3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope
Abstract
1. Introduction
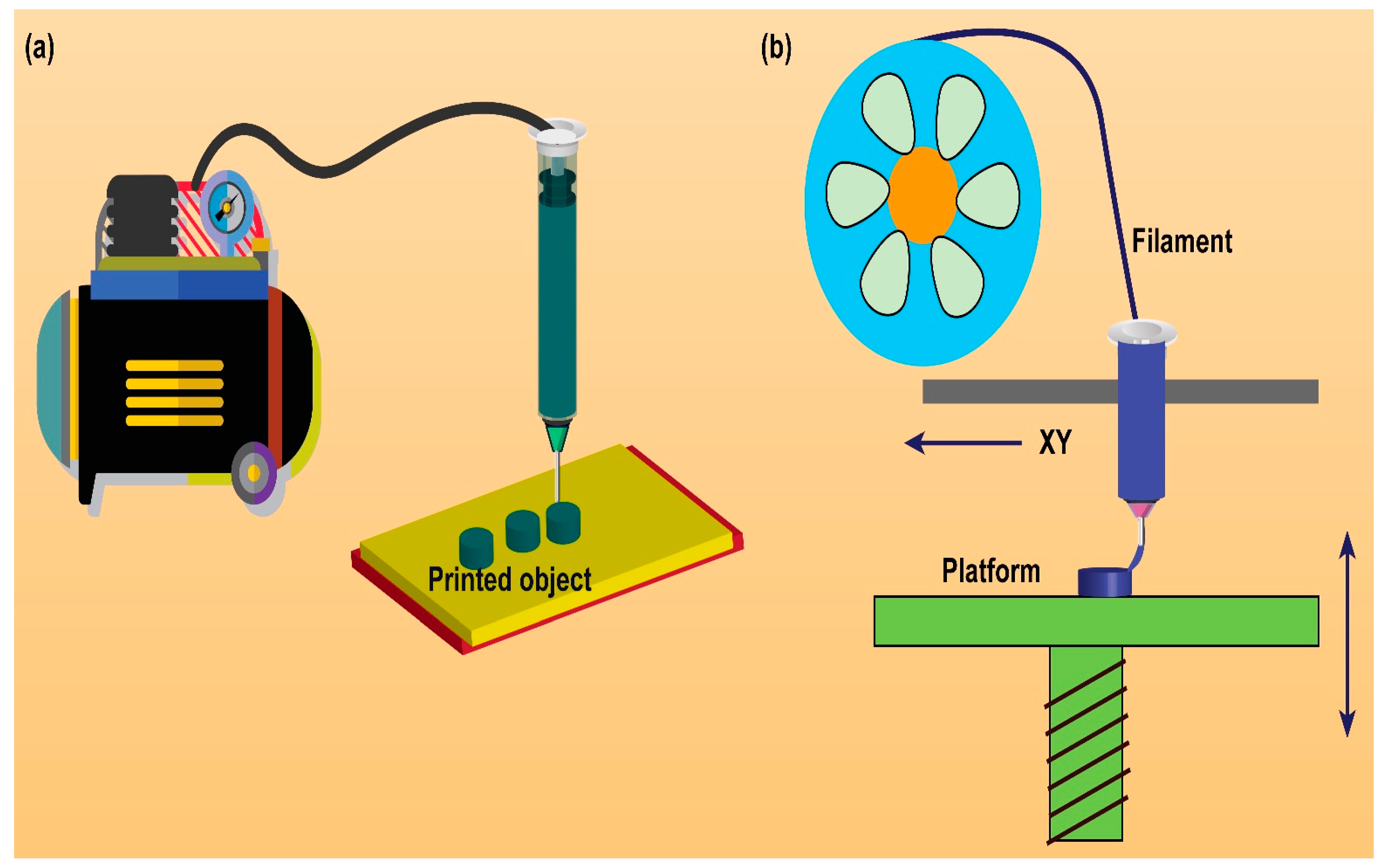
2. Rationale/Significance of 3D Printing Technology to Design Solid Dosage Forms
3. 3D Printing Technology to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research
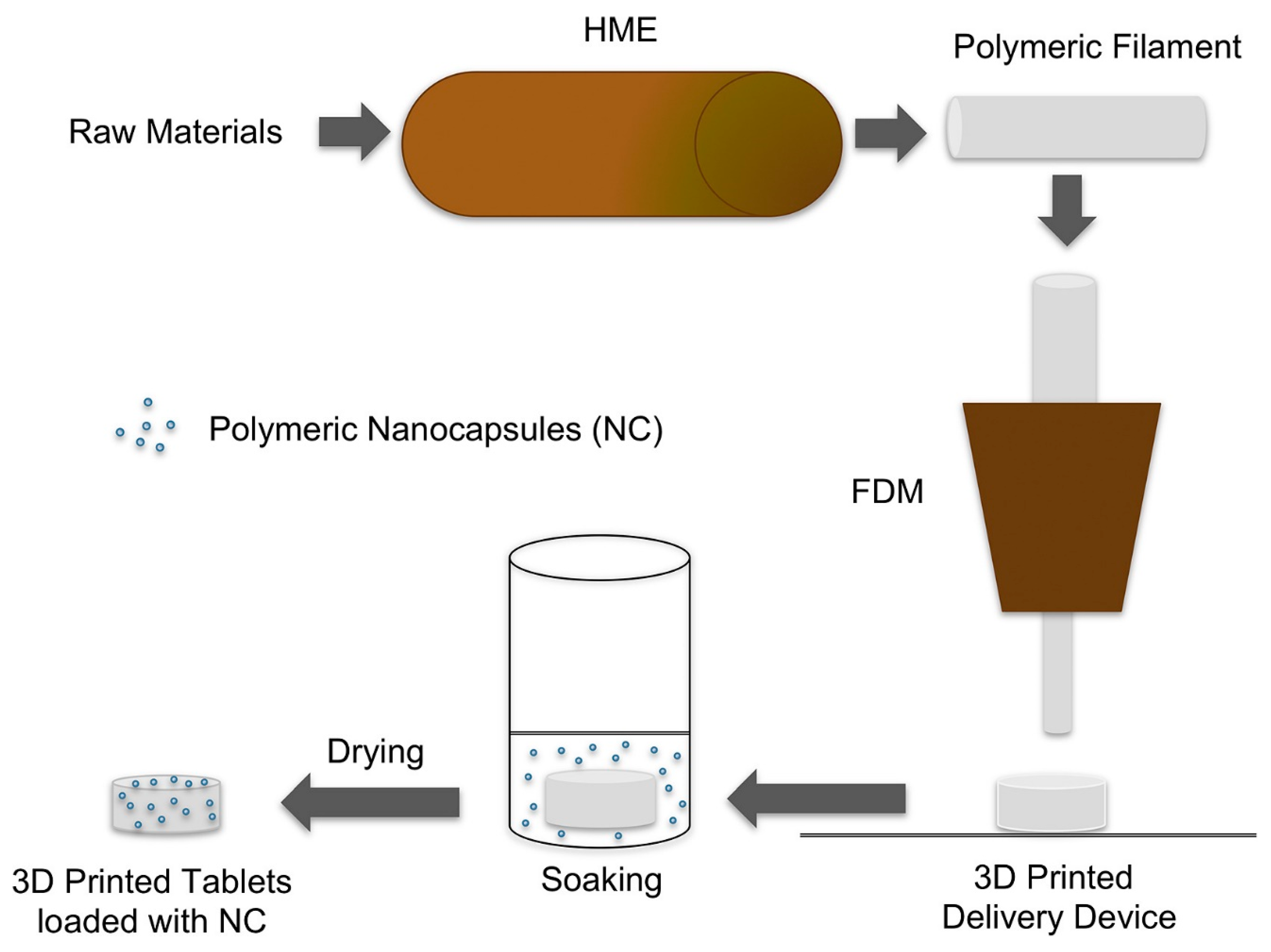
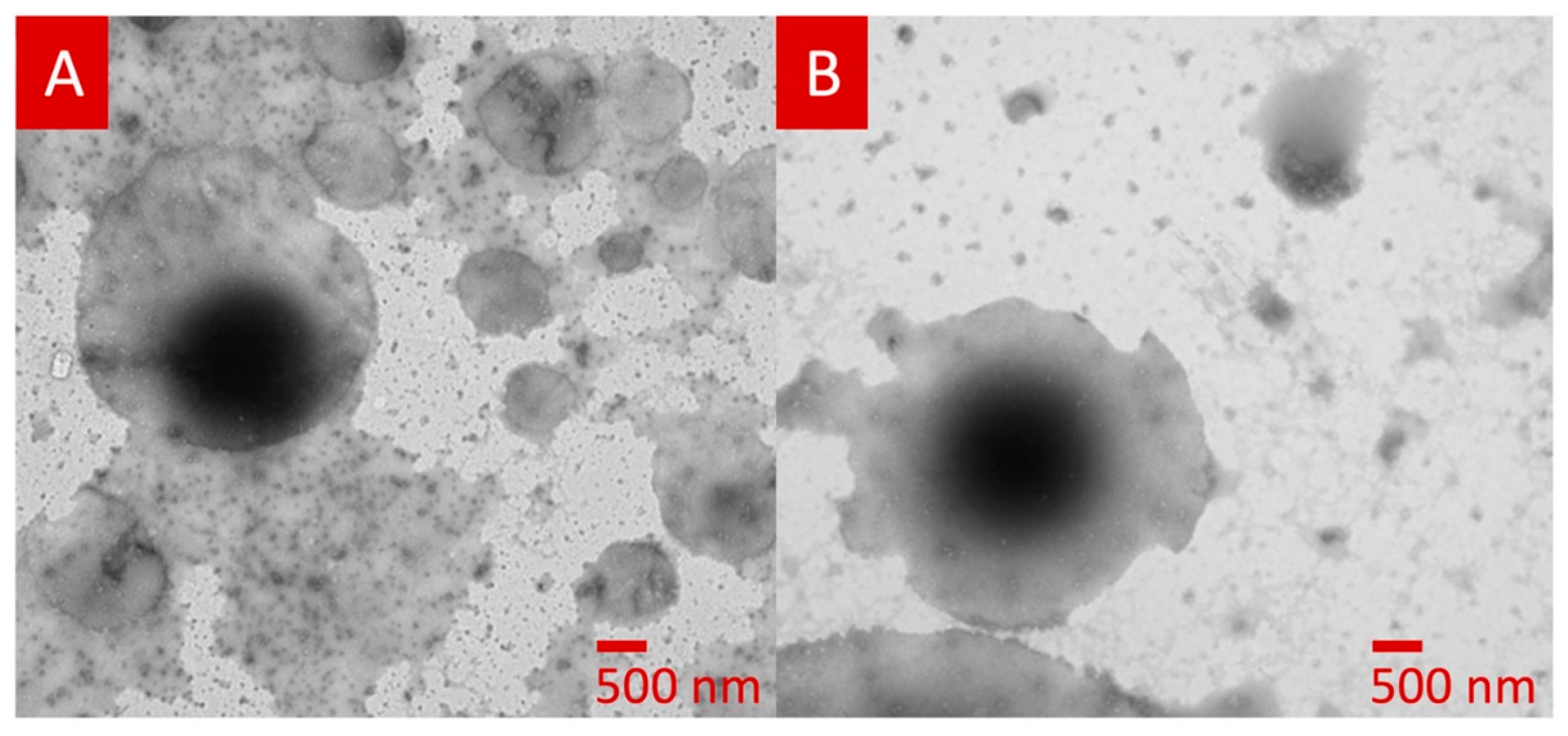
3.1. 3D Printed Tablets Loaded with Polymeric Nanocapsules
