Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems
Abstract
1. Introduction
2. Methods and Metrics Used to Construct the Database
2.1. Preparation of the Database
2.2. Drug Loading and Release Metrics
3. Database of Carbon-Nanomaterial-Based Cancer Therapeutics
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-FU | 5-Fluorouracil | HPMC | Hydroxypropyl methylcellulose |
| 6-MP | 6-Mercaptopurine | HSA | Human serum albumin |
| A1 | A549-cell-targeting oligonucleotide | HYD | Hydrazone |
| ADA | Adamantane | IM | Imatinib |
| ADH | Adipic acid dihydrazide | iRGD | PEGylated RGD peptide |
| AF | Alexa Fluor, AF488/647 | LA | Lactobionic acid |
| AL | Alendronate | LE | Lentinan |
| Anti-EpCAM | Epithelial cell adhesion molecule antibody | LIN | Linoleic acid |
| APA | Aspartic acid | MA | Mannose |
| Apt | Aptamer | mAb | Anti-VEGF monoclonal antibody |
| AQ4N | Banoxantrone dihydrochloride | mCNF | Mesoporous carbon nanoframe |
| Aso | Bcl-2 phosphorothioate antisense deoxyoligonucleotide | MET | Metformin |
| ATRA | All-trans retinoic acid | MitP | Mitochondrion-targeting peptide |
| BSA | Bovine serum albumin | MMC | Mitomycin C |
| BT | Biotin | MMT | Montmorillonite |
| CB[7] | Cucurbit[7]uril | MMT7 | Macrophage membrane hybridized with T7 peptide |
| CD | Carbon dot | MPA | Mercaptopropionic acid |
| CDT | Cyclodextrin | MSCD | Mesoporous silica carbon dot |
| Ce6 | Chlorin e6 | MTX | Mitoxantrone |
| CisP | Cisplatin | METX | Methotrexate |
| CMC | Carboxymethyl cellulose | MWCNT | Multiwalled carbon nanotube |
| CNP | Carbon nanoparticle | NCC | Nanocrystalline cellulose |
| CNH | Carbon nanohorn | NCNC | Nitrogen-doped carbon nanotube cup |
| CNM | Carbon nanomaterial | ND | Nanodiamond |
| CNR | Carbon nanoring | N-GO | Nitrogen-doped graphene oxide |
| CNT | Carbon nanotube | NGR | Aspargine–glycine–arginine peptide |
| CO | Chito oligosaccharide | NIR | Near-infrared |
| CP | Carboplatin | NMCS | Nitrogen-doped mesoporous carbon sphere |
| CQD | Carbon quantum dot | N-prGO | Nitrogen-doped porous reduced graphene oxide |
| cRGD | Cyclic RGD motif | OP | Oxaliplatin |
| CS | Chitosan | PA | P-gp monoclonal antibody |
| CNS | Carbon nanosphere | PAA | Poly(acrylic acid) |
| CUR | Curcumin | PAMAM | Poly(amidoamine) |
| CWKG(KWKG)6 | H-(-CysTrp-Lys-Gly-)(-Lys-Trp-Lys-Gly-)6-OH peptide | PANI | Poly(aniline) |
| CβCD | Carboxymethyl β-cyclodextrin | PC | Phosphatidylcholine |
| DCA-HPCHS | Deoxycholic acid-modified hydroxypropyl chitosan | PDA | Polydopamine |
| DES | Deep eutectic solvent | PDT | Photodynamic therapy |
| DLC | Drug loading content | PEG | Polyethylene glycol |
| DLE | Drug loading efficiency | PEI | Polyethyleneimine |
| DMPE | 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine | PF68 | Pluronic F68 |
| DOA | 2-(2-(docosyloxy)-2-oxoethoxy)acetic acid | PGA | Poly(glycolide) |
| DOX | Doxorubicin | P-gp | P-glycoprotein antibodies |
| DPPTE | 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanol | PHEA | Ethanolamine |
| DRE | Drug release efficiency | PHEMA | Polyhydroxyethyl methacrylate |
| DSPE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine | PLA | Poly(lactic acid) |
| DTX | Docetaxel | PLGA | Poly(lactic-co-glycolic acid) |
| E2 | β-estradiol | PMAA | Poly(methacrylic acid) |
| EGF | Epidermal growth factor | PNM | Poly(N-isopropylacrylamide) |
| EPI | Epirubicin | PNVCL | Poly(N-vinylcaprolactam) |
| Et | Etoposide | POEGMEA | Poly(oligoethylene glycol methyl ether acrylate) |
| Exo | Cancer cell exosomes | PRM | Peptide protamine sulphate |
| FA | Folic acid | PTT | Photothermal therapy |
| FCDb | Biotinylated Fe2+-doped carbon dot | PTX | Paclitaxel |
| f-CNTs | Functionalised carbon nanotubes | PVA | Poly(vinyl acetate) |
| FITC | Fluorescein isothiocyanate | PVP | Polyvinylpyrrolidone |
| FSCNO | Fucoidan-decorated silica–carbon nano-onion | RBC | Red blood cell membrane |
| GA | Galactose | Rf | Riboflavin |
| GE11 | EGFR antagonist peptide | rGO | Reduced graphene oxide |
| GEF | Gefitinib | ROS | Reactive oxygen species |
| GEM | Gemcitabine | SA | Sodium alginate |
| GFLG | Gly-phe-leu-gly enzyme-sensitive peptide | SB | SB-431542 (TGF-β inhibitor) |
| GGN | Graphene–gold nanocomposites | SLP2 | shRNA plasmid DNA |
| GlcN | Glucosamine | SWCNT | Single-walled carbon nanotube |
| GNR | Gold nanorod | TAM | Tamoxifen |
| GO | Graphene oxide | TAT | Trans-activating transcriptional activator peptide |
| GOQD | Graphene oxide quantum dot | TAU | Taurine |
| GOx | Glucose oxidase | TEG | Tetra(ethylene glycol) |
| GQD | Graphene quantum dot | Tf | Transferrin |
| GRP | Gastrin-releasing peptide | TM | Temozolomide |
| HA | Hyaluronic acid | TP | Tumour-targeting peptide (CKQFSALPFNFYT) |
| HIF | Anti-hypoxia-inducible factor 1α antibody | TR | Transferrin |
| HM | HM30181A transmembrane P-glycoprotein inhibitor | TT | Topotecan |
| HMCS | Hollow mesoporous carbon sphere | UA | Urocanic acid |
| HPMC | Hydroxypropyl methylcellulose | VEGF | Vascular endothelial growth factor |
| HSA | Human serum albumin | γPGA | γ-polyglutamic acid |
References
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A Decade of Graphene Research: Production, Applications and Outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Seifalian, A. A New Era of Cancer Treatment: Carbon Nanotubes as Drug Delivery Tools. Int. J. Nanomed. 2011, 2011, 2963–2979. [Google Scholar] [CrossRef] [PubMed]
- Lukyanov, A.N.; Torchilin, V.P. Micelles from Lipid Derivatives of Water-Soluble Polymers as Delivery Systems for Poorly Soluble Drugs. Adv. Drug Deliv. Rev. 2004, 56, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotech. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- POTOČNIK, J. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial Text with EEA Relevance. Off. J. Eur. Union 2011, 275, 38–40. [Google Scholar]
- Yang, S.; Liu, J.; Ping, Y.; Wang, Z.; Zhang, J.; Zhang, L.; Cui, L.; Xiao, Y.; Qu, L. Multi-Functionalized Single-Walled Carbon Nanotubes as Delivery Carriers: Promote the Targeting Uptake and Antitumor Efficacy of Doxorubicin. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 801–817. [Google Scholar] [CrossRef]
- Mohan, H.; Bincoletto, V.; Arpicco, S.; Giordani, S. Supramolecular Functionalisation of B/N Co-Doped Carbon Nano-Onions for Novel Nanocarrier Systems. Materials 2022, 15, 5987. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Han, H.; Li, H.; Jin, Q.; Wu, Y.; Chakrabortty, S.; Weil, T.; Ji, J. Polymer Coated Nanodiamonds as Gemcitabine Prodrug with Enzymatic Sensitivity for Pancreatic Cancer Treatment. Prog. Nat. Sci. Mater. Int. 2020, 30, 711–717. [Google Scholar] [CrossRef]
- Ma, X.; Shu, C.; Guo, J.; Pang, L.; Su, L.; Fu, D.; Zhong, W. Targeted Cancer Therapy Based on Single-Wall Carbon Nanohorns with Doxorubicin in Vitro and in Vivo. J. Nanopart. Res. 2014, 16, 2497. [Google Scholar] [CrossRef]
- Bartkowski, M.; Giordani, S. Carbon Nano-Onions as Potential Nanocarriers for Drug Delivery. Dalton Trans. 2021, 50, 2300–2309. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced Permeability and Retention Effect: A Key Facilitator for Solid Tumor Targeting by Nanoparticles. Photodiagn. Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Song, M.; Chen, C.; Du, Y.; Li, C.; Han, Y.; Yan, F.; Shi, Z.; Feng, S. Bortezomib-Encapsulated CuS/Carbon Dot Nanocomposites for Enhanced Photothermal Therapy via Stabilization of Polyubiquitinated Substrates in the Proteasomal Degradation Pathway. ACS Nano 2020, 14, 10688–10703. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ghosh, A.K.; Chowdhury, M.; Das, P.K. Folic Acid-Functionalized Carbon Dot-Enabled Starvation Therapy in Synergism with Paclitaxel against Breast Cancer. ACS Appl. Bio Mater. 2022, 5, 2389–2402. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, N.; Liu, B.; Du, Y.; Li, R.; Meng, Y.; Feng, Y.; Shan, Z.; Meng, S. A Phototheranostic Nanoparticle for Cancer Therapy Fabricated by BODIPY and Graphene to Realize Photo-Chemo Synergistic Therapy and Fluorescence/Photothermal Imaging. Dye Pigment. 2020, 177, 108262. [Google Scholar] [CrossRef]
- Fiekkies, J.T.R.; Fourie, E.; Erasmus, E. Cisplatin-Functionalized Nanodiamonds: Preparation and Characterization, with Potential Antineoplastic Application. Appl. Nanosci. 2021, 11, 2235–2245. [Google Scholar] [CrossRef]
- Keklikcioglu Cakmak, N.; Eroglu, A. Doxorubicin and Tamoxifen Loaded Graphene Oxide Nanoparticle Functionalized with Chitosan and Folic Acid for Anticancer Drug Delivery. Polym. Bull. 2023, 80, 2171–2185. [Google Scholar] [CrossRef]
- Bartelmess, J.; Giordani, S. Carbon Nano-Onions (Multi-Layer Fullerenes): Chemistry and Applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Bartkowski, M.; Giordani, S. Supramolecular Chemistry of Carbon Nano-Onions. Nanoscale 2020, 12, 9352–9358. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Seyedhosseini Ghaheh, H.; Ghasemi, F.; Shariati, L.; Rafienia, M.; Bidram, E.; Zarrabi, A. Graphene Oxide Quantum Dot-Chitosan Nanotheranostic Platform as a PH-Responsive Carrier for Improving Curcumin Uptake Internalization: In Vitro & in Silico Study. Biomater. Adv. 2022, 139, 213017. [Google Scholar] [CrossRef]
- Zhang, G.; Chang, H.; Amatore, C.; Chen, Y.; Jiang, H.; Wang, X. Apoptosis Induction and Inhibition of Drug Resistant Tumor Growth in Vivo Involving Daunorubicin-Loaded Graphene–Gold Composites. J. Mater. Chem. B 2013, 1, 493–499. [Google Scholar] [CrossRef]
- Gao, Q.; Gao, J.; Ding, C.; Li, S.; Deng, L.; Kong, Y. Construction of a PH- and near-Infrared Irradiation-Responsive Nanoplatform for Chemo-Photothermal Therapy. Int. J. Pharm. 2021, 593, 120112. [Google Scholar] [CrossRef] [PubMed]
- Arpicco, S.; Bartkowski, M.; Barge, A.; Zonari, D.; Serpe, L.; Milla, P.; Dosio, F.; Stella, B.; Giordani, S. Effects of the Molecular Weight of Hyaluronic Acid in a Carbon Nanotube Drug Delivery Conjugate. Front. Chem. 2020, 8, 578008. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, S.; Kumar, M.; Sharma, G.; Kumar, R.; Singh, B.; Raza, K. Fullerenol-Based Intracellular Delivery of Methotrexate: A Water-Soluble Nanoconjugate for Enhanced Cytotoxicity and Improved Pharmacokinetics. AAPS PharmSciTech 2018, 19, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- CAS SciFindern. Available online: https://scifinder-n.cas.org/ (accessed on 12 April 2023).
- Wang, R.; Cui, H.; Wang, J.; Li, N.; Zhao, Q.; Zhou, Y.; Lv, Z.; Zhong, W. Enhancing the Antitumor Effect of Methotrexate in Intro and in Vivo by a Novel Targeted Single-Walled Carbon Nanohorn-Based Drug Delivery System. RSC Adv. 2016, 6, 47272–47280. [Google Scholar] [CrossRef]
- Zavareh, H.S.; Pourmadadi, M.; Moradi, A.; Yazdian, F.; Omidi, M. Chitosan/Carbon Quantum Dot/Aptamer Complex as a Potential Anticancer Drug Delivery System towards the Release of 5-Fluorouracil. Int. J. Biol. Macromol. 2020, 165, 1422–1430. [Google Scholar] [CrossRef]
- Kaur, S.; Mehra, N.K.; Jain, K.; Jain, N.K. Development and Evaluation of Targeting Ligand-Anchored CNTs as Prospective Targeted Drug Delivery System. Artif. Cells Nanomed. Biotechnol. 2017, 45, 242–250. [Google Scholar] [CrossRef]
- Nivethaa, E.A.K.; Dhanavel, S.; Narayanan, V.; Narayana Kalkura, S.; Sivasankari, J.; Sivanandham, N.; Stephen, A. CS/Au/MWCNT Nanohybrid as an Efficient Carrier for the Sustained Release of 5-FU and a Study of Its Cytotoxicity on MCF-7. RSC Adv. 2021, 11, 4584–4592. [Google Scholar] [CrossRef]
- Garg, S.; Garg, A.; Sahu, N.K.; Yadav, A.K. Synthesis and Characterization of Nanodiamond-Anticancer Drug Conjugates for Tumor Targeting. Diam. Relat. Mater. 2019, 94, 172–185. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Li, S.; Chen, X.; Zhang, M.; Wang, C.; Wang, T.; Li, L. A Designed Synthesis of Multifunctional Carbon Nanoframes for Simultaneous Imaging and Synergistic Chemo-Photothermal Cancer Therapy. New J. Chem. 2018, 42, 923–929. [Google Scholar] [CrossRef]
- Dou, Z.; Xu, Y.; Sun, H.; Liu, Y. Synthesis of PEGylated Fullerene–5-Fluorouracil Conjugates to Enhance the Antitumor Effect of 5-Fluorouracil. Nanoscale 2012, 4, 4624. [Google Scholar] [CrossRef]
- Fu, D.; You, J.; Guo, R.; Zhang, J.; Li, Q.; Wen, J.; Wang, H.; Yan, H. Preparation of Nanostructured Graphene Oxide and Its Application in Drug Loading and Sustained Release. ChemistrySelect 2022, 7, e202200670. [Google Scholar] [CrossRef]
- Hassani, S.; Gharehaghaji, N.; Divband, B. Chitosan-Coated Iron Oxide/Graphene Quantum Dots as a Potential Multifunctional Nanohybrid for Bimodal Magnetic Resonance/Fluorescence Imaging and 5-Fluorouracil Delivery. Mater. Today Commun. 2022, 31, 103589. [Google Scholar] [CrossRef]
- Hasanin, M.S.; El-Sakhawy, M.; Ahmed, H.Y.; Kamel, S. Hydroxypropyl Methylcellulose/Graphene Oxide Composite as Drug Carrier System for 5-fluorouracil. Biotechnol. J. 2022, 17, 2100183. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Yu, Y.; Li, L.; Liu, B.; Liu, Y. Fabrication and Characterization of Taurine Functionalized Graphene Oxide with 5-Fluorouracil as Anticancer Drug Delivery Systems. Nanoscale Res. Lett. 2021, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Horo, H.; Saha, M.; Das, H.; Mandal, B.; Kundu, L.M. Synthesis of Highly Fluorescent, Amine-Functionalized Carbon Dots from Biotin-Modified Chitosan and Silk-Fibroin Blend for Target-Specific Delivery of Antitumor Agents. Carbohydr. Polym. 2022, 277, 118862. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S. DSPE-PEG-Coated Uniform Nitrogen-Doped Carbon Capsules for NIR-Mediated Synergistic Chemophototherapy of Skin Cancer. ACS Appl. Bio Mater. 2021, 4, 7059–7069. [Google Scholar] [CrossRef]
- Talib, A.B.; Mohammed, M.H. Preparation, Characterization and Preliminary Cytotoxic Evaluation of 6-Mercaptopurine-Coated Biotinylated Carbon Dots Nanoparticles as a Drug Delivery System. Mater. Today Proc. 2021, 80, 2327–2333. [Google Scholar] [CrossRef]
- Chaudhari, N.S.; Pandey, A.P.; Patil, P.O.; Tekade, A.R.; Bari, S.B.; Deshmukh, P.K. Graphene Oxide Based Magnetic Nanocomposites for Efficient Treatment of Breast Cancer. Mater. Sci. Eng. C 2014, 37, 278–285. [Google Scholar] [CrossRef]
- González-Domínguez, J.M.; Grasa, L.; Frontiñán-Rubio, J.; Abás, E.; Domínguez-Alfaro, A.; Mesonero, J.E.; Criado, A.; Ansón-Casaos, A. Intrinsic and Selective Activity of Functionalized Carbon Nanotube/Nanocellulose Platforms against Colon Cancer Cells. Colloids Surf. B Biointerfaces 2022, 212, 112363. [Google Scholar] [CrossRef]
- Randive, D.S.; Gavade, A.S.; Shejawal, K.P.; Bhutkar, M.A.; Bhinge, S.D.; Jadhav, N.R. Colon Targeted Dosage Form of Capecitabine Using Folic Acid Anchored Modified Carbon Nanotube: In Vitro Cytotoxicity, Apoptosis and in Vivo Roentgenographic Study. Drug Dev. Ind. Pharm. 2021, 47, 1401–1412. [Google Scholar] [CrossRef]
- Makharza, S.; Cirillo, G.; Bachmatiuk, A.; Vittorio, O.; Mendes, R.G.; Oswald, S.; Hampel, S.; Rümmeli, M.H. Size-Dependent Nanographene Oxide as a Platform for Efficient Carboplatin Release. J. Mater. Chem. B 2013, 1, 6107. [Google Scholar] [CrossRef] [PubMed]
- Makharza, S.; Vittorio, O.; Cirillo, G.; Oswald, S.; Hinde, E.; Kavallaris, M.; Büchner, B.; Mertig, M.; Hampel, S. Graphene Oxide-Gelatin Nanohybrids as Functional Tools for Enhanced Carboplatin Activity in Neuroblastoma Cells. Pharm. Res. 2015, 32, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Salas-Treviño, D.; Saucedo-Cárdenas, O.; Montes-de-Oca-Luna, R.; Rodríguez-Rocha, H.; García-García, A.; Montes-de-Oca-Luna, R.; Piña-Mendoza, E.I.; Contreras-Torres, F.F.; García-Rivas, G.; Soto-Domínguez, A. Hyaluronate Functionalized Multi-Wall Carbon Nanotubes Filled with Carboplatin as a Novel Drug Nanocarrier against Murine Lung Cancer Cells. Nanomaterials 2019, 9, 1572. [Google Scholar] [CrossRef] [PubMed]
