Crotamine/siRNA Nanocomplexes for Functional Downregulation of Syndecan-1 in Renal Proximal Tubular Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Crotamine–siRNA Nanocomplexes
2.2. Preparation and Characterization of Crotamine–siRNA Nanocomplexes
2.3. Crotamine–siRNA Complex Binding and Uptake in Cultured Cells
2.4. Downmodulation of Syndecan-1 Expression by the Crotamine–siRNA Complex in HK-2 Cells
2.5. Crotamine/siRNA Complex Administration in Mice
2.6. Statistical Analysis
3. Results
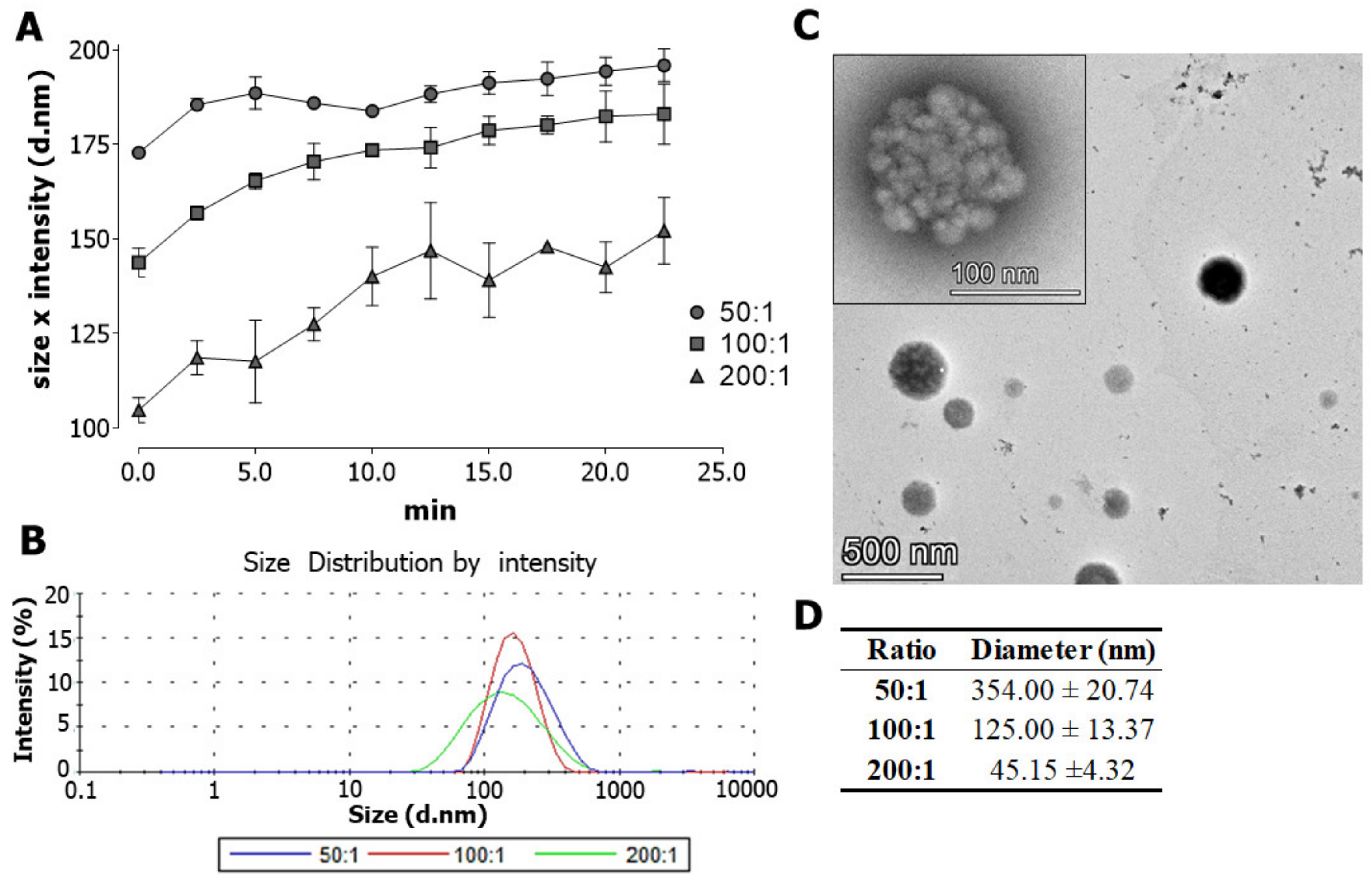
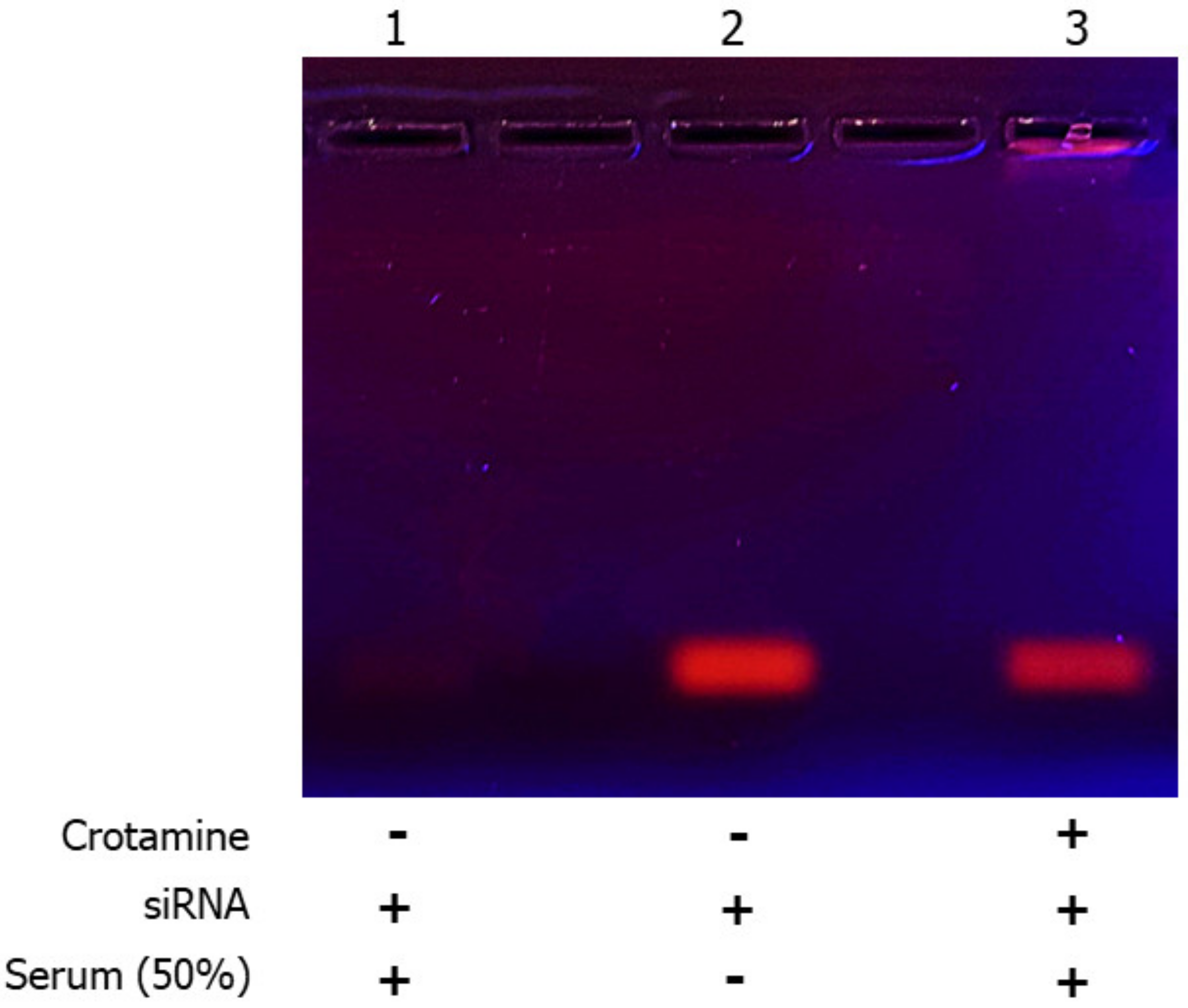
3.1. Characterization of Crotamine/siRNA Nanocomplexes
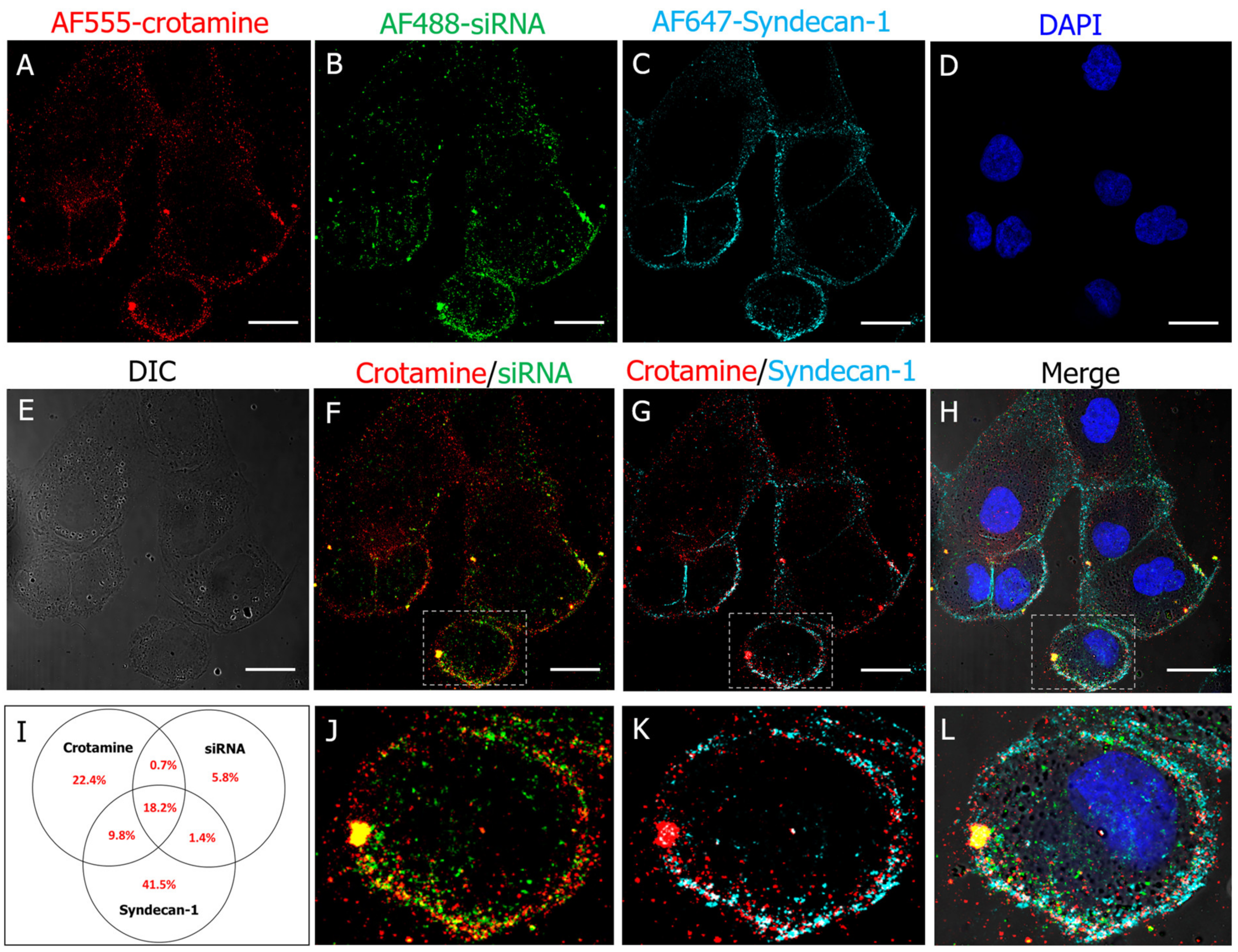
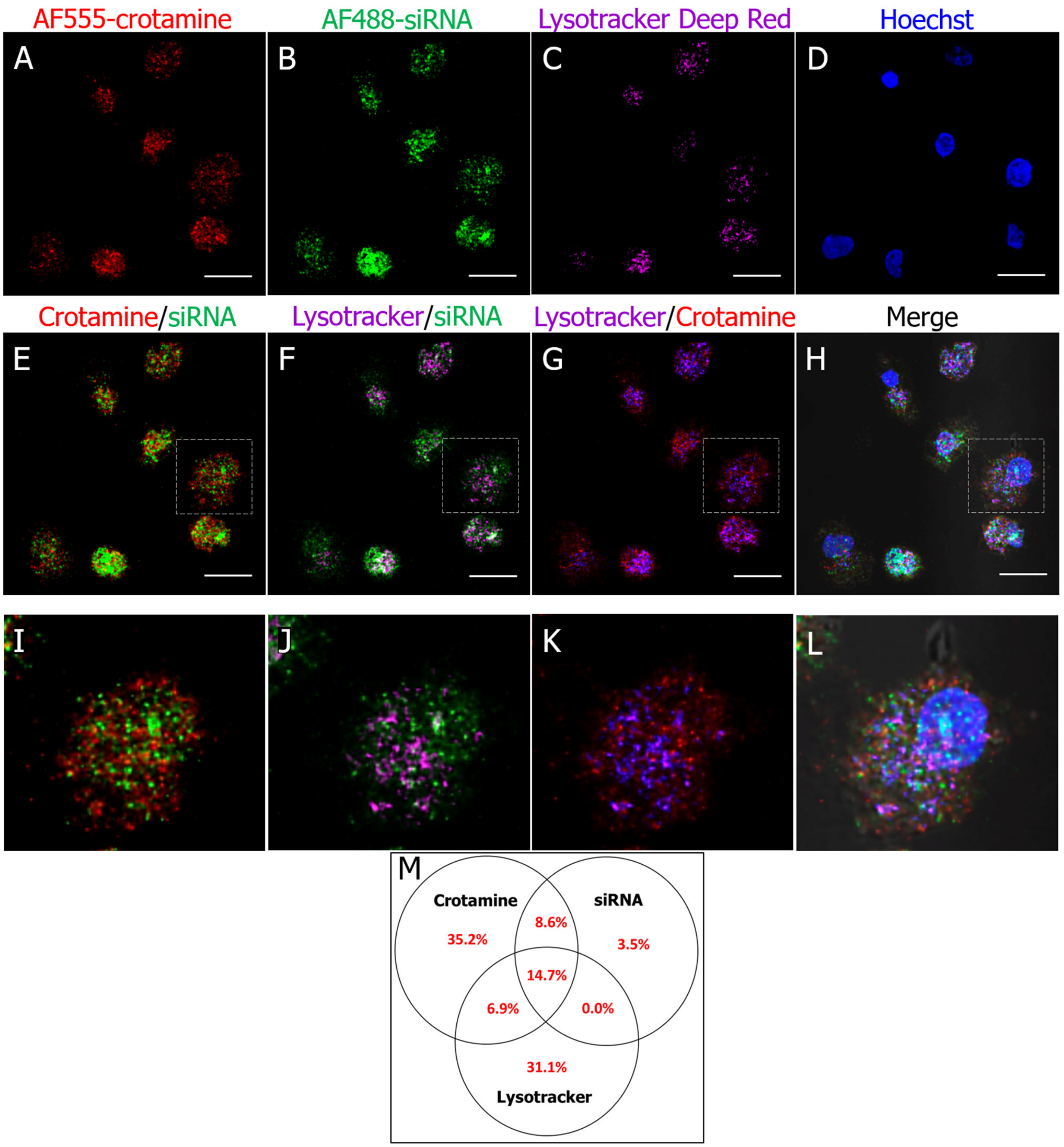
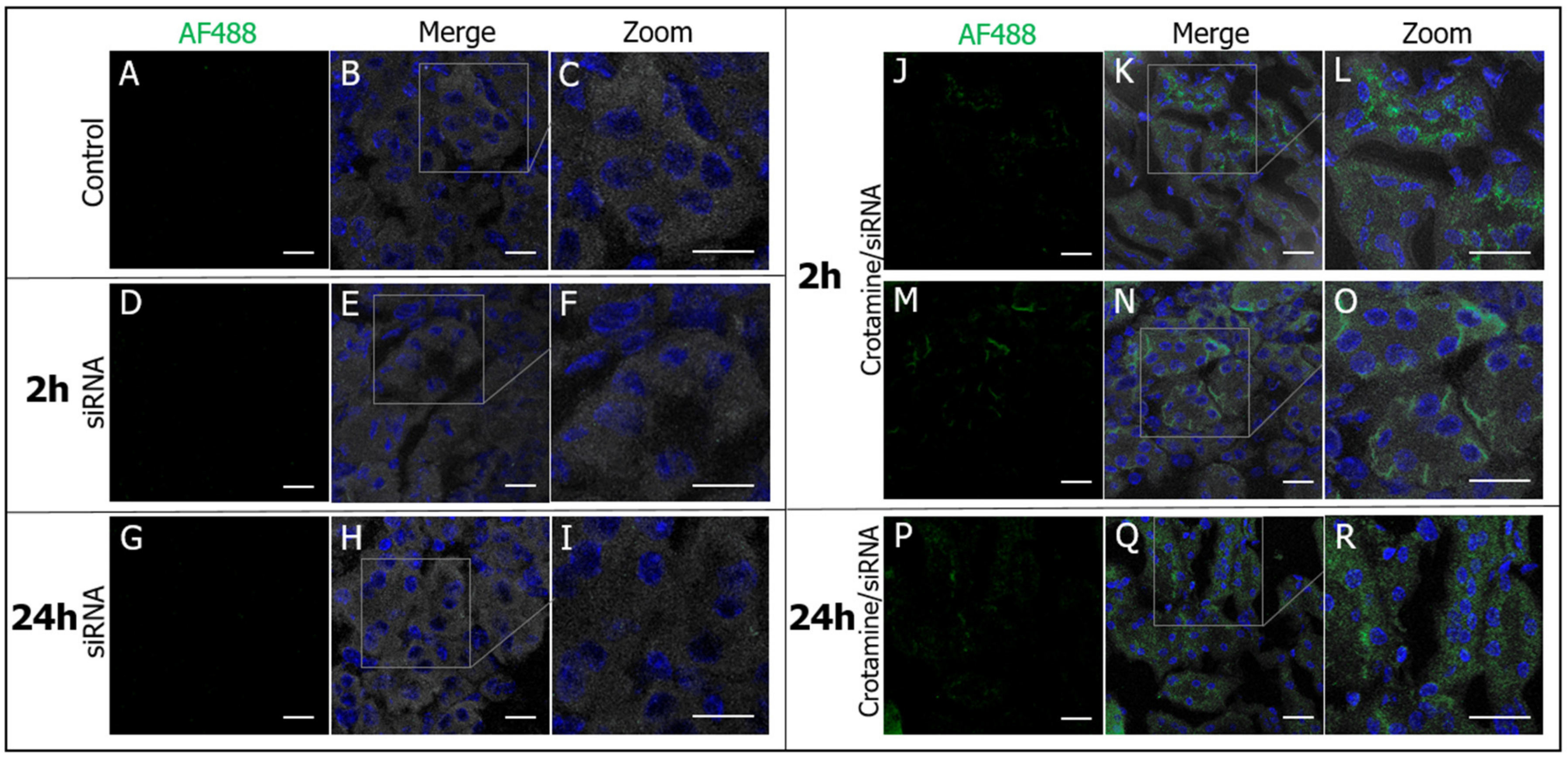
3.2. Crotamine/siRNA Nanocomplexes’ Binding and Internalization into the PTEC HK2 Cell Line In Vitro and In Vivo
3.3. Downregulation of Syndecan-1 by Crotamine/siRNA Complexes Reduces Properdin Binding and Complement C3 Deposition in HK-2 Cells under Healthy and Diseased Conditions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodgkins, K.S.; Schnaper, H.W. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr. Nephrol. 2012, 27, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhuang, S. Recent advances in renal interstitial fibrosis and tubular atrophy after kidney transplantation. Fibrogenesis Tissue Repair 2014, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, R.L. The proximal tubule is the primary target of injury and progression of kidney disease: Role of the glomerulotubular junction. Am. J. Physiol. Renal Physiol. 2016, 311, F145–F161. [Google Scholar] [CrossRef]
