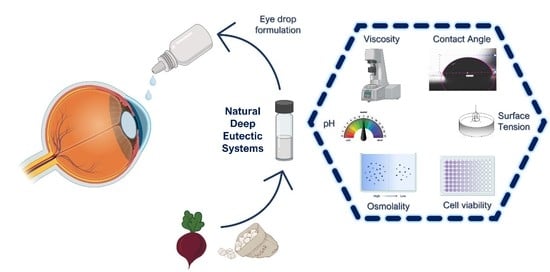
Using Natural Deep Eutectic Systems as Alternative Media for Ocular Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of NADES
2.2.2. Preparation of NADES Solutions
2.2.3. Rheological Studies
2.2.4. Osmolarity, pH, Density, Surface Tension, Contact Angle, and Refractive Index
2.2.5. Solubility Studies of ACZ in NADES
2.2.6. In Vitro Cytotoxicity Evaluation
2.2.7. Statistical Analysis
3. Results and Discussion
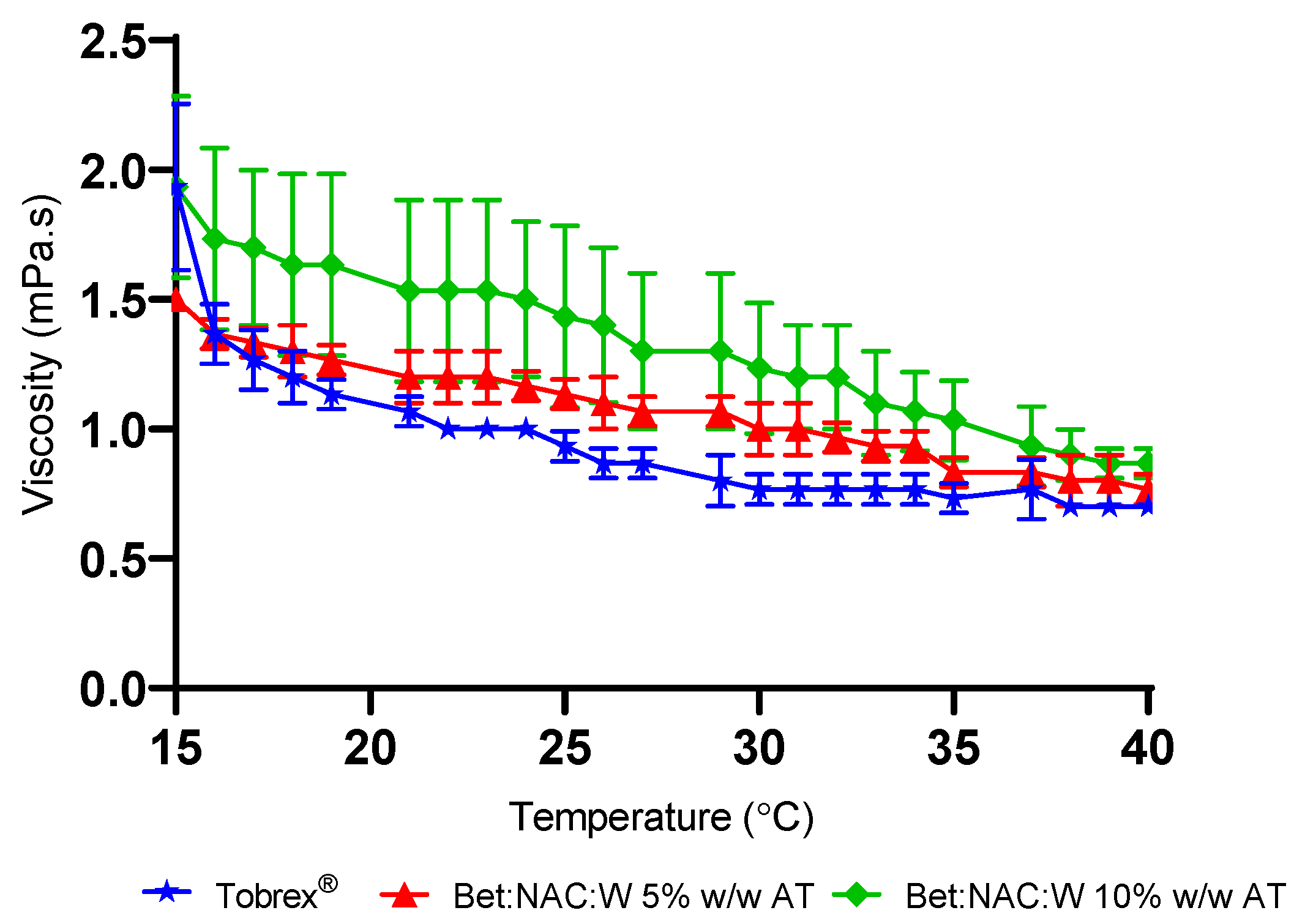
3.1. Rheological Studies
3.2. Osmolality, pH, and Refractive Index
3.3. Density, Surface Tension, and Contact Angle
3.4. Acetazolamide Solubility Studies
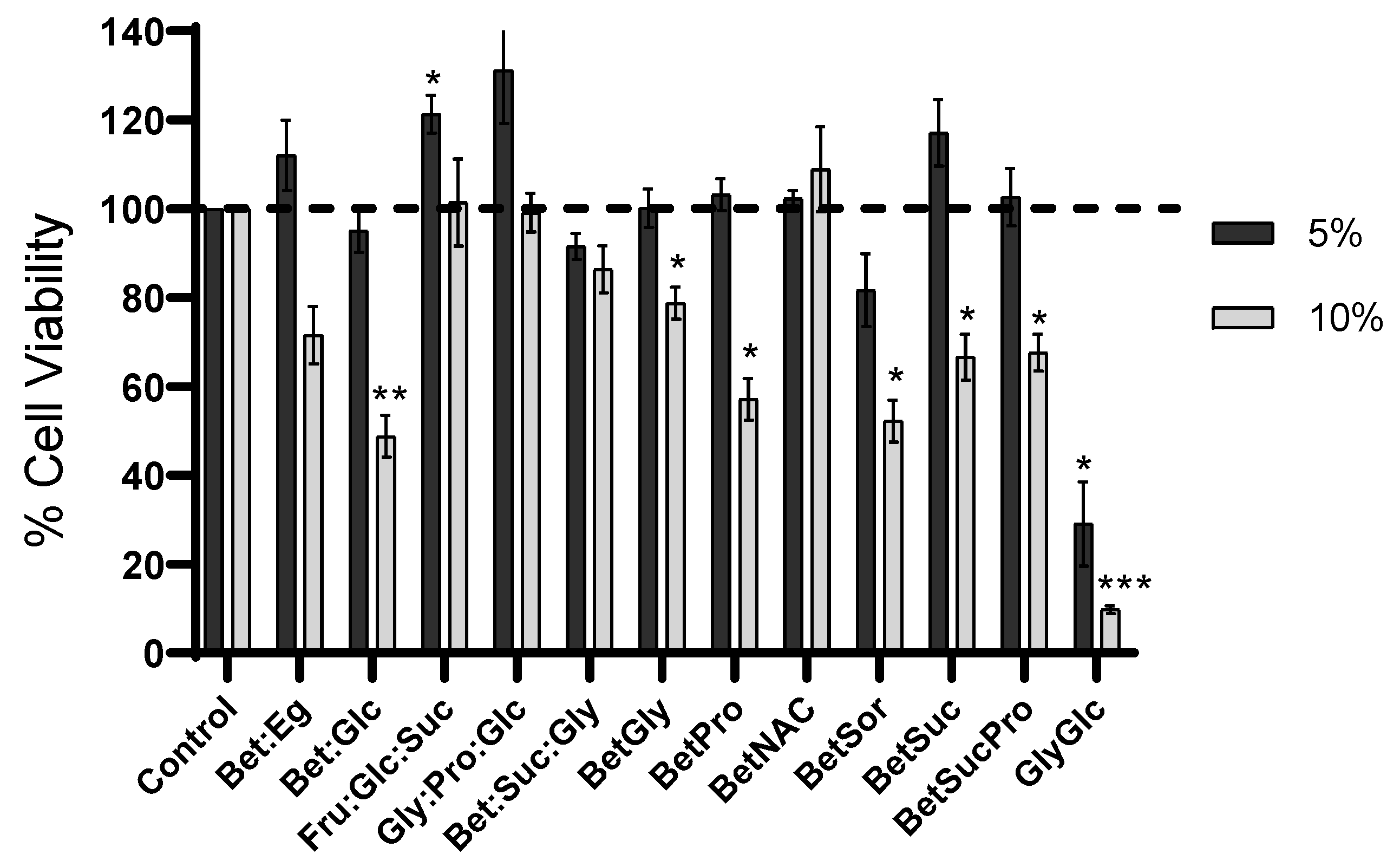
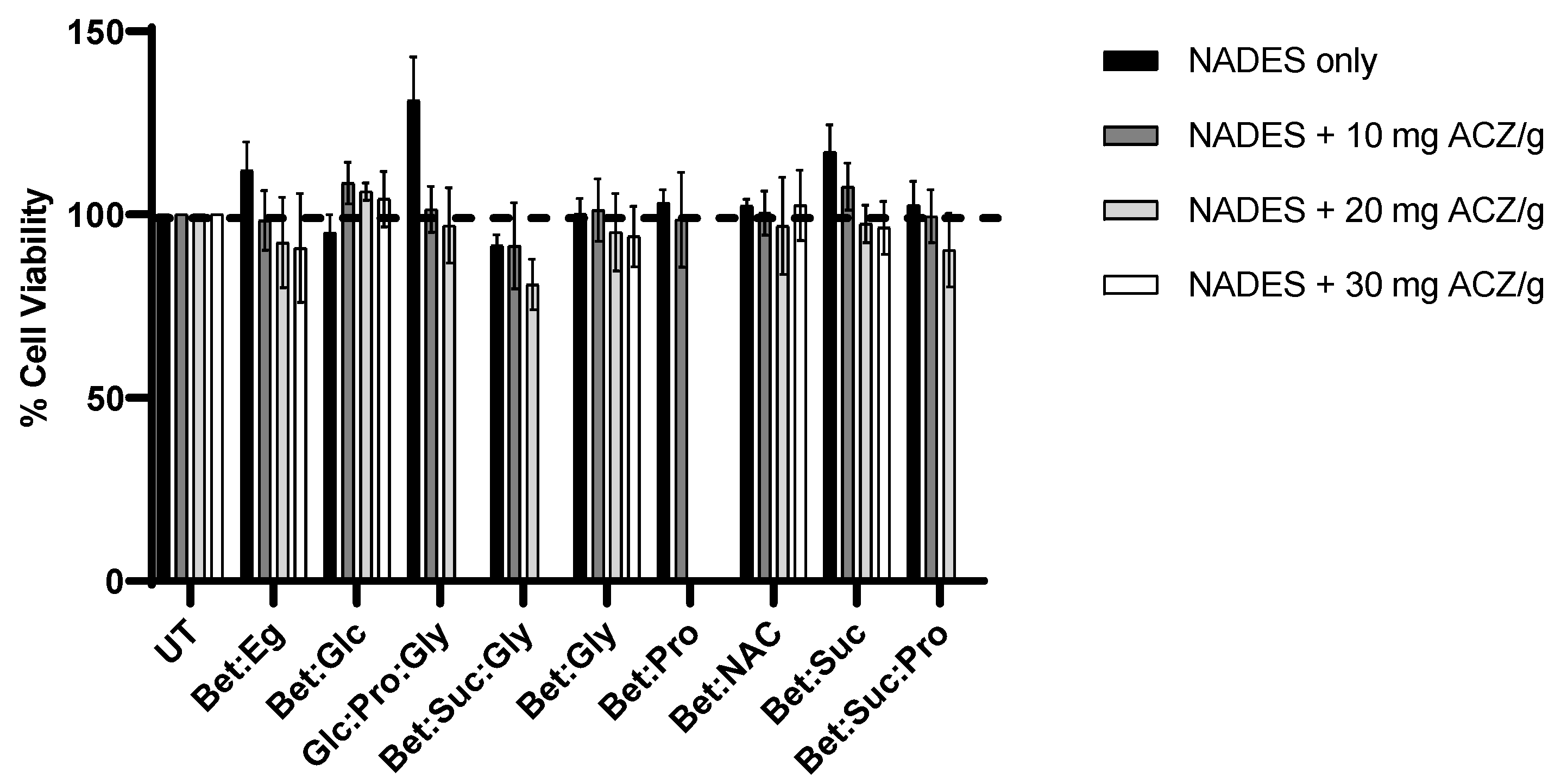
3.5. Cytotoxicity Evaluation of NADES and ACZ/NADES Mixtures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Blindness and Vision Impairment. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 12 March 2023).
- Bourne, R.R.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob. Heal. 2017, 5, e888–e897. [Google Scholar] [CrossRef] [PubMed]
- Yanai, R.; Okunuki, Y.; Park, D.H.; Zunaina, E. Editorial: Next Therapeutic Targets in Ocular Diseases. Front. Med. 2022, 9, 953377. [Google Scholar] [CrossRef] [PubMed]
- Short, B.G. Safety evaluation of ocular drug delivery formulations: Techniques and practical considerations. Toxicol. Pathol. 2008, 36, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef]
- Santos, F.; PS Leitão, M.I.C.; Duarte, A.R. Properties of Therapeutic Deep Eutectic Solvents of l-Arginine and Ethambutol for Tuberculosis Treatment. Molecules 2018, 24, 55. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Santos, F.; Duarte, A.R.C. Therapeutic Deep Eutectic Systems towards the Treatment of Tuberculosis and Colorectal Cancer: Opportunities and Challenges. Molecules 2021, 26, 7022. [Google Scholar] [CrossRef]