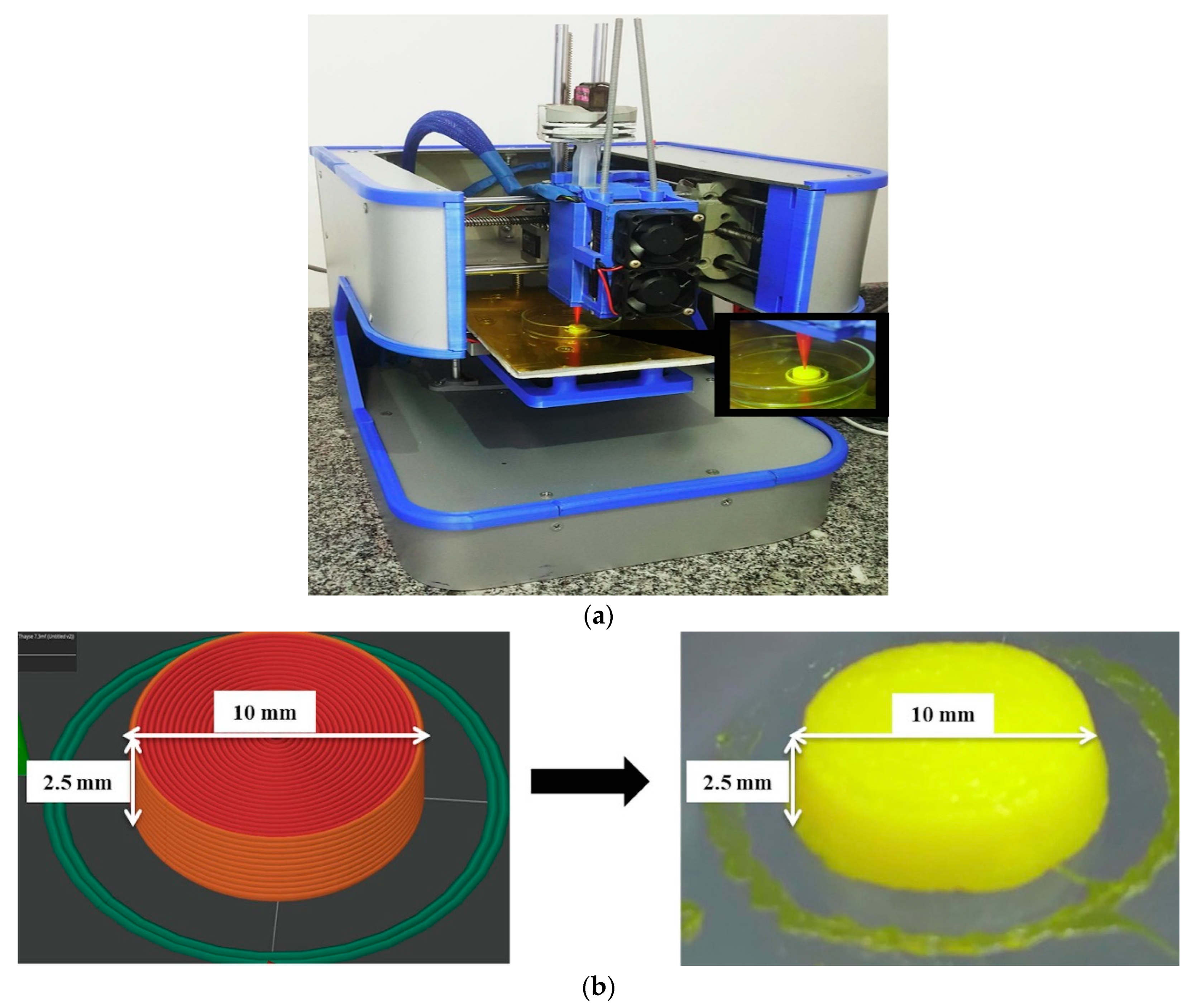
3.2. 3D Printing of Self-Nanoemulsifying Tablets
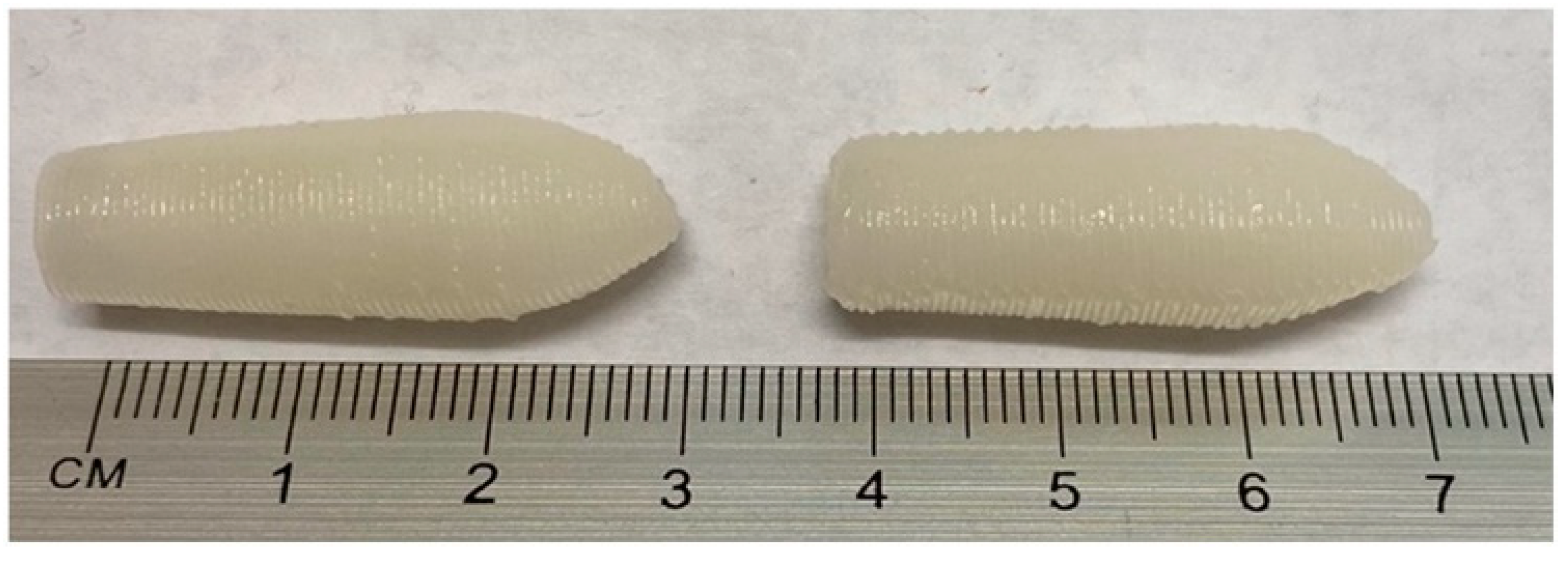
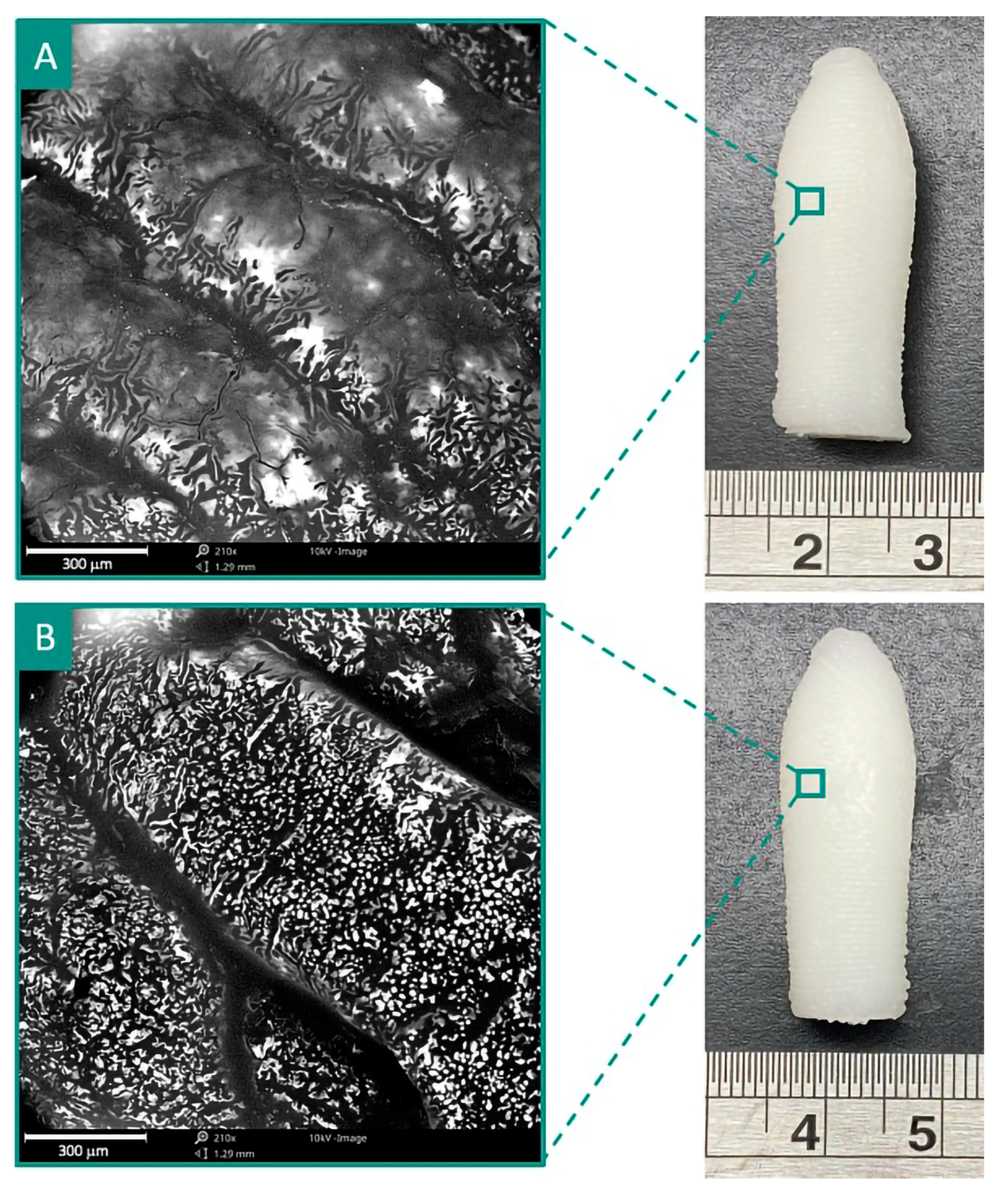
3.3. 3D Printing of SNEDDS-Based Suppositories
4. Opportunity, Challenges, and Future Scope
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arshad, M.S.; Zafar, S.; Yousef, B.; Alyassin, Y.; Ali, R.; AlAsiri, A.; Pitt, K. A review of emerging technologies enabling improved solid oral dosage form manufacturing and processing. Adv. Drug Deliv. Rev. 2021, 178, 113840. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P. Advances in solid dosage form manufacturing technology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 2935–2949. [Google Scholar] [CrossRef]
- Hermann, J.; Hermann, T.W. Bioavailability of drugs from suppositories in clinical practice after 1995. Acta Pol. Pharm. Drug Res. 2020, 77, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery–A review. Pharm. Sci. Technol. Today 2000, 3, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Graham, E. Improving outcomes through personalised medicine. NHS Engl. 2016, 6–10. Available online: https://www.england.nhs.uk/publication/improving-outcomes-through-personalised-medicine/ (accessed on 5 February 2023).