- Balas, M.; Constanda, S.; Duma-Voiculet, A.; Prodana, M.; Hermenean, A.; Pop, S.; Demetrescu, I.; Dinischiotu, A. Fabrication and Toxicity Characterization of a Hybrid Material Based on Oxidized and Aminated MWCNT Loaded with Carboplatin. Toxicol. Vitr. 2016, 37, 189–200. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, Y.; Sun, L.; Yao, H.; Zhao, B.; Zhang, R.; Zhang, Y. Folic Acid Functionalized γ Cyclodextrin C60, A Novel Vehicle for Tumor-Targeted Drug Delivery. J. Biomed. Nanotechnol. 2016, 12, 1393–1403. [Google Scholar] [CrossRef]
- Ramadan, I.; Nassar, M.Y.; Gomaa, A. In-Vitro Investigation of the Anticancer Efficacy of Carboplatin-Loaded Chitosan Nanocomposites Against Breast and Liver Cancer Cell Lines. J. Polym. Env. 2022, 31, 1102–1115. [Google Scholar] [CrossRef]
- Wei, L.; Li, G.; Lu, T.; Wei, Y.; Nong, Z.; Wei, M.; Pan, X.; Qin, Q.; Meng, F.; Li, X. Functionalized Graphene Oxide as Drug Delivery Systems for Platinum Anticancer Drugs. J. Pharm. Sci. 2021, 110, 3631–3638. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Meng, F.-Y.; Li, X.-H.; Wu, N.-N.; Deng, Y.-H.; Wei, L.-Y.; Zeng, X.-P. Magnetic Graphene Oxide-Fe3O4 -PANI Nanoparticle Adsorbed Platinum Drugs as Drug Delivery Systems for Cancer Therapy. J. Nanosci. Nanotechnol. 2019, 19, 7517–7525. [Google Scholar] [CrossRef]
- Chen, J.-P.; Lu, Y.-J.; Hung, S.-C.; Chen, J.-P.; Wei, K.-C. Improving Thermal Stability and Efficacy of BCNU in Treating Glioma Cells Using PAA-Functionalized Graphene Oxide. Int. J. Nanomed. 2012, 2020, 1737–1747. [Google Scholar] [CrossRef]
- Salazar, A.; Pérez-de la Cruz, V.; Muñoz-Sandoval, E.; Chavarria, V.; García Morales, M.d.L.; Espinosa-Bonilla, A.; Sotelo, J.; Jiménez-Anguiano, A.; Pineda, B. Potential Use of Nitrogen-Doped Carbon Nanotube Sponges as Payload Carriers Against Malignant Glioma. Nanomaterials 2021, 11, 1244. [Google Scholar] [CrossRef]
- Singh, G.; Nenavathu, B.P.; Imtiyaz, K.; Moshahid A Rizvi, M. Fabrication of Chlorambucil Loaded Graphene- Oxide Nanocarrier and Its Application for Improved Antitumor Activity. Biomed. Pharmacother. 2020, 129, 110443. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, N.; Mahjoub, A.R.; Shokrollahi, S.; Amiri, A.; Abolhosseini Shahrnoy, A. Novel Biocompatible Amino Acids-Functionalized Three-Dimensional Graphene Foams: As the Attractive and Promising Cisplatin Carriers for Sustained Release Goals. Int. J. Pharm. 2020, 589, 119857. [Google Scholar] [CrossRef] [PubMed]
- Ajima, K.; Yudasaka, M.; Murakami, T.; Maigné, A.; Shiba, K.; Iijima, S. Carbon Nanohorns as Anticancer Drug Carriers. Mol. Pharm. 2005, 2, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Isaac, K.M.; Sabaraya, I.V.; Ghousifam, N.; Das, D.; Pekkanen, A.M.; Romanovicz, D.K.; Long, T.E.; Saleh, N.B.; Rylander, M.N. Functionalization of Single-Walled Carbon Nanohorns for Simultaneous Fluorescence Imaging and Cisplatin Delivery in Vitro. Carbon 2018, 138, 309–318. [Google Scholar] [CrossRef]
- Guven, A.; Villares, G.J.; Hilsenbeck, S.G.; Lewis, A.; Landua, J.D.; Dobrolecki, L.E.; Wilson, L.J.; Lewis, M.T. Carbon Nanotube Capsules Enhance the in Vivo Efficacy of Cisplatin. Acta Biomater. 2017, 58, 466–478. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, Y.; Zhang, J.; Kong, H.; Tang, X.; Pan, L.; Xia, K.; Aldalbahi, A.; Li, A.; Tai, R.; et al. Sodium Alginate-Functionalized Nanodiamonds as Sustained Chemotherapeutic Drug-Release Vectors. Carbon 2016, 97, 78–86. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.; Wang, H.; Jiao, Y.; Wang, H.; Yu, Y.; Zhi, J. Fabrication of an EGF Modified Nanodiamonds-Based Anti-Cancer Drug Targeted Delivery System and Drug Carrier Uptake Visualization by 3D Raman Microscopy. RSC Adv. 2016, 6, 44543–44551. [Google Scholar] [CrossRef]
- Lynchak, O.; Byelinska, I.; Dziubenko, N.; Kuznietsova, H.; Abramchuk, O.; Prylutska, S. Acute Toxicity of C60–Cis-Pt Nanocomplex in Vivo. Appl. Nanosci. 2022, 12, 439–447. [Google Scholar] [CrossRef]
- Prylutska, S.; Grynyuk, I.; Skaterna, T.; Horak, I.; Grebinyk, A.; Drobot, L.; Matyshevska, O.; Senenko, A.; Prylutskyy, Y.; Naumovets, A.; et al. Toxicity of C60 Fullerene–Cisplatin Nanocomplex against Lewis Lung Carcinoma Cells. Arch. Toxicol. 2019, 93, 1213–1226. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Chen, J.; Mao, L.; Zeng, G.; Wen, Y.; Tian, J.; Zhou, N.; Zhang, X.; Wei, Y. Facile Fabrication of Carboxyl Groups Modified Fluorescent C 60 through a One-Step Thiol-Ene Click Reaction and Their Potential Applications for Biological Imaging and Intracellular Drug Delivery. J. Taiwan Inst. Chem. Eng. 2018, 86, 192–198. [Google Scholar] [CrossRef]
- Prylutska, S.; Panchuk, R.; Gołuński, G.; Skivka, L.; Prylutskyy, Y.; Hurmach, V.; Skorohyd, N.; Borowik, A.; Woziwodzka, A.; Piosik, J.; et al. C60 Fullerene Enhances Cisplatin Anticancer Activity and Overcomes Tumor Cell Drug Resistance. Nano Res. 2017, 10, 652–671. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Rejeeth, C.; Vivek, R.; Ponraj, T.; Jayaraman, K.; Anandasadagopan, S.K.; Vinayaga Moorthi, P. Design of Bio-Graphene-Based Multifunctional Nanocomposites Exhibits Intracellular Drug Delivery in Cervical Cancer Treatment. ACS Appl. Bio Mater. 2022, 5, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Long, Z.; Qiu, Q.; Liu, F.; Xu, Y.; Zhang, T.; Guo, R.; Zhong, W.; Huang, S.; Chen, S. Graphene Quantum Dots-Based Targeted Nanoprobes Detecting Drug Delivery, Imaging, and Enhanced Chemotherapy of Nasopharyngeal Carcinoma. Bioeng. Transl. Med. 2022, 7, e10270. [Google Scholar] [CrossRef]
- Giusto, E.; Žárská, L.; Beirne, D.F.; Rossi, A.; Bassi, G.; Ruffini, A.; Montesi, M.; Montagner, D.; Ranc, V.; Panseri, S. Graphene Oxide Nanoplatforms to Enhance Cisplatin-Based Drug Delivery in Anticancer Therapy. Nanomaterials 2022, 12, 2372. [Google Scholar] [CrossRef]
- Liu, P.; Xie, X.; Liu, M.; Hu, S.; Ding, J.; Zhou, W. A Smart MnO2-Doped Graphene Oxide Nanosheet for Enhanced Chemo-Photodynamic Combinatorial Therapy via Simultaneous Oxygenation and Glutathione Depletion. Acta Pharm. Sin. B 2021, 11, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Astani, S.; Salehi, R.; Massoumi, B.; Massoudi, A. Co-Delivery of Cisplatin and Doxorubicin by Carboxylic Acid Functionalized Poly (Hydroxyethyl Methacrylate)/Reduced Graphene Nanocomposite for Combination Chemotherapy of Breast Cancer Cells. J. Biomater. Sci. Polym. Ed. 2021, 32, 657–677. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, T.; Han, Y.; Yang, Z.; Wei, J.; Jin, L.; Fan, H. A Convergent Synthetic Platform for Dual Anticancer Drugs Functionalized by Reduced Graphene Nanocomposite Delivery for Hepatocellular Cancer. Drug Deliv. 2021, 28, 1982–1994. [Google Scholar] [CrossRef]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated Nano-Graphene Oxide as a Nanocarrier for Delivering Mixed Anticancer Drugs to Improve Anticancer Activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef]
- Dučić, T.; Alves, C.S.; Vučinić, Ž.; Lázaro-Martínez, J.M.; Petković, M.; Soto, J.; Mutavdžić, D.; Valle Martínez de Yuso, M.; Radotić, K.; Algarra, M. S, N-Doped Carbon Dots-Based Cisplatin Delivery System in Adenocarcinoma Cells: Spectroscopical and Computational Approach. J. Colloid Interface Sci. 2022, 623, 226–237. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Liu, Y.; Wang, K. Design and Synthesis of Dual-Responsive Carbon Nanodots Loaded with Cisplatin for Targeted Therapy of Lung Cancer Therapy and Nursing Care. J. Clust. Sci. 2022, 33, 331–338. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, W.; Liu, S.; Han, F.; Wang, H.; Zhao, Y.; Zhou, Y.; Zhou, D. Cisplatin Loaded Multiwalled Carbon Nanotubes Reverse Drug Resistance in NSCLC by Inhibiting EMT. Cancer Cell Int. 2021, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ni, J.; Liu, L.; Gu, L.; Wu, Z.; Li, T.; Ivanovich, K.I.; Zhao, W.; Sun, T.; Wang, T. Imaging-Guided Chemo–Photothermal Polydopamine Carbon Dots for EpCAM-Targeted Delivery toward Liver Tumor. ACS Appl. Mater. Interfaces 2021, 13, 29340–29348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Duan, Q.; Li, Y.; Wang, J.; Zhang, W.; Sang, S. PH and Redox Dual-Sensitive Drug Delivery System Constructed Based on Fluorescent Carbon Dots. RSC Adv. 2021, 11, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Jin, R.; Mu, W. Near-Infrared Mediated Polymer-Coated Carbon Nanodots Loaded Cisplatin for Targeted Care Management of Lung Cancer Therapy. Process Biochem. 2020, 99, 27–35. [Google Scholar] [CrossRef]
- Razaghi, M.; Ramazani, A.; Khoobi, M.; Mortezazadeh, T.; Aksoy, E.A.; Küçükkılınç, T.T. Highly Fluorinated Graphene Oxide Nanosheets for Anticancer Linoleic-Curcumin Conjugate Delivery and T2-Weighted Magnetic Resonance Imaging: In Vitro and in Vivo Studies. J. Drug Deliv. Sci. Technol. 2020, 60, 101967. [Google Scholar] [CrossRef]
- Ghanbari, N.; Salehi, Z.; Khodadadi, A.A.; Shokrgozar, M.A.; Saboury, A.A. Glucosamine-Conjugated Graphene Quantum Dots as Versatile and PH-Sensitive Nanocarriers for Enhanced Delivery of Curcumin Targeting to Breast Cancer. Mater. Sci. Eng. C 2021, 121, 111809. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Hao, Y.; Wang, Y.; Jia, S.; Zhang, H.; Zhang, Y.; Zhang, Z. Formulation of Curcumin Delivery with Functionalized Single-Walled Carbon Nanotubes: Characteristics and Anticancer Effects in Vitro. Drug Deliv. 2014, 21, 379–387. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Hao, Y.; Wang, Y.; Jia, S.; Zhang, H. Enhancement of Curcumin Antitumor Efficacy and Further Photothermal Ablation of Tumor Growth by Single-Walled Carbon Nanotubes Delivery System in Vivo. Drug Deliv. 2019, 26, 1017–1026. [Google Scholar] [CrossRef]
- Consoli, G.M.L.; Giuffrida, M.L.; Satriano, C.; Musumeci, T.; Forte, G.; Petralia, S. A Novel Facile One-Pot Synthesis of Photothermally Responsive Carbon Polymer Dots as Promising Drug Nanocarriers. Chem. Commun. 2022, 58, 3126–3129. [Google Scholar] [CrossRef]
- Arvapalli, D.M.; Sheardy, A.T.; Allado, K.; Chevva, H.; Yin, Z.; Wei, J. Design of Curcumin Loaded Carbon Nanodots Delivery System: Enhanced Bioavailability, Release Kinetics, and Anticancer Activity. ACS Appl. Bio Mater. 2020, 3, 8776–8785. [Google Scholar] [CrossRef]
- Daneshmoghanlou, E.; Miralinaghi, M.; Moniri, E.; Sadjady, S.K. Fabrication of a PH-Responsive Magnetic Nanocarrier Based on Carboxymethyl Cellulose-Aminated Graphene Oxide for Loading and In-Vitro Release of Curcumin. J. Polym. Env. 2022, 30, 3718–3736. [Google Scholar] [CrossRef]
- Vahedi, N.; Tabandeh, F.; Mahmoudifard, M. Hyaluronic Acid–Graphene Quantum Dot Nanocomposite: Potential Target Drug Delivery and Cancer Cell Imaging. Biotech. App. Biochem. 2022, 69, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Baneshi, M.; Dadfarnia, S.; Haji Shabani, A.M.; Sabbagh, S.K.; Bardania, H. AS1411 Aptamer-Functionalized Graphene Oxide-Based Nano-Carrier for Active-Target and PH-Sensitive Delivery of Curcumin. J. Iran. Chem. Soc. 2022, 19, 2367–2376. [Google Scholar] [CrossRef]
- Kazemi, S.; Pourmadadi, M.; Yazdian, F.; Ghadami, A. The Synthesis and Characterization of Targeted Delivery Curcumin Using Chitosan-Magnetite-Reduced Graphene Oxide as Nano-Carrier. Int. J. Biol. Macromol. 2021, 186, 554–562. [Google Scholar] [CrossRef]
- Gui, X.; Chen, Y.; Zhang, Z.; Lei, L.; Zhu, F.; Yang, W.; Guo, Y.; Chu, M. Fluorescent Hollow Mesoporous Carbon Spheres for Drug Loading and Tumor Treatment through 980-Nm Laser and Microwave Co-Irradiation. Biomaterials 2020, 248, 120009. [Google Scholar] [CrossRef]
- Azqhandi, M.H.A.; Farahani, B.V.; Dehghani, N. Encapsulation of Methotrexate and Cyclophosphamide in Interpolymer Complexes Formed between Poly Acrylic Acid and Poly Ethylene Glycol on Multi-Walled Carbon Nanotubes as Drug Delivery Systems. Mater. Sci. Eng. C 2017, 79, 841–847. [Google Scholar] [CrossRef]
- Sheng, Y.; Dai, W.; Gao, J.; Li, H.; Tan, W.; Wang, J.; Deng, L.; Kong, Y. PH-Sensitive Drug Delivery Based on Chitosan Wrapped Graphene Quantum Dots with Enhanced Fluorescent Stability. Mater. Sci. Eng. C 2020, 112, 110888. [Google Scholar] [CrossRef]
- Sima, L.E.; Chiritoiu, G.; Negut, I.; Grumezescu, V.; Orobeti, S.; Munteanu, C.V.A.; Sima, F.; Axente, E. Functionalized Graphene Oxide Thin Films for Anti-Tumor Drug Delivery to Melanoma Cells. Front. Chem. 2020, 8, 184. [Google Scholar] [CrossRef]
- Moore, T.L.; Grimes, S.W.; Lewis, R.L.; Alexis, F. Multilayered Polymer-Coated Carbon Nanotubes to Deliver Dasatinib. Mol. Pharm. 2014, 11, 276–282. [Google Scholar] [CrossRef]
- Hosseini, L.; Mahboobnia, K.; Irani, M. Fabrication of PLA/MWCNT/Fe3O4 Composite Nanofibers for Leukemia Cancer Cells. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 176–182. [Google Scholar] [CrossRef]
- Man, H.B.; Kim, H.; Kim, H.-J.; Robinson, E.; Liu, W.K.; Chow, E.K.-H.; Ho, D. Synthesis of Nanodiamond–Daunorubicin Conjugates to Overcome Multidrug Chemoresistance in Leukemia. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, S.; Barzegar, A.; Mahnam, K. Absorption of Daunorubicin and Etoposide Drugs by Hydroxylated and Carboxylated Carbon Nanotube for Drug Delivery: Theoretical and Experimental Studies. J. Biomol. Struct. Dyn. 2022, 40, 10057–10064. [Google Scholar] [CrossRef] [PubMed]
- Sheykhisarem, R.; Dehghani, H. In Vitro Biocompatibility Evaluations of PH-Sensitive Bi2MoO6/NH2-GO Conjugated Polyethylene Glycol for Release of Daunorubicin in Cancer Therapy. Colloids Surf. B Biointerfaces 2023, 221, 113006. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, P.; Yin, Y.; Zhang, H.; Cai, C. Aptamer-Functionalized Graphene Oxide for Highly Efficient Loading and Cancer Cell-Specific Delivery of Antitumor Drug. J. Mater. Chem. B 2014, 2, 3849–3859. [Google Scholar] [CrossRef]
- Tas, A.; Keklikcioglu Cakmak, N. Synthesis of PEGylated Nanographene Oxide as a Nanocarrier for Docetaxel Drugs and Anticancer Activity on Prostate Cancer Cell Lines. Hum. Exp. Toxicol. 2021, 40, 172–182. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.-X.; Huang, H.-Y.; Chen, L.-Q.; Cui, J.-H.; Liu, Y.; Jin, H.; Lee, B.-J.; Cao, Q.-R. Effective Deactivation of A549 Tumor Cells in Vitro and in Vivo by RGD-Decorated Chitosan-Functionalized Single-Walled Carbon Nanotube Loading Docetaxel. Int. J. Pharm. 2018, 543, 8–20. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, N.; Shu, C.; Li, R.; Ma, X.; Li, X.; Wang, R.; Zhong, W. Docetaxel-Loaded Single-Wall Carbon Nanohorns Using Anti-VEGF Antibody as a Targeting Agent: Characterization, in Vitro and in Vivo Antitumor Activity. J. Nanopart. Res. 2015, 17, 207. [Google Scholar] [CrossRef]
- Raza, K.; Thotakura, N.; Kumar, P.; Joshi, M.; Bhushan, S.; Bhatia, A.; Kumar, V.; Malik, R.; Sharma, G.; Guru, S.K.; et al. C60 -Fullerenes for Delivery of Docetaxel to Breast Cancer Cells: A Promising Approach for Enhanced Efficacy and Better Pharmacokinetic Profile. Int. J. Pharm. 2015, 495, 551–559. [Google Scholar] [CrossRef]
- Liu, W.; Ye, X.; He, L.; Cheng, J.; Luo, W.; Zheng, M.; Hu, Y.; Zhang, W.; Cao, Y.; Ran, H.; et al. A Novel Targeted Multifunctional Nanoplatform for Visual Chemo-Hyperthermia Synergy Therapy on Metastatic Lymph Nodes via Lymphatic Delivery. J. Nanobiotechnol. 2021, 19, 432. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, Y.; He, L.; Liu, W.; Chen, Y.; Liu, F.; Guo, Y.; Ran, H.; Yang, L. Novel Multifunctional Nanoagent for Visual Chemo/Photothermal Therapy of Metastatic Lymph Nodes via Lymphatic Delivery. ACS Omega 2020, 5, 3194–3206. [Google Scholar] [CrossRef]
- Kim, D.; Kyung, H.; Lee, S.S.; Park, S.Y.; Yoo, W.C.; Lee, H.J. In Vitro Evaluation of Carbon-Based Nanospheres as Drug Delivery Vesicles in Breast Cancer Cell. Bull. Korean Chem. Soc. 2020, 41, 112–116. [Google Scholar] [CrossRef]