- Gouveia, S.M.; Tiffany, J.M. Human tear viscosity: An interactive role for proteins and lipids. Biochim. Biophys. Acta-Proteins Proteomics 2005, 1753, 155–163. [Google Scholar] [CrossRef]
- Tiffany, J.M. The viscosity of human tears. Int. Ophthalmol. 1991, 15, 371–376. [Google Scholar] [CrossRef]
- Owen, S.C.; Kuo, J.W.; Prestwich, G.D. 2.14 Hyaluronic acid. Compr. Biomater. II 2016, 2, 306–331. [Google Scholar] [CrossRef]
- Jesus, A.R.; Meneses, L.; Duarte, A.R.C.; Paiva, A. Natural Deep Eutectic Systems—A New Era of Cryopreservation; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2021; Volume 97. [Google Scholar]
- Jesus, A.R.; Meneses, L.; Duarte, A.R.C.; Paiva, A. Natural deep eutectic systems, an emerging class of cryoprotectant agents. Cryobiology 2021, 101, 95–104. [Google Scholar] [CrossRef]
- Meneses, L.; Santos, F.; Gameiro, A.R.; Paiva, A.; Duarte, A.R.C. Preparation of Binary and Ternary Deep Eutectic Systems. JoVE 2019, 152, e60326. [Google Scholar] [CrossRef]
- Trombino, S.; Siciliano, C.; Procopio, D.; Curcio, F.; Laganà, A.S.; Di Gioia, M.L.; Cassano, R. Deep Eutectic Solvents for Improving the Solubilization and Delivery of Dapsone. Pharmaceutics 2022, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. Deep Eutectic Solvents and Pharmaceuticals. Encyclopedia 2021, 1, 942–963. [Google Scholar] [CrossRef]