- Schnaper, H.W. The Tubulointerstitial Pathophysiology of Progressive Kidney Disease. Adv. Chronic Kidney Dis. 2017, 24, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-C.; Tang, T.-T.; Lv, L.-L.; Lan, H.-Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, M.; Jalkanen, M.; Firestone, J.H.; Trelstad, R.L.; Bernfield, M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J. Histochem. Cytochem. 1987, 35, 1079–1088. [Google Scholar] [CrossRef]
- Celie, J.W.A.M.; Reijmers, R.M.; Slot, E.M.; Beelen, R.H.J.; Spaargaren, M.; Ter Wee, P.M.; Florquin, S.; Born, J.V.D. Tubulointerstitial heparan sulfate proteoglycan changes in human renal diseases correlate with leukocyte influx and proteinuria. Am. J. Physiol. Renal Physiol. 2008, 294, F253–F263. [Google Scholar] [CrossRef]
- Zaferani, A.; Vivès, R.R.; van der Pol, P.; Hakvoort, J.J.; Navis, G.J.; van Goor, H.; Daha, M.R.; Lortat-Jacob, H.; Seelen, M.A.; Born, J.V.D. Identification of tubular heparan sulfate as a docking platform for the alternative complement component properdin in proteinuric renal disease. J. Biol. Chem. 2011, 286, 5359–5367. [Google Scholar] [CrossRef]
- Kwon, M.-J.; Jang, B.; Yi, J.Y.; Han, I.-O.; Oh, E.S. Syndecans play dual roles as cell adhesion receptors and docking receptors. FEBS Lett. 2012, 586, 2207–2211. [Google Scholar] [CrossRef]
- ur Rehman, Z.; Sjollema, K.A.; Kuipers, J.; Hoekstra, D.; Zuhorn, I.S. Nonviral gene delivery vectors use syndecan-dependent transport mechanisms in filopodia to reach the cell surface. ACS Nano 2012, 6, 7521–7532. [Google Scholar] [CrossRef]
- Christianson, H.C.; Belting, M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014, 35, 51–55. [Google Scholar] [CrossRef]
