A Novel Approach for the Treatment of Aerobic Vaginitis: Azithromycin Liposomes-in-Chitosan Hydrogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Chitosan-HG
2.3. Preparation and Characterization of AZM-Liposomes
2.4. Preparation of AZM-Liposomal Hydrogels
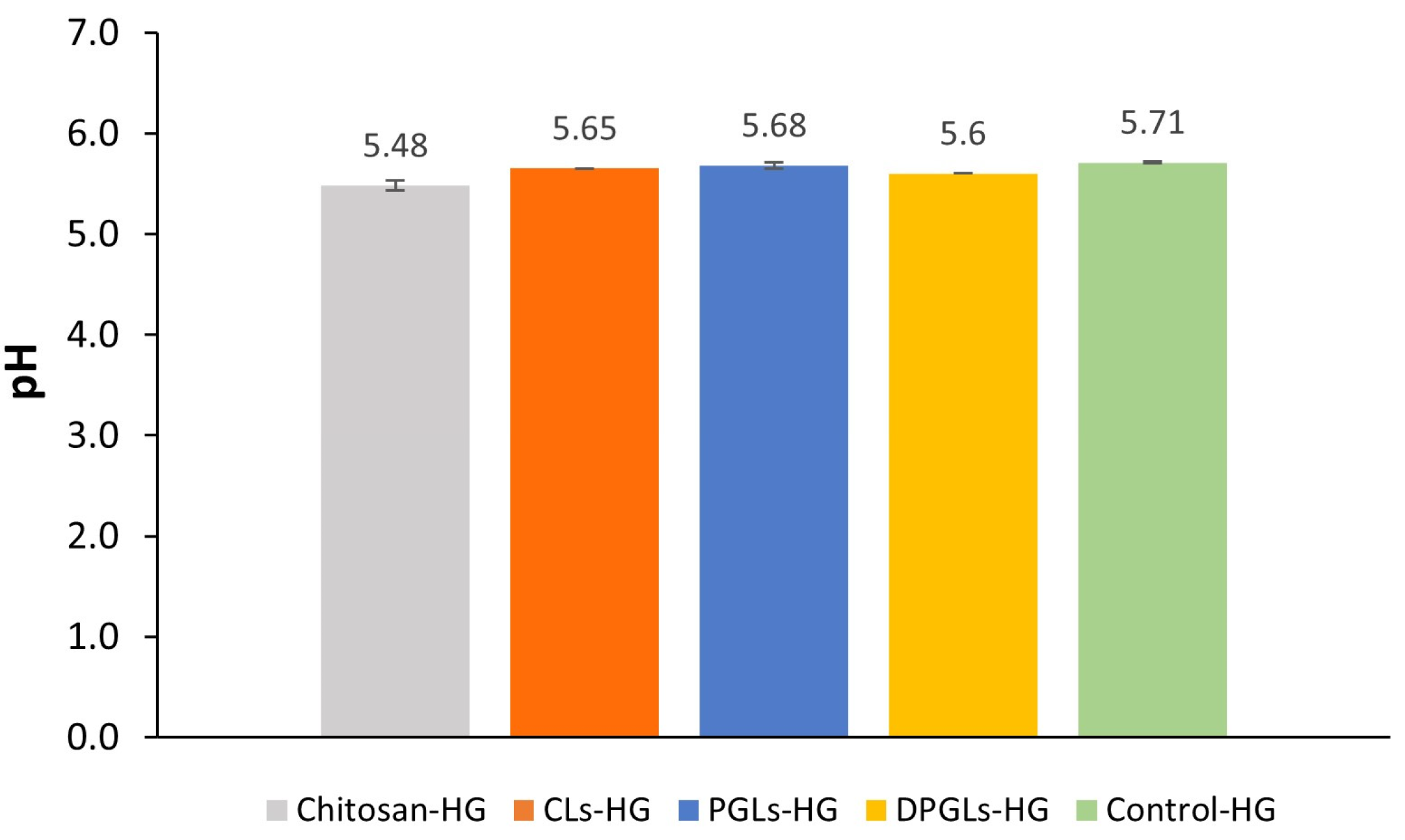
2.5. Measurements of the Hydrogels’ pH Values
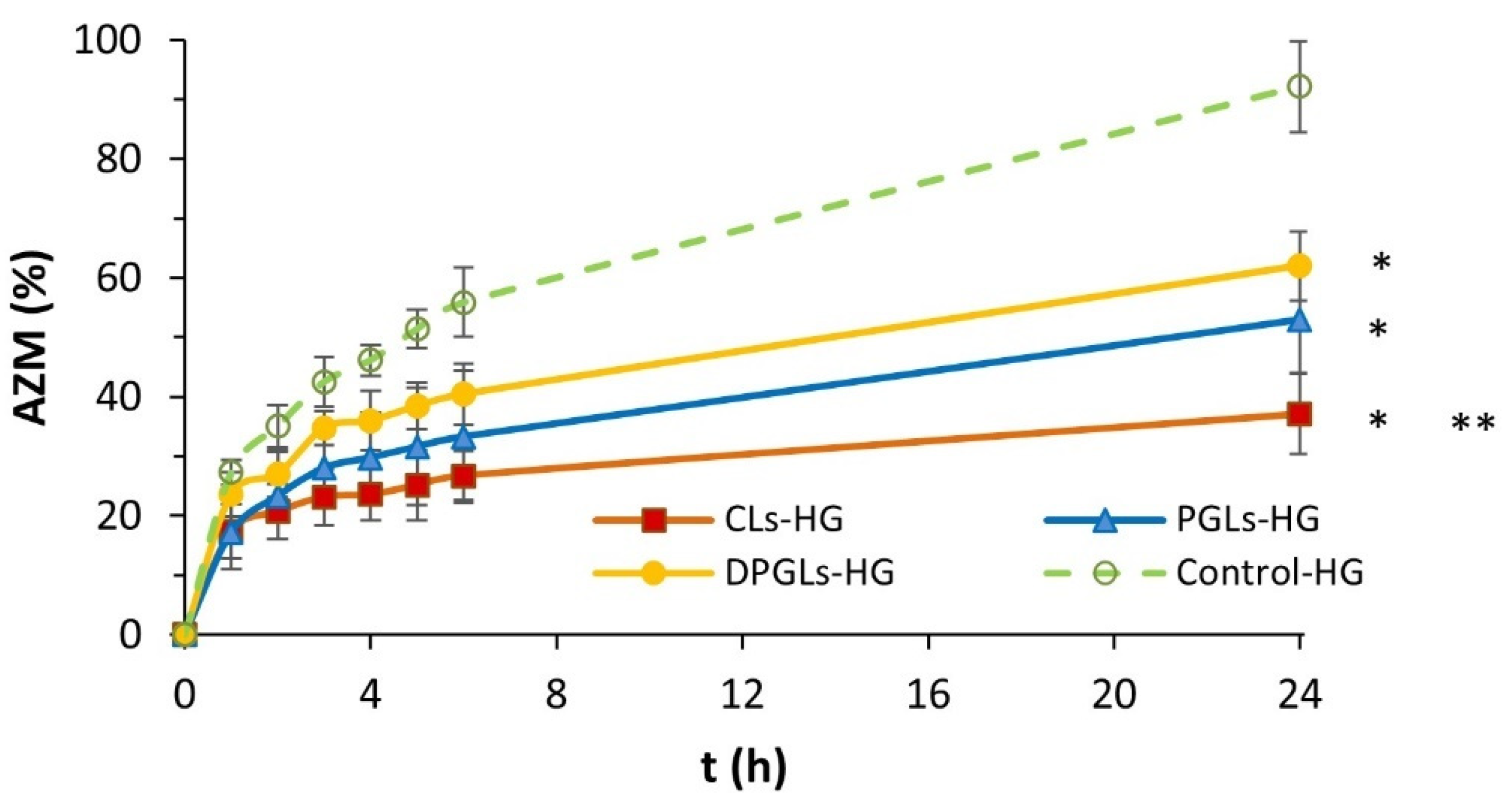
2.6. In Vitro Release Studies
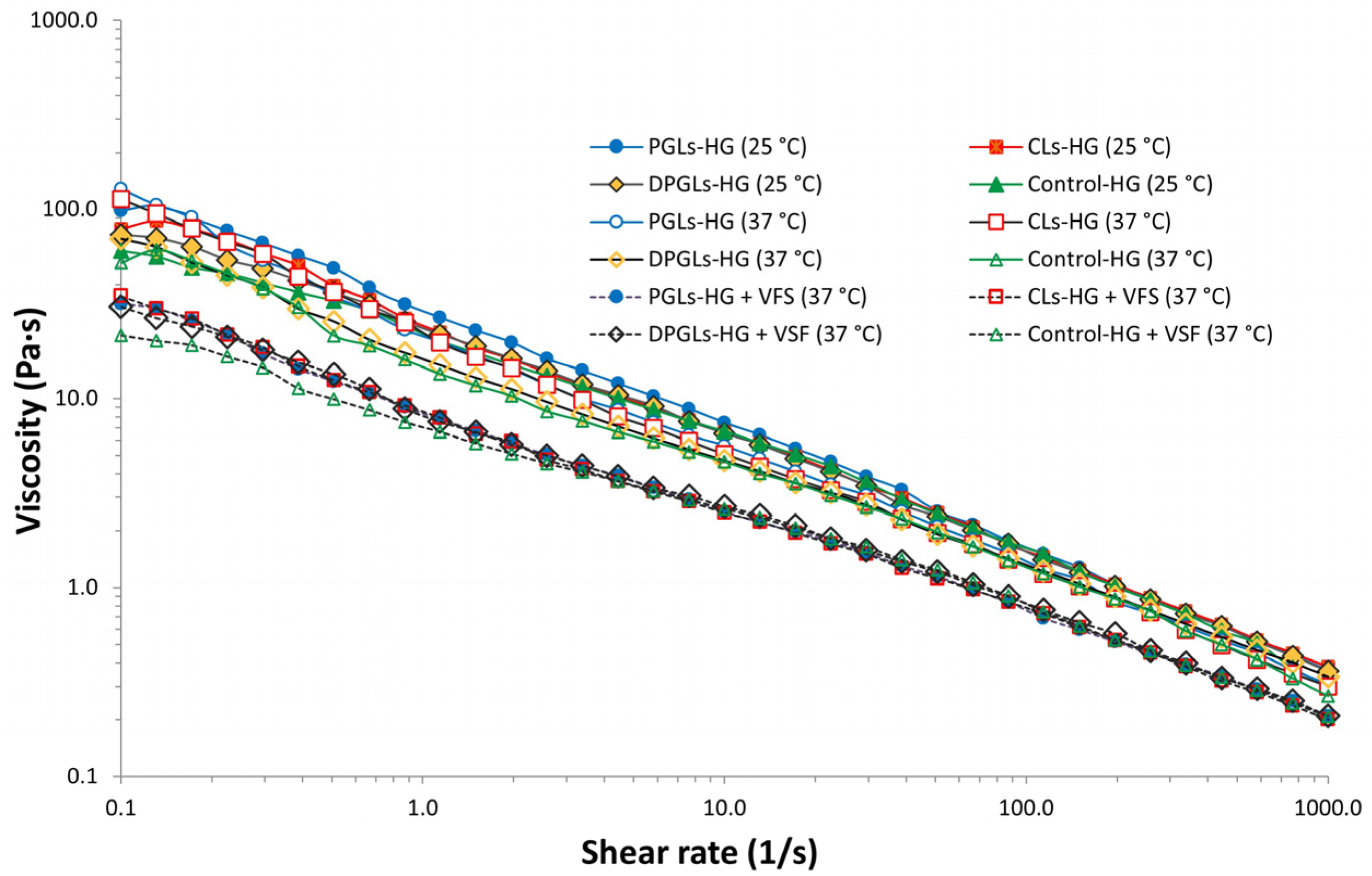
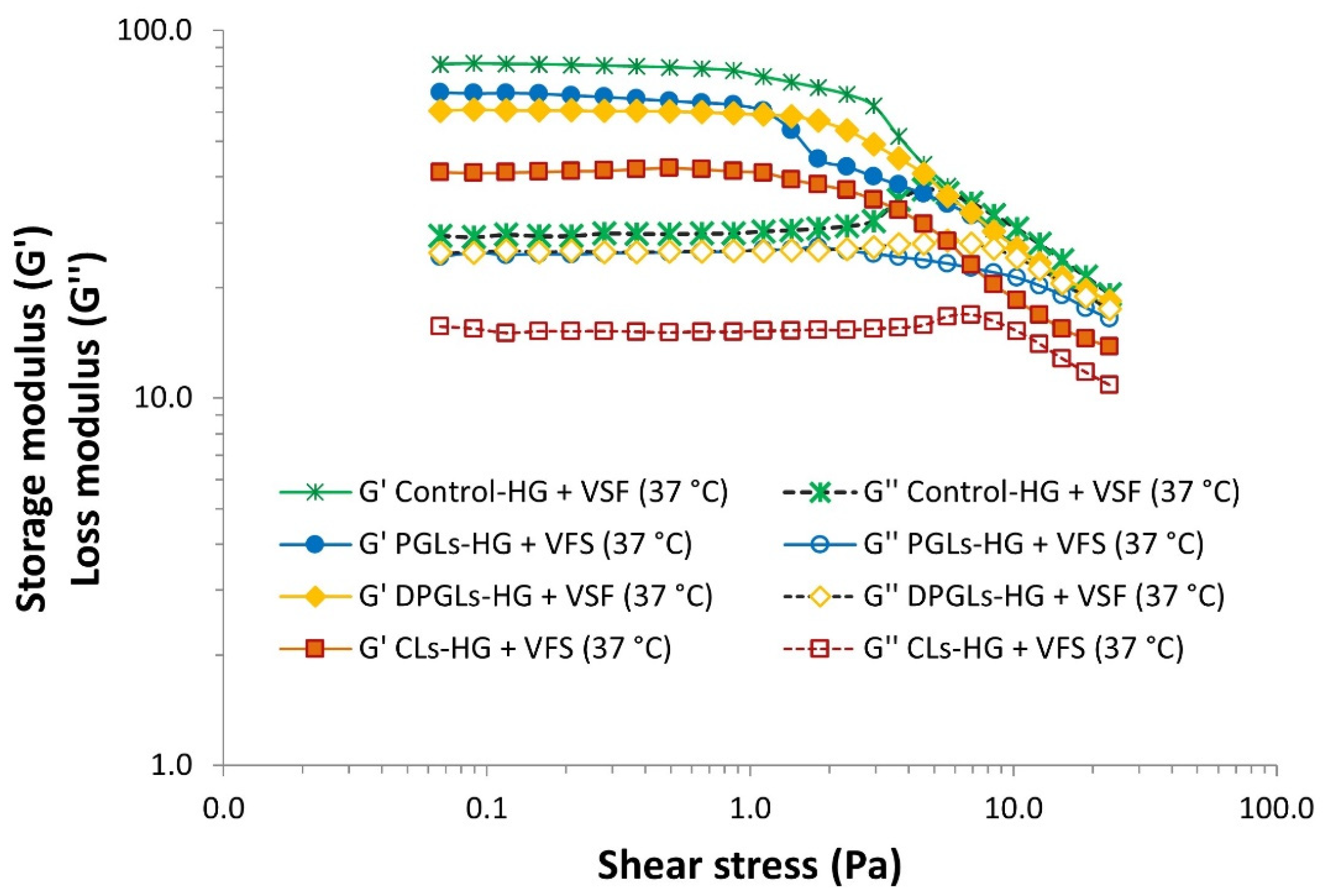
2.7. Rheological Assessment of the AZM-Liposomal Hydrogels
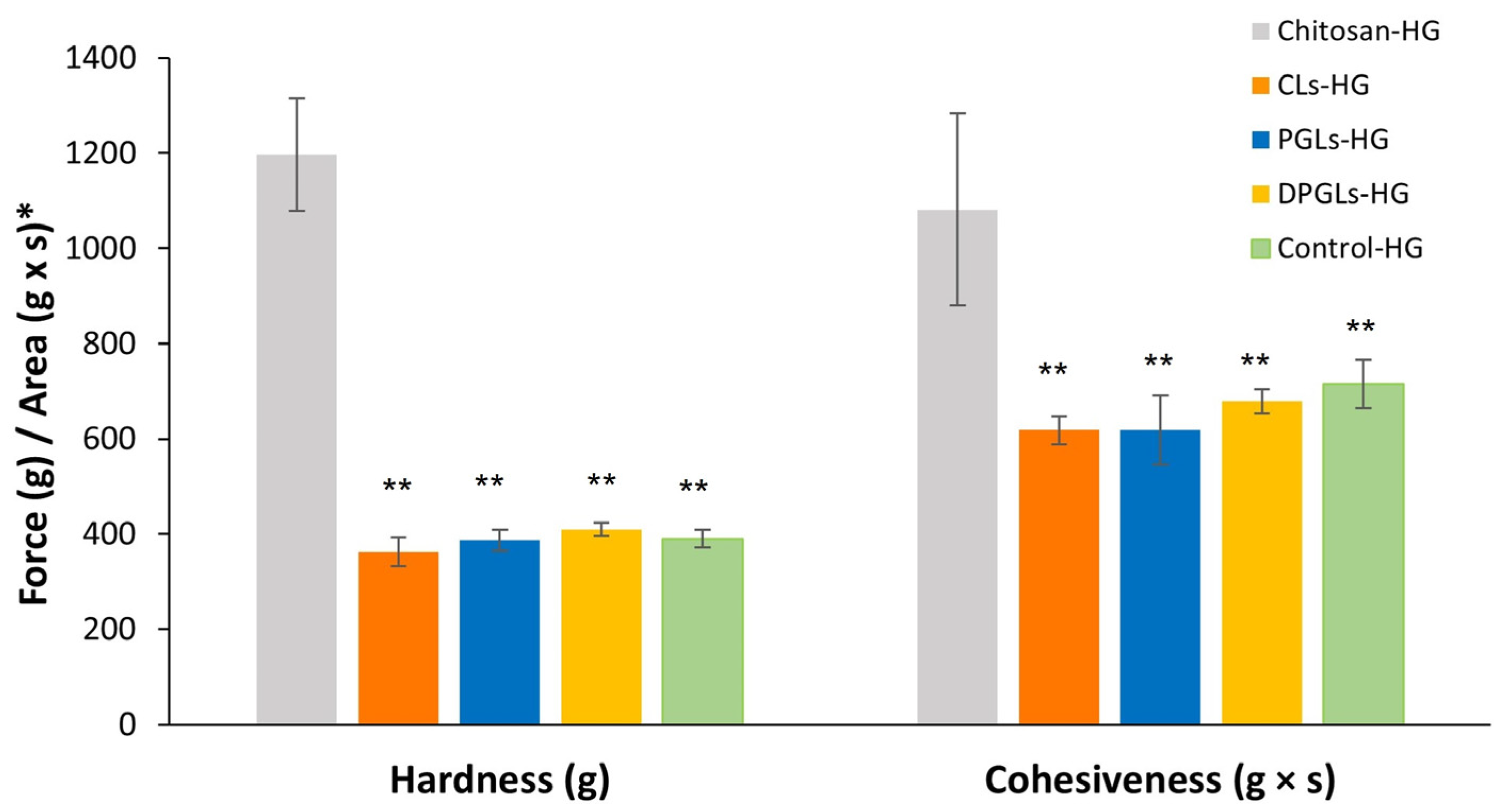
2.8. Texture Analysis of the AZM-Liposomal Hydrogels
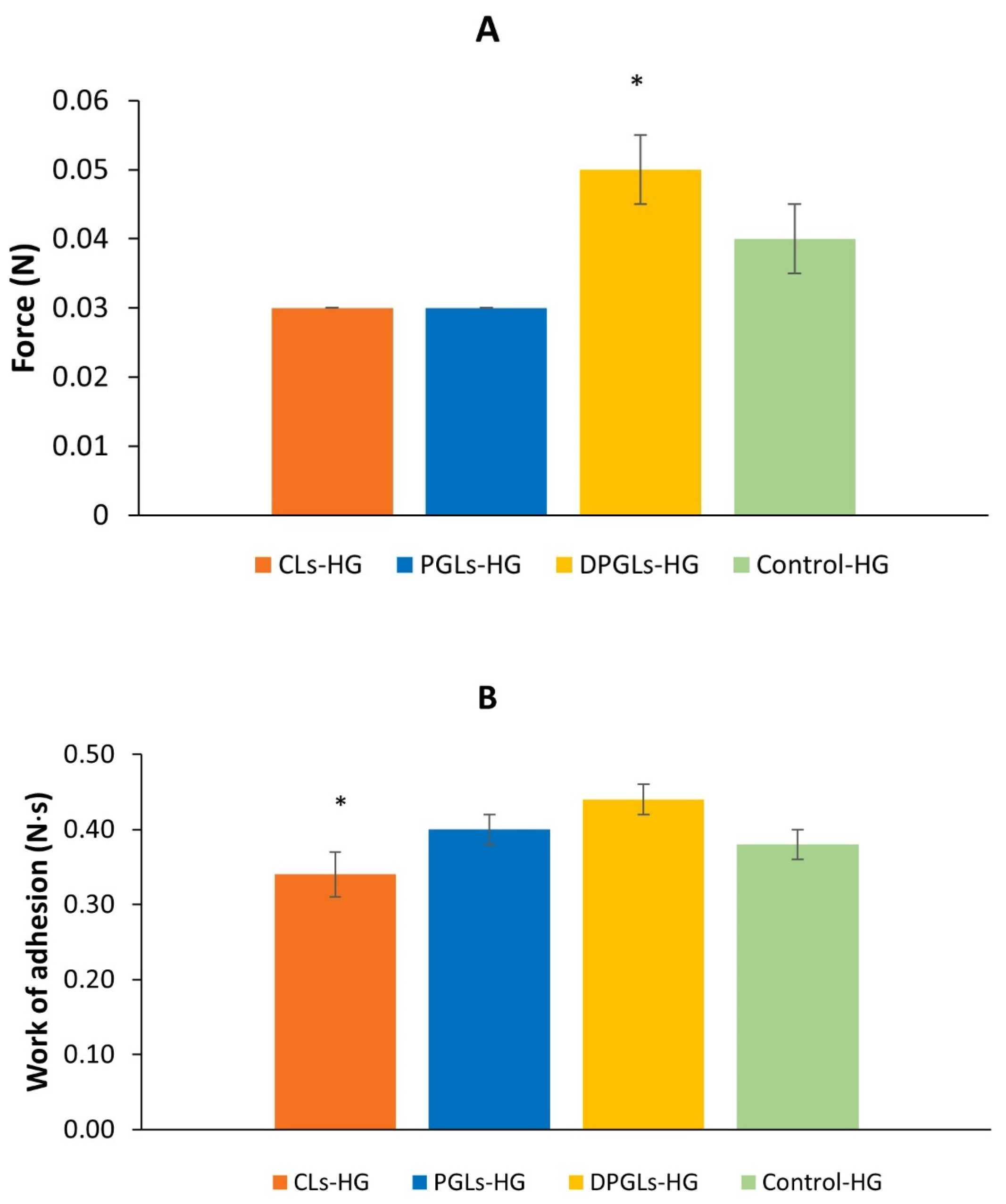
2.9. Mucoadhesion Test
2.10. Antimicrobial Evaluation of the Chitosan-HG
2.11. Antibacterial Activity of the AZM-Liposomal Hydrogels
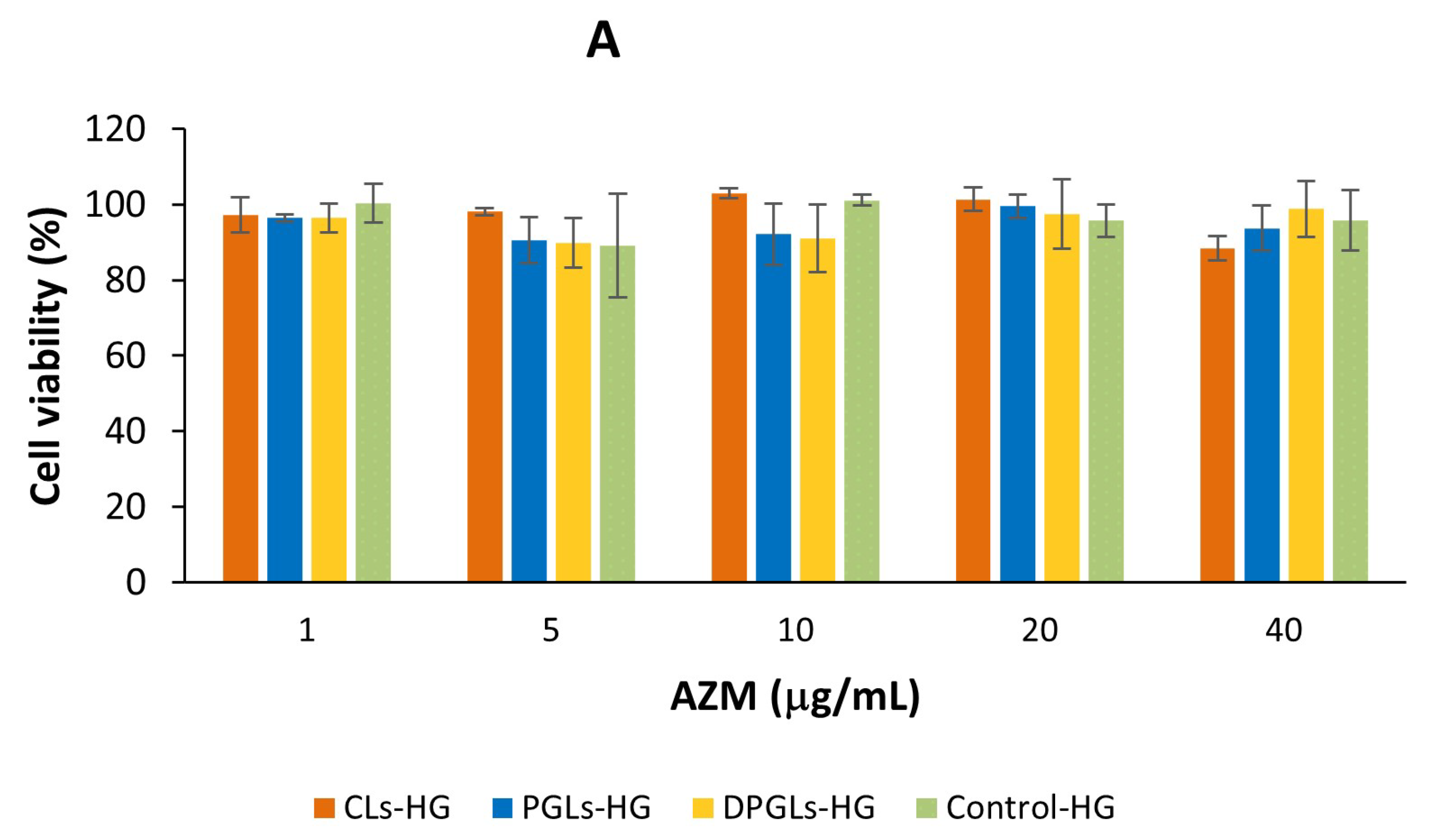
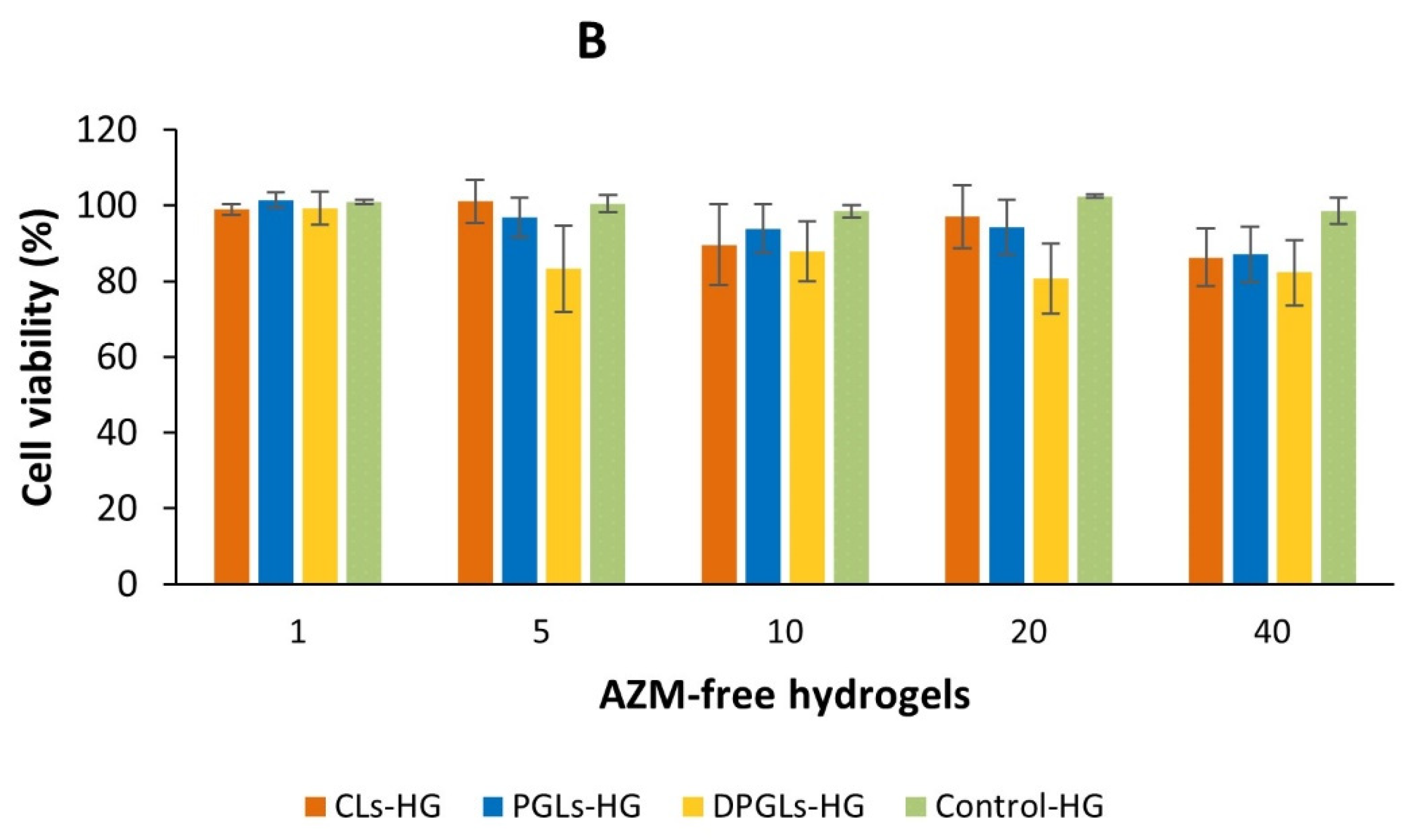
2.12. In Vitro Biocompatibility Assessment
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of AZM-Liposomal Hydrogels
3.2. Antimicrobial Potential of the Chitosan-HG
3.3. Antimicrobial Activity of the AZM-Liposomal Hydrogels against S. aureus
3.4. In Vitro Biocompatibility Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Donders, G.G.; Vereecken, A.; Bosmans, E.; Dekeersmaecker, A.; Salembier, G.; Spitz, B. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: Aerobic vaginitis. BJOG 2002, 109, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.G.; Bellen, G.; Grinceviciene, S.; Ruban, K.; Vieira-Baptista, P. Aerobic vaginitis: No longer a stranger. Res. Microbiol. 