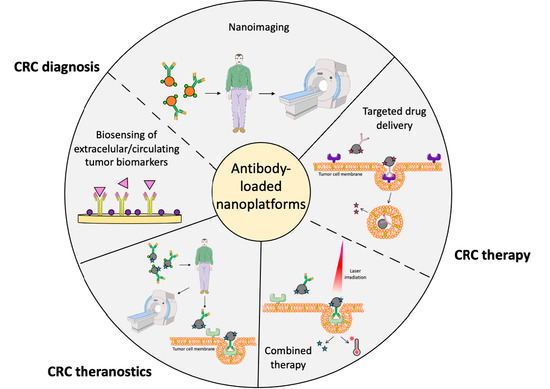
Antibody-Loaded Nanoplatforms for Colorectal Cancer Diagnosis and Treatment: An Update
Abstract
1. Introduction
2. Current Strategies for Colorectal Cancer Diagnosis and Therapy
2.1. Current Methods for CRC Screening and Detection
2.2. Current Options for CRC Therapy
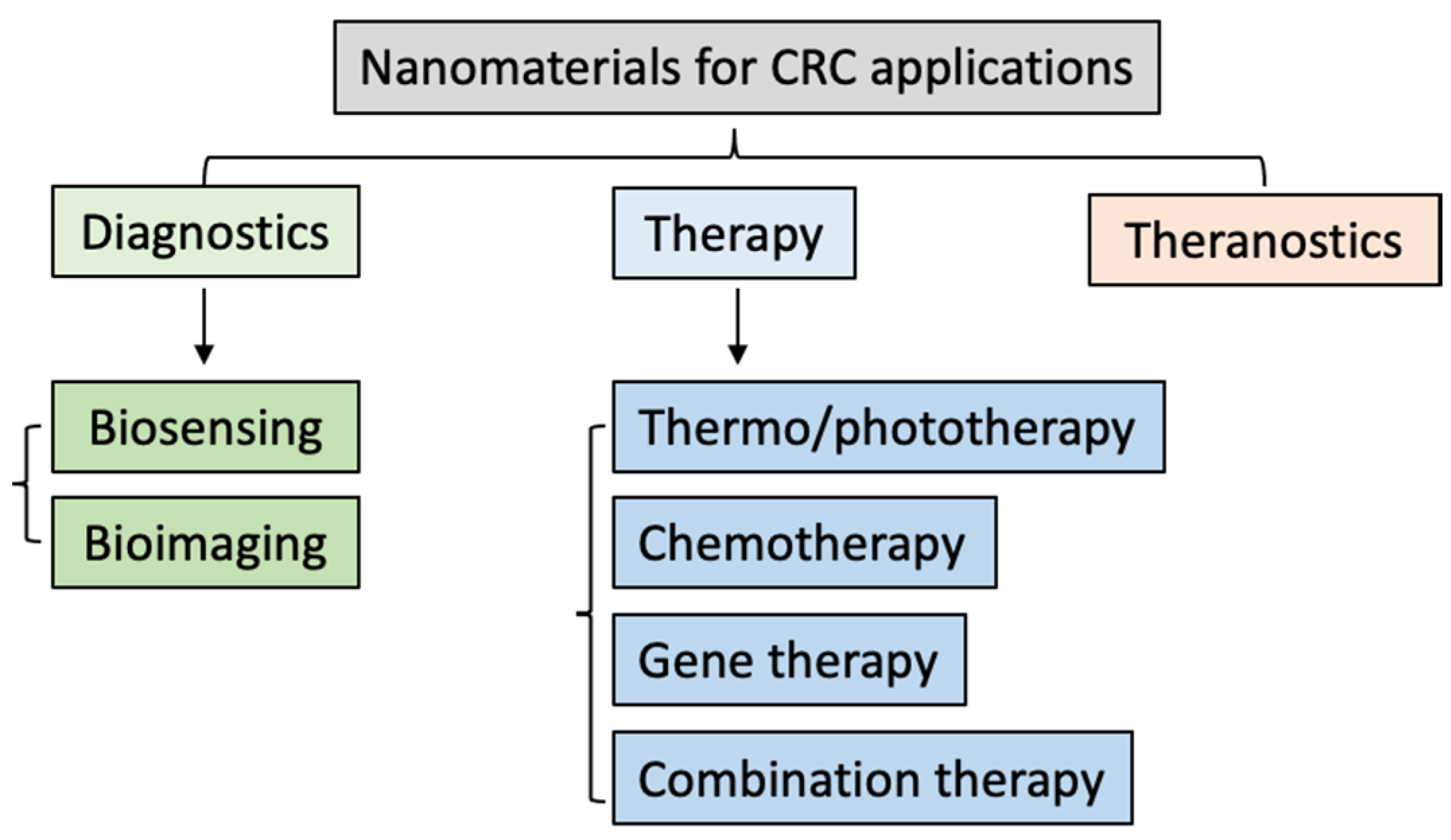
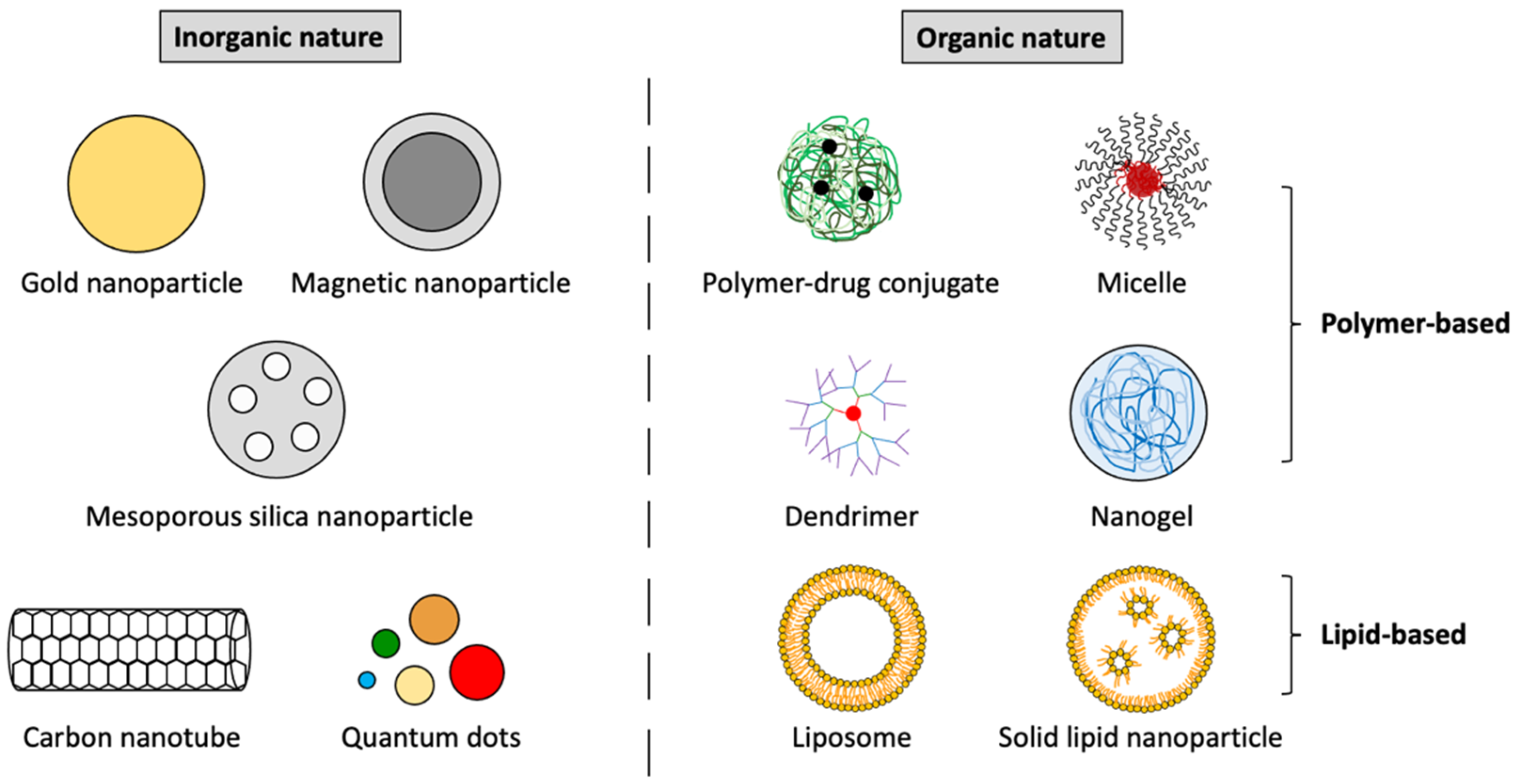
3. Most Researched Nanosystems for the Diagnosis and Treatment of CRC
3.1. The Most Investigated Inorganic Nanoplatforms for CRC Applications
3.2. The Most Investigated Organic Nanoplatforms for CRC Applications
4. Nanoplatform Pathways for Targeting Tumor Tissues
4.1. Passive Targeting of Tumor Tissues
4.2. Active Targeting of Tumor Tissues
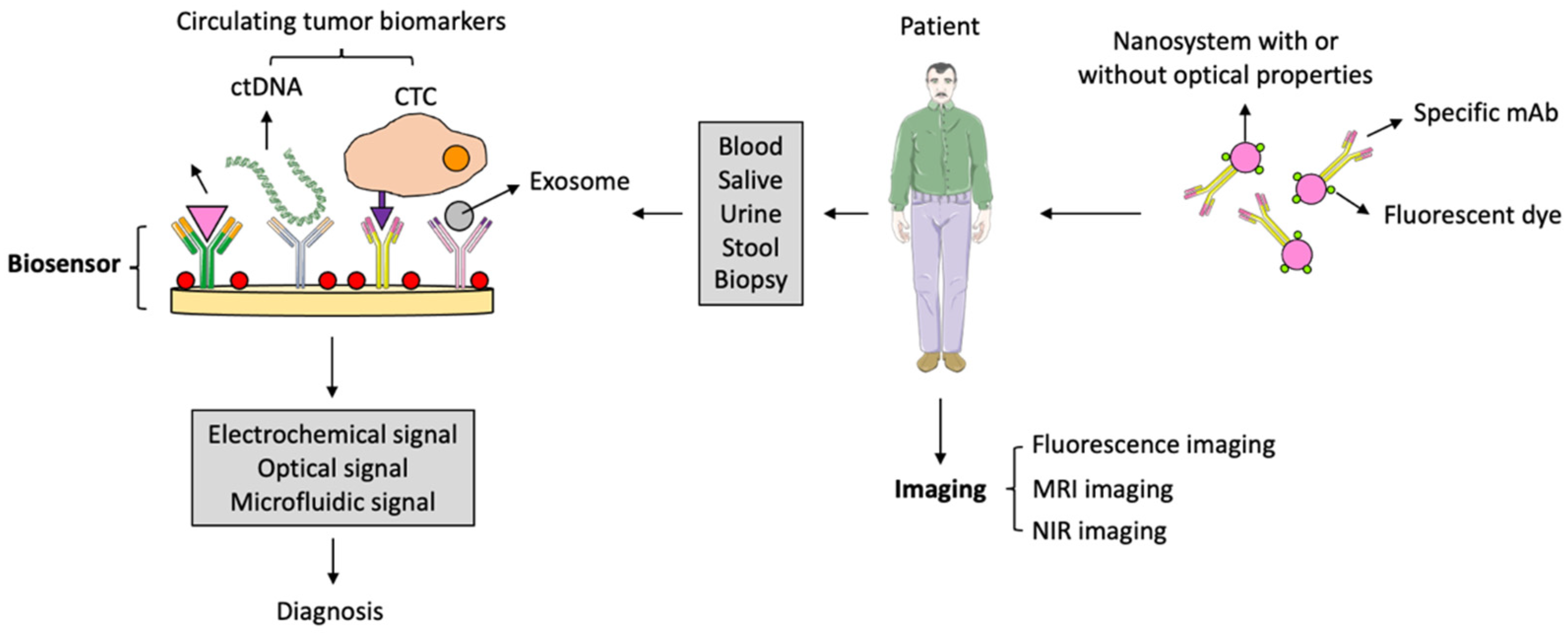
5. Antibody-Loaded Nanosystems for CRC Diagnosis
5.1. Antibody-Loaded Nanosystems for the Detection of Extracellular CRC Biomarkers
5.2. Antibody-Loaded Nanosystems for the Detection of Circulating CRC Cells and Tumor DNA and microRNA
5.3. Antibody-Loaded Nanosystems for CRC Imaging
6. Antibody-Loaded Nanosystems for the Therapy of CRC
6.1. Targeted DDS for the Treatment of CRC
6.2. Antibody-Loaded Nanosystems for CRC Combination Therapy
7. Antibody-Loaded Nanosystems for CRC Theranostic Applications
8. Nanoplatforms for CRC Therapy in Clinical Trials or Recently Patented
9. Conclusions and Suggestions for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| ADCP | Antibody-dependent cellular phagocytosis |
| BSA | Bovine serum albumin |
| CNTs | Carbon nanotubes |
| CEA | Carcinoembryonic agent |
| CTC | Circulating tumor cell |
| CRC | Colorectal cancer |
| CDCC | Complement-dependent cellular cytotoxicity |
| CDC | Complement-dependent cytotoxicity |
| CT | Computed tomography |
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 |
| DDSs | Drug delivery systems |
| ECL | Electrochemiluminiscence |
| EPR | Enhanced permeability and retention |
| ELISA | Enzyme-linked immunosorbent assay |
| EGFR | Epidermal growth factor receptor |
| EpCAM | Epithelial cell adhesion molecule |
| EMA | European Medicines Agency |
| GI | Gastrointestinal |
| AuNPs | Gold nanoparticles |
| HRP | Horseradish peroxidase |
| HA | Hyaluronic acid |
| IFP | Interstitial fluid pressure |
| CPT-11 | Irinotecan |
| QDs | Quantum dots |
| LV | Leucovorin |
| MNPs | Magnetic nanoparticles |
| MRI | Magnetic resonance imaging |
| MSNs | Mesoporous silica nanoparticles |
| mCRC | Metastatic colorectal cancer |
| miRNA | MicroRNA |
| mAb | Monoclonal antibody |
| MDR | Multidrug resistance |
| NPs | Nanoparticles |
| NIR | Near-infrared |
| OS | Overall survival |
| PEG | Polyethylene glycol |
| PGA | Polyglycolic acid |
| PLA | Polylactic acid |
| PLGA | Polylactic-co-glycolic acid |
| PCR | Polymerase chain reaction |
| PLC | Poly (ε-caprolactone) |
| PD-1 | Programmed death 1 molecule |
| RTK | Receptor tyrosine kinase |
| SLNs | Solid lipid nanoparticles |
| TGF-β | Transforming growth factor-β |
| FDA | U.S. Food and Drug Administration |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| WHO | World Health Organization |
| DTTC | 3-3′-Diethylthiatricarbocyaniniodid |
| ALA | 5-Aminolevulinic acid |
| 5-FU | 5-Fluoracil |
| 5-mC | 5-Mehtylcytosinine |
References
- Xi, Y.; Xu, P. Global colorrectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Pavitra, E.; Dariya, B.; Srivani, G.; Kang, S.-M.; Alam, A.; Sudhir, P.-R.; Kamal, M.A.; Raju, G.S.R.; Han, Y.-K.; Lakkakula, B.V.K.S.; et al. Engineered nanoparticles for imaging and drug delivery in colorectal cancer. Semin. Cancer Biol. 2021, 69, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Mahasneh, A.; Al-Shaheri, F.; Jamal, E. Molecular biomarkers for an early diagnosis, effective treatment, and prognosis of colorectal cancer: Current updates. Exp. Mol. Pathol. 2017, 102, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-H.; Chen, Y.-X.; Fan, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target Ther. 2020, 5, 22. [Google Scholar]