- Gaarkeuken, H.; Siezenga, M.A.; Zuidwijk, K.; van Kooten, C.; Rabelink, T.J.; Daha, M.R.; Berger, S.P. Complement activation by tubular cells is mediated by properdin binding. Am. J. Physiol. Renal Physiol. 2008, 295, F1397–F1403. [Google Scholar] [CrossRef] [PubMed]
- Celie, J.W.A.M.; Katta, K.K.; Adepu, S.; Melenhorst, W.B.; Reijmers, R.M.; Slot, E.M.; Beelen, R.H.; Spaargaren, M.; Ploeg, R.J.; Navis, G.; et al. Tubular epithelial syndecan-1 maintains renal function in murine ischemia/reperfusion and human transplantation. Kidney Int. 2012, 81, 651–661. [Google Scholar] [CrossRef]
- Adepu, S.; Rosman, C.W.K.; Dam, W.; van Dijk, M.C.R.F.; Navis, G.; van Goor, H.; Bakker, S.J.L.; Born, J.V.D. Incipient renal transplant dysfunction associates with tubular syndecan-1 expression and shedding. Am. J. Physiol. Renal Physiol. 2015, 309, F137–F145. [Google Scholar] [CrossRef] [PubMed]
- Abosalha, A.K.; Ahmad, W.; Boyajian, J.; Islam, P.; Ghebretatios, M.; Schaly, S.; Thareja, R.; Arora, K.; Prakash, S. A comprehensive update of siRNA delivery design strategies for targeted and effective gene silencing in gene therapy and other applications. Expert Opin. Drug Discov. 2023, 18, 149–161. [Google Scholar] [CrossRef]
- Tang, W.; Panja, S.; Jogdeo, C.M.; Tang, S.; Ding, L.; Yu, A.; Foster, K.W.; Dsouza, D.L.; Chhonker, Y.S.; Jensen-Smith, H.; et al. Modified chitosan for effective renal delivery of siRNA to treat acute kidney injury. Biomaterials 2022, 285, 121562. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, Y.; Jang, H.