2017, 168, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.S. Vaginal microbiomes associated with aerobic vaginitis and bacterial vaginosis. Front. Public Health 2018, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.; Bellen, G.; Rezeberg, D. Aerobic vaginitis in pregnancy. BJOG 2011, 118, 1163–1170. [Google Scholar] [CrossRef]
- Han, C.; Wu, W.; Fan, A.; Wang, Y.; Zhang, H.; Chu, Z.; Wang, C.; Xue, F. Diagnostic and therapeutic advancements for aerobic vaginitis. Arch. Gynecol. Obstet. 2015, 291, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.J.; Winter, A.J. How to diagnose and treat aerobic and desquamative inflammatory vaginitis. Sex. Transm. Infect. 2017, 93, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Jøraholmen, M.W.; Škalko-Basnet, N. Nanomedicines for the topical treatment of vulvovaginal infections: Addressing the challenges of antimicrobial resistance. Adv. Drug Deliv. Rev. 2021, 178, 113855. [Google Scholar] [CrossRef] [PubMed]
- Caramella, C.M.; Rossi, S.; Ferrari, F.; Bonferoni, M.C.; Sandri, G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv. Drug Deliv. Rev. 2015, 92, 39–52. [Google Scholar] [CrossRef]
- Martínez-Pérez, B.; Quintanar-Guerrero, D.; Tapia-Tapia, M.; Cisneros-Tamayo, R.; Zambrano-Zaragoza, M.L.; Alcalá-Alcalá, S.; Mendoza-Muñoz, N.; Piñón-Segundo, E. Controlled-release biodegradable nanoparticles: From preparation to vaginal applications. Eur. J. Pharm. Sci. 2018, 115, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Hurler, J.; Ferderber, K.; Golja Gašparović, P.; Škalko-Basnet, N.; Filipović-Grčić, J. Novel vaginal drug delivery system: Deformable propylene glycol liposomes-in-hydrogel. J. Liposome Res. 2014, 24, 27–36. [Google Scholar] [CrossRef]
- Ferreira, M.; Pinto, S.N.; Aires-da-Silva, F.; Bettencourt, A.; Aguiar, S.I.; Gaspar, M.M. Liposomes as a nanoplatform to improve the delivery of antibiotics into Staphylococcus aureus biofilms. Pharmaceutics 2021, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Liposomes as a delivery system for the treatment of biofilm-mediated infections. J. Appl. Microbiol. 2021, 131, 2626–2639. [Google Scholar] [CrossRef] [PubMed]
- Rukavina, Z.; Šegvić Klarić, M.; Filipović-Grčić, J.; Lovrić, J.; Vanić, Ž. Azithromycin-loaded liposomes for enhanced topical treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. Int. J. Pharm. 2018, 553, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan loaded into a hydrogel delivery system as a strategy to treat vaginal co-infection. Pharmaceutics 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Giunchedi, P.; Scalia, S.; Rossi, S.; Sandri, G.; Caramella, C. Chitosan gels for the vaginal delivery of lactic acid: Relevance of formulation parameters to mucoadhesion and release mechanisms. AAPS PharmSciTech 2006, 7, 104. [Google Scholar] [CrossRef]
