Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications
Abstract
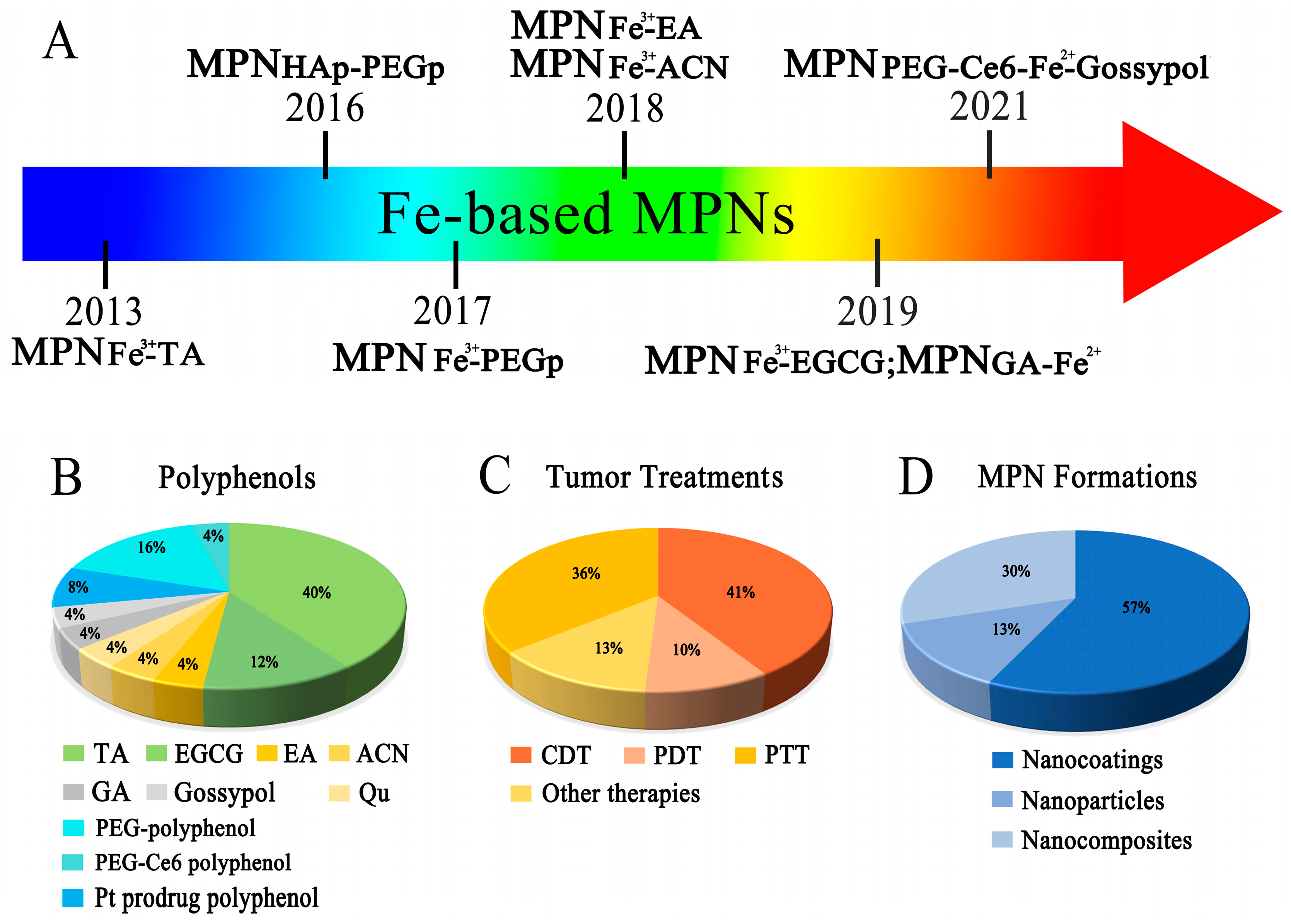
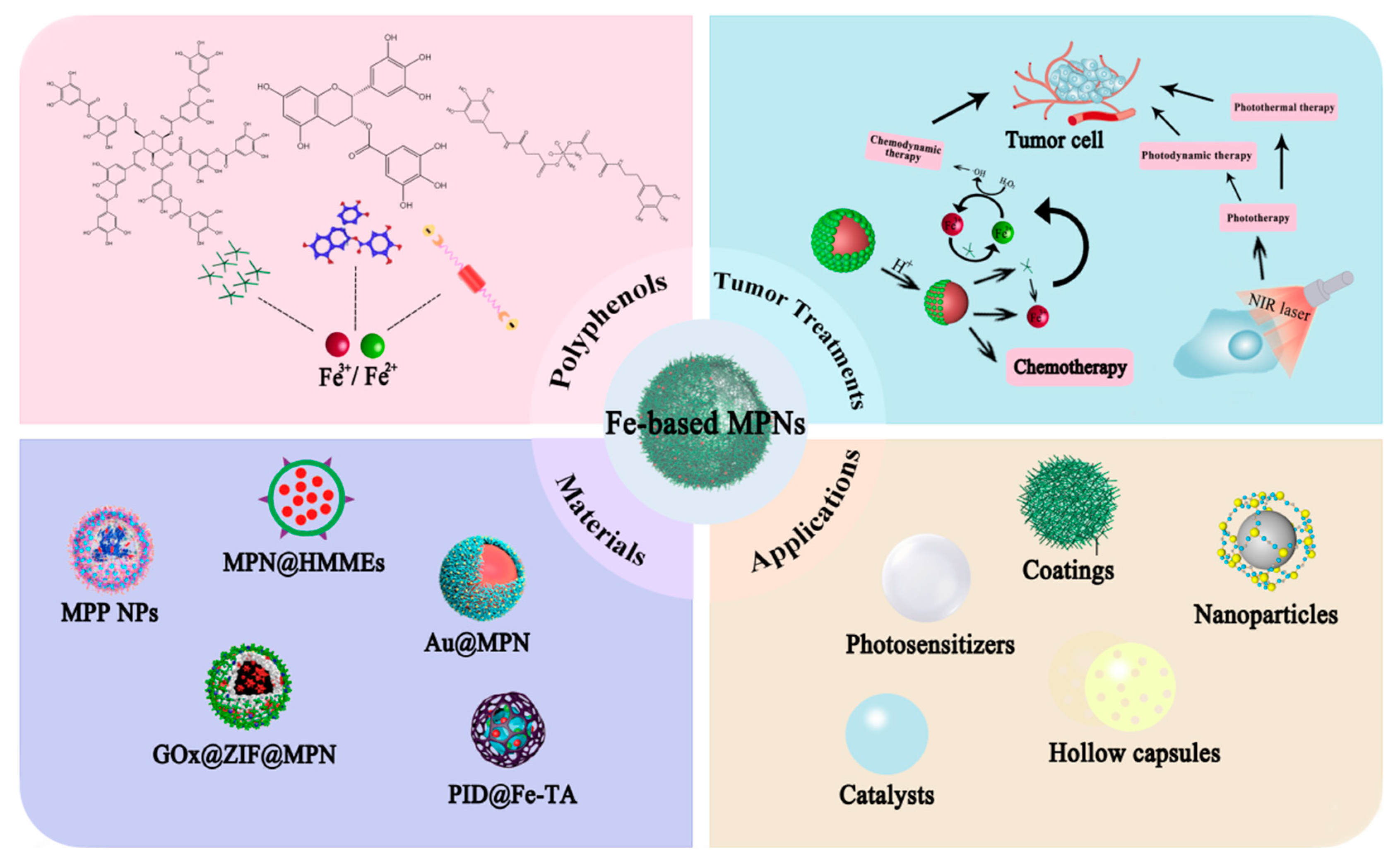
1. Introduction
2. Characteristics and Designing Strategies for Fe-Based MPNs
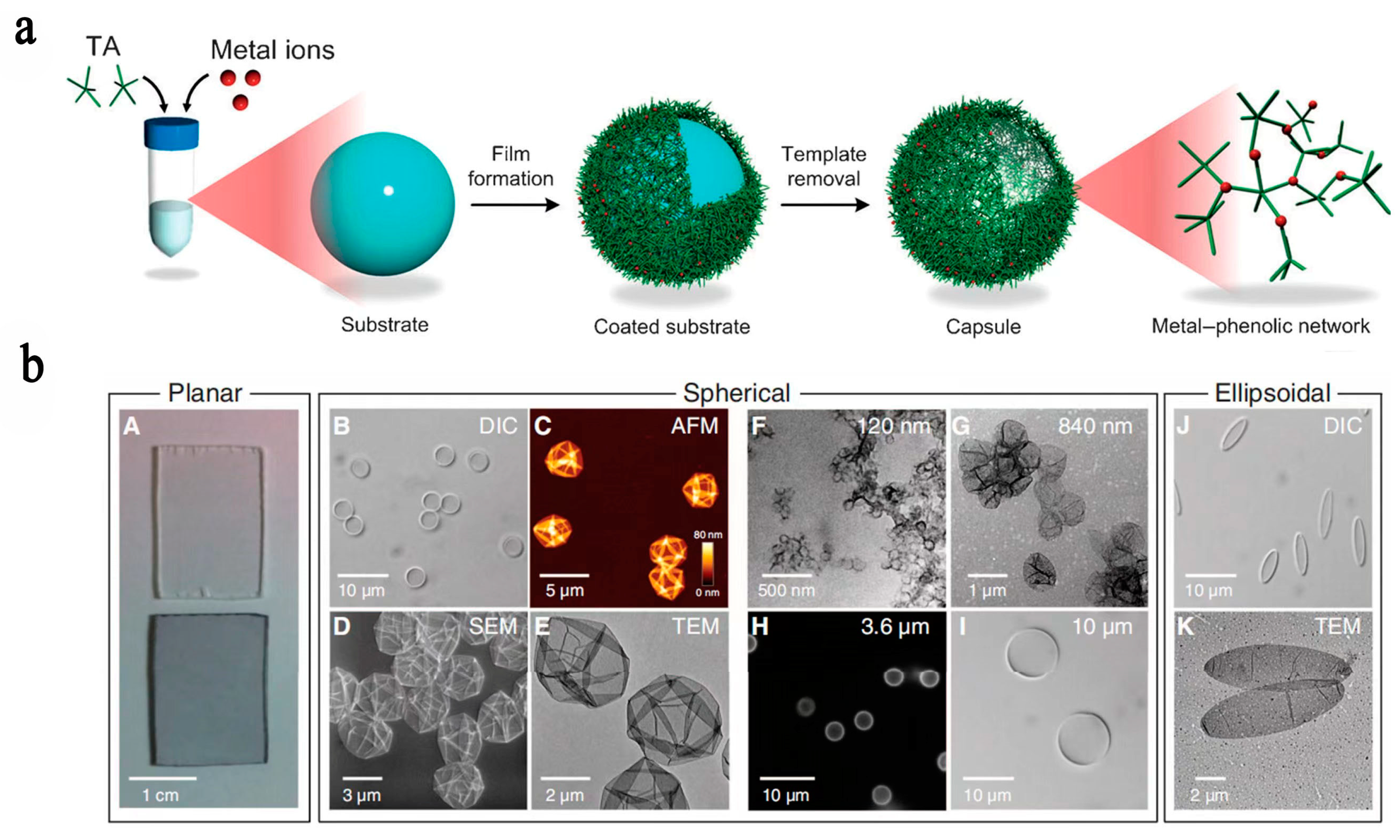
2.1. Fe with TA
2.2. Fe with GA
2.3. Fe with EGCG
2.4. Fe with ACN
2.5. Fe with EA
2.6. Fe with Qu
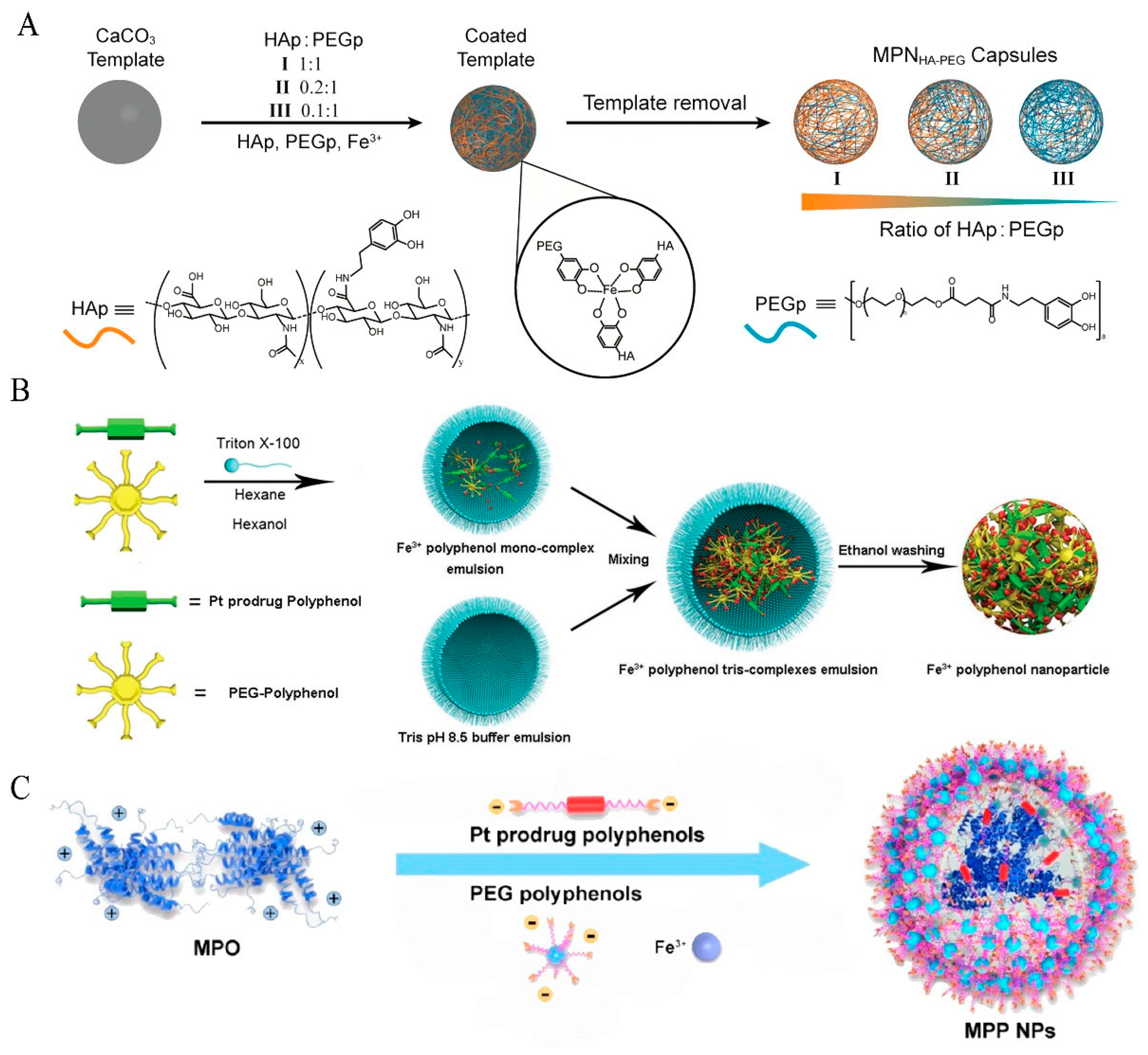
2.7. Fe with PEG-Polyphenol
2.8. Fe with Pt Prodrug-Polyphenol
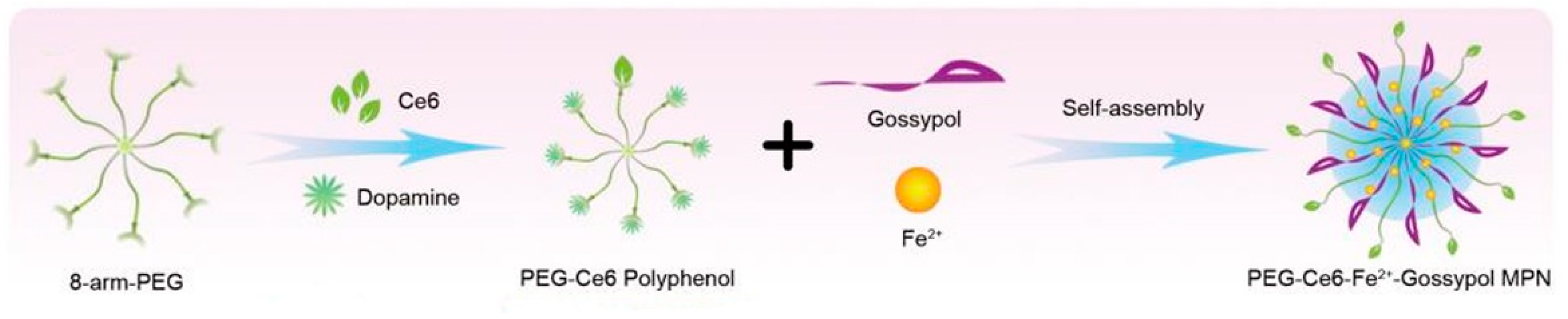
2.9. Fe with Gossypol and PEG-Ce6-Polyphenol
3. Fe-Based MPNs in CDT
3.1. Fe with TA
3.2. Fe with EGCG
3.3. Fe with PEG-Polyphenol
3.4. Fe with Hybrid Polyphenol
4. Fe-Based MPNs in PTT
4.1. Fe with TA
4.2. Fe with EGCG
4.3. Fe with ACN
4.4. Fe with Qu
4.5. Fe with EA
4.6. Fe with PEG-Polyphenol
4.7. Fe with PEG-Ce6-Polyphenol and Gossypol
5. Fe-Based MPNs in Other Therapies
5.1. Fe with TA
5.2. Fe with GA
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer. J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Yin, S.H.; Lin, M.Z.; Chen, X.L.; Pan, Y.; Peng, Y.Q.; Sun, J.B.; Kumar, A.; Liu, J.Q. Current status and prospects of metal-organic frameworks for bone therapy and bone repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Yan, Q.W.; Xia, C.; Wang, X.X.; Kumar, A.; Wang, Y.; Liu, Y.W.; Pan, Y. Recent advances in cell membrane coated metal–organic frameworks (MOFs) for tumor therapy. J. Mater. Chem. B 2021, 9, 4459–4474. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhao, S.J.; Li, B.L.; Wang, B.H.; Lan, M.H.; Song, X.Z. Low temperature photothermal therapy: Advances and perspectives. Coordin. Chem. Rev. 2022, 454, 214330. [Google Scholar] [CrossRef]
- Cui, D.; Huang, J.G.; Zhen, X.; Li, J.C.; Jiang, Y.Y.; Pu, K.Y. A Semiconducting Polymer Nano-prodrug for Hypoxia-Activated Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2019, 58, 5920–5924. [Google Scholar] [CrossRef]
- Li, Y.; Miao, Y.; Yang, L.N.; Zhao, Y.T.; Wu, K.K.; Lu, Z.H.; Hu, Z.Q.; Guo, J.S. Recent Advances in the Development and Antimicrobial. Adv. Sci. 2022, 9, 2202684. [Google Scholar] [CrossRef]
- Li, Q.G.; Dong, Z.L.; Chen, M.W.; Feng, L.Z. Phenolic molecules constructed nanomedicine for innovative cancer treatment. Coordin. Chem. Rev. 2021, 439, 213912. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Dabbaghi, A.; Kabiri, K.; Ramazani, A.; Zohuriaan-Mehr, M.J.; Jahandideh, A. Synthesis of bio-based internal and external cross-linkers based on tannic acid for preparation of antibacterial superabsorbents. Polym. Adv. Technol. 2019, 30, 2894–2905. [Google Scholar] [CrossRef]
- Choubey, S.; Goyal, S.; Varughese, L.R.; Kumar, V.; Sharma, A.K.; Beniwal, V. Probing Gallic Acid for Its Broad Spectrum Applications. Mini-Rev. Med. Chem. 2018, 18, 1283–1293. [Google Scholar] [CrossRef]
- Guo, J.L.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; Elverfeldt, D.V.; Hagemeyer, C.E.; et al. Engineering Multifunctional Capsules through the Assembly of Metal-Phenolic Networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef]
- Xie, L.; Li, J.; Wang, L.Y.; Dai, Y.L. Engineering metal-phenolic networks for enhancing cancer therapy by tumor microenvironment modulation. Wires. Nanomed. Nanobi 2022, e1864. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.W.; Caruso, F. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Ghafarifar, F.; Molaie, S.; Abazari, R.; Hasan, Z.M.; Foroutan, M. Fe3O4@Bio-MOF Nanoparticles Combined with Artemisinin, Glucantime®, or Shark Cartilage Extract on Iranian Strain of Leishmania major (MRHO/IR/75/ER): An In-Vitro and In-Vivo Study. Iran. J. Parasitol. 2020, 15, 537–548. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.H.; Wen, N.C.; Xiong, H.J.; Cai, S.D.; He, Q.Y.; Hu, Y.Q.; Peng, D.M.; Liu, Z.B.; Liu, Y.F. Metal-organic frameworks for stimuli-responsive drug delivery. Biomaterials 2020, 230, 119619. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Caruso, F. Metal-phenolic networks as a versatile platform to engineer nanomaterials and biointerfaces. Nano Today 2017, 12, 136–148. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Shen, L.B.; Zhong, Q.Z.; Li, J.H. Metal-Phenolic Network Coatings for Engineering Bioactive Interfaces. Colloid. Surfaces B 2021, 205, 111851. [Google Scholar] [CrossRef]
- Wang, R.X.; Zhao, X.T.; Jia, N.; Cheng, L.J.; Liu, L.F.; Gao, C.J. Superwetting Oil/Water Separation Membrane Constructed from In Situ Assembled Metal-Phenolic Networks and Metal-Organic Frameworks. ACS. Appl. Mater. Interfaces 2020, 12, 10000–10008. [Google Scholar] [CrossRef]
