Exploring the Contribution of Curcumin to Cancer Therapy: A Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Quality Assessment
2.4. Data Extraction and Synthesis
3. Results
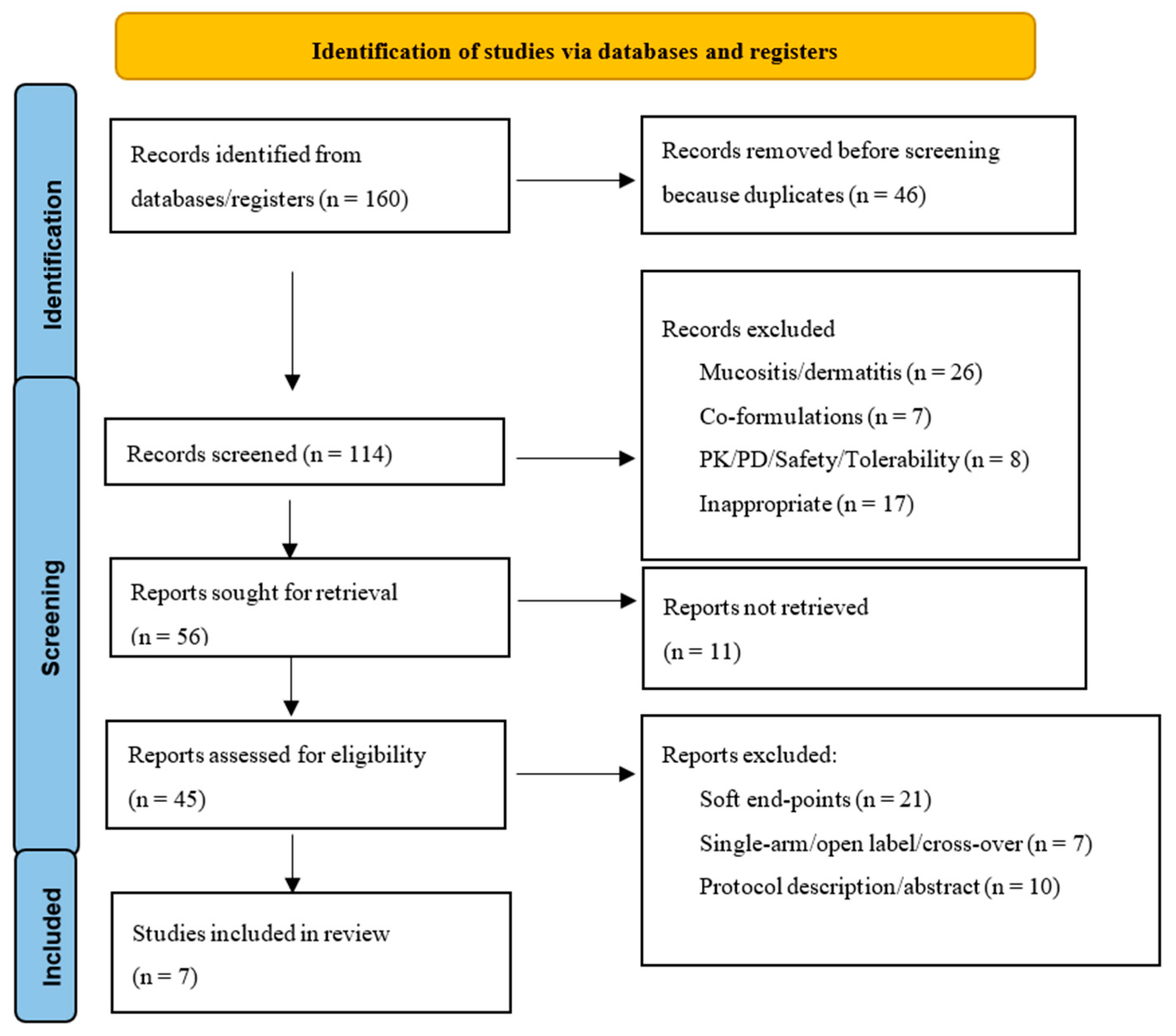
3.1. Study Search and Selection
3.2. Study Characteristics and Synthesis
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Siciliano, R.; Barone, E.; Preziosi, P. Natural substances and Alzheimer’s disease: From preclinical studies to evidence based medicine. Biochim. Biophys. Acta 2012, 1822, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Mhillaj, E.; Tarozzi, A.; Pruccoli, L.; Cuomo, V.; Trabace, L.; Mancuso, C. Curcumin and heme oxygenase: Neuroprotection and beyond. Int. J. Mol. Sci. 2019, 20, 2419. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Nurcahyanti, A.D.R.; Cokro, F.; Wulanjati, M.P.; Mahmoud, M.F.; Wink, M.; Sobeh, M. Curcuminoids for metabolic syndrome: Meta-analysis evidences toward personalized prevention and treatment management. Front. Nutr. 2022, 9, 891339. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, S.; Sindona, C.; Bramanti, P.; Mazzon, E. A state of the art of antioxidant properties of curcuminoids in neurodegenerative diseases. Int. J. Mol. Sci. 2021, 22, 3168. [Google Scholar] [CrossRef]
- Hay, E.; Lucariello, A.; Contieri, M.; Esposito, T.; De Luca, A.; Guerra, G.; Perna, A. Therapeutic effects of turmeric in several diseases: An overview. Chem. Biol. Interact. 2019, 310, 108729. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2016, 7, 205–233. [Google Scholar] [CrossRef]
- Li, C.; Miao, X.; Li, F.; Adhikari, B.K.; Liu, Y.; Sun, J.; Zhang, R.; Cai, L.; Liu, Q.; Wang, Y. Curcuminoids: Implication for inflammation and oxidative stress in cardiovascular diseases. Phytother. Res. 2019, 33, 1302–1317. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; He, L.; Liu, L.; Cheng, B.; Zhou, F.; Cao, D.; He, Y. A comprehensive review on the benefits and problems of curcumin with respect to human health. Molecules 2022, 27, 4400. [Google Scholar] [CrossRef]
- Scuto, M.C.; Mancuso, C.; Tomasello, B.; Ontario, M.L.; Cavallaro, A.; Frasca, F.; Maiolino, L.; Trovato Salinaro, A.; Calabrese, E.J.; Calabrese, V. Curcumin, hormesis and the nervous system. Nutrients 2019, 11, 2417. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, X.; Du, C.; Su, Y.; Yin, L.; Tan, X.; Liu, H.; Wang, Y.; Xu, L.; Xu, X. An examination of the protective effects and molecular mechanisms of curcumin, a polyphenol curcuminoid in diabetic nephropathy. Biomed. Pharmacother. 2022, 153, 113438. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.N.; Xie, Y.; Moaddel, R.; Sanghvi, M.; Dossou, K.S.; Kashuba, A.D.; Sandler, R.S.; Hawke, R.L. Randomized pharmacokinetic crossover study comparing 2 curcumin preparations in plasma and rectal tissue of healthy human volunteers. J. Clin. Pharmacol. 2017, 57, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef]
