Membranolytic Activity Profile of Nonyl 3,4-Dihydroxybenzoate: A New Anti-Biofilm Compound for the Treatment of Dermatophytosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Dermatophytes Tested
2.2. Synthesis of Nonyl 3,4-Dihydroxybenzoate
2.3. Preparation of Conventional Antifungals and Nonyl Protocatechuate (Nonyl 3,4-Dihydroxybenzoate)
2.4. Effects of Antifungal Drugs and Nonyl 3,4-Dihydroxybenzoate on T. rubrum and T. mentagrophytes, Planktonic Cells, and Mature Biofilms
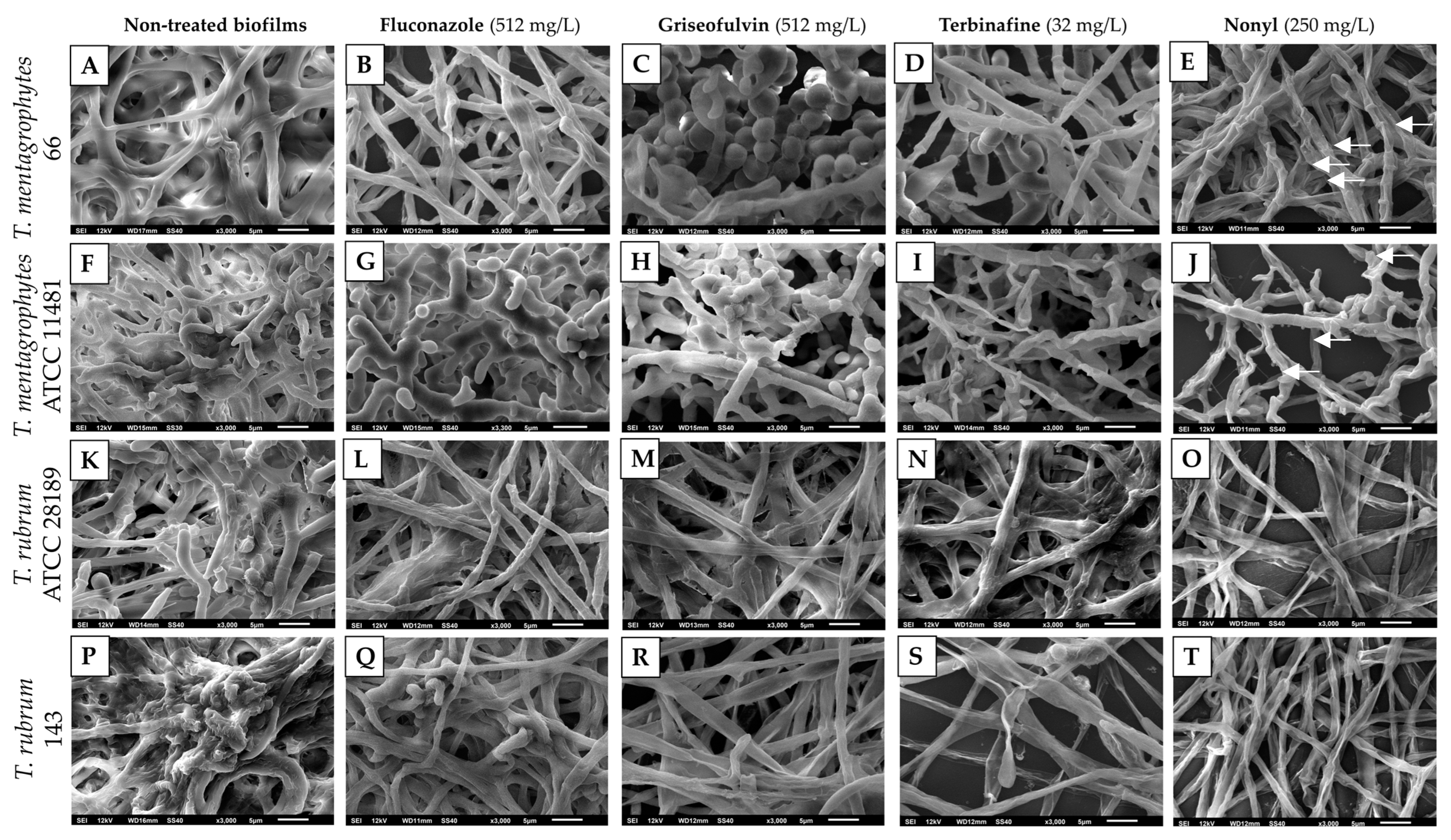
2.5. Scanning Electron Microscopy (SEM) of Treated Biofilms
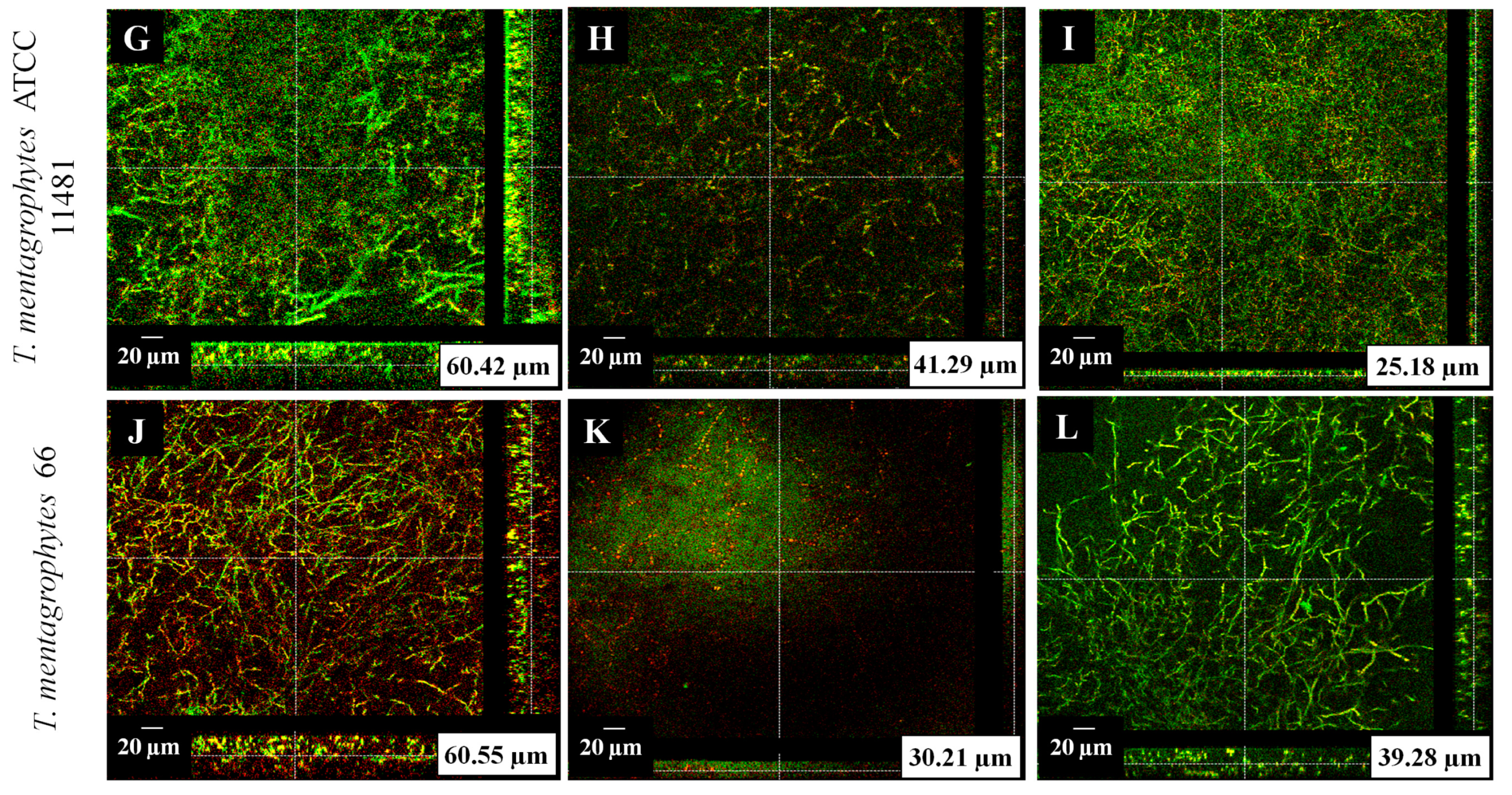
2.6. Confocal Microscopy of Treated Biofilms
2.7. Determination of the Mechanism of Action of Nonyl
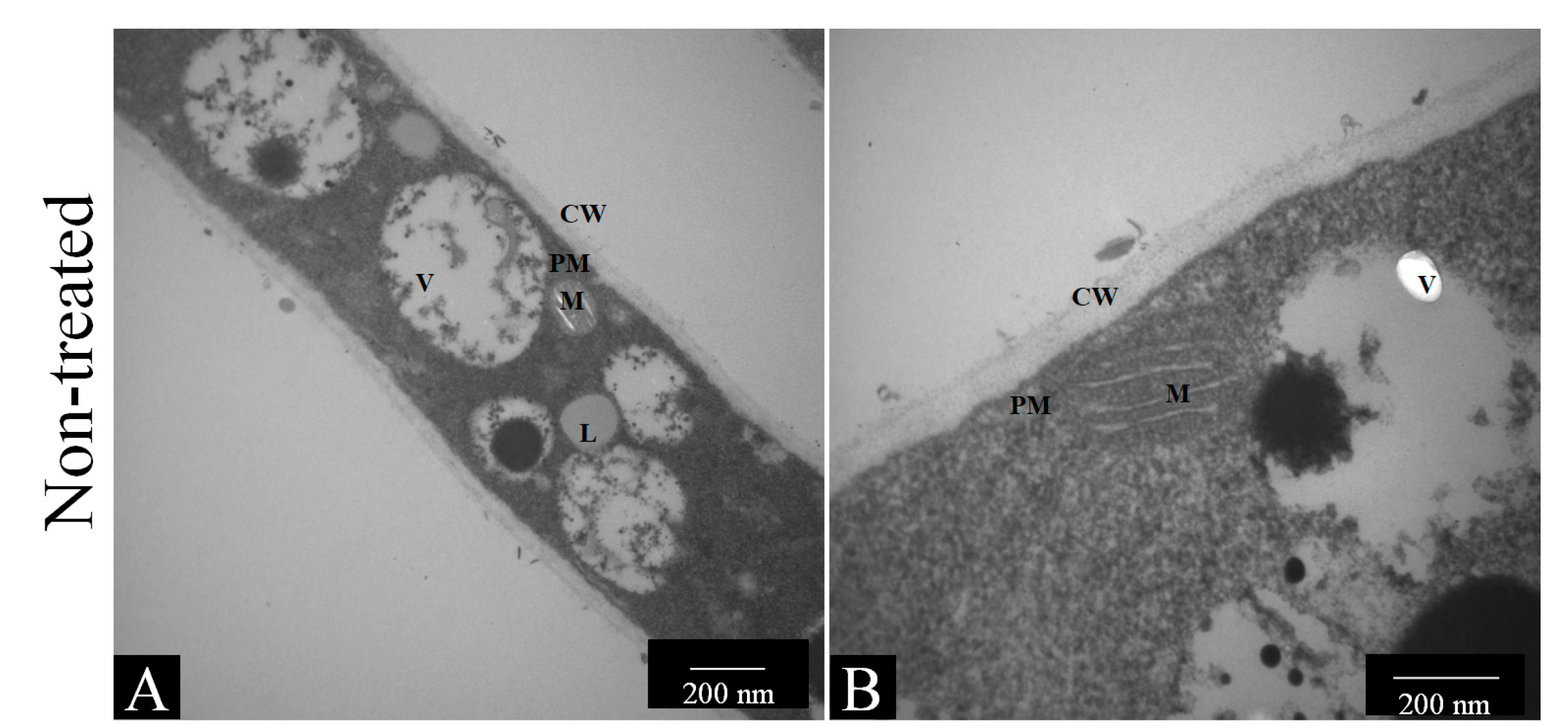
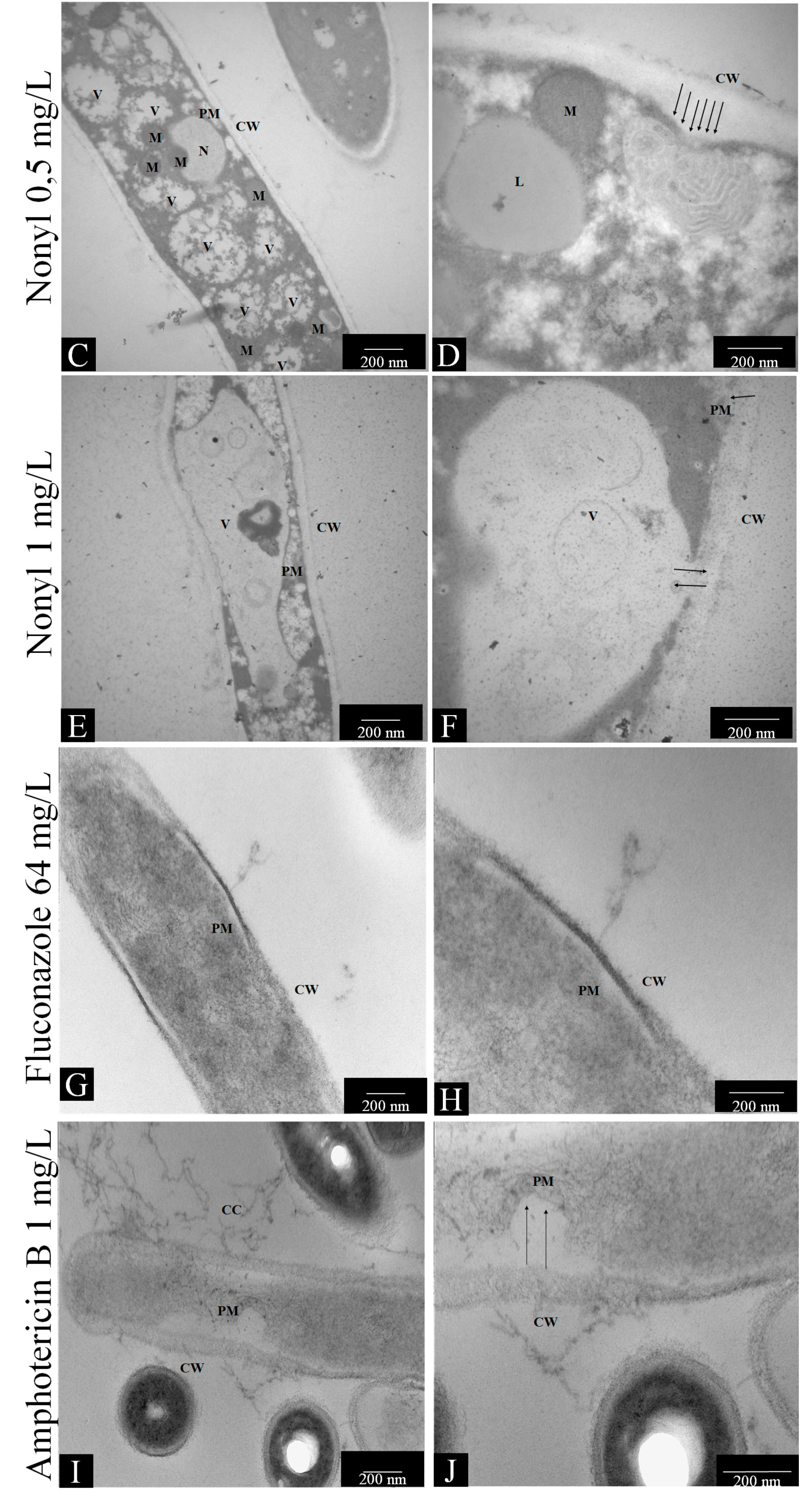
2.7.1. Transmission Electron Microscopy
2.7.2. Quantification of Fungal Membrane Sterols
2.7.3. Confocal Laser Scanning Microscopy after Staining with Calcofluor White
2.7.4. Quantification of Reactive Oxygen Species (ROS)
2.7.5. Apoptosis/Necrosis
2.7.6. Analysis of Differential Gene Expression of T. rubrum ATCC 28189 Treated with Nonyl by Real-Time PCR
2.8. Statistical Analysis
3. Results
3.1. Susceptibilities of T. rubrum and T. mentagrophytes Planktonic and Biofilm Forms to Antifungal Drugs and Nonyl 3, 4-Dihydroxybenzoate
3.2. Scanning Electron Microscopy of Treated Biofilms
3.3. Confocal Microscopy of Treated Biofilms
3.4. Determination of the Mechanism of Action of Nonyl Protocatechuate
3.4.1. Transmission Electron Microscopy (TEM)
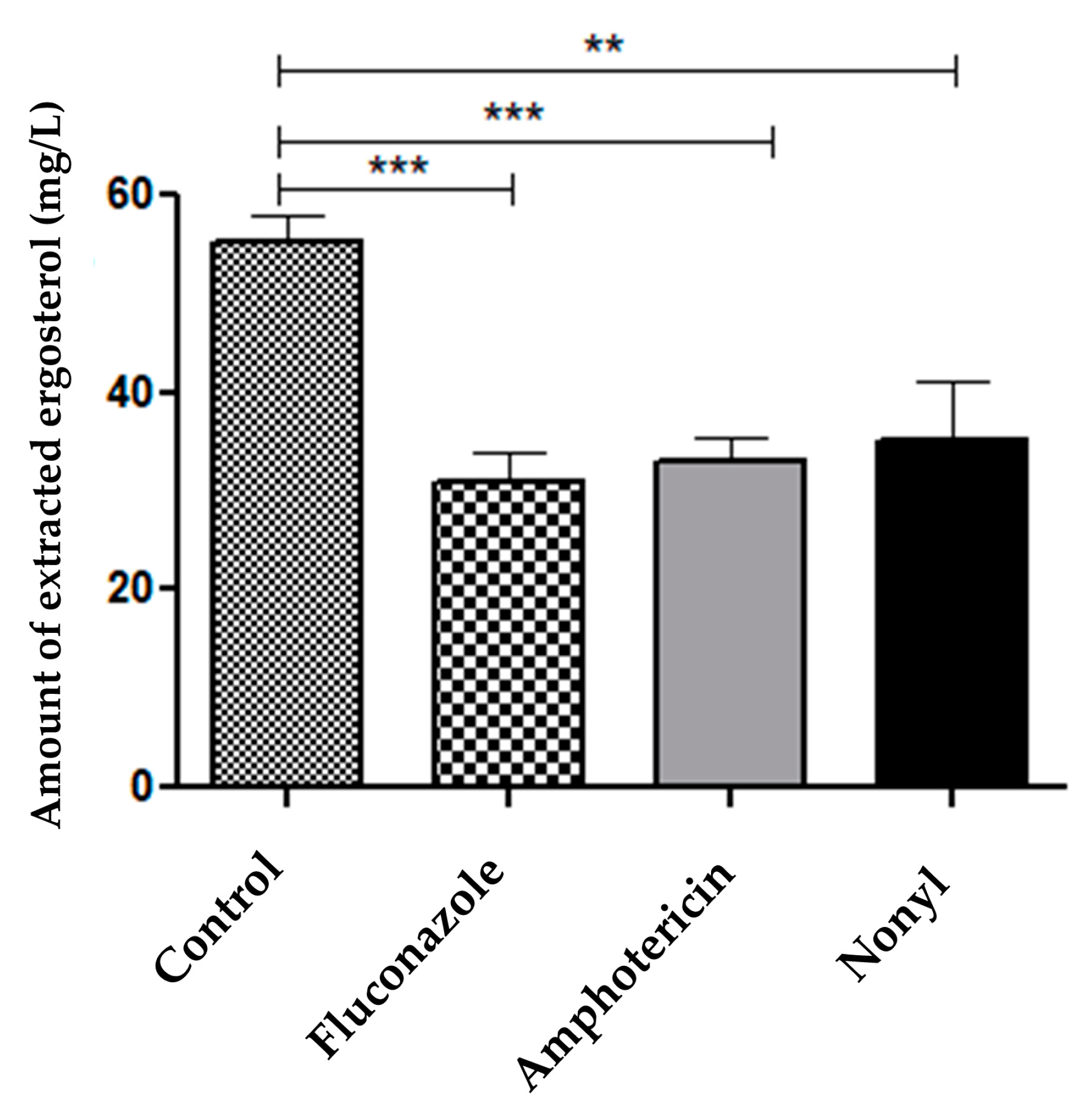
3.4.2. Quantification of Fungal Membrane Sterols