- Thotakura, N.; Sharma, S.; Khurana, R.K.; Babu, P.V.; Chitkara, D.; Kumar, V.; Singh, B.; Raza, K. Aspartic Acid Tagged Carbon Nanotubols as a Tool to Deliver Docetaxel to Breast Cancer Cells: Reduced Hemotoxicity with Improved Cytotoxicity. Toxicol. Vitr. 2019, 59, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-Y.; Su, Y.-L.; Cheng, W.; Hu, P.-F.; Chiang, C.-S.; Chen, W.-T.; Hu, S.-H. Graphene Quantum Dots-Mediated Theranostic Penetrative Delivery of Drug and Photolytics in Deep Tumors by Targeted Biomimetic Nanosponges. Nano Lett. 2019, 19, 69–81. [Google Scholar] [CrossRef]
- Thotakura, N.; Sharma, G.; Singh, B.; Kumar, V.; Raza, K. Aspartic Acid Derivatized Hydroxylated Fullerenes as Drug Delivery Vehicles for Docetaxel: An Explorative Study. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Tao, L.; Annie Bligh, S.W.; Yang, H.; Pan, Q.; Zhu, L. Targeted Delivery and Controlled Release of Doxorubicin into Cancer Cells Using a Multifunctional Graphene Oxide. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, F.; Liu, L.; Zhang, Y.; Li, Y.; Li, H.; Xie, J. Surface Modification of Graphene Oxide Nanosheets by Protamine Sulfate/Sodium Alginate for Anti-Cancer Drug Delivery Application. Appl. Surf. Sci. 2018, 440, 853–860. [Google Scholar] [CrossRef]
- Lu, T.; Nong, Z.; Wei, L.; Wei, M.; Li, G.; Wu, N.; Liu, C.; Tang, B.; Qin, Q.; Li, X.; et al. Preparation and Anti-Cancer Activity of Transferrin/Folic Acid Double-Targeted Graphene Oxide Drug Delivery System. J. Biomater. Appl. 2020, 35, 15–27. [Google Scholar] [CrossRef]
- Qi, Z.; Shi, J.; Zhu, B.; Li, J.; Cao, S. Gold Nanorods/Graphene Oxide Nanosheets Immobilized by Polydopamine for Efficient Remotely Triggered Drug Delivery. J. Mater. Sci. 2020, 55, 14530–14543. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.; Jiang, F.; Liu, Z.; Shu, C.; Wan, L.-J. In Vitro and in Vivo Photothermally Enhanced Chemotherapy by Single-Walled Carbon Nanohorns as a Drug Delivery System. J. Mater. Chem. B 2014, 2, 4726–4732. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, A.C.; Rakhra, K.; Sherlock, S.; Goodwin, A.; Chen, X.; Yang, Q.; Felsher, D.W.; Dai, H. Supramolecular Stacking of Doxorubicin on Carbon Nanotubes for In Vivo Cancer Therapy. Angew. Chem. Int. Ed. 2009, 48, 7668–7672. [Google Scholar] [CrossRef]
- Grebinyk, A.; Prylutska, S.; Grebinyk, S.; Prylutskyy, Y.; Ritter, U.; Matyshevska, O.; Dandekar, T.; Frohme, M. Complexation with C60 Fullerene Increases Doxorubicin Efficiency against Leukemic Cells In Vitro. Nanoscale Res. Lett. 2019, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Jović, D.S.; Seke, M.N.; Djordjevic, A.N.; Mrđanović, J.Ž.; Aleksić, L.D.; Bogdanović, G.M.; Pavić, A.B.; Plavec, J. Fullerenol Nanoparticles as a New Delivery System for Doxorubicin. RSC Adv. 2016, 6, 38563–38578. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Tan, L. A Novel Fullerene-Based Drug Delivery System Delivering Doxorubicin for Potential Lung Cancer Therapy. J. Nanosci. Nanotechnol. 2017, 17, 5147–5154. [Google Scholar] [CrossRef]
- Xiao, J.; Duan, X.; Yin, Q.; Zhang, Z.; Yu, H.; Li, Y. Nanodiamonds-Mediated Doxorubicin Nuclear Delivery to Inhibit Lung Metastasis of Breast Cancer. Biomaterials 2013, 34, 9648–9656. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.; Fu, C.; Feng, L.; Ji, Y.; Tao, L.; Li, S.; Wei, Y. PolyPEGylated Nanodiamond for Intracellular Delivery of a Chemotherapeutic Drug. Polym. Chem. 2012, 3, 2716–2719. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Wu, T.; Ran, G.; Song, Q. Template-Free Synthesis of Porous Fluorescent Carbon Nanomaterials with Gluten for Intracellular Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2022, 14, 21310–21318. [Google Scholar] [CrossRef]
- Thakur, C.K.; Neupane, R.; Karthikeyan, C.; Ashby, C.R.; Babu, R.J.; Boddu, S.H.S.; Tiwari, A.K.; Moorthy, N.S.H.N. Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells. Molecules 2022, 27, 7461. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.; Yin, Y.; Zhang, J.; Su, W.; White, A.M.; Jiang, B.; Xu, J.; Zhang, Y.; Stewart, S.; et al. Carbon Nano-Onion-Mediated Dual Targeting of P-Selectin and P-Glycoprotein to Overcome Cancer Drug Resistance. Nat. Commun. 2021, 12, 312. [Google Scholar] [CrossRef]
- Li, H.; Zeng, D.; Wang, Z.; Fang, L.; Li, F.; Wang, Z. Ultrasound-Enhanced Delivery of Doxorubicin/All-Trans Retinoic Acid-Loaded Nanodiamonds into Tumors. Nanomedicine 2018, 13, 981–996. [Google Scholar] [CrossRef]
- Du, X.; Li, L.; Wei, S.; Wang, S.; Li, Y. A Tumor-Targeted, Intracellular Activatable and Theranostic Nanodiamond Drug Platform for Strongly Enhanced in Vivo Antitumor Therapy. J. Mater. Chem. B 2020, 8, 1660–1671. [Google Scholar] [CrossRef]
- Falank, C.; Tasset, A.W.; Farrell, M.; Harris, S.; Everill, P.; Marinkovic, M.; Reagan, M.R. Development of Medical-Grade, Discrete, Multi-Walled Carbon Nanotubes as Drug Delivery Molecules to Enhance the Treatment of Hematological Malignancies. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102025. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-Synthesized Polysaccharide-Derived Carbon Dots as Therapeutic Cargoes and Toughening Agents for Elastomeric Gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, J.; Gong, C.; Wang, Y.; Gao, Y.; Yuan, Y. Intravenous Delivery of Enzalutamide Based on High Drug Loading Multifunctional Graphene Oxide Nanoparticles for Castration-Resistant Prostate Cancer Therapy. J. Nanobiotechnol. 2020, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.J.; Sun, L.; Liu, Y.; Jiang, S.; Pu, Y.; Li, J.; Zhang, Y. Monodistearoylphosphatidylethanolamine-Hyaluronic Acid Functionalization of Single-Walled Carbon Nanotubes for Targeting Intracellular Drug Delivery to Overcome Multidrug Resistance of Cancer Cells. Carbon 2016, 96, 362–376. [Google Scholar] [CrossRef]
- Suo, N.; Wang, M.; Jin, Y.; Ding, J.; Gao, X.; Sun, X.; Zhang, H.; Cui, M.; Zheng, J.; Li, N.; et al. Magnetic Multiwalled Carbon Nanotubes with Controlled Release of Epirubicin: An Intravesical Instillation System for Bladder Cancer. Int. J. Nanomed. 2019, 14, 1241–1254. [Google Scholar] [CrossRef]
- Wang, X.; Low, X.C.; Hou, W.; Abdullah, L.N.; Toh, T.B.; Mohd Abdul Rashid, M.; Ho, D.; Chow, E.K.-H. Epirubicin-Adsorbed Nanodiamonds Kill Chemoresistant Hepatic Cancer Stem Cells. ACS Nano 2014, 8, 12151–12166. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple Conjugated Carbon Dots as a Nano-Drug Delivery Model for Glioblastoma Brain Tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Wu, Y.-F.; Lan, T.-J.; Chen, Y.; Su, S.-H. Codelivery of Anticancer Drug and Photosensitizer by PEGylated Graphene Oxide and Cell Penetrating Peptide Enhanced Tumor-Suppressing Effect on Osteosarcoma. Front. Mol. Biosci. 2021, 7, 618896. [Google Scholar] [CrossRef]
- Lan, M.-Y.; Hsu, Y.-B.; Lan, M.-C.; Chen, J.-P.; Lu, Y.-J. Polyethylene Glycol-Coated Graphene Oxide Loaded with Erlotinib as an Effective Therapeutic Agent for Treating Nasopharyngeal Cancer Cells. Int. J. Nanomed. 2020, 15, 7569–7582. [Google Scholar] [CrossRef]
- Lam, A.T.N.; Yoon, J.-H.; Ly, N.H.; Joo, S.-W. Electrostatically Self-Assembled Quinazoline-Based Anticancer Drugs on Negatively-Charged Nanodiamonds for Overcoming the Chemoresistances in Lung Cancer Cells. BioChip J. 2018, 12, 163–171. [Google Scholar] [CrossRef]
- Li, L.; Pan, C.; Guo, Z.; Liu, B.; Pan, H.; Wu, H.; Liu, Y. Exploration on the Adsorption Mechanism of Aminopyrimidines and Quinazoline Compounds on Graphene Oxide: Hydrophobicity and Structure-Controlled Release Process. Mat. Express 2019, 9, 419–428. [Google Scholar] [CrossRef]
- Gholami, A.; Emadi, F.; Nazem, M.; Aghayi, R.; Khalvati, B.; Amini, A.; Ghasemi, Y. Expression of Key Apoptotic Genes in Hepatocellular Carcinoma Cell Line Treated with Etoposide-Loaded Graphene Oxide. J. Drug Deliv. Sci. Technol. 2020, 57, 101725. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Zhang, F.; Yin, Y.; Mei, L.; Song, F.; Tao, M.; Yue, W.; Zhong, W. Overcoming Multidrug Resistance by a Combination of Chemotherapy and Photothermal Therapy Mediated by Carbon Nanohorns. J. Mater. Chem. B 2016, 4, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.P.; Rezaei, B.; Khayamian, T.; Dinari, M.; Shamili, F.H.; Ramezani, M.; Alibolandi, M. Ingenious PH-Sensitive Etoposide Loaded Folic Acid Decorated Mesoporous Silica-Carbon Dot with Carboxymethyl-Βcyclodextrin Gatekeeper for Targeted Drug Delivery and Imaging. Mater. Sci. Eng. C 2018, 92, 892–901. [Google Scholar] [CrossRef]
- Heger, Z.; Polanska, H.; Krizkova, S.; Balvan, J.; Raudenska, M.; Dostalova, S.; Moulick, A.; Masarik, M.; Adam, V. Co-Delivery of VP-16 and Bcl-2-Targeted Antisense on PEG-Grafted OMWCNTs for Synergistic in Vitro Anti-Cancer Effects in Non-Small and Small Cell Lung Cancer. Colloids Surf. B Biointerfaces 2017, 150, 131–140. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized Graphene Oxide as a Nanocarrier for Dual Drug Delivery Applications: The Synergistic Effect of Quercetin and Gefitinib against Ovarian Cancer Cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef]
- Gautam, A.; Pal, K. Gefitinib Conjugated PEG Passivated Graphene Quantum Dots Incorporated PLA Microspheres for Targeted Anticancer Drug Delivery. Heliyon 2022, 8, e12512. [Google Scholar] [CrossRef]
- More, M.P.; Deshmukh, P.K. Quality by Design Approach for the Synthesis of Graphene Oxide Nanosheets Using Full Factorial Design with Enhanced Delivery of Gefitinib Nanocrystals. Mater. Res. Express 2021, 8, 075602. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, D.; Lian, S.; Zheng, J.; Li, B.; Li, T.; Jia, L. Redox-Responsive Hyaluronic Acid-Functionalized Graphene Oxide Nanosheets for Targeted Delivery of Water-Insoluble Cancer Drugs. Int. J. Nanomed. 2018, 13, 7457–7472. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Jain, A.; Shrivastava, C.; Jain, A.K. Hyaluronic Acid Conjugated Multi-Walled Carbon Nanotubes for Colon Cancer Targeting. Int. J. Biol. Macromol. 2019, 123, 691–703. [Google Scholar] [CrossRef]
- Razzazan, A.; Atyabi, F.; Kazemi, B.; Dinarvand, R. In Vivo Drug Delivery of Gemcitabine with PEGylated Single-Walled Carbon Nanotubes. Mater. Sci. Eng. C 2016, 62, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Tehrani, Z.M.; Moghanaki, A.A. Folate-Conjugated PH-Responsive Nanocarrier Designed for Active Tumor Targeting and Controlled Release of Gemcitabine. Pharm. Res. 2016, 33, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, Y.-K.; Zhao, J.; Lu, H.; Stenzel, M.H.; Xiao, P. PEG Grafted-Nanodiamonds for the Delivery of Gemcitabine. Macromol. Rapid Commun. 2016, 37, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Lu, M.; Lu, H.; Stenzel, M.H.; Xiao, P. PH-Triggered Release of Gemcitabine from Polymer Coated Nanodiamonds Fabricated by RAFT Polymerization and Copper Free Click Chemistry. Polym. Chem. 2016, 7, 6220–6230. [Google Scholar] [CrossRef]
- Farshi Azhar, F.; Rezaei, M.; Olad, A.; Mousazadeh, H. The Effect of Montmorillonite in Graphene Oxide/Chitosan Nanocomposite on Controlled Release of Gemcitabine. Polym. Bull. 2022, 79, 5861–5883. [Google Scholar] [CrossRef]
- Ashuri, A.; Miralinaghi, M.; Moniri, E. Evaluation of Folic Acid-Conjugated Chitosan Grafted Fe3O4/Graphene Oxide as a PH- and Magnetic Field-Responsive System for Adsorption and Controlled Release of Gemcitabine. Korean J. Chem. Eng. 2022, 39, 1880–1890. [Google Scholar] [CrossRef]
- Wei, X.; Li, P.; Zhou, H.; Hu, X.; Liu, D.; Wu, J.; Wang, Y. Engineering of Gemcitabine Coated Nano-Graphene Oxide Sheets for Efficient near-Infrared Radiation Mediated in Vivo Lung Cancer Photothermal Therapy. J. Photochem. Photobiol. B Biol. 2021, 216, 112125. [Google Scholar] [CrossRef]
- Yunus, U.; Zulfiqar, M.A.; Ajmal, M.; Bhatti, M.H.; Chaudhry, G.-S.; Muhammad, T.S.T.; Sung, Y.Y. Targeted Drug Delivery Systems: Synthesis and in Vitro Bioactivity and Apoptosis Studies of Gemcitabine-Carbon Dot Conjugates. Biomed. Mater. 2020, 15, 065004. [Google Scholar] [CrossRef]
- Zhang, P.; Yi, W.; Hou, J.; Yoo, S.; Jin, W.; Yang, Q. A Carbon Nanotube-Gemcitabine-Lentinan Three-Component Composite for Chemo-Photothermal Synergistic Therapy of Cancer. Int. J. Nanomed. 2018, 13, 3069–3080. [Google Scholar] [CrossRef]
- Tehrani, N.S.; Masoumi, M.; Chekin, F.; Baei, M.S. Nitrogen Doped Porous Reduced Graphene Oxide Hybrid as a Nanocarrier of Imatinib Anticancer Drug. Russ. J. Appl. Chem. 2020, 93, 1221–1228. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; Safaroghli-Azar, A.; Fakhroueian, Z.; Bashash, D. ZnO/CNT@Fe3O4 Induces ROS-Mediated Apoptosis in Chronic Myeloid Leukemia (CML) Cells: An Emerging Prospective for Nanoparticles in Leukemia Treatment. Artif. Cells Nanomed. Biotechnol. 2020, 48, 735–745. [Google Scholar] [CrossRef]
- Felix, D.M.; Rebelo Alencar, L.M.; Duarte de Menezes, F.; Midlej, V.d.V.P.; Aguiar, L.; Gemini Piperni, S.; Zhang, J.; Liu, Y.; Ricci-Junior, E.; Alexis, F.; et al. Graphene Quantum Dots Decorated with Imatinib for Leukemia Treatment. J. Drug Deliv. Sci. Technol. 2021, 61, 102117. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Lu, Y.-J.; Chen, J.-P. Magnetic Graphene Oxide as a Carrier for Targeted Delivery of Chemotherapy Drugs in Cancer Therapy. J. Magn. Magn. Mater. 2017, 427, 34–40. [Google Scholar] [CrossRef]
- Nicosia, A.; Cavallaro, G.; Costa, S.; Utzeri, M.; Cuttitta, A.; Giammona, G.; Mauro, N. Carbon Nanodots for On Demand Chemophotothermal Therapy Combination to Elicit Necroptosis: Overcoming Apoptosis Resistance in Breast Cancer Cell Lines. Cancers 2020, 12, 3114. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; Lan, Y.-H.; Lu, Y.-J.; Weng, Y.-L.; Chen, J.-P. Targeted Delivery of Irinotecan and SLP2 ShRNA with GRP-Conjugated Magnetic Graphene Oxide for Glioblastoma Treatment. Biomater. Sci. 2022, 10, 3201–3222. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Chen, L.; Li, Q.; Du, J.; Gao, Y.; Zhang, L.; Yang, Y. Antitumor Effects of Carbon Nanotube-drug Complex against Human Breast Cancer Cells. Exp. Ther. Med. 2018, 16, 1103–1110. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Wang, J.; Du, J.; Gao, Y.; Zhang, L.; Chen, L.; Yang, Y.; Liu, X. A Targeted Drug Delivery System Based on Carbon Nanotubes Loaded with Lobaplatin toward Liver Cancer Cells. J. Mater. Res. 2018, 33, 2565–2575. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, B.; Li, C.; Kuang, W.; Zhang, J.; Xiong, Y.; Tan, S.; Cai, X.; Huang, L. Carboxymethyl Cellulose-Grafted Graphene Oxide for Efficient Antitumor Drug Delivery. Nanotechnol. Rev. 2018, 7, 291–301. [Google Scholar] [CrossRef]
- Das, M.; Datir, S.R.; Singh, R.P.; Jain, S. Augmented Anticancer Activity of a Targeted, Intracellularly Activatable, Theranostic Nanomedicine Based on Fluorescent and Radiolabeled, Methotrexate-Folic Acid-Multiwalled Carbon Nanotube Conjugate. Mol. Pharm. 2013, 10, 2543–2557. [Google Scholar] [CrossRef]
- Shan, S.; Jia, S.; Lawson, T.; Yan, L.; Lin, M.; Liu, Y. The Use of TAT Peptide-Functionalized Graphene as a Highly Nuclear-Targeting Carrier System for Suppression of Choroidal Melanoma. Int. J. Mol. Sci. 2019, 20, 4454. [Google Scholar] [CrossRef]
- D’souza, S.L.; Chettiar, S.S.; Koduru, J.R.; Kailasa, S.K. Synthesis of Fluorescent Carbon Dots Using Daucus Carota Subsp. Sativus Roots for Mitomycin Drug Delivery. Optik 2018, 158, 893–900. [Google Scholar] [CrossRef]
- Ohta, T.; Hashida, Y.; Yamashita, F.; Hashida, M. Sustained Release of Mitomycin C from Its Conjugate with Single-Walled Carbon Nanotubes Associated by Pegylated Peptide. Biol. Pharm. Bull. 2016, 39, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, B.; Zhu, N.; Li, M.; Yu, Q. Mitochondrion Targeting Peptide-Modified Magnetic Graphene Oxide Delivering Mitoxantrone for Impairment of Tumor Mitochondrial Functions. Chin. Chem. Lett. 2021, 32, 1220–1223. [Google Scholar] [CrossRef]