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Pritchard, D.E.; Moeckel, F.; Villa, M.S.; Housman, L.T.; McCarty, C.A.; McLeod, H.L. Strategies for integrating personalized medicine into healthcare practice. Pers. Med. 2017, 14, 141–152. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef]
- Alqahtani, A.A.; Ahmed, M.M.; Mohammed, A.A.; Ahmad, J. 3D Printed Pharmaceutical Systems for Personalized Treatment in Metabolic Syndrome. Pharmaceutics 2023, 15, 1152. [Google Scholar] [CrossRef]
- Reddy, C.V.; Venkatesh, M.P.; Kumar, P. First FDA approved 3D printed drug paved new path for increased precision in patient care. Appl. Clin. Res. Clin. Trials Regul. Aff. 2020, 7, 93–103. [Google Scholar] [CrossRef]
- Reddy, R.D.P.; Sharma, V. Additive manufacturing in drug delivery applications: A review. Int. J. Pharm. 2020, 589, 119820. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical applications of 3D printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Cerda, J.R.; Fernández-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef]
- Zhang, J.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Structure-function correlation and personalized 3D printed tablets using a quality by design (QbD) approach. Int. J. Pharm. 2020, 590, 119945. [Google Scholar] [CrossRef] [PubMed]
- Buanz, A.B.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current trends on medical and pharmaceutical applications of inkjet printing technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Mukherjee, R.; Sansare, S.; Halder, A.; Kashi, H.; Ma, A.W.; Chaudhuri, B. Impact of powder-binder interactions on 3D printability of pharmaceutical tablets using drop test methodology. Eur. J. Pharm. Sci. 2021, 160, 105755. [Google Scholar] [CrossRef]
- Shi, K.; Tan, D.K.; Nokhodchi, A.; Maniruzzaman, M. Drop-on-powder 3D printing of tablets with an anti-cancer drug, 5-fluorouracil. Pharmaceutics 2019, 11, 150. [Google Scholar] [CrossRef]
- Francis, V.; Garg, S.; Saxena, K.K.; Jain, P.K.; Lade, J.; Kumar, D. Effect of chemical and heat treatment on 3D printed parts: Nanoparticles embedment approach. Adv. Mater. Process. Technol. 2022, 8, 2277–2288. [Google Scholar] [CrossRef]
- Macedo, J.; Marques, R.; Vervaet, C.; Pinto, J.F. Production of Bi-Compartmental Tablets by FDM 3D Printing for the Withdrawal of Diazepam. Pharmaceutics 2023, 15, 538. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D printed coating shell to control the drug release of encapsulated immediate-release tablets. Polymers 2020, 12, 1395. [Google Scholar] [CrossRef]
- Yadav, A.K.; Awasthi, A.; Saxena, K.K.; Agrawal, M.K. Critical Review on 3D Scaffolds Materials. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2022; Volume 1065, pp. 129–143. [Google Scholar]
- Manmadhachary, A.; Siva Rama Krishana, L.; Saxena, K.K. Quantification of the accuracy of additive manufactured (3D printed) medical models. Int. J. Interact. Des. Manuf. 2022. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, O.A.; Masood, S.H.; Bhowmik, J.L. Optimization of fused deposition modeling process parameters: A review of current research and future prospects. Adv. Manuf. 2015, 3, 42–53. [Google Scholar] [CrossRef]
- Ozbolat, I.T. 3D Bioprinting: Fundamentals, Principles and Applications; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Xu, W.; Jambhulkar, S.; Zhu, Y.; Ravichandran, D.; Kakarla, M.; Vernon, B.; Lott, D.G.; Cornella, J.L.; Shefi, O.; Miquelard-Garnier, G.; et al. 3D printing for polymer/particle-based processing: A review. Compos. B Eng. 2021, 223, 109102. [Google Scholar] [CrossRef]
- Sarkar, N.; Bose, S. Liposome-encapsulated curcumin-loaded 3D printed scaffold for bone tissue engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Johannesson, J.; Khan, J.; Hubert, M.; Teleki, A.; Bergström, C.A. 3D-printing of solid lipid tablets from emulsion gels. Int. J. Pharm. 2021, 597, 120304. [Google Scholar] [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Combining inkjet printing and amorphous nanonization to prepare personalized dosage forms of poorly-soluble drugs. Eur. J. Pharm. Biopharm. 2015, 96, 314–321. [Google Scholar] [CrossRef]
- Karalia, D.; Siamidi, A.; Karalis, V.; Vlachou, M. 3D-Printed oral dosage forms: Mechanical properties, computational approaches and applications. Pharmaceutics 2021, 13, 1401. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, J. 3D printing technique in the development of self-nanoemulsifying drug delivery system: Scope and future prospects. Ther. Deliv. 2022, 13, 135–139. [Google Scholar] [CrossRef]
- Persaud, S.; Eid, S.; Swiderski, N.; Serris, I.; Cho, H. Preparations of rectal suppositories containing artesunate. Pharmaceutics 2020, 12, 222. [Google Scholar] [CrossRef]
- Fernández-García, R.; Prada, M.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Oral fixed-dose combination pharmaceutical products: Industrial manufacturing versus personalized 3D printing. Pharm. Res. 2020, 37, 132. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Ourique, A.F.; Guterres, S.S.; Pohlmann, A.R. Spray-dried polymeric nanoparticles for pharmaceutics: A review of patents. Recent Pat. Drug Deliv. Formul. 2012, 6, 195–208. [Google Scholar] [CrossRef]
- Schaffazick, S.R.; Pohlmann, A.R.; Dalla-Costa, T.C.; Guterres, S.S. Freeze- drying polymeric colloidal suspensions: Nanocapsules, nanospheres and nanodispersion. A comparative study. Eur. J. Pharm. Biopharm. 2003, 56, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.B.; Fontana, M.C.; Bastos, M.O.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Drying polymeric drug-loaded nanocapsules: The wet granulation process as a promising approach. J. Nanosci. Nanotechnol. 2010, 10, 616–662. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.B.; Bastos, M.O.; Fontana, M.C.; Ourique, A.F.; Beck, R.C.R. Tablets containing drug-loaded polymeric nanocapsules: An innovative platform. J. Nanosci. Nanotechnol. 2010, 10, 5885–5888. [Google Scholar] [CrossRef]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef]
