Recent Advances in the Development of Liquid Crystalline Nanoparticles as Drug Delivery Systems
Abstract
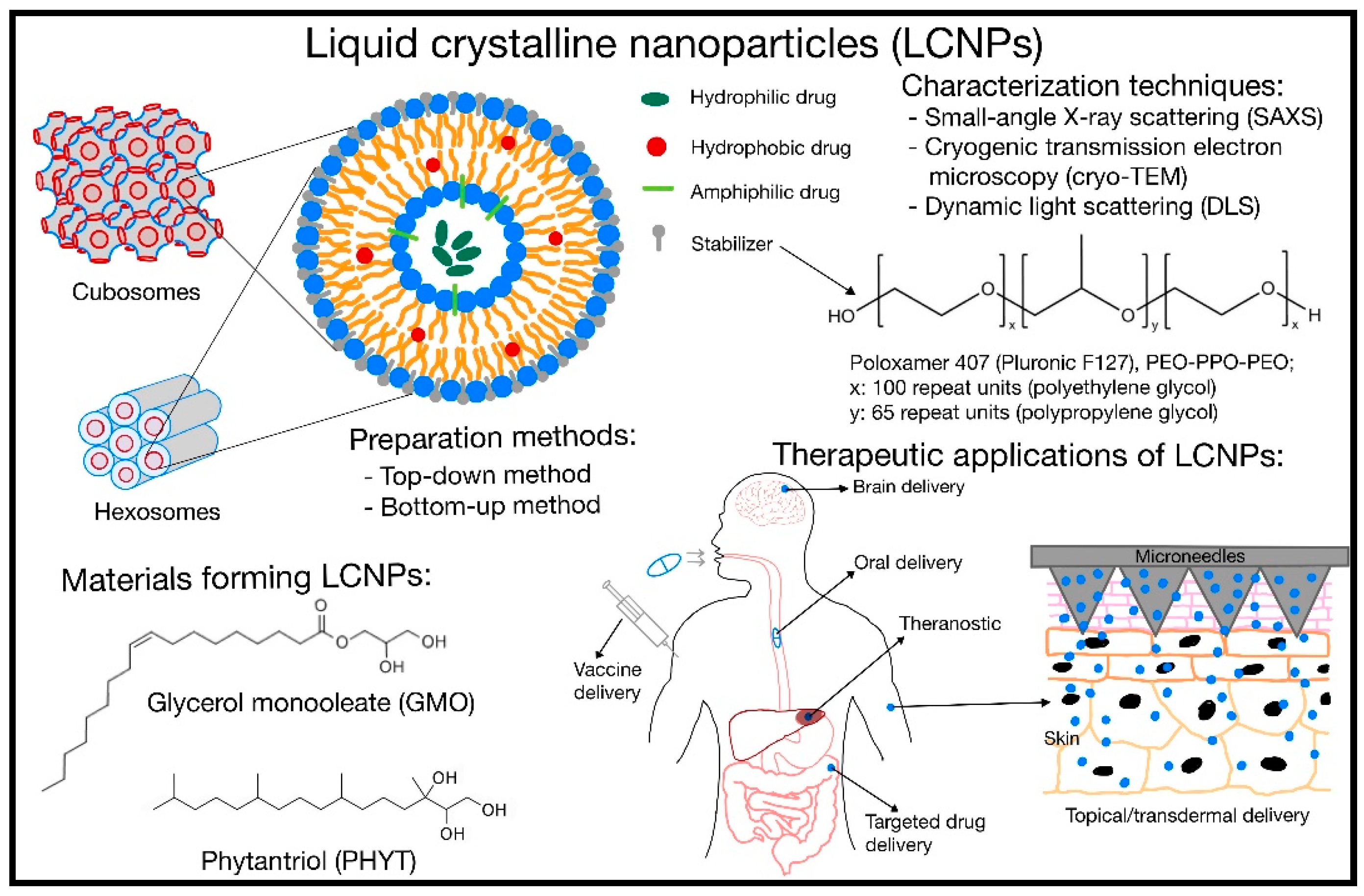
1. Introduction
2. Materials, Preparation Method, and Stabilizers
3. Characterization Techniques
4. Pharmacokinetic Modulation Using LCNPs
5. Therapeutics Application
5.1. Oral Delivery
5.2. Topical/Transdermal Delivery
5.3. Brain Delivery
5.4. Targeted Drug Delivery
5.5. Theranostic Application
5.6. Vaccine Delivery
5.7. Challenges and Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Madheswaran, T.; Kandasamy, M.; Bose, R.J.; Karuppagounder, V. Current Potential and Challenges in the Advances of Liquid Crystalline Nanoparticles as Drug Delivery Systems. Drug Discov. Today 2019, 24, 1405–1412. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2018, 10, 2958–2978. [Google Scholar] [CrossRef]
- Alimohamadi, H.; Vasan, R.; Hassinger, J.E.; Stachowiak, J.C.; Rangamani, P. The Role of Traction in Membrane Curvature Generation. Mol. Biol. Cell 2018, 29, 2024–2035. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Rajak, P.; Nath, L.K.; Bhuyan, B. Liquid Crystals: An Approach in Drug Delivery. Indian J. Pharm. Sci. 2019, 81, 11–21. [Google Scholar] [CrossRef]
- Popov, P.; Mann, E.K.; Jákli, A. Thermotropic Liquid Crystal Films for Biosensors and Beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef] [PubMed]
- Zare, A.; Montané, X.; Reina, J.A.; Giamberini, M. 7 Applications of Membranes in Sustainable Energy Systems: Energy Production and Storage. In Polymer Engineering; Tylkowski, B., Wieszczycka, K., Jastrząb, R., Montane, X., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2022; pp. 219–248. ISBN 9783110733822. [Google Scholar]
- Kim, D.H.; Jahn, A.; Cho, S.J.; Kim, J.S.; Ki, M.H.; Kim, D.D. Lyotropic Liquid Crystal Systems in Drug Delivery: A Review. J. Pharm. Investig. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Lee, D.R.; Park, J.S.; Bae, I.H.; Lee, Y.; Kim, B.M. Liquid Crystal Nanoparticle Formulation as an Oral Drug Delivery System for Liver-Specific Distribution. Int. J. Nanomed. 2016, 11, 853–871. [Google Scholar] [CrossRef]
- Zaki, R.M.; El Sayeh Abou El Ela, A.; Almurshedi, A.S.; Aldosari, B.N.; Aldossari, A.A.; Ibrahim, M.A. Fabrication and Assessment of Orodispersible Tablets Loaded with Cubosomes for the Improved Anticancer Activity of Simvastatin against the MDA-MB-231 Breast Cancer Cell Line. Polymers 2023, 15, 1774. [Google Scholar] [CrossRef]
- Lai, J.; Lu, Y.; Yin, Z.; Hu, F.; Wu, W. Pharmacokinetics and Enhanced Oral Bioavailability in Beagle Dogs of Cyclosporine A Encapsulated in Glyceryl Monooleate/Poloxamer 407 Cubic Nanoparticles. Int. J. Nanomed. 2010, 5, 13–23. [Google Scholar] [CrossRef]
- Chang, C.; Meikle, T.G.; Drummond, C.J.; Yang, Y.; Conn, C.E. Comparison of Cubosomes and Liposomes for the Encapsulation and Delivery of Curcumin. Soft Matter 2021, 17, 3306–3313. [Google Scholar] [CrossRef]
- Swarnakar, N.K.; Thanki, K.; Jain, S. Bicontinuous Cubic Liquid Crystalline Nanoparticles for Oral Delivery of Doxorubicin: Implications on Bioavailability, Therapeutic Efficacy, and Cardiotoxicity. Pharm. Res. 2014, 31, 1219–1238. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Younis, M.M.; Salama, A.; Darwish, A.B. Cubosomes as a Potential Oral Drug Delivery System for Enhancing the Hepatoprotective Effect of Coenzyme Q10. J. Pharm. Sci. 2021, 110, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, M.; Yang, M.; Chen, J.; Fang, W.; Xu, P. Evaluating the Potential of Cubosomal Nanoparticles for Oral Delivery of Amphotericin B in Treating Fungal Infection. Int. J. Nanomed. 2014, 9, 327–336. [Google Scholar] [CrossRef]
- Nasr, M.; Almawash, S.; Al Saqr, A.; Bazeed, A.Y.; Saber, S.; Elagamy, H.I. Bioavailability and Antidiabetic Activity of Gliclazide-Loaded Cubosomal Nanoparticles. Pharmaceuticals 2021, 14, 786. [Google Scholar] [CrossRef]
- Elfaky, M.A.; Sirwi, A.; Tolba, H.H.; Shaik, R.A.; Selmi, N.M.; Alattas, A.H.; Albreki, R.S.; Alshreef, N.M.; Gad, H.A. Development, Optimization, and Antifungal Assessment of Ocular Gel Loaded with Ketoconazole Cubic Liquid Crystalline Nanoparticles. J. Pharm. Sci. 2021, 110, 2210–2220. [Google Scholar] [CrossRef]
- Bessone, C.D.V.; Akhlaghi, S.P.; Tártara, L.I.; Quinteros, D.A.; Loh, W.; Allemandi, D.A. Latanoprost-Loaded Phytantriol Cubosomes for the Treatment of Glaucoma. Eur. J. Pharm. Sci. 2021, 160, 2210–2220. [Google Scholar] [CrossRef]
