistic effects, increasing OSCC apoptosis. | [56] |
| Gimeracil combined with Cisplatin | Preclinical trial | N/A | Inhibited in vitro and in vivo OSCC tumor growth | [64] |
| Combination of 5-FU with Juniperus communis extract | Preclinical trial | N/A | Exerted synergistic effects, potentiating proliferative inhibition. | [65] |
| Azurin combined with 5-FU or Etoposide | Preclinical trial | N/A | Increased OSCC sensibility, improving anticancer response. | [66] |
| Propofol combined with 5-FU | Preclinical trial | N/A | Reduced pharmacological resistance of oral cancer cells. | [70] |
| Combination of Cisplatin with PI3K/AKT/mTOR pathway inhibitors and radiotherapy | Phase 1 clinical trials - | LA-HNSCC ≥III | Exhibited satisfactory OS and PFS rates. | [76,77] |
| Combination of S-1 with Bevacizumab | Preclinical trial | N/A | Exerted synergistic effects, causing in vitro cell proliferation inhibition, and exacerbated apoptosis in vivo. | [83] |
| Combination of 5-FU with Bevacizumab | Preclinical trial | N/A | Exerted no synergistic effects, but was able to reduce cell proliferation. | [83] |
| Bevacizumab combined with Cisplatin and Gemcitabine | Phase 2 clinical trial - | LA NPC III–IVc | Displayed anticancer activity and was well tolerated. | [84] |
| Xevinapant, Cisplatin, and radiotherapy | Phase 1/2 clinical trials - | LA-HNSCC III/IVa/IVb | Showed improved efficacy. A 5-year follow-up demonstrated improved 5-year OS and 3-year PFS, and was deemed safe. | [90,91] |
| Dichloroacetate, Cisplatin, and radiotherapy | Phase 2 clinical trial 1st line | LA-HNSCC III/IVa/IVb | The treatment was safe, but the efficacy could not be determined. | [92] |
| Gemcitabine in combination with Nedaplatin and radiotherapy | Clinical trial - | R/M or LA-HNSCC III–IV | Can be a therapeutic option for HNSCC, although high number of adverse events highlights the need for the optimization of dose and schedule. | [93] |
| Combination of Bortezomib, Camptothecin, and Doxorubicin | Preclinical trial | N/A | Potentiated cytotoxicity only in KB oral cancer cells and not in non-cancerous cells. | [94] |
| Postoperative weekly administration of Cisplatin plus radiation | Phase 2/3 clinical trial Postoperative | LA-HNSCC III/IVa/IVb | Similar efficacy, but less toxicity when compared with 3-weekly Cisplatin administration. | [95] |
| One cycle chemoselection split-dose TPF IC before two cycles of split TPF followed by curative surgery combined with postoperative radiotherapy | Phase 2 clinical trial - | LA OPSCC and LA OCC III/IVa | Well tolerated and a good strategy to select patients that will benefit with TPF treatment. | [96] |
| TPF as induction chemotherapy | Phase III clinical trial 1st line (IC) | LA OSCC - | Did not improve survival of unselected patients, but patients which achieved FPR had good OS and PFS. | [97] |
3.2. Epidermal Growth Factor Receptor Inhibition-Based Combination Therapies
| Combination Therapies | Study Design/ Treatment Type | Cancer Type and Stage | Main Reported Outcome | References |
|---|---|---|---|---|
| EGFR inhibition combined with radiotherapy | Phase 3 clinical trial - | LA-HNSCC III/IV | Exerted supra-additive effects, increasing OS. | [121] |
| Retrospective study 1st and 2nd lines | OCSCC ≥I | Slightly less effective than when assessed for HNSCC patients, but deemed safe and with good efficacy. | [122] | |
| Combination of EGFR and PI3K inhibitors | Phase 2 clinical trial 2nd and 3rd lines | R/M HNSCC II/III/IV | Exhibited no improvement of PFS, ORR, and OS. | [128] |
| Phase 1 clinical trial Most patients previously treated | R/M HNSCC - | Associated with high toxicity and poor efficacy. | [129] | |
| Alpelisib combined with Cetuximab and IMRT | Phase 1b clinical trial - | LA-HNSCC III/IVa/IVb | This treatment modality was considered safe. | [131] |
| Combination of EGFR inhibitors with drugs that target DNA repair defective tumors | Preclinical trial | N/A | Improved cytotoxicity and increased in vitro and in vivo radiation effects. | [135] |
| EGFR mAb combined with pan-aurora inhibitors | Preclinical trial | N/A | Exerted additive effects in inhibiting cell growth. | [138] |
| Combination of HER-3 and EGFR inhibitors | Preclinical trial | N/A | Enhanced the suppression of cell proliferation in vitro and in vivo. | [106] |
| Phase 1/1b clinical trials Most patients previously treated | R/M HNSCC - | Showed great anticancer activity and was well tolerated. | [130,146] | |
| Patritumab combined with Cetuximab and platinum | Phase 2 clinical trial 1st line | R/M HNSCC III/IVa-c | Exhibited good tolerability, but did not improve combination of Cetuximab and platinum. | [147] |
| Combination of EGFR and ERK inhibitors | Preclinical trial | N/A | Exerted synergistic effects, enhancing anticancer activity. | [148] |
| Phase 1b clinical trial Patients previously treated | SGC - | Exerted no synergistic effects with limited efficacy and poor tolerability. | [149] | |
| Combination of EGFR and c-Met inhibitors | Preclinical trial | N/A | Enhanced anticancer efficacy in in vitro and in vivo models. | [5] |
| Phase 2 clinical trial ≥1st line | R/M HNSCC - | Displayed high toxicity associated with no improvement in tumor response or OS. | [152] | |
| Combination of EGFR and VEGF inhibitors | Preclinical and phase 2 clinical trial Patients previously treated | R/M HNSCC - | Delayed tumor growth and inhibited tumor angiogenesis in vitro and in vivo. Deemed safe and showed activity in previously treated patients. | [155] |
| Cetuximab combined with Pazopanib | Phase 1b clinical trial ≥1st line | R/M HNSCC - | Showed safety and a good antitumor response. | [158] |
| Cetuximab combined with Sorafenib | Phase 2 clinical trial - | R/M HNSCC - | Exerted no significant survival response and showed high toxicity when compared to Cetuximab monotherapy. | [153] |
| Combination of Cetuximab, Bevacizumab, and Temsirolimus | Phase 1 clinical trial Mostly patients previously treated | HNSCC - | Exhibited good anticancer response; however, high toxicity was observed. | [161] |
| Combined Cetuximab and Temsirolimus | Phase 2 clinical trial R/M HNSCC | - - | Did not improve PFS, but induced response in Cetuximab refractory patients with good safety profile. | [162] |
| Everolimus in combination with Erlotinib | Phase 2 clinical trial Patients previously treated and untreated | R/M HNSCC - | Was deemed safe, but no benefit was observed for this combination. | [163] |
| Bevacizumab combined with platinum-based chemotherapy | Phase 3 clinical trial Patients previously treated and untreated | R/M HNSCC - | Exhibited no significant OS improvement and increased toxicity. Nonetheless, PFS and ORR improved. | [164] |
| Bevacizumab in combination with Cisplatin and IMRT | Phase 2 clinical trial 1st line | LA-HNSCC III/IVa/IVb | Slightly increased toxicity and promising efficacy were observed. | [165] |
| Combination of Bevacizumab, Cetuximab, Cisplatin, and IMRT | Phase 2 clinical trial - | LA-HNSCC III/IVa/IVb | Exhibited good tolerability and anticancer activity. | [166] |
| Dasatinib combined with EGFR inhibitor | Clinical trial - | HNSCC I–IV | Showed no clinical benefits. | [170] |
| Phase II clinical trial - | R/M HNSCC - | Showed clinical relevance in patients with low serum IL-6 levels. | [171] | |
| Combination of anti-IGF1R with Cetuximab | Phase 2 clinical trial - | R/M HNSCC - | Exhibited no significant improvement of OS and PFS. | [175] |
| Combination of Paclitaxel and Cetuximab | Preclinical trial | N/A | Exerted synergistic effects, increasing anticancer response. | [176] |
| Phase 2 clinical trial 1st line | R/M HNSCC - | Exhibited antitumoral activity and good tolerability. | [191] | |
| Combination of Tipifarnib and Cetuximab | Preclinical trial | N/A | Enhanced cell inhibitory activity. | [179] |
| Combination of Docetaxel and Trastuzumab | Phase 2 clinical trial Patients previously treated | SDC - | Showed an acceptable toxicity profile and promising efficacy for HER-2-positive SDC patients | [184] |
| Afatinib combined with Docetaxel and postoperative radiation therapy | Phase 1 clinical trial - | LA-HNSCC II/III/IV | Exhibited high toxicity. | [185] |
| Panitumumab in combination with Paclitaxel | Phase 2 clinical trial Patients previously treated | R/M HNSCC - | Exhibited good anticancer activity and was considered safe. | [186] |
| Panitumumab combined with Paclitaxel followed by radiotherapy and Panitumumab | Phase 2 clinical trial 1st line (IC) | LA-HNSCC III/IVa/IVb | Toxicity worse than expected; however, led to a higher ORR. | [187] |
| Cetuximab combined with a platinum and 5-FU | Phase 3 clinical trial 1st line | R/M HNSCC - | Displayed good anticancer response in OSCC patients, improving OS. | [188] |
| Combination of Cetuximab with TPF | Phase 2 clinical trial 1st line (IC) | LA OCSCC IV | Exhibited a high ORR, was well tolerated and effective; however, no significant improvement of OS was observed. | [189] |
| Combination of Cetuximab, Paclitaxel, and Carboplatin | Phase 2 clinical trial 1st line | R/M HNSCC III/IV | Showed good anticancer activity with acceptable toxicity. | [192] |
| Phase 2 clinical trial 1st line (IC) | LA-HNSCC IVa/IVb | Good anticancer activity and promising survival was observed. | [193] | |
| Combination of Cetuximab, Paclitaxel, and Cisplatin | Phase 2 clinical trial 1st line | R/M HNSCC - | Exhibited a moderate OS and low toxicity. | [194] |
| Phase 2 clinical trial 1st line | R/M HNSCC - | No significant change in OS with the addition of Paclitaxel. | [195] | |
| Phase 2b clinical trial 1st line | R/M HNSCC - | Addition of Paclitaxel did not improve patient’s outcome. | [196] | |
| Gefitinib combined with Cisplatin | Preclinical trial | N/A | Exacerbated in vitro anticancer activity. | [197] |
| Combination of Cetuximab, Docetaxel, and Cisplatin | Phase 2 clinical trial 1st line | R/M HNSCC - | Exhibited no significant improvement of OS when compared to EXTREME regime. However, can be an alternative in first-line treatment. | [198] |
| Methotrexate combined with Cetuximab | Phase 1b/2 clinical trial 1st line | R/M HNSCC - | Improved PFS and clinical efficacy. | [199] |
| Retrospective study ≥1st line | R/M HNSCC - | The treatment was deemed safe and is an option for palliative treatment. | [200] | |
| Concurrent radiotherapy, Dacomitinib, and Cisplatin | Phase 1 clinical trial - | LA-HNSCC III/IVa/IVb | Tolerable side effects, but the study was early terminated since other studies showed high toxicity profiles and no improvement in the outcomes. | [201] |
| Vandetanib combined with Cisplatin and radiotherapy | Phase 1 clinical trial 1st line | LA-HNSCC III/IV | Well tolerated. | [202] |
| Combination of Nimotuzumab with Cisplatin and radiotherapy | Phase 3 clinical trial - | LA-HNSCC III/IV | Improved PFS and DFS. | [203] |
| Phase 2 clinical trial 1st line | LA-HNSCC III/IV | Exhibited good tolerability, with high response rates. | [115] | |
| Addition of Ipilimumab to concurrent Cetuximab and radiotherapy | Phase 1 clinical trial 1st line | LA-HNSCC III/IVa/IVb | Well tolerated and showed clinical activity. However, it did not meet PFS endpoint. | [116] |
| Combination of histone deacetylase inhibitor with radiotherapy and EGFR or HER-2 inhibitors | Phase 1 clinical trial - | LA-HNSCC III/IV | Exhibited good tolerability at biologically effective doses. | [206] |
3.3. Cyclin-Dependent Kinase Inhibition-Based Combination Therapies
3.4. Bromodomain and Extra-Terminal Domain (BET) Proteins Inhibition-Based Combination Therapies
3.5. PD-1 and PD-L1 Inhibition-Based Combination Therapies
| Combination Therapies | Study Design/Treatment Type | Cancer Type and Stage | Main Reported Outcome | References |
|---|---|---|---|---|
| Sitravatinib combined with Nivolumab | Clinical trial Preoperative | LA-HNSCC III/IVa | Exerted synergistic/addictive effects, promoting tumor reduction, and showed good safety. | [244] |
| Combination of PD-1 and B7-H3 inhibitors | Phase 1/2 clinical trial - | R/M HNSCC - | Showed good tolerance, acceptable safety profile, and antitumoral activity. | [245] |
| Combination of PD-1 and CTLA-4 inhibitors | Phase 2 clinical trial - | LA-OCSCC II/III/IVa | Exhibited high pathologic response and good safety profile. | [247] |
| Phase 3 clinical trial 1st line | R/M HNSCC - | Better toxicity profile than the EXTREME regimen, but did not meet endpoint of OS. | [248] | |
| Phase 3 clinical trial 1st line | R/M HNSCC - | Did not improve OS in patients with high expression of PD-L1. Led to lower median PFS, but with less grade 3/4 adverse events. | [249] | |
| Phase 3 clinical trial 2nd line | R/M HNSCC - | Showed no significant improvement of OS comparing to standard therapies. | [250] | |
| VEGFR inhibitors with anti-PD-1 mAbs | Phase 1 clinical trial Neoadjuvant | LA-OSCC III/IVa | Well tolerated and showed a major pathological response rate of 40% | [257] |
| Phase 1b/2 clinical trial ≥1st line | R/M HNSCC - | Deemed tolerable and displayed promising anticancer activity. | [258] | |
| Nivolumab in combination with Tadalafil | Clinical trial Neoadjuvant | HNSCC - | The treatment was safe, with 50% of the patients showing pathological treatment response. | [263] |
| Pembrolizumab plus Acalabrutinib | Phase 2 clinical trial - | R/M HNSCC - | Showed no clinical benefit with high toxicity. | [252] |
| Pembrolizumab combined with SD-101 | Phase 2 clinical trial ≥1st line | R/M HNSCC - | Overall, 24% of the patients showed objective response but did not reach the threshold. | [254] |
| Combination of Nivolumab and Lirilumab | Phase 2 clinical trial Neoadjuvant/ adjuvant | LRR HNSCC I–IV | Led to high 2-year OS and a pathological response rate of 43%. | [256] |
| Pembrolizumab plus Docetaxel | Phase 1/2 clinical trial ≥1st line | R/M HNSCC - | Achieved a median PFS of 5.8 months and a median OS of 21.3 with manageable toxicity. | [264] |
| Camrelizumab, Paclitaxel or Docetaxel and Cisplatin | Phase 2 clinical trial Neoadjuvant | LA-HNSCC III/IVa/IVb | Led to high ORR and was well tolerated. | [265] |
| TPF combined with an anti-PD-1 | Clinical trial 1st (IC) | LA-HNSCC III/IV | Displayed greater ORR and PFS than monotherapies, with no significant increase in adverse effects; however, no improvement of OS was observed. | [266] |
| Addition of Toripalimab to Gemcitabine and Cisplatin | Phase 1b clinical trial Neoadjuvant | LA-HNSCC III/IVa/IVb | The therapy was considered safe and led to an increase in CD20 expression. | [267] |
| Combination of Pembrolizumab with a platinum and 5-FU | Phase 3 clinical trial 1st line | R/M HNSCC - | Appropriate first-line treatment for R/M HNSCC. Four-year follow up showed continued survival benefit. | [28,268] |
| Addition of Avelumab to standard-of-care chemoradiotherapy | Phase 3 clinical trial - | LA-HNSCC III/IVa/IVb | The objective of prolonging PFS was not achieved. | [269] |
| Avelumab combined with Cetuximab and radiotherapy | Phase 2 clinical trial - | LA-HNSCC III/IV | Deemed tolerable. Improved PFS, but did not meet the endpoint | [270] |
| Nivolumab in combination with Cetuximab | Phase 2 clinical trial Patients previously treated and untreated | R/M HNSCC - | Demonstrated manageable toxicity, with promising anticancer activity for both previously treated and untreated patients. | [271] |
| Combination of Avelumab, Cetuximab, and Palbociclib | Phase 1 clinical trial 1st line | R/M HNSCC - | Showed good tolerability and clinical responses. | [272] |
| Combination of Cetuximab and Pembrolizumab | Phase 2 clinical trial ≥1st line | R/M HNSCC - | Improved ORR and slightly increased toxicity when compared to single-drug therapies. | [273] |
| Afatinib in combination with Pembrolizumab | Phase 2 clinical trial ≥1st line | R/M HNSCC - | The combination improved ORR. | [274] |
| Cetuximab combined with Nivolumab | Phase 1/2 clinical trial 2nd line | R/M HNSCC - | Exhibited good safety; however, no improvement of OS was observed. | [275] |
| Combination of Vorinostat and Pembrolizumab | Phase 2 clinical trial - | R/M HNSCC and SGC - | Exerted synergistic effects, increasing anticancer activity. Higher toxicity than Pembrolizumab monotherapy. | [276] |
| Resveratrol combined with PD-L1 Inhibition | Preclinical trial | N/A | Exhibited in vitro and in vivo antiproliferative effects. | [277] |
| Atezolizumab plus Cobimetinib | Phase 2 clinical trial - | HNSCC - | Moderate activity was observed for patients who had not previously been treated with PD-1/PD-L1 inhibitors. | [281] |
| Navoximod combined with Atezolizumab | Phase 1 clinical trial - | HNSCC - | Showed acceptable safety and tolerability profile and antitumoral activity, but there was no evidence of a benefit of the combination. | [284] |
| Epacadostat combined with Pembrolizumab | Phase 1/2 clinical trial - | HNSCC - | Exhibited good safety and moderate anticancer response. | [285] |
3.6. Microtubule Inhibition-Based Combination Therapies
| Combination Therapies | Study Design/Treatment Type | Cancer Type and Stage | Main Reported Outcome | References |
|---|---|---|---|---|
| Combination of Buparlisib and Paclitaxel | Phase 2 clinical trial 2nd line | R/M HNSCC - | Increased PFS, OS, and ORR when compared to single paclitaxel therapy; however, higher toxicity was observed. | [292] |
| Combination of Docetaxel and PX-866 | Phase 2 clinical trial 2nd and 3rd line | R/M HNSCC - | Exerted no improvement in PFS, ORR, or OS when compared to docetaxel monotherapy. | [293] |
| Gemcitabine combined with Paclitaxel | Phase 2 clinical trial 1st line | R/M HNSCC - | Exhibited satisfactory efficacy and good safety, with no treatment related deaths. | [294] |
| Combination of Cisplatin with Genexol-PM | Phase 2 clinical trial 1st line (IC) | LA-HNSCC III/IVa/IVb | Promoted tumor reduction in 48 of the 52 patients | [296] |
| Combination of Temsirolimus with low-dose weekly Carboplatin and Paclitaxel | Phase 2 clinical trial ≥1st line | R/M HNSCC - | Exerted synergistic effects, with manageable toxicity profile. | [297] |
| Combination of Docetaxel, Cetuximab, 5-FU, and Cisplatin | Phase 2 clinical trial 1st line | R/M HNSCC - | Did not improve efficacy and showed high toxicity profile and mortality rate. | [298] |
| AZD1775 combined with Cisplatin, Gemcitabine, or Carboplatin | Phase 1 clinical trial - | HNSCC - | Exhibited good tolerability in patients with advanced solid tumors. | [300] |
| AZD1775 combined with neoadjuvant weekly Docetaxel and Cisplatin | Phase 1 clinical trial - | LA-HNSCC III/IVb | Exhibited a good safety, efficiency, and tolerability profile. | [299] |
| Combination of WEE1 and PARP inhibitors | Preclinical trial | N/A | Exerted no synergistic effects | [224] |
3.7. Other Target Inhibition-Based Combination Therapies
| Combination Therapies | Study Design/Treatment Type | Cancer Type and Stage | Main Reported Outcome | References |
|---|---|---|---|---|
| Combination of α-Mangostin with TRAIL treatment | Preclinical trial | N/A | Inhibited cell proliferation and promoted tumor apoptosis in OSCC. | [304] |
| Solid lipid nanoparticles containing Paclitaxel and AA | Preclinical trial | N/A | Exerted high efficacy, leading to moderate dysplasia in vivo. | [9] |
| Combination of heteronemin with tetrac | Preclinical trial | N/A | Exerted synergistic effects potentiating anticancer activity. | [10] |
| Navitoclax combined with NOXA inducer | Preclinical trial | N/A | Efficiently promoted HNSCC cell death by apoptosis. | [314] |
| Navitoclax combined with MCL-1 inhibitor | Preclinical trial | N/A | The combination exhibited synergistic activity. | [318] |
| Combination of PI3K and autophagy inhibitors | Preclinical trial | N/A | Exerted synergistic effects, decreasing cancer cells proliferation. | [321] |
| Combination of an anti-IGF1R with an Src inhibitor | Preclinical trial | N/A | Exerted synergistic effects, enhancing anticancer response in HNSCC cells. | [322] |
| Nanoparticle albumin-bound Paclitaxel combined with Cetuximab and Carboplatin | Phase 2 clinical trial 1st line | R/M HNSCC - | Improved ORR and OS and induced tumor reduction; however, no improvement in PFS was observed. | [323] |
| Irinotecan combined with Bortezomib | Phase 2 clinical trial - | LA-HNSCC - | Reduced OS when compare with other therapies. | [326] |
| Combination of Ridaforolimus and MK-0752 | Phase 1 clinical trial - | R/M HNSCC - | Showed activity in HNSCC patients, but considerably increased the side effects in patients with advanced solid tumors. | [327] |
| Combination of Motolimod with the EXTREME regimen | Phase 2 clinical trial 1st line | R/M HNSCC - | Exerted good safety; however, no improvements of PFS and OS in HNSCC patients were observed. Led to enhanced outcomes for HPV-positive oropharyngeal cancer patients. | [330] |
| Combination of LSD1 and YAP inhibition | Preclinical trail | N/A | Exerted additive effects in inhibiting cell proliferation. | [334] |
| Combination of HIF-1α and Trx-1 inhibitor | Preclinical trail | N/A | Exerted synergistic effect under hypoxia condition and addictive effects in normoxia. | [335] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.; Hallworth, P.; Abbas Tahami Monfared, A.; Moshyk, A.; Shaw, J.W. Real-World Systemic Therapy Treatment Patterns for Squamous Cell Carcinoma of the Head and Neck in Canada. Curr. Oncol. 2019, 26, e167–e174. [Google Scholar] [CrossRef] [PubMed]
- Nandini, D.B.; Rao, R.S.; Hosmani, J.; Khan, S.; Patil, S.; Awan, K.H. Novel Therapies in the Management of Oral Cancer: An Update. Disease-a-Month 2020, 66, 101036. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.J. Perspectives on Oral Squamous Cell Carcinoma Prevention—Proliferation, Position, Progression and Prediction. J. Oral Pathol. Med. 2018, 47, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, Y.; Nannapaneni, S.; Griffith, C.C.; Steuer, C.; Qian, G.; Wang, X.; Chen, Z.; Patel, M.; El-Deiry, M.; et al. Combinatorial Approaches Targeting the EGFR Family and C-Met in SCCHN. Oral Oncol. 2021, 112, 105074. [Google Scholar] [CrossRef]
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic Aspects of Oral Cancer. Disease-a-Month 2020, 66, 100988. [Google Scholar] [CrossRef]
- Tsai, W.C.; Kung, P.T.; Wang, Y.H.; Huang, K.H.; Liu, S.A. Influence of Time Interval from Diagnosis to Treatment on Survival for Oral Cavity Cancer: A Nationwide Cohort Study. PLoS ONE 2017, 12, e0175148. [Google Scholar] [CrossRef]
- Bugter, O.; van Iwaarden, D.L.P.; Dronkers, E.A.C.; de Herdt, M.J.; Wieringa, M.H.; Verduijn, G.M.; Mureau, M.A.M.; ten Hove, I.; van Meerten, E.; Hardillo, J.A.; et al. Survival of Patients with Head and Neck Cancer with Metachronous Multiple Primary Tumors Is Surprisingly Favorable. Head Neck 2019, 41, 1648–1655. [Google Scholar] [CrossRef]
- Bharadwaj, R.; Sahu, B.P.; Haloi, J.; Laloo, D.; Barooah, P.; Keppen, C.; Deka, M.; Medhi, S. Combinatorial Therapeutic Approach for Treatment of Oral Squamous Cell Carcinoma. Artif. Cells Nanomed. Biotechnol. 2019, 47, 572–585. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, T.Y.; Chang, W.J.; Pan, Y.S.; Chu, H.R.; Li, Z.L.; Unson, S.; Chin, Y.T.; Lin, C.Y.; Huang, H.M.; et al. Combined Treatment of Heteronemin and Tetrac Induces Antiproliferation in Oral Cancer Cells. Mar. Drugs 2020, 18, 348. [Google Scholar] [CrossRef]
- National Cancer Institute. PDQ Adult Treatment Editorial Board Lip and Oral Cavity Cancer Treatment (Adult) (PDQ®): Health Professional Version; National Cancer Institute: Bethesda, MD, USA, 2002.