-S.; Hang, Y.; Jogdeo, C.M.; Li, J.; Ding, L.; Zhang, C.; Yu, A.; Yu, F.; et al. Preferential siRNA delivery to injured kidneys for combination treatment of acute kidney injury. J. Control. Release 2022, 341, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Alidori, S.; Akhavein, N.; Thorek, D.L.J.; Behling, K.; Romin, Y.; Queen, D.; Beattie, B.J.; Manova-Todorova, K.; Bergkvist, M.; Scheinberg, D.A.; et al. Targeted fibrillar nanocarbon RNAi treatment of acute kidney injury. Sci. Transl. Med. 2016, 8, 331ra39. [Google Scholar] [CrossRef]
- Han, S.J.; Williams, R.M.; D’agati, V.; Jaimes, E.A.; Heller, D.A.; Lee, H.T. Selective nanoparticle-mediated targeting of renal tubular Toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int. 2020, 98, 76–87. [Google Scholar] [CrossRef]
- de Carvalho Porta, L.; Fadel, V.; D’Arc Campeiro, J.; Oliveira, E.B.; Godinho, R.O.; Hayashi, M.A.F. Biophysical and pharmacological characterization of a full-length synthetic analog of the antitumor polypeptide crotamine. J. Mol. Med. 2020, 98, 1561–1571. [Google Scholar] [CrossRef]
- Pei, D.; Buyanova, M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjug. Chem. 2019, 30, 273–283. [Google Scholar] [CrossRef]
- Teo, S.L.Y.; Rennick, J.J.; Yuen, D.; Al-Wassiti, H.; Johnston, A.P.R.; Pouton, C.W. Unravelling cytosolic delivery of cell penetrating peptides with a quantitative endosomal escape assay. Nat. Commun. 2021, 12, 3721. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Oliveira, E.B.; Kerkis, I.; Karpel, R.L. Crotamine: A novel cell-penetrating polypeptide nanocarrier with potential anti-cancer and biotechnological applications. Methods Mol. Biol. 2012, 906, 337–352. [Google Scholar] [PubMed]