- Osmałek, T.; Froelich, A.; Jadach, B.; Tatarek, A.; Gadziński, P.; Falana, A.; Gralińska, K.; Ekert, M.; Puri, V.; Wrotyńska-Barczyńska, J.; et al. Recent advances in polymer-based vaginal drug delivery systems. Pharmaceutics 2021, 13, 884. [Google Scholar] [CrossRef]
- Araujo, V.H.S.; de Souza, M.P.C.; Carvalho, G.C.; Duarte, J.L.; Chorilli, M. Chitosan-based systems aimed at local application for vaginal infections. Carbohydr. Polym. 2021, 261, 117919. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F.; Saar, S.; Kara, A.A.; Yıldız, A.; Tunçel, E.; Acartürk, F. Development and characterization of chitosan nanoparticles loaded nanofiber hybrid system for vaginal controlled release of benzydamine. Eur. J. Pharm. Sci. 2021, 161, 105801. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Rukavina, Z.; Manner, S.; Fallarero, A.; Uzelac, L.; Kralj, M.; Amidžić Klarić, D.; Bogdanov, A.; Raffai, T.; Virok, D.P.; et al. Azithromycin-liposomes as a novel approach for localized therapy of cervicovaginal bacterial infections. Int. J. Nanomed. 2019, 14, 5957–5976. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial effectiveness testing. In United States Pharmacopeia; The United States Pharmacopeial Convention: Rockville, MD, USA, 2018; USP41-NF36, Chapter 51.
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Hemmingsen, L.M.; Giordani, B.; Pettersen, A.K.; Vitali, B.; Basnet, P.; Škalko-Basnet, N. Liposomes-in-chitosan hydrogel boosts potential of chlorhexidine in biofilm eradication in vitro. Carbohydr. Polym. 2021, 262, 117939. [Google Scholar] [CrossRef]