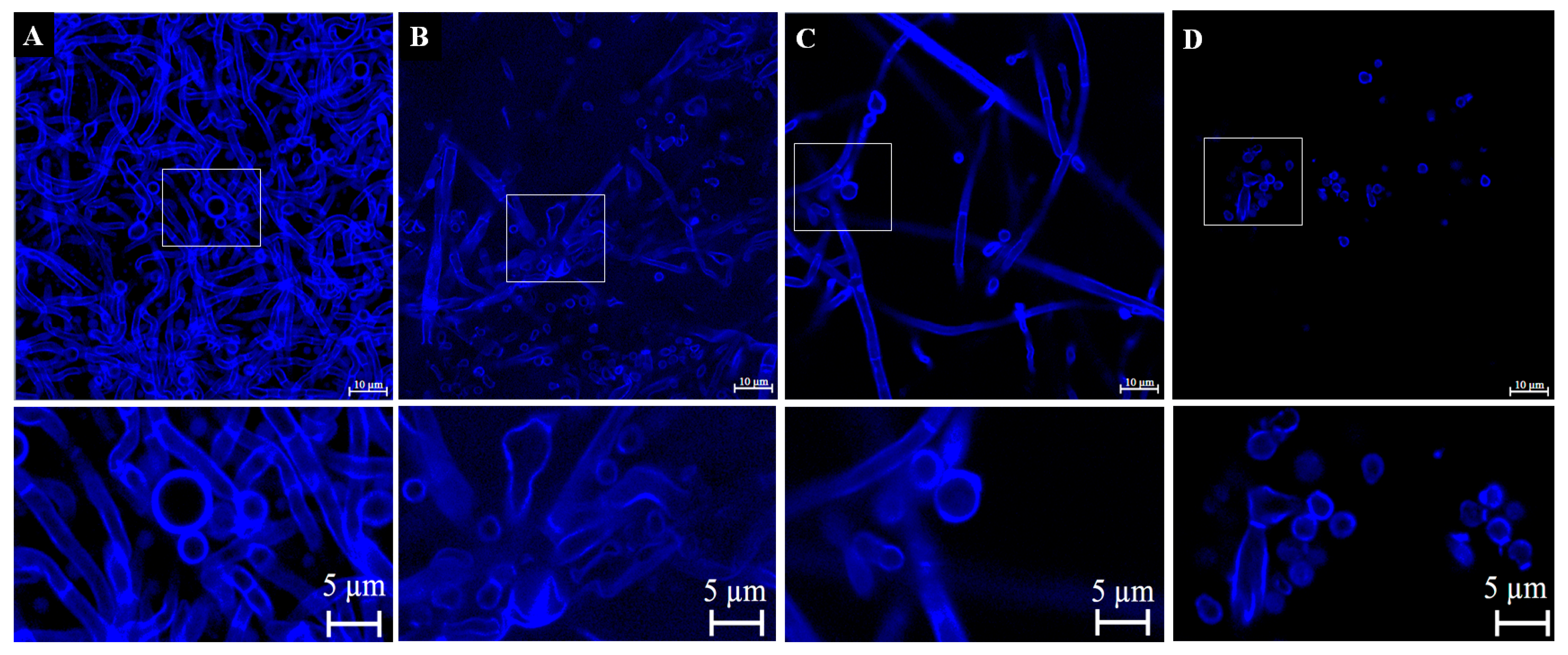
3.4.3. Verification of Cell Wall Morphology and Damage by Calcofluor White Staining and Confocal Microscopy
3.4.4. ROS Quantification
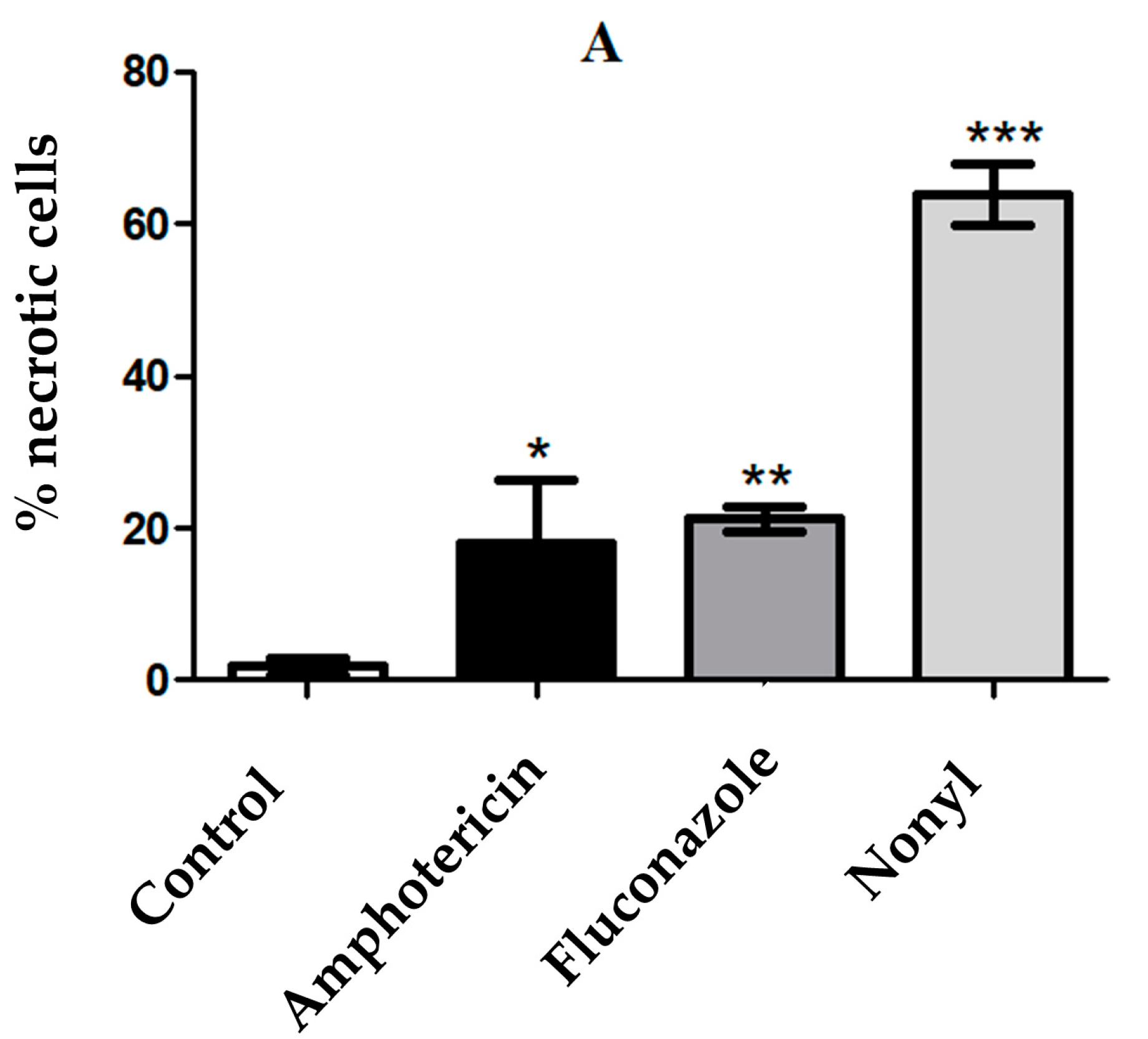
3.4.5. Determination of the Mechanisms of Cell Death by Apoptosis/Necrosis
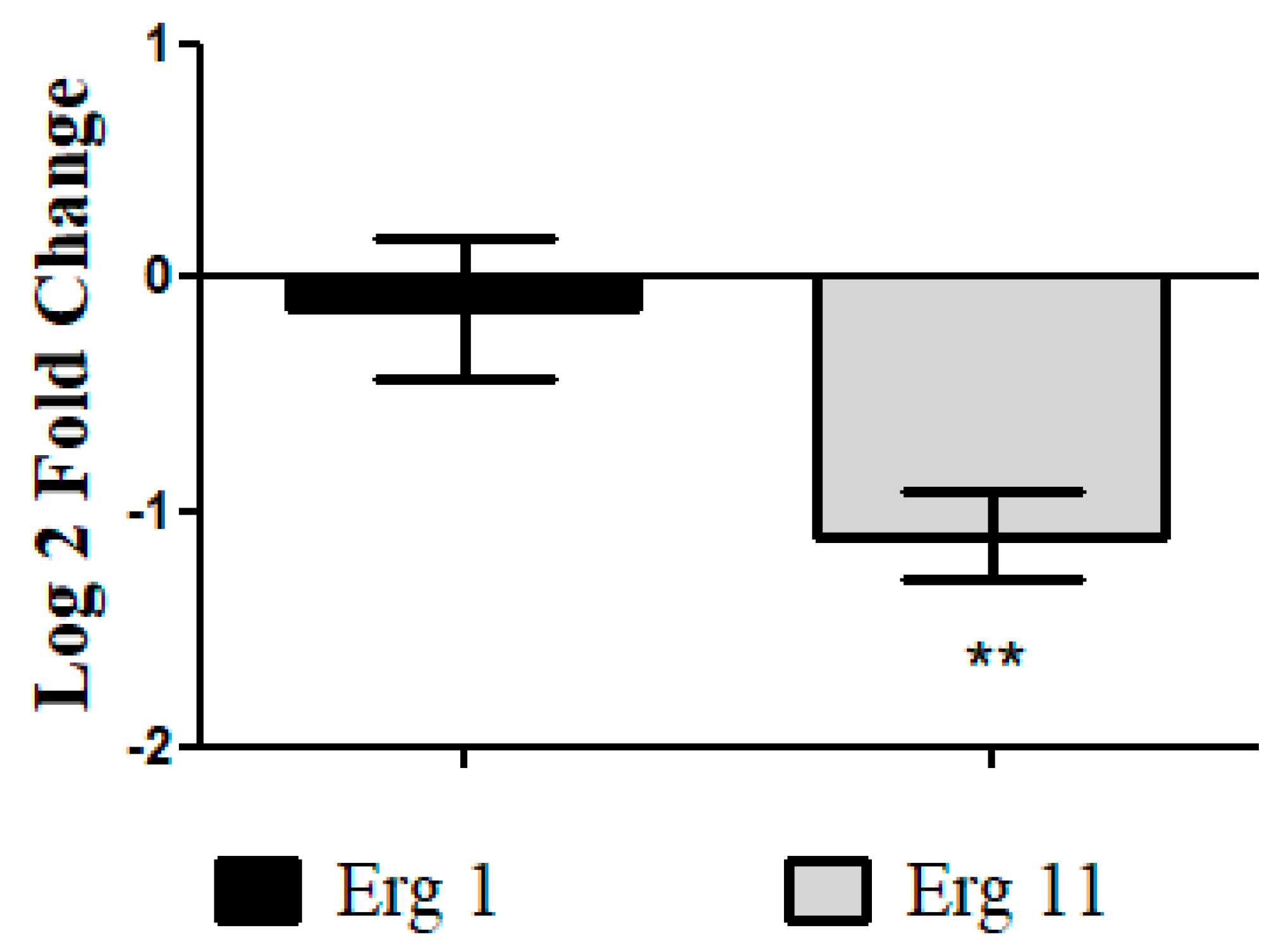
3.4.6. Analysis of Differential Gene Expression of T. rubrum ATCC MYA-4438 Treated with Nonyl Protocatechuate by Real-Time PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51 (Suppl. 4), 2–15. [Google Scholar] [CrossRef]
- Zhan, P.; Liu, W. The Changing Face of Dermatophytic Infections Worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Magalhães, G.M.; Oliveira, M.B.; Taylor, E.L.; Marques, C.R.; de Resende-Stoianoff, M.A. Prevalence of dermatomycosis in a Brazilian tertiary care hospital. Mycopathologia 2012, 174, 489–497. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Martinez, L.R.; Bila, N.M.; Friedman, J.M.; Friedman, A.J.; Mendes-Giannini, M.J.S.; Nosanchuk, J.D. Nitric Oxide-Releasing Nanoparticles Are Similar to Efinaconazole in Their Capacity to Eradicate Trichophyton rubrum Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 684150. [Google Scholar] [CrossRef]
- Gupta, A.K.; Taborda, V.B.A.; Taborda, P.R.O.; Shemer, A.; Summerbell, R.C.; Nakrieko, K.A. High prevalence of mixed infections in global onychomycosis. PLoS ONE 2020, 15, e0239648. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1972–1990. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, C.B.; Serafim-Pinto, A.; da Silva, P.B.; Bila, N.M.; Bonatti, J.L.d.C.; Scorzoni, L.; Singulani, J.d.L.; Santos, C.T.d.; Nazaré, A.C.; Chorilli, M.; et al. Incorporation of Nonyl 3,4-Dihydroxybenzoate Into Nanostructured Lipid Systems: Effective Alternative for Maintaining Anti-Dermatophytic and Antibiofilm Activities and Reducing Toxicity at High Concentrations. Front. Microbiol. 2020, 11, 1154. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Cooper, E.A. Dermatophytomas: Clinical Overview and Treatment. J. Fungi 2022, 8, 742. [Google Scholar] [CrossRef]
- Gupta, A.K.; Foley, K.A. Evidence for biofilms in onychomycosis. G. Ital. Dermatol. Venereol. 2019, 154, 50–55. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Sardi, J.C.; Santos, C.T.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. In vitro characterization of Trichophyton rubrum and T. mentagrophytes biofilms. Biofouling 2014, 30, 719–727. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cooper, E.A. Update in antifungal therapy of dermatophytosis. Mycopathologia 2008, 166, 353–367. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Peres, N.T.; Rossi, A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia 2008, 166, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Foley, K.A.; Versteeg, S.G. New Antifungal Agents and New Formulations Against Dermatophytes. Mycopathologia 2017, 182, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Paquet, M.; Simpson, F.C. Therapies for the treatment of onychomycosis. Clin. Dermatol. 2013, 31, 544–554. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mays, R.R.; Versteeg, S.G.; Shear, N.H.; Piguet, V. Update on current approaches to diagnosis and treatment of onychomycosis. Expert Rev. Anti-Infect. Ther. 2018, 16, 929–938. [Google Scholar] [CrossRef]