- Gaballa, S.A.; El Garhy, O.H.; Moharram, H.; Abdelkader, H. Preparation and Evaluation of Cubosomes/Cubosomal Gels for Ocular Delivery of Beclomethasone Dipropionate for Management of Uveitis. Pharm. Res. 2020, 37, 198. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; El-Zahaby, S.A. Norfloxacin Loaded Nano-Cubosomes for Enhanced Management of Otitis Externa: In Vitro and in Vivo Evaluation. Int. J. Pharm. 2021, 600, 120490. [Google Scholar] [CrossRef]
- Peng, X.; Wen, X.; Pan, X.; Wang, R.; Chen, B.; Wu, C. Design and in Vitro Evaluation of Capsaicin Transdermal Controlled Release Cubic Phase Gels. AAPS PharmSciTech 2010, 11, 1405–1410. [Google Scholar] [CrossRef]
- Ramalheiro, A.; Paris, J.L.; Silva, B.F.B.; Pires, L.R. Rapidly Dissolving Microneedles for the Delivery of Cubosome-like Liquid Crystalline Nanoparticles with Sustained Release of Rapamycin. Int. J. Pharm. 2020, 591, 119942. [Google Scholar] [CrossRef]
- Elakkad, Y.E.; Younis, M.K.; Allam, R.M.; Mohsen, A.F.; Khalil, I.A. Tenoxicam Loaded Hyalcubosomes for Osteoarthritis. Int. J. Pharm. 2021, 601, 120483. [Google Scholar] [CrossRef]
- Nasr, M.; Younes, H.; Abdel-Rashid, R.S. Formulation and Evaluation of Cubosomes Containing Colchicine for Transdermal Delivery. Drug. Deliv. Transl. Res. 2020, 10, 1302–1313. [Google Scholar] [CrossRef]
- Nithya, R.; Jerold, P.; Siram, K. Cubosomes of Dapsone Enhanced Permeation across the Skin. J. Drug. Deliv. Sci. Technol. 2018, 48, 75–81. [Google Scholar] [CrossRef]
- Mohammad, Y.; Prentice, R.N.; Boyd, B.J.; Rizwan, S.B. Comparison of Cubosomes and Hexosomes for the Delivery of Phenytoin to the Brain. J. Colloid Interface Sci. 2022, 605, 146–154. [Google Scholar] [CrossRef]
- Azhari, H.; Younus, M.; Hook, S.M.; Boyd, B.J.; Rizwan, S.B. Cubosomes Enhance Drug Permeability across the Blood–Brain Barrier in Zebrafish. Int. J. Pharm. 2021, 600, 120411. [Google Scholar] [CrossRef]
- Gelperina, S.; Maksimenko, O.; Khalansky, A.; Vanchugova, L.; Shipulo, E.; Abbasova, K.; Berdiev, R.; Wohlfart, S.; Chepurnova, N.; Kreuter, J. Drug Delivery to the Brain Using Surfactant-Coated Poly(Lactide-Co-Glycolide) Nanoparticles: Influence of the Formulation Parameters. Eur. J. Pharm. Sci. 2010, 74, 157–163. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Zhang, Q.; Yan, X.; Guo, L.; Gao, X.; Qiu, M.; Jiang, X.; Lai, R.; Chen, H. A Novel Small Odorranalectin-Bearing Cubosomes: Preparation, Brain Delivery and Pharmacodynamic Study on Amyloid-β25-35-Treated Rats Following Intranasal Administration. Eur. J. Pharm. Biopharm. 2012, 80, 368–378. [Google Scholar] [CrossRef]
- Hong, L.; Gontsarik, M.; Amenitsch, H.; Salentinig, S. Human Antimicrobial Peptide Triggered Colloidal Transformations in Bacteria Membrane Lipopolysaccharides. Small 2022, 18, 2104211. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Bilosomes as a Novel Carrier for the Cutaneous Delivery for Dapsone as a Potential Treatment of Acne: Preparation, Characterization and in Vivo Skin Deposition Assay. J. Liposome Res. 2020, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Fan, B.; Thang, S.H.; Drummond, C.J.; Tran, N.; Zhai, J. Paclitaxel-Loaded Cubosome Lipid Nanocarriers Stabilised with PH and Hydrogen Peroxide-Responsive Steric Stabilisers as Drug Delivery Vehicles. J. Mater. Chem. B 2023, 11, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abdel-Bar, H.; Elsayed Khater, S.; Mahmoud Ghorab, D.; Mohsen Al-mahallawi, A. Hexosomes as Efficient Platforms for Possible Fluoxetine Hydrochloride Repurposing with Improved Cytotoxicity against HepG2 Cells. ACS Omega 2020, 5, 26697–26709. [Google Scholar] [CrossRef]
- Saber, M.M.; Al-mahallawi, A.M.; Nassar, N.N.; Stork, B.; Shouman, S.A. Targeting Colorectal Cancer Cell Metabolism through Development of Cisplatin and Metformin Nano-Cubosomes. BMC Cancer 2018, 18, 822. [Google Scholar] [CrossRef]
- Saber, S.; Nasr, M.; Saad, A.S.; Mourad, A.A.E.; Gobba, N.A.; Shata, A.; Hafez, A.M.; Elsergany, R.N.; Elagamy, H.I.; El-Ahwany, E.; et al. Albendazole-Loaded Cubosomes Interrupt the ERK1/2-HIF-1α-P300/CREB Axis in Mice Intoxicated with Diethylnitrosamine: A New Paradigm in Drug Repurposing for the Inhibition of Hepatocellular Carcinoma Progression. Biomed. Pharmacother. 2021, 142, 112029. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.K.; Choi, J.Y.; Poudel, B.K.; Hiep, T.T.; Pathak, S.; Gupta, B.; Choi, H.G.; Yong, C.S.; Kim, J.O. Multilayer-Coated Liquid Crystalline Nanoparticles for Effective Sorafenib Delivery to Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2015, 7, 20360–20368. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, U.A.; Fahmy, O.; Alhakamy, N.A. Optimized Icariin Cubosomes Exhibit Augmented Cytotoxicity against SKOV-3 Ovarian Cancer Cells. Pharmaceutics 2021, 13, 20. [Google Scholar] [CrossRef]
