Metal and Metal Oxides Nanoparticles and Nanosystems in Anticancer and Antiviral Theragnostic Agents
Abstract
1. Introduction
2. Applications of MNPs for MRI in Cancer Diagnostics
2.1. MRI Contrast Agents
2.2. T1-Contrast Agents
2.3. T2-Contrast Agents
2.4. Targeted Contrast Agents
2.5. Ultrasmall Superparamagnetic Iron Oxides Nanoparticles (USSPIONPs) as T1 and T1/T2 Contrasts Agents
- By evading the nonspecific absorption by mononuclear phagocytes, these NPs can circulate within the body for an extended period, making them suitable for targeted imaging, steady-state imaging, and high-resolution imaging.
- Appropriate surface modifications enable these particles to clear through the kidneys, which reduces the risk of iron overload in patients with iron metabolism disorders, thereby improving biocompatibility and ensuring biosafety.
- Assembly/disassembly can be utilized to produce T2/T1 switchable contrast enhancement effects, thus improving the accuracy and sensitivity of MRI, making them a viable option.
2.6. Mixed Oxides as Contrasts Agents
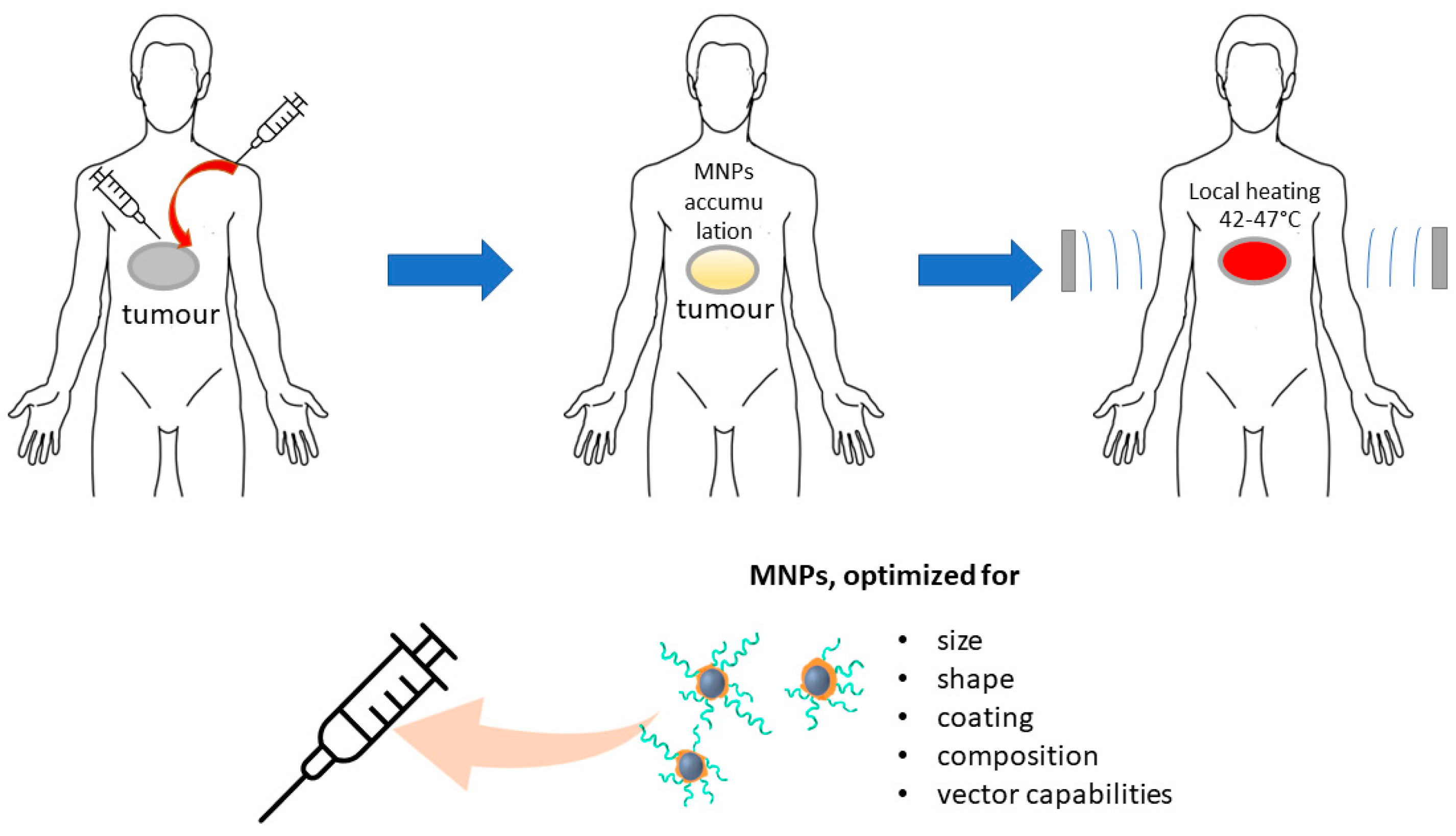
3. Applications of MNPs for Cancer Heating Therapy
3.1. HT Types
- Whole body (WB) HT.