- Falotico, J.M.; Lipner, S.R. Updated Perspectives on the Diagnosis and Management of Onychomycosis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1933–1957. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Stec, N. Recent advances in therapies for onychomycosis and its management. F1000Research 2019, 8, 968. [Google Scholar] [CrossRef]
- Feng, X.; Xie, J.; Zhuang, K.; Ran, Y. Efficacy and tolerability of luliconazole cream 1% for dermatophytoses: A meta-analysis. J. Dermatol. 2014, 41, 779–782. [Google Scholar] [CrossRef]
- Zane, L.T.; Plattner, J.; Chanda, S.; Coronado, D.; Merchant, T.; Alley, M.R.; Gupta, A.K. Tavaborole topical solution, 5% for the treatment of toenail onychomycosis. Drugs Today 2015, 51, 599–607. [Google Scholar] [CrossRef]
- Bila, N.M.; Costa-Orlandi, C.B.; Vaso, C.O.; Bonatti, J.L.C.; de Assis, L.R.; Regasini, L.O.; Fontana, C.R.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. 2-Hydroxychalcone as a Potent Compound and Photosensitizer Against Dermatophyte Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 679470. [Google Scholar] [CrossRef]
- Zeichner, J.A. New Topical Therapeutic Options in the Management of Superficial Fungal Infections. J. Drugs Dermatol. 2015, 14, s35–s41. [Google Scholar]
- Gupta, A.K.; Daigle, D.; Carviel, J.L. The role of biofilms in onychomycosis. J. Am. Acad. Dermatol. 2016, 74, 1241–1246. [Google Scholar] [CrossRef]
- Ali, F.A.; Bakir, S.H.; Haji, S.H.; Hussen, B.M. Evaluation of blaGES-5 and bla veb-1 genes with multidrug-resistant extend, pandrug resistance patterns (MDR, XDR, PDR), and biofilm formation in Pseudomonas aeruginosa isolates. Cell. Mol. Biol. 2021, 67, 52–60. [Google Scholar] [CrossRef]