- Nascimento, F.D.; Hayashi, M.A.F.; Kerkis, A.; Oliveira, V.; Oliveira, E.B.; Rádis-Baptista, G.; Nader, H.B.; Yamane, T.; dos Santos Tersariol, I.L.; Kerkis, I. Crotamine mediates gene delivery into cells through the binding to heparan sulfate proteoglycans. J. Biol. Chem. 2007, 282, 21349–21360. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.A.F.; Nascimento, F.D.; Kerkis, A.; Oliveira, V.; Oliveira, E.B.; Pereira, A.; Rádis-Baptista, G.; Nader, H.B.; Yamane, T.; Kerkis, I.; et al. Cytotoxic effects of crotamine are mediated through lysosomal membrane permeabilization. Toxicon 2008, 52, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Dal Mas, C.; Pinheiro, D.A.; Campeiro, J.D.; Mattei, B.; Oliveira, V.; Oliveira, E.; Miranda, A.; Perez, K.; Hayashi, M. Biophysical and biological properties of small linear peptides derived from crotamine, a cationic antimicrobial/antitumoral toxin with cell penetrating and cargo delivery abilities. Biochim. Biophys. Acta 2017, 1859, 2340–2349. [Google Scholar] [CrossRef]
- Campeiro, J.D.; Dam, W.; Monte, G.G.; Porta, L.C.; de Oliveira, L.C.G.; Nering, M.B.; Viana, G.M.; Carapeto, F.C.; Oliveira, E.B.; van den Born, J.; et al. Long term safety of targeted internalization of cell penetrating peptide crotamine into renal proximal tubular epithelial cells in vivo. Sci. Rep. 2019, 9, 3312. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Campeiro, J.D.; Porta, L.C.; Szychowski, B.; Alves, W.A.; Oliveira, E.B.; Kerkis, I.; Daniel, M.-C.; Karpel, R.L. Crotamine Cell-Penetrating Nanocarriers: Cancer-Targeting and Potential Biotechnological and/or Medical Applications. Methods Mol. Biol. 2020, 2118, 61–89. [Google Scholar]
- Chen, P.-C.; Hayashi, M.A.F.; Oliveira, E.B.; Karpel, R.L. DNA-interactive properties of crotamine, a cell-penetrating polypeptide and a potential drug carrier. PLoS ONE 2012, 7, e48913. [Google Scholar] [CrossRef]