- Ping, Y.; Guo, J.J.; Ejima, H.; Chen, X.; Richardson, J.J.; Sun, H.L.; Caruso, F. pH-Responsive Capsules Engineered from Metal-Phenolic Networks for Anticancer Drug Delivery. Small 2015, 11, 2032–2036. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Wei, Z.Z.; Luo, P.C.; Wei, W.; Liu, S.Q. pH-sensitive metal-phenolic network capsules for targeted photodynamic therapy against cancer cells. Artif. Cells Nanomed. B 2018, 46, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Abazari, R.; Ataei, F.; Morsali, A.; Slawin, A.M.Z.; Carpenter-Warren, C.L. A Luminescent Amine-Functionalized Metal-Organic Framework Conjugated with Folic Acid as a Targeted Biocompatible pH-Responsive Nanocarrier for Apoptosis Induction in Breast Cancer. ACS. Appl. Mater. Interfaces 2019, 11, 45442–45454. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.S.; Guo, Z.H.; Zhao, L.Y.; Wei, Y. Metal-phenolic networks: Facile assembled complexes for cancer theranostics. Theranostics 2021, 11, 6407–6426. [Google Scholar] [CrossRef] [PubMed]
- Yun, G.; Pan, S.J.; Wang, T.Y.; Guo, J.L.; Richardson, J.J.; Caruso, F. Synthesis of Metal Nanoparticles in Metal-Phenolic Networks: Catalytic and Antimicrobial Applications of Coated Textiles. Adv. Healthc. Mater. 2018, 7, 1700934. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New. Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Zhong, Y.Y.; Liu, W.C.; Rao, C.Y.; Li, B.H.; Wang, X.X.; Liu, D.; Pan, Y.; Liu, J.Q. Recent Advances in Fe-MOF Compositions for Biomedical Applications. Curr. Med. Chem. 2021, 28, 6179–6198. [Google Scholar] [CrossRef]
- Guo, Y.X.; Sun, Q.; Wu, F.G.; Dai, Y.L.; Chen, X.Y. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, 2007356. [Google Scholar] [CrossRef]
- Abazari, R.; Mahjoub, A.R.; Ataei, F.; Morsali, A.; Carpenter-Warren, C.L.; Mehdizadeh, K.; Slawin, A.M.Z. Chitosan Immobilization on Bio-MOF Nanostructures: A Biocompatible pH-Responsive Nanocarrier for Doxorubicin Release on MCF-7 Cell Lines of Human Breast Cancer. Inorg. Chem. 2018, 57, 13364–13379. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.L.; Zhang, M.K.; Yu, W.Y.; Gao, F.; Li, C.X.; Wang, S.B.; Feng, J.; Zhang, X.Z. Ferrous-Supply-Regeneration Nanoengineering for Cancer Cell Specific Ferroptosis in Combination with Imaging-Guided Photodynamic Therapy. ACS Nano 2018, 12, 12181–12192. [Google Scholar] [CrossRef]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; McAinsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2015, 66, 37–46. [Google Scholar] [CrossRef]
- Gao, Y.H.; Yang, S.C.; Zhu, M.H.; Zhu, X.D.; Luan, X.; Liu, X.L.; Lai, X.; Yuan, Y.H.; Lu, Q.; Sun, P.; et al. Metal phenolic network-integrated multistage nanosystem for enhanced drug delivery to solid tumors. Small 2021, 17, 2100789. [Google Scholar] [CrossRef]
- Liu, J.J.; Shi, J.J.; Nie, W.M.; Wang, S.J.; Liu, G.H.; Cai, K.Y. Recent Progress in the Development of Multifunctional Nanoplatform for Precise Tumor Phototherapy. Adv. Healthc. Mater. 2021, 10, 2001207. [Google Scholar] [CrossRef]
- Dong, Z.L.; Feng, L.Z.; Chao, Y.; Chen, M.C.; Gong, F.; Han, X.; Zhang, R.; Cheng, L.; Liu, Z. Amplification of Tumor Oxidative Stresses with Liposomal Fenton Catalyst and Glutathione Inhibitor for Enhanced Cancer Chemotherapy and Radiotherapy. Nano. Lett. 2019, 19, 805–815. [Google Scholar] [CrossRef]
- Shan, L.L.; Gao, G.Z.; Wang, W.W.; Tang, W.; Wang, Z.T.; Yang, Z.; Fan, W.P.; Zhu, G.Z.; Zhai, K.F.; Jacobson, O.; et al. Self-assembled green tea polyphenol-based coordination nanomaterials to improve chemotherapy efficacy by inhibition of carbonyl reductase 1. Biomaterials 2019, 210, 62–69. [Google Scholar] [CrossRef]
- Xu, C.N.; Wang, Y.B.; Yu, H.Y.; Tian, H.Y.; Chen, X.S. Multifunctional Theranostic Nanoparticles Derived from Fruit Extracted Anthocyanins with Dynamic Disassembly and Elimination Abilities. ACS Nano 2018, 12, 8255–8265. [Google Scholar] [CrossRef]
- Zhao, G.Z.; Wu, H.H.; Feng, R.L.; Wang, D.D.; Xu, P.P.; Jiang, P.; Yang, K.; Guo, Z.; Wang, H.B.; Chen, Q.W. Novel metal polyphenol framework for MR imaging-guided photothermal therapy. ACS. Appl. Mater. Interfaces 2018, 10, 3295–3304. [Google Scholar] [CrossRef]
- Ju, Y.; Cui, J.W.; Sun, H.L.; Mu, M.; Dai, Y.L.; Guo, J.L.; Bertleff-Zieschang, N.; Suma, T.; Richardson, J.J.; Caruso, F. Engineered Metal-Phenolic Capsules Show Tunable Targeted Delivery to Cancer Cells. Biomacromolecules 2016, 17, 2268–2276. [Google Scholar] [CrossRef]
- Dai, Y.L.; Guo, J.L.; Wang, T.Y.; Ju, Y.; Mitchell, A.J.; Bonnard, T.; Cui, J.W.; Richardson, J.J.; Hagemeyer, C.E.; Alt, K.; et al. Self-Assembled Nanoparticles from Phenolic Derivatives for Cancer Therapy. Adv. Healthc. Mater. 2017, 6, 1700467. [Google Scholar] [CrossRef]