- Mehanna, M.M.; Sarieddine, R.; Alwattar, J.K.; Chouaib, R.; Gali-Muhtasib, H. Anticancer Activity of Thymoquinone Cubic Phase Nanoparticles against Human Breast Cancer: Formulation, Cytotoxicity and Subcellular Localization. Int. J. Nanomed. 2020, 15, 9557–9570. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic Combinatorial Drug-Loaded Coated Cubosomes for Enhanced Targeting and Efficacy against Cancer Cells. Cell Death Dis. 2020, 11, 1. [Google Scholar] [CrossRef]
- Meli, V.; Caltagirone, C.; Sinico, C.; Lai, F.; Falchi, A.M.; Monduzzi, M.; Obiols-Rabasa, M.; Picci, G.; Rosa, A.; Schmidt, J.; et al. Theranostic Hexosomes for Cancer Treatments: An in Vitro Study. New J. Chem. 2017, 41, 1558–1565. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, L.; Zheng, S.; Niu, Y.; Bo, R.; Huang, Y.; Xing, J.; Li, Z.; Wang, D. Cubosome Nanoparticles Potentiate Immune Properties of Immunostimulants. Int. J. Nanomed. 2016, 11, 3571–3583. [Google Scholar] [CrossRef]
- Von Halling Laier, C.; Gibson, B.; van de Weert, M.; Boyd, B.J.; Rades, T.; Boisen, A.; Hook, S.; Nielsen, L.H. Spray Dried Cubosomes with Ovalbumin and Quil-A as a Nanoparticulate Dry Powder Vaccine Formulation. Int. J. Pharm. 2018, 550, 35–44. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, H.; Yu, L.; Xu, S.; Bo, R.; Qiu, T.; Gu, P.; Zhu, T.Y.; He, J.; Wusiman, A.; et al. Adjuvant Activities of CTAB-Modified Polygonatum Sibiricum Polysaccharide Cubosomes on Immune Responses to Ovalbumin in Mice. Int. J. Biol. Macromol. 2020, 148, 793–801. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. J. Chem. Soc. Faraday Trans. II Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Hyde, S.T. Identification of Lyotropic Liquid Crystalline Mesophases. In Handbook of Applied Surface and Colloid Chemistry; John Wiley & Sons: West Sussex, UK, 2001; pp. 300–332. [Google Scholar]
- Gagliardi, A.; Cosco, D.; Udongo, B.P.; Dini, L.; Viglietto, G.; Paolino, D. Design and Characterization of Glyceryl Monooleate-Nanostructures Containing Doxorubicin Hydrochloride. Pharmaceutics 2020, 12, 1017. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Cao, F.; Lee, R.J.; Zhai, G. Lyotropic Liquid Crystal Systems in Drug Delivery. Drug Discov. Today 2010, 15, 1032–1040. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Boyd, B.J.; Drummond, C.J. Steric Stabilizers for Cubic Phase Lyotropic Liquid Crystal Nanodispersions (Cubosomes). In Advances in Planar Lipid Bilayers and Liposomes; Academic Press: Cambridge, MA, USA, 2015; Volume 21, pp. 131–187. [Google Scholar] [CrossRef]
- Wan Iskandar, W.F.N.; Salim, M.; Hashim, R.; Zahid, N.I. Stability of Cubic Phase and Curvature Tuning in the Lyotropic System of Branched Chain Galactose-Based Glycolipid by Amphiphilic Additives. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126697. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pispas, S.; Tseti, I.K.; Demetzos, C. Lyotropic Liquid Crystalline Nanostructures as Drug Delivery Systems and Vaccine Platforms. Pharmaceuticals 2022, 15, 429. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Anwar, A.; Ayish, A.; Elliott, J.M.; Squires, A.M. Phytantriol Based Smart Nano-Carriers for Drug Delivery Applications. Eur. J. Pharm. Sci. 2017, 101, 31–42. [Google Scholar] [CrossRef]
- Rizwan, S.B.; Assmus, D.; Boehnke, A.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Preparation of Phytantriol Cubosomes by Solvent Precursor Dilution for the Delivery of Protein Vaccines. Eur. J. Pharm. Biopharm. 2011, 79, 15–22. [Google Scholar] [CrossRef]
- Sharma, P.; Dhawan, S.; Nanda, S. Cubosome: A Potential Liquid Crystalline Carrier System. Curr. Pharm. Des. 2020, 26, 3300–3316. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Dawoud, M. Sorbitol Based Powder Precursor of Cubosomes as an Oral Delivery System for Improved Bioavailability of Poorly Water Soluble Drugs. J. Drug. Deliv. Sci. Technol. 2016, 35, 106–113. [Google Scholar] [CrossRef]