- Boni-Mitake, M.; Costa, H.; Vassilieff, V.S.; Rogero, J.R. Distribution of (125)I-labeled crotamine in mice tissues. Toxicon 2006, 48, 550–555. [Google Scholar] [CrossRef]
- Yen, A.; Cheng, Y.; Sylvestre, M.; Gustafson, H.H.; Puri, S.; Pun, S.H. Serum Nuclease Susceptibility of mRNA Cargo in Condensed Polyplexes. Mol. Pharm. 2018, 15, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Scindia, Y.; Deshmukh, U.; Thimmalapura, P.-R.; Bagavant, H. Anti-α8 integrin immunoliposomes in glomeruli of lupus-susceptible mice: A novel system for delivery of therapeutic agents to the renal glomerulus in systemic lupus erythematosus. Arthritis Rheum. 2008, 58, 3884–3891. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; Shah, J.; Ng, B.D.; Minton, D.R.; Gudas, L.J.; Park, C.Y.; Heller, D.A. Mesoscale nanoparticles selectively target the renal proximal tubule epithelium. Nano Lett. 2015, 15, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; He, J.C.; Ausiello, D.A.; Farokhzad, O.C. Nanomedicines for renal disease: Current status and future applications. Nat. Rev. Nephrol. 2016, 12, 738–753. [Google Scholar] [CrossRef]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 2020, 321, 442–462. [Google Scholar] [CrossRef]
- Harding, S.E.; Jumel, K. Light Scattering. Curr. Protoc. Protein Sci. 1998, 11, 7.8.1–7.8.14. [Google Scholar] [CrossRef]
- Pabisch, S.; Feichtenschlager, B.; Kickelbick, G.; Peterlik, H. Effect of interparticle interactions on size determination of zirconia and silica based systems—A comparison of SAXS, DLS, BET, XRD and TEM. Chem. Phys. Lett. 2012, 521, 91–97. [Google Scholar] [CrossRef]