- Murta, E.F.; Perfeito, P.B.; Oliveira, T.M.; Michelin, M.A.; Maluf, P.J. Relation between vaginal and endocervical pH in patients undergoing cold-knife conization and hysterectomy. Arch. Gynecol. Obstet. 2008, 277, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Billard, A.; Pourchet, L.; Malaise, S.; Alcouffe, P.; Montembault, A.; Ladavière, C. Liposome-loaded chitosan physical hydrogel: Toward a promising delayed-release biosystem. Carbohydr. Polym. 2015, 115, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.; Miranda, M.; Cardoso, C.; Vitorino, F.V.A. Rheology by design: A regulatory tutorial for analytical method validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, J.Y.; Lee, E.J.; Park, S.K. Rheological properties and microstructures of Carbopol gel network system. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Mourtas, S.; Haikou, M.; Theodoropoulou, M.; Tsakiroglou, C.; Antimisiaris, S.G. The effect of added liposomes on the rheological properties of a hydrogel: A systematic study. J. Colloid Interface Sci. 2008, 317, 611–619. [Google Scholar] [CrossRef]
- Das Neves, J.; Amaral, M.H.; Bahia, M.F. Performance of an in vitro mucoadhesion testing method for vaginal semisolids: Influence of different testing conditions and instrumental parameters. Eur. J. Pharm. Biopharm. 2008, 69, 622–632. [Google Scholar] [CrossRef]