- Matos, A.I.; Carreira, B.; Peres, C.; Moura, L.I.F.; Conniot, J.; Fourniols, T.; Scomparin, A.; Martínez-Barriocanal, A.; Arango, D.; Conde, J.P.; et al. Nanotechnology is an important strategy for combinational innovative chemo-immunotherapies against colorectal cancer. J. Control. Release 2019, 307, 108–138. [Google Scholar] [CrossRef] [PubMed]
- Brar, B.; Ranjan, K.; Palria, A.; Kumar, R.; Ghosh, H.; Sihag, S.; Minakshi, P. Nanotechnology in CRC for precision diagnosis and therapy. Front. Nanotechnol. 2021, 3, 699266. [Google Scholar] [CrossRef]
- Ghorbani, F.; Kokhaei, P.; Ghorbani, M.; Eslami, M. Application of different nanoparticles in the diagnosis of colorectal cancer. Gene Rep. 2020, 21, 100896. [Google Scholar] [CrossRef]
- Shin, N.; Son, G.M.; Shin, D.-H.; Kwon, M.-S.; Park, B.-S.; Kim, H.-S.; Ryu, D.; Kang, C.-D. Cancer associated fibroblast and desmoplastic reactions related to cancer invasiveness in patients with colorectal cancer. Ann. Coloproctol. 2019, 35, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current trends and future perspectives of nanomedicine for the management of colon cancer. Eur. J. Pharm. 2021, 910, 174464. [Google Scholar] [CrossRef]
- Banerjee, A.; Pathak, S.; Subramanium, V.D.; Dharanivasan, D.; Murugesan, R.; Verma, R.S. Strategies for targeted drug delivery in treatment of colon-cancer: Current trends and future perspectives. Drug Discov. 2017, 22, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
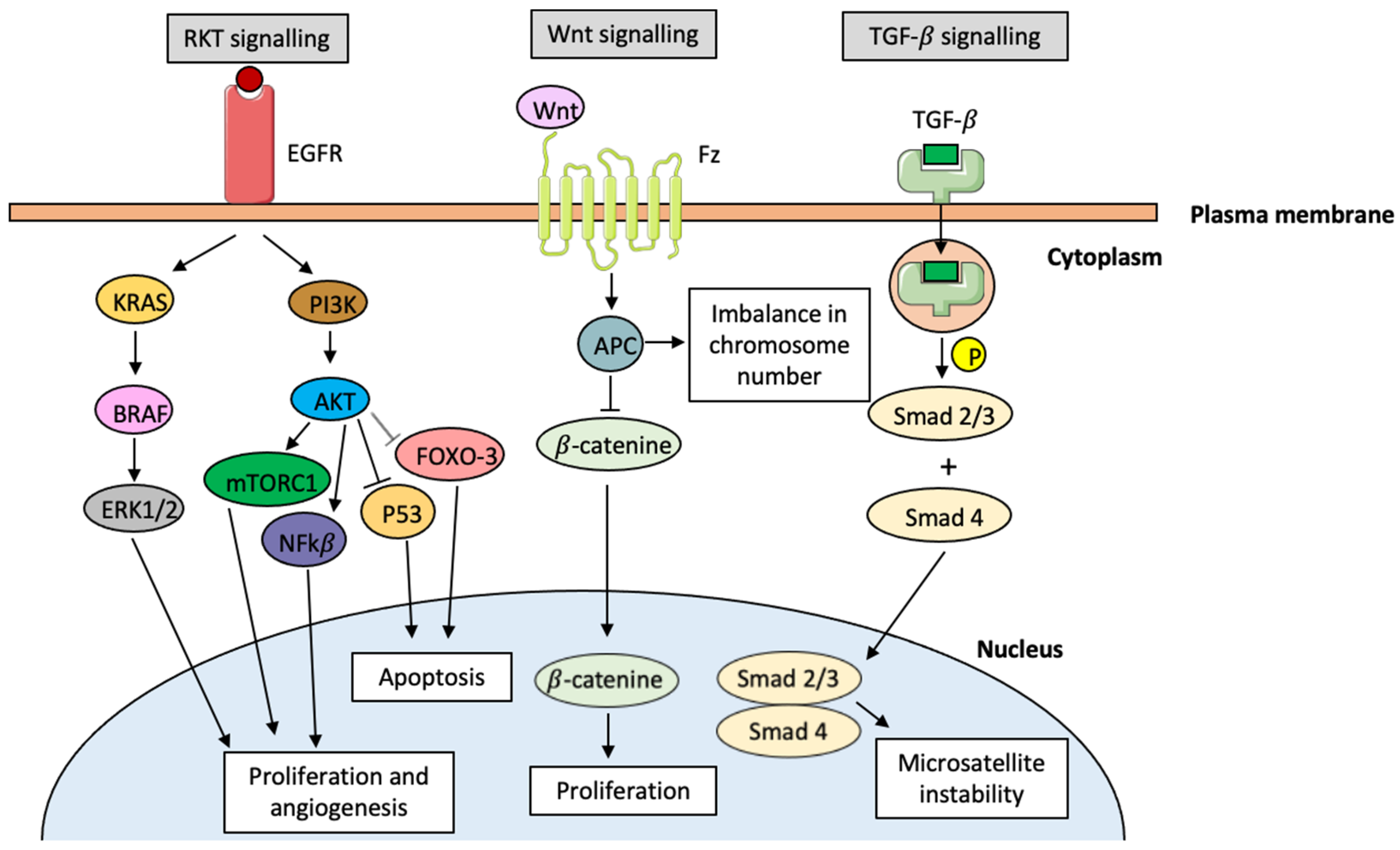
- Al-Sohaily, S.; Biankin, A.; Leong, R.; Kohonen-Corish, M.; Warusavitarne, J. Molecular pathways in colorectal cancer. J. Gastroenterol. Hepatol. 2012, 27, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Malki, A.; ElRuz, R.A.; Gupta, I.; Allouch, A.; Vranic, S.; Al Moustafa, A.-E. Molecular mechanisms of colon cancer progression and metastasis: Recent insights and advacements. Int. J. Mol. Sci. 2021, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Forootan, F.S.; Esfahani, M.H.N.; Ghaedi, K. Signaling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, N.A.; Kumar, L. Treating colon cancers with a non-conventional yet strategic approach: An overview of various nanoparticulate systems. J. Control. Release 2021, 336, 16–39. [Google Scholar] [CrossRef]
- Liu, Q.; Duo, Y.; Fu, J.; Quiu, M.; Sun, Z.; Adah, D.; Kang, J.; Xie, Z.; Fan, T.; Bao, S.; et al. Nano-immunotherapy: Unique mechanisms of nanomaterials in synergizing cancer immunotherapy. Nanotoday 2021, 36, 101023. [Google Scholar] [CrossRef]
- Aldahhan, R.; Almohazey, D.; Khan, F.A. Emerging trends in the application of gold nanoformulations in colon cancer diagnosis and treatment. Semin. Cancer Biol. 2022, 86, 1056–1065. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, K.; Chen, K.; Xu, C.; Ma, P.; Dang, G.; Yang, Y.; Lei, Q.; Huang, H.; Yu, Y. Nanoparticle-based medicines in clinical cancer therapy. Nanotoday 2022, 45, 101512. [Google Scholar] [CrossRef]
- Kotlevets, L.; Chastre, E.; Desmaïle, D.; Couvreur, P. Nanotechnologies for the treatment of colon cancer: From old drugs to new hope. Int. J. Pharm. 2016, 514, 24–40. [Google Scholar] [CrossRef]
- Viswanath, B.; Kim, S.; Lee, K. Recent insights into nanotechnology development for detection and treatment of colorectal cancer. Int. J. Nanomed. 2016, 11, 2491–2504. [Google Scholar]
- Shrivastava, P.; Sharma, R.; Gautam, L.; Vyas, S.; Vyas, S.P. Nano Drug Delivery Strategies for the Treatment of Cancers, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 191–223. [Google Scholar]
- Zhang, X.; Zhang, H.; Shen, B.; Sun, X.-F. Chromogranin-A expression as a novel biomarker for early diagnosis of colon cancer patients. Int. J. Mol. Sci. 2019, 20, 2019. [Google Scholar] [CrossRef]