- Lammerts, R.G.M.; Talsma, D.T.; Dam, W.A.; Daha, M.R.; Seelen, M.A.J.; Berger, S.P.; Born, J.V.D. Properdin Pattern Recognition on Proximal Tubular Cells Is Heparan Sulfate/Syndecan-1 but Not C3b Dependent and Can Be Blocked by Tick Protein Salp20. Front. Immunol. 2020, 11, 1643. [Google Scholar] [CrossRef]
- Zaferani, A.; Vivès, R.R.; van der Pol, P.; Navis, G.J.; Daha, M.R.; van Kooten, C.; Lortat-Jacob, H.; Seelen, M.A.; Born, J.V.D. Factor H and properdin recognize different epitopes on renal tubular epithelial heparan sulfate. J. Biol. Chem. 2012, 287, 31471–31481. [Google Scholar] [CrossRef]
- Yu, H.; Lin, T.; Chen, W.; Cao, W.; Zhang, C.; Wang, T.; Ding, M.; Zhao, S.; Wei, H.; Guo, H.; et al. Size and temporal-dependent efficacy of oltipraz-loaded PLGA nanoparticles for treatment of acute kidney injury and fibrosis. Biomaterials 2019, 219, 119368. [Google Scholar] [CrossRef]
- McAnuff, M.A.; Rettig, G.R.; Rice, K.G. Potency of siRNA versus shRNA mediated knockdown in vivo. J. Pharm. Sci. 2007, 96, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Park, C.W. Catalytic Antioxidants in the Kidney. Antioxidants 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Matoba, K.; Kawanami, D.; Okada, R.; Tsukamoto, M.; Kinoshita, J.; Ito, T.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Murai, N.; et al. Rho-kinase inhibition prevents the progression of diabetic nephropathy by downregulating hypoxia-inducible factor 1α. Kidney Int. 2013, 84, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; Awad, A.M.; Ibrahim, T.M.; Abu-Elsaad, N.M. Small-Dose Sunitinib Modulates p53, Bcl-2, STAT3, and ERK1/2 Pathways and Protects against Adenine-Induced Nephrotoxicity. Pharmaceuticals 2020, 13, 397. [Google Scholar] [CrossRef]