- Fança-Berthon, P.; Tenon, M.; Bouter-Banon, S.L.; Manfré, A.; Maudet, C.; Dion, A.; Chevallier, H.; Laval, J.; van Breemen, R.B. Pharmacokinetics of a single dose of turmeric curcuminoids depends on formulation: Results of a human crossover study. J. Nutr. 2021, 151, 1802–1816. [Google Scholar] [CrossRef]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. 2012, 69, 65–70. [Google Scholar] [CrossRef]
- Chen, W.F.; Deng, S.L.; Zhou, B.; Yang, L.; Liu, Z.L. Curcumin and its analogues as potent inhibitors of low density lipoprotein oxidation: H-atom abstraction from the phenolic groups and possible involvement of the 4-hydroxy-3-methoxyphenyl groups. Free Radic. Biol. Med. 2006, 40, 526–535. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Vrânceanu, M.; Galimberti, D.; Banc, R.; Dragoş, O.; Cozma-Petruţ, A.; Hegheş, S.C.; Voştinaru, O.; Cuciureanu, M.; Stroia, C.M.; Miere, D.; et al. The anticancer potential of plant-derived nutraceuticals via the modulation of gene expression. Plants 2022, 11, 2524. [Google Scholar] [CrossRef]
- Younes, M.; Mardirossian, R.; Rizk, L.; Fazlian, T.; Khairallah, J.P.; Sleiman, C.; Naim, H.Y.; Rizk, S. The synergistic effects of curcumin and chemotherapeutic drugs in inhibiting metastatic, invasive and proliferative pathways. Plants 2022, 11, 2137. [Google Scholar] [CrossRef] [PubMed]
- Dharman, S.; Maragathavalli, G.; Karpagavalli, S.; Kumar Sampath, R. A systematic review and meta-analysis on the efficacy of curcumin/turmeric for the prevention and amelioration of radiotherapy/radiochemotherapy induced oral mucositis in head and neck cancer patients. Asian Pac. J. Cancer Prev. 2021, 22, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Kumar, N.; Sharma, S.; Parveen, S.; Rasheed, A. Turmeric in the management of oral submucous fibrosis: A systematic review and meta-analysis. J. Cancer Res. Ther. 2021, 17, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ginex, P.K.; Backler, C.; Croson, E.; Horrell, L.N.; Moriarty, K.A.; Maloney, C.; Vrabel, M.; Morgan, R.L. Radiodermatitis in patients with cancer: Systematic review and meta-analysis. Oncol. Nurs. Forum. 2020, 47, E225–E236. [Google Scholar] [CrossRef]
- Costa Normando, A.G.; de Menêses, A.G.; de Toledo, I.P.; Borges, G.Á.; de Lima, C.L.; Diniz Dos Reis, P.E.; Silva Guerra, E.N. Effects of turmeric and curcumin on oral mucositis: A systematic review. Phytother. Res. 2019, 33, 1318–1329. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Gunther, J.R.; Chadha, A.S.; Guha, S.; Raju, G.S.; Maru, D.M.; Munsell, M.F.; Jiang, Y.; Yang, P.; Felix, E.; Clemons, M.; et al. A phase II randomized double blinded trial evaluating the efficacy of curcumin with pre-operative chemoradiation for rectal cancer. J. Gastrointest. Oncol. 2022, 13, 2938–2950. [Google Scholar] [CrossRef]