- HT by wireless applicators (WA).
- HT by heating source insertion (HIS).
- Magnetic hyperthermia (MHT).
3.2. Types of MNPs Suitable for HT and Their Requirements
3.3. Effect of Size and Shape on MHT Properties
3.4. Effect of Coating on HT Properties
- Preserving MNPs’ physiochemical properties and composition.
- Enhancing MNPs’ biocompatibility while also reducing toxicity since their surface comes into direct contact with blood and tissues.
- Inserting hydrophilic molecules on the surface to enhance the dispersity of SPMNPs, which in turn prevents agglomeration, controls particle size, reduces the possibility of blood capillary obstruction, and improves blood circulation by transporting SPMNPs to targeted areas.
- Altering the surface to create a more suitable platform for further functionalization and protein absorption.
- Preventing the SPMNPs opsonization.
3.5. Targeting Cancer Cells with MNPs for MHT
4. Photo-Thermal Therapy and Photo-Dynamic Therapy (PTT and PDT)
4.1. PTT
- Precise targeting of therapy to particular tissues to prevent unintended effects due to high radiation absorption caused by the resonance wavelength of NPs.
- The ability of NPs to reach tumors located in deeper tissues through attachment of specific agents to the surface of Au NPs that augment their specificity for selected tumor cells.
- Possibility of merging NPs PTT with drug treatment as NPs serve as carriers for drugs to designated tissue locations, thereby amplifying their curative potential.
4.2. PDT
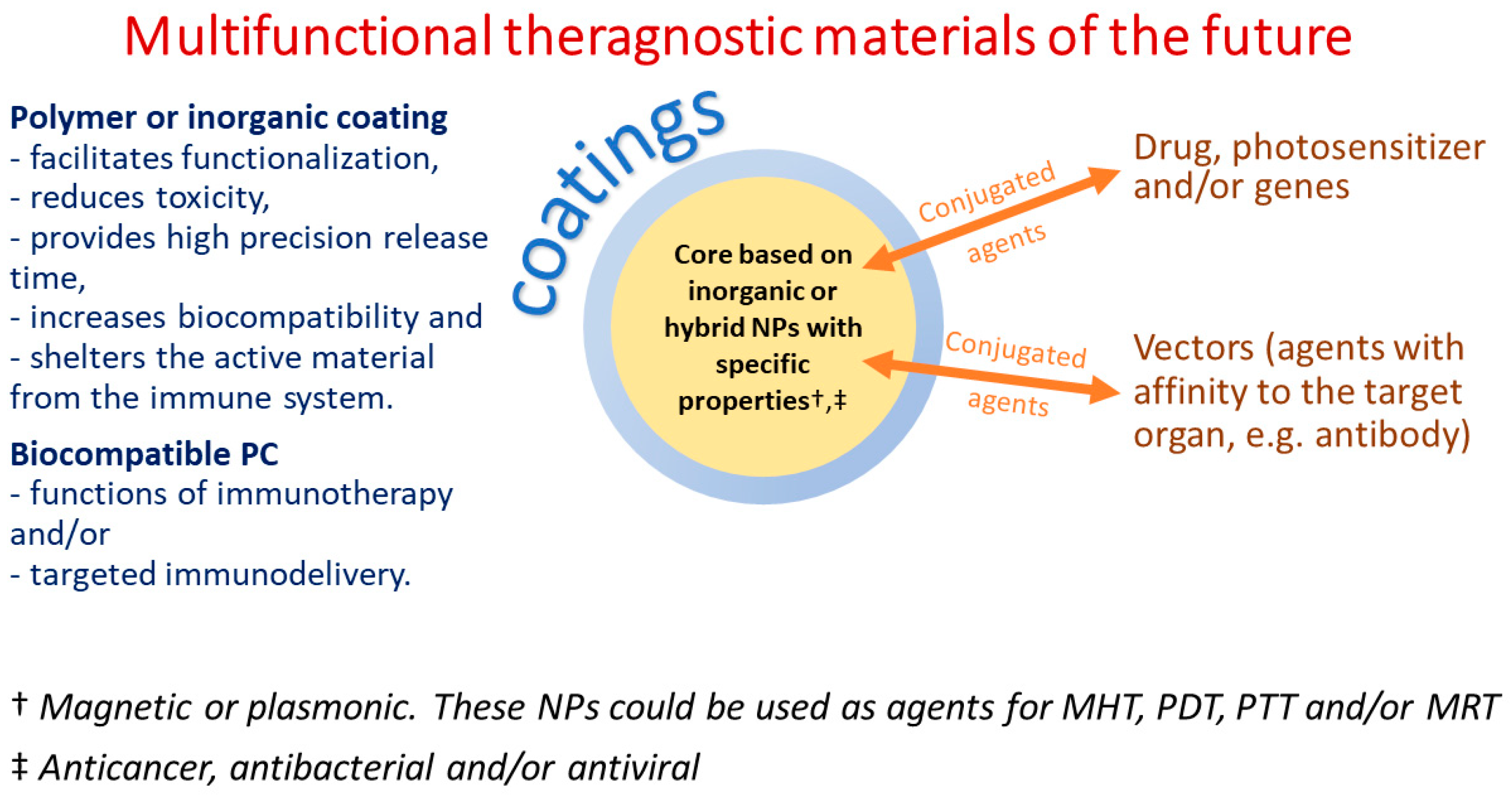
5. Applications of INPs for Drug Delivery
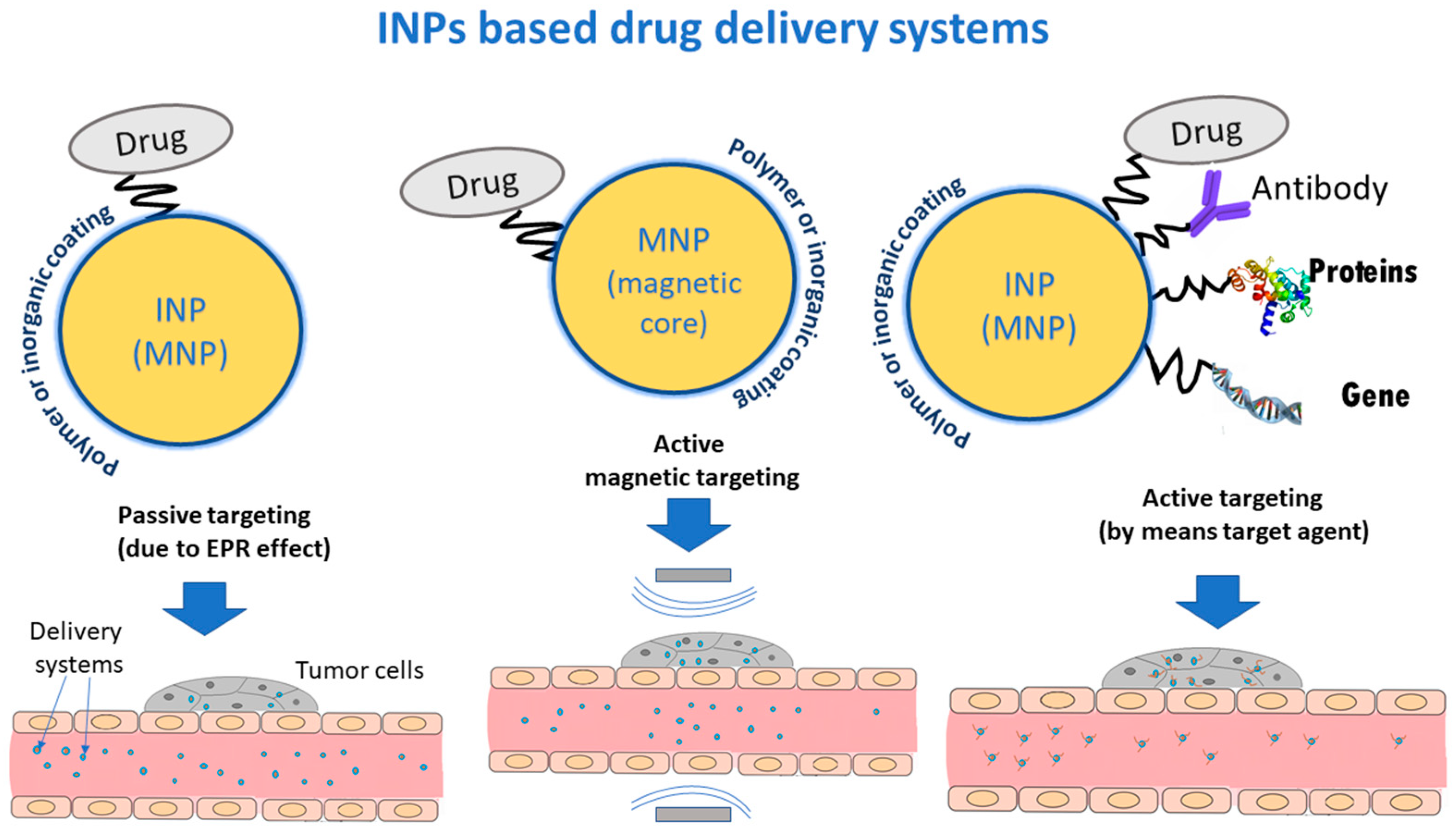
5.1. NPs Based Delivery Systems
- Visualized (utilizing ferro-, ferri-, and superparamagnetic NPs for MRI).
- Controlled or fixed in position via a magnetic field.