- Spicer, P.T.; Small, W.B.; Lynch, M.L.; Burns, J.L. Dry Powder Precursors of Cubic Liquid Crystalline Nanoparticles (Cubosomes). J. Nanoparticle Res. 2002, 4, 297–311. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Keddie, D.J.; Waddington, L.; Mudie, S.T.; Boyd, B.J.; Drummond, C.J. Novel Steric Stabilizers for Lyotropic Liquid Crystalline Nanoparticles: PEGylated-Phytanyl Copolymers. Langmuir 2015, 31, 2615–2629. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. High-Throughput Discovery of Novel Steric Stabilizers for Cubic Lyotropic Liquid Crystal Nanoparticle Dispersions. Langmuir 2012, 28, 9223–9232. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Gui, S.; Huang, J.; Cao, J.; Li, Z.; Li, Q.; Chu, X. Characterization of Lipid-Based Lyotropic Liquid Crystal and Effects of Guest Molecules on Its Microstructure: A Systematic Review. AAPS PharmSciTech 2018, 19, 2023–2040. [Google Scholar] [CrossRef] [PubMed]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro Junior, J.A.; Chiavacci, L.A. Physicochemical Characterization of Drug Nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for Physicochemical Characterization of Nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Chavda, V.P.; Dawre, S.; Pandya, A.; Vora, L.K.; Modh, D.H.; Shah, V.; Dave, D.J.; Patravale, V. Lyotropic Liquid Crystals for Parenteral Drug Delivery. J. Control. Release 2022, 349, 533–549. [Google Scholar] [CrossRef]
- Lancelot, A.; Sierra, T.; Serrano, J.L. Nanostructured Liquid-Crystalline Particles for Drug Delivery. Expert Opin. Drug. Deliv. 2014, 11, 547–564. [Google Scholar] [CrossRef]
- Zahid, N.I.; Salim, M.; Liew, C.Y.; Boyd, B.J.; Hashim, R. Structural Investigation and Steric Stabilisation of Guerbet Glycolipid-Based Cubosomes and Hexosomes Using Triblock Polyethylene Oxide-Polypropylene Oxide-Polyethylene Oxide Copolymers. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129212. [Google Scholar] [CrossRef]
- Frost, K.; Kaminski, D.; Kirwan, G.; Lascaris, E.; Shanks, R. Crystallinity and Structure of Starch Using Wide Angle X-Ray Scattering. Carbohydr. Polym. 2009, 78, 543–548. [Google Scholar] [CrossRef]
- Nilsson, C.; Østergaard, J.; Larsen, S.W.; Larsen, C.; Urtti, A.; Yaghmur, A. PEGylation of Phytantriol-Based Lyotropic Liquid Crystalline Particles-the Effect of Lipid Composition, PEG Chain Length, and Temperature on the Internal Nanostructure. Langmuir 2014, 30, 6398–6407. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.D.M.; Østergaard, J.; Stürup, S.; Gammelgaard, B.; Urtti, A.; Moghimi, S.M.; Yaghmur, A. Cisplatin Encapsulation Generates Morphologically Different Multicompartments in the Internal Nanostructures of Nonlamellar Liquid-Crystalline Self-Assemblies. Langmuir 2018, 34, 6570–6581. [Google Scholar] [CrossRef]
- Pham, A.C.; Hong, L.; Montagnat, O.; Nowell, C.J.; Nguyen, T.H.; Boyd, B.J. In Vivo Formation of Cubic Phase in Situ after Oral Administration of Cubic Phase Precursor Formulation Provides Long Duration Gastric Retention and Absorption for Poorly Water-Soluble Drugs. Mol. Pharm. 2016, 13, 280–286. [Google Scholar] [CrossRef]
- Yaghmur, A.; Rappolt, M.; Jonassen, A.L.U.; Schmitt, M.; Larsen, S.W. In Situ Monitoring of the Formation of Lipidic Non-Lamellar Liquid Crystalline Depot Formulations in Synovial Fluid. J. Colloid Interface Sci. 2021, 582, 773–778. [Google Scholar] [CrossRef]
- Tan, A.; Hong, L.; Du, J.D.; Boyd, B.J. Self-Assembled Nanostructured Lipid Systems: Is There a Link between Structure and Cytotoxicity? Adv. Sci. 2019, 6, 1801223. [Google Scholar] [CrossRef]
- Waghule, T.; Laxmi Swetha, K.; Roy, A.; Narayan Saha, R.; Singhvi, G. Quality by Design Assisted Optimization of Temozolomide Loaded PEGylated Lyotropic Liquid Crystals: Investigating Various Formulation and Process Variables along with in-Vitro Characterization. J. Mol. Liq. 2022, 352, 18–29. [Google Scholar] [CrossRef]
- Dyett, B.P.; Yu, H.; Strachan, J.; Drummond, C.J.; Conn, C.E. Fusion Dynamics of Cubosome Nanocarriers with Model Cell Membranes. Nat. Commun. 2019, 10, 4492. [Google Scholar] [CrossRef]
- Jain, S.; Yadav, P.; Swami, R.; Swarnakar, N.K.; Kushwah, V.; Katiyar, S.S. Lyotropic Liquid Crystalline Nanoparticles of Amphotericin B: Implication of Phytantriol and Glyceryl Monooleate on Bioavailability Enhancement. AAPS PharmSciTech 2018, 19, 1699–1711. [Google Scholar] [CrossRef]
- Deshpande, S.; Singh, N. Influence of Cubosome Surface Architecture on Its Cellular Uptake Mechanism. Langmuir 2017, 33, 3509–3516. [Google Scholar] [CrossRef] [PubMed]
- Helvig, S.Y.; Andersen, H.; Antopolsky, M.; Airaksinen, A.J.; Urtti, A.; Yaghmur, A.; Moghimi, S.M. Hexosome Engineering for Targeting of Regional Lymph Nodes. Materialia 2020, 11, 100705. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Perinelli, D.R.; Pippa, N.; Chrysostomou, V.; Forys, A.; Otulakowski, L.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Physicochemical, Morphological and Thermal Evaluation of Lyotropic Lipidic Liquid Crystalline Nanoparticles: The Effect of Stimuli-Responsive Polymeric Stabilizer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124678. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, R.; Heimann, K.; Wang, Z.; Wang, J.; Liu, F. Temperature-Sensitive Lyotropic Liquid Crystals as Systems for Transdermal Drug Delivery. J. Mol. Liq. 2021, 326, 115310. [Google Scholar] [CrossRef]