- Santosa, D.; Suharti, C.; Riwanto, I.; Dharmana, E.; Pangarsa, E.A.; Setiawan, B.; Suyono, S.; Tobing, M.L.; Suhartono, S.; Hadisapurto, S. Curcumin as adjuvant therapy to improve remission in myeloma patients: A pilot randomized clinical trial. Casp. J. Intern. Med. 2022, 13, 375–384. [Google Scholar] [CrossRef]
- Passildas-Jahanmohan, J.; Eymard, J.C.; Pouget, M.; Kwiatkowski, F.; Van Praagh, I.; Savareux, L.; Atger, M.; Durando, X.; Abrial, C.; Richard, D.; et al. Multicenter randomized phase II study comparing docetaxel plus curcumin versus docetaxel plus placebo in first-line treatment of metastatic castration-resistant prostate cancer. Cancer Med. 2021, 10, 2332–2340. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.R.; et al. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Han, D.H.; Kim, S.W.; Kim, M.J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate 2019, 79, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, M.A.; Ramdas, K.; Dey, B.; Iyer, S.; Rajan, G.; Elango, K.K.; Suresh, A.; Ravindran, D.; Kumar, R.R.; Prathiba, R.; et al. A randomized double-blind placebo-controlled phase IIB trial of curcumin in oral leukoplakia. Cancer Prev. Res. 2016, 9, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.R.; Iwuji, C.O.; Morgan, B.; Berry, D.P.; Steward, W.P.; Thomas, A.; Brown, K.; Howells, L.M. Combining curcumin (C3-complex, Sabinsa) with standard care FOLFOX chemotherapy in patients with inoperable colorectal cancer (CUFOX): Study protocol for a randomised control trial. Trials 2015, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Barik, A.; Mishra, B.; Rathinasamy, K.; Pandey, R.; Priyadarsini, K.I. Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells. Biochim. Biophys. Acta 2008, 1780, 673–679. [Google Scholar] [CrossRef]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar] [CrossRef]
- Mancuso, C.; Siciliano, R.; Barone, E. Curcumin and Alzheimer disease: This marriage is not to be performed. J. Biol. Chem. 2011, 286, le3. [Google Scholar] [CrossRef]
- Mancuso, C.; Barone, E. Curcumin in clinical practice: Myth or reality? Trends Pharmacol. Sci. 2009, 30, 333–334. [Google Scholar] [CrossRef]
- Correia, M.A. Drug Biotransformation. In Basic & Clinical Pharmacology, 15th ed.; Katzung, B.G., Vanderah, T.W., Eds.; McGraw Hill: New York, NY, USA, 2021; pp. 57–76. [Google Scholar]
| Formulation | Pharmacokinetic Parameters | Refs. | |||
|---|---|---|---|---|---|
| AUC0–24h (ng/mL h) | Tmax (h) | Cmax (ng/mL) | T1/2 (h) | ||
| Curcumin C3 a (Sabinsa Corporation, East Windsor, NJ, USA) | 731.6 e | 2–7 | 32–103 e | - | [13,14] |
| BCM-95 CG b (Arjuna Natural Extracts, Ltd., Aluva, India) | 3201.3 f | 3.5 | 456.88 f | 5 | [15] |
| PC-curcumin c (Meriva, Indena S.p.A., Milan, Italy) | 669.4 g | 0.5–2 | 42–119 g | 22.8 ± 34.2 | [13,14,16] |