- Subjected to a magnetic field for heat-induced drug release or tissue hyperthermia/ablation.
5.2. Delivery Strategies for Drug Systems
5.2.1. Passive Targeting
5.2.2. Magnetic Targeting
- Fastening a biocompatible MNPs carrier to a cytotoxic drug.
- Infusing these hybrid nanosystems as a colloidal suspension through an intravenous injection.
- Employing a magnetic field gradient to steer these hybrid nanosystems towards the affected area.
- Directed release the medical substance from the drug system.
5.2.3. Active Targeting
6. Applications of Metal and Metal Oxides NPs for Delivery of Anticancer Agents
6.1. Targeted Delivery Systems of Different Anticancer Agents Based on MNPS
6.1.1. Conventional Chemotherapeutic Agents
6.1.2. Genes
6.1.3. Proteins and Peptides
6.2. Targeted Delivery Systems of Different Anticancer Agents Based on Non-Magnetic NPS
7. INPs in Antiviral Therapy
7.1. INPs Antiviral Application
7.2. INPs-Based Nanomaterials for Antiviral Drug Delivery
8. The Effect of Protein Corona on Targeted Theragnostic Nanosystems
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shabatina, T.; Vernaya, O.; Shumilkin, A.; Semenov, A.; Melnikov, M. Nanoparticles of Bioactive Metals/Metal Oxides and Their Nanocomposites with Antibacterial Drugs for Biomedical Applications. Materials 2022, 15, 3602. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.M.; Sait, S.M.; Ellahi, R. Magnetic Nanoparticles for Drug Delivery through Tapered Stenosed Artery with Blood Based Non-Newtonian Fluid. Pharmaceuticals 2022, 15, 1352. [Google Scholar] [CrossRef] [PubMed]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Deliv. Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Batlle, X.; Moya, C.; Escoda-Torroella, M.; Iglesias, Ò.; Rodríguez, A.F.; Labarta, A. Magnetic nanoparticles: From the nanostructure to the physical properties. J. Magn. Magn. Mater. 2022, 543, 168594. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Deo, S.V.S.; Sharma, J.; Kumar, S. Globocan 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef]