- Rikiishi, H.; Shinohara, F.; Sato, T.; Sato, Y.; Suzuki, M.; Echigo, S. Chemosensitization of Oral Squamous Cell Carcinoma Cells to Cisplatin by Histone Deacetylase Inhibitor, Suberoylanilide Hydroxamic Acid. Int. J. Oncol. 2007, 30, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Patel, V.; Shyur, L.F.; Lee, W.L. Copper Supplementation Amplifies the Anti-Tumor Effect of Curcumin in Oral Cancer Cells. Phytomedicine 2016, 23, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Mohs, R.C.; Greig, N.H. Drug Discovery and Development: Role of Basic Biological Research. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination Therapy in Combating Cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Bozic, I.; Nowak, M.A. Resisting Resistance. Annu. Rev. Cancer Biol. 2017, 1, 203–221. [Google Scholar] [CrossRef]
- Pinto, B.; Novais, P.; Henriques, A.C.; Carvalho-Tavares, J.; Silva, P.M.A.; Bousbaa, H. Navitoclax Enhances the Therapeutic Effects of PLK1 Targeting on Lung Cancer Cells in 2D and 3D Culture Systems. Pharmaceutics 2022, 14, 1209. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck Cancers-Major Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Patel, S.G.; Shah, J.P. Changes in the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer: Rationale and Implications. Curr. Oncol. Rep. 2019, 21, 52. [Google Scholar] [CrossRef]
- Dong, H.; Shu, X.; Xu, Q.; Zhu, C.; Kaufmann, A.M.; Zheng, Z.-M.; Albers, A.E.; Qian, X. Current Status of Human Papillomavirus-Related Head and Neck Cancer: From Viral Genome to Patient Care. Virol. Sin. 2021, 36, 1284–1302. [Google Scholar] [CrossRef]
- Hartner, L. Chemotherapy for Oral Cancer. Dent. Clin. N. Am. 2018, 62, 87–97. [Google Scholar] [CrossRef]
- Henriques, A.C.; Ribeiro, D.; Pedrosa, J.; Sarmento, B.; Silva, P.M.A.; Bousbaa, H. Mitosis Inhibitors in Anticancer Therapy: When Blocking the Exit Becomes a Solution. Cancer Lett. 2019, 440–441, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2022. [Google Scholar] [CrossRef]
- Taberna, M.; Oliva, M.; Mesía, R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Lacas, B.; Pignon, J.P.; Le, Q.T.; Grégoire, V.; Grau, C.; Hackshaw, A.; Zackrisson, B.; Parmar, M.K.B.; Lee, J.W.; et al. Chemotherapy and Radiotherapy in Locally Advanced Head and Neck Cancer: An Individual Patient Data Network Meta-Analysis. Lancet Oncol. 2021, 22, 727–736. [Google Scholar] [CrossRef]
- Argiris, A.; Harrington, K.J.; Tahara, M.; Schulten, J.; Chomette, P.; Castro, A.F.; Licitra, L. Evidence-Based Treatment Options in Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2017, 7, 72. [Google Scholar] [CrossRef]
- Mody, M.D.; Rocco, J.W.; Yom, S.S.; Haddad, R.I.; Saba, N.F. Head and Neck Cancer. Lancet 2021, 398, 2289–2299. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Golusiński, W.; Golusińska-Kardach, E. Current Role of Surgery in the Management of Oropharyngeal Cancer. Front. Oncol. 2019, 9, 388. [Google Scholar] [CrossRef]
- Ribeiro-Rotta, R.F.; Rosa, E.A.; Milani, V.; Dias, N.R.; Masterson, D.; da Silva, E.N.; de Sene Amâncio Zara, A.L. The Cost of Oral Cancer: A Systematic Review. PLoS ONE 2022, 17, e0266346. [Google Scholar] [CrossRef]
- Lang, K.; Baur, M.; Held, T.; El Shafie, R.; Moratin, J.; Freudlsperger, C.; Zaoui, K.; Bougatf, N.; Hoffmann, J.; Plinkert, P.K.; et al. Definitive Radiotherapy for Squamous Cell Carcinoma of the Oral Cavity: A Single-Institution Experience. Radiol. Oncol. 2021, 55, 467–473. [Google Scholar] [CrossRef]
- Dhawan, A. Adjunctive Therapy in Oral Cancer. In Oral and Maxillofacial Surgery for the Clinician; Springer Nature: Singapore, 2021; pp. 1903–1913. [Google Scholar]
- Carneiro-Neto, J.; De-Menezes, J.; Moura, L.; Massucato, E.; De-Andrade, C. Protocols for Management of Oral Complications of Chemotherapy and/or Radiotherapy for Oral Cancer: Systematic Review and Meta-Analysis Current. Med. Oral Patol. Oral Y Cir. Bucal. 2016, 22, e15–e23. [Google Scholar] [CrossRef]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA Damage and the Balance between Survival and Death in Cancer Biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.K. DNA Damage Repair: Historical Perspectives, Mechanistic Pathways and Clinical Translation for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Velic, D.; Couturier, A.M.; Ferreira, M.T.; Rodrigue, A.; Poirier, G.G.; Fleury, F.; Masson, J.Y. DNA Damage Signalling and Repair Inhibitors: The Long-Sought-after Achilles’ Heel of Cancer. Biomolecules 2015, 5, 3204–3259. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Yan, W.; Chen, Y. Antitumor Effect of Poly Lactic Acid Nanoparticles Loaded with Cisplatin and Chloroquine on the Oral Squamous Cell Carcinoma. Aging 2021, 13, 2593–2603. [Google Scholar] [CrossRef]
- Abd Rashid, N.; Abd Halim, S.A.S.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Adib Ridzuan, N.R.; Abdul Jalil, N.A. The Role of Natural Antioxidants in Cisplatin-Induced Hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Li, S.; Gao, L.; Zhi, K.; Ren, W. The Molecular Basis and Therapeutic Aspects of Cisplatin Resistance in Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 761379. [Google Scholar] [CrossRef] [PubMed]
- Riddell, I.A.; Lippard, S.J. Cisplatin and Oxaliplatin: Our Current Understanding of TheirActions. In Metallo-Drugs: Development and Action of Anticancer Agents Actions; De Gruyter: Berlin, Germany, 2018; Volume 18, ISBN 9783110470734. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Pozzi, C.; Lopresti, L.; Tassone, G.; Mangani, S. Targeting Methyltransferases in Human Pathogenic Bacteria: Insights into Thymidylate Synthase (TS) and Flavin-Dependent TS (FDTS). Molecules 2019, 24, 1638. [Google Scholar] [CrossRef]
- Hernando-Cubero, J.; Matos-García, I.; Alonso-Orduña, V.; Capdevila, J. The Role of Fluoropirimidines in Gastrointestinal Tumours: From the Bench to the Bed. J. Gastrointest. Cancer 2017, 48, 135–147. [Google Scholar] [CrossRef]
- Bollareddy, S.R.; Krishna, V.; Roy, G.; Dasari, D.; Dhar, A.; Vamsi, V.; Venuganti, K. Transfersome Hydrogel Containing 5-Fluorouracil and Etodolac Combination for Synergistic Oral Cancer Treatment. AAPS PharmSciTech 2022, 23, 70. [Google Scholar] [CrossRef]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; Van Der Burg, E.; Nygren, A.O.H.; Zander, S.A.L.; Derksen, P.W.B.; De Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High Sensitivity of BRCA1-Deficient Mammary Tumors to the PARP Inhibitor AZD2281 Alone and in Combination with Platinum Drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Wang, Y. PARP-1 and Its Associated Nucleases in DNA Damage Response. DNA Repair (Amst). 2019, 81, 102651. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, M.; Fujihara, H.; Fujimori, H.; Kawaguchi, K.; Yamada, H.; Nakayama, R.; Yamamoto, N.; Kishi, Y.; Hamada, Y.; Masutani, M. Synergetic Effects of PARP Inhibitor AZD2281 and Cisplatin in Oral Squamous Cell Carcinoma in Vitro and in Vivo. Int. J. Mol. Sci. 2016, 17, 272. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.J.; Foster, N.R.; Zoroufy, A.J.; Schwartz, G.K.; Munster, P.N.; Seiwert, T.Y.; de Souza, J.A.; Vokes, E.E. A Phase I Trial Adding Poly(ADP-Ribose) Polymerase Inhibitor Veliparib to Induction Carboplatin-Paclitaxel in Patients with Head and Neck Squamous Cell Carcinoma: Alliance A091101. Oral Oncol. 2021, 114, 105171. [Google Scholar] [CrossRef]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural Product Target Network Reveals Potential for Cancer Combination Therapies. Front. Pharmacol. 2019, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Molla, S.; Chatterjee, S.; Sethy, C.; Sinha, S.; Kundu, C.N. Olaparib Enhances Curcumin-Mediated Apoptosis in Oral Cancer Cells by Inducing PARP Trapping through Modulation of BER and Chromatin Assembly. DNA Repair 2021, 105, 103157. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Sinha, S.; Molla, S.; Hembram, K.C.; Kundu, C.N. PARP Inhibitor Veliparib (ABT-888) Enhances the Anti-Angiogenic Potentiality of Curcumin through Deregulation of NECTIN-4 in Oral Cancer: Role of Nitric Oxide (NO). Cell. Signal. 2021, 80, 109902. [Google Scholar] [CrossRef]
- Chatterjee, S.; Dhal, A.K.; Paul, S.; Sinha, S.; Das, B.; Dash, S.R.; Kundu, C.N. Combination of Talazoparib and Olaparib Enhanced the Curcumin-Mediated Apoptosis in Oral Cancer Cells by PARP-1 Trapping. J. Cancer Res. Clin. Oncol. 2022, 148, 3521–3535. [Google Scholar] [CrossRef]
- Kim, H.-R. Combination Treatment with Arsenic Trioxide and Sulindac Enhances Apoptotic Cell Death in Lung Cancer Cells via Activation of Oxidative Stress and Mitogen-Activated Protein Kinases. Oncol. Rep. 2008, 20, 379–384. [Google Scholar] [CrossRef]
- Liu, B.; Pan, S.; Dong, X.; Qiao, H.; Jiang, H.; Krissansen, G.W.; Sun, X. Opposing Effects of Arsenic Trioxide on Hepatocellular Carcinomas in Mice. Cancer Sci. 2006, 97, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Chen, Z. Acute Promyelocytic Leukemia: From Highly Fatal to Highly Curable. Blood 2008, 111, 2505–2515. [Google Scholar] [CrossRef]
- Nakaoka, T.; Ota, A.; Ono, T.; Karnan, S.; Konishi, H.; Furuhashi, A.; Ohmura, Y.; Yamada, Y.; Hosokawa, Y.; Kazaoka, Y. Combined Arsenic Trioxide-Cisplatin Treatment Enhances Apoptosis in Oral Squamous Cell Carcinoma Cells. Cell. Oncol. 2014, 37, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.C.; Teo, W.H.; Huang, T.F.; Lee, T.C.; Lo, J.F. Combinatorial Low Dose Arsenic Trioxide and Cisplatin Exacerbates Autophagy via AMPK/STAT3 Signaling on Targeting Head and Neck Cancer Initiating Cells. Front. Oncol. 2020, 10, 463. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Chang, M.-Y.; Shiau, J.-P.; Farooqi, A.A.; Huang, Y.-H.; Tang, J.-Y.; Chang, H.-W. Antiproliferation- and Apoptosis-Inducible Effects of a Novel Nitrated [6,6,6]Tricycle Derivative (SK2) on Oral Cancer Cells. Molecules 2022, 27, 1576. [Google Scholar] [CrossRef]
- Wang, S.-C.; Yen, C.-Y.; Shiau, J.-P.; Chang, M.-Y.; Hou, M.-F.; Jeng, J.-H.; Tang, J.-Y.; Chang, H.-W. Synergistic Antiproliferation of Cisplatin and Nitrated [6,6,6]Tricycle Derivative (SK2) for a Combined Treatment of Oral Cancer Cells. Antioxidants 2022, 11, 926. [Google Scholar] [CrossRef]
- Lü, L.; Liu, X.; Wang, C.; Hu, F.; Wang, J.; Huang, H. Dissociation of E-Cadherin/β-Catenin Complex by MG132 and Bortezomib Enhances CDDP Induced Cell Death in Oral Cancer SCC-25 Cells. Toxicol. Vitr. 2015, 29, 1965–1976. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Chen, D. Proteasome Inhibitor MG132 Enhances the Sensitivity of Human OSCC Cells to Cisplatin via a ROS/DNA Damage/P53 Axis. Exp. Ther. Med. 2023, 25, 224. [Google Scholar] [CrossRef]
- Takagi, M.; Sakata, K.; Someya, M.; Matsumoto, Y.; Tauchi, H.; Hareyama, M.; Fukushima, M. The Combination of Hyperthermia or Chemotherapy with Gimeracil for Effective Radiosensitization. Strahlenther. Und Onkol. 2012, 188, 255–261. [Google Scholar] [CrossRef]
- Harada, K.; Ferdous, T.; Ueyama, Y. Gimeracil Exerts Radiosensitizing Effects on Oral Squamous Cell Carcinoma Cells in Vitro and in Vivo. Anticancer Res. 2016, 36, 5923–5930. [Google Scholar] [CrossRef]
- Harada, K.; Ferdous, T.; Harada, T.; Takenawa, T.; Ueyama, Y. Gimeracil Enhances the Antitumor Effect of Cisplatin in Oral Squamous Cell Carcinoma Cells in Vitro and in Vivo. Oncol. Lett. 2017, 14, 3349–3356. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Hsiao, C.Y.; Lee, S.C.; Huang, X.F.; Chang, K.F.; Lee, M.S.; Hsieh, M.C.; Tsai, N.M. Suppression of Oral Cancer by Induction of Cell Cycle Arrest and Apoptosis Using Juniperus Communis Extract. Biosci. Rep. 2020, 40, BSR20202083. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, M.H.; Cho, Y.J.; Park, B.S.; Kim, S.; Kim, G.C. The Bacterial Protein AAzurin Enhances Sensitivity of Oral Squamous Carcinoma Cells to Anticancer Drugs. Yonsei Med. J. 2011, 52, 773–778. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, L.Y.; dos Moreira, F.S.; dos Santos, G.A.S.; Cuadra Zelaya, F.J.M.; Ortiz, C.A.; Agostini, M.; Mariano, F.S.; Bastos, D.C.; Daher, U.R.N.; Kowalski, L.P.; et al. FASN Inhibition Sensitizes Metastatic OSCC Cells to Cisplatin and Paclitaxel by Downregulating Cyclin B1. Oral Dis. 2021, 29, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dong, T.; Sheng, J. Propofol Suppresses Gastric Cancer Progression by Regulating Circpdss1/Mir-1324/Sox4 Axis. Cancer Manag. Res. 2021, 13, 6031–6043. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, J.; Bie, S.; Kang, L.; Mao, Q.; Liu, W.; Guo, J.; Lu, J.; Xia, R. Propofol Inhibits Wnt Signaling and Exerts Anticancer Activity in Glioma Cells. Oncol. Lett. 2018, 16, 402–408. [Google Scholar] [CrossRef]
- Yang, K.-S.; Che, P.-C.; Hsieh, M.-J.; Lee, I.-N.; Wu, Y.-P.; Chen, M.-S.; Chen, J.-C. Propofol Induces Apoptosis and Ameliorates 5-fluorouracil Resistance in OSCC Cells by Reducing the Expression and Secretion of Amphiregulin. Mol. Med. Rep. 2021, 25, 36. [Google Scholar] [CrossRef]
- Al-majed, A.A.; Bakheit, A.H.H.; Aziz, H.A.A.; Alajmi, F.M.; Alrabiah, H. Propranolol. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2017; Volume 42, pp. 287–338. [Google Scholar] [CrossRef]
- Ashrafi, S.; Shapouri, R.; Shirkhani, A.; Mahdavi, M. Anti-Tumor Effects of Propranolol: Adjuvant Activity on a Transplanted Murine Breast Cancer Model. Biomed. Pharmacother. 2018, 104, 45–51. [Google Scholar] [CrossRef]