- Zhai, J.; Yap, S.L.; Drummond, C.J.; Tran, N. Controlling the PH Dependent Transition between Monoolein Fd3m Micellar Cubosomes and Hexosomes Using Fatty Acetate and Fatty Acid Additive Mixtures. J. Colloid. Interface Sci. 2022, 607, 848–856. [Google Scholar] [CrossRef]
- Pramanik, A.; Xu, Z.; Shamsuddin, S.H.; Khaled, Y.S.; Ingram, N.; Maisey, T.; Tomlinson, D.; Coletta, P.L.; Jayne, D.; Hughes, T.A.; et al. Affimer Tagged Cubosomes: Targeting of Carcinoembryonic Antigen Expressing Colorectal Cancer Cells Using In Vitro and In Vivo Models. ACS Appl. Mater. Interfaces 2022, 14, 11078–11091. [Google Scholar] [CrossRef]
- Dully, M.; Brasnett, C.; Djeghader, A.; Seddon, A.; Neilan, J.; Murray, D.; Butler, J.; Soulimane, T.; Hudson, S.P. Modulating the Release of Pharmaceuticals from Lipid Cubic Phases Using a Lipase Inhibitor. J. Colloid Interface Sci. 2020, 573, 176–192. [Google Scholar] [CrossRef]
- Dully, M.; Bhattacharya, S.; Verma, V.; Murray, D.; Thompson, D.; Soulimane, T.; Hudson, S.P. Balanced Lipase Interactions for Degradation-Controlled Paclitaxel Release from Lipid Cubic Phase Formulations. J. Colloid Interface Sci. 2022, 607, 978–991. [Google Scholar] [CrossRef]
- Freag, M.S.; Saleh, W.M.; Abdallah, O.Y. Self-Assembled Phospholipid-Based Phytosomal Nanocarriers as Promising Platforms for Improving Oral Bioavailability of the Anticancer Celastrol. Int. J. Pharm. 2018, 535, 18–26. [Google Scholar] [CrossRef]
- Jain, S.; Heeralal, B.; Swami, R.; Swarnakar, N.K.; Kushwah, V. Improved Oral Bioavailability, Therapeutic Efficacy, and Reduced Toxicity of Tamoxifen-Loaded Liquid Crystalline Nanoparticles. AAPS PharmSciTech 2018, 19, 9452–9464. [Google Scholar] [CrossRef]
- Yasser, M.; Teaima, M.; El-Nabarawi, M.; El-Monem, R.A. Cubosomal Based Oral Tablet for Controlled Drug Delivery of Telmisartan: Formulation, in-Vitro Evaluation and in-Vivo Comparative Pharmacokinetic Study in Rabbits. Drug. Dev. Ind. Pharm. 2019, 45, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Hexagonal Liquid Crystalline Nanodispersions Proven Superiority for Enhanced Oral Delivery of Rosuvastatin: In Vitro Characterization and In Vivo Pharmacokinetic Study. J. Pharm. Sci. 2017, 106, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, W.R.; Oshizaka, T.; Ichiro, H.; Todo, H.; Sugibayashi, K. Usefulness of Liquid-Crystal Oral Formulations to Enhance the Bioavailability and Skin Tissue Targeting of p-Amino Benzoic Acid as a Model Compound. Eur. J. Pharm. Sci. 2016, 88, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.W.; Jin, H.S.; Park, Y.J. Formation of Self-Assembled Liquid Crystalline Nanoparticles and Absorption Enhancement of Ω-3s by Phospholipids and Oleic Acids. Pharmaceutics 2022, 14, 68. [Google Scholar] [CrossRef]
- Shiadeh, S.N.R.; Khodaverdi, E.; Maleki, M.F.; Eisvand, F.; Nazari, A.; Zarqi, J.; Hadizadeh, F.; Kamali, H. A Sustain-Release Lipid-Liquid Crystal Containing Risperidone Based on Glycerol Monooleate, Glycerol Dioleate, and Glycerol Trioleate: In-Vitro Evaluation and Pharmacokinetics in Rabbits. J. Drug. Deliv. Sci. Technol. 2022, 70, 103257. [Google Scholar] [CrossRef]
- Shiadeh, S.N.R.; Khodaverdi, E.; Maleki, M.F.; Eisvand, F.; Boujaran, H.; Zarei, H.; Vosooghi, R.; Hadizadeh, F.; Kamali, H. Lipid-Liquid Crystals for 2 Months Controlled Risperidone Release: In-Vitro Evaluation and Pharmacokinetics in Rabbits. Int. J. Pharm. 2022, 618, 121649. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In Vitro and in Vivo Evaluation of Cubosomes Containing 5-Fluorouracil for Liver Targeting. Acta. Pharm. Sin. B 2015, 5, 79–88. [Google Scholar] [CrossRef]
- Otte, A.; Báez-Santos, Y.M.; Mun, E.A.; Soh, B.K.; Lee, Y.; Park, K. The in Vivo Transformation and Pharmacokinetic Properties of a Liquid Crystalline Drug Delivery System. Int. J. Pharm. 2017, 532, 345–351. [Google Scholar] [CrossRef]
- Kamali, H.; Karimi, M.; Abbaspour, M.; Nadim, A.; Hadizadeh, F.; Khodaverdi, E.; Eisvand, F. Comparison of Lipid Liquid Crystal Formulation and Vivitrol® for Sustained Release of Naltrexone: In Vitro Evaluation and Pharmacokinetics in Rats. Int. J. Pharm. 2022, 611, 121275. [Google Scholar] [CrossRef]