- Lomba, L.; García, C.B.; Ribate, M.P.; Giner, B.; Zuriaga, E. Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals. Appl. Sci. 2021, 11, 10156. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 116. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Ferreira, I.J.; Meneses, L.; Paiva, A.; Diniz, M.; Duarte, A.R.C. Duarte. Assessment of deep eutectic solvents toxicity in zebrafish (Danio rerio). Chemosphere 2022, 299, 134415. [Google Scholar] [CrossRef]
- Hua, X.; Su, Z.; Deng, R.; Lin, J.; Li, D.Q.; Pflugfelder, S.C. Effects of L-carnitine, erythritol and betaine on pro-inflammatory markers in primary human corneal epithelial cells exposed to hyperosmotic stress. Curr. Eye Res. 2015, 40, 657–667. [Google Scholar] [CrossRef]
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the Anticancer Potential of Limomene Based Therapeutic Deep Eutectic Solvents. Sci. Rep. 2019, 9, 14926. [Google Scholar] [CrossRef]
- Centre for Industrial Rheology. Not a Dry Eye in the House: Eye Drop Rheology. Available online: https://www.rheologylab.com/articles/pharmaceutical-rheology/rheology-lubricating-eye-drops/ (accessed on 1 March 2023).
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents from choline chloride and betaine—Physicochemical properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Aroso, I.M.A.; Craveiro, R.; Dionisio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Fundamental Studies on Natural Deep Eutectic Solvents: Physico-Chemical, Thermal and Rheological Properties. Conference Procedings. 2015. Available online: https://hdl.handle.net/1822/40945 (accessed on 18 May 2023).
- Altamash, T.; Nasser, M.S.; Elhamarnah, Y.; Magzoub, M.; Ullah, R.; Anaya, B.; Aparicio, S.; Atilhan, M. Gas Solubility and Rheological Behavior of Natural Deep Eutectic Solvents (NADES) via Combined Experimental and Molecular Simulation Techniques. ChemistrySelect 2017, 2, 7278–7295. [Google Scholar] [CrossRef]