- Shibuya, C.M.; Tjioe, K.C.; Oliveira, S.H.P.; Bernabé, D.G. Propranolol Inhibits Cell Viability and Expression of the Pro-Tumorigenic Proteins Akt, NF-ĸB, and VEGF in Oral Squamous Cell Carcinoma. Arch. Oral Biol. 2022, 136, 105383. [Google Scholar] [CrossRef]
- Li, C.; Feng, Y.; Shao, W. Changes of Serum MiR-223-3p in Patients with Oral Cancer Treated with TPF Regimen and the Prognosis. Oncol. Lett. 2020, 19, 2527–2532. [Google Scholar] [CrossRef]
- Ju, W.; Ma, H.; Zhao, T.; Liang, S.; Zhu, D.; Wang, L.; Li, J.; Zhang, Z.; Zhou, G.; Zhong, L. Stathmin Guides Personalized Therapy in Oral Squamous Cell Carcinoma. Cancer Sci. 2020, 111, 1303–1313. [Google Scholar] [CrossRef]
- Gulati, S.; Desai, J.; Palackdharry, S.M.; Morris, J.C.; Zhu, Z.; Jandarov, R.; Riaz, M.K.; Takiar, V.; Mierzwa, M.; Gutkind, J.S.; et al. Phase 1 Dose-Finding Study of Metformin in Combination with Concurrent Cisplatin and Radiotherapy in Patients with Locally Advanced Head and Neck Squamous Cell Cancer. Cancer 2020, 126, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Day, D.; Prawira, A.; Spreafico, A.; Waldron, J.; Karithanam, R.; Giuliani, M.; Weinreb, I.; Kim, J.; Cho, J.; Hope, A.; et al. Phase I Trial of Alpelisib in Combination with Concurrent Cisplatin-Based Chemoradiotherapy in Patients with Locoregionally Advanced Squamous Cell Carcinoma of the Head and Neck. Oral Oncol. 2020, 108, 104753. [Google Scholar] [CrossRef]
- O-charoenrat, P.; Rhys-Evans, P.; Eccles, S.A. Expression of Vascular Endothelial Growth Factor Family Members in Head and Neck Squamous Cell Carcinoma Correlates with Lymph Node Metastasis. Cancer 2001, 92, 556–568. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Smith, G.L.; Carter, D.; Sasaki, C.T.; Haffty, B.G. Prognostic Significance of Vascular Endothelial Growth Factor Protein Levels in Oral and Oropharyngeal Squamous Cell Carcinoma. J. Clin. Oncol. 2000, 18, 2046–2052. [Google Scholar] [CrossRef]
- Nie, K.-K.; Geng, C.-X.; Zhang, L.; Liu, S.-C.; Zhang, Z.-F.; Wang, R.; Zou, X.; Ji, Y.-X. Clinical Observation of Bevacizumab Combined with S-1 in the Treatment of Pretreated Advanced Esophageal Carcinoma. Chin. Med. Sci. J. 2016, 31, 221–227. [Google Scholar] [CrossRef]
- Yoshida, M.; Takagane, A.; Miyake, Y.; Shimada, K.; Nagata, N.; Sato, A.; Ogata, Y.; Fukunaga, M.; Otsuka, K.; Takahashi, T.; et al. A Phase II Study of Third-Line Combination Chemotherapy with Bevacizumab Plus S-1 for Metastatic Colorectal Cancer with Mutated KRAS (SAVIOR Study). Oncology 2016, 91, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Kubota, A.; Hasegawa, Y.; Takemura, H.; Terada, T.; Taguchi, T.; Nagahara, K.; Nakatani, H.; Yoshino, K.; Higaki, Y.; et al. Randomized Phase III Trial of Adjuvant Chemotherapy with S-1 after Curative Treatment in Patients with Squamous-Cell Carcinoma of the Head and Neck (ACTS-HNC). PLoS ONE 2015, 10, e0116965. [Google Scholar] [CrossRef]
- Itashiki, Y.; Harada, K.; Takenawa, T.; Ferdous, T.; Ueyama, Y.; Mishima, K. Antitumor Effects of Bevacizumab in Combination with Fluoropyrimidine Drugs on Human Oral Squamous Cell Carcinoma. Oncol. Lett. 2021, 22, 730. [Google Scholar] [CrossRef]
- Chong, W.Q.; Lim, C.M.; Sinha, A.K.; Tan, C.S.; Hui, G.; Chan, J.; Huang, Y.; Kumarakulasinghe, N.B.; Sundar, R.; Jeyasekharan, A.D.; et al. Integration of Antiangiogenic Therapy with Cisplatin and Gemcitabine Chemotherapy in Patients with Nasopharyngeal Carcinoma. Clin. Cancer Res. 2020, 26, 5320–5328. [Google Scholar] [CrossRef]
- Thoppil, R.J.; Cappelli, H.C.; Adapala, R.K.; Kanugula, A.K.; Paruchuri, S.; Thodeti, C.K. TRPV4 Channels Regulate Tumor Angiogenesis via Modulation of Rho/Rho Kinase Pathway. Oncotarget 2016, 7, 25849–25861. [Google Scholar] [CrossRef] [PubMed]
- Yahya, F.; Mohd Bakri, M.; Hossain, M.; Syed Abdul Rahman, S.; Mohammed Alabsi, A.; Ramanathan, A. Combination Treatment of TRPV4 Agonist with Cisplatin Promotes Vessel Normalization in an Animal Model of Oral Squamous Cell Carcinoma. Medicina 2022, 58, 1229. [Google Scholar] [CrossRef] [PubMed]
- Michinaga, S.; Tanabe, A.; Nakaya, R.; Fukutome, C.; Inoue, A.; Iwane, A.; Minato, Y.; Tujiuchi, Y.; Miyake, D.; Mizuguchi, H.; et al. Angiopoietin-1/Tie-2 Signal after Focal Traumatic Brain Injury Is Potentiated by BQ788, an ET B Receptor Antagonist, in the Mouse Cerebrum: Involvement in Recovery of Blood–Brain Barrier Function. J. Neurochem. 2020, 154, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Affara, N.I.; Ruffell, B.; Medler, T.R.; Gunderson, A.J.; Johansson, M.; Bornstein, S.; Bergsland, E.; Steinhoff, M.; Li, Y.; Gong, Q.; et al. B Cells Regulate Macrophage Phenotype and Response to Chemotherapy in Squamous Carcinomas. Cancer Cell 2014, 25, 809–821. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Lien, M.-Y.; Lin, C.-Y.; Lo, W.-J.; Hua, C.-H.; Chang, W.-C.; Chiu, C.-F.; Lin, C.-C. Rituximab in Combination with Gemcitabine plus Cisplatin in Patients with Recurrent and Metastatic Head and Neck Squamous Cell Carcinoma: A Phase I Trial. BMC Cancer 2022, 22, 169. [Google Scholar] [CrossRef]
- Sun, X.-S.; Tao, Y.; Le Tourneau, C.; Pointreau, Y.; Sire, C.; Kaminsky, M.-C.; Coutte, A.; Alfonsi, M.; Boisselier, P.; Martin, L.; et al. Debio 1143 and High-Dose Cisplatin Chemoradiotherapy in High-Risk Locoregionally Advanced Squamous Cell Carcinoma of the Head and Neck: A Double-Blind, Multicentre, Randomised, Phase 2 Study. Lancet Oncol. 2020, 21, 1173–1187. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, X.-S.; Pointreau, Y.; Le Tourneau, C.; Sire, C.; Kaminsky, M.-C.; Coutte, A.; Alfonsi, M.; Calderon, B.; Boisselier, P.; et al. Extended Follow-up of a Phase 2 Trial of Xevinapant plus Chemoradiotherapy in High-Risk Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomised Clinical Trial. Eur. J. Cancer 2023, 183, 24–37. [Google Scholar] [CrossRef]
- Powell, S.F.; Mazurczak, M.; Dib, E.G.; Bleeker, J.S.; Geeraerts, L.H.; Tinguely, M.; Lohr, M.M.; McGraw, S.C.; Jensen, A.W.; Ellison, C.A.; et al. Phase II Study of Dichloroacetate, an Inhibitor of Pyruvate Dehydrogenase, in Combination with Chemoradiotherapy for Unresected, Locally Advanced Head and Neck Squamous Cell Carcinoma. Investig. New Drugs 2022, 40, 622–633. [Google Scholar] [CrossRef]
- Huo, R.-X.; Jin, Y.-Y.; Zhuo, Y.-X.; Ji, X.-T.; Cui, Y.; Wu, X.-J.; Wang, Y.-J.; Zhang, L.; Zhang, W.-H.; Cai, Y.-M.; et al. Concurrent Chemoradiotherapy Using Gemcitabine and Nedaplatin in Recurrent or Locally Advanced Head and Neck Squamous Cell Carcinoma. World J. Clin. Cases 2022, 10, 3414–3425. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Matsuo, K.; Xu, L.; Yangd, J.; Zheng, L. Optimized Combinations of Bortezomib, Camptothecin, and Doxorubicin Show Increased Efficacy and Reduced Toxicity in Treating Oral Cancer. Anticancer Drugs 2015, 26, 547–554. [Google Scholar] [CrossRef]
- Kiyota, N.; Tahara, M.; Mizusawa, J.; Kodaira, T.; Fujii, H.; Yamazaki, T.; Mitani, H.; Iwae, S.; Fujimoto, Y.; Onozawa, Y.; et al. Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Inhestern, J.; Schmalenberg, H.; Dietz, A.; Rotter, N.; Maschmeyer, G.; Jungehülsing, M.; Grosse-Thie, C.; Kuhnt, T.; Görner, M.; Sudhoff, H.; et al. A Two-Arm Multicenter Phase II Trial of One Cycle Chemoselection Split-Dose Docetaxel, Cisplatin and 5-Fluorouracil (TPF) Induction Chemotherapy before Two Cycles of Split TPF Followed by Curative Surgery Combined with Postoperative Radiotherapy in Patien. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Liu, Y.; Wang, L.; Li, J.; Ren, G.; Sun, J.; Tu, W.; Hu, Y.; Ji, T.; Yang, W.; et al. Phase III Trial of Docetaxel Cisplatin 5-Fluorouracil Induction Chemotherapy for Resectable Oral Cancer Suggests Favorable Pathological Response as a Surrogate Endpoint for Good Therapeutic Outcome. Cancer Commun. 2021, 41, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.Y.; Yue, X.H.; Dong, M.J.; Li, J.; Zhang, C.P. Assessment of Neoadjuvant Chemotherapy with Docetaxel, Cisplatin, and Fluorouracil in Patients with Oral Cavity Cancer. Cancer Med. 2023, 12, 2417–2426. [Google Scholar] [CrossRef]
- Bei, R.; Budillon, A.; Masuelli, L.; Cereda, V.; Vitolo, D.; Di Gennaro, E.; Ripavecchia, V.; Palumbo, C.; Ionna, F.; Losito, S.; et al. Frequent Overexpression of Multiple ErbB Receptors by Head and Neck Squamous Cell Carcinoma Contrasts with Rare Antibody Immunity in Patients. J. Pathol. 2004, 204, 317–325. [Google Scholar] [CrossRef]
- Ongkeko, W.M.; Altuna, X.; Weisman, R.A.; Wang-Rodriguez, J. Expression of Protein Tyrosine Kinases in Head and Neck Squamous Cell Carcinomas. Am. J. Clin. Pathol. 2005, 124, 71–76. [Google Scholar] [CrossRef]
- Jiang, N.; Saba, N.F.; Chen, Z.G. Advances in Targeting HER3 as an Anticancer Therapy. Chemother. Res. Pract. 2012, 2012, 817304. [Google Scholar] [CrossRef]
- Dokala, A.; Thakur, S.S. Extracellular Region of Epidermal Growth Factor Receptor: A Potential Target for Anti-EGFR Drug Discovery. Oncogene 2017, 36, 2337–2344. [Google Scholar] [CrossRef]
- Wieduwilt, M.J.; Moasser, M.M. The Epidermal Growth Factor Receptor Family: Biology Driving Targeted Therapeutics. Cell. Mol. Life Sci. 2011, 65, 1566–1584. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Wang, D.; Qian, G.; Zhang, H.; Magliocca, K.R.; Nannapaneni, S.; Amin, A.R.M.R.; Rossi, M.; Patel, M.; El-Deiry, M.; Wadsworth, J.T.; et al. HER3 Targeting Sensitizes HNSCC to Cetuximab by Reducing HER3 Activity and HER2/HER3 Dimerization: Evidence from Cell Line and Patient-Derived Xenograft Models. Clin. Cancer Res. 2017, 23, 677–686. [Google Scholar] [CrossRef]
- Mock, A.; Plath, M.; Moratin, J.; Tapken, M.J.; Jäger, D.; Krauss, J.; Fröhling, S.; Hess, J.; Zaoui, K. EGFR and PI3K Pathway Activities Might Guide Drug Repurposing in HPV-Negative Head and Neck Cancers. Front. Oncol. 2021, 11, 678966. [Google Scholar] [CrossRef]
- Rebucci, M.; Peixoto, P.; Dewitte, A.; Wattez, N.; De Nuncques, M.-A.; Rezvoy, N.; Vautravers-Dewas, C.; Buisine, M.-P.; Guerin, E.; Peyrat, J.-P.; et al. Mechanisms Underlying Resistance to Cetuximab in the HNSCC Cell Line: Role of AKT Inhibition in Bypassing This Resistance. Int. J. Oncol. 2010, 38, 189–200. [Google Scholar] [CrossRef]
- Thomas, R.; Weihua, Z. Rethink of EGFR in Cancer With Its Kinase Independent Function on Board. Front. Oncol. 2019, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Gharwan, H.; Groninger, H. Kinase Inhibitors and Monoclonal Antibodies in Oncology: Clinical Implications. Nat. Rev. Clin. Oncol. 2016, 13, 209–227. [Google Scholar] [CrossRef]
- Golay, J.; Taylor, R.P. The Role of Complement in the Mechanism of Action of Therapeutic Anti-Cancer MAbs. Antibodies 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Markovic, A.; Chung, C.H. Current Role of EGF Receptor Monoclonal Antibodies and Tyrosine Kinase Inhibitors in the Management of Head and Neck Squamous Cell Carcinoma. Expert Rev. Anticancer Ther. 2012, 12, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Dassonville, O.; Bozec, A.; Fischel, J.L.; Milano, G. EGFR Targeting Therapies: Monoclonal Antibodies versus Tyrosine Kinase Inhibitors. Crit. Rev. Oncol. Hematol. 2007, 62, 53–61. [Google Scholar] [CrossRef]
- Hutchinson, R.A.; Adams, R.A.; McArt, D.G.; Salto-Tellez, M.; Jasani, B.; Hamilton, P.W. Epidermal Growth Factor Receptor Immunohistochemistry: New Opportunities in Metastatic Colorectal Cancer. J. Transl. Med. 2015, 13, 217. [Google Scholar] [CrossRef]
- Ang, M.K.; Montoya, J.E.; Tharavichitkul, E.; Lim, C.; Tan, T.; Wang, L.Y.; Wee, J.; Soong, Y.L.; Fong, K.W.; Ng, Q.S.; et al. Phase II Study of Nimotuzumab (TheraCim-HR3) Concurrent with Cisplatin/Radiotherapy in Patients with Locally Advanced Head and Neck Squamous Cell Carcinoma. Head Neck 2021, 43, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Moskovitz, J.; Kunning, S.; Ruffin, A.T.; Reeder, C.; Ohr, J.; Gooding, W.E.; Kim, S.; Karlovits, B.J.; Vignali, D.A.A.; et al. Phase I Trial of Cetuximab, Radiotherapy, and Ipilimumab in Locally Advanced Head and Neck Cancer. Clin. Cancer Res. 2022, 28, 1335–1344. [Google Scholar] [CrossRef]
- Pogorzelski, M.; Hilser, T.; Ting, S.C.; Kansy, B.; Gauler, T.C.; Stuschke, M.; Schmid, K.W.; Lang, S.; Grünwald, V.; Schuler, M.; et al. Identification of a Prognostic Clinical Score for Patients With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck Treated With Systemic Therapy Including Cetuximab. Front. Oncol. 2021, 11, 635096. [Google Scholar] [CrossRef] [PubMed]
- Jie, H.-B.; Srivastava, R.M.; Argiris, A.; Bauman, J.E.; Kane, L.P.; Ferris, R.L. Increased PD-1+ and TIM-3+ TILs during Cetuximab Therapy Inversely Correlate with Response in Head and Neck Cancer Patients. Cancer Immunol. Res. 2017, 5, 408–416. [Google Scholar] [CrossRef]
- Fenoglio, D.; Belgioia, L.; Parodi, A.; Missale, F.; Bacigalupo, A.; Tarke, A.; Incandela, F.; Negrini, S.; Vecchio, S.; Altosole, T.; et al. Development of Exhaustion and Acquisition of Regulatory Function by Infiltrating CD8+CD28− T Lymphocytes Dictate Clinical Outcome in Head and Neck Cancer. Cancers 2021, 13, 2234. [Google Scholar] [CrossRef] [PubMed]
- Bozec, A.; Ebran, N.; Radosevic-Robin, N.; Chamorey, E.; Yahia, H.B.; Marcie, S.; Gautier, M.; Penault-Llorca, F.; Milano, G. Combination of Phosphotidylinositol-3-Kinase Targeting with Cetuximab and Irradiation: A Preclinical Study on an Orthotopic Xenograft Model of Head and Neck Cancer. Head Neck 2017, 39, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus Cetuximab for Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Otsuru, M.; Yanamoto, S.; Yamada, S.; Nakashiro, K.; Harazono, Y.; Kohgo, T.; Nakamura, M.; Nomura, T.; Kasamatsu, A.; Tanaka, S.; et al. Radiotherapy Plus Cetuximab for Squamous Cell Carcinoma of the Oral Cavity: A Multicenter Retrospective Study of 79 Patients in Japan. Int. J. Environ. Res. Public Health 2023, 20, 4545. [Google Scholar] [CrossRef]