- Zhang, Z.; Sang, W.; Xie, L.S.; Li, W.X.; Li, B.; Li, J.; Tian, H.; Yuan, Z.; Zhao, Q.; Dai, Y.L. Polyphenol-Based Nanomedicine Evokes Immune Activation for Combination Cancer Treatment. Angew. Chem. Int. Ed. 2020, 60, 1967–1975. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhang, X.D.; Sun, W.; Jia, H.R.; Zhu, Y.X.; Zhang, X.P.; Zhou, N.X.; Wu, F.G. Metal-Phenolic Network-Based Nanocomplexes that Evoke Ferroptosis by Apoptosis: Promoted Nuclear Drug Influx and Reversed Drug Resistance of Cancer. Chem. Mater. 2019, 31, 10071–10084. [Google Scholar] [CrossRef]
- Liu, J.J.; Jin, Y.J.; Song, Z.; Xu, L.H.; Yang, Y.; Zhao, X.; Wang, B.H.; Liu, W.; Zhang, K.X.; Zhang, Z.Z.; et al. Boosting Tumor Treatment by dredging the Hurdles of Chemodynamic Therapy synergistic Ion Therapy-ScienceDirect. Chem. Eng. J. 2021, 411, 128440. [Google Scholar] [CrossRef]
- Peng, M.R.; Ju, E.G.; Xu, Y.T.; Wang, Y.Q.; Lv, S.X.; Shao, D.; Wang, H.X.; Tao, Y.; Zheng, Y.; Li, M.Q. Dual-responsive disassembly of core-shell nanoparticles with self-supplied H2O2 and autocatalytic Fenton reaction for enhanced chemodynamic therapy. NPG Asia Mater. 2022, 14, 95. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Guo, Y.Q.; Fan, Y.; Chen, J.W.; Wang, H.; Shen, M.W.; Shi, X.Y. Metal-Phenolic-Network-Coated Dendrimer-Drug Conjugates for Tumor MR Imaging and Chemo/Chemodynamic Therapy via Amplification of Endoplasmic Reticulum Stress. Adv. Mater. 2022, 34, 2107009. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.X.; Jia, H.R.; Zhang, X.D.; Zhang, X.P.; Sun, Q.; Wang, S.Z.; Zhao, J.; Wu, F.G. A Glucose/Oxygen-Exhausting Nanoreactor for Starvation- and Hypoxia-Activated Sustainable and Cascade Chemo-Chemodynamic Therapy. Small 2020, 16, 2000897. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Y.; Chen, L.Z.; Lv, R.M.; Liu, M.; He, N.Y.; Wang, Z.F. A metal-phenolic network-based multifunctional nanocomposite with pH-responsive ROS generation and drug release for synergistic chemodynamic/photothermal/chemo-therapy. J. Mater. Chem. B 2020, 8, 2177–2188. [Google Scholar] [CrossRef]
- Liu, P.; Shi, X.Y.; Peng, Y.; Hu, J.M.; Ding, J.S.; Zhou, W.H. Anti-PD-L1 DNAzyme Loaded Photothermal Mn2+/Fe3+ Hybrid Metal-Phenolic Networks for Cyclically Amplified Tumor Ferroptosis-Immunotherapy. Adv. Healthc. Mater. 2022, 11, 21023. [Google Scholar] [CrossRef]
- Zhou, K.Y.; Xu, W.N.; Zhang, X.T.; Wang, S.S.; Li, Y.Q.; Yang, L.; Cai, R.; Xu, Q.; Li, G.Z.; Guo, X.H. Complex of nanocarriers based on the metal polyphenol network: Multi-modal synergistic inhibition of tumor cell proliferation by inducing ferroptosis and photodynamic effect. New J. Chem. 2022, 46, 21962–21967. [Google Scholar] [CrossRef]
- Yi, X.Q.; Zeng, W.J.; Wang, C.; Chen, Y.; Zheng, L.Y.; Zhu, X.L.; Ke, Y.Q.; He, X.Y.; Kuang, Y. A step-by-step multiple stimuli-responsive metal-phenolic network prodrug nanoparticles for chemotherapy. Nano Rev. 2022, 15, 1205–1212. [Google Scholar] [CrossRef]
- Dai, X.; Zhu, Y.Q.; Su, M.; Chen, J.B.; Shen, S.; Xu, C.F.; Yang, X.Z. Rigid Shell Decorated Nanodevice with Fe/H2O2 Supply and Glutathione Depletion Capabilities for Potentiated Ferroptosis and Synergized Immunotherapy. Adv. Funct. Mater. 2023, 2215022. [Google Scholar] [CrossRef]
- Yu, H.Z.; Ma, M.; Liang, K.C.; Shen, J.; Lan, Z.Y.; Chen, H.R. A self-assembled metal-polyphenolic nanomedicine for mild photothermal-potentiated chemodynamic therapy of tumors. Appl. Mater. Today 2021, 25, 101235. [Google Scholar] [CrossRef]
- Yu, C.X.; Can, Y.Z.C.G.; Min, M.X.; Wen, S.; Xing, C.X.; Dong, L.X. DOX-assisted functionalization of green tea polyphenol nanoparticles for effective chemo-photothermal cancer therapy. J. Mater. Chem. B 2019, 7, 4066. [Google Scholar]
- Xie, Q.; Li, S.C.; Feng, X.X.; Shi, J.Y.; Li, Y.; Yuan, G.J.; Yang, C.L.; Shen, Y.Q.; Kong, L.; Zhang, Z.P. All-in-one approaches for triple-negative breast cancer therapy: Metal-phenolic nanoplatform for MR imaging-guided combinational therapy. J. Nanobiotechnol. 2022, 20, 226. [Google Scholar] [CrossRef]
- Yang, G.G.; Zhou, D.J.; Pan, Z.Y.; Yang, J.; Zhang, D.Y.; Cao, Q.; Ji, L.N.; Mao, Z.W. Multifunctional low-temperature photothermal nanodrug with in vivo clearance, ROS-Scavenging and anti-inflammatory abilities. Biomaterials 2019, 216, 119280. [Google Scholar] [CrossRef]
- Ren, Z.G.; Sun, S.C.; Sun, R.R.; Cui, G.Y.; Hong, L.J.; Rao, B.C.; Li, A.; Yu, Z.J.; Kan, Q.C.; Mao, Z.W. A Metal-Polyphenol-Coordinated Nanomedicine for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Adv. Mater. 2019, 32, 1906024. [Google Scholar] [CrossRef]
- Chen, X.L.; Ma, R.Y.; Fu, Z.M.; Su, Q.H.; Luo, X.Y.; Han, Y.C.; Yang, Y.; Deng, Q.C. Metal-phenolic networks-encapsulated cascade amplification delivery nanoparticles overcoming cancer drug resistance via combined starvation/chemodynamic/chemo therapy. Chem. Eng. J. 2022, 442, 136221. [Google Scholar] [CrossRef]