- Altamash, T.; Nasser, M.S.; Elhamarnah, Y.; Magzoub, M.; Ullah, R.; Qiblawey, H.; Aparicio, S.; Atilhan, M. Gas solubility and rheological behavior study of betaine and alanine based natural deep eutectic solvents (NADES). J. Mol. Liq. 2018, 256, 286–295. [Google Scholar] [CrossRef]
- Tomlinson, A.; Khanal, S. Assessment of tear film dynamics: Quantification approach. Ocul. Surf. 2005, 3, 81–95. [Google Scholar] [CrossRef]
- Craig, J.P.; Simmons, P.A.; Patel, S.; Tomlinson, A. Refractive index and osmolality of human tears. Optom. Vis. Sci. 1995, 72, 718–724. [Google Scholar] [CrossRef]
- Dutescu, R.M.; Panfil, C.; Schrage, N. Osmolarity of prevalent eye drops, side effects, and therapeutic approaches. Cornea 2015, 34, 560–566. [Google Scholar] [CrossRef]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- D’Souza, S.; Annavajjhala, S.; Thakur, P.; Mullick, R.; Tejal, S.J.; Shetty, N. Study of tear film optics and its impact on quality of vision. Indian J. Ophthalmol. 2020, 68, 2899. [Google Scholar] [CrossRef]
- Pierscionek, B.K. Refractive index of the human lens surface measured with an optic fibre sensor. Ophthalmic Res. 1994, 26, 32–35. [Google Scholar] [CrossRef]
- Patel, S.; Tutchenko, L. The refractive index of the human cornea: A review. Contact Lens Anterior Eye 2019, 42, 575–580. [Google Scholar] [CrossRef]
- Bock, U.; Von Deylen, D.; Jochner, M.; Doerr, M.; Stäbler, C.; Reichl, S. Development of In Vitro Methodologies to Investigate Binding by Sodium Hyaluronate in Eye Drops to Corneal Surfaces. Open Ophthalmol. J. 2018, 12, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Grgurević, M.H.; Juretić, M.; Hafner, A.; Lovrić, J.; Pepić, I. Tear fluid-eye drops compatibility assessment using surface tension. Drug Dev. Ind. Pharm. 