- Perri, F.; Pacelli, R.; Della Vittoria Scarpati, G.; Cella, L.; Giuliano, M.; Caponigro, F.; Pepe, S. Radioresistance in Head and Neck Squamous Cell Carcinoma: Biological Bases and Therapeutic Implications. Head Neck 2015, 37, 763–770. [Google Scholar] [CrossRef]
- Horn, D.; Hess, J.; Freier, K.; Hoffmann, J.; Freudlsperger, C. Targeting EGFR-PI3K-AKT-MTOR Signaling Enhances Radiosensitivity in Head and Neck Squamous Cell Carcinoma. Expert Opin. Ther. Targets 2015, 19, 795–805. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Morse, N.; Krigsfeld, G.; Gupta, G.; Higginson, D.S.; Lee, N.Y.; Morris, L.; Ganly, I.; Shiao, S.L.; Powell, S.N.; et al. Taselisib (GDC-0032), a Potent β-Sparing Small Molecule Inhibitor of PI3K, Radiosensitizes Head and Neck Squamous Carcinomas Containing Activating PIK3CA Alterations. Clin. Cancer Res. 2016, 22, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; McKenna, W.G.; Weber, C.N.; Machtay, M.; Rosenthal, D.I.; Bakanauskas, V.J.; Cerniglia, G.J.; Bernhard, E.J.; Feldman, M.D.; Goldsmith, J.D.; et al. Local Recurrence in Head and Neck Cancer: Relationship to Radiation Resistance and Signal Transduction. Clin. Cancer Res. 2002, 8, 885–892. [Google Scholar] [PubMed]
- Leiker, A.J.; DeGraff, W.; Choudhuri, R.; Sowers, A.L.; Thetford, A.; Cook, J.A.; Van Waes, C.; Mitchell, J.B. Radiation Enhancement of Head and Neck Squamous Cell Carcinoma by the Dual PI3K/MTOR Inhibitor PF-05212384. Clin. Cancer Res. 2015, 21, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, A.; Shirai, K.; Choi, M.; Laskin, J.; Kochenderfer, M.; Spira, A.; Winquist, E.; Hausman, D.; Walker, L.; Cohen, R.B. A Randomized, Phase II Trial of Cetuximab with or without PX-866, an Irreversible Oral Phosphatidylinositol 3-Kinase Inhibitor, in Patients with Relapsed or Metastatic Head and Neck Squamous Cell Cancer. Ann. Oncol. 2015, 26, 556–561. [Google Scholar] [CrossRef]
- Marret, G.; Isambert, N.; Rezai, K.; Gal, J.; Saada-Bouzid, E.; Rolland, F.; Chausson, M.; Borcoman, E.; Alt, M.; Klijanienko, J.; et al. Phase I Trial of Copanlisib, a Selective PI3K Inhibitor, in Combination with Cetuximab in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Investig. New Drugs 2021, 39, 1641–1648. [Google Scholar] [CrossRef]
- Gazzah, A.; Boni, V.; Soria, J.; Calles, A.; Even, C.; Doger, B.; Mahjoubi, L.; Bahleda, R.; Ould-kaci, M.; Esler, A.; et al. A Phase 1b Study of Afatinib in Combination with Standard-Dose Cetuximab in Patients with Advanced Solid Tumours. Eur. J. Cancer 2018, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.A.; Riaz, N.; Fury, M.G.; McBride, S.M.; Michel, L.; Lee, N.Y.; Sherman, E.J.; Baxi, S.S.; Haque, S.S.; Katabi, N.; et al. A Phase 1b Study of Cetuximab and BYL719 (Alpelisib) Concurrent with Intensity Modulated Radiation Therapy in Stage III-IVB Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2020, 106, 564–570. [Google Scholar] [CrossRef]
- Leonard, B.; Brand, T.M.; O’Keefe, R.A.; Lee, E.D.; Zeng, Y.; Kemmer, J.D.; Li, H.; Grandis, J.R.; Bhola, N.E. BET Inhibition Overcomes Receptor Tyrosine Kinase–Mediated Cetuximab Resistance in HNSCC. Cancer Res. 2018, 78, 4331–4343. [Google Scholar] [CrossRef]
- Stuhlmiller, T.J.; Miller, S.M.; Zawistowski, J.S.; Nakamura, K.; Beltran, A.S.; Duncan, J.S.; Angus, S.P.; Collins, K.A.L.; Granger, D.A.; Reuther, R.A.; et al. Inhibition of Lapatinib-Induced Kinome Reprogramming in ERBB2-Positive Breast Cancer by Targeting BET Family Bromodomains. Cell Rep. 2015, 11, 390–404. [Google Scholar] [CrossRef]
- Stratikopoulos, E.E.; Dendy, M.; Szabolcs, M.; Khaykin, A.J.; Lefebvre, C.; Zhou, M.-M.; Parsons, R. Kinase and BET Inhibitors Together Clamp Inhibition of PI3K Signaling and Overcome Resistance to Therapy. Cancer Cell 2015, 27, 837–851. [Google Scholar] [CrossRef]
- Frederick, B.A.; Gupta, R.; Atilano-Roque, A.; Su, T.T.; Raben, D. Combined EGFR1 and PARP1 Inhibition Enhances the Effect of Radiation in Head and Neck Squamous Cell Carcinoma Models. Radiat. Res. 2020, 194, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Bai, C.; Xie, D.; Ma, T.; Zhou, P.K. DNA-PKcs: A Multi-Faceted Player in DNA Damage Response. Front. Genet. 2020, 11, 607428. [Google Scholar] [CrossRef] [PubMed]
- Nowsheen, S.; Bonner, J.A.; LoBuglio, A.F.; Trummell, H.; Whitley, A.C.; Dobelbower, M.C.; Yang, E.S. Cetuximab Augments Cytotoxicity with Poly (ADP-Ribose) Polymerase Inhibition in Head and Neck Cancer. PLoS ONE 2011, 6, e24148. [Google Scholar] [CrossRef]
- Hoellein, A.; Pickhard, A.; von Keitz, F.; Schoeffmann, S.; Piontek, G.; Rudelius, M.; Baumgart, A.; Wagenpfeil, S.; Peschel, C.; Dechow, T.; et al. Aurora Kinase Inhibition Overcomes Cetuximab Resistance in Squamous Cell Cancer of the Head and Neck. Oncotarget 2011, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Rabinowits, G.; Haddad, R.I. Overcoming Resistance to EGFR Inhibitor in Head and Neck Cancer: A Review of the Literature. Oral Oncol. 2012, 48, 1085–1089. [Google Scholar] [CrossRef]
- Quartuccio, S.M.; Schindler, K. Functions of Aurora Kinase C in Meiosis and Cancer. Front. Cell Dev. Biol. 2015, 3, 50. [Google Scholar] [CrossRef]
- Lai, C.H.; Tseng, J.T.; Lee, Y.C.; Chen, Y.J.; Lee, J.C.; Lin, B.W.; Huang, T.C.; Liu, Y.W.; Leu, T.H.; Liu, Y.W.; et al. Translational Up-Regulation of Aurora-A in EGFR-Overexpressed Cancer. J. Cell. Mol. Med. 2010, 14, 1520–1531. [Google Scholar] [CrossRef]
- González-Loyola, A.; Fernández-Miranda, G.; Trakala, M.; Partida, D.; Samejima, K.; Ogawa, H.; Cañamero, M.; de Martino, A.; Martínez-Ramírez, Á.; de Cárcer, G.; et al. Aurora B Overexpression Causes Aneuploidy and P21 Cip1 Repression during Tumor Development. Mol. Cell. Biol. 2015, 35, 3566–3578. [Google Scholar] [CrossRef]
- Furqan, M.; Huma, Z.; Ashfaq, Z.; Nasir, A.; Ullah, R.; Bilal, A.; Iqbal, M.; Khalid, M.H.; Hussain, I.; Faisal, A. Identification and Evaluation of Novel Drug Combinations of Aurora Kinase Inhibitor CCT137690 for Enhanced Efficacy in Oral Cancer Cells. Cell Cycle 2019, 18, 2281–2292. [Google Scholar] [CrossRef]
- Chen, C.F.; Lu, C.C.; Chiang, J.H.; Chiu, H.Y.; Yang, J.S.; Lee, C.Y.; Der Way, T.; Huang, H.J. Synergistic Inhibitory Effects of Cetuximab and Curcumin on Human Cisplatin-Resistant Oral Cancer CAR Cells through Intrinsic Apoptotic Process. Oncol. Lett. 2018, 16, 6323–6330. [Google Scholar] [CrossRef]
- Jozkowiak, M.; Dyszkiewicz-Konwinska, M.; Ramlau, P.; Kranc, W.; Spaczynska, J.; Wierzchowski, M.; Kaczmarek, M.; Jodynis-Liebert, J.; Piotrowska-Kempisty, H. Individual and Combined Treatments with Methylated Resveratrol Analogue Dmu-214 and Gefitinib Inhibit Tongue Cancer Cells Growth via Apoptosis Induction and Egfr Inhibition. Int. J. Mol. Sci. 2021, 22, 6180. [Google Scholar] [CrossRef] [PubMed]
- Deeken, J.F.; Wang, H.; Subramaniam, D.; He, A.R.; Hwang, J.; Marshall, J.L.; Urso, C.E.; Wang, Y.; Ramos, C.; Steadman, K.; et al. A Phase 1 Study of Cetuximab and Lapatinib in Patients with Advanced Solid Tumor Malignancies. Cancer 2015, 121, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Forster, M.D.; Dillon, M.T.; Kocsis, J.; Remenár, É.; Pajkos, G.; Rolland, F.; Greenberg, J.; Harrington, K.J. Patritumab or Placebo, with Cetuximab plus Platinum Therapy in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: A Randomised Phase II Study. Eur. J. Cancer 2019, 123, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Yee, P.S.; Zainal, N.S.; Gan, C.P.; Lee, B.K.B.; Mun, K.S.; Abraham, M.T.; Ismail, S.M.; Abdul Rahman, Z.A.; Patel, V.; Cheong, S.C. Synergistic Growth Inhibition by Afatinib and Trametinib in Preclinical Oral Squamous Cell Carcinoma Models. Target. Oncol. 2019, 14, 223–235. [Google Scholar] [CrossRef]
- Lieu, C.H.; Hidalgo, M.; Berlin, J.D.; Ko, A.H.; Cervantes, A.; LoRusso, P.; Gerber, D.E.; Eder, J.P.; Eckhardt, S.G.; Kapp, A.V.; et al. A Phase Ib Dose-Escalation Study of the Safety, Tolerability, and Pharmacokinetics of Cobimetinib and Duligotuzumab in Patients with Previously Treated Locally Advanced or Metastatic Cancers with Mutant KRAS. Oncologist 2017, 22, 1024-e89. [Google Scholar] [CrossRef]
- Madoz-Gúrpide, J.; Zazo, S.; Chamizo, C.; Casado, V.; Caramés, C.; Gavín, E.; Cristóbal, I.; García-Foncillas, J.; Rojo, F. Activation of MET Pathway Predicts Poor Outcome to Cetuximab in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Transl. Med. 2015, 13, 282. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Stabile, L.P. Hepatocyte Growth Factor/c-Met Signaling in Head and Neck Cancer and Implications for Treatment. Cancers 2017, 9, 39. [Google Scholar] [CrossRef]
- Kochanny, S.E.; Worden, F.P.; Adkins, D.R.; Lim, D.W.; Bauman, J.E.; Wagner, S.A.; Brisson, R.J.; Karrison, T.G.; Stadler, W.M.; Vokes, E.E.; et al. A Randomized Phase 2 Network Trial of Tivantinib plus Cetuximab versus Cetuximab in Patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. Cancer 2020, 126, 2146–2152. [Google Scholar] [CrossRef]
- Gilbert, J.; Schell, M.J.; Zhao, X.; Murphy, B.; Tanvetyanon, T.; Leon, M.E.; Neil Hayes, D.; Haigentz, M.; Saba, N.; Nieva, J.; et al. A Randomized Phase II Efficacy and Correlative Studies of Cetuximab with or without Sorafenib in Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2015, 51, 376–382. [Google Scholar] [CrossRef]
- Viloria-Petit, A.M.; Kerbel, R.S. Acquired Resistance to EGFR Inhibitors: Mechanisms and Prevention Strategies. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 914–926. [Google Scholar] [CrossRef]
- Argiris, A.; Kotsakis, A.P.; Hoang, T.; Worden, F.P.; Savvides, P.; Gibson, M.K.; Gyanchandani, R.; Blumenschein, G.R.; Chen, H.X.; Grandi, J.R.; et al. Cetuximab and Bevacizumab: Preclinical Data and Phase II Trial in Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck. Ann. Oncol. 2013, 24, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Trigo, J.; Hitt, R.; Koralewski, P.; Diaz-Rubio, E.; Rolland, F.; Knecht, R.; Amellal, N.; Schueler, A.; Baselga, J. Open-Label, Uncontrolled, Multicenter Phase II Study to Evaluate the Efficacy and Toxicity of Cetuximab as a Single Agent in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck Who Failed to Respond to Platinum-Based The. J. Clin. Oncol. 2007, 25, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Clement, P.M.; Cupissol, D.; Del Campo, J.; de Mont-Serrat, H.; Thurm, H.C.; Blackman, A.S.; Cohen, E.E. BIBW 2992 versus Cetuximab in Patients with Metastatic or Recurrent Head and Neck Cancer (SCCHN) after Failure of Platinum-Containing Therapy with a Cross-over Period for Progressing Patients: Preliminary Results of a Randomized, Open-Label Phase II Study. J. Clin. Oncol. 2010, 28, 5501. [Google Scholar] [CrossRef]
- Adkins, D.; Mehan, P.; Ley, J.; Siegel, M.J.; Siegel, B.A.; Dehdashti, F.; Jiang, X.; Salama, N.N.; Trinkaus, K.; Oppelt, P. Pazopanib plus Cetuximab in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: An Open-Label, Phase 1b and Expansion Study. Lancet Oncol. 2018, 19, 1082–1093. [Google Scholar] [CrossRef]
- Hartwich, J.; Orr, W.S.; Ng, C.Y.; Spence, Y.; Morton, C.; Davidoff, A.M. HIF-1α Activation Mediates Resistance to Anti-Angiogenic Therapy in Neuroblastoma Xenografts. J. Pediatr. Surg. 2013, 48, 39–46. [Google Scholar] [CrossRef]
- Hudes, G.R.; Berkenblit, A.; Feingold, J.; Atkins, M.B.; Rini, B.I.; Dutcher, J. Clinical Trial Experience With Temsirolimus in Patients With Advanced Renal Cell Carcinoma. Semin. Oncol. 2009, 36, S26–S36. [Google Scholar] [CrossRef]
- Liu, X.; Kambrick, S.; Fu, S.; Naing, A.; Subbiah, V.; Blumenschein, G.R.; Glisson, B.S.; Kies, M.S.; Tsimberidou, A.M.; Wheler, J.J.; et al. Advanced Malignancies Treated with a Combination of the VEGF Inhibitor Bevacizumab, Anti-EGFR Antibody Cetuximab, and the MTOR Inhibitor Temsirolimus. Oncotarget 2016, 7, 23227–23238. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Kochanny, S.; Wood, K.; Worden, F.P.; Adkins, D.; Wade, J.L.; Sleckman, B.G.; Anderson, D.; Brisson, R.J.; Karrison, T.; et al. A Randomized Phase 2 Study of Temsirolimus and Cetuximab versus Temsirolimus Alone in Recurrent/Metastatic, Cetuximab-resistant Head and Neck Cancer: The MAESTRO Study. Cancer 2020, 126, 3237–3243. [Google Scholar] [CrossRef]
- Massarelli, E.; Lin, H.; Ginsberg, L.E.; Tran, H.T.; Lee, J.J.; Canales, J.R.; Williams, M.D.; Jr, G.R.B.; Lu, C.; Heymach, J.V.; et al. Phase II Trial of Everolimus and Erlotinib in Patients with Platinum-Resistant Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Ann. Oncol. 2015, 26, 1476–1480. [Google Scholar] [CrossRef]
- Argiris, A.; Li, S.; Savvides, P.; Ohr, J.P.; Gilbert, J.; Levine, M.A.; Chakravarti, A.; Haigentz, M.; Saba, N.F.; Ikpeazu, C.V.; et al. Phase III Randomized Trial of Chemotherapy with or without Bevacizumab in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Clin. Oncol. 2019, 37, 3266–3274. [Google Scholar] [CrossRef]
- Fury, M.G.; Lee, N.Y.; Sherman, E.; Lisa, D.; Kelly, K.; Lipson, B.; Carlson, D.; Stambuk, H.; Haque, S.; Shen, R.; et al. A Phase 2 Study of Bevacizumab with Cisplatin plus Intensity-Modulated Radiation Therapy for Stage III/IVB Head and Neck Squamous Cell Cancer. Cancer 2012, 118, 5008–5014. [Google Scholar] [CrossRef]