- Suwal, N.; Subba, R.K.; Paudyal, P.; Khanal, D.P.; Panthi, M.; Nassan, M.A.; Alqarni, M.; Batiha, G.E.; Koirala, N. Antimicrobial and antibiofilm potential of Curcuma longa Linn. Rhizome extract against biofilm producing Staphylococcus aureus and Pseudomonas aeruginosa isolates. Cell. Mol. Biol. 2021, 67, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, M.; Sun, M. Inhibition characteristics of biofilm structure of Staphylococcus aureus. Cell. Mol. Biol. 2020, 66, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, K.I.; Najmuldeen, H.H.R.; Rachid, S.K. Physiological regulation for enhancing biosynthesis of biofilm-inhibiting secondary metabolites in Streptomyces cellulosae. Cell. Mol. Biol. 2022, 68, 33–46. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Bila, N.M.; Vaso, C.O.; da Silva Pires, A.C.M.; de Matos Silva, S.; Medina Alarcón, K.P.; Marcos, C.M.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Chapter 31—Polymicrobial biofilms: Impact on fungal pathogenesis. In Understanding Microbial Biofilms; Das, S., Kungwani, N.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 521–567. [Google Scholar]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal biofilm resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef] [PubMed]
- Costa-Orlandi, B.C.; Sardi, C.J.; Pitangui, S.N.; de Oliveira, C.H.; Scorzoni, L.; Galeane, C.M.; Medina-Alarcón, P.K.; Melo, C.W.; Marcelino, Y.M.; Braz, D.J.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.; Gullo, F.; Sardi, J.; Pitangui, N.; Costa-Orlandi, C.; Sangalli-Leite, F.; Scorzoni, L.; Regasini, L.; Petônio, M.; Souza, F.; et al. Anti-Trichophyton Activity of Protocatechuates and Their Synergism with Fluconazole. Evid.-Based Complement. Altern. Med. 2014, 2014, 957860. [Google Scholar] [CrossRef]