2017, 43, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, G.; Alvarez, E.; Navaza, J.M. Surface Tension of Alcohol Water + Water from 20 to 50.degree. C. J. Chem. Eng. Data 1995, 40, 611–614. [Google Scholar] [CrossRef]
- Van Santvliet, L.; Caljon, K.; Pieters, L.; Ludwig, A. Physicochemical properties, NMR spectroscopy and tolerance of inclusion complexes of antazoline and tetracaine with hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 1998, 171, 147–156. [Google Scholar] [CrossRef]
- Han, K.; Woghiren, O.E.; Priefer, R. Surface tension examination of various liquid oral, nasal, and ophthalmic dosage forms. Chem. Cent. J. 2016, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yasueda, S.I.; Inada, K.; Matsuhisa, K.; Terayama, H.; Ohtori, A. Evaluation of ophthalmic suspensions using surface tension. Eur. J. Pharm. Biopharm. 2004, 57, 377–382. [Google Scholar] [CrossRef]
- Becker, B.; Middleton, W.H. Long-Term Acetazoleamide (Diamox) Administration in Therapy of Glaucomas. AMA. Arch. Ophthalmol. 1955, 54, 187–192. [Google Scholar] [CrossRef]
- Granero, G.E.; Longhi, M.R.; Becker, C.; Junginger, H.E.; Kopp, S.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; Barends, D.M. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Acetazolamide. J. Pharm. Sci. 2008, 97, 3691–3699. [Google Scholar] [CrossRef]


| NADES | Components | Molar Ratio | Water Content (%) | ||
|---|---|---|---|---|---|
| A | B | C | |||
| Bet:Eg | Betaine | Ethylene glycol | - | 1:3 | 0.80 ± 0.1 |
| Bet:Glc | Betaine | Glucose | - | 5:2 | 16.4 ± 0.1 |
| Fru:Glc:Suc | Frutose | Glucose | Sucrose | 1:1:1 | 19.4 ± 2.4 |