- Marzo, T.; Ferraro, G.; Cucci, L.M.; Pratesi, A.; Hansson, O.; Satriano, C.; Merlino, A.; La Mendola, D. Oxaliplatin inhibits angiogenin proliferative and cell migration effects in prostate cancer cells. J. Inorg. Biochem. 2022, 226, 111657. [Google Scholar] [CrossRef] [PubMed]
- Souglakos, J.; Androulakis, N.; Syrigos, K.; Polyzos, A.; Ziras, N.; Athanasiadis, A.; Kakolyris, S.; Tsousis, S.; Kouroussis, C.; Vamvakas, L.; et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs. FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): A multicentre randomized phase III trial from the Hellenic Oncology Research Group (HORG). Br. J. Cancer 2006, 94, 798–805. [Google Scholar] [PubMed]
- Ibrahim, B.; Mady, O.Y.; Tambuwala, M.M.; Muggag, Y.A. pH-sensitive nanoparticles containing 5-fluorouracil and leucovorin as an improved anti-cancer option for colon cancer. Nanomedicine 2022, 17, 367–381. [Google Scholar] [CrossRef]
- Souglakos, J.; Kalykaki, A.; Vamvakas, L.; Androulakis, N.; Kalbakis, K.; Agelaki, S.; Vardakis, N.; Tzardi, M.; Kotsakis, A.P.; Gioulbasanis, J.; et al. Phase II trial of capecitabine and oxaliplatin (CAPOX) plus cetuximab in patients with metastatic colorectal cancer who progressed after oxaliplatin-based chemotherapy. Ann. Oncol. 2007, 18, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Mitchell, E.P.; Hoff, P.M. Irinotecan in the treatment of colorectal cancer. Cancer Treat. Rev. 2006, 32, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.A.; Osman, M.A.; El-Gizawy, S.A.; Goda, A.E.; Shamloula, M.M.; Faheem, A.M.; McCarron, P.A. Polymeric nano-encapsulation of 5-fluorouracil enhances anti-cancer activity and ameliorates side effects in solid Erlich carcinoma-bearing mice. Biomed. Pharm. 2018, 105, 215–224. [Google Scholar] [CrossRef]
- Cassidy, J.; Misset, J.L. Oxaliplatin-related side effects: Characteristics and management. Semin. Oncol. 2002, 29, 11–20. [Google Scholar] [CrossRef]
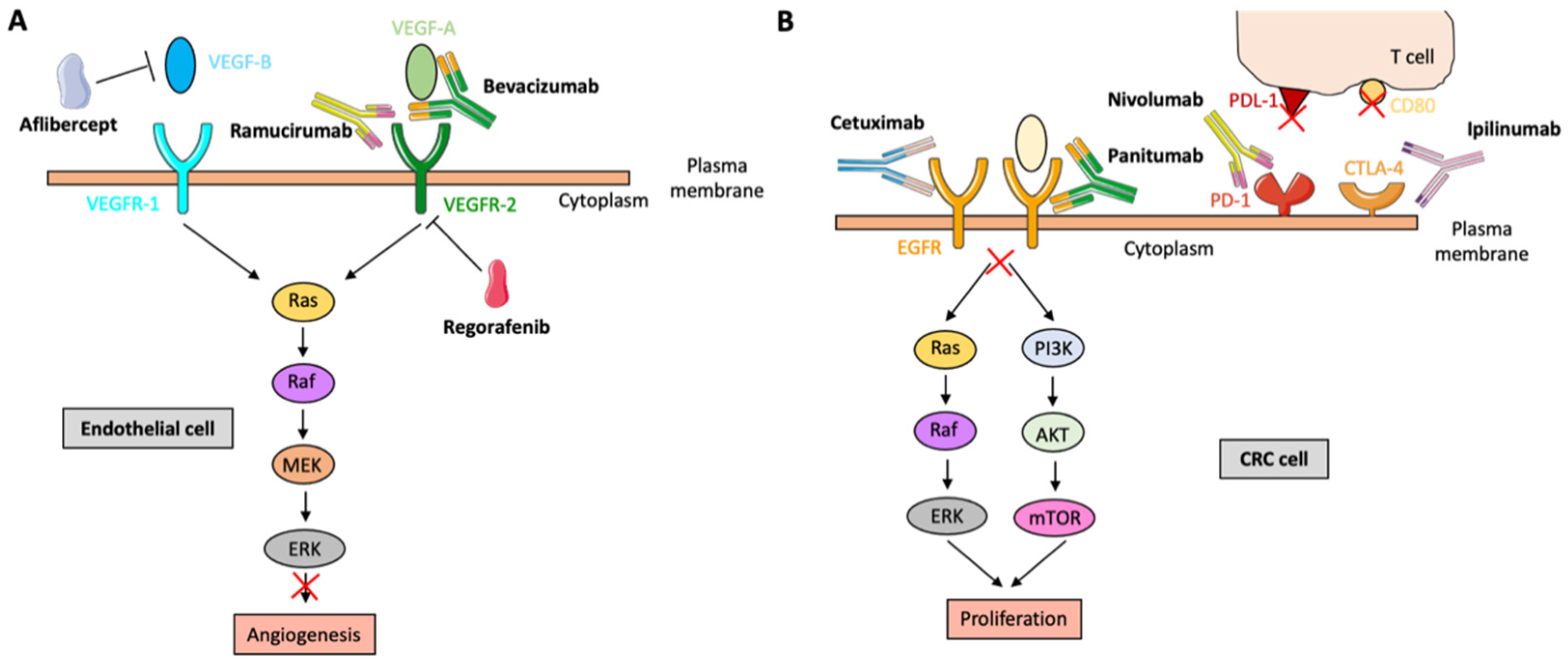
- Folkman, J. Endogeneous angiogenesis inhibitors. APMIS 2004, 112, 496–507. [Google Scholar] [CrossRef]
- Françoso, A.; Simione, P.U. Immunotherapy for the treatment of colorectal tumors: Focus on approved and in-clinical-trial monoclonal antibodies. Drug Des. Develop. Ther. 2017, 11, 177–184. [Google Scholar] [CrossRef]
- Rodríguez, M. Ziv-Aflibercept use in metastatic colorectal cancer. J. Adv. Pract. Oncol. 2013, 4, 348–352. [Google Scholar]
- Arai, H.; Battaglin, F.; Wang, J.; Lo, J.H.; Soni, S.; Zhang, W.; Lenz, H.-J. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat. Rev. 2019, 81, 101912. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Dheer, D.; Samykutty, A.; Shankar, R. Antibody drug conjugates in gastrointestinal cancer: From lab to clinical development. J. Control. Release 2021, 340, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Ghani, S.; Bahrami, S.; Rafiee, B.; Eyvazi, S.; Yarian, F.; Ahangarzadeh, S.; Khalili, S.; Shahzamani, K.; Jofarisani, M.; Bandehpour, M.; et al. Recent developments in antibody derivatives against colorectal cancer: A review. Life Sci. 2021, 265, 118791. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M. Targeted therapies in colorectal cancer: The dos, don’ts, and future directions. J. Gastrointest. Oncol. 2013, 4, 239–244. [Google Scholar]
- Smith, K.M.; Desai, J. Nivolumab for the treatment of colorectal cancer. Expert Rev. Anticancer Ther. 2018, 18, 611–618. [Google Scholar] [CrossRef]
- Wyss, J.; Dislich, B.; Koelzer, V.H.; Galván, J.A.; Dawson, H.; Hädrich, M.; Inderbitzin, D.; Lugli, A.; Zlobec, I.; Berger, M.D. Stromal PD-1/PD-L1 expression predicts outcome in colon cancer patients. Clin. Color. Cancer 2019, 18, e20–e38. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Song, Q.; Sun, X.; Xue, M.; Yang, Z.; Shang, J. Comprehensive analysis of CTLA-4 in the tumor microenvironment of 33 cancer types. Int. Immunopharmacol. 2020, 85, 106633. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Martín del Valle, E.M. Trastuzumab: More than a guide in HER2-positive cancer nanomedicine. Nanomaterials 2020, 10, 1674. [Google Scholar] [CrossRef]