- Kalepu, S.; Manthina, M.; Padavala, V. Oral lipid-based drug delivery systems–An overview. Acta Pharm. Sin. B 2013, 3, 361–372. [Google Scholar] [CrossRef]
- Nazzal, S.; Khan, M.A. Controlled release of a self-emulsifying formulation from a tablet dosage form: Stability assessment and optimization of some processing parameters. Int. J. Pharm. 2006, 315, 110–121. [Google Scholar] [CrossRef]
- Agarwal, V.; Siddiqui, A.; Ali, H.; Nazzal, S. Dissolution and powder flow characterization of solid self-emulsified drug delivery system (SEDDS). Int. J. Pharm. 2009, 366, 44–52. [Google Scholar] [CrossRef]
- Barber, B.W.; Dumont, C.; Caisse, P.; Simon, G.P.; Boyd, B.J. A 3D-printed polymer–lipid-hybrid tablet towards the development of bespoke SMEDDS formulations. Pharmaceutics 2021, 13, 2107. [Google Scholar] [CrossRef]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An overview of 3D printing technologies for soft materials and potential opportunities for lipid-based drug delivery systems. Pharm. Res. 2019, 36, 4. [Google Scholar] [CrossRef] [PubMed]
- van Mourik, I.D.; Thomson, M.; Kelly, D.A. Comparison of pharmacokinetics of Neoral and Sandimmune in stable pediatric liver transplant recipients. Liver Transplant. Surg. 1999, 5, 107–111. [Google Scholar] [CrossRef]
- Alomari, M.; Mohamed, F.H.; Basit, A.W.; Gaisford, S. Personalised dosing: Printing a dose of one’s own medicine. Int. J. Pharm. 2015, 494, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Madla, C.M.; Umerji, A.; Piñeiro, G.D.; Montero, J.M.G.; Diaz, M.J.L.; Barcia, M.G.; Taherali, F.; Sánchez-Pintos, P.; Couce, M.-L.; et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: First single-centre, prospective, crossover study in patients. Int. J. Pharm. 2019, 567, 118497. [Google Scholar] [CrossRef]
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I spy with my little eye: A paediatric visual preferences survey of 3D printed tablets. Pharmaceutics 2020, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J.; Kotta, S. 3D printing in medicine: Technology overview and drug delivery applications. Ann. 3D Print. Med. 2021, 4, 100037. [Google Scholar] [CrossRef]
- Serrano, D.R.; Kara, A.; Yuste, I.; Luciano, F.C.; Ongoren, B.; Anaya, B.J.; Molina, G.; Diez, L.; Ramirez, B.I.; Ramirez, I.O.; et al. 3D printing technologies in personalized medicine, nanomedicines, and biopharmaceuticals. Pharmaceutics 2023, 15, 313. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Sekar, R.; Gomez, L.A.; Sekhar, M.R.; Hiruthyaswamy, S.P.; Basavegowda, N.; Somu, P. Recent Developments of Silk-Based Scaffolds for Tissue Engineering and Regenerative Medicine Applications: A Special Focus on the Advancement of 3D Printing. Biomimetics 2023, 8, 16. [Google Scholar] [CrossRef]
- Pourmasoumi, P.; Moghaddam, A.; Nemati Mahand, S.; Heidari, F.; Salehi Moghaddam, Z.; Arjmand, M.; Kühnert, I.; Kruppke, B.; Wiesmann, H.-P.; Khonakdar, H.A. A review on the recent progress, opportunities, and challenges of 4D printing and bioprinting in regenerative medicine. J. Biomater. Sci. Polym. Ed. 2023, 34, 108–146. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Tzimtzimis, E.; Katsamenis, O.L.; Dourou, A.; Markopoulou, C.; Bouropoulos, N.; Fatouros, D.G. Fabrication of an osmotic 3D printed solid dosage form for controlled release of active pharmaceutical ingredients. Eur. J. Pharm. Sci. 2020, 143, 105176. [Google Scholar] [CrossRef]
- Pradeep, P.V.; Paul, L. Review on novel biomaterials and innovative 3D printing techniques in biomedical applications. Mater. Today Proc. 2022, 58, 96–103. [Google Scholar] [CrossRef]
- Ikeda, S.; Kobayashi, M.; Aoki, S.; Terukina, T.; Kanazawa, T.; Kojima, H.; Kondo, H. 3D-Printed Fast-Dissolving Oral Dosage Forms via Fused Deposition Modeling Based on Sugar Alcohol and Poly (Vinyl Alcohol)—Preparation, Drug Release Studies and In Vivo Oral Absorption. Pharmaceutics 2023, 15, 395. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing–An overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Okafor-Muo, O.L.; Hassanin, H.; Kayyali, R.; ElShaer, A. 3D printing of solid oral dosage forms: Numerous challenges with unique opportunities. J. Pharm. Sci. 2020, 109, 3535–3550. [Google Scholar] [CrossRef] [PubMed]
- Kulinowski, P.; Malczewski, P.; Łaszcz, M.; Baran, E.; Milanowski, B.; Kuprianowicz, M.; Dorożyński, P. Development of Composite, Reinforced, Highly Drug-Loaded Pharmaceutical Printlets Manufactured by Selective Laser Sintering—In Search of Relevant Excipients for Pharmaceutical 3D Printing. Materials 2022, 15, 2142. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Lui, Y.S.; Sow, W.T.; Tan, L.P.; Wu, Y.; Lai, Y.; Li, H. 4D printing and stimuli-responsive materials in biomedical aspects. Acta Biomater. 2019, 92, 19–36. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Breakthrough in the printing tactics for stimuli-responsive materials: 4D printing. Chem. Eng. J. 2019, 366, 264–304. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, Y.; Wang, M.; Liang, Y.; Ren, L.; Ren, L. Recent progress in 4D printing of stimuli-responsive polymeric materials. Sci. China Technol. Sci. 2020, 63, 532–544. [Google Scholar] [CrossRef]
- Bawa, P.; Pillay, V.; Choonara, Y.E.; Du Toit, L.C. Stimuli-responsive polymers and their applications in drug delivery. Biomed. Mater. 2009, 4, 022001. [Google Scholar] [CrossRef]
- Cook, A.B.; Decuzzi, P. Harnessing endogenous stimuli for responsive materials in theranostics. ACS Nano 2021, 15, 2068–2098. [Google Scholar] [CrossRef]
- Sorrenti, M.; Catenacci, L.; Bonferoni, M.; Sandri, G.; Caramella, C.; Bettinetti, G. Thermal characterization of diltiazem and λ-carrageenan binary systems. J. Therm. Anal. Calorim. 2010, 102, 337–342. [Google Scholar] [CrossRef]
- McClelland, G.A.; Sutton, S.C.; Engle, K.; Zentner, G.M. The solubility-modulated osmotic pump: In vitro/in vivo release of diltiazem hydrochloride. Pharm. Res. 1991, 8, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Gioumouxouzis, C.I.; Katsamenis, O.L.; Bouropoulos, N.; Fatouros, D.G. 3D printed oral solid dosage forms containing hydrochlorothiazide for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 164–171. [Google Scholar] [CrossRef]