| Glc:Pro:Gly | Glucose | Proline | Glycerol | 3:5:3 | 20.1 ± 0.6 |
| Bet:Suc:Gly | Betaine | Sucrose | Glycerol | 2:1:3 | 10.0 ± 0.1 |
| Bet:Gly | Betaine | Glycerol | - | 1:2 | 1.70 ± 0.1 |
| Bet:Pro | Betaine | Proline | - | 1:2 | 12.9 ± 2.9 |
| Bet:NAC | Betaine | N-acetyl cysteine | - | 1:1 | 17.7 ± 1.1 |
| Bet:Sorb | Betaine | Sorbitol | - | 1:1 | 13.8 ± 1.5 |
| Bet:Suc | Betaine | Sucrose | - | 4:1 | 16.7 ± 0.4 |
| Bet:Suc:Pro | Betaine | Sucrose | Proline | 5:2:2 | 18.5 ± 0.3 |
| Gly:Glc | Glycerol | Glucose | - | 4:1 | 0.20 ± 1.0 |
| Sample | Osmolality [mOsmKg−1] | pH | Refractive Index | |||
|---|---|---|---|---|---|---|
| Tobrex® | 214 ± 1 | 7.47 | 1.3379 | |||
| Artificial Tears (AT) | 286 ± 1 | 7.42 | 1.3350 | |||
| HPMC 0.3% in PBS | 280 ± 1 | - | 1.3359 | |||
| NADES | 5% w/w AT | 10% w/w AT | 5% w/w AT | 10% w/w AT | 5% w/w AT | 10% w/w AT |
| Bet:Eg | 962 ± 3 | 1883 ± 1 | 7.43 | 7.52 | 1.3402 | 1.3466 |
| Bet:Glc | 602 ± 4 | 947 ± 3 | 7.32 | 7.29 | 1.3459 | 1.3463 |
| Fru:Glc:Suc | 412 ± 2 | 558 ± 2 | 7.32 | 7.29 | 1.3459 | 1.3463 |
| Glc:Pro:Gly | 538 ± 2 | 825 ± 2 | 7.28 | 7.20 | 1.3416 | 1.3470 |
| Bet:Suc:Gly | 639 ± 2 | 1032 ± 4 | 7.37 | 7.37 | 1.3397 | 1.3491 |
| Bet:Gly | 432 ± 4 | 1504 ± 4 | 7.46 | 7.42 | 1.3416 | 1.372 |
| Bet:Pro | 587 ± 2 | 957 ± 1 | 7.38 | 7.33 | 1.3433 | 1.3460 |
| Bet:NAC | 563 ± 2 | 900 ± 4 | 2.65 | 2.64 | 1.3407 | 1.3455 |
| Bet:Sorb | 585 ± 1 | 922 ± 7 | 7.43 | 7.43 | 1.3405 | 1.3460 |
| Bet:Suc | 554 ± 2 | 875 ± 3 | 7.46 | 7.45 | 1.3411 | 1.3456 |
| Bet:Suc:Pro | 541 ± 5 | 833 ± 2 | 7.42 | 7.42 | 1.3405 | 1.3460 |
| Gly:Glc | 709 ± 3 | 1207 ± 8 | 7.38 | 7.28 | 1.3411 | 1.3456 |
| Sample | Density (g/cm3) | Surface Tension (mN/m) | Contact Angle (θ) | |