- Khan, N.; Ruchika; Dhritlahre, R.K.; Saneja, A. Recent advances in dual-ligand targeted nanocarriers for cancer therapy. Drug Discov. Today 2022, 27, 2288–2299. [Google Scholar] [CrossRef]
- Alhaj-Suliman, S.D.; Wafa, E.I.; Salem, A.K. Engineering nanosystems to overcome barriers to cancer diagnosis and treatment. Adv. Drug Delivery Rev. 2022, 189, 114482. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Chen, L.; Zhang, Y.; Yuan, Y. Emerging role of nanoparticles in the diagnostic imaging of gastrointestinal cancer. Sem. Cancer Biol. 2022, 86, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Khizar, S.; Alrushaid, N.; Khan, F.A.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Nanocarriers based novel and effective drug delivery system. Int. J. Pharm. 2023, 632, 122570. [Google Scholar] [CrossRef] [PubMed]
- Khiavi, M.A.; Safary, A.; Aghanejad, A.; Barar, J.; Rasta, S.H.; Golchin, A.; Omidi, Y.; Somi, M.H. Enzyme-conjugated gold nanoparticles for combined enzyme and photothermal therapy of colon cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 333–344. [Google Scholar] [CrossRef]
- Moskvin, M.; Babic, M.; Reis, S.; Cruz, M.M.; Ferreira, L.P.; Carvalho, M.D.; Lima, S.A.C.; Morák, D. Biological evaluation of surface-modified magnetic nanoparticles as a platform for colon cancer theranostics. Colloids Surf. B Biointerfaces 2018, 161, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Jia, T.-t.; Huang, Q.-x; Qiu, Y.-y.; Xu, J.; Yin, P.-h.; Liu, T. Mesoporous silica nanoparticles (MSNs)-based organic/inorganic hybrid nanocarriers loading 5-Fluorouracil for the treatment of colon cancer with improved anticancer efficacy. Colloids Surf. B Biointerfaces 2017, 159, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, A.; Shrivastava, C.; Jain, A.K. Hyaluronic acid conjugated multi-walled carbon nanotubes for colon cancer targeting. Int. J. Biol. Macromol. 2019, 123, 691–703. [Google Scholar] [CrossRef]
- Majumder, J.; Tarutala, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Delevery Rev. 2019, 144, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, A.O.; Lebelo, S.L.; Msagati, T.A.M. Nanocarrier design-function relationship: The prodigious role of properties in regulating biocompatibility for drug delivery applications. Chem. Biol. Interact. 2023, in press. [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Khurana, R.K.; Kesharwani, P. Strategizing biodegradable polymeric nanoparticles to cross the biological barriers for cancer targeting. Int. J. Pharm. 2019, 565, 509–522. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Sheoran, S.; Arora, S.; Samsonray, R.; Govindaiah, P.; Vuree, S. Lipid-based nanoparticles for treatment of cancer. Heliyon 2022, 8, e09403. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, D.G.; Ghosh, D.; Das, A. Recent developments in nanocarriers for cancer chemotherapy. OpenNano 2022, 8, 100080. [Google Scholar] [CrossRef]
- Graván, P.; Aguilera-Garrido, A.; Marchal, J.A.; Navarro-Marchal, S.A.; Galisteo-González, F. Lipid-core nanoparticles: Classification, preparation methods, routes of administration and recent advances in cancer treatment. Adv. Colloid Interface Sci. 2023, 314, 102871. [Google Scholar] [CrossRef] [PubMed]
- Vyas, K.; Rathod, M.; Patel, M.M. Insight on nano drug delivery systems with targeted therapy in treatment of oral cancer. Nanomed. Nanotechnol. Biol. Med. 2023, 49, 102662. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer therapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Sun, R.; Xiang, J.; Zhou, Q.; Piao, Y.; Tang, J.; Shao, S.; Zhou, Z.; Bae, Y.H.; Shen, Y. The tumor EPR effect for cancer drug delivery: Current status, limitations, and alternatives. Adv. Drug Delivery Rev. 2022, 191, 114614. [Google Scholar] [CrossRef]