| NP-curcumin d (Theracurmin, Theravalues Corporation, Tokyo, Japan) | 2649 ± 350 h 3649 ± 430 i | 1–6 h 2–6 i | 189 ± 48 h 275 ± 67 i | 9.7 ± 2.1 h 13 ± 3.3 i | [17] |
| First Author, Year | Country | Study Design | Duration of Study | Sample Size | Study Population | Age (Years Range) | Males (%) | Intervention | Study Endpoints (Time) | Experimental Arm N° (%) | Control Arm N° (%) | Hazard Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gunther et al. [28] | Germany | Phase II RCT | 13 years | 22 (E: 15;C: 7) | Patients with either T3/T4 or T2 and node-positive locally advanced CRC | 28–75 | 59% | E: capecitabine (825 mg/m2 per os + RT (50.4 Gy in 28 fractions) + curcumin C3 complex (8 g/day per os during RT and for 6 weeks after its completion) C: capecitabine + RT as above + placebo | Pathologic complete response (at the time of surgery) | 1 (7%) | 2 (33%) * | |
| Overall survival (5 years) | 85.7% | 85.7% | ||||||||||
| Progression-free survival (5 years) | 66.7% | 71.4% | ||||||||||
| Cumulative incidence of local regional failure (5 years) | 6.7% | 14.3% | ||||||||||
| Cumulative incidence of distant failure (5 years) | 33.3% | 28.6% | ||||||||||
| Santosa et al. [29] | Indonesia | Pilot RCT | 16 weeks | 33 (E: 17; C: 16) | New patients with MM who were ineligible for transplant and were not previously treated | 31–77 | 60.6% | E: MP regimen (melphalan 4 mg/m2 per os, prednisone 40 mg/m2 per os for 7 days) + curcumin (BCM-95 CG 8 g/day per os for 28 days) C: MP + placebo | Remission (4 months) | 9 out of 12 patients who completed the follow-up (75%) | 4 out of 12 patients who completed the follow-up (33.3%) | |
| Passildas-Jahanmohan et al. [30] | France | Phase II RCT | 18 weeks | 50 (44 in the ITT) [E: 26 (22); C: 24 (22)] | Patients with stage IV PC with documented castration resistance who were previously submitted to surgery, therapy, or hormonotherapy | 44–87 | 100% | E: docetaxel (75 mg/m2 IV on day 1 every 3 weeks for 6 cycles) + prednisone/prednisolone (10 mg/day per os) + curcumin (6 g/day per os for 7 days every 3 weeks for 6 months) C: docetaxel and prednisone/prednisolone as above + placebo | Progression-free survival | 5.3 months | 3.7 months | N.A. |
| Cumulative progression-free survival (6 months) | 31.8% | 45.5% | ||||||||||
| Overall survival | 15.8 months | 19.8 months | N.A. | |||||||||
| Cumulative survival (12 months) Cumulative survival (24 months) | 60.1% 20% | 80% 29.3% | ||||||||||
| Grade 3 or 4 adverse events | 10 | 6 | ||||||||||