- Fury, M.G.; Xiao, H.; Sherman, E.J.; Baxi, S.; Smith-Marrone, S.; Schupak, K.; Gewanter, R.; Gelblum, D.; Haque, S.; Schoder, H.; et al. Phase II Trial of Bevacizumab + Cetuximab + Cisplatin with Concurrent Intensity-Modulated Radiation Therapy for Patients with Stage III/IVB Head and Neck Squamous Cell Carcinoma. Head Neck 2016, 38, E566–E570. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Yasui, H.; Ohnishi, Y. Ligand-Independent EGFR Activation by Anchorage-Stimulated Src Promotes Cancer Cell Proliferation and Cetuximab Resistance via ErbB3 Phosphorylation. Cancers 2019, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yoshida, T.; Kawakami, H.; Takegawa, N.; Tanizaki, J.; Hayashi, H.; Takeda, M.; Yonesaka, K.; Tsurutani, J.; Nakagawa, K. T790M-Selective EGFR-TKI Combined with Dasatinib as an Optimal Strategy for Overcoming EGFR-TKI Resistance in T790M-Positive Non–Small Cell Lung Cancer. Mol. Cancer Ther. 2017, 16, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Dosch, A.R.; Dai, X.; Reyzer, M.L.; Mehra, S.; Srinivasan, S.; Willobee, B.A.; Kwon, D.; Kashikar, N.; Caprioli, R.; Merchant, N.B.; et al. Combined Src/EGFR Inhibition Targets STAT3 Signaling and Induces Stromal Remodeling to Improve Survival in Pancreatic Cancer. Mol. Cancer Res. 2020, 18, 623–631. [Google Scholar] [CrossRef]
- Bauman, J.E.; Duvvuri, U.; Gooding, W.E.; Rath, T.J.; Gross, N.D.; Song, J.; Jimeno, A.; Yarbrough, W.G.; Johnson, F.M.; Wang, L.; et al. Randomized, Placebo-Controlled Window Trial of EGFR, Src, or Combined Blockade in Head and Neck Cancer. JCI Insight 2017, 2, e90449. [Google Scholar] [CrossRef]
- Stabile, L.P.; Egloff, A.M.; Gibson, M.K.; Gooding, W.E.; Ohr, J.; Zhou, P.; Rothenberger, N.J.; Wang, L.; Geiger, J.L.; Flaherty, J.T.; et al. IL6 Is Associated with Response to Dasatinib and Cetuximab: Phase II Clinical Trial with Mechanistic Correlatives in Cetuximab-Resistant Head and Neck Cancer. Oral Oncol. 2017, 69, 38–45. [Google Scholar] [CrossRef]
- Sinto, M.S.; Thomas, S.; Kannan, S. Combinatorial Treatment with Gefitinib and Bay11-7085 Sensitizes Primary Gefitinib-Resistant OSCC Cells by Influencing the EGFR- NFκB Signaling Axis. Med. Oncol. 2021, 38, 110. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like Growth Factor Receptor Signaling in Tumorigenesis and Drug Resistance: A Challenge for Cancer Therapy. J. Hematol. Oncol. 2020, 13, 64. [Google Scholar] [CrossRef]
- Jameson, M.J.; Beckler, A.D.; Taniguchi, L.E.; Allak, A.; VanWagner, L.B.; Lee, N.G.; Thomsen, W.C.; Hubbard, M.A.; Thomas, C.Y. Activation of the Insulin-like Growth Factor-1 Receptor Induces Resistance to Epidermal Growth Factor Receptor Antagonism in Head and Neck Squamous Carcinoma Cells. Mol. Cancer Ther. 2011, 10, 2124–2134. [Google Scholar] [CrossRef]
- Ferrarotto, R.; William, W.N.; Tseng, J.E.; Marur, S.; Shin, D.M.; Murphy, B.; Cohen, E.E.W.; Thomas, C.Y.; Willey, R.; Cosaert, J.; et al. Randomized Phase II Trial of Cixutumumab Alone or with Cetuximab for Refractory Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2018, 82, 83–90. [Google Scholar] [CrossRef]
- Sawatani, Y.; Komiyama, Y.; Nakashiro, K.I.; Uchida, D.; Fukumoto, C.; Shimura, M.; Hasegawa, T.; Kamimura, R.; Hitomi-Koide, M.; Hyodo, T.; et al. Paclitaxel Potentiates the Anticancer Effect of Cetuximab by Enhancing Antibody-Dependent Cellular Cytotoxicity on Oral Squamous Cell Carcinoma Cells in Vitro. Int. J. Mol. Sci. 2020, 21, 6292. [Google Scholar] [CrossRef]
- Monteverde, M.; Milano, G.; Strola, G.; Maffi, M.; Lattanzio, L.; Vivenza, D.; Tonissi, F.; Merlano, M.; Lo Nigro, C. The Relevance of ADCC for EGFR Targeting: A Review of the Literature and a Clinically-Applicable Method of Assessment in Patients. Crit. Rev. Oncol. Hematol. 2015, 95, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK Cell-Mediated Antibody-Dependent Cellular Cytotoxicity in Cancer Immunotherapy. Front. Immunol. 2015, 6, 368. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Wang, D.; Nannapaneni, S.; Sun, Y.; Griffith, C.C.; Wang, X.; Chen, Z.; Patel, M.; El-deiry, M.; Shin, D.M.; et al. Tipifarnib Enhances Anti-EGFR Activity of Cetuximab in Non-HRas Mutated Head and Neck Squamous Cell Carcinoma Cancer (HNSCC). Oral Oncol. 2021, 122, 105546. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.G.; Somani, R.R. Farnesyltransferase Inhibitor as Anticancer Agent. Mini Rev. Med. Chem. 2009, 9, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Whyte, D.B.; Kirschmeier, P.; Hockenberry, T.N.; Nunez-Oliva, I.; James, L.; Catino, J.J.; Bishop, W.R.; Pai, J.K. K- and N-Ras Are Geranylgeranylated in Cells Treated with Farnesyl Protein Transferase Inhibitors. J. Biol. Chem. 1997, 272, 14459–14464. [Google Scholar] [CrossRef]
- Bullock, K.; Blackwell, K. Clinical Efficacy of Taxane–Trastuzumab Combination Regimens for HER-2–Positive Metastatic Breast Cancer. Oncologist 2008, 13, 515–525. [Google Scholar] [CrossRef]
- De Block, K.; Vander Poorten, V.; Dormaar, T.; Nuyts, S.; Hauben, E.; Floris, G.; Deroose, C.M.; Schöffski, P.; Clement, P.M. Metastatic HER-2-Positive Salivary Gland Carcinoma Treated with Trastuzumab and a Taxane: A Series of Six Patients. Acta Clin. Belgica Int. J. Clin. Lab. Med. 2016, 71, 383–388. [Google Scholar] [CrossRef]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II Trial of Trastuzumab and Docetaxel in Patients with Human Epidermal Growth Factor Receptor 2-Positive Salivary Duct Carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef]
- Margalit, D.N.; Haddad, R.I.; Tishler, R.B.; Chau, N.G.; Schoenfeld, J.D.; Bakst, R.L.; Misiukiewicz, K.J.; Gupta, V.; Posner, M.; Hanna, G.J.; et al. A Phase 1 Study of Afatinib in Combination with Postoperative Radiation Therapy with and Without Weekly Docetaxel in Intermediate- and High-Risk Patients with Resected Squamous Cell Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 132–139. [Google Scholar] [CrossRef] [PubMed]
- del Barco Morillo, E.; Mesía, R.; Adansa Klain, J.C.; Vázquez Fernández, S.; Martínez-Galán, J.; Pastor Borgoñon, M.; González-Rivas, C.; Caballero Daroqui, J.; Berrocal, A.; Martínez-Trufero, J.; et al. Phase II Study of Panitumumab and Paclitaxel as First-Line Treatment in Recurrent or Metastatic Head and Neck Cancer. TTCC-2009-03/VECTITAX Study. Oral Oncol. 2016, 62, 54–59. [Google Scholar] [CrossRef]
- Martínez-Trufero, J.; Lozano Borbalas, A.; Pajares Bernad, I.; Taberna Sanz, M.; Ortega Izquierdo, E.; Cirauqui Cirauqui, B.; Rubió-Casadevall, J.; Plana Serrahima, M.; Ponce Ortega, J.M.; Planas Toledano, I.; et al. Sequential Chemotherapy Regimen of Induction with Panitumumab and Paclitaxel Followed by Radiotherapy and Panitumumab in Patients with Locally Advanced Head and Neck Cancer Unfit for Platinum Derivatives. The Phase II, PANTERA/TTCC-2010-06 Study. Clin. Transl. Oncol. 2021, 23, 1666–1677. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.-R.; Cupissol, D.; et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.M.H.; Lu, H.J.; Wang, L.W.; Tai, S.K.; Chen, M.H.; Chu, P.Y.; Yang, M.H. Effectiveness of Incorporating Cetuximab into Docetaxel/Cisplatin/Fluorouracil Induction Chemotherapy and Chemoradiotherapy for Inoperable Squamous Cell Carcinoma of the Oral Cavity: A Phase II Study. Head Neck 2017, 39, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Adkins, D.; Ley, J.; Oppelt, P.; Wildes, T.M.; Gay, H.A.; Daly, M.; Rich, J.; Paniello, R.C.; Jackson, R.; Pipkorn, P.; et al. Nab-Paclitaxel-Based Induction Chemotherapy with or without Cetuximab for Locally Advanced Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2017, 72, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hitt, R.; Irigoyen, A.; Cortes-Funes, H.; Grau, J.J.; García-Sáenz, J.A.; Cruz-Hernandez, J.J. Phase II Study of the Combination of Cetuximab and Weekly Paclitaxel in the First-Line Treatment of Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of Head and Neck. Ann. Oncol. 2012, 23, 1016–1022. [Google Scholar] [CrossRef]
- Tahara, M.; Kiyota, N.; Yokota, T.; Hasegawa, Y.; Muro, K.; Takahashi, S.; Onoe, T.; Homma, A.; Taguchi, J.; Suzuki, M.; et al. Phase II Trial of Combination Treatment with Paclitaxel, Carboplatin and Cetuximab (PCE) as First-Line Treatment in Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck (CSPOR-HN02). Ann. Oncol. 2018, 29, 1004–1009. [Google Scholar] [CrossRef]
- Enokida, T.; Ogawa, T.; Homma, A.; Okami, K.; Minami, S.; Nakanome, A.; Shimizu, Y.; Maki, D.; Ueda, Y.; Fujisawa, T.; et al. A Multicenter Phase II Trial of Paclitaxel, Carboplatin, and Cetuximab Followed by Chemoradiotherapy in Patients with Unresectable Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Cancer Med. 2020, 9, 1671–1682. [Google Scholar] [CrossRef]
- William, W.N.; Tsao, A.S.; Feng, L.; Ginsberg, L.E.; Lee, J.J.; Kies, M.S.; Glisson, B.S.; Kim, E.S. Single Arm, Phase II Study of Cisplatin, Docetaxel, and Erlotinib in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinomas. Oncologist 2018, 23, 526-e49. [Google Scholar] [CrossRef]
- Bossi, P.; Miceli, R.; Locati, L.D.; Ferrari, D.; Vecchio, S.; Moretti, G.; Denaro, N.; Caponigro, F.; Airoldi, M.; Moro, C.; et al. A Randomized, Phase 2 Study of Cetuximab plus Cisplatin with or without Paclitaxel for the First-Line Treatment of Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck. Ann. Oncol. 2017, 28, 2820–2826. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dou, H.; Li, Q.; Sun, Y.; Wang, Y.; Zhang, W. Efficacy and Safety of Cetuximab Plus Cisplatin Alone or in Combination With Paclitaxel in Patients With Head and Neck Squamous Cell Carcinoma: A Randomized Trial. Cancer Control 2021, 28, 1073274821997444. [Google Scholar] [CrossRef]
- Khalil, A.; Jameson, M.J. The EGFR Inhibitor Gefitinib Enhanced the Response of Human Oral Squamous Cell Carcinoma to Cisplatin In Vitro. Drugs R D 2017, 17, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Guigay, J.; Aupérin, A.; Fayette, J.; Saada-Bouzid, E.; Lafond, C.; Taberna, M.; Geoffrois, L.; Martin, L.; Capitain, O.; Cupissol, D.; et al. Cetuximab, Docetaxel, and Cisplatin versus Platinum, Fluorouracil, and Cetuximab as First-Line Treatment in Patients with Recurrent or Metastatic Head and Neck Squamous-Cell Carcinoma (GORTEC 2014-01 TPExtreme): A Multicentre, Open-Label, Randomised, Phas. Lancet Oncol. 2021, 22, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Ham, J.C.; van Meerten, E.; Fiets, W.E.; Beerepoot, L.V.; Jeurissen, F.J.F.; Slingerland, M.; Jonker, M.A.; Husson, O.; van der Graaf, W.T.A.; van Herpen, C.M.L. Methotrexate plus or Minus Cetuximab as First-Line Treatment in a Recurrent or Metastatic (R/M) Squamous Cell Carcinoma Population of the Head and Neck (SCCHN), Unfit for Cisplatin Combination Treatment, a Phase Ib-Randomized Phase II Study Commence. Head Neck 2020, 42, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Sukari, A.; Nagasaka, M.; Diab, M.; Al Sibai, K.; Atassi, B.; Elayoubi, J.A.; Kim, S.; Küçük, Ö. Cetuximab and Methotrexate in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma—A Single Institution Analysis of 54 Patients. Clin. Otolaryngol. 2019, 44, 639–643. [Google Scholar] [CrossRef]
- Prawira, A.; Brana-Garcia, I.; Spreafico, A.; Hope, A.; Waldron, J.; Razak, A.R.A.; Chen, E.X.; Jang, R.; O’Sullivan, B.; Giuliani, M.; et al. Phase I Trial of Dacomitinib, a Pan-Human Epidermal Growth Factor Receptor (HER) Inhibitor, with Concurrent Radiotherapy and Cisplatin in Patients with Locoregionally Advanced Squamous Cell Carcinoma of the Head and Neck (XDC-001). Investig. New Drugs 2016, 34, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Papadimitrakopoulou, V.A.; Frank, S.J.; Cohen, E.W.; Hirsch, F.R.; Myers, J.N.; Heymach, J.V.; Lin, H.; Tran, H.T.; Chen, C.R.; Jimeno, A.; et al. Phase I Study of Vandetanib with Radiation Therapy with or without Cisplatin in Locally Advanced Head and Neck Squamous Cell Carcinoma. Head Neck 2016, 38, 439–447. [Google Scholar] [CrossRef]
- Patil, V.M.; Noronha, V.; Joshi, A.; Agarwal, J.; Ghosh-Laskar, S.; Budrukkar, A.; Murthy, V.; Gupta, T.; Mahimkar, M.; Juvekar, S.; et al. A Randomized Phase 3 Trial Comparing Nimotuzumab Plus Cisplatin Chemoradiotherapy Versus Cisplatin Chemoradiotherapy Alone in Locally Advanced Head and Neck Cancer. Cancer 2019, 125, 3184–3197. [Google Scholar] [CrossRef]
- Jie, H.-B.; Schuler, P.J.; Lee, S.C.; Srivastava, R.M.; Argiris, A.; Ferrone, S.; Whiteside, T.L.; Ferris, R.L. CTLA-4+ Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015, 75, 2200–2210. [Google Scholar] [CrossRef]
- Karagiannis, T.C.; El-Osta, A. Modulation of Cellular Radiation Responses by Histone Deacetylase Inhibitors. Oncogene 2006, 25, 3885–3893. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.J.; Wirth, L.J.; Colevas, A.D.; Gilbert, J.; Bauman, J.E.; Saba, N.F.; Raben, D.; Mehra, R.; Ma, A.W.; Atoyan, R.; et al. A Phase I Study of CUDC-101, a Multitarget Inhibitor of HDACs, EGFR, and HER2, in Combination with Chemoradiation in Patients with Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015, 21, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Schoenwaelder, N.; Salewski, I.; Engel, N.; Krause, M.; Schneider, B.; Müller, M.; Riess, C.; Lemcke, H.; Skorska, A.; Grosse-Thie, C.; et al. The Individual Effects of Cyclin-Dependent Kinase Inhibitors on Head and Neck Cancer Cells—A Systematic Analysis. Cancers 2021, 13, 2396. [Google Scholar] [CrossRef]