- Rieken, S.; Herroeder, S.; Sassmann, A.; Wallenwein, B.; Moers, A.; Offermanns, S.; Wettschureck, N. Lysophospholipids control integrin-dependent adhesion in splenic B cells through G(i) and G(12)/G(13) family G-proteins but not through G(q)/G(11). J. Biol. Chem. 2006, 281, 36985–63992. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial activity of chitosan-based systems. In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar]
- Divya, K.; Vijayan, S.; Tijith, K.G.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.; Papp, I.; Zichar, M.; Regdon, G., Jr.; Béres, M.; Szalóki, M.; Kovács, R.; Fehér, P.; Ujhelyi, Z.; Vecsernyés, M.; et al. Manufacturing and examination of vaginal drug delivery system by FDM 3D printing. Pharmaceutics 2021, 13, 1714. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Sánchez, B.; Suárez, J.E.; Urdaci, M.C. Characterization of the adherence properties of human Lactobacilli strains to be used as vaginal probiotics. FEMS Microbiol. Lett. 2012, 328, 166–173. [Google Scholar] [CrossRef]
- Banjanac, M.; Munić Kos, V.; Nujić, K.; Vrančić, M.; Belamarić, D.; Crnković, S.; Hlevnjak, M.; Eraković Haber, V. Anti-inflammatory mechanism of action of azithromycin in LPS-stimulated J774A.1 cells. Pharmacol. Res. 2012, 66, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Venditto, V.J.; Haydar, D.; Abdel-Latif, A.; Gensel, J.C.; Anstead, M.I.; Pitts, M.G.; Creameans, J.; Kopper, T.J.; Peng, C.; Feola, D.J. Immunomodulatory effects of azithromycin revisited: Potential applications to COVID-19. Front. Immunol. 2021, 12, 574425. [Google Scholar] [CrossRef]