- Risi, G.; Bloise, N.; Merli, D.; Icaro-Cornaglia, A.; Profumo, A.; Fagnoni, M.; Quartarone, E.; Imbriani, M.; Visai, L. Invitro Study of Multiwall Carbon Nanotubes (MWCNTs) with Adsorbed Mitoxantrone (MTO) as a Drug Delivery System to Treat Breast Cancer. RSC Adv. 2014, 4, 18683–18693. [Google Scholar] [CrossRef]
- Toh, T.-B.; Lee, D.-K.; Hou, W.; Abdullah, L.N.; Nguyen, J.; Ho, D.; Chow, E.K.-H. Nanodiamond–Mitoxantrone Complexes Enhance Drug Retention in Chemoresistant Breast Cancer Cells. Mol. Pharm. 2014, 11, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Heister, E.; Neves, V.; Lamprecht, C.; Silva, S.R.P.; Coley, H.M.; McFadden, J. Drug Loading, Dispersion Stability, and Therapeutic Efficacy in Targeted Drug Delivery with Carbon Nanotubes. Carbon 2012, 50, 622–632. [Google Scholar] [CrossRef]
- Chen, Q.; Che, C.; Liu, J.; Gong, Z.; Si, M.; Yang, S.; Yang, G. Construction of an Exosome-Functionalized Graphene Oxide Based Composite Bionic Smart Drug Delivery System and Its Anticancer Activity. Nanotechnology 2022, 33, 175101. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, M.; Luo, T.; Qu, J.; Chen, W.R. Photo-Activated Chemo-Immunotherapy for Metastatic Cancer Using a Synergistic Graphene Nanosystem. Biomaterials 2021, 265, 120421. [Google Scholar] [CrossRef]
- Kazempour, M.; Edjlali, L.; Akbarzadeh, A.; Davaran, S.; Farid, S.S. Synthesis and Characterization of Dual PH-and Thermo-Responsive Graphene-Based Nanocarrier for Effective Anticancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2019, 54, 101158. [Google Scholar] [CrossRef]
- Farnaz, R.; Maryam, S.; Masoumeh, J.; Parvaneh, S. Colloidal HSA—Graphene Oxide Nanosheets for Sustained Release of Oxaliplatin: Preparation, Release Mechanism, Cytotoxicity and Electrochemical Approaches. Colloids Surf. B Biointerfaces 2018, 171, 10–16. [Google Scholar] [CrossRef]
- Wu, L.; Man, C.; Wang, H.; Lu, X.; Ma, Q.; Cai, Y.; Ma, W. PEGylated Multi-Walled Carbon Nanotubes for Encapsulation and Sustained Release of Oxaliplatin. Pharm. Res. 2013, 30, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.-F.; Kwong, C.H.T.; Li, S.; Pan, Y.-T.; Wei, J.; Wang, L.-H.; Mok, G.S.P.; Wang, R. Supramolecular Nanomedicine Derived from Cucurbit[7]Uril-Conjugated Nano-Graphene Oxide for Multi-Modality Cancer Therapy. Biomater. Sci. 2021, 9, 3804–3813. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wang, N.; He, L.; Shi, C.; Zhang, D.; Liu, Y.; Luo, L.; Chen, T. Designing Dual-Functionalized Carbon Nanotubes with High Blood–Brain-Barrier Permeability for Precise Orthotopic Glioma Therapy. Dalton Trans. 2019, 48, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Liu, S.; Li, J.; Qu, D.; Zhao, H.; Guan, X.; Hu, X.; Xie, Z.; Jing, X.; Sun, Z. Integrating Oxaliplatin with Highly Luminescent Carbon Dots: An Unprecedented Theranostic Agent for Personalized Medicine. Adv. Mater. 2014, 26, 3554–3560. [Google Scholar] [CrossRef]
- Vinothini, K.; Rajendran, N.K.; Ramu, A.; Elumalai, N.; Rajan, M. Folate Receptor Targeted Delivery of Paclitaxel to Breast Cancer Cells via Folic Acid Conjugated Graphene Oxide Grafted Methyl Acrylate Nanocarrier. Biomed. Pharmacother. 2019, 110, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Paul, A.; Zhao, B.; Lee, C.; Rodes, L.; Prakash, S. Carbon Nanotube Lipid Drug Approach for Targeted Delivery of a Chemotherapy Drug in a Human Breast Cancer Xenograft Animal Model. Biomaterials 2013, 34, 10109–10119. [Google Scholar] [CrossRef]
- Liu, W.; Wei, J.; Chen, Y. Electrospun Poly(l-Lactide) Nanofibers Loaded with Paclitaxel and Water-Soluble Fullerenes for Drug Delivery and Bioimaging. New J. Chem. 2014, 38, 6223–6229. [Google Scholar] [CrossRef]
- Singh, S.; Mehra, N.K.; Jain, N.K. Development and Characterization of the Paclitaxel Loaded Riboflavin and Thiamine Conjugated Carbon Nanotubes for Cancer Treatment. Pharm. Res. 2016, 33, 1769–1781. [Google Scholar] [CrossRef]
- Burkert, S.C.; Shurin, G.V.; White, D.L.; He, X.; Kapralov, A.A.; Kagan, V.E.; Shurin, M.R.; Star, A. Targeting Myeloid Regulators by Paclitaxel-Loaded Enzymatically Degradable Nanocups. Nanoscale 2018, 10, 17990–18000. [Google Scholar] [CrossRef]
- Moore, T.L.; Podilakrishna, R.; Rao, A.; Alexis, F. Systemic Administration of Polymer-Coated Nano-Graphene to Deliver Drugs to Glioblastoma. Part. Part. Syst. Charact. 2014, 31, 886–894. [Google Scholar] [CrossRef]
- Han, M.; Zhou, Z.-H.; Luo, Y.-L.; Xu, F.; Chen, Y.-S. PH-Sensitive Carbon Nanotubes Graft Polymethylacrylic Acid Self-Assembly Nanoplatforms for Cellular Drug Release. J. Biomater. Appl. 2022, 37, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.; Kumar Das, P. Paclitaxel-Loaded Biotinylated Fe2+ -Doped Carbon Dot: Combination Therapy in Cancer Treatment. ACS Appl. Bio Mater. 2021, 4, 5132–5144. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tang, X.; Wu, X.; Feng, X. Biosynthesis of Sorafenib Coated Graphene Nanosheets for the Treatment of Gastric Cancer in Patients in Nursing Care. J. Photochem. Photobiol. B Biol. 2019, 191, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ruman, U.; Buskaran, K.; Pastorin, G.; Masarudin, M.J.; Fakurazi, S.; Hussein, M.Z. Synthesis and Characterization of Chitosan-Based Nanodelivery Systems to Enhance the Anticancer Effect of Sorafenib Drug in Hepatocellular Carcinoma and Colorectal Adenocarcinoma Cells. Nanomaterials 2021, 11, 497. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Mostafa, M.E.; Alaaeldin, E.; Sarhan, H.A.A.; Shaykoon, M.S.A.; Allam, S.; Ahmed, A.R.H.; Elsadek, B.E.M. Design and Characterisation of Novel Sorafenib-Loaded Carbon Nanotubes with Distinct Tumour-Suppressive Activity in Hepatocellular Carcinoma. Int. J. Nanomed. 2019, 14, 8445–8467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Niu, B.; Chen, J.; He, X.; Bao, X.; Zhu, J.; Yu, H.; Li, Y. The Use of Lipid-Coated Nanodiamond to Improve Bioavailability and Efficacy of Sorafenib in Resisting Metastasis of Gastric Cancer. Biomaterials 2014, 35, 4565–4572. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Li, Z.; Xia, N.; Yu, H.; Zhang, Y. Synthesis and Characterization of Tamoxifen Citrate Modified Reduced Graphene Oxide Nano Sheets for Breast Cancer Therapy. J. Photochem. Photobiol. B Biol. 2018, 180, 68–71. [Google Scholar] [CrossRef]
- Chen, C.; Hou, L.; Zhang, H.; Zhu, L.; Zhang, H.; Zhang, C.; Shi, J.; Wang, L.; Jia, X.; Zhang, Z. Single-Walled Carbon Nanotubes Mediated Targeted Tamoxifen Delivery System Using Aspargine-Glycine-Arginine Peptide. J. Drug Target. 2013, 21, 809–821. [Google Scholar] [CrossRef]
- Yi, W.; Zhang, P.; Hou, J.; Chen, W.; Bai, L.; Yoo, S.; Khalid, A.; Hou, X. Enhanced Response of Tamoxifen toward the Cancer Cells Using a Combination of Chemotherapy and Photothermal Ablation Induced by Lentinan-Functionalized Multi-Walled Carbon Nanotubes. Int. J. Biol. Macromol. 2018, 120, 1525–1532. [Google Scholar] [CrossRef]
- Misra, C.; Kumar, M.; Sharma, G.; Kumar, R.; Singh, B.; Katare, O.P.; Raza, K. Glycinated Fullerenes for Tamoxifen Intracellular Delivery with Improved Anticancer Activity and Pharmacokinetics. Nanomedicine 2017, 12, 1011–1023. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, G.; Misra, C.; Kumar, R.; Singh, B.; Katare, O.P.; Raza, K. N-Desmethyl Tamoxifen and Quercetin-Loaded Multiwalled CNTs: A Synergistic Approach to Overcome MDR in Cancer Cells. Mater. Sci. Eng. C 2018, 89, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Potentiating the Anti-Cancer Profile of Tamoxifen-Loaded Graphene Using Deep Eutectic Solvents as Functionalizing Agents. Appl. Nanosci. 2020, 10, 293–304. [Google Scholar] [CrossRef]
- Wang, L.-H.; Sui, L.; Zhao, P.-H.; Ma, H.-D.; Liu, J.-Y.; Wei, Z.; Zhan, Z.-J.; Wang, Y.-L. A Composite of Graphene Oxide and Iron Oxide Nanoparticles for Targeted Drug Delivery of Temozolomide. Die Pharm.—Int. J. Pharm. Sci. 2020, 75, 313–317. [Google Scholar] [CrossRef]
- Reddy, S.; Song, L.; Zhao, Y.; Zhao, R.; Wu, D.; He, L.; Ramakrishana, S. Reduced Graphene Oxide-Based Electrochemically Stimulated Method for Temozolomide Delivery. Med. Devices Sens. 2018, 1, e10014. [Google Scholar] [CrossRef]
- Nandi, A.; Ghosh, C.; Bajpai, A.; Basu, S. Graphene Oxide Nanocells for Impairing Topoisomerase and DNA in Cancer Cells. J. Mater. Chem. B 2019, 7, 4191–4197. [Google Scholar] [CrossRef]
- Mallick, A.; Nandi, A.; Basu, S. Polyethylenimine Coated Graphene Oxide Nanoparticles for Targeting Mitochondria in Cancer Cells. ACS Appl. Bio Mater. 2019, 2, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Peng, J.; Wang, S.; Xie, B.; Lei, L.; Zhao, D.; Nie, H. Fabrication of Graphene Oxide-β-Cyclodextrin Nanoparticle Releasing Doxorubicin and Topotecan for Combination Chemotherapy. Mater. Technol. 2015, 30, 242–249. [Google Scholar] [CrossRef]
- Au, T.H.; Nguyen, B.N.; Nguyen, P.H.; Pethe, S.; Vo-Thanh, G.; Vu Thi, T.H. Vinblastine Loaded on Graphene Quantum Dots and Its Anticancer Applications. J. Microencapsul. 2022, 39, 239–251. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Q.; Shu, C.; Ma, X.; Li, R.; Shen, H.; Zhong, W. Targeted Killing of Cancer Cells in Vivo and in Vitro with IGF-IR Antibody-Directed Carbon Nanohorns Based Drug Delivery. Int. J. Pharm. 2015, 478, 644–654. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef] [PubMed]
- Nocito, G.; Calabrese, G.; Forte, S.; Petralia, S.; Puglisi, C.; Campolo, M.; Esposito, E.; Conoci, S. Carbon Dots as Promising Tools for Cancer Diagnosis and Therapy. Cancers 2021, 13, 1991. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Saravanan, A.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Naturally Derived Carbon Dots In Situ Confined Self-Healing and Breathable Hydrogel Monolith for Anomalous Diffusion-Driven Phytomedicine Release. ACS Appl. Bio Mater. 2022, 5, 5617–5633. [Google Scholar] [CrossRef]
- Consoli, G.M.L.; Giuffrida, M.L.; Zimbone, S.; Ferreri, L.; Maugeri, L.; Palmieri, M.; Satriano, C.; Forte, G.; Petralia, S. Green Light-Triggerable Chemo-Photothermal Activity of Cytarabine-Loaded Polymer Carbon Dots: Mechanism and Preliminary In Vitro Evaluation. ACS Appl. Mater. Interfaces 2023, 15, 5732–5743. [Google Scholar] [CrossRef]
- Forte, G.; Consiglio, G.; Satriano, C.; Maugeri, L.; Petralia, S. A Nanosized Photothermal Responsive Core-Shell Carbonized Polymer Dots Based on Poly(N-Isopropylacrylamide) for Light-Triggered Drug Release. Colloids Surf. B Biointerfaces 2022, 217, 112628. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Hosseini Fouladi, M.; Yong, P.V.C.; Chinna, K.; Palanisamy, N.K.; Wong, E.H. Toxicity of Carbon Nanotubes: Molecular Mechanisms, Signaling Cascades, and Remedies in Biomedical Applications. Chem. Res. Toxicol. 2021, 34, 24–46. [Google Scholar] [CrossRef]
- Kolosnjaj, J.; Szwarc, H.; Moussa, F. Toxicity Studies of Fullerenes and Derivatives. In Bio-Applications of Nanoparticles; Advances in Experimental Medicine and Biology; Chan, W.C.W., Ed.; Springer: New York, NY, USA, 2007; pp. 168–180. ISBN 978-0-387-76713-0. [Google Scholar]


| Chemotherapeutic—Drug Class | CNM-Based Nanocarrier | Biological Study Models | Drug Loading and Release Metrics | Experimental Results | Ref. |
|---|---|---|---|---|---|
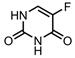 5-fluorouracil (5-FU)— pyrimidine antimetabolite | CS-carbon quantum dot (CQD)-Apt | in vitro: MCF-7 cells | 32% DLC, approximately 100% DRE, and pH-sensitive, controlled 5-FU release | The unloaded nanocarrier is biocompatible, and the use of an aptamer increases uptake and cytotoxicity in breast cancer cells. | [26] |
| FA-PEG-bis-amine multiwalled carbon nanotube (MWCNT) | in vitro: MCF-7 cells | 99% DLE, ~90% DRE, with pH-triggered drug release sustained over 900 min | This nanocarrier increases circulation time, half-life, and accumulation of 5-FU in target tissues, and this leads to the effective killing of breast cancer cells in vitro. | [27] | |
| CS/Au/MWCNT | in vitro: MCF-7 cells | 43% DLC, 59% DRE, with prolonged, sustained drug release | Reduced potential side effects and increased efficacy compared to free 5-FU were observed. A reduction in cancer cell viability was observed at low nanocarrier concentrations. | [28] | |
| Nanodiamond (ND)-ADH | in vitro: MCF-7 and HepG2 cells | 88% DLE, 35% DRE, with pH-mediated, sustained drug release | This nanocarrier showed potent anticancer effects with low haemolytic toxicity in human blood. | [29] | |
| Mesoporous carbon nanoframe (mCNF) | in vitro: HeLa cells | 31% DLC, 80% DRE, with dual pH/NIR-triggered drug release | This system displayed excellent photothermal efficiency with the NIR pulse-triggered burst release of 5-FU. The photothermal conversion efficiency of this system was found to be 21%. This synergistic chemo–photothermal therapy combined with photoacoustic imaging capabilities can effectively treat cancer in vitro. | [30] | |
| PEG-C60 fullerene–alanine | in vitro: MCF-7 and BGC-823 cells | 1% DLC, with no quantitative drug release data | The unloaded nanocarrier displays good biocompatibility and the system is stable in murine serum for over 24 h. This formulation results in the significantly better inhibition of cancer cells compared to free 5-FU. | [31] | |
| Graphene oxide (GO) | in vitro: A549 cells | 31% DLC, 35% DRE, with pH-triggered drug release | The blank nanocarrier is biocompatible and the loaded system improved the stability of 5-FU. | [32] | |
| CS-coated Fe3O4–NH2/graphene quantum dot (GQD) nanohybrid | in vitro: A549 cells | 90% DLC, 84% DRE, with pH-dependent drug release | This system has magnetic resonance/fluorescence imaging capabilities and displayed significantly higher cytotoxicity than free 5-FU, whilst the unloaded nanocarrier is biocompatible. | [33] | |
| HPMC/GO | in vitro: Vero, HepG2, and A549 cells | No quantitative drug loading/release studies were performed. The blank nanocarrier displays high biocompatibility in normal cells, whilst the drug-loaded system displays a higher antitumour efficacy than free 5-FU. A green synthesis method was used. | [34] | ||
| TAU-GO | in vitro: HepG2 cells; in vivo: SD rats | 50% DLE, 90% DRE, with pH-triggered 5-FU release | This biocompatible nanocarrier improved the circulation time and anticancer efficacy of 5-FU. | [35] | |
| Carbon dot (CD)-BT | in vitro: MCF-7, HeLa, and HEK-296 cells | 35% DLE, 81% DRE, with pH-triggered drug release | An initial burst of 5-FU is followed by sustained release; this nanocarrier also displays fluorescence imaging capabilities. BT-mediated targeting of cancer cells resulted in high cytotoxicity towards neoplastic cells and increased cellular uptake due to biotin-receptor-mediated endocytosis. | [36] | |
| N-doped mesoporous carbon sphere (NMCS)-DSPE-PEG | in vitro: B16F0 cells | 38% DLC, 78% DRE, with dual pH/NIR-triggered drug release | This nanocarrier produces reactive oxygen species when irradiated with an NIR laser, and the resulting PDT/PTT/chemotherapeutic combination therapy effectively kills melanoma cells much more efficiently than 5-FU alone. | [37] | |
 6-mercaptopurine (6-MP)— purine antimetabolite | CD-BT | in vitro: CHO, MCF-7, and HepG2 cells | 5% DLC, 79% DRE, with dual pH- and redox-sensitive drug release | Comparable anticancer activity to free 6-MP (in cancer cells) with much lower cytotoxicity (in healthy cells). A GSH-sensitive carbonyl vinyl sulphide group was used to bind 6-MP to BT. | [38] |
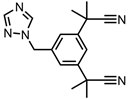 Anastrozole—aromatase inhibitor | GO-Fe nanoparticles | in vitro: MCF-7 cells | 84% DLE | No qualitative drug release data shown. The system displays higher cytotoxicity than the free drug for cancer cells, and it has magnetic properties. | [39] |