- Nihei, K.; Nihei, A.; Kubo, I. Rational design of antimicrobial agents: Antifungal activity of alk(en)yl dihydroxybenzoates and dihydroxyphenyl alkanoates. Bioorg. Med. Chem. Lett. 2003, 13, 3993–3996. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.; Nihei, K. Molecular design of multifunctional antibacterial agents against methicillin resistant Staphylococcus aureus (MRSA). Bioorg. Med. Chem. 2003, 11, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Hsu, C.C.; Yin, M.C. In vitro anti-Helicobacter pylori activity of diallyl sulphides and protocatechuic acid. Phytother. Res. 2008, 22, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Chao, C.Y. Anti-Campylobacter, anti-aerobic, and anti-oxidative effects of roselle calyx extract and protocatechuic acid in ground beef. Int. J. Food Microbiol. 2008, 127, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Medina-Alarcon, K.P.; Singulani, J.L.; Voltan, A.R.; Sardi, J.C.O.; Petronio, M.S.; Santos, M.B.; Polaquini, C.R.; Regasini, L.O.; Bolzani, V.S.; da Silva, D.H.S.; et al. Alkyl Protocatechuate-Loaded Nanostructured Lipid Systems as a Treatment Strategy for Paracoccidioides brasiliensis and Paracoccidioides lutzii In Vitro. Front. Microbiol. 2017, 8, 1048. [Google Scholar] [CrossRef] [PubMed]
- CLSI Document M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Aproved Standard—Second Edition. CLSI: Wayne, PA, USA, 2008.