- Dolman, M.E.M.; Harmsen, S.; Pieters, E.H.E.; Sparidans, R.W.; Lacombe, M.; Szokol, B.; Orfi, L.; Keri, G.; Storm, G.; Hennink, W.E.; et al. Targeting of a platinum-bound sunitinib analog to renal proximal tubular cells. Int. J. Nanomed. 2012, 7, 417–433. [Google Scholar]
- Barreto-Vieira, D.F.; Barth, O.M. Negative and Positive Staining in Transmission Electron Microscopy for Virus Diagnosis. In Microbiology in Agriculture and Human Health; Shah, M.M., Ed.; InTech: London, UK, 2015. [Google Scholar]
- Bolte, S.; Cordelières, F.P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campeiro, J.D.; Dam, W.A.; Hayashi, M.A.F.; van den Born, J. Crotamine/siRNA Nanocomplexes for Functional Downregulation of Syndecan-1 in Renal Proximal Tubular Epithelial Cells. Pharmaceutics 2023, 15, 1576. https://doi.org/10.3390/pharmaceutics15061576
Campeiro JD, Dam WA, Hayashi MAF, van den Born J. Crotamine/siRNA Nanocomplexes for Functional Downregulation of Syndecan-1 in Renal Proximal Tubular Epithelial Cells. Pharmaceutics. 2023; 15(6):1576. https://doi.org/10.3390/pharmaceutics15061576
Chicago/Turabian StyleCampeiro, Joana D’Arc, Wendy A. Dam, Mirian A. F. Hayashi, and Jacob van den Born. 2023. "Crotamine/siRNA Nanocomplexes for Functional Downregulation of Syndecan-1 in Renal Proximal Tubular Epithelial Cells" Pharmaceutics 15, no. 6: 1576. https://doi.org/10.3390/pharmaceutics15061576
APA StyleCampeiro, J. D., Dam, W. A., Hayashi, M. A. F., & van den Born, J. (2023). Crotamine/siRNA Nanocomplexes for Functional Downregulation of Syndecan-1 in Renal Proximal Tubular Epithelial Cells. Pharmaceutics, 15(6), 1576. https://doi.org/10.3390/pharmaceutics15061576