- Kaul, S.; Nagaich, U.; Verma, N. Preclinical Assessment of Nanostructured Liquid Crystalline Particles for the Management of Bacterial Keratitis: In Vivo and Pharmacokinetics Study. Drug. Deliv. Transl. Res. 2022, 12, 1719–1737. [Google Scholar] [CrossRef]
- Said, M.; Aboelwafa, A.A.; Elshafeey, A.H.; Elsayed, I. Central Composite Optimization of Ocular Mucoadhesive Cubosomes for Enhanced Bioavailability and Controlled Delivery of Voriconazole. J. Drug. Deliv. Sci. Technol. 2021, 61, 102075. [Google Scholar] [CrossRef]
- Bu, M.; Tang, J.; Wei, Y.; Sun, Y.; Wang, X.; Wu, L.; Liu, H. Enhanced Bioavailability of Nerve Growth Factor with Phytantriol Lipid-Based Crystalline Nanoparticles in Cochlea. Int. J. Nanomed. 2015, 10, 6879–6889. [Google Scholar] [CrossRef]
- Mahmood, A.; Rapalli, V.K.; Waghule, T.; Gorantla, S.; Singhvi, G. Luliconazole Loaded Lyotropic Liquid Crystalline Nanoparticles for Topical Delivery: QbD Driven Optimization, in-Vitro Characterization and Dermatokinetic Assessment. Chem. Phys. Lipids 2021, 234, 105028. [Google Scholar] [CrossRef]
- Waghule, T.; Patil, S.; Rapalli, V.K.; Girdhar, V.; Gorantla, S.; Kumar Dubey, S.; Saha, R.N.; Singhvi, G. Improved Skin-Permeated Diclofenac-Loaded Lyotropic Liquid Crystal Nanoparticles: QbD-Driven Industrial Feasible Process and Assessment of Skin Deposition. Liq. Cryst. 2021, 48, 991–1009. [Google Scholar] [CrossRef]
- Gorantla, S.; Saha, R.N.; Singhvi, G. Spectrophotometric Method to Quantify Tofacitinib in Lyotropic Liquid Crystalline Nanoparticles and Skin Layers: Application in Ex Vivo Dermal Distribution Studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 255, 119719. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Saha, R.N.; Singhvi, G. Exploring the Affluent Potential of Glyceryl Mono Oleate—Myristol Liquid Crystal Nanoparticles Mediated Localized Topical Delivery of Tofacitinib: Study of Systematic QbD, Skin Deposition and Dermal Pharmacokinetics Assessment. J. Mol. Liq. 2022, 346, 117053. [Google Scholar] [CrossRef]
- Bakr, M.M.; Shukr, M.H.; ElMeshad, A.N. In Situ Hexosomal Gel as a Promising Tool to Ameliorate the Transnasal Brain Delivery of Vinpocetine: Central Composite Optimization and In Vivo Biodistribution. J. Pharm. Sci. 2020, 109, 2213–2223. [Google Scholar] [CrossRef]
- Sugibayashi, K.; Yamamoto, N.; Itakura, S.; Okada, A.; Hijikuro, I.; Todo, H. Development of Spray Formulations Applied to the Oral Mucosa Using Non-Lamellar Liquid Crystal-Forming Lipids. Chem. Pharm. Bull. 2020, 68, 1025–1033. [Google Scholar] [CrossRef]
- Kawai, M.; Ibaraki, H.; Takashima, Y.; Kanazawa, T.; Okada, H. Development of a Liquid Crystal Formulation That Can Penetrate the Stratum Corneum for Intradermal Delivery of Small Interfering RNA. Mol. Pharm. 2021, 18, 1025–1033. [Google Scholar] [CrossRef]
- Kozaka, S.; Wakabayashi, R.; Kamiya, N.; Goto, M. Design of Swollen Lipidic Cubic Phase to Increase Transcutaneous Penetration of Biomacromolecules. ACS Appl. Mater. Interfaces 2021, 13, 54753–54761. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, W. Liquid Crystalline Phases for Enhancement of Oral Bioavailability. AAPS PharmSciTech 2021, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Mu, H. Recent Advances in Drug Delivery Applications of Cubosomes, Hexosomes, and Solid Lipid Nanoparticles. Acta. Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Mertins, O.; Mathews, P.D.; Angelova, A. Advances in the Design of Ph-Sensitive Cubosome Liquid Crystalline Nanocarriers for Drug Delivery Applications. Nanomaterials 2020, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Salmazi, R.; Calixto, G.; Bernegossi, J.; Aparecido Dos, M.; Ramos, S.; Bauab, T.M.; Chorilli, M. A Curcumin-Loaded Liquid Crystal Precursor Mucoadhesive System for the Treatment of Vaginal Candidiasis. Int. J. Nanomed. 2015, 10, 4815–24824. [Google Scholar] [CrossRef]
- Lai, J.; Chen, J.; Lu, Y.; Sun, J.; Hu, F.; Yin, Z.; Wu, W. Glyceryl Monooleate/Poloxamer 407 Cubic Nanoparticles as Oral Drug Delivery Systems: I. In Vitro Evaluation and Enhanced Oral Bioavailability of the Poorly Water-Soluble Drug Simvastatin. AAPS PharmSciTech 2009, 10, 960–966. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug. Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Sayed, S.; Abdel-Moteleb, M.; Amin, M.M.; Khowessah, O.M. Cubogel as Potential Platform for Glaucoma Management. Drug Deliv. 2021, 28, 293–305. [Google Scholar] [CrossRef]
- Sadhu, V.R.; Beram, N.S.; Kantamneni, P. A Review on Cubosome: The Novel Drug Delivery System. GSC Biol. Pharm. Sci. 2018, 5, 76–81. [Google Scholar] [CrossRef]