- de Faria, C.M.; Nazaré, A.C.; Petrônio, M.S.; Paracatu, L.C.; Zeraik, M.L.; Regasini, L.O.; Silva, D.H.; da Fonseca, L.M.; Ximenes, V.F. Protocatechuic acid alkyl esters: Hydrophobicity as a determinant factor for inhibition of NADPH oxidase. Curr. Med. Chem. 2012, 19, 4885–4893. [Google Scholar] [CrossRef]
- Garcia, L.M.; Costa-Orlandi, C.B.; Bila, N.M.; Vaso, C.O.; Gonçalves, L.N.C.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. A Two-Way Road: Antagonistic Interaction Between Dual-Species Biofilms Formed by Candida albicans/Candida parapsilosis and Trichophyton rubrum. Front. Microbiol. 2020, 11, 1980. [Google Scholar] [CrossRef]
- Vaso, C.O.; Bila, N.M.; Pandolfi, F.; De Vita, D.; Bortolami, M.; Bonatti, J.L.C.; De Moraes Silva, R.A.; Gonçalves, L.N.C.; Tudino, V.; Costi, R.; et al. Evaluation of the Anti-Histoplasma capsulatum Activity of Indole and Nitrofuran Derivatives and Their Pharmacological Safety in Three-Dimensional Cell Cultures. Pharmaceutics 2022, 14, 1043. [Google Scholar] [CrossRef]
- Martinez, L.R.; Casadevall, A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 2006, 50, 1021–1033. [Google Scholar] [CrossRef]
- Ghannoum, M.; Isham, N.; Henry, W.; Kroon, H.A.; Yurdakul, S. Evaluation of the morphological effects of TDT 067 (terbinafine in Transfersome) and conventional terbinafine on dermatophyte hyphae in vitro and in vivo. Antimicrob. Agents Chemother. 2012, 56, 2530–2534. [Google Scholar] [CrossRef]
- Arthington-Skaggs, B.A.; Jradi, H.; Desai, T.; Morrison, C.J. Quantitation of ergosterol content: Novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 1999, 37, 3332–3337. [Google Scholar] [CrossRef]
- Ram, A.F.; Klis, F.M. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, L.M.; Krausz, A.E.; Souza, A.C.; Adler, B.L.; Landriscina, A.; Musaev, T.; Nosanchuk, J.D.; Friedman, A.J. Trichophyton rubrum is inhibited by free and nanoparticle encapsulated curcumin by induction of nitrosative stress after photodynamic activation. PLoS ONE 2015, 10, e0120179. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Román, E.; Sánchez-Fresneda, R.; Casas, C.; Herrero, E.; Argüelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.R.; Peres, N.T.; Persinoti, G.F.; Silva, L.G.; Mazucato, M.; Rossi, A.; Martinez-Rossi, N.M. rpb2 is a reliable reference gene for quantitative gene expression analysis in the dermatophyte Trichophyton rubrum. Med. Mycol. 2012, 50, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Komoto, T.T.; Bitencourt, T.A.; Silva, G.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Gene Expression Response of Trichophyton rubrum during Coculture on Keratinocytes Exposed to Antifungal Agents. Evid.-Based Complement. Altern. Med. 2015, 2015, 180535. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, W.; Wang, L.; Yang, J.; Liu, T.; Peng, J.; Leng, W.; Chen, L.; Li, R.; Jin, Q. Transcriptional profiles of the response to ketoconazole and amphotericin B in Trichophyton rubrum. Antimicrob. Agents Chemother. 2007, 51, 144–153. [Google Scholar] [CrossRef]
- Martins, M.P.; Silva, L.G.; Rossi, A.; Sanches, P.R.; Souza, L.D.R.; Martinez-Rossi, N.M. Global Analysis of Cell Wall Genes Revealed Putative Virulence Factors in the Dermatophyte Trichophyton rubrum. Front. Microbiol. 2019, 10, 2168. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Harrington, B.J.; Hageage, G.J., Jr. Calcofluor White: A Review of its Uses and Applications in Clinical Mycology and Parasitology. Lab. Med. 2003, 34, 361–367. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Tragiannidis, A.; Munchen, S.; Groll, A.H. Clinical hepatotoxicity associated with antifungal agents. Expert Opin. Drug Saf. 2017, 16, 149–165. [Google Scholar] [CrossRef]
- Lam, P.L.; Wong, M.M.; Hung, L.K.; Yung, L.H.; Tang, J.C.O.; Lam, K.H.; Chung, P.Y.; Wong, W.Y.; Ho, Y.W.; Wong, R.S.M.; et al. Miconazole and terbinafine induced reactive oxygen species accumulation and topical toxicity in human keratinocytes. Drug Chem. Toxicol. 2022, 45, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, J.W.; Lipner, S.R. Safety of current therapies for onychomycosis. Expert Opin. Drug Saf. 2020, 19, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Correia, E.E.M.; Guedes, G.M.M.; de Oliveira, J.S.; Castelo-Branco, D.; Cordeiro, R.A.; Pinheiro, A.Q.; Chaves, L.J.Q.; Pereira Neto, W.A.; Sidrim, J.J.C.; et al. In vitro activity of azole derivatives and griseofulvin against planktonic and biofilm growth of clinical isolates of dermatophytes. Mycoses 2018, 61, 449–454. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Dias-Souza, M.V. Effectiveness of five antidandruff cosmetic formulations against planktonic cells and biofilms of dermatophytes. Saudi J. Biol. Sci. 2017, 24, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.d.O. A review of recent research on antifungal agents against dermatophyte biofilms. Med. Mycol. 2021, 59, 313–326. [Google Scholar] [CrossRef]
- Coelho, L.M.; Aquino-Ferreira, R.; Maffei, C.M.; Martinez-Rossi, N.M. In vitro antifungal drug susceptibilities of dermatophytes microconidia and arthroconidia. J. Antimicrob. Chemother. 2008, 62, 758–761. [Google Scholar] [CrossRef]
- Hashimoto, T.; Blumenthal, H.J. Survival and resistance of Trichophyton mentagrophytes arthrospores. Appl. Environ. Microbiol. 1978, 35, 274–277. [Google Scholar] [CrossRef]
- Merkl, R.; HRádkoVá, I.; FIlIp, V.; ŠMIdRkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar] [CrossRef]
- Wolf, J.M.; Espadas, J.; Luque-Garcia, J.; Reynolds, T.; Casadevall, A. Lipid Biosynthetic Genes Affect Candida albicans Extracellular Vesicle Morphology, Cargo, and Immunostimulatory Properties. Eukaryot. Cell 2015, 14, 745–754. [Google Scholar] [CrossRef]
- Vargas, G.; Rocha, J.D.; Oliveira, D.L.; Albuquerque, P.C.; Frases, S.; Santos, S.S.; Nosanchuk, J.D.; Gomes, A.M.; Medeiros, L.C.; Miranda, K.; et al. Compositional and immunobiological analyses of extracellular vesicles released by Candida albicans. Cell. Microbiol. 2015, 17, 389–407. [Google Scholar] [CrossRef]
- Wolf, J.M.; Espadas-Moreno, J.; Luque-Garcia, J.L.; Casadevall, A. Interaction of Cryptococcus neoformans extracellular vesicles with the cell wall. Eukaryot. Cell 2014, 13, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.C.; Nakayasu, E.S.; Rodrigues, M.L.; Frases, S.; Casadevall, A.; Zancope-Oliveira, R.M.; Almeida, I.C.; Nosanchuk, J.D. Vesicular transport in Histoplasma capsulatum: An effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell. Microbiol. 2008, 10, 1695–1710. [Google Scholar] [CrossRef]