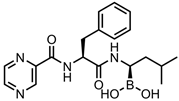 Bortezomib—proteasome inhibitor | CD-CuS NPs-MMT7 | in vitro: U251 MG cells | Synergistic drug delivery and PTT platform that specifically targets cancer cells. No qualitative drug loading/release data shown. This innovative nanocarrier combines immune system evasion capabilities with the enhanced suppression of tumour growth and metastasis to provide excellent control over cancer growth and metastasis. | [12] | |
 Capecitabine—pyrimidine antimetabolite | Single-walled carbon nanotube (SWCNT)-FL-FA-NCC | in vitro: Caco-2/TC7 cells | No quantitative drug loading/release data shown. This nanocarrier is nontoxic and has fluorescence imaging capabilities. The effective targeting of colon cancer cells leads to an increase in anticancer activity compared to the free drug. | [40] | |
| oxiSWCNT-CS-FA | in vitro: COLO320DM and HT29 cells; in vivo: albino rabbits | 94% DLE, 89% DRE | An increase in cytotoxicity compared to free drug was noticed during in vitro experiments. The capsule formulation of this nanocarrier is exclusively released in the colon in vivo, avoiding premature release in the stomach. Active targeting of cancer cells was achieved via the FA-targeting ligand. | [41] | |
 Carboplatin (CP)—DNA alkylating agent | GO-PAMAM | in vitro: hMSC and HeLa cells | No quantitative drug loading/release data shown. The 100 nm width GO (unloaded) was found to be the least toxic. This system displayed enhanced anticancer activity compared to free CP, with decreased cytotoxicity. | [42] | |
| GO-gelatine | in vitro: IMR-32 and hMSC cells | 99% DLE, with no quantitative drug release data shown | This formulation displays effective CP delivery and uptake in vitro, resulting in a higher potency than free CP. Excellent biocompatibility and stability were observed in vitro. | [43] | |
| oxiMWCNT-HA | in vitro: TC–1 and NIH/3T3 cells | No quantitative drug loading/release data shown | This system displayed the selective uptake and targeting of cancer cells over healthy cells, resulting in significantly higher cytotoxic effects in neoplastic cells and lower side effects in healthy cells. | [44] | |
| Aminated MWCNT | in vitro: MDA-MB-23 and MCF-12A | 89% DLC, 21% DRE | This formulation provided increased cancer cell death compared to free CP and killed cells via an ROS-triggered autophagy mechanism. | [45] | |
| FA-CDT-C60 fullerene | in vitro: HeLa, HeLa-RFP, and A549 cells; in vivo: Danio rerio, both healthy and bearing HeLa tumours | 37% DLC, ~80% DRE, with pH-triggered drug release | This system displayed increased anticancer effects compared to the free drug alone due to the active targeting of folate-receptor-overexpressing cancer cells and improved cellular uptake. Low toxicity and improved antitumour effects compared to the free drug were also seen in vivo. | [46] | |
| CS-Fe3O4-GO | in vitro: HepG2 and MCF-7 cells | 74% DLE, 90% DRE, with pH-triggered drug release | A very high amount of CP was released at neutral pH. Despite this, an increase in CP potency and a reduction in systemic toxicity was observed. | [47] | |
| GO-CS-FA | in vitro: LX-2 and SKOV3 cells | 14% DLC, ~90% DRE | CP release was similar in neutral and acidic environments; hence, this system is unsuitable for pH-triggered drug release via noncovalent drug attachment. The system showed slightly lower cancer cell inhibition than free CP. | [48] | |
| GO-Fe3O4-PANI | in vitro: SMMC-7721, HepG2, and HL-7702 cells | ~95% DRE; the qualitative drug loading data provided does not account for unbound CP that was removed | The blank nanocarrier showed efficient cellular uptake and negligible cytotoxicity. This nanocarrier has magnetic properties and pH-triggered drug release. | [49] | |
 Carmustine—DNA alkylating agent | GO-PAA | in vitro: GL261 cells | 19% DLC, with no quantitative drug release data shown | A significant increase in half-life, >70% decrease in IC50 value, and 30% increase in inter-strand DNA crosslinking was observed compared to the free drug in vitro. | [50] |
| N-doped carbon nanotube (CNT) sponges | in vitro: rat astrocytes, C6, RG2, and U87 cells | ~90% DRE, with no quantitative drug loading data | This nanocarrier displayed similar cytotoxicity to the free drug, with a sustained-release profile. The sponges appear to be more biocompatible than CNTs alone; hence, the blank nanocarrier showed low cytotoxicity, whilst the drug-loaded system displayed strong anticancer effects. | [51] | |
 Chlorambucil—DNA alkylating agent | Reduced graphene oxide (rGO)-FA-gelatine | in vitro: Siha cells | 35% DLC, 82% DRE, with pH-triggered drug release | A significant decrease in IC50 value compared to the free drug was observed. The use of gelatine facilitated sustained drug release. This system is a promising treatment for cervical adenocarcinoma. | [52] |
 Cisplatin (CisP)—DNA alkylating agent | GO-Ala | in vitro: MCF-7 and HepG2 cells | 4% DLC and ~70% DRE, with sustained drug release at neutral pH | The blank nanocarrier is biocompatible, whilst the CisP-loaded material is effective at killing cancer cells in vitro. | [53] |
| Oxidised carbon nanohorn (CNH) | in vitro: NCI-H460 | Approximately 1% DLC and 80% DRE, with sustained CisP release | This nanosystem displayed similar anticancer effects to the free drug in vitro. | [54] | |
| CNH-quantum dot | in vitro: AY-27 cells | 18% DLC and 70% DRE, with a sustained drug release profile | This theragnostic system displayed a significant reduction in anticancer potency compared to free CisP; however, it has imaging capabilities arising from the inclusion of CdSe quantum dots. | [55] | |
| Ultra-short SWCNT | in vivo: SCID/beige mice bearing MCF-7, BCM-4272, and MDA-MB-231 tumours | This nanoformulation effectively treated CisP-resistant breast cancer in a xenograft mouse model. The nanocarrier also displays an enhanced circulation time and increased tumour localisation, leading to increased potency compared to the free drug. | [56] | ||
| Silane-modified ND | in vitro: HeLa cells | This unique system has a Pt loading of 0.25 mmol/g ND, and CisP is not released from the conjugate. Despite this, the system displayed a similar IC50 value to CisP. The main advantage of this system is the prevention of CisP isomerisation, leading to enhanced aqueous stability. No quantitative drug loading/release data were given. | [15] | ||
| SA/ND | in vitro: HepG2, HeLa, A549, and RAW264.7 cells | This sustained-release drug platform improved the CisP accumulation in cancer cells, with improved drug safety. Whilst no quantitative drug loading or release data were given, no change in the antitumour mechanism was observed compared to the free drug. | [57] | ||
| EGF-ND | in vitro: HepG2 cells | 1% DLC, with no quantitative drug release data shown | This nanoformulation was capable of selectively killing liver cancer cells and displayed increased potency compared to free CisP. This was due to the EGF-mediated targeting of cancer cells. In addition, the NDs are probes for 3D Raman microscopy imaging. This nanocarrier system induces morphological changes in cancer cells, resulting in higher surface areas for CisP absorption with a lower risk of adverse side effects. | [58] | |
| C60 fullerene | in vivo: BALB/c mice | 50% DLC, with no quantitative drug release data shown | A two-fold decrease in systemic toxicity (LD50) compared to free CisP was observed. Specifically, the nanocarrier decreased drug-induced leukopenia, anaemia, thrombocytosis, and inflammation. | [59] | |
| C60 fullerene | in vitro: LLC cells | No quantitative drug loading or release data given | A 4.5× decrease in IC50 value compared to the free drug was observed in vivo. The fullerene itself was found to increase the cellular uptake and accumulation of CisP. | [60] | |
| oxiC60 fullerene | in vitro: L929 cells | 16% DLC, 60% DRE, with pH-triggered drug release | This nontoxic nanocarrier displayed outstanding fluorescence properties for cellular imaging experiments. | [61] | |
| C60 fullerene | in vitro: HCT-116, HeLa, HL-60, HL-60/adr, and HL-60/vinc cells; in vivo: C57BL/6J mice bearing LLC tumours | No quantitative drug loading/release data shown | This nanocarrier killed chemotherapy-resistant leukaemia cells in vitro and exhibited effective lung cancer tumour growth inhibition in vivo. Molecular docking studies suggested that the fullerene binds to proteins involved in chemotherapy resistance. | [62] | |
| CS-GO | in vitro: HeLa cells | 71% DLE, 88% DRE, with pH-triggered drug release | The functionalisation of GO with CS and CisP dramatically reduced protein binding. This biocompatible nanocarrier triggered apoptosis in drug-resistant cancer cells. | [63] | |
| CQD-GE11-DOX | in vitro: CNE-2 cells; in vivo: BALB/c mice bearing CNE-2 tumours | 5% DLC and 57% DRE, with pH-triggered drug release | Specific tumour targeting and inhibition was observed in vivo, and the effective killing of nasopharyngeal carcinoma cells was exhibited in vitro. This nanocarrier has fluorescence imaging capabilities and showed no obvious side effects in vivo. | [64] | |
| GO-PEG | in vitro: MG63, SAOS-2, U2-OS, MDA-MB-231, MDA-MB468, U118, and U87 cells | 64% DLE, with no quantitative drug release data shown and redox-sensitive drug delivery using a CisP prodrug | This nanoformulation displayed high uptake and proliferation inhibition in osteosarcoma cells, and effective internalisation, but reduced potency in glioblastoma cells. This system is capable of inhibiting cell migration in highly invasive breast carcinoma. | [65] | |
| MnO2-GO-Ce6-HA | in vitro: MDA-MB-231 and RLE-6TN cells; in vivo: BALB/c mice bearing MDA-MB-231 tumours | 7% DLC and 60% DRE, with pH-triggered drug release | Combination therapy of (1) MnO2 to regulate the tumour microenvironment, enhancing the anticancer effect of (2) CisP chemotherapy and (3) Ce6 PDT. The incorporation of HA facilitates tumour targeting for a true theragnostic system. This system also shows excellent biocompatibility and antitumour efficacy in vivo. | [66] | |
| rGO-PHEMA-DOX | in vitro: MCF-7 cells | 82% DLE and 64% DRE, with pH-triggered drug release | A significant decrease in IC50 value compared to free CisP and DOX was observed. This biocompatible nanocarrier displayed efficient cellular uptake and a synergistic effect between the two loaded drugs, resulting in the effective killing of breast cancer cells. | [67] | |
| Fe3O4-rGO-PHEMA-MET | in vitro: HepG2 and Caco-2 cells; in vivo: BALB/c mice, both healthy and bearing HepG2 tumours | 82% DLE, 60% DRE, with pH-triggered drug release | No side effects and potent antitumour efficacy was noted in vivo. This highly biocompatible nanosystem effectively killed hepatocellular carcinoma in vitro. | [68] | |
| GO-PEG-DOX | in vitro: CAL-27, L929, and MCF-7 cells; in vivo: nude mice carrying CAL-27 tumours | 37% DLC and 65% DRE, with pH-triggered drug release | A 2× increase in cancer cell apoptosis and necrosis compared to the single-drug-loaded nanocarrier was observed. An attenuation of toxicity and enhanced anticancer effects compared to free DOX/CisP were observed. | [69] | |
| S-doped CD | in vitro: A2780 and A2780 cells | No quantitative drug loading/release data shown | The unloaded CDs were found to be biocompatible and could interact with proteins and lipids on the surfaces of cancer cells. A similar IC50 value to the free drug was seen in normal ovarian cancer cells, and the nanoformulation could kill drug-resistant cancer cells. | [70] | |
| CD-iRGD | in vitro: A549, HUVEC, and HEL-299 | No quantitative drug loading/release data shown | This nanocarrier destroyed lung cancer cells whilst leaving healthy cells unharmed. | [71] | |
| MWCNT | in vitro: A549 and A549/DDP cells; in vivo: BALB/c mice carrying A549/DDP tumours | No quantitative drug loading/release data given | The unloaded MWCNTs were found to be biocompatible, whilst the loaded nanocarrier had higher cytotoxicity against cancer cells than free CisP. This nanoformulation could effectively treat a drug-resistant lung cancer in vivo model. | [72] | |
| PDA CD-anti-EpPCAN | in vitro: HepG2 cells; in vivo: BALB/c mice bearing HepG2 tumours | This synergistic nanocarrier combined a cisplatin prodrug with significant PTT and fluorescence imaging capabilities for effective image-guided chemo–photothermal therapy. This biocompatible system exhibited excellent antitumour effects in vitro and in vivo. | [73] | ||
| CD-PEG | in vitro: GES-1 and MGC-803 cells | 5% DLC, with no quantitative drug release data given and pH/redox-mediated drug release achieved via a hydrolysable benzoic imine bond | A CisP prodrug was used, and the resulting system had comparable anticancer efficacy to free CisP, with reduced side effects. This system also exhibited fluorescence imaging capabilities. | [74] | |
| CD-PEG | in vitro: A549, HUVEC, and HEL-299 cells | No quantitative drug loading data given, 86% DRE and redox-sensitive drug release | This fluorescent nanocarrier could effectively kill cancer cells whilst leaving healthy cells unharmed. | [75] | |
 Curcumin (CUR)—ROS scavenger and lipid peroxidation inhibitor | Fluorinated GO-LIN | in vitro: MCF-7 and MCF10A cells; in vivo: BALB/c mice with 4T1-induced breast cancer | 61% DLC, 95% DRE, with pH-sensitive drug release | This simple, low-cost system acts as a contrast agent for magnetic resonance imaging. It also displays improved cytotoxicity, tumour suppression, and reduced side effects compared to free CUR due to effective cancer cell targeting. | [76] |
| GQD-GlcN | in vitro: MCF-7 cells | No quantitative drug loading information, 37% DRE with pH-triggered, sustained drug release | The unloaded nanocarrier is biocompatible, whilst the loaded system exhibits effective cancer cell targeting and internalisation in vitro. This system also has fluorescence imaging capabilities. | [77] | |
| SWCNT | in vitro: PC-3 cells | 94% DLE, 95% DRE, with pH-triggered drug release | The combination of efficient CUR delivery and SWCNT-mediated PTT successfully inhibited tumour cell growth. This nanocarrier also reduced CUR biodegradation and increased its solubility. | [78] | |
| SWCNT-PC-PVP | in vitro: PC-3 and S180 cells; in vivo: Kunming mice bearing S180 tumours | No quantitative drug loading/release data. This biocompatible nanocarrier increased CUR cellular uptake, plasma concentration, and bioavailability. The system overcomes the main barrier to the low anticancer effect of free CUR (low plasma concentration) whilst displaying low in vivo toxicity. This is a combination therapy with the SWCNT-mediated photothermal ablation of cancer cells. | [79] | ||
| oxiND-ADH | in vitro: MCF-7 and HepG2 cells | 93% DLE, 36% DRE, with pH-triggered, sustained drug release | The use of a pH-sensitive amide bond to bind CUR slows release and increases stability, resulting in potent cytotoxicity. | [29] | |
| Graphene oxide quantum dot (GOQD)-CS-PEG-MUC-1 aptamer | in vitro: MCF-7 and HT-2 cells | 99% DLC, 64% DRE, with pH-responsive drug release | This system effectively targets MUC-1-overexpressing cancer cells whilst displaying photoluminescence imaging and cancer detection abilities. An increase in therapeutic efficacy and cellular uptake compared to free CUR and low haemolysis with human blood was observed with this system. | [19] | |
| CD-PNM | in vitro: SH-SY5Y cells | No qualitative CUR loading information, 82% DRE | This formulation resulted in a 10× enhancement of CUR solubility whilst displaying excellent photophysical properties and low toxicity. | [80] | |
| CD | in vitro: HepG2 and A549 cells | 3% DLC, ~90% DRE, with pH-mediated drug release | This cost-effective, photoluminescent nanocarrier is nontoxic to normal cells and displayed potent anticancer effects with enhanced CUR bioavailability and a small size. | [81] | |