|---|---|---|---|---|
| Tobrex® | n.a. | n.a. | 74.7 ± 5.0 | |
| HPMC 0.3% in PBS | n.a. | 43.82 ± 0.02 | 57.7 ± 1.4 | |
| Bet:Eg | 5% w/w in PBS 10% w/w in PBS | 1.0118 1.0172 | 68.18 ± 0.03 69.49 ± 0.01 | 105.3 ± 1.9 101.7 ± 0.8 |
| Bet:Glc | 5% w/w in PBS 10% w/w in PBS | 1.0150 1.0209 | 71.57 ± 0.01 70.01 ± 0.08 | 105.9 ± 1.7 106.9 ± 1.2 |
| Fru:Glc:Suc | 5% w/w in PBS 10% w/w in PBS | 1.0192 1.0328 | 70.71 ± 0.01 70.68 ± 0.08 | 104.3 ± 1.3 103.0 ± 3.1 |
| Glc:Pro:Gly | 5% w/w in PBS 10% w/w in PBS | 1.0171 1.0287 | 63.28 ± 0.02 60.28 ± 0.05 | 104.2 ± 0.2 100.7 ± 3.0 |
| Bet:Suc:Gly | 5% w/w in PBS 10% w/w in PBS | 1.0165 1.0277 | 70.31 ± 0.04 68.96 ± 0.09 | 102.2 ± 0.9 104.4 ± 0.1 |
| Bet:Gly | 5% w/w in PBS 10% w/w in PBS | 1.0148 1.0239 | 66.23 ± 0.02 66.68 ± 0.07 | 106.3 ± 2.0 107.3 ± 0.2 |
| Bet:Pro | 5% w/w in PBS 10% w/w in PBS | 1.0129 1.0197 | 69.24 ± 0.03 68.13 ± 0.09 | 109.9 ± 0.1 108.0 ± 3.4 |
| Bet:NAC | 5% w/w in PBS 10% w/w in PBS | 1.0148 1.0230 | 68.51 ± 0.03 67.09 ± 0.05 | 105.7 ± 3.0 103.0 ± 3.1 |
| Bet:Sorb | 5% w/w in PBS 10% w/w in PBS | 1.0167 1.0221 | 68.44 ± 0.07 66.30 ± 0.08 | 108.8 ± 2.0 103.2 ± 3.0 |
| Bet:Suc | 5% w/w in PBS 10% w/w in PBS | 1.0150 1.0246 | 70.94 ± 0.02 69.10 ± 0.04 | 103.3 ± 2.5 104.9 ± 1.6 |
| Bet:Suc:Pro | 5% w/w in PBS 10% w/w in PBS | 1.0162 1.0258 | 66.30 ± 0.05 66.51 ± 0.04 | 101.9 ± 2.3 108.0 ± 1.3 |
| Gly:Glc | 5% w/w in PBS 10% w/w in PBS | 1.0189 1.0316 | 70.70 ± 0.02 64.17 ± 0.06 | 103.0 ± 2.9 103.9 ± 1.1 |
| NADES | Solubility in Pure NADES | Solubility in 5% Aqueous Solutions | ||||
|---|---|---|---|---|---|---|
| 10 mg/g | 20 mg/g | 30 mg/g | 10 mg/g (0.5 mg ACZ/mL) | 20 mg/g (1 mg ACZ/mL) | 30 mg/g (1.5 mg ACZ/mL) | |