| Saghatelyan et al. [31] | Armenia | Phase II RCT | 23 weeks | 150 (E: 75; C: 75) | Patients with progressive, locally advanced, or MBC after at least one prior chemotherapy regimen who had progressed during or within 12 months of completing adjuvant or neoadjuvant chemotherapy or other cases of BC in which weekly paclitaxel was considered an adequate approach | 28–75 | 0% | E: paclitaxel (80 mg/m2 IV once every 7 days for 12 weeks) + curcumin [(CUC-01) 300 mg IV once every 7 days for 12 weeks] C: paclitaxel as above + placebo | Objective response rate (16 weeks) Objective response rate (24 weeks) | 38 (all PR, 50,7% ITT and 61.3% among 47 patients who completed the treatment) 22 [1 CoR and 21 PR, (29% in ITT, 44.9% among 47 patients who completed the treatment)] | 25 (all PR, 33.3% in ITT and 38.5% among 46 patients who completed the treatment) 15 (all PR, 20% in ITT, 27.8 among 46 patients who completed the treatment) | |
| Progression-free survival | 27.0 weeks | 24.6 weeks | 1.28 (95% CI: 0.765–3.135) | |||||||||
| Stable disease (16 weeks) Stable disease (24 weeks) | 18 (24% in ITT) 12 (16% in ITT) | 26 (34.77% in ITT) 15 (20% in ITT) | ||||||||||
| Progressive disease | 5 (6.7%) | 14 (18.7%) | ||||||||||
| Patients with any adverse event | 39 (54%) | 42 (56%) | ||||||||||
| Patients with grade 3–4 adverse event | 3 (4%) | 2 (2.7%) | ||||||||||
| Howells et al. [32] | United Kingdom | Phase IIa RCT | 24 weeks | 27 (E: 18; C: 9) | Patients with stage IV CRC without previous treatment | 53–78 | N.A. | E: FOLFOX ± bevacizumab (80 mg/m2 once every 7 days for 12 weeks) + curcumin C3 complex (2 g per os, once every 2 weeks for 12 cycles) C: FOLFOX ± bevacizumab (once every 2 weeks for ≤12 cycles or until patient progression, unacceptable toxicity, death, or withdrawal) + placebo | Overall survival (ITT) | N.A. | N.A. | 0.339 (95% CI: 0.141; 0.815) |
| Overall survival (PP) | 596 days | 200 days | 0.271 (95% CI: 0.106; 0.697) | |||||||||
| Cumulative overall survival (PP) (6 months) | 93.3% | 55.6% | ||||||||||
| Progression-free survival (ITT) | N.A. | N.A. | 0.571 (95% CI: 0.24; 1.36) | |||||||||
| Progression-free survival (PP) | 320 days | 171 days | 0.549 (95% CI: 0.225; 1.34) | |||||||||
| Cumulative progression-free survival (PP) (6 months) | 73.3% | 33.3% | ||||||||||
| Objective response (6 cycles) Objective response (12 cycles) | 66.7% 53.3% | 44.4% 11.1% | ||||||||||
| Total adverse events | 282 | 103 | ||||||||||
| Choi et al. [33] | South Korea | RCT | 36 months | 97 (E: 49; C: 48) | Patients with stage IV PC with BCR after localized treatments or metastatic PC at initial diagnosis who received LHRH agonist and anti-androgens for at least 6 months with a subsequent ADT withdrawal period | E (mean ± SD): 71.5 ± 9.0) C (mean ± SD): 72.9 ± 6.0) | 100% | E: ADT withdrawal (deprivation after LHRH agonist and anti-androgens for at least 6 months) + curcumin (1440 mg/day per os, 2 capsules for 3 times/day for 6 months) C: ADT withdrawal (as above) + placebo | Off-treatment # | 16.3 months | 18.5 months | N.A. |
| PSA progression (6 months) | 10.3% | 30.2% | ||||||||||