| CoFe2O4/GO-ADH-CMC | in vitro: MDA-MB-231 and MCF-10A cells | 2% DLC, 86% DRE, with pH-triggered, controlled drug release | A decrease in cancer cell viability compared to free CUR was noted when using this system. | [82] | |
| GQD-HA | in vitro: HeLa and L929 cells | 98% DLE, ~100% DRE, with pH-triggered drug release | This nanoformulation displayed excellent anticancer activity compared to CUR alone and has no toxic effect on healthy cells. The system is also highly fluorescent when CUR is released. | [83] | |
| GO-BSA-AS1411 aptamer | in vitro: MCF-7 and SKBR3 cells | 9% DLC, 70% DRE, with pH-triggered drug release | Efficient targeting of nucleolin-overexpressing MCF7 cancer cells was achieved, facilitated by aptamer attachment. This resulted in increased CUR antitumour activity. BSA decoration was found to improve nanocarrier biostability and slow CUR degradation. | [84] | |
| CS-Fe3O4-RGO | in vitro: MCF-7 cells | 95% DLE, 96% DRE | This system successfully targeted and induced apoptosis in breast cancer cells. The superparamagnetic nanocarrier increased the rate of CUR delivery compared to the free drug. | [85] | |
 Cyclophosphamide—DNA alkylating agent | Hollow mesoporous carbon sphere (HMCS) | in vitro: CNE cells; in vivo: nude mice with CNE tumours | 20% DLC, with no release experiments carried out with PTX | The HMCSs themselves exhibited strong antitumour effects through PTT. | [86] |
| PAA/PEG/CNT/MTX | Approximately 80% DRE, with no quantitative drug loading data shown | The system displayed dual pH- and temperature-triggered drug release, with an initial burst of drug followed by sustained delivery. | [87] | ||
 Cytarabine—DNA polymerase inhibitor | GQD-CS | 73% DLE, 72% DRE, with pH-sensitive drug release via a hydrolysable amide linkage | This nanosystem possesses remarkable fluorescence stability, and CS wrapping enhanced the water solubility of this system. | [88] | |
| Au/GQD/MPA/PEI | in vitro: HL-60 cells | 68% DLE, 78% DRE, with dual pH- and NIR-triggered drug release | Cytarabine was attached via charge–dipole interactions. The chemo–photothermal combination therapy had higher efficacy than PTT alone. | [21] | |
 Dabrafenib—reversible ATP-competitive kinase inhibitor | GO-BSA | in vitro: A375, HDF, SKmel28, SKmel23, MelJuSo, MNT-1, and NHEM cells | No quantitative drug loading/release data, with pH-triggered drug release | The potency of dabrafenib was retained, with effective BRAF and HDAC inhibition in human melanoma cells. | [89] |
 Dasatinib—tyrosine kinase inhibitor | PLA-PGA-PEG-CNT | in vitro: U-87 cells | 4% DLC, ~65% DRE | This nanocarrier system was synthesised via a simple one-pot method and demonstrated improved therapeutic efficacy compared to the free drug in vitro. The drug release profile of this system can be controlled by varying the composition of the polymer coating. | [90] |
 Daunorubicin (DNR)— topoisomerase II inhibitor | P-gp-GGN | in vitro: adriamycin-resistant leukaemia cell lines KA and K562/A02 cells; in vivo: KA nude mice with drug-resistant leukaemia-cell-induced tumours | 32% DLC, roughly 45% DRE, with redox-triggered DNR release facilitated by increasing glutathione concentration | This nanocarrier overrides the cell‘s drug resistance to facilitate DNR uptake, resulting in a remarkable inhibition of tumour growth in vivo. | [20] |
| PLA/MWCNT/FE3O4 | in vitro: K562 cells | 96% DLE, roughly 55% DRE, with dual magnetic field- and pH-mediated drug release | The most effective killing of leukaemia cells was observed at a 20 µg/mL nanocarrier concentration. | [91] | |
| ND | in vitro: K562 cells | ~95% DLC, most of the bound DNR was released | This formulation achieved a three-fold reduction in the IC-50 value compared to free DNR for the treatment of drug-resistant K562 cells. | [92] | |
| f-CNTs | 94% DLE at 3:1 DNR:f-CNT ratio, with no quantitative drug release data provided | Hydroxylated CNTs provided the best DNR binding (through electrostatic interactions). | [93] | ||
| Bi2MoO6/NH2-GO/PEG | in vitro: HUVEC and MCF-7 cells | 33% DLC, 86% DRE, with pH-triggered drug release | DNR was selectively released in cancer cells. Haemolysis and coagulation tests prove system has no negative effects on the blood. | [94] | |
 Decitabine—DNA methyltransferase inhibitor | A1-GO | in vitro: A549, NCI-H157, NCI-H520, NCI-H1299, NCI-H446, MCF-7, and HeLa cells | 64% DLE, 75% DRE, with pH-dependent drug release | Specific recognition and targeting of lung cancer cells over other cancer cells was achieved by using the A1 aptamer. This system achieved much higher anticancer efficacy than the free drug. | [95] |
 Docetaxel (DTX)—microtubule growth inhibitor | GO-PEG | in vitro: DU-145 cells | No quantitative drug loading or release data provided | This system was highly effective at killing prostate cancer cells due to a decrease in IC50 compared to free DTX. The nanocarrier displayed low dispersion stability in biological fluids. | [96] |
| RGD-CS-SWCNT | in vitro: A549 and MCF-7 cells; in vivo: BALB/c mice inoculated with A549 tumours | 32% DLC, 68% DRE, with pH-triggered drug release | Significant drug uptake and growth inhibition in A549 cells was observed. The system entered cells via clathrin- and caveolin-mediated endocytosis and displayed strong tumour targeting, growth inhibition, and biosafety in vivo. | [97] | |
| Oxi-carbon nano-horn (CNH)-PEG-mAb | in vitro: MCF-7; in vivo: ICR mice xenografted with H22 tumours | 74% DLE, 59% DRE | The adsorption of DTX to the nanohorns was achieved via π–π stacking. Prolonged diffusion-controlled DTX release was achieved. The use of mAb resulted in the selective killing of cancer cells in vitro and in vivo and a lower IC50 and no significant side effects compared to free DTX in vivo. This nanocarrier also leveraged the enhanced permeability and retention effect. | [98] | |
| Acylated C60 fullerene | in vitro: MCF-7 and MDA-MB-23 cells; in vivo: Wistar rats | 81% DLC, 84% DRE | This system achieved 4.2× higher bioavailability and 50% lower drug clearance compared to free DTX. This resulted in enhanced cancer cell cytotoxicity, low haemolysis, and high erythrocyte compatibility. | [99] | |
| Hexagonal nanostructured GO | in vitro: A549 cells | 41% DLE and approximately 20% DRE, with pH-mediated drug release | The nanostructured material improved the drug loading capacity compared to pristine GO and displayed good biocompatibility. | [32] | |
| Carbon nanoparticle (CNP)-HIF-PLGA | in vitro: Walker256 cells; in vivo: rats xenografted with Walker256 tumours | 16% DLC, with no qualitative release data shown and NIR-activated drug release | This nanocarrier displayed photothermal properties. The synergistic effect of chemotherapy and image-guided NIR PTT gave this system the ability to effectively target and treat metastatic lymph nodes both in vitro and in vivo, with minimal side effects. | [100] | |
| CNP-PLGA | in vitro: MDA-MB-231 and HUVEC cells; in vivo: New Zealand white rabbits bearing VX2 liver tumours | NIR-triggered drug release, with no quantitative drug loading/release data shown | This system relied on a combination of PTT and photoacoustic imaging to treat cancer in vitro and in vivo. Highly targeted drug delivery was achieved by transport through the lymphatic system to produce an excellent therapeutic effect on metastatic lymph nodes with favourable biocompatibility and biosafety. | [101] | |
| CNS | in vitro: MDA-MB 231 cells | 92% DLE, with no quantitative release data shown | CS nanopores substantially assisted in drug loading to give this system favourable anticancer properties. | [102] | |
| Hydroxylated CNT-APA | in vitro: MDA MB-231 cells; in vivo: Wistar rats | 51% DLE, with no quantitative drug release data shown and pH-triggered drug release in cancer cytosol | This formulation achieved a 2.8× enhancement in cytotoxicity and superior pharmacokinetics compared to free DTX, with substantial hemocompatibility with human blood and reduced side effects compared to the drug alone in vivo. | [103] | |
| RBC@GQD | in vitro: A549 cells; in vivo: CAnN.CgFoxn mice carrying A549-induced tumours; C57BL/6J mice with ALTS1C1 intracranial tumours | Approximately 40% DRE, with no qualitative drug loading data and NIR-triggered drug release | This system achieved an eight-fold increase in the accumulation in tumour tissues compared to the free drug. The synergy between the chemotherapy and photolytic properties of the nanocarrier allowed for deep penetration into tumours and effective treatment in vivo. | [104] | |
| C60 fullerene-APA | in vitro: MDA MB-231 cells; in vivo: Wistar rats | 48% DLE, 96% DRE, with pH-triggered drug release | A substantial decrease in haemolysis (human blood) and protein binding (BSA) compared to free DTX was observed. The nanoformulation also increased bioavailability and potency compared to free DTX. Fullerenes display partial P-gp efflux inhibition. | [105] | |
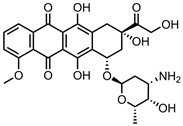 Doxorubicin (DOX)— topoisomerase II inhibitor | GO/PEI.Ac-FITC-PEG-LA | in vitro: SMMC-7721 and PIEC cells | 85% DLC, ~80% DRE, with pH-dependent drug release | The nanocarrier shows specificity for ASGPR-receptor-containing cancer cells whilst retaining DOX therapeutic efficacy. | [106] |
| GO-PRM/SA | in vitro: MCF-7 cells | 29% DLC, 49% DRE, with pH-dependent drug release | Protein adsorption in physiological environments was suppressed and the system showed enhanced cytotoxicity compared to GO-DOX alone. | [107] | |
| Tf/FA/GO/PF68 | in vitro: SMMC-7721 and L-02 cells | 96% DLC, 55% DRE, with pH-dependent drug release | The nanocarrier displayed low toxicity and high specificity due to the synergistic effect of Tf- and FA-targeting ligands on cancer cell targeting. The DOX-loaded nanosystem was not tested on healthy L-02 cells. | [108] | |
| GNRs/GO@PDA nanosheets | in vitro: MCF-7 cells | 81% DLE, 49% DRE | This system displayed dual pH/NIR-responsive drug release. The GNRs act as a probe for PTT. | [109] | |
| CNH/DCA-HPCHS | in vitro: 4T1 cells; in vivo: mice bearing 4T1 tumours | This system combines chemotherapy with NIR-mediated PTT for the treatment of cancer in vitro and in vivo. The nanocarrier displays pH-dependent drug release; however, no DOX quantification for drug loading/release was included. | [110] | ||
| oxiCNH/SA-mAb | in vitro: MCF-7 and HEK293 cells; in vivo: CR male mice bearing subcutaneous hepatic H22 tumours | 100% DLC, 67% DRE, with pH-dependent drug release | DOX is released in the endosomes of MCF-7 cells. Specific targeting of VEGF-containing cancer cells over healthy HEK293 cells was achieved by using mAb as a targeting ligand. This nanocarrier was more effective than free DOX both in vivo and in vitro, whilst showing reduced liver and cardiac toxicity. | [9] | |
| PEG-SWCNT | in vivo: SCID mice bearing Raji lymphoma xenografts | 100% DRE, with pH-dependent drug release | Increased tumour inhibition and reduced systemic toxicity compared to free DOX was determined through in vivo experiments. | [111] | |
| C60 fullerene | in vitro: CCRF-CEM, Jurkat, THP1, and Molt-16 cells | Long-term nanocarrier stability was observed in physiological saline solution. Complexation with C60 fullerene promoted Dox entry into leukemic cells, resulting in ≤ 3.5 higher cytotoxicity compared to free DOX. | [112] | ||
| Fullerenol | in vitro: MCF-7 and MDA-MB-231 cells; in vivo: zebrafish embryo (Danio rerio, wild type) | This formulation resulted in enhanced uptake, decreased proliferation, and remarkable cytotoxicity of DOX in breast cancer cells. A decrease in zebrafish embryotoxicity compared to DOX alone was noted. No drug loading or release analysis was included in this study. | [113] | ||
| C82 fullerene-cRGD | in vitro: NCl-H2135 cells | Redox-dependent DOX release, triggered by increasing glutathione concentration | Significant cytotoxicity at low doses was observed due to enhanced cellular uptake relative to free DOX. | [114] | |
| DSPE-PEG-ND | in vitro: 4T1 cells; in vivo: BALB/c mice injected with 4T1 cells and SD rats | Approximately 95% DLE and 34% DRE | Favourable circulation time, accumulation in tumour tissues, and ability to deliver DOX to tumour cell nucleus was observed. This leads to a significant enhancement of DOX efficacy and biocompatibility. | [115] | |
| ND-PEG | in vitro: A549 cells | 65% DLC, approximately 60% DRE | The nanocarrier was readily soluble in PBS and water, and unloaded ND-PEG displayed excellent biocompatibility. This system was able to deliver DOX directly to cancer cells. | [116] | |
| Carbon nanoring (CNR) | in vitro: A549, Hela, L929, and BEAS-2B cells | 51% DLE, approximately 80% DRE, with pH-mediated drug release | Enhanced antitumor activity compared to free DOX was achieved. The DOX-CNR system was highly selective, with much higher cytotoxicity for cancerous cells than normal cells. DOX was released in the nuclei of cancer cells. | [117] | |
| GA-MWCNT MA-MWCNT | in vitro: MDA-MB-231 and MCF-7 cells | 97% DLE and 71% DRE for MA-MWCNTs, and 96% DLE and 72% DRE for GA-MWCNTs, with pH-controlled drug release | The unloaded nanocarrier displayed high biocompatibility, whilst the drug-loaded systems showed slightly higher cytotoxicity than DOX in cancerous cells. Overall, the systems could effectively target cancer cells and deliver DOX to them. | [118] | |
| SWCNT-PEG-PEI-FA-CS | in vitro: MCF-7 cells | 74% DLE and approximately 60% DRE, with pH-mediated drug release | Excellent dispersibility, cellular uptake, and antitumor activity was observed in this system. This nanocarrier caused apoptosis in cancer cells by triggering ROS overproduction. | [6] | |
| Fucoidan-decorated silica–carbon nano-onion (FSCNO)-HM | in vitro: HUVEC, NCI/ADR-RES, A2780ADR, and OVCAR-8 cells; in vivo: NU/NU nude mice xenografted with NCI/ADR-RES, A2780ADR, and OVCAR-8 cells | 4% DLC and approximately 60% DRE for DOX, with NIR-triggered drug release | The co-delivery of DOX and P-gp pump inhibitor (HM) to counteract chemotherapy resistance increased DOX bioavailability and cytotoxicity. The nanosystem could effectively target and treat both drug- and non-drug-resistant tumour models with decreased side effects. FSCNOs also display photothermal capabilities. | [119] | |
| ATRA-ND | in vitro: HepG2, MCF-7, and CRL1730; in vivo: HepG2- and MCF-7-induced tumour-bearing BALB/c nude mice | 25% DLC and >80% DRE, with pH-triggered drug release | Co-delivery of DOX and ATRA in conjunction with ultrasound treatment. ATRA enhances DOX cytotoxicity, whilst ultrasound enhances nanocarrier permeability into tumour blood vessels. This approach resulted in effective DOX delivery and dramatic tumour growth inhibition. | [120] | |
| ND-PEG-HYD-FA | in vitro: HeLa, HepG2, MCF-7, and CHO cells; in vivo: BALB/c mice inoculated with HepG2 cells | 8% DLC, 85% DRE, with pH-triggered drug release | This nanocarrier has fluorescence imaging capabilities. Low drug release at pH 7 was due to the use of a cleavable hydrazone linkage. The FA-targeting ligand facilitated endocytosis and the rapid build-up of nanocarrier in cells. In vivo, experiments showed better tumour inhibition along with minimal cardiotoxicity, hepatotoxicity and nephrotoxicity when compared to free DOX. | [121] | |
| CNT/HA-DMPE | in vitro: MDA-MB-231 and A2780 cells | 20% DLC, 18% DRE, with pH-triggered drug release | This biocompatible nanocarrier is highly stable in biological buffers. The system is easy to prepare and exhibits good targetability towards CD44-overexpressing cancer cells, resulting in a remarkable increase in DOX efficacy. | [22] | |