- Xie, L.S.; Li, J.; Wang, G.H.; Sang, W.; Xu, M.Z.; Li, W.X.; Yan, J.; Li, B.; Zhang, Z.; Zhao, Q.; et al. Phototheranostic Metal-Phenolic Networks with Antiexosomal PD-L1 Enhanced Ferroptosis for Synergistic Immunotherapy. J. Am. Chem. Soc. 2022, 144, 787–797. [Google Scholar] [CrossRef]
- Tang, Z.M.; Liu, Y.Y.; He, M.Y.; Bu, W.B. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Zhang, S.C.; Chai, Q.H.; Man, Z.T.; Tang, C.W.; Li, Z.Y.; Zhang, J.; Xu, H.L.; Xu, X.X.; Chen, C.; Liu, Y.; et al. Bioinspired nano-painting on orthopedic implants orchestrates periprosthetic anti-infection and osseointegration in a rat model of arthroplasty. Chem. Eng. J. 2022, 435, 134848. [Google Scholar] [CrossRef]
- Han, J.H.; Shin, H.E.; Lee, J.Y.; Kang, J.M.; Park, J.H.; Park, C.G.; Han, D.K.; Kim, I.H.; Park, W. Combination of Metal-Phenolic Network-Based Immunoactive Nanoparticles and Bipolar Irreversible Electroporation for Effective Cancer Immunotherapy. Small 2022, 18, 2200316. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Yang, D.Y.; Yu, L.D.; Jing, X.X.; Chen, Y. Catalytic chemistry of iron-free Fenton nanocatalysts for versatile radical nanotherapeutics. Mater. Horiz. 2020, 7, 317–337. [Google Scholar] [CrossRef]
- Zhou, J.; Lei, M.; Peng, X.L.; Wei, D.X.; Yan, L.K. Fenton Reaction Induced by Fe-Based Nanoparticles for Tumor Therapy. J. Biomed. Nanotechnol. 2021, 17, 1510–1524. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, L.S.; Ju, Y.; Dai, Y.L. Recent Advances in Metal-Phenolic Networks for Cancer Theranostics. Small 2021, 17, 2100314. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Xie, W.S.; Lu, J.S.; Guo, X.X.; Xu, J.Z.; Xu, W.L.; Chi, Y.J.; Takuya, N.; Wu, H.; Zhao, L.Y. Tannic Acid-based Metal Phenolic Networks for Bio-applications: A Review. J. Mater. Chem. B 2021, 9, 4098–4110. [Google Scholar] [CrossRef] [PubMed]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer Preventive and Therapeutic Effects of EGCG, the Major Polyphenol in Green Tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23. [Google Scholar] [CrossRef]
- Pastore, R.L.; Fratellone, P. Potential Health Benefits of Green Tea (Camellia sinensis): A Narrative Review. Explore 2006, 2, 531–539. [Google Scholar] [CrossRef]
- Shen, G.Z.; Xing, R.R.; Zhang, N.; Chen, C.J.; Ma, G.H.; Yan, X.H. Interfacial Cohesion and Assembly of Bioadhesive Molecules for Design of Long-Term Stable Hydrophobic Nanodrugs toward Effective Anticancer Therapy. ACS. Nano 2016, 10, 5720–5729. [Google Scholar] [CrossRef]
- Chen, J.L.; Xu, B.J.; Sun, J.X.; Jiang, X.W.; Bai, W.B. Anthocyanin supplement as a dietary strategy in cancer prevention and management: A comprehensive review. Crit. Rev. Food Sci. 2021, 26, 7242–7254. [Google Scholar] [CrossRef]
- Ríos, J.L.; Giner, R.M.; Marín, M.; Recio, M.C. A Pharmacological Update of Ellagic Acid. Planta Med. 2018, 84, 1068–1093. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.F.; Wang, J.Y.; Tang, N. Antioxidative and anti-tumour activities of solid quercetin metal (II) complexes. Transit. Metal. Chem. 2001, 26, 57–63. [Google Scholar] [CrossRef]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar]
- Goddard, J.M.; Hotchkiss, J. Polymer surface modifification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Sang, W.; Zhang, Z.; Wang, G.; Xie, L.S.; Li, L.; Li, W.X.; Tian, H.; Dai, Y.L. A Triple-Kill Strategy for Tumor Eradication Reinforced by Metal-Phenolic Network Nanopumps. Adv. Funct. Mater. 2022, 32, 2113168. [Google Scholar] [CrossRef]
- Dai, Y.L.; Cheng, S.Y.; Wang, Z.L.; Zhang, R.L.; Yang, Z.; Wang, J.J.; Yung, B.C.; Wang, Z.T.; Jacobson, O.; Xu, C.; et al. Hypochlorous Acid Promoted Platinum Drug Chemotherapy by Myeloperoxidase Encapsulated Therapeutic Metal Phenolic Nanoparticles. ACS Nano 2018, 12, 455–463. [Google Scholar] [CrossRef]
- Zeng, Y.; Ma, J.W.; Xu, L.; Wu, D.C. Natural Product Gossypol and its Derivatives in Precision Cancer Medicine. Curr. Med. Chem. 2019, 26, 1849–1873. [Google Scholar] [CrossRef]
- Liu, P.; Peng, Y.; Ding, J.S.; Zhou, W.H. Fenton metal nanomedicines for imaging-guided combinatorial chemodynamic therapy against cancer. Asian J. Pharm. Sci. 2021, 17, 177–192. [Google Scholar] [CrossRef]
- Sun, Q.Q.; Wang, Z.; Liu, B.; He, F.; Gai, S.L.; Yang, P.P.; Yang, D.; Li, C.X.; Lin, J. Recent advances on endogenous/exogenous stimuli-triggered nanoplatforms for enhanced chemodynamic therapy. Coordin. Chem. Rev. 2022, 451, 214267. [Google Scholar] [CrossRef]