- Huda, S.; Alam, M.A.; Sharma, P.K. Smart nanocarriers-based drug delivery for cancer therapy: An innovative and developing strategy. J. Drug Delivery Sci. Tech. 2020, 6, 102018. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor-microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Delivery Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing nanoparticles with cancer-targeting antibodies: A comparison of strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Chen, Z.; Kankala, R.K.; Long, L.; Xie, S.; Chen, A.; Zou, L. Current understanding of passive and active targeting nanomedicines to enhance tumor accumulation. Coord. Chem. Rev. 2023, 481, 215051. [Google Scholar] [CrossRef]
- Seledtsov, V.I.; Seledtova, G.V. Attaining threshold antibody cytotoxicity for selective tumor cell destruction: An opinion article. Oncotarget 2018, 9, 35790–35794. [Google Scholar] [CrossRef] [PubMed]
- Golay, J.; Taylor, R.P. The role of complement in the mechanism of action of therapeutic anti-cancer mAbs. Antibodies 2020, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Pahl, J.; Schottelius, A.; Carter, P.; Koch, J. Reimagining antibody-dependent cellular cytotoxicity in cancer: The potential of natural killer cell engagers. Trends Immunol. 2022, 43, 932–946. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, R.; Najali, R.; Molaei, P.; Amini, R.; Pecic, S. Nanoparticles: The future of effective diagnosis and treatment of colorectal cancer? Eur. J. Pharm. 2022, 936, 175350. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Zhang, G.; Zhang, L. Progress in Molecular Biology and Translational Science, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–227. [Google Scholar]
- Zhang, A.; Huang, C.; Shi, H.; Guo, W.; Zhang, X.; Xiang, H.; Jia, T.; Miao, F.; Jia, N. Electrochemiluminescence immunosensor for sensitive determination of tumor biomarker CEA based on multifunctionalized Flower-like Au@BSA nanoparticles. Sens. Actuactors B Chem. 2017, 238, 24–31. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Srivastava, S.K. A study on surface plasmon resonance biosensor for the detection of CEA biomarker using 2D materials graphene, Mxene and MoS2. Optik 2022, 258, 168885. [Google Scholar] [CrossRef]
- Zheng, D.; Yang, J.; Zheng, Z.; Peng, M.; Chen, J.; Chen, Y.; Gao, W. A highly sensitive photoelectrochemical biosensor for CEA analysis based on hollow NiS@NiO/TiO2 composite with typal p-n heterostructure. Talanta 2022, 246, 123523. [Google Scholar] [CrossRef]
- Jozghorbani, M.; Fathi, M.; Kazemi, S.H.; Alinejadian, N. Determination of carcinoembryonic antigen as a tumor marker using a novel graphene-based label-free electrochemical immunosensor. Anal. Biochem. 2021, 613, 114017. [Google Scholar] [CrossRef]
- Sattar, R.S.A.; Verma, R.; Nimisha; Kumar, A.; Dar, G.M.; Apurva; Sharma, A.K.; Kumari, I.; Ahmad, E.; Ali, A.; et al. Diagnostic and prognostic biomarkers in colorrectal cancer and the potential of exosomes in drug delivery. Cel. Signal. 2022, 99, 110413. [Google Scholar]
- Pallares, R.M.; Thanh, N.T.K.; Su, X. Sensing of circulating cancer biomarkers with metal nanoparticles. Nanoscale 2019, 11, 22152–22171. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, M.; Vymetalkova, V.; Neves, R.P.L.; Duran-Sanchon, S.; Vedeld, H.M.; Tham, E.; van Dalum, G.; Flügen, G.; Garcia-Barberan, V.; Fijneman, R.J.A.; et al. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol. Aspects Med. 2019, 69, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Ribeira-Samy, S.; Oliveira, M.I.; Pereira-Veiga, T.; Muinelo-Romay, L.; Carvalho, S.; Gaspar, J.; Freitas, P.P.; López-López, R.; Costa, C.; Diéguez, L. Fast and efficient microfluidic cell filter for isolation of circulating tumor cells from unprocessed whole blood of colorectal patients. Sci. Rep. 2019, 9, 8032. [Google Scholar] [CrossRef] [PubMed]