- McDonagh, T.; Belton, P.; Qi, S. An investigation into the effects of geometric scaling and pore structure on drug dose and release of 3D printed solid dosage forms. Eur. J. Pharm. Biopharm. 2022, 177, 113–125. [Google Scholar] [CrossRef]
- Bogdahn, M.; Torner, J.; Krause, J.; Grimm, M.; Weitschies, W. Influence of the geometry of 3D printed solid oral dosage forms on their swallowability. Eur. J. Pharm. Biopharm. 2021, 167, 65–72. [Google Scholar] [CrossRef]
- Pyteraf, J.; Pacławski, A.; Jamróz, W.; Mendyk, A.; Paluch, M.; Jachowicz, R. Application and Multi-Stage Optimization of Daylight Polymer 3D Printing of Personalized Medicine Products. Pharmaceutics 2022, 14, 843. [Google Scholar] [CrossRef]
- de Oliveira, T.V.; de Oliveira, R.S.; Dos Santos, J.; Funk, N.L.; Petzhold, C.L.; Beck, R.C.R. Redispersible 3D printed nanomedicines: An original application of the semisolid extrusion technique. Int. J. Pharm. 2022, 624, 122029. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Abdullah, M.M.; Saleh, E. 3D printing of dapagliflozin containing self-nanoemulsifying tablets: Formulation design and in vitro characterization. Pharmaceutics 2021, 13, 993. [Google Scholar] [CrossRef]
- Ito, Y.; Kusawake, T.; Ishida, M.; Tawa, R.; Shibata, N.; Takada, K. Oral solid gentamicin preparation using emulsifier and adsorbent. J. Control. Release 2005, 105, 23–31. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Wang, Y.; Liu, X.; Liu, Y.; Fu, Q.; Meng, P.; He, Z. Solid self-emulsifying nitrendipine pellets: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2010, 383, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ansen, T.; Holm, P.; Schultz, K. Process characteristics and compaction of spray-dried emulsions containing a drug dissolved in lipid. Int. J. Pharm. 2004, 287, 55–66. [Google Scholar] [CrossRef]
- Buya, A.B.; Beloqui, A.; Memvanga, P.B.; Preat, V. Self-Nano-Emulsifying Drug-Delivery Systems: From the Development to the Current Applications and Challenges in Oral Drug Delivery. Pharmaceutics 2020, 12, 1194. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J.H. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Jannin, V.; Musakhanian, J.; Marchaud, D. Approaches for the development of solid and semi-solid lipid-based formulations. Adv. Drug Deliv. Rev. 2008, 60, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bajpai, M.; Mishra, P. Self-emulsifying drug delivery system (SEDDS): An emerging dosage form to improve the bioavailability of poorly absorbed drugs. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 305–329. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Shrivastava, N.; Kumar, S.; Singh, A.K.; Ali, J.; Baboota, S. Designing and development of omega-3 fatty acid based self-nanoemulsifying drug delivery system (SNEDDS) of docetaxel with enhanced biopharmaceutical attributes for management of breast cancer. J. Drug Deliv. Sci. Technol. 2022, 68, 103117. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: Design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Brüsewitz, C.; Schendler, A.; Funke, A.; Wagner, T.; Lipp, R. Novel poloxamer-based nanoemulsions to enhance the intestinal absorption of active compounds. Int. J. Pharm. 2007, 329, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.R.; Kazi, M.; Shahba, A.A.W.; Radhanpuri, A.; Maniruzzaman, M. Three-Dimensional Printing of a Container Tablet: A New Paradigm for Multi-Drug-Containing Bioactive Self-Nanoemulsifying Drug-Delivery Systems (Bio-SNEDDSs). Pharmaceutics 2022, 14, 1082. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Felimban, R.I.; Tayeb, H.H.; Rizg, W.Y.; Alnadwi, F.H.; Alotaibi, H.A.; Alhakamy, N.A.; Abd-Allah, F.I.; Mohamed, G.A.; Zidan, A.S.; et al. Development of multi-compartment 3d-printed tablets loaded with self-nanoemulsified formulations of various drugs: A new strategy for personalized medicine. Pharmaceutics 2021, 13, 1733. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M.; Felimban, R.I.; Tayeb, H.H.; Chaudhary, A.G.; Omar, A.M.; Rizg, W.Y.; Alnadwi, F.H.; Abd-Allah, F.I.; Ahmed, T.A. Pairing 3D-Printing with Nanotechnology to Manage Metabolic Syndrome. Int. J. Nanomed. 2022, 17, 1783–1801. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Alotaibi, H.A.; Alharbi, W.S.; Safo, M.K.; El-Say, K.M. Development of 3D-Printed, Liquisolid and Directly Compressed Glimepiride Tablets, Loaded with Black Seed Oil Self-Nanoemulsifying Drug Delivery System: In Vitro and In Vivo Characterization. Pharmaceuticals 2022, 15, 68. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Tsongas, K.; Tzimtzimis, E.K.; Tzetzis, D.; Bouropoulos, N.; Barmpalexis, P.; Eleftheriadis, G.K.; Fatouros, D.G. 3D printing of patient-tailored SNEDDS-based suppositories of lidocaine. J. Drug Deliv. Sci. Technol. 2021, 61, 102292. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Ong, J.J.; Luzardo-Álvarez, A.; González-Barcia, M.; Basit, A.W.; Otero-Espinar, F.J.; Goyanes, A. 3D printed tacrolimus suppositories for the treatment of ulcerative colitis. Asian J. Pharm. Sci. 2021, 16, 110–119. [Google Scholar] [CrossRef]
- Awad, A.; Goyanes, A.; Orlu, M.; Gaisford, S.; Basit, A.W. 3D printed infliximab suppositories for rectal biologic delivery. Int. J. Pharm. X 2023, 5, 100176. [Google Scholar] [CrossRef]
- Awad, A.; Hollis, E.; Goyanes, A.; Orlu, M.; Gaisford, S.; Basit, A.W. 3D printed multi-drug-loaded suppositories for acute severe ulcerative colitis. Int. J. Pharm. X 2023, 5, 100165. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef]
- Sankar, P.L.; Parker, L.S. The Precision Medicine Initiative’s All of Us Research Program: An agenda for research on its ethical, legal, and social issues. Genet. Med. 2017, 19, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Genome UK: The Future of Healthcare. Available online: https://www.gov.uk/government/publications/genome-uk-the-future-of-healthcare (accessed on 5 February 2023).