- Tian, Q.W.; Xue, F.F.; Wang, Y.R.; Cheng, Y.Y.; An, L.; Yang, S.P.; Chen, X.Y.; Huang, G. Recent advances in enhanced chemodynamic therapy strategies. Nano Today 2021, 39, 101162. [Google Scholar] [CrossRef]
- Zhao, R.B.; Liu, X.Y.; Yang, X.Y.; Jin, B.; Shao, C.Y.; Kang, W.J.; Tang, R.K. Nanomaterial-Based Organelles Protect Normal Cells against Chemotherapy-Induced Cytotoxicity. Adv. Mater. 2018, 30, 1801304. [Google Scholar] [CrossRef]
- Shi, J.J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Liu, X.; Jin, Y.L.; Liu, T.T.; Yang, S.J.; Zhou, M.X.; Wang, W.Q.; Yu, H.J. Iron-Based Theranostic Nanoplatform for Improving Chemodynamic Therapy of Cancer. ACS Biomater. Sci. Eng. 2020, 6, 4834–4845. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, F.; Lu, G.H.; Nie, W.D.; Zhang, L.J.; Lv, Y.L.; Bao, W.E.; Gao, X.Y.; Wei, W.; Pu, K.Y.; et al. Engineering Magnetosomes for Ferroptosis/Immunomodulation Synergism in Cancer. ACS Nano 2019, 13, 5662–5673. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.Y.; Song, J.B.; Yung, B.C.; Zhou, Z.J.; Wu, A.G.; Chen, X.Y. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv. Mater. 2018, 30, 1704007. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Liu, T.; Li, Y.; Lau, J.; Yang, Z.; Fan, W.P.; Zhou, Z.J.; Shi, C.R.; Ke, C.M.; Bregadze, V.I.; et al. Fenton-Reaction-Accelerable Magnetic Nanoparticles for Ferroptosis Therapy of Orthotopic Brain Tumors. ACS Nano 2018, 12, 11355–11365. [Google Scholar] [CrossRef]
- Liu, M.; Liu, B.; Liu, Q.Q.; Du, K.K.; Wang, Z.F.; He, N. Nanomaterial-induced ferroptosis for cancer specific therapy. Coordin. Chem. Rev. 2019, 382, 160–180. [Google Scholar] [CrossRef]
- Li, K.; Dai, Y.L.; Chen, W.; Yu, K.; Xiao, G.; Richardson, J.J.; Huang, W.; Guo, J.L.; Liao, X.P.; Shi, B. Self-Assembled Metal-Phenolic Nanoparticles for Enhanced Synergistic Combination Therapy against Colon Cancer. Adv. Biosyst. 2019, 3, 1800241. [Google Scholar] [CrossRef]
- Blum, R.H.; Carter, S.K. A New Anticancer Drug with Significant Clinical Activity. Ann. Intern. Med. 1974, 80, 249–259. [Google Scholar] [CrossRef]
- Lee, D.; Khaja, S.; Velasquez-Castano, J.C.; Dasari, M.; Sun, C.; Petros, J.; Taylor, W.R.; Murthy, N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat. Mater. 2007, 6, 765–769. [Google Scholar] [CrossRef]
- Zhang, C.; Bu, W.B.; Ni, D.L.; Zhang, S.J.; Li, Q.; Yao, Z.W.; Zhang, J.W.; Yao, H.L.; Wang, Z.; Shi, J.L. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew. Chem. Int. Ed. 2015, 55, 2101–2106. [Google Scholar] [CrossRef]
- Huo, M.F.; Wang, L.Y.; Chen, Y.; Shi, J.L. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017, 8, 357. [Google Scholar] [CrossRef]
- Northup, A.; Cassidy, D. Calcium peroxide (CaO2) for use in modified Fenton chemistry. J. Hazard. Mater. 2008, 152, 1164–1170. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.Y.; Kim, D.Y.; Wu, Z.Q.; Wang, W.Z.; Xiao, Y.M.; Chow, P.; Meng, Y.; Prakapenka, V.B.; Mao, H.K.; et al. Hydrogen-bearing iron peroxide and the origin of ultralow-velocity zones. Nature 2017, 551, 494–497. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal-Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, S.S.; Li, C.X.; Xu, L.; Cheng, H.; Zhang, X.Z. An Adenosine Triphosphate-Responsive Autocatalytic Fenton Nanoparticle for Tumor Ablation with Self-Supplied H2O2 and Acceleration of Fe(III)/Fe(II) Conversion. Nano Lett. 2018, 18, 7609–7618. [Google Scholar] [CrossRef]
- Ding, Y.; Xu, H.; Xu, C.; Tong, Z.R.; Zhang, S.T.; Bai, Y.; Chen, Y.N.; Xu, Q.H.; Zhou, L.Z.; Ding, H.; et al. A Nanomedicine Fabricated from Gold Nanoparticles-Decorated Metal-Organic Framework for Cascade Chemo/Chemodynamic Cancer Therapy. Adv. Sci. 2020, 7, 2001060. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.; Jahangirian, H.; Rafifiee, R.; Rafiee-Moghaddam, R.; Webster, T.J. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Gandara, D.R.; Lara, P.N.; Goldberg, Z.; Le, Q.T.; Mack, P.C.; Lau, D.H.M.; Gumerlock, P.H. Tirapazamine: Prototype for a Novel Class of Therapeutic Agents Targeting Tumor Hypoxia. Semin. Oncol. 2002, 29, 102–109. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.W.; Sun, W.; Guo, Y.X.; Jia, H.R.; Yu, X.W.; Pan, G.Y.; Wu, F.G. Molecular Targeting-Mediated Mild-Temperature Photothermal Therapy with a Smart Albumin-Based Nanodrug. Small 2019, 15, 1900501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Chen, X.K.; Wang, H.Y.; Jia, H.R.; Wu, F.G. Supramolecular Nanogel-Based Universal Drug Carriers Formed by “Soft-Hard” Co-Assembly: Accurate Cancer Diagnosis and Hypoxia-Activated Cancer Therapy. Adv. Ther. 2019, 2, 1800140. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.Y.; Xu, W.J.; Dong, C.C.; Bai, Y.C.; Zeng, J.B.; Zhou, Y.; Zhang, J.L.; Yin, Y.D. Metal Sulfides as Excellent Co-catalysts for H2O2 Decomposition in Advanced Oxidation Processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef]