- Chaudhary, S.; Pothuraju, R.; Rachagani, S.; Siddiqui, J.A.; Atri, P.; Mallya, K.; Nasser, M.W.; Sayed, Z.; Lyden, E.R.; Smith, L.; et al. Dual Blockade of EGFR and CDK4/6 Delays Head and Neck Squamous Cell Carcinoma Progression by Inducing Metabolic Rewiring. Cancer Lett. 2021, 510, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Cai, X.; Yao, Z.; Huang, J. Progress in Targeted Therapeutic Drugs for Oral Squamous Cell Carcinoma. Surg. Oncol. 2019, 31, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Cyclin-Dependent Kinases. Genome Biol. 2014, 15, 122. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Zangardi, M.L.; Moy, B.; Bardia, A. Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations. Oncologist 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- van Caloen, G.; Schmitz, S.; van Marcke, C.; Caignet, X.; Mendola, A.; Ruys, S.P.D.; Roger, P.P.; Vertommen, D.; Machiels, J.P. Preclinical Evaluation of the Association of the Cyclin-Dependent Kinase 4/6 Inhibitor, Ribociclib, and Cetuximab in Squamous Cell Carcinoma of the Head and Neck. Cancers 2021, 13, 1251. [Google Scholar] [CrossRef]
- van Caloen, G.; Schmitz, S.; El Baroudi, M.; Caignet, X.; Pyr dit Ruys, S.; Roger, P.P.; Vertommen, D.; Machiels, J.P. Preclinical Activity of Ribociclib in Squamous Cell Carcinoma of the Head and Neck. Mol. Cancer Ther. 2020, 19, 777–789. [Google Scholar] [CrossRef]
- Seront, E.; Schmitz, S.; Papier, M.; Van Maanen, A.; Henry, S.; Lonchay, C.; Rottey, S.; Van Caloen, G.; Machiels, J.P. Phase 1 Study Evaluating the Association of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib and Cetuximab in Recurrent/Metastatic P16-Negative Squamous Cell Carcinoma of the Head and Neck. Front. Oncol. 2019, 9, 155. [Google Scholar] [CrossRef]
- Michel, L.; Ley, J.; Wildes, T.M.; Schaffer, A.; Robinson, A.; Chun, S.-E.; Lee, W.; Lewis, J.; Trinkaus, K.; Adkins, D. Phase I Trial of Palbociclib, a Selective Cyclin Dependent Kinase 4/6 Inhibitor, in Combination with Cetuximab in Patients with Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2016, 58, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Adkins, D.R.; Lin, J.C.; Sacco, A.; Ley, J.; Oppelt, P.; Vanchenko, V.; Komashko, N.; Yen, C.J.; Wise-Draper, T.; Lopez-Picazo Gonzalez, J.; et al. Palbociclib and Cetuximab Compared with Placebo and Cetuximab in Platinum-Resistant, Cetuximab-Naïve, Human Papillomavirus-Unrelated Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Double-Blind, Randomized, Phase 2 Trial. Oral Oncol. 2021, 115, 105192. [Google Scholar] [CrossRef]
- Oppelt, P.; Ley, J.C.; Worden, F.; Palka, K.; Maggiore, R.; Liu, J.; Adkins, D. Palbociclib and Cetuximab in Cetuximab-Resistant Human Papillomavirus-Related Oropharynx Squamous-Cell Carcinoma: A Multicenter Phase 2 Trial. Oral Oncol. 2021, 114, 105164. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Lui, V.W.Y.; Li, Y.; Jia, L.; You, C.; Li, X.; Piao, W.; Yuan, H.; Khong, P.L.; Lo, K.W.; et al. Therapeutic Evaluation of Palbociclib and Its Compatibility with Other Chemotherapies for Primary and Recurrent Nasopharyngeal Carcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 262. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Swiecicki, P.L.; Durm, G.; Bellile, E.; Bhangale, A.; Brenner, J.C.; Worden, F.P. A Multi-Center Phase II Trial Evaluating the Efficacy of Palbociclib in Combination with Carboplatin for the Treatment of Unresectable Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Investig. New Drugs 2020, 38, 1550–1558. [Google Scholar] [CrossRef]
- Robinson, A.M.; Rathore, R.; Redlich, N.J.; Adkins, D.R.; VanArsdale, T.; Van Tine, B.A.; Michel, L.S. Cisplatin Exposure Causes C-Myc-Dependent Resistance to CDK4/6 Inhibition in HPV-Negative Head and Neck Squamous Cell Carcinoma. Cell Death Dis. 2019, 10, 867. [Google Scholar] [CrossRef]
- Taylor-Harding, B.; Aspuria, P.J.; Agadjanian, H.; Cheon, D.J.; Mizuno, T.; Greenberg, D.; Allen, J.R.; Spurka, L.; Funari, V.; Spiteri, E.; et al. Cyclin E1 and RTK/RAS Signaling Drive CDK Inhibitor Resistance via Activation of E2F and ETS. Oncotarget 2015, 6, 696–714. [Google Scholar] [CrossRef]
- Zainal, N.S.; Lee, B.K.B.; Wong, Z.W.; Chin, I.S.; Yee, P.S.; Gan, C.P.; Mun, K.S.; Rahman, Z.A.A.; Silvio Gutkind, J.; Patel, V.; et al. Effects of Palbociclib in Oral Squamous Cell Carcinoma and the Role of PIK3CA in Conferring Resistance. Cancer Biol. Med. 2019, 16, 264–275. [Google Scholar] [CrossRef]
- Kostopoulou, O.N.; Zupancic, M.; Pont, M.; Papin, E.; Lukoseviciute, M.; Mikelarena, B.A.; Holzhauser, S.; Dalianis, T. Targeted Therapy of HPV Positive and Negative Tonsillar Squamous Cell Carcinoma Cell Lines Reveals Synergy between CDK4/6, PI3K and Sometimes FGFR Inhibitors, but Rarely between PARP and WEE1 Inhibitors. Viruses 2022, 14, 1372. [Google Scholar] [CrossRef]
- Holzhauser, S.; Wild, N.; Zupancic, M.; Ursu, R.G.; Bersani, C.; Näsman, A.; Kostopoulou, O.N.; Dalianis, T. Targeted Therapy With PI3K and FGFR Inhibitors on Human Papillomavirus Positive and Negative Tonsillar and Base of Tongue Cancer Lines With and Without Corresponding Mutations. Front. Oncol. 2021, 11, 1723. [Google Scholar] [CrossRef]
- Weiss, J.M.; Csoszi, T.; Maglakelidze, M.; Hoyer, R.J.; Beck, J.T.; Domine Gomez, M.; Lowczak, A.; Aljumaily, R.; Rocha Lima, C.M.; Boccia, R.V.; et al. Myelopreservation with the CDK4/6 Inhibitor Trilaciclib in Patients with Small-Cell Lung Cancer Receiving First-Line Chemotherapy: A Phase Ib/Randomized Phase II Trial. Ann. Oncol. 2019, 30, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, N.; Chavez, C.G.; Li, D.; Mehta, V.; Thomas, C.; Fulcher, C.D.; Kawachi, N.; Bottalico, D.M.; Prystowsky, M.B.; Basu, I.; et al. CDK4/6 Inhibition Induces Senescence and Enhances Radiation Response by Disabling DNA Damage Repair in Oral Cavity Squamous Cell Carcinoma. Cancers 2023, 15, 2005. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Q.; Huang, P.; Tong, D.; Wu, H.; Zhang, F. EGFR-Mediated Signaling Pathway Influences the Sensitivity of Oral Squamous Cell Carcinoma to JQ1. J. Cell. Biochem. 2018, 119, 8368–8377. [Google Scholar] [CrossRef] [PubMed]
- Hajmirza, A.; Emadali, A.; Gauthier, A.; Casasnovas, O.; Gressin, R.; Callanan, M. BET Family Protein BRD4: An Emerging Actor in NFκB Signaling in Inflammation and Cancer. Biomedicines 2018, 6, 16. [Google Scholar] [CrossRef]
- Donati, B.; Lorenzini, E.; Ciarrocchi, A. BRD4 and Cancer: Going beyond Transcriptional Regulation. Mol. Cancer 2018, 17, 164. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Huang, P.; Zhang, F. JQ1 and PI3K Inhibition Synergistically Reduce Salivary Adenoid Cystic Carcinoma Malignancy by Targeting the C-Myc and EGFR Signaling Pathways. J. Oral Pathol. Med. 2019, 48, 43–51. [Google Scholar] [CrossRef]
- Xu, B.; Lefringhouse, J.; Liu, Z.; West, D.; Baldwin, L.A.; Ou, C.; Chen, L.; Napier, D.; Chaiswing, L.; Brewer, L.D.; et al. Inhibition of the Integrin/FAK Signaling Axis and c-Myc Synergistically Disrupts Ovarian Cancer Malignancy. Oncogenesis 2017, 6, e295. [Google Scholar] [CrossRef] [PubMed]
- Rathert, P.; Roth, M.; Neumann, T.; Muerdter, F.; Roe, J.S.; Muhar, M.; Deswal, S.; Cerny-Reiterer, S.; Peter, B.; Jude, J.; et al. Transcriptional Plasticity Promotes Primary and Acquired Resistance to BET Inhibition. Nature 2015, 525, 543–547. [Google Scholar] [CrossRef]
- Iniguez, A.B.; Alexe, G.; Wang, E.J.; Roti, G.; Patel, S.; Chen, L.; Kitara, S.; Conway, A.; Robichaud, A.L.; Stolte, B.; et al. Resistance to Epigenetic-Targeted Therapy Engenders Tumor Cell Vulnerabilities Associated with Enhancer Remodeling. Cancer Cell 2018, 34, 922–938.e7. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, H.; Jiang, Y.; Huang, R.; Wu, Y.; Wang, D.; Guo, S.; Li, S.; Wang, Y.; Jiang, H.; et al. Combinational Therapeutic Targeting of BRD4 and CDK7 Synergistically Induces Anticancer Effects in Head and Neck Squamous Cell Carcinoma. Cancer Lett. 2020, 469, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, S.; Sakashita, A.; Yukawa, M.; Chen, X.; Takahashi, K.; Alavattam, K.G.; Nakata, I.; Weirauch, M.T.; Barski, A.; Namekawa, S.H. Super-Enhancer Switching Drives a Burst in Gene Expression at the Mitosis-to-Meiosis Transition. Nat. Struct. Mol. Biol. 2020, 27, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.Y.; Seo, Y.; Oh, S.J.; Ahn, J.S.; Song, M.; Kang, M.J.; Oh, J.M.; Lee, D.; Kim, Y.H.; Sung, E.S.; et al. Melatonin and Verteporfin Synergistically Suppress the Growth and Stemness of Head and Neck Squamous Cell Carcinoma through the Regulation of Mitochondrial Dynamics. J. Pineal Res. 2022, 72, e12779. [Google Scholar] [CrossRef]
- Penke, L.R.; Speth, J.M.; Dommeti, V.L.; White, E.S.; Bergin, I.L.; Peters-Golden, M. FOXM1 Is a Critical Driver of Lung Fibroblast Activation and Fibrogenesis. J. Clin. Investig. 2018, 128, 2389–2405. [Google Scholar] [CrossRef]
- Wang, W.; Tan, J. Co-Inhibition of BET Proteins and PD-L1 as a Potential Therapy for OSCC through Synergistic Inhibition of FOXM1 and PD-L1 Expressions. J. Oral Pathol. Med. 2019, 48, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, H.M. Forkhead Box M1 Transcription Factor: A Novel Target for Pulmonary Arterial Hypertension Therapy. World J. Pediatr. 2020, 16, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, F.; Kong, L.; Yu, J. PD-L1 Expression in Human Cancers and Its Association with Clinical Outcomes. Onco. Targets. Ther. 2016, 9, 5023–5039. [Google Scholar] [CrossRef]
- Li, B.; Jin, J.; Guo, D.; Tao, Z.; Hu, X. Immune Checkpoint Inhibitors Combined with Targeted Therapy: The Recent Advances and Future Potentials. Cancers 2023, 15, 2858. [Google Scholar] [CrossRef]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors: Recent Progress and Potential Biomarkers. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Oliva, M.; Chepeha, D.; Araujo, D.V.; Diaz-Mejia, J.J.; Olson, P.; Prawira, A.; Spreafico, A.; Bratman, S.V.; Shek, T.; De Almeida, J.; et al. Antitumor Immune Effects of Preoperative Sitravatinib and Nivolumab in Oral Cavity Cancer: SNOW Window-of-Opportunity Study. J. Immunother. Cancer 2021, 9, e003476. [Google Scholar] [CrossRef]
- Aggarwal, C.; Prawira, A.; Antonia, S.; Rahma, O.; Tolcher, A.; Cohen, R.B.; Lou, Y.; Hauke, R.; Vogelzang, N.; Zandberg, D.P.; et al. Dual Checkpoint Targeting of B7-H3 and PD-1 with Enoblituzumab and Pembrolizumab in Advanced Solid Tumors: Interim Results from a Multicenter Phase I/II Trial. J. Immunother. Cancer 2022, 10, e004424. [Google Scholar] [CrossRef] [PubMed]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.-J.; Hanna, E.; et al. Neoadjuvant Immune Checkpoint Blockade in High-Risk Resectable Melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Hanna, G.J.; Jo, V.Y.; Rawal, B.; Chen, Y.H.; Catalano, P.S.; Lako, A.; Ciantra, Z.; Weirather, J.L.; Criscitiello, S.; et al. Neoadjuvant Nivolumab or Nivolumab plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.I.; Harrington, K.; Tahara, M.; Ferris, R.L.; Gillison, M.; Fayette, J.; Daste, A.; Koralewski, P.; Zurawski, B.; Taberna, M.; et al. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J. Clin. Oncol. 2023, 41, 2166–2180. [Google Scholar] [CrossRef]
- Psyrri, A.; Fayette, J.; Harrington, K.; Gillison, M.; Ahn, M.-J.; Takahashi, S.; Weiss, J.; Machiels, J.-P.; Baxi, S.; Vasilyev, A.; et al. Durvalumab with or without Tremelimumab versus the EXTREME Regimen as First-Line Treatment for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: KESTREL, a Randomized, Open-Label, Phase III Study. Ann. Oncol. 2023, 34, 262–274. [Google Scholar] [CrossRef]
- Ferris, R.L.; Haddad, R.; Even, C.; Tahara, M.; Dvorkin, M.; Ciuleanu, T.E.; Clement, P.M.; Mesia, R.; Kutukova, S.; Zholudeva, L.; et al. Durvalumab with or without Tremelimumab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: EAGLE, a Randomized, Open-Label Phase III Study. Ann. Oncol. 2020, 31, 942–950. [Google Scholar] [CrossRef]
- Gunderson, A.J.; Kaneda, M.M.; Tsujikawa, T.; Nguyen, A.V.; Affara, N.I.; Ruffell, B.; Gorjestani, S.; Liudahl, S.M.; Truitt, M.; Olson, P.; et al. Bruton Tyrosine Kinase–Dependent Immune Cell Cross-Talk Drives Pancreas Cancer. Cancer Discov. 2016, 6, 270–285. [Google Scholar] [CrossRef]
- Taylor, M.H.; Betts, C.B.; Maloney, L.; Nadler, E.; Algazi, A.; Guarino, M.J.; Nemunaitis, J.; Jimeno, A.; Patel, P.; Munugalavadla, V.; et al. Safety and Efficacy of Pembrolizumab in Combination with Acalabrutinib in Advanced Head and Neck Squamous Cell Carcinoma: Phase 2 Proof-of-Concept Study. Clin. Cancer Res. 2022, 28, 903–914. [Google Scholar] [CrossRef]
- Suek, N.; Campesato, L.F.; Merghoub, T.; Khalil, D.N. Targeted APC Activation in Cancer Immunotherapy to Enhance the Abscopal Effect. Front. Immunol. 2019, 10, 604. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Nabell, L.; Wong, D.J.; Day, T.; Daniels, G.A.; Milhem, M.; Deva, S.; Jameson, M.; Guntinas-Lichius, O.; Almubarak, M.; et al. Intralesional SD-101 in Combination with Pembrolizumab in Anti-PD-1 Treatment-Naïve Head and Neck Squamous Cell Carcinoma: Results from a Multicenter, Phase II Trial. Clin. Cancer Res. 2022, 28, 1157–1166. [Google Scholar] [CrossRef]