- Life Sciences Vision. Available online: https://www.gov.uk/government/publications/life-sciences-vision (accessed on 5 February 2023).
- 3D Printed Drugs Market to Reach US$ 2064.8 Million by 2027 Globally|CAGR: 15.2%|UnivDatos Market Insights. Available online: https://www.prnewswire.com/in/news-releases/3d-printed-drugs-market-to-reach-us-2-064-8-million-by-2027-globally-cagr-15-2-univdatos-market-insights-866286870.html (accessed on 5 February 2023).
- Mohapatra, S.; Kar, R.K.; Biswal, P.K.; Bindhani, S. Approaches of 3D printing in current drug delivery. Sens. Int. 2022, 3, 100146. [Google Scholar] [CrossRef]


| 3D Technique | Process Parameters | Type of Solid Dosage Forms | Dimension and Other Characteristics of 3D Printed Dosage Forms | Ref. |
|---|---|---|---|---|
| FDM |
| Tablet |
| [73] |
| PAM |
| Tablet | 3D Tab A: 8 mm × 3 mm 3D Tab B: 10 mm × 3 mm 3D Tab C: 12 mm × 3 mm | [74] |
| FDM |
| Tablet | FEN S-SMEDDS: (L × W × H × Layers)
| [85] |
| FDM |
| Tablet |
| [86] |
| FDM |
| Liqui-Solid tablet |
| [87] |
| FDM |
| Liqui-Solid tablet |
| [88] |
| PAM |
| Suppository |
| [89] |
| PAM |
| Suppository |
| [90] |
| Types of Nanocarriers | Drugs | Route of Administration | Outcome/Purpose | Ref. |
|---|---|---|---|---|
| Nanocapsules | Deflazacort | Oral | Partially hollow core with higher drug loading and faster drug release rate. | [73] |
| SNEDDS | Dapagliflozin | Oral | PAM-based 3D printing technique was found effective to print a self-nanoemulsifying tablet dosage form with an immediate-release drug profile for poorly water-soluble drugs. | [74] |
| SMEDDS | Fenofibrate or cinnarizine | Oral | The kinetics of dispersion depended on the SA/V ratio values. The digestion process was affected by the initial geometry of the dosage form by virtue of the kinetics of dispersion of the dosage forms into the digestion medium | [85] |
| SNEEDS | Glimepirideand rosuvastatin | Oral | Enhancement in the pharmacokinetic behavior when compared to the commercial tablets | [86] |
| SNEEDS | Glimepiride (GMD) and rosuvastatin | Oral | 3D printed polypills improved the pharmacokinetics of both glimepiride and rosuvastatin with AUC values of 16,035.25 ng/mL × h and 67,811.75 ng/mL × h respectively. | [87] |
| SNEEDS | Glimepiride | Oral | SNEDDS formulation in the prepared paste lubricates the solid particles and prevents water loss. Fast in vitro drug release and controlled drug release behavior. 3D tablets showed an improvement in pharmacokinetic parameters compared to the directly compressed tablets with higher relative bioavailability. | [88] |
| SNEDDS | Lidocaine | Rectal | Work highlighted the potential of 3D printing as an alternative pathway to formulate personalized LID-loaded suppositories for local anesthesia | [89] |
| SNEDDS | Tacrolimus | Rectal | Fabrication of 3D printed self-supporting suppositories to deliver personalized doses of a narrow therapeutic index drug, with potential benefits for patients with ulcerative colitis | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, J.; Garg, A.; Mustafa, G.; Mohammed, A.A.; Ahmad, M.Z. 3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope. Pharmaceutics 2023, 15, 1448. https://doi.org/10.3390/pharmaceutics15051448
Ahmad J, Garg A, Mustafa G, Mohammed AA, Ahmad MZ. 3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope. Pharmaceutics. 2023; 15(5):1448. https://doi.org/10.3390/pharmaceutics15051448
Chicago/Turabian StyleAhmad, Javed, Anuj Garg, Gulam Mustafa, Abdul Aleem Mohammed, and Mohammad Zaki Ahmad. 2023. "3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope" Pharmaceutics 15, no. 5: 1448. https://doi.org/10.3390/pharmaceutics15051448
APA StyleAhmad, J., Garg, A., Mustafa, G., Mohammed, A. A., & Ahmad, M. Z. (2023). 3D Printing Technology as a Promising Tool to Design Nanomedicine-Based Solid Dosage Forms: Contemporary Research and Future Scope. Pharmaceutics, 15(5), 1448. https://doi.org/10.3390/pharmaceutics15051448