| AL-PEG-MWCNTs | in vitro: OPM2, HOS, MCF-7, 3T3-L1, and Raji Burritt’s lymphoma cells; in vivo: CD-1, Ath/nu, and CB.17 SCID mice; ex vivo: C57BL/6J bones | 35% DLC, 51% DRE | This system uses individual MWCNTs to improve DOX pharmacokinetics. Its biocompatibility was confirmed through neoplastic transformation, chromosomal aberration, and cytotoxicity assays. Treatment-related weight loss was eliminated in a lymphoma in vivo model, whilst retaining DOX efficacy. | [122] | |
| Alginate–urea CDs | in vitro: MFC-7 cells | >70% DLE, ~45% DRE, with pH-triggered drug release | This fluorescent CD system is nontoxic and could be incorporated into hydrogels as a toughening agent. The nanohybrid displayed controlled drug release. | [123] | |
 Enzalutamide—androgen receptor antagonist | TP-PEG-Aminated GQD | in vitro: C4-2B and LNCaP cells; in vivo: BALB/c nude mice bearing C4-2B tumours | 60% DLE, 95% DRE, with redox-sensitive drug release | This nanocarrier was rapidly uptaken by prostate cancer cells via endocytosis. This caused the effective inhibition of said prostate cancer cell growth in vitro and enhanced targetability and reduced side effects compared to the free drug in vivo. | [124] |
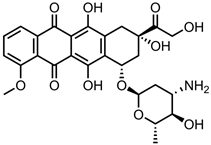 Epirubicin (EPI)— topoisomerase II inhibitor | SWCNT-DSPE-HA | in vitro: A549, AGS, and Taxol-resistant A549 cells | 47% DLC, 60% DRE, with pH-mediated drug release | This system displayed high cancer cell targetability and biosafety. By facilitating the accumulation of EPI in cells by CD44-receptor-mediated endocytosis and preventing EPI efflux by P-gp, this system markedly improved the EPI anticancer efficacy in drug-resistant cancer cells. | [125] |
| Fe3O4-MWCNTs | in vitro: T24 and 5637 cells; in vivo: MNU-induced rat-bladder tumour model | 40% DLE, 100% DRE, with magnetic-field-triggered EPI release | This system displayed prolonged retention, enhanced antitumor activity, and enhanced cytotoxicity compared to free EPI. Low systemic toxicity was also seen in vivo. The nanocarrier was also simple and quick to make. | [126] | |
| ND | in vitro: LT2-MYC cells; in vivo: FVB/N mice with MYC-induced tumours | 48% DLE, >80% DRE, with pH- and intracellular charged protein-triggered release | This nanocarrier prevents the efflux of EPI by ABC transporters to counter chemoresistance in cancer stem cells. | [127] | |
| CD-TR-TM | in vitro: SJGBM2, CHLA266, CHLA200, and U87 cells | 75% DRE with no information provided on drug release | No reference study was performed with healthy cells. The synergistic anticancer effect of the EPI/TM combination, combined with TR-facilitated targeting, drastically reduces cancer cell viability, even at low concentrations. | [128] | |
| HPPH/CPP-PEG-GO | in vitro: MG-63 cells; in vivo: osteosarcoma xenograft nude mouse model | Approximately 70% DRE, with no quantitative drug loading information provided | The synergistic effect of HPPH-mediated PDT and EPI chemotherapy allows for control over cancer cell growth. The incorporation of CPP further increases nanocarrier effectiveness by improving cancer cell targeting and internalisation. | [129] | |
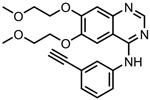 Erlotinib—tyrosine kinase inhibitor | GO-PEG | in vitro: NPC TW01 cells | 38% DLC, 98% DRE, with pH-mediated drug release | This nanoformulation achieved suppression of nasopharyngeal cancer migration, proliferation, and invasion via several molecular mechanisms. | [130] |
| Carboxylated NDs | in vitro: A549, NCI-H460, and NCI-H1975 cells | ~57% DLE, no quantitative drug release data shown | This nanocarrier caused a decrease in drug-resistant cancer cell viability. Efficient uptake of nanocarrier via clathrin-dependent endocytosis was the key to its effectiveness, and the system was preferentially consumed by cancer cells. | [131] | |
| GO | in vivo: mice | 30% DLC, 80% DRE, with pH-triggered drug release | The drug was released in a quick-burst fashion. | [132] | |
 Etoposide (Et)— topoisomerase II inhibitor | GO-COOH | in vitro: HepG2 and RPMI-1640 cells | 83% DLE, 98% DRE | The nanocarrier improved the cytotoxic effect of Et, with no change to its apoptosis pathway. The sustained release of Et offered by this formulation allowed for higher cytotoxicity and efficiency compared to the free drug. | [133] |
| oxiCNH/PEG-PA | in vitro: A549 and A549R cells; in vivo: BALB/c nude mice inoculated with A549R cells | 39% DLC, 81% DRE, with NIR-triggered Et release | Slow and steady delivery of Et was observed at neutral pH, which was accelerated 1.5× upon NIR irradiation. CNHs are also photosensitizers, and the synergistic effect of PTT and Et chemotherapy killed multidrug-resistant cells by combating P-gp-mediated drug efflux. | [134] | |
| FA-CβCDT-MSCD | in vitro: HeLa and HepG2 cells | 14% DLC, 25% DRE, with pH-mediated drug release | This nanocarrier displayed preferential targeting of folate-receptor-overexpressing cells. The encapsulation of Et in cyclodextrin prevented premature drug release. | [135] | |
| oxiMWCNT-PEG-Aso | in vitro: DMS53 and NCIH2135 cells | 45% DLC, 88% DRE, with pH-sensitive drug release | This biocompatible nanocarrier allowed for the cellular internalisation of negatively charged nucleic acids, such as Aso. Aso binding increased the chemosensitivity of drug-resistant lung cancer cells, leading to superior cytostatic efficacy compared to the free drug. This system also displayed good aqueous dispersibility and low haemolytic activity. | [136] | |
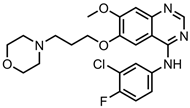 Gefitinib (GEF)—tyrosine kinase inhibitor | GO-PVP | in vitro: PA-1 and IOSE-364 cells | 46% DLE, 35% DRE, with GEF release at neutral pH | This biocompatible nanocarrier is a combination therapy with quercetin and was found to enter cells via receptor-mediated endocytosis. The synergistic effect of GEF and quercetin results in significant therapeutic efficacy with ovarian cancer cells, higher than that of drugs delivered separately. | [137] |
| PEG-CQD-PVA-PLA | in vitro: NCI–H522 cells | ~65% DRE, with no quantitative drug loading data shown | The PLA microspheres degrade via a hydrolytic reaction in acidic conditions, releasing GEF. The nanosystem delivered the drug directly to lung cancer cells, resulting in a significant decrease in the IC50 value compared to free GEF. | [138] | |
| GO nanosheets | 43% DLC, 51% DRE, with pH-triggered drug release | Effective control over GO nanosheet size was achieved; GEF was converted to nanocrystals and then loaded onto GO. | [139] | ||
| GO-HA | in vitro: A549 and HELF cells; in vivo: BALB/c nude mice bearing A549 tumours | 13% DLC, 60% DRE, with redox-dependent GET release (glutathione-mediated) | This nanocarrier displayed efficient cancer cell uptake via CD44-receptor-mediated endocytosis, resulting in a significant enhancement of the GEF efficacy. The system showed stronger tumour inhibition than the free drug in vivo, with no obvious side effects. | [140] | |
 Gemcitabine (GEM)— ribonucleotide reductase inhibitor | HA-PEG-MWCNT | in vitro: HT-29 cells; in vivo: SD rats bearing HT-29 tumours | 90% DLE, ~85% DRE, with a pH-mediated, sustained drug release profile | A reduction in haemolytic activity and remarkable improvement in pharmacokinetic parameters compared to free GEM was observed. This was due to improved cellular internalisation, leading to enhanced anticancer effects. | [141] |
| SWCNT-PEG | in vitro: A549 and MIA PaCa-2 cells; in vivo: B6 athymic nude mice (both healthy and with A549 tumours) | 37% DLC, ~80% DRE, with pH-triggered drug release, via a hydrolysable ester bond | This nanocarrier system accumulates in tumour cells and releases considerable amounts of GEM, resulting in the inhibition of tumour growth and a reduction in GEM side effects. This system also improved the stability of GEM. | [142] | |
| PEG-Fe3O4@GO@ mSiO2-FA | in vitro: A431 cells | 14% DLC, 85% DRE, with pH-triggered drug release | This system demonstrated enhanced GEM cytotoxicity and cellular uptake. | [143] | |
| ND-PEI-PAA-PEG-GFLG | in vitro: BxPC-3; in vivo: BALB/c nude mice xenografted with BxPC-3 tumours | No quantitative drug loading/release studies were performed | Significant nanocarrier stability was observed in physiological conditions, with long-term circulation due to PEG attachment and enzyme-sensitive GEM release. This system showed similar anticancer effects in vitro and a significant increase in antitumour effects in vivo compared to free GEM. | [8] | |
| ND-PEG | in vitro: AsPC-1 cells | No quantitative drug loading/release data were provided | The fluorescent NDs provide imaging and cell-tracking capabilities, and whilst no cytotoxicity enhancement compared to free GEM was seen, the nanocarrier successfully delivered GEM directly to pancreas cancer cells. | [144] | |
| ND@PHEA-co-POEGMEA | in vitro: AsPC-1 cells | 7% DLC, ~100% DRE, with pH-triggered drug release | GEM was incorporated into HEA polymer and then loaded onto NDs. This resulted in slow, sustained GEM release delivered directly to cancer cells, with a similar IC50 value to free GEM. | [145] | |
| GO/MMT/CS | in vitro: MDA-MB-231 cells | 99% DRE, with no qualitative drug loading data shown and pH-triggered drug release | GEM intercalated between MMT silicate layers, preventing burst release. The unloaded nanocarrier is nontoxic, and the sustained release of GEM from the system results in the excellent growth inhibition of breast cancer cells. | [146] | |
| FA-CS/Fe3O4/GO | 22% DLC, 83% DRE, with pH-triggered drug release | This system was tested in simulated cancer fluid and simulated human blood, and it is also responsive to external magnetic fields. | [147] | ||
| rGO | in vitro: A549, HEL-299, and NIH-3 T3 cells; in vivo: BALB/c mice (both healthy and xenografted with A549 tumours) | No quantitative drug loading/release data shown. NIR-triggered drug release | This system displayed excellent in vitro cytotoxicity against multiple lung cancer cell lines and low systemic toxicity, and a significant enhancement in antitumour activity compared to the free drug in vivo. | [148] | |
| CD | in vitro: MCF-7 and HeLa cells | 34% DLC, 54% DRE, with pH-mediated drug release | This highly fluorescent nanosystem increased the potency of GEM with minimal cytotoxic effects on healthy cells. This was due to the effective transport and delivery of GEM to cancer cells. | [149] | |
| MWCNT-LE | in vitro: MCf-7 cells; in vivo: BALB/c nude mice bearing MCf-7 tumours | Approx. 31% DLE, with no qualitative drug release data | This combination chemotherapy and PTT nanocarrier achieved good antitumour activity in vitro and in vivo, with reduced side effects seen in animal studies. | [150] | |
 Imatinib—tyrosine kinase inhibitor | N-prGO-CMC | 74% DLC, 58% DRE, with pH-triggered IM release | The drug is bound to the nanocarrier via π–π stacking and hydrogen-bonding interactions. | [151] | |
| ZnO/CNT@Fe3O4 | in vitro: CML-derived K562 cells | No quantitative drug loading/release data shown | The system causes a significant decrease in leukaemia cell viability and metabolism. Cell death was caused by apoptosis via an ROS-dependent mechanism. | [152] | |
| GQD | in vitro: RPMI 8226, MDA-MB-231, and NCI-ADR/RES cells | No quantitative drug loading/release data given | The system demonstrated efficient internalisation and remarkable cytotoxicity for cancer cells and was shown to localise in nuclei of neoplastic cells. | [153] | |
 Irinotecan— topoisomerase I inhibitor | Fe3O4-GO-CS-PEG | 3% DLC, 90% DRE, with pH-triggered drug release | A large percentage of drug bound to this nanocarrier was released at neutral pH. This system has magnetic targeting capabilities. | [154] | |
| CD-PEG-BT | in vitro: MDA-MB231 and MCF-7 cells | 23% DLC, 90% DRE, with NIR-triggered drug release | This combination chemotherapy/PTT nanosystem caused drug-resistant breast cancer cell death via necrosis and apoptosis pathways. The CDs also have fluorescence imaging capabilities. | [155] | |
| Fe3O4-GO-CS-UA-GRP-SLP2 | in vitro: U87 cells; in vivo: BALB/c nude mice with U87 tumours | 58% DLC, 62% DRE, with pH-responsive drug release | This system displayed excellent targeted drug delivery, antitumour efficacy, and prolonged animal survival in brain tumour models using intravenous administration coupled with magnetic guidance. A 4.9× increase in drug uptake compared to the free drug was measured in vitro. A 6.5× enhancement in the ability to cross the blood–brain barrier compared to the free drug was seen in vivo. Highly biocompatibility was also seen in vivo. | [156] | |
 Lobaplatin—DNA alkylating agent | E2-PEG-CNT | in vitro: MCF-7 cells; in vivo: healthy C57BL/6 mice | No qualitative drug loading/release data shown | This nanocarrier has sustained release properties, with no obvious side effects and an increase in retention time compared to the free drug in vivo. The effective killing of cancer cells was seen in vitro due to E2-mediated targeting. | [157] |
| PEG-CNT-FITC | in vitro: HepG2 cells | 72% DLC, 80% DRE, with pH-triggered, sustained drug release and fluorescence imaging properties | This nanocarrier can effectively enter and kill liver cancer cells, with increased cytotoxicity observed up to 72 h. | [158] | |
 Methotrexate (METX)— nucleotide synthesis inhibitor | oxiSWNH-PEG-Tf | in vitro: MAD-MB-231 and HepG2 cells; in vivo: ICR mice carrying H22 tumours | 15% DLC, 55% DRE; this system utilises pH-triggered drug release | The drug is released quickly at neutral pH, which could cause toxicity due to premature leakage. This system displayed favourable tumour targeting, cytotoxicity, and accumulation. | [25] |
| CMC-GO | in vitro: NIH-3T3 and HT-29 cells; in vivo: BALB/c mice and nude mice xenografted with HT-29 tumours | 39% DLC, 82% DRE, with pH-triggered drug release | This system reduced drug toxicity against healthy cells and facilitated a higher plasma concentration, superior tumour cytotoxicity, and liver cancer metastasis inhibition compared to free METX. | [159] | |
| Hydroxylated C60 fullerene | in vitro: MDA-MB-231 cells; in vivo: Wistar rats | No quantitative drug loading data shown, with 85% DRE and pH-sensitive drug release | This nanosystem drastically increased plasma half-life and AUC compared to the free drug, resulting in a large reduction in its IC50 value. Enhanced bioavailability, erythrocyte compatibility, protein binding, and haemolysis in human blood compared to free METX were also observed. | [23] | |
| AF-FA-99mTc-MWCNT | in vitro: A549 and MCF 7 cells; in vivo: New Zealand rabbits and FR+ EAT-bearing mice | 33% DLC, >85% DRE, with pH-triggered drug release achieved via a cleavable ester linkage | Effective targeting and treatment of folate-receptor-overexpressing cancer cells with reduced side effects and increased efficacy in vivo was observed. This nanocarrier also had fluorescence imaging and radio-tracing capabilities. | [160] | |
 Mitomycin C (MMC)— DNA alkylating agent | TAT-graphene | in vitro: OCM-1 and ARPE-19 cells | 22% DLC, 45% DRE, with pH-triggered drug release; however, the release in acidic and neutral environments was very similar | This system could specifically target cancer cells over healthy cells in a co-culture environment. The nanocarrier localised in the cancer cell nuclei, resulting in strong growth suppression. | [161] |
| CD | in vitro: MCF-7 cells | Approximately 80% DRE, with no quantitative drug loading information provided and pH-mediated MMC release | MMC was bound to the CDs via hydrogen bonding. This nanocarrier showed high affinity towards cancer cell membranes and could effectively enter them and accumulate. This resulted in a significant improvement in anticancer potency compared to free MMC. | [162] | |
| SWCNT-PEG-CWKG(KWKG)6 | in vitro: A549 cells | Approximately 80% DRE, with pH-mediated MMC release | The unloaded nanocarrier showed high biocompatibility whilst the drug-loaded system exhibited similar anticancer efficacy to free MMC. | [163] | |