| Bet:Eg | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Bet:Glc | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Fru:Glc:Suc | n.s. | n.s. | n.s | n.a. | n.a. | n.a. |
| Glc:Pro:Gly | ++++ | ++++ | n.s. | ++++ | ++++ | n.a. |
| Bet:Suc:Gly | ++++ | ++++ | n.s. | ++++ | ++++ | n.a. |
| Bet:Gly | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Bet:Pro | n.s. | n.s. | n.s. | n.a. | n.a. | n.a. |
| Bet:NAC | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Bet:Sorb | n.s. | n.s. | n.s. | n.a. | n.a. | n.a. |
| Bet:Suc | ++++ | ++++ | ++++ | ++++ | ++++ | ++ |
| Bet:Suc:Pro | ++++ | ++++ | n.s. | ++++ | ++++ | n.a. |
| Gly:Glc | ++++ | n.s. | n.s. | ++++ | n.a. | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarmento, C.; Monteiro, H.; Paiva, A.; Duarte, A.R.C.; Jesus, A.R. Using Natural Deep Eutectic Systems as Alternative Media for Ocular Applications. Pharmaceutics 2023, 15, 1553. https://doi.org/10.3390/pharmaceutics15051553
Sarmento C, Monteiro H, Paiva A, Duarte ARC, Jesus AR. Using Natural Deep Eutectic Systems as Alternative Media for Ocular Applications. Pharmaceutics. 2023; 15(5):1553. https://doi.org/10.3390/pharmaceutics15051553
Chicago/Turabian StyleSarmento, Célia, Hugo Monteiro, Alexandre Paiva, Ana Rita C. Duarte, and Ana Rita Jesus. 2023. "Using Natural Deep Eutectic Systems as Alternative Media for Ocular Applications" Pharmaceutics 15, no. 5: 1553. https://doi.org/10.3390/pharmaceutics15051553
APA StyleSarmento, C., Monteiro, H., Paiva, A., Duarte, A. R. C., & Jesus, A. R. (2023). Using Natural Deep Eutectic Systems as Alternative Media for Ocular Applications. Pharmaceutics, 15(5), 1553. https://doi.org/10.3390/pharmaceutics15051553