- Kaul, S.; Nagaich, U.; Verma, N. Investigating Nanostructured Liquid Crystalline Particles as Prospective Ocular Delivery Vehicle for Tobramycin Sulfate: Ex Vivo and in Vivo Studies. J. Adv. Pharm. Technol. Res. 2021, 12, 356–361. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for Topical Delivery of the Antimicrobial Peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, J.; He, Y.; Lin, K.; Li, S.; Zhang, Y.; Song, P.; Zhou, Y.; Chen, X. Nanocarriers for Inner Ear Disease Therapy. Front Cell. Neurosci. 2021, 15, 475. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef]
- Garg, T.; Bhandari, S.; Rath, G.; Goyal, A.K. Current Strategies for Targeted Delivery of Bio-Active Drug Molecules in the Treatment of Brain Tumor. J. Drug Target 2015, 23, 865–887. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–Brain Barrier Delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the Blood-Brain Barrier with Nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, T.T.D.; Tran, N.M.A.; Van Vo, G. Lipid-Based Nanocarriers via Nose-to-Brain Pathway for Central Nervous System Disorders. Neurochem. Res. 2022, 47, 552–573. [Google Scholar] [CrossRef]
- Zhai, J.; Fan, B.; Thang, S.H.; Drummond, C.J. Novel Amphiphilic Block Copolymers for the Formation of Stimuli-Responsive Non-Lamellar Lipid Nanoparticles. Molecules 2021, 26, 3648. [Google Scholar] [CrossRef]
- Alavi, M.; Nokhodchi, A. Micro- and Nanoformulations of Paclitaxel Based on Micelles, Liposomes, Cubosomes, and Lipid Nanoparticles: Recent Advances and Challenges. Drug Discov. Today 2022, 27, 576–584. [Google Scholar] [CrossRef]
- Zhai, J.; Tan, F.H.; Luwor, R.B.; Srinivasa Reddy, T.; Ahmed, N.; Drummond, C.J.; Tran, N. In Vitro and in Vivo Toxicity and Biodistribution of Paclitaxel-Loaded Cubosomes as a Drug Delivery Nanocarrier: A Case Study Using an A431 Skin Cancer Xenograft Model. ACS Appl. Bio. Mater 2020, 3, 4198–4207. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Luwor, R.B.; Ahmed, N.; Escalona, R.; Tan, F.H.; Fong, C.; Ratcliffe, J.; Scoble, J.A.; Drummond, C.J.; Tran, N. Paclitaxel-Loaded Self-Assembled Lipid Nanoparticles as Targeted Drug Delivery Systems for the Treatment of Aggressive Ovarian Cancer. ACS Appl. Mater Interfaces 2018, 10, 25174–25185. [Google Scholar] [CrossRef] [PubMed]
- Freag, M.S.; Elnaggar, Y.S.R.; Abdelmonsif, D.A.; Abdallah, O.Y. Layer-by-Layer-Coated Lyotropic Liquid Crystalline Nanoparticles for Active Tumor Targeting of Rapamycin. Nanomedicine 2016, 11, 2975–2996. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. PH Responsiveness of Hexosomes and Cubosomes for Combined Delivery of Brucea Javanica Oil and Doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef]
- Aleandri, S.; Bandera, D.; Mezzenga, R.; Landau, E.M. Biotinylated Cubosomes: A Versatile Tool for Active Targeting and Codelivery of Paclitaxel and a Fluorescein-Based Lipid Dye. Langmuir 2015, 31, 12770–12776. [Google Scholar] [CrossRef]
- Ding, Y.; Chow, S.H.; Chen, J.; Brun, A.P.L.; Wu, C.M.; Duff, A.P.; Wang, Y.; Song, J.; Wang, J.H.; Wong, V.H.Y.; et al. Targeted Delivery of LM22A-4 by Cubosomes Protects Retinal Ganglion Cells in an Experimental Glaucoma Model. Acta. Biomater. 2021, 126, 433–444. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Du, S. Production of Gold/Silver Doped Carbon Nanocomposites for Effective Photothermal Therapy of Colon Cancer. Sci. Rep. 2020, 10, 7618. [Google Scholar] [CrossRef]
- Mulet, X.; Boyd, B.J.; Drummond, C.J. Advances in Drug Delivery and Medical Imaging Using Colloidal Lyotropic Liquid Crystalline Dispersions. J. Colloid Interface Sci. 2013, 393, 1–20. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Gu, P.; Bo, R.; Xu, S.; Wusiman, A.; Liu, J.; Hu, Y.; Wang, D. Surface-Engineered Cubosomes Serve as a Novel Vaccine Adjuvant to Modulate Innate Immunity and Improve Adaptive Immunity in Vivo. Int. J. Nanomed. 2020, 15, 8595–8608. [Google Scholar] [CrossRef]
- Rizwan, S.B.; McBurney, W.T.; Young, K.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Cubosomes Containing the Adjuvants Imiquimod and Monophosphoryl Lipid A Stimulate Robust Cellular and Humoral Immune Responses. J. Control. Release 2013, 165, 16–21. [Google Scholar] [CrossRef]
- Qiu, T.; Gu, P.; Wusiman, A.; Ni, H.; Xu, S.; Zhang, Y.; Zhu, T.; He, J.; Liu, Z.; Hu, Y.; et al. Immunoenhancement Effects of Chitosan-Modified Ginseng Stem-Leaf Saponins-Encapsulated Cubosomes as an Ajuvant. Colloids Surf. B Biointerfaces 2021, 204, 111799. [Google Scholar] [CrossRef] [PubMed]