- Xiao, S.T.; Lu, Y.; Feng, M.; Dong, M.; Cao, Z.; Zhang, X.G.; Chen, Y.; Liu, J. Multifunctional FeS2 theranostic nanoparticles for photothermal-enhanced chemodynamic/photodynamic cancer therapy and photoacoustic imaging. Chem. Eng. J. 2020, 396, 125294. [Google Scholar] [CrossRef]
- Besford, Q.A.; Ju, Y.; Wang, T.Y.; Yun, G.; Cherepanov, P.V.; Hangemeyer, C.E.; Cavalieri, F.; Caruso, F. Self-Assembled Metal-Phenolic Networks on Emulsions as Low-Fouling and pH-Responsive Particles. Small 2018, 14, 1802342. [Google Scholar] [CrossRef]
- Browning, R.J.; Reardon, P.J.T.; Parhizkar, M.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C.; Stride, E. Drug Delivery Strategies for Platinum-Based Chemotherapy. ACS Nano 2017, 11, 8560–8578. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, Y.; Wang, J.N.; Wei, T.X.; Dai, Z.H. Mild Hyperthermia-Enhanced Enzyme-Mediated Tumor Cell Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 23065–23071. [Google Scholar] [CrossRef]
- Hou, Z.; Sang, S.M.; You, H. Mechanism of Action of (−)-Epigallocatechin-3-Gallate: Auto-oxidation-Dependent Inactivation of Epidermal Growth Factor Receptor and Direct Effects on Growth Inhibition in Human Esophageal Cancer KYSE 150 Cells. Cancer Res. 2005, 65, 8049–8056. [Google Scholar] [CrossRef]
- El-Mowafy, A.M.; Al-Gayyar, M.M.; Salem, H.A.; El-Mesery, M.E.; Darweish, M.M. Novel chemotherapeutic and renal protective effects for the green tea (EGCG): Role of oxidative stress and inflammatory-cytokine signaling. Phytomedicine 2010, 17, 1067–1075. [Google Scholar] [CrossRef]
- Tynga, I.M.; Abrahamse, H. Nano-Mediated Photodynamic Therapy for Cancer: Enhancement of Cancer Specificity and Therapeutic Effects. Nanomaterials 2018, 8, 923. [Google Scholar] [CrossRef]
- Xu, T.; Ma, Y.Y.; Yuan, Q.L.; Hu, H.X.; Hu, X.K.; Qian, Z.Y.; Rolle, J.K.; Gu, Y.Q.; Li, S.W. Enhanced Ferroptosis by Oxygen-Boosted Phototherapy Based on a 2-in-1 Nanoplatform of Ferrous Hemoglobin for Tumor Synergistic Therapy. ACS Nano 2020, 14, 3414–3425. [Google Scholar] [CrossRef]
- Felsher, D.W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer 2003, 3, 375–380. [Google Scholar] [CrossRef]
- Hu, J.J.; Lei, Q.; Zhang, X.Z. Recent advances in photonanomedicines for enhanced cancer photodynamic therapy. Prog. Mater. Sci. 2020, 114, 100685. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Z.L.; Zhao, K.J.; Zhang, P.Y.; Zhong, Q.Z.; Yu, Q.; Zhai, S.M.; Cui, J.W. Co-delivery of enzymes and photosensitizers via metal-phenolic network capsules for enhanced photodynamic therapy. Chin. Chem. Lett. 2022, 33, 1917–1922. [Google Scholar] [CrossRef]
- Lee, H.; Dey, D.K.; Kim, K.; Kim, S.; Kim, E.; Kang, S.C.; Bajpai, V.K.; Huh, Y.S. Hypoxia-responsive nanomedicine to overcome tumor microenvironment-mediated resistance to chemo-photodynamic therapy. Mater. Today Adv. 2022, 14, 100218. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, S.; Zeng, J.; Zhang, L.P.; Zhang, R.H.; Liang, K.; Xie, L.; Shao, B.; Song, S.L.; Huang, G.; et al. Super-assembled core-shell mesoporous silica-metal-phenolic network nanoparticles for combinatorial photothermal therapy and chemotherapy. Nano Res. 2020, 13, 1013–1019. [Google Scholar] [CrossRef]
- Fan, G.; Cottet, J.; Rodriguez-Otero, M.R.; Wasuwanich, P.; Furst, A.L. Metal-Phenolic Networks as Versatile Coating Materials for Biomedical Applications. ACS Appl. Bio Mater. 2022, 5, 4687–4695. [Google Scholar] [CrossRef]
- Wang, H.C.; Wang, D.Y.; Yu, J.Z.; Zhang, Y.D.; Zhou, Y.M. Applications of metal phenolic networks in nanomedicine: A review. Biomater. Sci. 2022, 10, 5786–5808. [Google Scholar] [CrossRef]
- Rahim, M.A.; Kempe, K.; Müllner, M.; Ejima, H.; Ju, Y.; van Koeverden, M.P.; Suma, T.; Braunger, J.A.; Leeming, M.G.; Abrahams, B.F.; et al. Surface-Confined Amorphous Films from Metal-Coordinated Simple Phenolic Ligands. Chem. Mater. 2015, 27, 5825–5832. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Liu, W.; Zeng, X.; Song, X.; Yang, X.; Zhang, X.; Feng, J. Metal Ion/Tannic Acid Assembly as a Versatile Photothermal Platform in Engineering Multimodal Nanotheranostics for Advanced Applications. ACS Nano 2018, 12, 3917. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.B.; Kang, R.; Klionsky, D.J.; Tang, D.L. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Liu, J.; Ren, J.; Huang, F.; Ou, H.; Ding, Y.; Zhang, Y.; Ma, R.; An, Y.; Liu, J.; et al. Green Tea Catechin-Based Complex Micelles Combined with Doxorubicin to Overcome Cardiotoxicity and Multidrug Resistance. Theranostics 2016, 6, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Meng, Q.; Zhou, J.; Chen, B.; Xi, J.; Long, P.; Zhang, L.; Hou, R. Nanoemulsion Delivery System of Tea Polyphenols Enhanced the Bioavailability of Catechins in Rats. Food Chem. 2018, 242, 527–532. [Google Scholar] [CrossRef]