- Tietze, R.; Zaloga, J.; Unterweger, H.; Lyer, S.; Friedrich, R.P.; Janko, C.; Pöttler, M.; Dürr, S.; Alexiou, C. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem. Biophys. Res. Com. 2015, 468, 463–470. [Google Scholar] [CrossRef]
- Renella, P.; Li, J.; Prosper, A.E.; Finn, J.P.; Nguyen, K.-L. Ferumoxytol-Enhanced Cardiac Magnetic Resonance Angiography and 4D Flow: Safety and Utility in Pediatric and Adult Congenital Heart Disease. Children 2022, 9, 1810. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Alonso, V.; Pratt, E.C.; Zong, H.; Lara-Martinez, A.; Kaittanis, C.; Rabie, M.O.; Longo, V.; Becker, M.W.; Roboz, G.J.; Grimm, J.; et al. FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nat. Nanotechnol. 2019, 14, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.H.; Alizadeh, M.; Derakhshan, P.; Babazadeh, P.; Jahandideh, A. Understanding Epidemic Data and Statistics: A case study of COVID-19. J. Med. Virol. 2020, 92, 868–882. [Google Scholar] [CrossRef]
- Hawkes, N. Cancer survival data emphasise importance of early diagnosis. BMJ 2019, 364, l408. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.F.; de Lange, S.V.; Pijnappel, R.M.; Mann, R.M.; Peeters, P.H.M.; Monninkhof, E.M.; Emaus, M.J.; Loo, C.E.; Bisschops, R.H.C.; Lobbes, M.B.I.; et al. Supple-mental MRI Screening for Women with Extremely Dense Breast Tissue. N. Engl. J. Med. 2019, 381, 2091–2102. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Hazhirkarzar, B.; Dublin, A.B. Gadolinium Magnetic Resonance Imaging, 1st ed.; StatPearls Publishing LLC: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482487/ (accessed on 4 July 2022).