| AZM-Liposomes | EPC (mg) | EPG (mg) | SLPC-80 (mg) | SPC-3 (mg) | PG (g) | AZM (mg) | Buffer, pH 7.5 (g) |
|---|---|---|---|---|---|---|---|
| CLs | 140 | 10 | - | 50 | - | 30 | 10 |
| PGLs | 190 | 10 | - | - | 1 | 30 | 9 |
| DPGLs | 160 | 10 | 30 | - | 1 | 30 | 9 |
| AZM- Liposomes | Mean Diameter (nm) | PDI | ZP (mV) | Degree of Elasticity | EE (µg AZM/mg Lipid) |
|---|---|---|---|---|---|
| CLs | 253 ± 3 | 0.17 ± 0.03 | −62.5 ± 0.8 | 0.5 ± 0.2 * | 6.4 ± 0.8 |
| PGLs | 216 ± 2 | 0.19 ± 0.02 | −56.2 ± 0.7 | 13.4 ± 0.5 | 8.0 ± 0.4 |
| DPGLs | 179 ± 1 * | 0.20 ± 0.02 | −54.3 ± 0.6 | 16.1 ± 0.7 | 7.4 ± 0.7 |
| Test Microbes | S. aureus ATCC 6538 | P. aeruginosa ATCC 9027 | E. coli ATCC 8739 | C. albicans ATCC 10231 | |
|---|---|---|---|---|---|
| Inoculum CFU/mL (log10) | 3.0 × 105 (5.5) | 4.3 × 105 (5.6) | 3.5 × 105 (5.5) | 4.2 × 105 (5.6) | |
| Log10 reduction | 2nd day | >4.5 | >4.6 | >4.5 | - |
| 7th day | >4.5 | >4.6 | >4.5 | - | |
| 14th day | >4.5 | >4.6 | >4.5 | >4.6 | |
| 28th day | NI | NI | NI | NI | |
| AZM-Sample | Zone of Inhibition (mm ± S.D.) | |
|---|---|---|
| AZM-Liposomes | AZM-Liposomal Hydrogel | |
| CLs | 18.47 ± 0.28 | 19.07 ± 0.60 |
| PGLs | 17.85 ± 0.24 | 18.50 ± 0.39 |
| DPGLs | 17.59 ± 0.22 | 18.46 ± 0.02 |