- Habli, Z.; Alchamaa, W.; Saab, R.; Kadara, H.; Khraiche, M.L. Circulating tumor cell detection technologies and clinical utility: Challenges and opportunities. Cancers 2020, 12, 1930. [Google Scholar] [CrossRef]
- Wang, K.; Peng, Z.; Lin, X.; Nian, W.; Zheng, X.; Wu, J. Electrochemical biosensors for circulating tumor DNA detection. Biosensors 2022, 12, 649. [Google Scholar] [CrossRef]
- Povedano, E.; Vargas, E.; Ruíz-Valdepeñas, V.; Torrente-Rodríguez, R.; Pedrero, M.; Barderas, R.; San Segundo-Acosta, P.; Peláez-García, A.; Mendiola, M.; Hardisson, D.; et al. Electrochemical affinity biosensors for fast detection of gene-specific methylations with no need fir bisulfite and amplification treatments. Sci. Rep. 2018, 8, 6418. [Google Scholar] [CrossRef]
- Hanoglu, S.B.; Man, E.; Harmanci, D.; Ruzgar, S.T.; Sanli, S.; Keles, N.A.; Tuna, B.G.; Duzgun, O.; Ozkan, O.F.; Dogan, S.; et al. Magnetic nanoparticle-based electrochemical sensing platform using ferrocene-labeled peptide nucleic acid for the early diagnosis of colorectal cancer. Biosensors 2022, 12, 736. [Google Scholar] [CrossRef]
- Tran, H.V.; Piro, B. Recent trends in application of nanomaterials for the development of electrochemical microRNA biosensors. Mikrochim. Acta 2021, 188, 128. [Google Scholar] [CrossRef]
- Ortega, F.G.; Gómez, G.E.; Boni, C.; Cañas, I.; Garrido, C.; D’vries, R.F.; Molina, M.P.; Serrano, M.J.; Messina, G.A.; Expósito, J.; et al. Electrochemical microfluidic immunosensor based on porous nanomaterial towards to claudin7 determination for colorectal cancer diagnosis. SSRN 2022, in press. [Google Scholar] [CrossRef]
- Gazouili, M.; Bouziotis, P.; Lyberopoulou, A.; Ikanomopoulos, J.; Papalois, A.; Anagnou, N.; Efstathopoulos, E. Quantum dots-bevacizumab complexes for in vivo imaging of tumors. In Vivo 2014, 28, 1091–1096. [Google Scholar]
- Cho, Y.-S.; Yoon, T.-J.; Jang, E.-S.; Hong, K.S.; Lee, S.Y.; Kim, O.R.; Park, C.; Kim, Y.-J.; Yi, G.-C.; Chang, K. Cetuximab-conjugated magneto-fluorescent silica nanoparticles for in vivo colon cancer targeting and imaging. Cancer Lett. 2010, 299, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, E.; Lecht, S.; Lazarovici, P.; Danino, D.; Magdassi, S. Cetuximab-labeled liposomes containing near-infrared probe for in vivo imaging. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Hedge, M.; Naliyadhara, N.; Unnikrishnan, J.; Alqahtani, M.S.; Abbas, M.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Nanoparticles in the diagnosis and treatment of cancer metastases: Current and future perspectives. Cancer Lett. 2023, 556, 216066. [Google Scholar]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Feng, Q.-P.; Zhu, Y.-T.; Yuan, Y.-Z.; Li, W.-J.; Yu, H.-H.; Hu, M.-Y.; Xiang, S.-Y.; Yu, S.-Q. Oral administration co-delivery nanoparticles of docetaxel and bevacizumab for improving intestinal absorption and enhancing anticancer activity. Mat. Sci. Eng. C 2021, 124, 112039. [Google Scholar] [CrossRef]
- Akborzadeh-Khiavi, M.; Farzi-Khajeh, H.; Somi, M.H.; Safary, A.; Barar, J.; Ansari, R.; Omidi, Y. Eradication of KRAS mutant colorectal adenocarcinoma by PEGylated gold nanoparticles-cetuximab conjugates through ROS-dependent apoptosis. Colloid Surf. A Physicochem. 2022, 653, 129980. [Google Scholar]
- Salim, E.I.; Hosbah, A.M.; Elhussiny, F.A.; Hanafy, N.A.N.; Abdou, Y. Preparation and characterization of cetuximab-loaded egg serum albumin nanoparticles and their uses as a drug delivery system against Caco-2 colon cancer cells. Cancer Nanotechnol. 2023, 14, 4. [Google Scholar] [CrossRef]
- Marcelo, G.A.; Montpeyó, D.; Galhano, J.; Martínez-Máñez, R.; Capelo-Martínez, J.L.; Lorenzo, J.; Lodeiro, C.; Oliveira, E. Development of new targeted nanotherapy combined with magneto-fluorescent nanoparticles against colorectar cancer. Int J. Mol. Sci. 2023, 24, 6612. [Google Scholar] [CrossRef]