| Patients who had adverse events | 7 (15.6% out of 45 patients who ingested the test food more than once after randomization) | 16 (34.8% out of 46 patients who ingested the test food more than once after randomization) | ||||||||||
| Kuriakose, et al. [34] | India | Phase IIb RCT | 12 months | 223 (E: 111; C: 112) | Patients with clinical and histologically confirmed oral leukoplakia of a size more than 15 mm2 in area, with any linear dimension more than 1 cm, and without previous treatment | 26–74 | 72.2% | E: curcumin BCM-95 CG (3.6 g/day per os for 6 months) C: placebo | Clinical response based on the lesion size (6 months) | 75 (67.5% out of 105 available at the end of 6 months for the evaluation of primary endpoints) | 62 (55.3% out of 108 available at the end of 6 months for the evaluation of primary endpoints) | |
| Histologic response (6 months) | 25 (22.32%) | 23 (20.53%) | ||||||||||
| Combined clinical and histologic response (6 months) | 65 (58%) | 50 (44.64%) | ||||||||||
| Clinical response based on the lesion size (12 months) | 29 (54.7% among 103 subjects with a 50% or greater decrease in the lesions at 6 months) | 30 (60% among 103 subjects with a 50% or greater decrease in the lesions at 6 months) | ||||||||||
| Subjects experiencing any adverse events | 26 (23.4%) | 35 (31.3%) | ||||||||||
| Subjects experiencing moderate/severe adverse events | 4 (3.6%) | 18 (16.1%) |
| Articles | D1 | D2 | D3 | D4 | D5 | O |
|---|---|---|---|---|---|---|
| Gunther et al. [28] |  |  |  |  |  |  |
| Santosa et al. [29] |  |  |  |  |  |  |
| Passildas-Jahanmohan et al. [30] |  |  |  |  |  |  |
| Saghatelyan et al. [31] |  |  |  |  |  |  |
| Howells et al. [32] |  |  |  |  |  |  |
| Choi et al. [33] |  |  |  |  |  |  |
| Kuriakose et al. [34] |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Waure, C.; Bertola, C.; Baccarini, G.; Chiavarini, M.; Mancuso, C. Exploring the Contribution of Curcumin to Cancer Therapy: A Systematic Review of Randomized Controlled Trials. Pharmaceutics 2023, 15, 1275. https://doi.org/10.3390/pharmaceutics15041275
de Waure C, Bertola C, Baccarini G, Chiavarini M, Mancuso C. Exploring the Contribution of Curcumin to Cancer Therapy: A Systematic Review of Randomized Controlled Trials. Pharmaceutics. 2023; 15(4):1275. https://doi.org/10.3390/pharmaceutics15041275
Chicago/Turabian Stylede Waure, Chiara, Carlotta Bertola, Gaia Baccarini, Manuela Chiavarini, and Cesare Mancuso. 2023. "Exploring the Contribution of Curcumin to Cancer Therapy: A Systematic Review of Randomized Controlled Trials" Pharmaceutics 15, no. 4: 1275. https://doi.org/10.3390/pharmaceutics15041275
APA Stylede Waure, C., Bertola, C., Baccarini, G., Chiavarini, M., & Mancuso, C. (2023). Exploring the Contribution of Curcumin to Cancer Therapy: A Systematic Review of Randomized Controlled Trials. Pharmaceutics, 15(4), 1275. https://doi.org/10.3390/pharmaceutics15041275