- Yi, Z.; Sun, Z.; Chen, G.; Zhang, H.; Ma, X.; Su, W.; Cui, X.; Li, X. Size-Controlled, Colloidally Stable and Functional Nanoparticles Based on the Molecular Assembly of Green Tea Polyphenols and Keratins for Cancer Therapy. J. Mater. Chem. B 2018, 6, 1373–1386. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, X.; Tian, X.; Zhang, P.; Chen, Z.; Hu, X.; Mei, X. GSH and Enzyme Responsive Nanospheres Based on Self-Assembly of Green Tea Polyphenols and Bsa Used for Target Cancer Chemotherapy. Colloids. Surf. B 2019, 173, 654–661. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, S.; Feng, Y.; Zou, P.; Wang, Y.; Qin, P.; Yue, J.; Liang, Y.; Wang, H.; Liu, L. Preparation of ChitosanEpigallocatechin-3-O-Gallate Nanoparticles and Their Inhibitory Effect on the Growth of Breast Cancer Cells. J. Innov. Opt. Health Sci. 2018, 11, 1850018. [Google Scholar] [CrossRef]
- Liang, K.; Chung, J.E.; Gao, S.J.; Yongvongsoontorn, N.; Kurisawa, M. Highly Augmented Drug Loading and Stability of Micellar Nanocomplexes Composed of Doxorubicin and Poly (Ethylene glycol)-Green Tea Catechin Conjugate for Cancer Therapy. Adv. Mater. 2018, 30, 1706963. [Google Scholar] [CrossRef]
- Chen, J.Y.; Stubbe, J. Bleomycins: Towards better therapeutics. Nat. Rev. Cancer 2005, 5, 102–112. [Google Scholar] [CrossRef]
- Han, J.Y.; Lin, Z.X.; Zhou, J.J.; Yun, G.; Guo, R.; Richardson, J.J.; Caruso, F. Polyphenol-Mediated Assembly of Proteins for Engineering Functional Materials. Angew. Chem. Int. Ed. 2022, 59, 15618–15625. [Google Scholar] [CrossRef]
- Taha, M.S.; Cresswell, G.M.; Park, J.; Lee, W.; Ratliff, T.L.; Yeo, Y. Sustained delivery of carfilzomib by tannic acid based nanocapsules helps develop antitumor immunity. Nano Lett. 2019, 19, 118333–118341. [Google Scholar] [CrossRef]
- Raza, A.; Xu, X.Q.; Xia, L.; Xia, C.K.; Tang, J.; Ouyang, Z. Quercetin-iron complex: Synthesis, characterization, antioxidant, DNA binding, DNA cleavage, and antibacterial activity studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef]
- AM, S.; TF, M.; MAM, M. Mohamed EA and Hasan HF, Ellagic and ferulic acids alleviate gamma radiation and aluminium chloride-induced oxidative damage. Life Sci. 2016, 160, 2–11. [Google Scholar]
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food. Chem. 2002, 50, 2200–2206. [Google Scholar] [CrossRef]
- Galano, A.; Márquez, M.F.; Pérez-González, A. Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef]
- Saha, P.; Yeoh, B.S.; Singh, R.; Chandrasekar, B.; Vemula, P.K.; Haribabu, B.; VijayKumar, M.; Jala, V.R. Gut microbiota conversion of dietary ellagic acid into bioactive phytoceutical urolithin A inhibits heme peroxidases. PLoS ONE 2016, 11, e0156811. [Google Scholar] [CrossRef]
- Allen, C.; Santos, N.D.; Gallagher, R.; Chiu, G.N.C.; Shu, Y.; Li, W.M.; Johnstone, S.A.; Janoff, A.S.; Mayer, L.D.; Webb, M.S. Controlling the physical behavior and biological performance of liposome formulations through use of surface grafted poly (ethylene glycol). Biosci. Rep. 2002, 22, 225–250. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug. Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.E.; Liu, X.W.; Lv, Y.L.; Lu, G.H.; Li, F.; Zhang, F.; Liu, B.; Li, D.; Wei, W.; Li, Y. Nanolongan with Multiple On-Demand Conversions for Ferroptosis-Apoptosis Combined Anticancer Therapy. ACS Nano 2019, 13, 260–273. [Google Scholar] [CrossRef]
- Chen, Q.F.; Zhou, J.; Chen, Z.; Luo, Q.; Xu, J.; Song, G.B. Tumor-Specific Expansion of Oxidative Stress by Glutathione Depletion and Use of a Fenton Nanoagent for Enhanced Chemodynamic Therapy. ACS Appl. Mater. Interface 2019, 11, 30551–30565. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Xu, Z.; Culbert, A.; Luo, T.; Guo, N.; Yang, K.; Pearson, E.; Preusser, B.; Wu, T.; La Riviere, P.; et al. Synergistic checkpoint-blockade and radiotherapy-radiodynamic therapy via an immunomodulatory nanoscale metal-organic framework. Nat. Biomed. Eng. 2022, 6, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, B.; Liu, Y.; Chen, J.; Sun, Y.; Pan, X.; Xu, J.; Xu, S.; Liu, Z.; Tan, W. DNA-based MXFs to enhance radiotherapy and stimulate robust antitumor immune responses. Nano Lett. 2022, 22, 2826–2834. [Google Scholar] [CrossRef] [PubMed]






| Polyphenol | Structure | Application |
|---|---|---|
| TA | 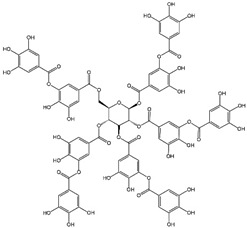 | CDT/PTT/PDT/chemotherapy/IT |
| GA |  | RT/chemotherapy |