| Control * | 15.55 ± 0.20 | 17.05 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čačić, A.; Amidžić Klarić, D.; Keser, S.; Radiković, M.; Rukavina, Z.; Jøraholmen, M.W.; Uzelac, L.; Kralj, M.; Škalko-Basnet, N.; Šegvić Klarić, M.; et al. A Novel Approach for the Treatment of Aerobic Vaginitis: Azithromycin Liposomes-in-Chitosan Hydrogel. Pharmaceutics 2023, 15, 1356. https://doi.org/10.3390/pharmaceutics15051356
Čačić A, Amidžić Klarić D, Keser S, Radiković M, Rukavina Z, Jøraholmen MW, Uzelac L, Kralj M, Škalko-Basnet N, Šegvić Klarić M, et al. A Novel Approach for the Treatment of Aerobic Vaginitis: Azithromycin Liposomes-in-Chitosan Hydrogel. Pharmaceutics. 2023; 15(5):1356. https://doi.org/10.3390/pharmaceutics15051356
Chicago/Turabian StyleČačić, Ana, Daniela Amidžić Klarić, Sabina Keser, Maja Radiković, Zora Rukavina, May Wenche Jøraholmen, Lidija Uzelac, Marijeta Kralj, Nataša Škalko-Basnet, Maja Šegvić Klarić, and et al. 2023. "A Novel Approach for the Treatment of Aerobic Vaginitis: Azithromycin Liposomes-in-Chitosan Hydrogel" Pharmaceutics 15, no. 5: 1356. https://doi.org/10.3390/pharmaceutics15051356
APA StyleČačić, A., Amidžić Klarić, D., Keser, S., Radiković, M., Rukavina, Z., Jøraholmen, M. W., Uzelac, L., Kralj, M., Škalko-Basnet, N., Šegvić Klarić, M., & Vanić, Ž. (2023). A Novel Approach for the Treatment of Aerobic Vaginitis: Azithromycin Liposomes-in-Chitosan Hydrogel. Pharmaceutics, 15(5), 1356. https://doi.org/10.3390/pharmaceutics15051356