- Kumar, B.; Murali, A.; Bharath, A.B.; Giri, S. Guar gum modified upconversion nanocomposites for colorectal cancer treatment through enzyme-responsive drug release and NIR-triggered photodynamic therapy. Nanotechnology 2019, 30, 315102. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, X.; Tang, J.; Wang, Y.; Xu, J.; Sun, Y. EGFR-targeted hybrid lipid nanoparticles for chemophotothermal therapy against colorectal cancer cells. Chem. Phys. Lipids 2023, 251, 105280. [Google Scholar] [CrossRef]
- Conde, J.; Oliva, N.; Zhang, Y.; Artzi, N. Local triple-combination therapy results in tumor regression and prevents recurrence in a colon cancer model. Nat. Mater. 2016, 15, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Borges, J.P. Recent advances in magnetic electrospun nanofibers for cancer theranostic applications. Prog. Nat. Sci. Mater. Int. 2021, 31, 835–844. [Google Scholar] [CrossRef]
- Rout, S.K.; Priya, V.; Setia, A.; Mehata, A.K.; Mohan, S.; Albratty, M.; Najmi, A.; Meraya, A.M.; Makeen, H.A.; Tambuwala, M.M.; et al. Mitochondrial targeting theranostic nanomedicine and molecular biomarkers for efficient cancer diagnosis and therapy. Biomed. Pharmacother. 2022, 153, 113451. [Google Scholar] [CrossRef] [PubMed]
- Shete, M.B.; Patil, T.S.; Deshpande, A.; Saraogi, G.; Vasdev, N.; Deshpande, M.; Rajpoot, K.; Tekade, R.K. Current trends in theranostic nanomedicines. J. Drug Delivery Sci. Tech. 2022, 71, 103280. [Google Scholar] [CrossRef]
- Kukkar, D.; Kukkar, P.; Kumar, V.; Hong, J.; Kim, K.-H.; Deep, A. Recent advances in nanoscale materials for antibody-based cancer theranostics. Biosens. Bioelectron. 2021, 173, 112787. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Bao, C.; Cui, D.; Baptista, P.V.; Tian, F. Antibody-drug gold nanoantennas with Raman spectroscopic fingerprints for in vivo tumor theranostics. J. Control. Release 2014, 183, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, X.; Li, Y.; Sun, T.; Xue, H.; Jin, Z.; Tian, J. Liposomal nanohybrid cerasomes targeted to PD-L1 enable dual-modality imaging and improve antitumor treatments. Cancer Lett. 2018, 414, 230–238. [Google Scholar] [CrossRef]
- Li, Y.; Du, Y.; Liang, X.; Sun, T.; Xue, H.; Tian, J.; Jin, Z. EGFR-targeted liposomal nanohybrid cerasomes: Theranostic function and immune checkpoint inhibition in a mouse model of colorectal cancer. Nanoscale 2018, 10, 16738–16749. [Google Scholar] [CrossRef]
- Hashemkhani, M.; Demirci, G.; Bayir, A.; Muti, A.; Sennaroglu, A.; Hadi, L.M.; Yaghini, E.; Loizidou, M.; McRobert, A.J.; Acar, H.Y. Cetuximab-Ag2S quantum dots for fluorescence imaging and highly effective combination of ACA-based photodynamic/chemo-therapy of colorectal cancer cells. Nanoscale 2021, 13, 14879–14899. [Google Scholar] [CrossRef]
- Cabeza, L.; Perazzoli, G.; Mesas, C.; Jiménez-Luna, C.; Prados, J.; Rama, A.R.; Melguizo, C. Nanoparticles in colorrectal cancer therapy: Latest in vivo assays, clinical trials and patents. AAPS PharmSciTech 2020, 21, 178. [Google Scholar] [CrossRef]



| Method | Advantages | Disadvantages |
|---|---|---|
| Stool tests |
|
|
| Colonoscopy |
|
|
| CT colonography |
|
|
| Sigmoidoscopy |
|
|
| Double-contrast barium enema |
|
|
| Compound | Category | Action Mechanism | Side Effects |
|---|---|---|---|
| 5-FU | Pyrimidine analogue | Inhibition of thymidylate synthase | Myelosuppression, diarrhea, skin toxicity, mucositis |
| CPT-11 | Camptothecin analogue | Inhibition of topoisomerase I | Neutropenia, hepatic dysfunction |
| Oxaliplatin | Platinum agent | Inhibition of DNA synthesis | Gastrointestinal alterations, hepatic encephalopathy |
| Celecoxib | Anti-inflammatory agent | Inhibition of COX-2 | Gastrointestinal alterations, ulceration, stomach or intestine perforation |
| Capecitabine | Pyrimidine analogue | Inhibition of thymidylate synthase | Hepatotoxicity |