| Graphene-BODIPY-PEG | in vitro: HeLa cells | 10% DLC, with no quantitative drug release data given | This nanocarrier possesses excellent photothermal conversion efficiency and ROS production capabilities for combination PTT/PDT. The system also has fluorescence and photothermal imaging capabilities and displayed outstanding anticancer effects. | [14] | |
 Mitoxantrone (MTX)—topoisomerase II inhibitor | GO-Fe2O3-MitP | in vitro: A549 cells | 19% DLC, 38% DRE, with magnetic-field-triggered MTX release | MitP grafting improves the drug loading capability of this nanocarrier. Successful targeting and disruption of tumour mitochondria was achieved, causing cell death. | [164] |
| oxiMWCNT | in vitro: NIH3T3 and MDA 231 cells | 95% DLC, 30% DRE | MTX was bound to oxiMWCNTs via electrostatic interactions. This formulation resulted in increased MTX efficacy; however, the system was more toxic to healthy cells than cancerous cells. | [165] | |
| ND | in vitro: MDA-MB-231 and MDA-MB231-ABCG2 cells | 87% DLE, 80% DRE, with pH- and soluble-protein-triggered MTX release | MTX release was found to be higher in FBS than in water, suggesting that the presence of soluble biological matter increases the DRE. A marked increase in MTX retention and efficacy was observed when using this nanocarrier. | [166] | |
| oxiSWCNT-PEG-FA | in vitro: HeLa cells | ~35% DLE, 55% DRE, with pH-mediated, sustained drug release | This system selectively targeted cancer cells. | [167] | |
| EXO-GO-CO-γPGA | in vitro: MDA-MB-231 and BEAS-2B cells | 73% DLE, 56% DRE, with pH-mediated, sustained drug release | This nanocarrier displays excellent cancer cell targetability. The attachment of exosomes was found to improve drug loading, pH response, and biocompatibility. | [168] | |
| rGO-PEG-SB | in vitro: 4T1, CT26, and bone marrow macrophages + DCs harvested from BALB/c mice; in vivo: BALB/c mice with 4T1 tumours | 48% DLE, with no quantitative drug release data provided and NIR-triggered drug release | The synergistic combination of PTT, chemotherapy, and immunotherapy facilitated the destruction of local primary tumours and distant metastases in an in vivo model. rGO acted as a photosensitizer, whilst the SB immunotherapeutic increased effectiveness of rGO and MTX by TGF-β inhibition. | [169] | |
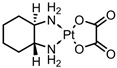 Oxaliplatin (OP)— DNA alkylating agent | GO-PNVCL-PGA | in vitro: MCF-7 cells | 12% DLC, 80% DRE, with pH- and thermal-responsive OP release | Improved cytotoxicity compared to free OP against breast cancer cells was observed. The blank nanocarrier is nontoxic. | [170] |
| GO-HSA NPs | in vitro: HFFF2 | 61% DLE, ~97% DRE, with pH-mediated, sustained drug release | The use of HSA nanoparticles increased nanocarrier biocompatibility. | [171] | |
| MWCNT-PEG | in vitro: HT29 cells | 43% DLC, no quantitative drug release data | A drastic increase in cytotoxicity towards human bowel cancer cells was observed. | [172] | |
| GO-CB [7]/Ce6/AQ4N/ADA-HA | in vitro: B16 and L02 cells; in vivo: C57BL/6 mice (both healthy and carrying B16 tumours) | 58% DRE, with spermine-triggered OX release | Significant antitumour efficacy was observed, resulting from synergistic PTT (GO)/PDT (Ce6), and chemotherapy (OP and AQ4N) properties. The enhanced hypoxia resulting from PTT bolsters the effects of chemotherapy drugs. This strategy has the benefit of being able to noncovalently attach nonaromatic drug molecules via host–guest complex formation. | [173] | |
| TAT-BT-PEI-MWCNT | in vitro: C6 glioma (cell and tumour spheroid) and CHEM-5 and L02 cells; in vivo: mice bearing C6 tumours | 19% DLC, with no quantitative drug release data shown | The nanocarrier shows enhanced blood–brain barrier penetration compared to free OX, resulting in a significant decrease in the IC50 value. The system shows low cytotoxicity towards healthy cells; however, a build-up of cerebrospinal fluid was noticed during treatment of in vivo models. | [174] | |
| GO-CS-FA | in vitro: LX-2 and SKOV3 cells | 34% DLC, ~80% DRE | This nanoformulation shows similar potency to free OX in ovarian cancer cells and good biocompatibility. | [48] | |
| CD | in vitro: L929, HeLa, and HepG2 cells | 4% DLC, with redox-sensitive drug release | The CDs have multicoloured emission capabilities and high fluorescence stability. This system shows good biocompatibility, bio-imaging, and anticancer effects both in vitro and in vivo. | [175] | |
 Paclitaxel (PTX)—microtubule growth inhibitor | GO-MA/FA | in vitro: MDA-MB-231 cells; in vivo: SD rats with DMBA-induced mammary carcinoma | 40% DLC, 71% DRE | PTX was attached to GO via π–π stacking and hydrophobic interactions. This system inhibited cancer cell growth in vitro and reduced tumour size in vivo via cell cycle arrest and apoptosis. Highly specific targeting and drug release for folate-receptor-overexpressing cancers was observed. | [176] |
| SWCNT/DOA-PEG-FA | in vitro: MCF-7 cells; in vivo: athymic nude mice with MFF-7-induced tumours and BALB/c mice | No quantitative drug loading or release data were provided | This system displayed high specificity, biocompatibility, and efficacy compared to free PTX in vitro. In vivo, studies revealed significant tumour growth inhibition, with no side effects on the blood and major organs of mice observed. | [177] | |
| PLA composite nanofibers/C70 fullerene | in vitro: HepG2 cells | No quantitative drug loading information, 72% DRE | Control of in vitro PTX release profile was achieved by varying fullerene content. Successful control of cancer cell growth was achieved using this nanoformulation. | [178] | |
| Rf-MWCNTs | in vitro: MCF-7 cells; in vivo: SD rats | 82% DLE, 99% DRE | Haemolysis in human blood was much lower than free PTX. This system also showed better cytotoxicity than free PTX, with low systemic toxicity, favourable biodistribution, and renal excretion observed in vivo. | [179] | |
| Au–N-doped carbon nanotube cup (NCNC) | in vitro: MDSC cells; in vivo: B16 melanoma cells inoculated into C57BL/6 mice | 36% DLE, with no quantitative drug release data shown | The PTX-containing NCNCs capped with Au nanoparticles exhibited strong surface-enhanced Raman scattering effects, allowing for extremely sensitive detection. A single injection of nanocarrier solution given to an in vivo model significantly reduced tumour growth and eliminated tumours in ~30% of mice. This system targeted lymphoid tissues surrounding tumours to boost the host immune system response. | [180] | |
| Graphene-PLA-PEG | in vitro: U-138 cells; in vivo: athymic nude Foxn1nu mice implanted with U-138 tumours | 4% DLC, 6% DRE | This nanocarrier is nontoxic and expressed long-term sustained drug release over 19 days. The system was found to be six times more potent than free PTX in vitro and is capable of reducing U-138 cell viability despite low drug loading and release. | [181] | |
| HMCS | in vitro: CNE cells; in vivo: nude mice with CNE tumours | 19% DLC, no release experiments were carried out with PTX | HMCSs themselves exhibited strong antitumour effects through PTT. | [86] | |
| CNT-PMAA self-assembled micelles | in vitro: L929 and HeLa cells | 36% DLE, 74% DRE, with pH-triggered drug release | The blank nanocarrier is nontoxic, with low haemolysis observed. Higher anticancer activity than free PTX was also noted. The self-assembly of CNT-PMAA is pH-dependant, and at low pH, the nanocarrier disassembles. | [182] | |
| FA-CD-GOx | in vitro: MDA-MB-468 (cells and tumour spheroids); TNBC and HEK293 cells | 82% DLE, 95% DRE, with GOx and PTX release occurring at pH 7.4 | GOx-induced cancer starvation, which had a synergistic effect with PTX chemotherapy, and resulted in significant cancer cell death. This biocompatible nanocarrier also efficiently targets cancer cells over normal cells. | [13] | |
| FCDb | in vitro: NIH3T3 and B16F10 cells | 82% DLC, with no quantitative release data given | This nanocarrier targeted cancer cells in a co-culture with healthy cells due to its biotin-targeting ligand. It caused the selective sensing and activation of H2O2, and it also has fluorescence imaging capabilities. | [183] | |
 Sorafenib—tyrosine kinase inhibitor | rGO | in vitro: SGC7901 cells | No quantitative drug loading/release data | A significant increase in cytotoxicity compared to the free drug in gastric cancer cells was observed, with apoptosis being the main mechanism of cell death. | [184] |
| CS nanoparticles-FA | in vitro: HepG2, HDFa, and HT29 cells | 19% DLC, 91% DRE, with pH-triggered, sustained drug release profile | This system showed negligible toxicity to healthy cells and enhanced anticancer properties compared to the free drug. | [185] | |
| CNT-PEG | in vitro: HepG2 cells; in vivo: Wistar rats, both healthy and with DENA-induced liver tumours | 95% DLC, ~100% DRE | A negligible change in potency or morphology was observed over a 3-month stability study. This system displayed superior anticancer abilities compared to the free drug both in vitro and in vivo. | [186] | |
| DSPE-PEG-ND | in vivo: healthy rats and BALB/c mice carrying BGC-823 tumours | ~90% DRE, with no quantitative drug loading data shown | A 14-fold increase in drug concentration in tumour tissues compared to the free drug was observed. This caused considerable growth inhibition for the in vivo gastric cancer model. A 7.64× increase in oral bioavailability compared to the free drug was also seen in vivo. | [187] | |
 Tamoxifen (TAM)—oestrogen receptor antagonist | rGO | in vitro: MCF-7 cells; in vivo: BALB/c mice bearing MCF-7 tumours | This system displayed lower cytotoxicity than free TAM but had promising photothermal properties. No quantitative drug loading/release data were shown. The nanocarrier has excellent stability. | [188] | |
| NGR-SWCNT-PF68 | in vitro: 4T1 cells; in vivo: BALB/c mice carrying 4T1 tumours | This targeted nanocarrier could effectively enter cancer cells whilst retaining TAM cytotoxicity. Receptor-mediated tumour targeting combined with PTT capabilities resulted in significant anticancer efficacy in vivo. No quantitative drug loading/release data shown. | [189] | ||
| MWCNT-LE | in vitro: MCF-7 cells | 28% DLC, no quantitative drug release data | Enhanced cellular uptake, apoptosis, and antitumour activity (compared to free TAM) were observed. This system also has PTT properties. LEN acts as both a dispersant and a potent anticancer agent itself. Combination chemo–PTT results in cancer cell destruction at low drug concentrations. | [190] | |
| C60 fullerene-Gly | in vitro: MCF-7 cells; in vivo: Wistar rats | 66% DLC, 85% DRE, with pH-triggered TAM release | This nanocarrier vastly improved the pharmacokinetic properties and cytotoxicity of TAM against breast cancer cells. The system displayed minimal haemolysis towards human blood. The bioavailability, half-life, and cancer cell penetration of the drug were all markedly improved. | [191] | |
| TEG-MWCNT-quercetin | in vitro: MDA-MB-231 cells; in vivo: Wistar rats | No quantitative drug loading info, 93% DRE, and pH-triggered drug release using a TAM prodrug | This haem-compatible formulation reduced the IC50 values and increased the uptake in drug-resistant cells. These favourable properties carried over to in vivo studies, resulting in enhanced efficacy, pharmacokinetics, and biocompatibility. | [192] | |
| DES-graphene | in vitro: MCF-7 cells | No quantitative drug loading/release data | Nanocarrier possesses acute, selective anticancer activity achieved through intracellular ROS-production-triggering cell cycle arrest. | [193] | |
 Temozolomide—DNA alkylating agent | GO-Fe3O4 | in vitro: rat glioma C6 cells | 90% DLC and 74% DRE, with pH-mediated drug release | The blank nanocarrier is biocompatible, whilst the loaded system showed better inhibitory effects than the free drug in rat glioma cells. This formulation also has strong magnetic properties. | [194] |
| rGO | in vitro: LN229 cells | 84% DLC and 83% DRE, with electrochemically controlled drug release | This system retained the anticancer potency of temozolomide. | [195] | |
 Topotecan—topisomerase I inhibitor | GO/TT/CisP/cholesterol-DOX encapsulated in a DSPE-PEG nanocell | in vitro: HeLa cells | 29% DLC and 40% DRE for TT, with pH-triggered drug release | This nanocarrier has fluorescence imaging capabilities and displays remarkably higher anticancer efficacy compared to the free-drug combination due to the synchronised targeting of multiple targets in cancer cells at once. | [196] |
| PEI-GO-TT-CisP | in vitro: HeLa cells | 51% DLC for TT, no quantitative drug release data | This nanocarrier has subcellular-organelle-targeting capabilities and can effectively impair the mitochondria of cervical cancer cells, leading to cell death. This resulted in a 4.4× decrease in IC50 compared to the free-drug cocktail. | [197] | |
| TT/GO-CD/DOX | in vitro: HeLa cells | 16% DLE and 77% DRE for TT, with sustained, pH-triggered drug release | This system demonstrated superior anticancer efficacy compared to free drugs and single-drug-loaded nanocarrier due to the synergistic effect between the drugs and GO. | [198] | |
 Vinblastine—mitosis inhibitor | CQD | in vitro: Hela, HGC-27, A549, MCF-7, CF-STTG, and Vero cells; in vivo: NOD-SCID mice carrying A549 tumours | 95% DLC, with no qualitative drug release data | The cytotoxicity of vinblastine was reduced in normal cells and increased in cancer cells compared to the free drug. Significant inhibition of tumour growth was observed in vivo, with no liver toxicity. The synergistic combination of chemotherapy and PTT allowed for the control of cancer cell growth. | [199] |
 Vincristine—mitosis inhibitor | oxiSWCNH-PEG-mAb | in vitro: MCF-7 and HUVEC cells; in vivo: ICR mice carrying H22 tumours | 35% DLC, 80% DRE, with pH-triggered, sustained drug release | Effective cancer cell killing in MCF-7 cells and treatment of liver cancer in mice with superior efficacy compared to the free drug. The system displayed reduced systemic toxicity compared to the free drug due to mAb-facilitated targeting. | [200] |
| Drug | Page | Drug | Page |
|---|---|---|---|
| 5-fluorouracil | 5 | epirubicin | 23 |
| 6-mercaptopurine | 6 | erlotinib | 24 |
| anastrozole | 7 | etoposide | 24 |
| bortezomib | 7 | gefitinib | 25 |
| capecitabine | 7 | gemcitabine | 26 |
| carboplatin | 7 | imatinib | 27 |
| carmustine | 9 | irinotecan | 28 |
| chlorambucil | 9 | lobaplatin | 29 |
| cisplatin | 9 | methotrexate | 29 |
| curcumin | 13 | mitomycin C | 30 |
| cyclophosphamide | 15 | mitoxantrone | 30 |
| cytarabine | 15 | oxaliplatin | 31 |
| dabrafenib | 16 | paclitaxel | 33 |
| dasatinib | 16 | sorafenib | 34 |
| daunorubicin | 16 | tamoxifen | 35 |
| decitabine | 17 | temozolomide | 36 |
| docetaxel | 17 | topotecan | 36 |
| doxorubicin | 19 | vinblastine | 37 |
| enzalutamide | 23 | vincristine | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, H.; Fagan, A.; Giordani, S. Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems. Pharmaceutics 2023, 15, 1545. https://doi.org/10.3390/pharmaceutics15051545
Mohan H, Fagan A, Giordani S. Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems. Pharmaceutics. 2023; 15(5):1545. https://doi.org/10.3390/pharmaceutics15051545
Chicago/Turabian StyleMohan, Hugh, Andrew Fagan, and Silvia Giordani. 2023. "Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems" Pharmaceutics 15, no. 5: 1545. https://doi.org/10.3390/pharmaceutics15051545
APA StyleMohan, H., Fagan, A., & Giordani, S. (2023). Carbon Nanomaterials (CNMs) in Cancer Therapy: A Database of CNM-Based Nanocarrier Systems. Pharmaceutics, 15(5), 1545. https://doi.org/10.3390/pharmaceutics15051545