- Pockley, A.G.; Vaupel, P.; Multhoff, G. NK Cell-Based Therapeutics for Lung Cancer. Expert Opin. Biol. Ther. 2020, 20, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hanna, G.J.; O’Neill, A.; Shin, K.-Y.; Wong, K.; Jo, V.Y.; Quinn, C.T.; Cutler, J.M.; Flynn, M.; Lizotte, P.H.; Annino, D.J.; et al. Neoadjuvant and Adjuvant Nivolumab and Lirilumab in Patients with Recurrent, Resectable Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2022, 28, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Xia, R.; Zhu, D.; Dou, S.; Zhu, G.; Dong, M.; Wang, L.; Sun, Q.; Zhao, T.; Zhou, Z.; et al. A Pilot Study of Neoadjuvant Combination of Anti-PD-1 Camrelizumab and VEGFR2 Inhibitor Apatinib for Locally Advanced Resectable Oral Squamous Cell Carcinoma. Nat. Commun. 2022, 13, 5378. [Google Scholar] [CrossRef]
- Taylor, M.H.; Lee, C.-H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef]
- Catalano, S.; Panza, S.; Augimeri, G.; Giordano, C.; Malivindi, R.; Gelsomino, L.; Marsico, S.; Győrffy, B.; Bonofiglio, D.; Andò, S.; et al. Phosphodiesterase 5 (PDE5) Is Highly Expressed in Cancer-Associated Fibroblasts and Enhances Breast Tumor Progression. Cancers 2019, 11, 1740. [Google Scholar] [CrossRef]
- Catalano, S.; Campana, A.; Giordano, C.; Győrffy, B.; Tarallo, R.; Rinaldi, A.; Bruno, G.; Ferraro, A.; Romeo, F.; Lanzino, M.; et al. Expression and Function of Phosphodiesterase Type 5 in Human Breast Cancer Cell Lines and Tissues: Implications for Targeted Therapy. Clin. Cancer Res. 2016, 22, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Sundquist, J.; Sundquist, K.; Ji, J. Phosphodiesterase-5 Inhibitors Use and Risk for Mortality and Metastases among Male Patients with Colorectal Cancer. Nat. Commun. 2020, 11, 3191. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.-L.; Yang, Y.; Zhang, Y.-J.; Li, Y.; Zhao, J.-M.; Qiu, J.-G.; Zhang, W.-J.; Jiang, Q.-W.; Xue, Y.-Q.; Zheng, D.-W.; et al. Sildenafil Inhibits the Growth of Human Colorectal Cancer in Vitro and in Vivo. Am. J. Cancer Res. 2015, 5, 3311–3324. [Google Scholar]
- Luginbuhl, A.J.; Johnson, J.M.; Harshyne, L.A.; Linnenbach, A.J.; Shukla, S.K.; Alnemri, A.; Kumar, G.; Cognetti, D.M.; Curry, J.M.; Kotlov, N.; et al. Tadalafil Enhances Immune Signatures in Response to Neoadjuvant Nivolumab in Resectable Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2022, 28, 915–927. [Google Scholar] [CrossRef]
- Fuereder, T.; Minichsdorfer, C.; Mittlboeck, M.; Wagner, C.; Heller, G.; Putz, E.M.; Oberndorfer, F.; Müllauer, L.; Aretin, M.-B.; Czerny, C.; et al. Pembrolizumab plus Docetaxel for the Treatment of Recurrent/Metastatic Head and Neck Cancer: A Prospective Phase I/II Study. Oral Oncol. 2022, 124, 105634. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, B.; Peng, G.; Xiao, G.; Huang, J.; Ding, Q.; Yang, C.; Xiong, X.; Ma, H.; Shi, L.; et al. Neoadjuvant Chemoimmunotherapy for the Treatment of Locally Advanced Head and Neck Squamous Cell Carcinoma: A Single-Arm Phase 2 Clinical Trial. Clin. Cancer Res. 2022, 28, 3268–3276. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, Q.; Du, W.; Zhang, X.; Dai, L.; Qiao, Y. Induction Chemotherapy Combined with Immunotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma. BMC Cancer 2021, 21, 622. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Q.; Zhong, G.; Peng, Y.; Liu, Y.; Liang, L.; Hong, H.; Feng, W.; Yang, S.; Zhang, Y.; et al. Neoadjuvant Toripalimab Combined with Gemcitabine and Cisplatin in Resectable Locally Advanced Head and Neck Squamous Cell Carcinoma (NeoTGP01): An Open Label, Single-Arm, Phase Ib Clinical Trial. J. Exp. Clin. Cancer Res. 2022, 41, 300. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Burtness, B.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Brana, I.; Basté, N.; Neupane, P.; et al. Pembrolizumab With or Without Chemotherapy in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: Updated Results of the Phase III KEYNOTE-048 Study. J. Clin. Oncol. 2023, 41, 790–802. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Psyrri, A.; Haddad, R.I.; Tahara, M.; Bourhis, J.; Harrington, K.; Chang, P.M.H.; Lin, J.C.; Razaq, M.A.; et al. Avelumab plus Standard-of-Care Chemoradiotherapy versus Chemoradiotherapy Alone in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomised, Double-Blind, Placebo-Controlled, Multicentre, Phase 3 Trial. Lancet Oncol. 2021, 22, 450–462. [Google Scholar] [CrossRef]
- Bourhis, J.; Tao, Y.; Sun, X.; Sire, C.; Martin, L.; Liem, X.; Coutte, A.; Pointreau, Y.; Thariat, J.; Miroir, J.; et al. LBA35 Avelumab-Cetuximab-Radiotherapy versus Standards of Care in Patients with Locally Advanced Squamous Cell Carcinoma of Head and Neck (LA-SCCHN): Randomized Phase III GORTEC-REACH Trial. Ann. Oncol. 2021, 32, S1310. [Google Scholar] [CrossRef]
- Chung, C.H.; Li, J.; Steuer, C.E.; Bhateja, P.; Johnson, M.; Masannat, J.; Poole, M.I.; Song, F.; Hernandez-Prera, J.C.; Molina, H.; et al. Phase II Multi-Institutional Clinical Trial Result of Concurrent Cetuximab and Nivolumab in Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2022, 28, 2329–2338. [Google Scholar] [CrossRef]
- Dennis, M.J.; Sacco, A.G.; Qi, Y.; Bykowski, J.; Pittman, E.; Chen, R.; Messer, K.; Cohen, E.E.W.; Gold, K.A. A Phase I Study of Avelumab, Palbociclib, and Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2022, 135, 106219. [Google Scholar] [CrossRef]
- Sacco, A.G.; Chen, R.; Worden, F.P.; Wong, D.J.L.; Adkins, D.; Swiecicki, P.; Chai-Ho, W.; Oppelt, P.; Ghosh, D.; Bykowski, J.; et al. Pembrolizumab plus Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: An Open-Label, Multi-Arm, Non-Randomised, Multicentre, Phase 2 Trial. Lancet Oncol. 2021, 22, 883–892. [Google Scholar] [CrossRef]
- Kao, H.; Liao, B.; Huang, Y.; Huang, H.; Chen, C.; Chen, T.; Hong, Y.; Chan, C.; Chia, J.; Hong, R. Afatinib and Pembrolizumab for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (ALPHA Study): A Phase II Study with Biomarker Analysis. Clin. Cancer Res. 2022, 28, 1560–1571. [Google Scholar] [CrossRef]
- Chung, C.H.; Bonomi, M.; Steuer, C.E.; Li, J.; Bhateja, P.; Johnson, M.; Masannat, J.; Song, F.; Hernandez-prera, J.C.; Wenig, B.M.; et al. Concurrent Cetuximab and Nivolumab as a Second-Line or beyond Treatment of Patients with Recurrent and / or Metastatic Head and Neck Squamous Cell Carcinoma: Results of Phase I / II Study. Cancers 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.P.; Wu, Q.; Voutsinas, J.; Fromm, J.R.; Jiang, X.; Pillarisetty, V.G.; Lee, S.M.; Santana-Davila, R.; Goulart, B.; Baik, C.S.; et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. 2020, 26, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; Wu, C.Y.; Chin, Y.T.; Li, Z.L.; Pan, Y.S.; Huang, T.Y.; Su, P.Y.; Lee, S.Y.; Crawford, D.R.; Su, K.W.; et al. NDAT Suppresses Pro-Inflammatory Gene Expression to Enhance Resveratrol-Induced Anti-Proliferation in Oral Cancer Cells. Food Chem. Toxicol. 2020, 136, 111092. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Kim, S.-S.; Park, S.; Kim, C.; Heo, S.J.; Lim, J.S.; Kim, H.; Kim, H.S.; Rha, S.Y.; Chung, H.C.; et al. Depth of Response Is a Significant Predictor for Long-Term Outcome in Advanced Gastric Cancer Patients Treated with Trastuzumab. Oncotarget 2017, 8, 31169–31179. [Google Scholar] [CrossRef] [PubMed]
- Recouvreux, M.V.; Commisso, C. Macropinocytosis: A Metabolic Adaptation to Nutrient Stress in Cancer. Front. Endocrinol. 2017, 8, 261. [Google Scholar] [CrossRef]
- Chiang, I.-T.; Lee, Y.-H.; Tan, Z.-L.; Hsu, F.-T.; Tu, H.-F. Regorafenib Enhances Antitumor Immune Efficacy of Anti-PD-L1 Immunotherapy on Oral Squamous Cell Carcinoma. Biomed. Pharmacother. 2022, 147, 112661. [Google Scholar] [CrossRef]
- Sherman, E.; Lee, J.L.; Debruyne, P.R.; Keam, B.; Shin, S.J.; Gramza, A.; Caro, I.; Amin, R.; Shah, K.; Yan, Y.; et al. Safety and Efficacy of Cobimetinib plus Atezolizumab in Patients with Solid Tumors: A Phase II, Open-Label, Multicenter, Multicohort Study. ESMO Open 2023, 8, 100877. [Google Scholar] [CrossRef] [PubMed]
- Spahn, J.; Peng, J.; Lorenzana, E.; Kan, D.; Hunsaker, T.; Segal, E.; Mautino, M.; Brincks, E.; Pirzkall, A.; Kelley, S.; et al. Improved Anti-Tumor Immunity and Efficacy upon Combination of the IDO1 Inhibitor GDC-0919 with Anti-PD-L1 Blockade versus Anti-PD-L1 Alone in Preclinical Tumor Models. J. Immunother. Cancer 2015, 3, P303. [Google Scholar] [CrossRef]
- Prendergast, G.C.; Malachowski, W.J.; Mondal, A.; Scherle, P.; Muller, A.J. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 336, pp. 175–203. ISBN 9780128146514. [Google Scholar]
- Jung, K.H.; LoRusso, P.; Burris, H.; Gordon, M.; Bang, Y.-J.; Hellmann, M.D.; Cervantes, A.; de Olza, M.O.; Marabelle, A.; Hodi, F.S.; et al. Phase I Study of the Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Navoximod (GDC-0919) Administered with PD-L1 Inhibitor (Atezolizumab) in Advanced Solid Tumors. Clin. Cancer Res. 2019, 25, 3220–3228. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.J.; Jason, J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results From a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018, 36, 3223–3230. [Google Scholar] [CrossRef]
- Čermák, V.; Dostál, V.; Jelínek, M.; Libusová, L.; Kovář, J.; Rösel, D.; Brábek, J. Microtubule-Targeting Agents and Their Impact on Cancer Treatment. Eur. J. Cell Biol. 2020, 99, 151075. [Google Scholar] [CrossRef]
- Thompson, K.N.; Ju, J.A.; Ory, E.C.; Pratt, S.J.P.; Lee, R.M.; Mathias, T.J.; Chang, K.T.; Lee, C.J.; Goloubeva, O.G.; Bailey, P.C.; et al. Microtubule Disruption Reduces Metastasis More Effectively than Primary Tumor Growth. Breast Cancer Res. 2022, 24, 13. [Google Scholar] [CrossRef]
- Van Vuuren, R.J.; Visagie, M.H.; Theron, A.E.; Joubert, A.M. Antimitotic Drugs in the Treatment of Cancer. Cancer Chemother. Pharmacol. 2015, 76, 1101–1112. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-Binding Agents: A Dynamic Field of Cancer Therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Novais, P.; Silva, P.M.A.; Amorim, I.; Bousbaa, H. Second-Generation Antimitotics in Cancer Clinical Trials. Pharmaceutics 2021, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hofmann, J.; Lu, Y.; Mills, G.B.; Jaffe, R.B. Inhibition of Phosphatidylinositol 3′-Kinase Increases Efficacy of Paclitaxel in in Vitro and in Vivo Ovarian Cancer Models. Cancer Res. 2002, 62, 1087–1092. [Google Scholar]
- Soulières, D.; Faivre, S.; Mesía, R.; Remenár, É.; Li, S.H.; Karpenko, A.; Dechaphunkul, A.; Ochsenreither, S.; Kiss, L.A.; Lin, J.C.; et al. Buparlisib and Paclitaxel in Patients with Platinum-Pretreated Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (BERIL-1): A Randomised, Double-Blind, Placebo-Controlled Phase 2 Trial. Lancet Oncol. 2017, 18, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, A.; Bauman, J.E.; Weissman, C.; Adkins, D.; Schnadig, I.; Beauregard, P.; Bowles, D.W.; Spira, A.; Levy, B.; Seetharamu, N.; et al. A Randomized, Phase 2 Trial of Docetaxel with or without PX-866, an Irreversible Oral Phosphatidylinositol 3-Kinase Inhibitor, in Patients with Relapsed or Metastatic Head and Neck Squamous Cell Cancer. Oral Oncol. 2015, 51, 383–388. [Google Scholar] [CrossRef]
- Malhotra, B.; Moon, J.; Kucuk, O.; Clark, J.I.; Urba, S.G.; Wolf, G.T.; Worden, F.P. Phase II Trial of Biweekly Gemcitabine and Paclitaxel with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Southwest Oncology Group Study S0329. Head Neck 2014, 36, 1712–1717. [Google Scholar] [CrossRef]
- Park, I.H.; Sohn, J.H.; Kim, S.B.; Lee, K.S.; Chung, J.S.; Lee, S.H.; Kim, T.Y.; Jung, K.H.; Cho, E.K.; Kim, Y.S.; et al. An Open-Label, Randomized, Parallel, Phase III Trial Evaluating the Efficacy and Safety of Polymeric Micelle-Formulated Paclitaxel Compared to Conventional Cremophor EL-Based Paclitaxel for Recurrent or Metastatic HER2-Negative Breast Cancer. Cancer Res. Treat. 2017, 49, 569–577. [Google Scholar] [CrossRef]
- Keam, B.; Lee, K.-W.; Lee, S.-H.; Kim, J.-S.; Kim, J.H.; Wu, H.-G.; Eom, K.-Y.; Kim, S.; Ahn, S.-H.; Chung, E.-J.; et al. A Phase II Study of Genexol-PM and Cisplatin as Induction Chemotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma. Oncologist 2019, 24, 751-e231. [Google Scholar] [CrossRef]
- Dunn, L.A.; Fury, M.G.; Xiao, H.; Baxi, S.S.; Sherman, E.J.; Korte, S.; Pfister, C.; Haque, S.; Katabi, N.; Ho, A.L.; et al. A Phase II Study of Temsirolimus Added to Low-Dose Weekly Carboplatin and Paclitaxel for Patients with Recurrent and/or Metastatic (R/M) Head and Neck Squamous Cell Carcinoma (HNSCC). Ann. Oncol. 2017, 28, 2533–2538. [Google Scholar] [CrossRef]
- Klinghammer, K.; Gauler, T.; Dietz, A.; Grünwald, V.; Stöhlmacher, J.; Knipping, S.; Schroeder, M.; Guntinas-Lichius, O.; Frickhofen, N.; Lindeman, H.W.; et al. Cetuximab, Fluorouracil and Cisplatin with or without Docetaxel for Patients with Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck (CeFCiD): An Open-Label Phase II Randomised Trial (AIO/IAG-KHT Trial 1108). Eur. J. Cancer 2019, 122, 53–60. [Google Scholar] [CrossRef]
- Méndez, E.; Rodriguez, C.P.; Kao, M.C.; Raju, S.; Diab, A.; Harbison, R.A.; Konnick, E.Q.; Mugundu, G.M.; Santana-Davila, R.; Martins, R.; et al. A Phase I Clinical Trial of AZD1775 in Combination with Neoadjuvant Weekly Docetaxel and Cisplatin before Definitive Therapy in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2018, 24, 2740–2748. [Google Scholar] [CrossRef] [PubMed]