- Vallejo, M.C.; Matsuo, A.L.; Ganiko, L.; Medeiros, L.C.; Miranda, K.; Silva, L.S.; Freymüller-Haapalainen, E.; Sinigaglia-Coimbra, R.; Almeida, I.C.; Puccia, R. The pathogenic fungus Paracoccidioides brasiliensis exports extracellular vesicles containing highly immunogenic α-Galactosyl epitopes. Eukaryot. Cell 2011, 10, 343–351. [Google Scholar] [CrossRef]
- Gehrmann, U.; Qazi, K.R.; Johansson, C.; Hultenby, K.; Karlsson, M.; Lundeberg, L.; Gabrielsson, S.; Scheynius, A. Nanovesicles from Malassezia sympodialis and host exosomes induce cytokine responses--novel mechanisms for host-microbe interactions in atopic eczema. PLoS ONE 2011, 6, e21480. [Google Scholar] [CrossRef]
- Bitencourt, T.A.; Rezende, C.P.; Quaresemin, N.R.; Moreno, P.; Hatanaka, O.; Rossi, A.; Martinez-Rossi, N.M.; Almeida, F. Extracellular Vesicles From the Dermatophyte Trichophyton interdigitale Modulate Macrophage and Keratinocyte Functions. Front. Immunol. 2018, 9, 2343. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the wall: Extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Hasumi, Y.; Ueda, K.; Uchida, K.; Yamaguchi, H. Effects of micafungin on the morphology of Aspergillus fumigatus. J. Electron. Microsc. 2005, 54, 67–77. [Google Scholar] [CrossRef]
- Czub, J.; Baginski, M. Modulation of amphotericin B membrane interaction by cholesterol and ergosterol a molecular dynamics study. J. Phys. Chem. 2006, 110, 16743–16753. [Google Scholar] [CrossRef]
- Croxtall, J.D.; Plosker, G.L. Sertaconazole: A review of its use in the management of superficial mycoses in dermatology and gynaecology. Drugs 2009, 69, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, J.; Li, X.; Xiao, E.; Lange, J.D.; Rienstra, C.M.; Burke, M.D.; Mitchell, D.A. Sterol Sponge Mechanism Is Conserved for Glycosylated Polyene Macrolides. ACS Cent. Sci. 2021, 7, 781–791. [Google Scholar] [CrossRef]
- Lewandowska, A.; Soutar, C.P.; Greenwood, A.I.; Nimerovsky, E.; De Lio, A.M.; Holler, J.T.; Hisao, G.S.; Khandelwal, A.; Zhang, J.; SantaMaria, A.M.; et al. Fungicidal amphotericin B sponges are assemblies of staggered asymmetric homodimers encasing large void volumes. Nat. Struct. Mol. Biol. 2021, 28, 972–981. [Google Scholar] [CrossRef]
- Victoria, G.S.; Kumar, P.; Komath, S.S. The Candida albicans homologue of PIG-P, CaGpi19p: Gene dosage and role in growth and filamentation. Microbiology 2010, 156, 3041–3051. [Google Scholar] [CrossRef]
- Singulani, J.L.; Galeane, M.C.; Ramos, M.; Gomes, P.C.; Santos, C.T.D.; Souza, B.M.d.; Palma, M.; Almeida, A.M.F.; Giannini, M.J.M.M. Antifungal Activity, Toxicity, and Membranolytic Action of a Mastoparan Analog Peptide. Front. Cell. Infect. Microbiol. 2019, 9, 419. [Google Scholar] [CrossRef]
- Sokol-Anderson, M.L.; Brajtburg, J.; Medoff, G. Amphotericin B-Induced Oxidative Damage and Killing of Candida albicans. J. Infect. Dis. 1986, 154, 76–83. [Google Scholar] [CrossRef]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Soares Mendes Gianinni, M.J.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef]
- Vandenbosch, D.; Braeckmans, K.; Nelis, H.J.; Coenye, T. Fungicidal activity of miconazole against Candida spp. biofilms. J. Antimicrob. Chemother. 2010, 65, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Sharon, A.; Finkelstein, A.; Shlezinger, N.; Hatam, I. Fungal apoptosis: Function, genes and gene function. FEMS Microbiol. Rev. 2009, 33, 833–854. [Google Scholar] [CrossRef] [PubMed]
- Proskuryakov, S.Y.; Gabai, V.L. Mechanisms of tumor cell necrosis. Curr. Pharm. Des. 2010, 16, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Tower, J. Programmed cell death in aging. Ageing Res. Rev. 2015, 23, 90–100. [Google Scholar] [CrossRef] [PubMed]



| Gene | 5′-3′ Oligonucleotides | Efficiency |
|---|---|---|
| Gapdh | F: GCGTGACCCAGCCAACA R: CGGTGGACTCGACGATGTAGT | 90.7 |
| Rpb2—DNA-dependent RNA polymerase II | F: TACAGGAAATTGGGGTGAGC R: GTTCGTCGAAGATGGGAAAG | 104.1 |
| ERG1 | F: GTGAAGATACCTTTCCCTAGCG R: TTATGGTAGAAACGGCCTTGG | 98.41 |
| ERG 11 | F: CACTTCCTTGCCCTGTAGAGATC R: GGAGTTTTCAATGTCAGCAAGGTTT | 98.85 |
| Compound/Drug | Phenotype | TM 66 | TM ATCC 11489 | TR ATCC 28189 | TR 143 |
|---|---|---|---|---|---|
| Fluconazole | Planktonic (MIC50) | 16 | 2 | 128 | 32 |
| Biofilm (SMIC50) | >512 | >512 | >512 | >512 | |
| Griseofulvin | Planktonic (MIC50) | 1 | 1 | 4 | 256 |
| Biofilm (SMIC50) | >512 | >512 | >512 | >512 | |
| Terbinafine | Planktonic (MIC50) | 0.06 | 0.015 | 0.125 | 0.125 |
| Biofilm (SMIC50) | >32 | 16 | >32 | 0.5 | |
| Nonyl 3, 4-dihydroxybenzoate | Planktonic (MIC50) | 15.6 | 7.8 | 15.6 | 15.6 |
| Biofilm (SMIC50) | 250 | 125 | 62.5 | 62.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-Orlandi, C.B.; Bila, N.M.; Bonatti, J.L.C.; Vaso, C.O.; Santos, M.B.; Polaquini, C.R.; Santoni Biasioli, M.M.; Herculano, R.D.; Regasini, L.O.; Fusco-Almeida, A.M.; et al. Membranolytic Activity Profile of Nonyl 3,4-Dihydroxybenzoate: A New Anti-Biofilm Compound for the Treatment of Dermatophytosis. Pharmaceutics 2023, 15, 1402. https://doi.org/10.3390/pharmaceutics15051402
Costa-Orlandi CB, Bila NM, Bonatti JLC, Vaso CO, Santos MB, Polaquini CR, Santoni Biasioli MM, Herculano RD, Regasini LO, Fusco-Almeida AM, et al. Membranolytic Activity Profile of Nonyl 3,4-Dihydroxybenzoate: A New Anti-Biofilm Compound for the Treatment of Dermatophytosis. Pharmaceutics. 2023; 15(5):1402. https://doi.org/10.3390/pharmaceutics15051402
Chicago/Turabian StyleCosta-Orlandi, Caroline B., Níura M. Bila, Jean Lucas C. Bonatti, Carolina O. Vaso, Mariana B. Santos, Carlos R. Polaquini, Mariana M. Santoni Biasioli, Rondinelli D. Herculano, Luis O. Regasini, Ana Marisa Fusco-Almeida, and et al. 2023. "Membranolytic Activity Profile of Nonyl 3,4-Dihydroxybenzoate: A New Anti-Biofilm Compound for the Treatment of Dermatophytosis" Pharmaceutics 15, no. 5: 1402. https://doi.org/10.3390/pharmaceutics15051402
APA StyleCosta-Orlandi, C. B., Bila, N. M., Bonatti, J. L. C., Vaso, C. O., Santos, M. B., Polaquini, C. R., Santoni Biasioli, M. M., Herculano, R. D., Regasini, L. O., Fusco-Almeida, A. M., & Mendes-Giannini, M. J. S. (2023). Membranolytic Activity Profile of Nonyl 3,4-Dihydroxybenzoate: A New Anti-Biofilm Compound for the Treatment of Dermatophytosis. Pharmaceutics, 15(5), 1402. https://doi.org/10.3390/pharmaceutics15051402