| EGCG | 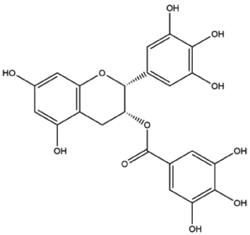 | CDT/PTT/chemotherapy |
| ACN |  | PTT |
| EA |  | PTT |
| Qu |  | PTT |
| Gossypol | 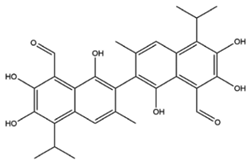 | IT/chemotherapy |
| PEG-polyphenol |  | CDT/PTT/chemotherapy |
| PEG-Ce6-polyphenol | 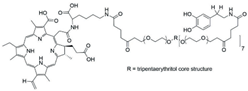 | IT/PDT |
| Pt prodrug-polyphenol |  | chemotherapy |
| Polyphenol | MPN Formation | Materials | Mechanism | Ref. |
|---|---|---|---|---|
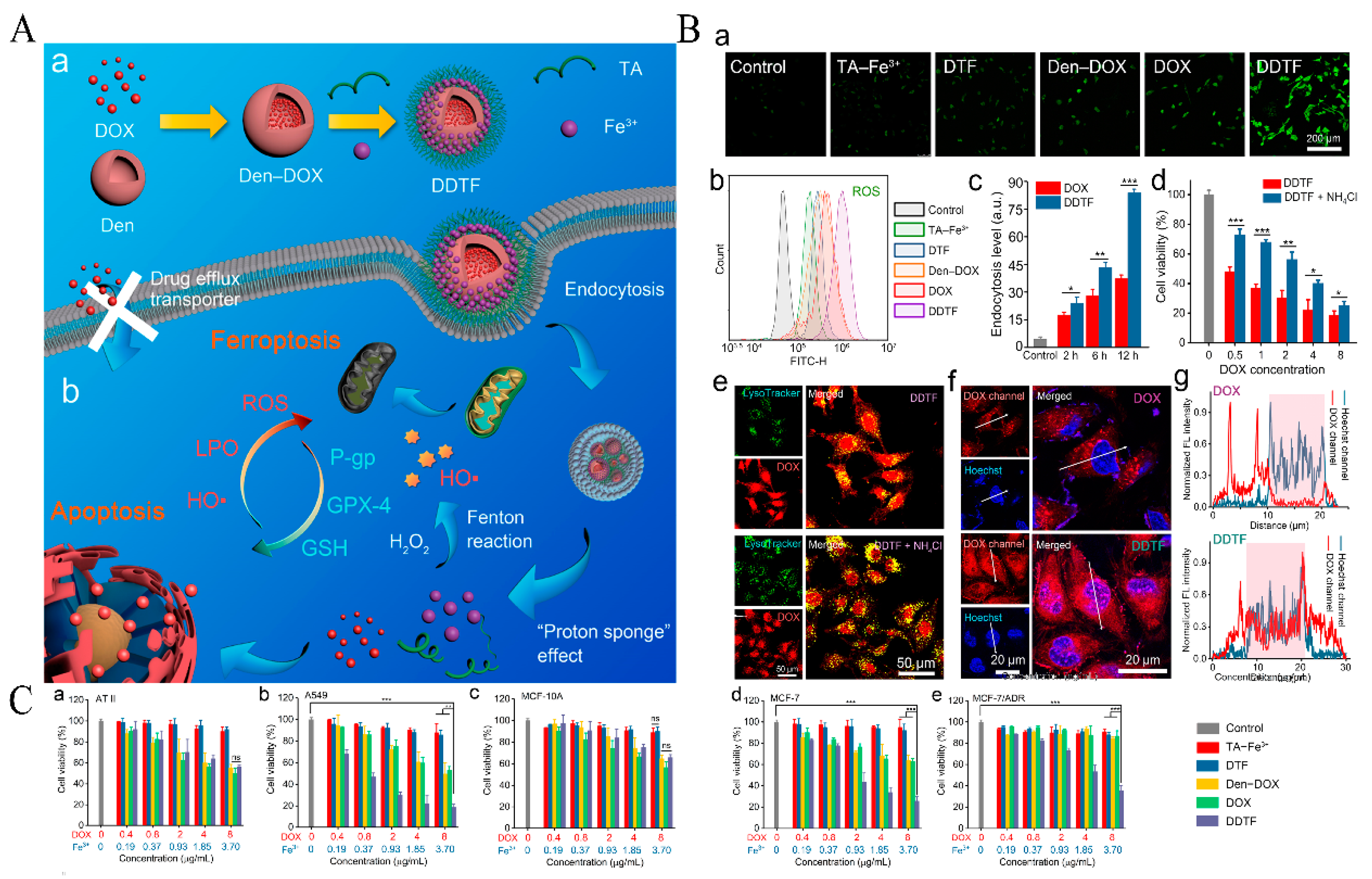
| TA | Nanocoating | Den-DOX-TA-Fe3+ | Induces ferroptosis and enhances ROS. | [40] |
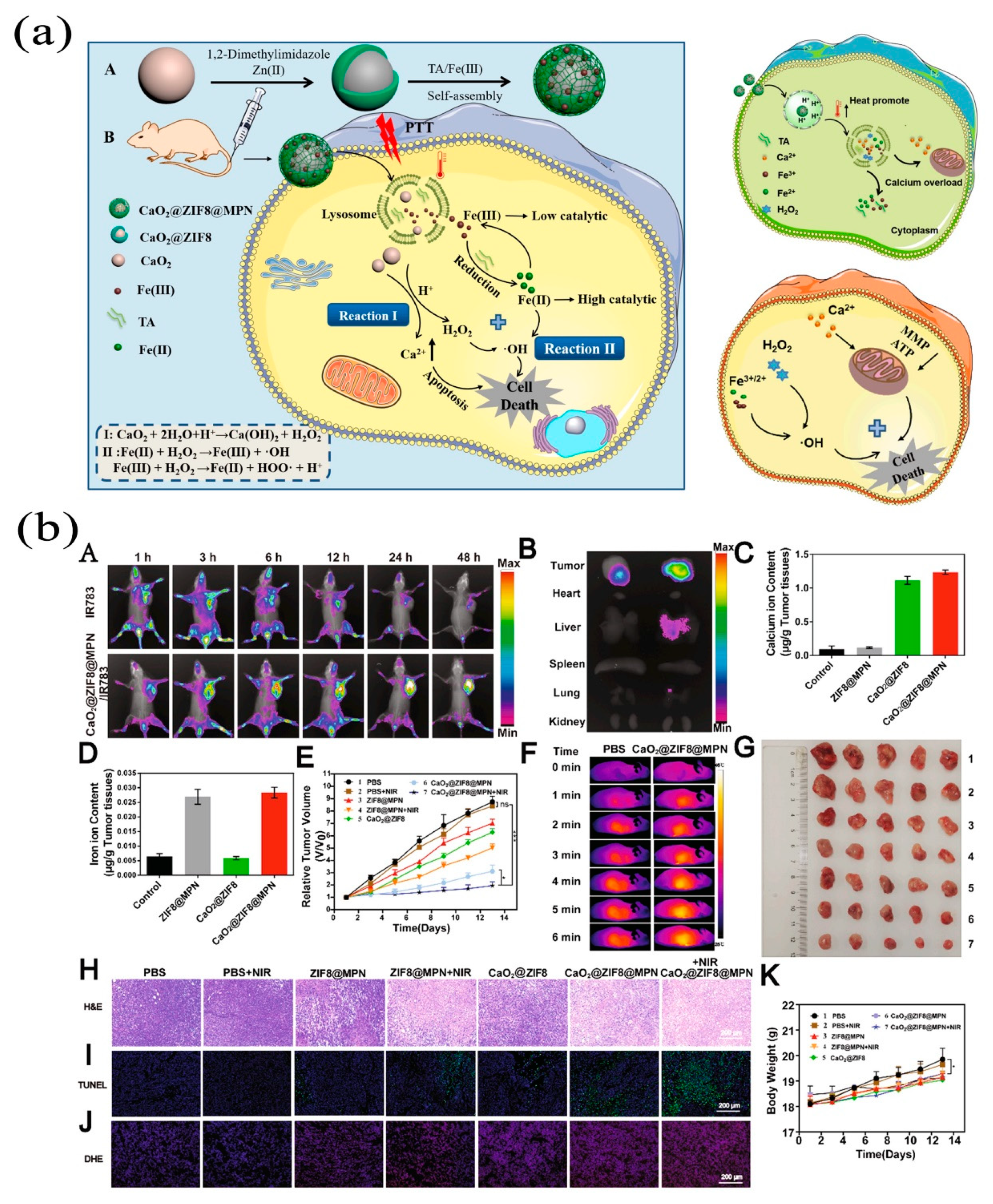
| Nanocoating | CaO2@ZIF8@MPN | Enhances Fenton reaction of Ca2+ react with H2O2. | [41] | |
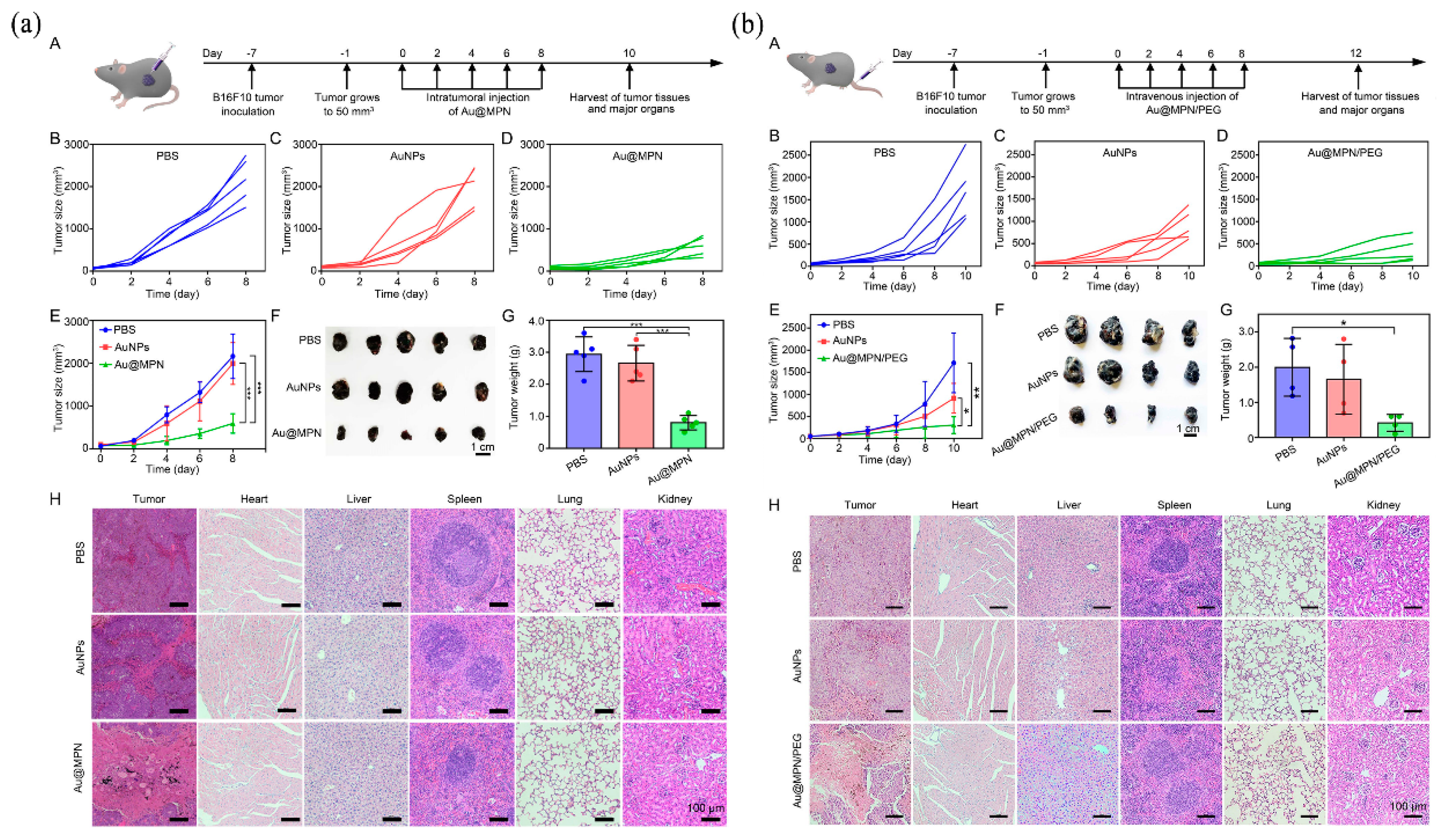
| Nanocoating | Au@MPN | Self-supplies H2O2 and autocatalytic Fenton reaction. | [42] | |
| Nanocoating | G5.NHAc-Toy@TF | Upregulates ROS, downregulates GSH and GPX-4, and allows LPO to accumulate in cells, leading to ferroptosis. | [43] | |
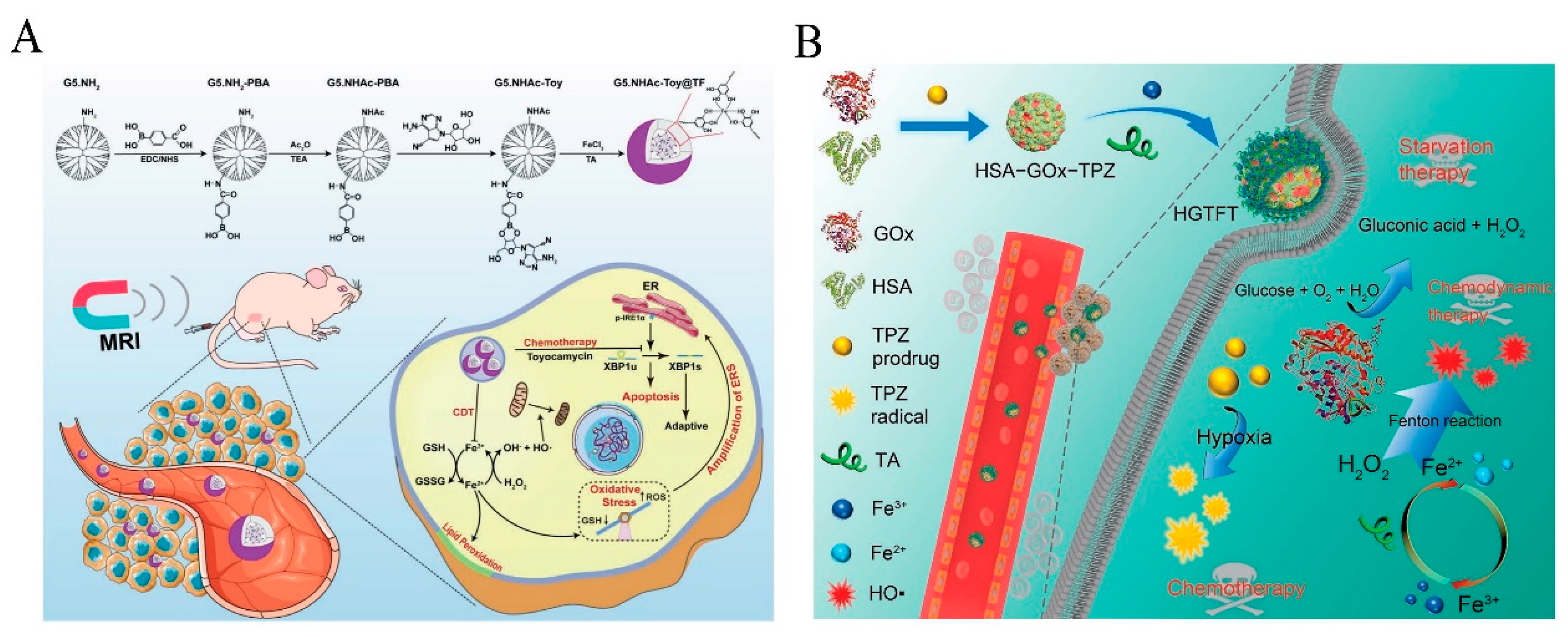
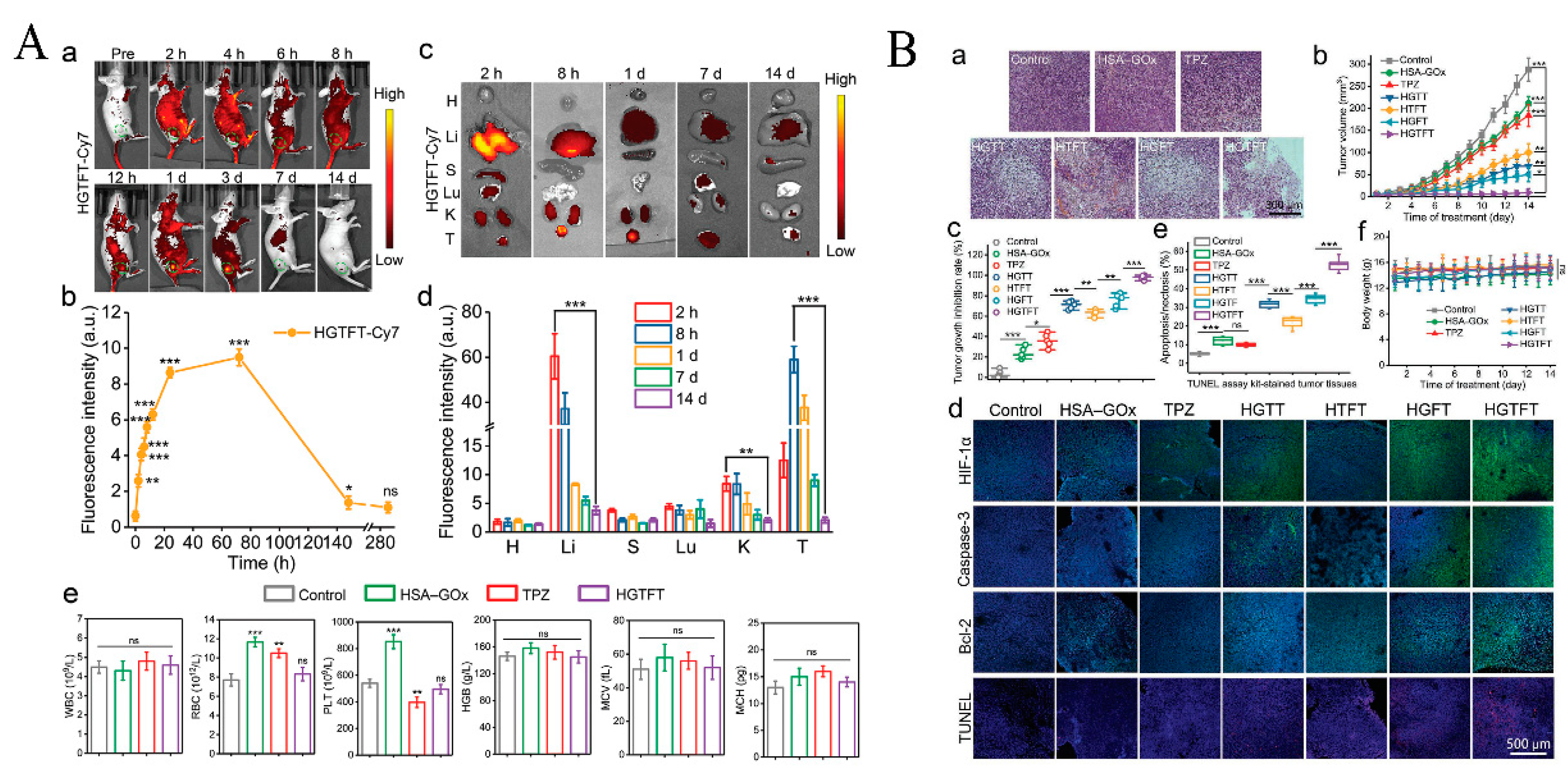
| Nanocoating | HSA-GOx-TPZ-Fe3+-TA (HGTFT) | Accelerates Fe3+/Fe2+ conversion, starvation therapy. | [44] | |
| Nanocoating | PID@Fe-TA | pH-responsiveness and NIR enhance ROS. | [45] | |
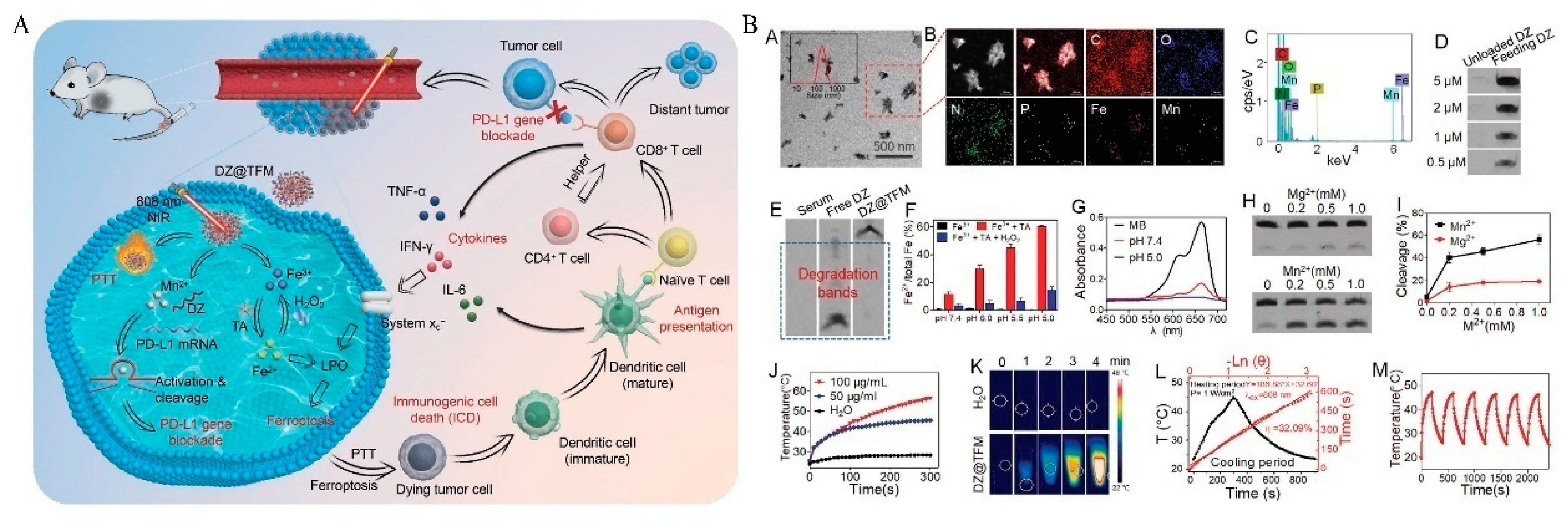
| Nanocoating of TA-Fe3+/Mn2+ | DZ@TFM | Induced ferroptosis and Mn2+-activate DA inhibit PD-L1. | [46] | |
| Nanocoating | SRF@FeIIITA-NAPP | Release of SRF induces ferroptosis while enhancing Fenton response and ROS level | [47] | |
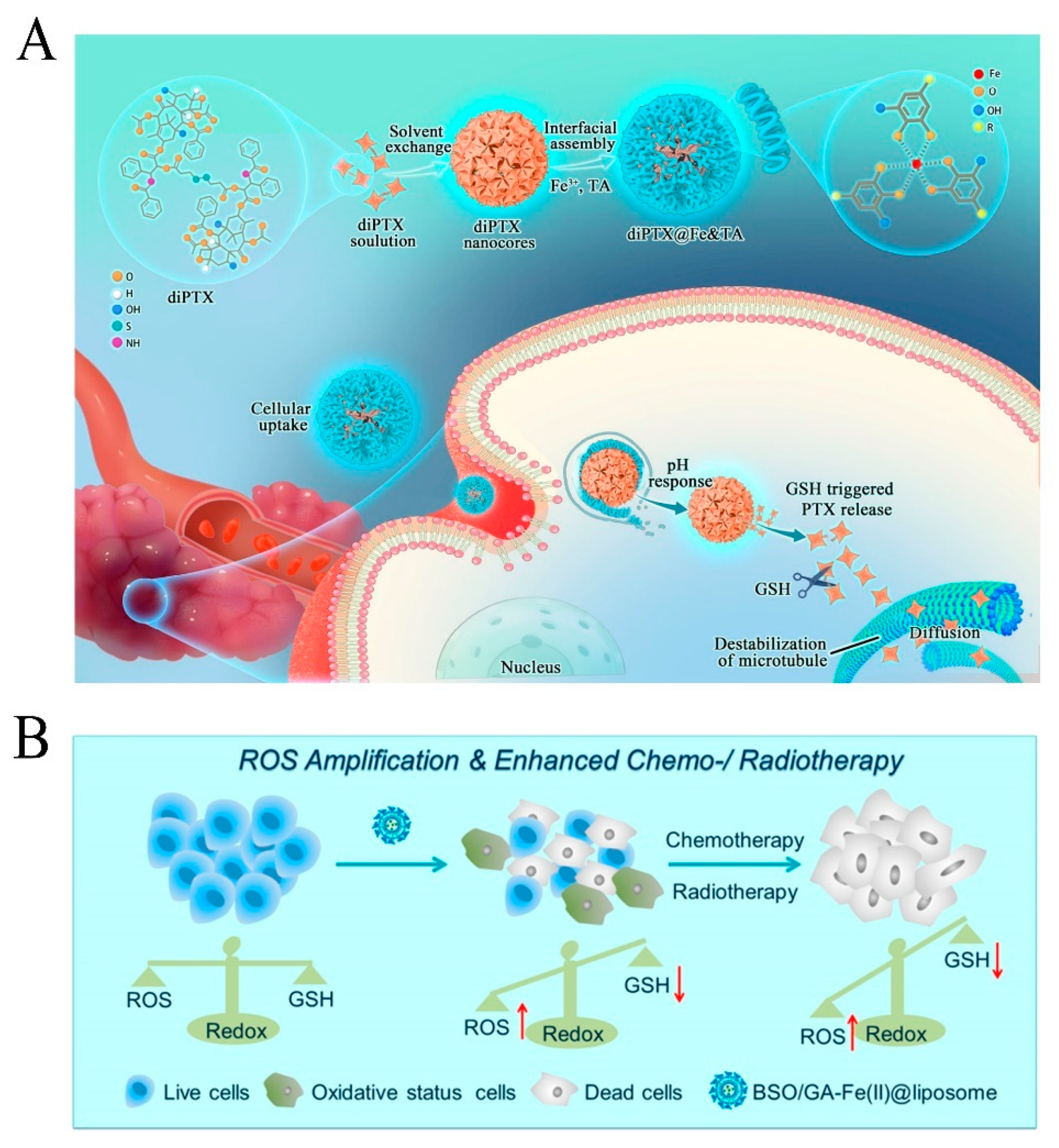
| Nanocoating | diPTX@Fe&TA | Convert diPTX into free PTX to diffuse to microtubules, disrupting the microtubule balance and leading to tumor cell apoptosis. | [48] | |
| Nanocoating | ssPPELap@Fe-TA | Increased Fe3+/H2O2 supply and GSH depletion, immunotherapy synergistic cellular ferroptosis | [49] | |
| GA | Nanoparticle | BSO/GA-Fe(II)@liposome | Generation of ROS and simultaneous consumption of GSH to co-amplify intracellular oxidative stress. | [33] |
| EGCG | Nanocoating of EGCG and PVP with Fe2+ | FeEP-NPs | Continuously supplies Fe2+ with mildly high temperature to promote Fenton reaction. | [50] |
| Nanocomposite | DOX@GTCs-Fe | Synergistic treatment with PTT and chemotherapy in the presence of NIR. | [51] | |
| Nanocoating | BFE@BSA NPs | BLM improves local temperature to enhance Fenton response and ROS level. | [52] | |
| ACN | Nanocomposite | FeAP-NPs | EPR effect, DFO-triggered in vivo dynamic disassembly | [35] |
| EA | Nanoparticle | Fe-EA | pH-activated amplification of MRI contrast effect and in vivo PTT for tumor ablation | [36] |
| Qu | Nanocomposite | Qu-FeIIP | EPR effect, inhibition of Hsp70 expression, ablation and elimination of tumors by low temperature PTT | [53] |
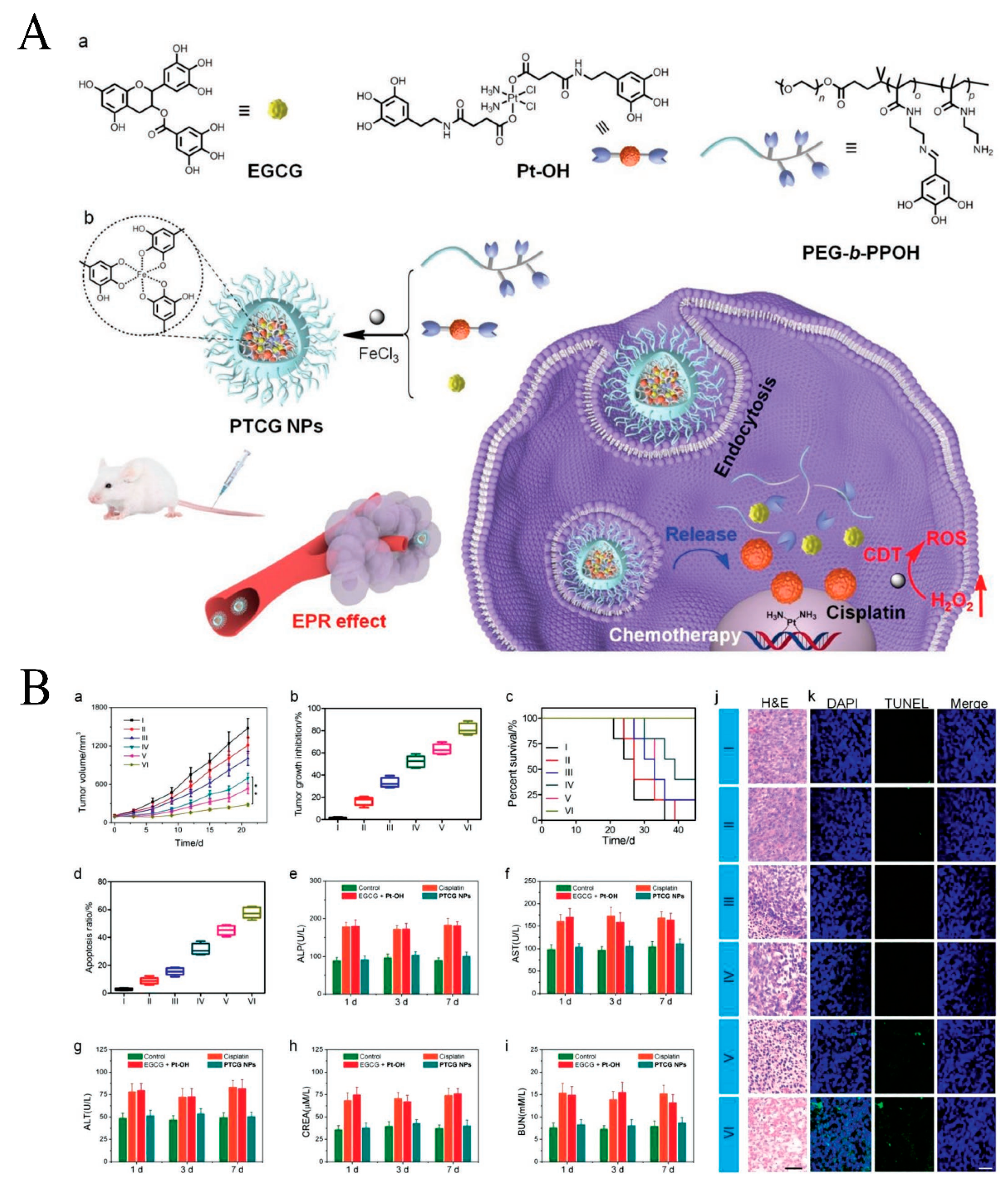
| Pt prodrug-polyphenol | Nanocomposite | PTCG NPs | Cascade reaction enhances H2O2 levels and catalyzes the production of toxic ROS. | [54] |
| PEG-polyphenol | Nanocoating/Nanocomposite | CADNs | GOx catalyzes the achievement of starvation therapy, killing tumor cells through Fe3+/Fe2+-mediated Fenton reaction in conjunction with chemotherapy. | [55] |
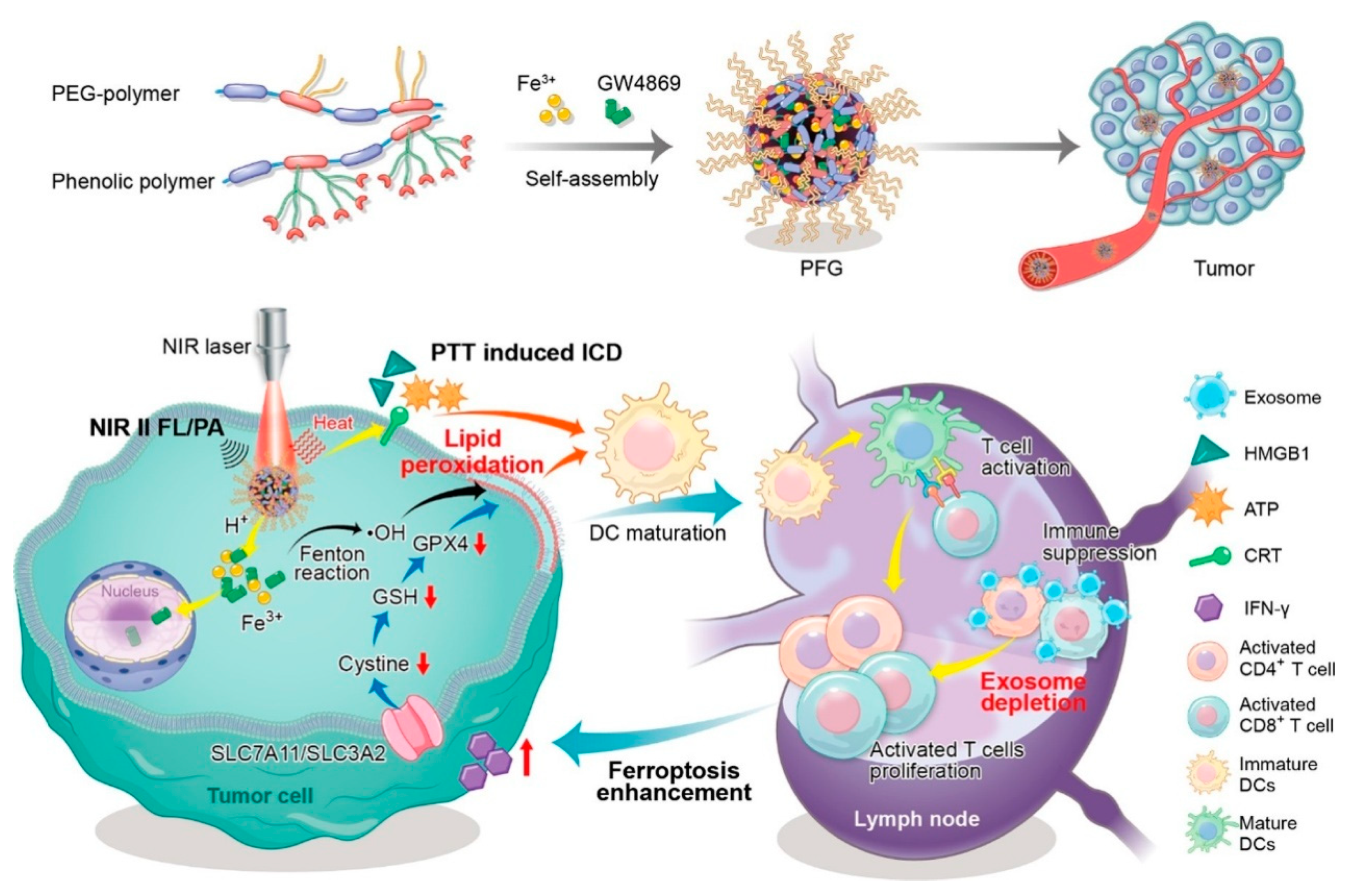
| Nanoparticle/Nanocomposite | PFG MPNs | Relief of exosomal immunosuppression, ferroptosis enhancement, and immune stimulation. | [56] | |
| Nanocomposite | MPN@HMME | HMME, as a photosensitizer, dissociated in lysosomes and killed carcinoma cells with a laser. | [21] | |
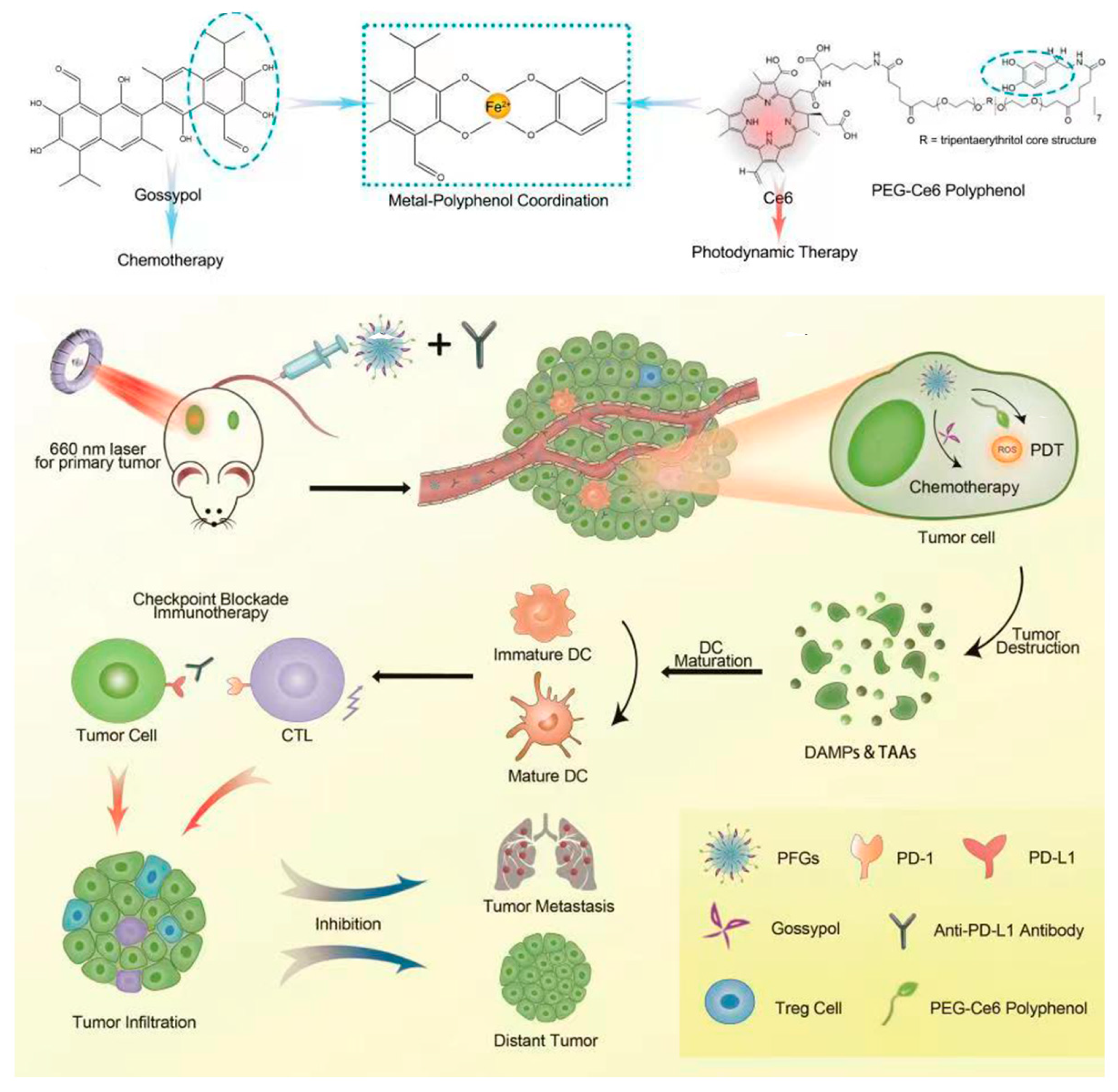
| Gossypol PEG-Ce6-polyphenol | Nanocomposite | PFGs | Ce6, as a photosensitizer induces a Fenton reaction for PDT to enhance the level of ICD. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Liu, M.; Yang, H.; Nezamzadeh-Ejhieh, A.; Lu, C.; Pan, Y.; Liu, J.; Bai, Z. Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications. Pharmaceutics 2023, 15, 1323. https://doi.org/10.3390/pharmaceutics15051323
Chen W, Liu M, Yang H, Nezamzadeh-Ejhieh A, Lu C, Pan Y, Liu J, Bai Z. Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications. Pharmaceutics. 2023; 15(5):1323. https://doi.org/10.3390/pharmaceutics15051323
Chicago/Turabian StyleChen, Weipeng, Miao Liu, Hanping Yang, Alireza Nezamzadeh-Ejhieh, Chengyu Lu, Ying Pan, Jianqiang Liu, and Zhi Bai. 2023. "Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications" Pharmaceutics 15, no. 5: 1323. https://doi.org/10.3390/pharmaceutics15051323
APA StyleChen, W., Liu, M., Yang, H., Nezamzadeh-Ejhieh, A., Lu, C., Pan, Y., Liu, J., & Bai, Z. (2023). Recent Advances of Fe(III)/Fe(II)-MPNs in Biomedical Applications. Pharmaceutics, 15(5), 1323. https://doi.org/10.3390/pharmaceutics15051323