- Leijen, S.; Van Geel, R.M.J.M.; Pavlick, A.C.; Tibes, R.; Rosen, L.; Razak, A.R.A.; Lam, R.; Demuth, T.; Rose, S.; Lee, M.A.; et al. Phase I Study Evaluating WEE1 Inhibitor AZD1775 as Monotherapy and in Combination with Gemcitabine, Cisplatin, or Carboplatin in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2016, 34, 4371–4380. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.; Sharpe, R.; Garcia-Murillas, I.; Gevensleben, H.; Hurd, M.S.; Shumway, S.D.; Toniatti, C.; Ashworth, A.; Turner, N.C. Forced Mitotic Entry of S-Phase Cells as a Therapeutic Strategy Induced by Inhibition of WEE1. Cancer Discov. 2012, 2, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Ayeni, J.O.; Varadarajan, R.; Mukherjee, O.; Stuart, D.T.; Sprenger, F.; Srayko, M.; Campbell, S.D. Dual Phosphorylation of Cdk1 Coordinates Cell Proliferation with Key Developmental Processes in Drosophila. Genetics 2014, 196, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL Death Receptor-Based Cancer Therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef]
- Fukuda, M.; Sakashita, H.; Hayashi, H.; Shiono, J.; Miyake, G.; Komine, Y.; Taira, F.; Sakashita, H. Synergism between α-Mangostin and TRAIL Induces Apoptosis in Squamous Cell Carcinoma of the Oral Cavity through the Mitochondrial Pathway. Oncol. Rep. 2017, 38, 3439–3446. [Google Scholar] [CrossRef]
- Lim, S.M.; Kim, T.H.; Jiang, H.H.; Park, C.W.; Lee, S.; Chen, X.; Lee, K.C. Improved Biological Half-Life and Anti-Tumor Activity of TNF-Related Apoptosis-Inducing Ligand (TRAIL) Using PEG-Exposed Nanoparticles. Biomaterials 2011, 32, 3538–3546. [Google Scholar] [CrossRef]
- Yuan, K.; Sun, Y.; Zhou, T.; McDonald, J.; Chen, Y. PARP-1 Regulates Resistance of Pancreatic Cancer to Trail Therapy. Clin. Cancer Res. 2013, 19, 4750–4759. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, M.; Shinohara, H.; Taniguchi, K.; Ueda, H.; Nishi, M.; Ryo, A.; Akao, Y. Understanding of Tolerance in TRAIL-Induced Apoptosis and Cancelation of Its Machinery by α-Mangostin, a Xanthone Derivative. Oncotarget 2015, 6, 25828–25842. [Google Scholar] [CrossRef] [PubMed]
- Ong, P.S.; Chan, S.Y.; Ho, P.C. Differential Augmentative Effects of Buthionine Sulfoximine and Ascorbic Acid in As2O3-Induced Ovarian Cancer Cell Death: Oxidative Stress-Independent and -Dependent Cytotoxic Potentiation. Int. J. Oncol. 2011, 38, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Espey, M.G.; Chen, P.; Chalmers, B.; Drisko, J.; Sun, A.Y.; Levine, M.; Chen, Q. Pharmacologic Ascorbate Synergizes with Gemcitabine in Preclinical Models of Pancreatic Cancer. Free Radic. Biol. Med. 2011, 50, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic Doses of Ascorbate Act as a Prooxidant and Decrease Growth of Aggressive Tumor Xenografts in Mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef]
- Chang, W.T.; Bow, Y.D.; Fu, P.J.; Li, C.Y.; Wu, C.Y.; Chang, Y.H.; Teng, Y.N.; Li, R.N.; Lu, M.C.; Liu, Y.C.; et al. A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways. Oxid. Med. Cell. Longev. 2021, 2021, 7689045. [Google Scholar] [CrossRef]
- Wu, J.C.; Wang, C.T.; Hung, H.C.; Wu, W.J.; Wu, D.C.; Chang, M.C.; Sung, P.J.; Chou, Y.W.; Wen, Z.H.; Tai, M.H. Heteronemin Is a Novel C-Met/STAT3 Inhibitor Against Advanced Prostate Cancer Cells. Prostate 2016, 76, 1469–1483. [Google Scholar] [CrossRef]
- Lin, H.Y.; Tey, S.L.; Ho, Y.; Chin, Y.T.; Wang, K.; Whang-Peng, J.; Shih, Y.J.; Chen, Y.R.; Yang, Y.N.; Chen, Y.C.; et al. Heteronemin Induces Anti-Proliferation in Cholangiocarcinoma Cells via Inhibiting TGF-β Pathway. Mar. Drugs 2018, 16, 489. [Google Scholar] [CrossRef]
- Britt, E.L.; Raman, S.; Leek, K.; Sheehy, C.H.; Kim, S.W.; Harada, H. Combination of Fenretinide and ABT-263 Induces Apoptosis through NOXA for Head and Neck Squamous Cell Carcinoma Treatment. PLoS ONE 2019, 14, e0219398. [Google Scholar] [CrossRef]
- Kim, E.M.; Jung, C.H.; Kim, J.; Hwang, S.G.; Park, J.K.; Um, H.D. The P53/P21 Complex Regulates Cancer Cell Invasion and Apoptosis by Targeting Bcl-2 Family Proteins. Cancer Res. 2017, 77, 3092–3100. [Google Scholar] [CrossRef]
- Xiang, W.; Yang, C.Y.; Bai, L. MCL-1 Inhibition in Cancer Treatment. Onco. Targets. Ther. 2018, 11, 7301–7314. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, K.; Kuwahara, Y.; Iehara, T.; Miyachi, M.; Katsumi, Y.; Tsuchiya, K.; Konishi, E.; Yanagisawa, A.; Hosoi, H. A NOXA/MCL-1 Imbalance Underlies Chemoresistance of Malignant Rhabdoid Tumor Cells. J. Cell. Physiol. 2016, 231, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Ow, T.J.; Fulcher, C.D.; Thomas, C.; Broin, P.; López, A.; Reyna, D.E.; Smith, R.V.; Sarta, C.; Prystowsky, M.B.; Schlecht, N.F.; et al. Optimal Targeting of BCL-Family Proteins in Head and Neck Squamous Cell Carcinoma Requires Inhibition of Both BCL-XL and MCL-1. Oncotarget 2019, 10, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, H.Y.; Fan, S.; Wang, J.; Shen, Y.; Gao, C.Y.; Wu, M.L.; Lu, S.M.; Zhang, S.Q.; Han, W. W941, a New PI3K Inhibitor, Exhibits Preferable Anti-Proliferative Activities against Nonsmall Cell Lung Cancer with Autophagy Inhibitors. Investig. New Drugs 2020, 38, 1218–1226. [Google Scholar] [CrossRef]
- Lamoureux, F.; Thomas, C.; Crafter, C.; Kumano, M.; Zhang, F.; Davies, B.R.; Gleave, M.E.; Zoubeidi, A. Blocked Autophagy Using Lysosomotropic Agents Sensitizes Resistant Prostate Tumor Cells to the Novel Akt Inhibitor AZD5363. Clin. Cancer Res. 2013, 19, 833–844. [Google Scholar] [CrossRef]
- Bernard, M.; Cardin, G.B.; Cahuzac, M.; Ayad, T.; Bissada, E.; Guertin, L.; Bahig, H.; Nguyen-Tan, P.F.; Filion, E.; Ballivy, O.; et al. Dual Inhibition of Autophagy and Pi3k/Akt/Mtor Pathway as a Therapeutic Strategy in Head and Neck Squamous Cell Carcinoma. Cancers 2020, 12, 2371. [Google Scholar] [CrossRef]
- Lehman, C.E.; Spencer, A.; Hall, S.; Shaw, J.J.P.; Wulfkuhle, J.; Petricoin, E.F.; Bekiranov, S.; Jameson, M.J.; Gioeli, D. IGF1R and Src Inhibition Induce Synergistic Cytotoxicity in HNSCC through Inhibition of FAK. Sci. Rep. 2021, 11, 10826. [Google Scholar] [CrossRef]
- Adkins, D.; Ley, J.; Atiq, O.; Powell, S.; Spanos, W.C.; Gitau, M.; Rigden, C.; Palka, K.; Liu, J.; Oppelt, P. Nanoparticle Albumin-Bound Paclitaxel with Cetuximab and Carboplatin as First-Line Therapy for Recurrent or Metastatic Head and Neck Cancer: A Single-Arm, Multicenter, Phase 2 Trial. Oral Oncol. 2021, 115, 105173. [Google Scholar] [CrossRef]
- Veiby, O.P.; Read, M.A. Chemoresistance: Impact of Nuclear Factor (NF)-ΚB Inhibition by Small Interfering RNA. Clin. Cancer Res. 2004, 10, 3262–3264. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, J.Y.; Choi, J.Y.; Han, J.Y. Simvastatin Enhances Irinotecan-Induced Apoptosis in Human Non-Small Cell Lung Cancer Cells by Inhibition of Proteasome Activity. Investig. New Drugs 2011, 29, 883–890. [Google Scholar] [CrossRef]
- Gilbert, J.; Lee, J.W.; Argiris, A.; Haigentz, M.; Feldman, L.E.; Jang, M.; Arun, P.; Van Waes, C.; Forastiere, A.A. Phase II 2-Arm Trial of the Proteasome Inhibitor, PS-341 (Bortezomib) in Combination with Irinotecan or PS-341 Alone Followed by the Addition of Irinotecan at Time of Progression in Patients with Locally Recurrent or Metastatic Squamous Cell Carcinoma. Head Neck 2012, 341, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Munster, P.N.; Hollebecque, A.; Argilés, G.; Dajani, O.; Cheng, J.D.; Wang, R.; Swift, A.; Tosolini, A.; Gupta, S. Results of a Phase 1 Trial Combining Ridaforolimus and MK-0752 in Patients with Advanced Solid Tumours. Eur. J. Cancer 2015, 51, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S. Unraveling the Complexity of γ-Secretase. Semin. Cell Dev. Biol. 2020, 105, 3–11. [Google Scholar] [CrossRef]
- Dietsch, G.N. Motolimod Effectively Drives Immune Activation in Advanced Cancer Patients. Oncoimmunology 2016, 5, e1126037. [Google Scholar] [CrossRef]
- Ferris, R.L.; Saba, N.F.; Gitlitz, B.J.; Haddad, R.; Sukari, A.; Neupane, P.; Morris, J.C.; Misiukiewicz, K.; Bauman, J.E.; Fenton, M.; et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients with Squamous Cell Carcinoma of the Head and Neck the ACTIVE8 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1583–1588. [Google Scholar] [CrossRef]
- Lei, Z.-J.; Wang, J.; Xiao, H.-L.; Guo, Y.; Wang, T.; Li, Q.; Liu, L.; Luo, X.; Fan, L.-L.; Lin, L.; et al. Lysine-Specific Demethylase 1 Promotes the Stemness and Chemoresistance of Lgr5+ Liver Cancer Initiating Cells by Suppressing Negative Regulators of β-Catenin Signaling. Oncogene 2015, 34, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Verigos, J.; Karakaidos, P.; Kordias, D.; Papoudou-Bai, A.; Evangelou, Z.; Harissis, H.V.; Klinakis, A.; Magklara, A. The Histone Demethylase LSD1/ΚDM1A Mediates Chemoresistance in Breast Cancer via Regulation of a Stem Cell Program. Cancers 2019, 11, 1585. [Google Scholar] [CrossRef]
- Sheng, W.; LaFleur, M.W.; Nguyen, T.H.; Chen, S.; Chakravarthy, A.; Conway, J.R.; Li, Y.; Chen, H.; Yang, H.; Hsu, P.-H.; et al. LSD1 Ablation Stimulates Anti-Tumor Immunity and Enables Checkpoint Blockade. Cell 2018, 174, 549–563.e19. [Google Scholar] [CrossRef]
- Alhousami, T.; Diny, M.; Ali, F.; Shin, J.; Kumar, G.; Kumar, V.; Campbell, J.D.; Noonan, V.; Hanna, G.J.; Denis, G.V.; et al. Inhibition of LSD1 Attenuates Oral Cancer Development and Promotes Therapeutic Efficacy of Immune Checkpoint Blockade and YAP/TAZ Inhibition. Mol. Cancer Res. 2022, 20, 712–721. [Google Scholar] [CrossRef]
- Akhlaq, R.; Khan, T.; Ahmed, T.; Musharraf, S.G.; Ali, A. PX-12 Synergistically Enhances the Therapeutic Efficacy of Vorinostat under Hypoxic Tumor Microenvironment in Oral Squamous Cell Carcinoma in Vitro. Drug Dev. Res. 2023, 84, 606–610. [Google Scholar] [CrossRef]
- Mohammadi, F.; Soltani, A.; Ghahremanloo, A.; Javid, H.; Hashemy, S.I. The Thioredoxin System and Cancer Therapy: A Review. Cancer Chemother. Pharmacol. 2019, 84, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Nakamura, H.; Masutani, H.; Yodoi, J. Anti-Oxidative, Anti-Cancer and Anti-Inflammatory Actions by Thioredoxin 1 and Thioredoxin-Binding Protein-2. Pharmacol. Ther. 2010, 127, 261–270. [Google Scholar] [CrossRef] [PubMed]
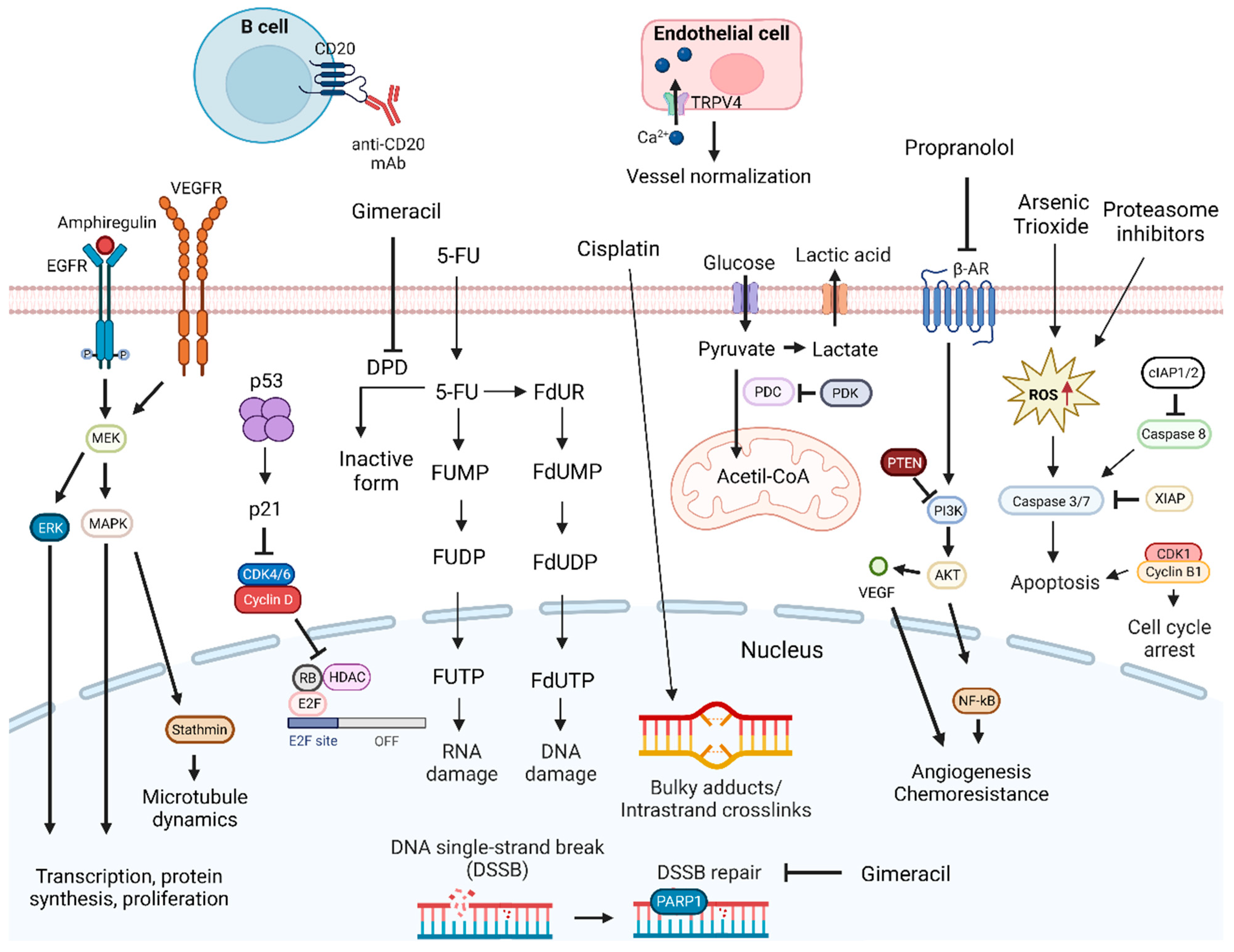
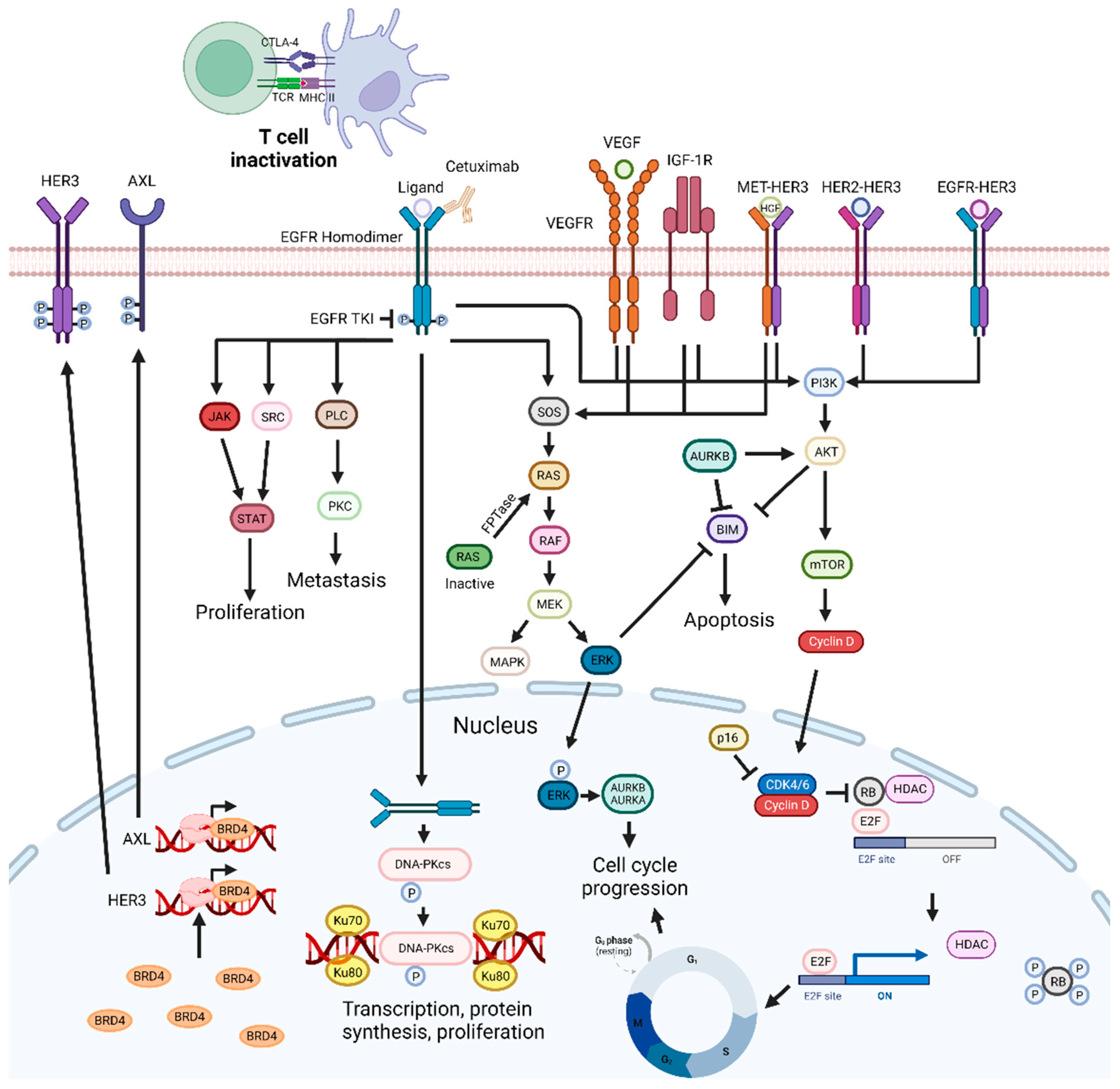
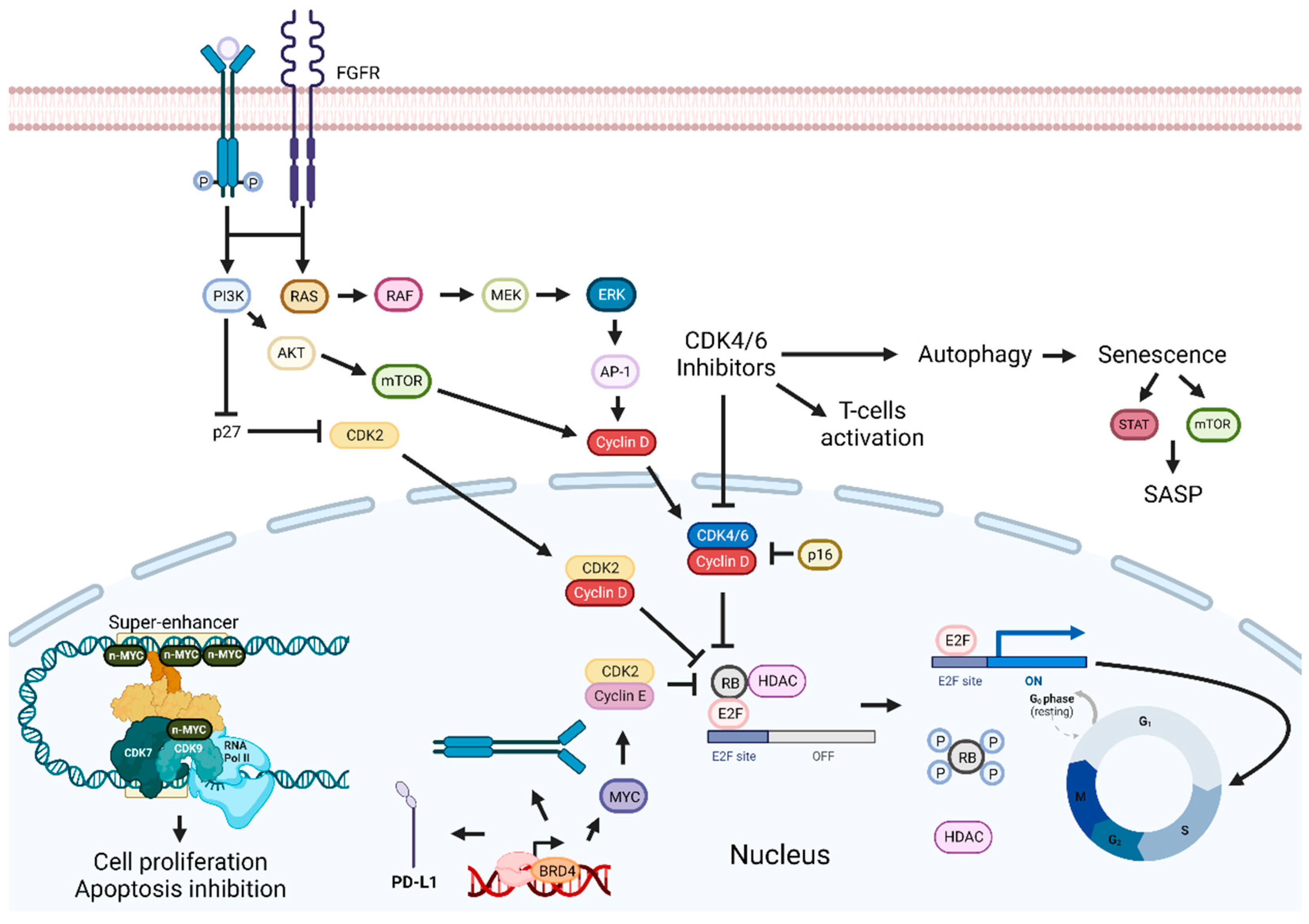
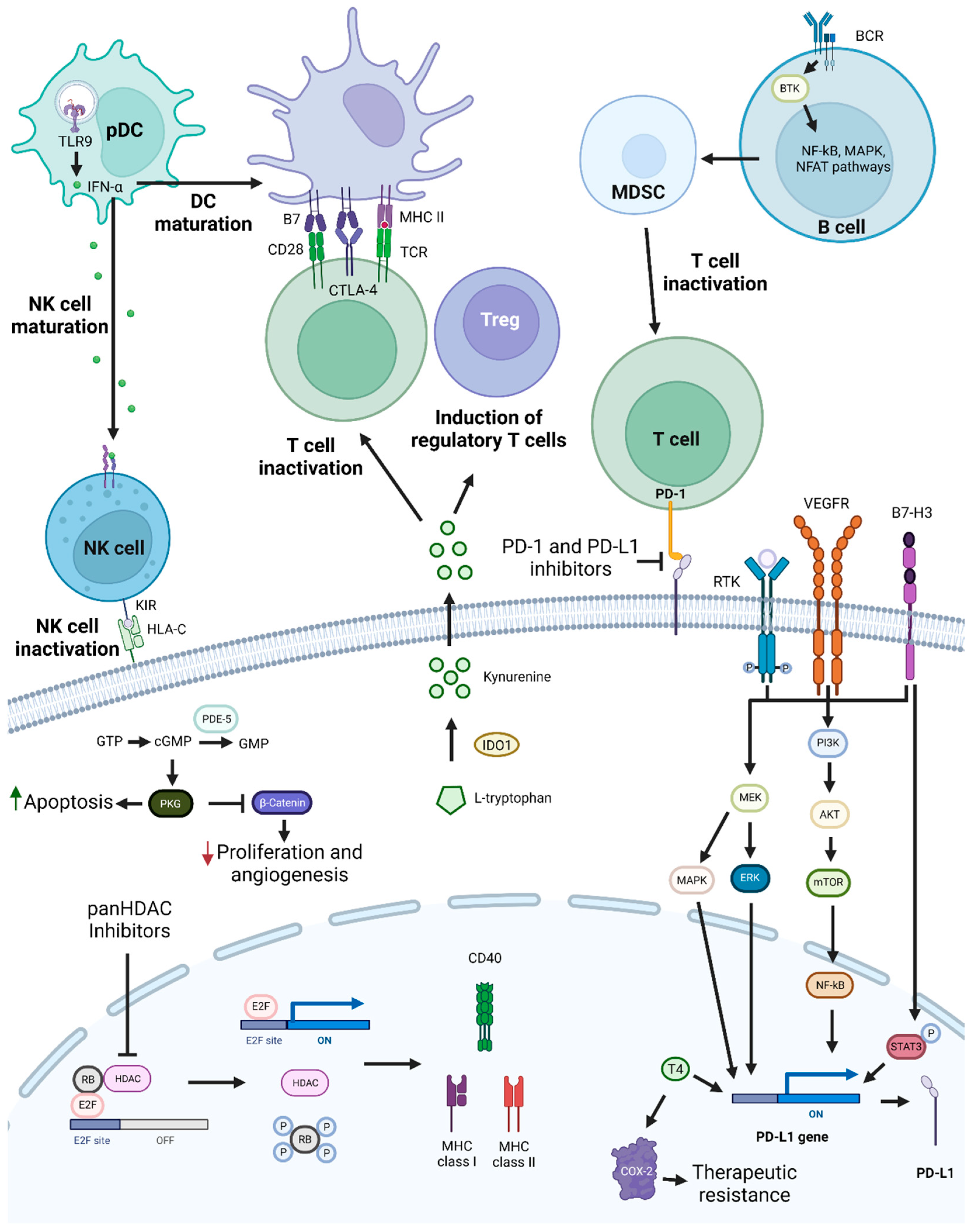
| Combination Therapies | Study Design/Treatment Type | Cancer Type and Stage | Main Reported Outcome | References |
|---|---|---|---|---|
| Palbociclib combined with Afatinib | Preclinical trial | N/A | Induced cellular senescence and inhibited tumor growth. | [208] |
| Combination of Cetuximab with Ribociclib or Palbociclib | Preclinical trial | N/A | Exhibited less effectiveness than Ribociclib monotherapy in Cetuximab-resistant PDTX models. No improvement in anticancer response was observed when compared with Cetuximab alone. | [213] |
| Phase 1 clinical trial - | R/M HNSCC - | Displayed good anticancer response, with tolerable side effects. | [214,215] | |
| Phase 2 clinical trial - | R/M HNSCC I–IV | Exhibited no significant improvement of OS and higher side effects than Cetuximab single therapy. | [216] | |
| Palbociclib combined with Cetuximab | Phase 2 clinical trial Patients previously treated | R/M OPSCC - | Did not meet primary endpoint. | [217] |
| The combination of a CDK4/6 inhibitor with Suberanilohydroxamic acid | Preclinical trial | N/A | Enhanced inhibition of NPC cell growth in vitro and in vivo. | [218] |
| Palbociclib combined with Cisplatin | Preclinical trial | N/A | Exerted antagonistic effects, reducing cancer cell cytotoxicity. | [218] |
| Addition of Pablociclib and Carboplatin | Phase 2 clinical trial - | R/M HNSCC - | Insufficient antitumoral activity and high toxicity. | [220] |
| Combination of JQ1 with Palbociclib | Preclinical trial | N/A | Exerted synergistic effects, reducing tumor volume in vivo. | [221] |
| PI3K inhibitor combined with Palbociclib | Preclinical trial | N/A | Exhibited in vitro and in vivo controlled tumor growth. | [223] |
| Palbociclib combined with Dinaciclib | Preclinical trial | N/A | Exerted synergistic effects in tongue squamous cell carcinoma. | [207] |
| Dinaciclib combined with Cisplatin | Preclinical trial | N/A | Increased tumor growth inhibition in vivo. | [207] |
| Combination Therapies | Study Design | Main Reported Outcome | References |
|---|---|---|---|
| JQ1 combined with PI3K inhibitor | Preclinical trial | Exerted synergistic effects, increasing treatment efficacy in vivo and in vitro. | [228] |
| Combination of BRD4 and CDK7 inhibitors | Preclinical trial | Exhibited in vitro and in vivo synergistic effects. | [235] |
| Combination of a YAP inhibitor with melatonin | Preclinical trial | Exerted synergistic effects, increasing apoptosis and reducing metastatic activity. | [237] |
| JQ1 combined with siPD-L1 | Preclinical trial | Improved in vitro and in vivo tumor growth inhibition. | [239] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.P.N.; Pinto, B.; Monteiro, L.; Silva, P.M.A.; Bousbaa, H. Combination Therapy as a Promising Way to Fight Oral Cancer. Pharmaceutics 2023, 15, 1653. https://doi.org/10.3390/pharmaceutics15061653
Silva JPN, Pinto B, Monteiro L, Silva PMA, Bousbaa H. Combination Therapy as a Promising Way to Fight Oral Cancer. Pharmaceutics. 2023; 15(6):1653. https://doi.org/10.3390/pharmaceutics15061653
Chicago/Turabian StyleSilva, João P. N., Bárbara Pinto, Luís Monteiro, Patrícia M. A. Silva, and Hassan Bousbaa. 2023. "Combination Therapy as a Promising Way to Fight Oral Cancer" Pharmaceutics 15, no. 6: 1653. https://doi.org/10.3390/pharmaceutics15061653
APA StyleSilva, J. P. N., Pinto, B., Monteiro, L., Silva, P. M. A., & Bousbaa, H. (2023). Combination Therapy as a Promising Way to Fight Oral Cancer. Pharmaceutics, 15(6), 1653. https://doi.org/10.3390/pharmaceutics15061653