| Types of LCNP | Applications | Therapeutic Molecules | Advantages | References |
|---|---|---|---|---|
| Cubosomes | Oral delivery | Cyclosporin A | Improved solubility, protection from harsh environments and enzymatic degradation | [11,12] |
| Simvastatin | ||||
| Curcumin | Improved cytotoxic activities | [13] | ||
| Doxorubicin | Improved antitumor efficacy and bioavailability, reduced risk of cardiotoxicity | [14] | ||
| Cubosomes | CoQ10 | Improved drug delivery, enhanced hepatoprotective effect | [15] | |
| Amphotericin B | Improved drug delivery | [16] | ||
| Gliclazide | Improved bioavailability, increased therapeutic effect | [17] | ||
| Topical (eyes) | Ketoconazole | Improved antifungal action, enhanced drug permeability | [18] | |
| Latanoprost | Enhanced effectiveness, improved sustained-release property | [19] | ||
| Beclomethasone dipropionate | Enhanced effectiveness | [20] | ||
| Topical (ears) | Norfloxacin | Enhanced permeation and accumulation of drugs at specific site | [21] | |
| Transdermal | Capsaicin | Improved sustained skin retention and sustained-release property | [22] | |
| Rapamycin | Enhanced efficiency and sustained-release property | [23] | ||
| Tenoxicam | Enhanced effectiveness | [24] | ||
| Colchicine | Increased drug absorption | [25] | ||
| Dapsone | Enhanced skin permeation | [26] | ||
| Cubosomes and Hexosomes | Brain (IV) | Phenytoin | Enhanced brain penetration of blood–brain barrier | [27] |
| Cubosomes | RhoB | Enhanced uptake of drug | [28] | |
| Doxorubicin and loperamide | Increased drug delivery | [29] | ||
| S14G-HN peptide | Increased drug delivery and effectiveness | [30] | ||
| Targeted delivery route | Antimicrobial peptides | Enhanced penetration of LPS layers | [31] | |
| Dapsone | Increased permeation of drug | [32] | ||
| Paclitaxel | Enhanced controlled release and cellular uptake | [33] | ||
| Fluoxetine hydrochloride | Prolonged in vitro drug release | [34] | ||
| Cisplatin | Better cytotoxic impact | [35] | ||
| Albendazole | Increased bioavailability of drug | [36] | ||
| Sorafenib | Increased cellular absorption and therapeutic anticancer activity | [37] | ||
| Icariin | Improved solubility and cellular permeability | [38] | ||
| Thymoquinone | Encapsulated drug and delivers anticancer molecule | [39] | ||
| Cubosomes | Theranostic | Cisplatin (coating: polylisine) | Prevents initial burst release of drugs/higher therapeutic efficacy | [40] |
| Paclitaxel (coating: polylisine) | Prevents initial burst release of drugs/higher therapeutic efficacy | [40] | ||
| Hexosomes | Theranostic | Docetaxel | Higher cytotoxicity against certain cell lines and able to monitor the extent of nanoparticle uptake | [41] |
| Cubosomes | Vaccine | Polysaccharide, promising adjuvant for vaccines | Enhanced ability of immunostimulants to generate an immune response | [42] |
| Ovalbumin | Producing nanoparticulate vaccine formulations in dry powder form | [43] | ||
| Ovalbumin (OVA) absorbed cetyltrimethylammonium bromide-modified polygonatum sibiricum polysaccharide cubosomes | Stimulates the cellular immune response and increases the level of humoral immunity | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leu, J.S.L.; Teoh, J.J.X.; Ling, A.L.Q.; Chong, J.; Loo, Y.S.; Mat Azmi, I.D.; Zahid, N.I.; Bose, R.J.C.; Madheswaran, T. Recent Advances in the Development of Liquid Crystalline Nanoparticles as Drug Delivery Systems. Pharmaceutics 2023, 15, 1421. https://doi.org/10.3390/pharmaceutics15051421
Leu JSL, Teoh JJX, Ling ALQ, Chong J, Loo YS, Mat Azmi ID, Zahid NI, Bose RJC, Madheswaran T. Recent Advances in the Development of Liquid Crystalline Nanoparticles as Drug Delivery Systems. Pharmaceutics. 2023; 15(5):1421. https://doi.org/10.3390/pharmaceutics15051421
Chicago/Turabian StyleLeu, Jassica S. L., Jasy J. X. Teoh, Angel L. Q. Ling, Joey Chong, Yan Shan Loo, Intan Diana Mat Azmi, Noor Idayu Zahid, Rajendran J. C. Bose, and Thiagarajan Madheswaran. 2023. "Recent Advances in the Development of Liquid Crystalline Nanoparticles as Drug Delivery Systems" Pharmaceutics 15, no. 5: 1421. https://doi.org/10.3390/pharmaceutics15051421
APA StyleLeu, J. S. L., Teoh, J. J. X., Ling, A. L. Q., Chong, J., Loo, Y. S., Mat Azmi, I. D., Zahid, N. I., Bose, R. J. C., & Madheswaran, T. (2023). Recent Advances in the Development of Liquid Crystalline Nanoparticles as Drug Delivery Systems. Pharmaceutics, 15(5), 1421. https://doi.org/10.3390/pharmaceutics15051421