| Targeted Agent | Category | Market Name | Target | First Approval Date |
|---|---|---|---|---|
| Bevacizumab | Human recombinant mAb (IgG1) | Avastin® | VEGF | 2004 FDA 2005 EMA |
| Cetuximab | Chimeric mAb (IgG1) | Erbitux® | EGFR | 2004 FDA 2004 EMA |
| Panitumab | Human mAb (IgG2) | Vectibix® | EGFR | 2006 FDA 2007 EMA |
| Ipilimumab | Human mAb (IgG1) | Yervoy® | PD-1 | 2011 FDA 2011 EMA |
| Aflibercept | Recombinant fusion protein | Eylea® | VEGF | 2011 FDA 2012 EMA |
| Regorafenib | Kinase inhibitor | Stivarga® | VEGFR-1 VEGFR-2 | 2012 FDA 2013 EMA |
| Ramucirumab | Human recombinant mAb (IgG1) | Cyramza® | VEGFR-2 | 2014 FDA 2014 EMA |
| Nivolumab | Human mAb (IgG4) | Opdivo® | PD-1 | 2014 FDA 2015 EMA |
| Prembrolizumab | Human mAb (IgG4) | Keytruda® | CTLA-4 | 2020 FDA 2021 EMA |
| Nanoplatform | Loaded Antibody | Payload | Application | References |
|---|---|---|---|---|
| Flower-like AuNPs coated with BSA | Anti-CEA | - | Biosensing | [68] |
| Porous hollow NiS@NiO/TiO2 spheres | Anti-CEA | - | Biosensing | [70] |
| Glass carbon electrode coated with reduced graphene oxide | Anti-CEA | - | Biosensing | [71] |
| Immunomagnetic beads | mAb cocktail | - | Biosensing | [76] |
| Electrochemical immunosensors and DNA sensors | Anti-5-mC | - | Biosensing | [78] |
| MNPs | Anti-methylated septin-9 gene | - | Biosensing | [79] |
| NPs with a metal–organic framework introduced into a microfluidic sensor | Anti-claudin-7 | - | Biosensing | [81] |
| QDs | Bevacizumab | - | Nanoimaging | [82] |
| Iron oxide NPs coated with silica | Cetuximab | Rhodamine B isothiocyanate | Nanoimaging | [83] |
| Liposomes | Cetuximab | Indocyanine green | Nanoimaging | [84] |
| Carboxymethyl chitosan/PLGA NPs and methoxy-PEG-poly(β-amino ester) NPs | Bevacizumab | Docetaxel | DDS development | [87] |
| PEGylated AuNPs | Cetuximab | - | DDS development | [88] |
| Egg serum albumin NPs | Cetuximab | - | DDS development | [89] |
| MSNs | Cetuximab | Doxorubicin and ofloxacin | DDS development | [90] |
| Hybrid lipid–polymer NPs | Cetuximab | Irinotecan | Combination therapy | [92] |
| Dextran hydrogel path with gold nanorods | Bevacizumab | siRNA against Kras | Combination therapy | [93] |
| AuNPs coated with DTTC molecules | Cetuximab | - | Nanotheranostics | [97,98] |
| Liposomal nanohybrid cerasomes | Anti-PD-L1 | Paclitaxel, IRDye800CW and DOTA | Nanotheranostics | [99] |
| Liposomal nanohybrid cerasomes | Cetuximab | IRDye800CW and DOTA | Nanotheranostics | [100] |
| Ag2S QDs | Cetuximab | 5-FU and ALA | Nanotheranostics | [101] |
| Nanosystem | Application | Clinical Trial Phase/Patented | Clinical Trial or Patent Number | Refs. |
|---|---|---|---|---|
| Polymeric NPs loaded with cetuximab and a somatostatin analogue | mCRC therapy | Phase I clinical trial (2018) | NTC03774680 | [6,102] |
| QDs of porphyrin carbon conjugated with cetuximab | CRC photodynamic therapy | Patented (2018) | US20180125976 | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djermane, R.; Nieto, C.; Vega, M.A.; del Valle, E.M.M. Antibody-Loaded Nanoplatforms for Colorectal Cancer Diagnosis and Treatment: An Update. Pharmaceutics 2023, 15, 1514. https://doi.org/10.3390/pharmaceutics15051514
Djermane R, Nieto C, Vega MA, del Valle EMM. Antibody-Loaded Nanoplatforms for Colorectal Cancer Diagnosis and Treatment: An Update. Pharmaceutics. 2023; 15(5):1514. https://doi.org/10.3390/pharmaceutics15051514
Chicago/Turabian StyleDjermane, Rania, Celia Nieto, Milena A. Vega, and Eva M. Martín del Valle. 2023. "Antibody-Loaded Nanoplatforms for Colorectal Cancer Diagnosis and Treatment: An Update" Pharmaceutics 15, no. 5: 1514. https://doi.org/10.3390/pharmaceutics15051514
APA StyleDjermane, R., Nieto, C., Vega, M. A., & del Valle, E. M. M. (2023). Antibody-Loaded Nanoplatforms for Colorectal Cancer Diagnosis and Treatment: An Update. Pharmaceutics, 15(5), 1514. https://doi.org/10.3390/pharmaceutics15051514