- Daldrup-Link, H.E.; Theruvath, A.J.; Rashidi, A.; Iv, M.; Majzner, R.G.; Spunt, S.L.; Goodman, S.; Moseley, M. How to stop using gadolinium chelates for magnetic resonance imaging: Clinical-translational experiences with ferumoxytol. Pediatr. Radiol. 2021, 52, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Qureshi, Z.P.; Sartor, A.O.; Norris, L.B.; Murday, A.; Xirasagar, S.; Thomsen, H.S. Gadolini-um-induced nephrogenic systemic fibrosis: The rise and fall of an iatrogenic disease. Clin. Kidney J. 2012, 5, 82–88. [Google Scholar] [CrossRef]
- Xia, Q.; Feng, X.; Huang, H.; Du, L.; Yang, X.; Wang, K. Gadolinium-induced oxidative stress triggers endoplas-mic reticulum stress in rat cortical neurons. J. Neurochem. 2011, 117, 38–47. [Google Scholar] [CrossRef]
- Chen, R.; Ling, D.; Zhao, L.; Wang, S.; Liu, Y.; Bai, R.; Baik, S.; Zhao, Y.; Chen, C.; Hyeon, T. Parallel comparative studies on mouse toxicity of oxide nanoparticle- and gadolinium-based T1 MRI contrast agents. ACS Nano 2015, 9, 12425–12435. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, A.; Chen, X. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2016, 14, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Siebenhandl-Wolff, P.; Tranquart, F.; Jones, P.; Evansl, P. Gadolinium: Pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol. 2022, 96, 403–429. [Google Scholar] [CrossRef] [PubMed]
- Geraldes, C.F.; Laurent, S. Classification and basic properties contrast agents for magnetic resonance imaging. Contrast Media Mol. Imaging 2009, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.J. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.M. Biomedical Nanomagnetics: A Spin Through Possibilities in Imaging, Diagnostics, and Therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, B.; van Veggel, F.C.J.M.; Tomanek, B. Applications of Nanoparticles for MRI Cancer Diagnosis and Therapy. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef] [PubMed]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.-A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Joh, D.Y.; Al-Zaki, A.; Stangl, M.; Murty, S.; Davis, J.J.; Baumann, B.C.; Alonso-Basanta, M.; Kao, G.D.; Tsourkas, A. Theranostic application of mixed gold and superparamagnetic iron oxide nanoparticle micelles in glioblastoma multiforme. J. Biomed. Nanotechnol. 2016, 12, 347–356. [Google Scholar] [CrossRef]
- Ding, N.; Sano, K.; Kanazaki, K.; Ohashi, M.; Deguchi, J.; Kanada, Y.; Ono, M.; Saji, H. In Vivo HER2-Targeted Magnetic Resonance Tumor Imaging Using Iron Oxide Nanoparticles Conjugated with Anti-HER2 Fragment An-tibody. Mol. Imaging Biol. 2016, 18, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Tsoukalas, C.; Psimadas, D.; Kastis, G.A.; Koutoulidis, V.; Harris, A.L.; Paravatou-Petsotas, M.; Karageorgou, M.; Furenlid, L.R.; Moulopoulos, L.A.; Stamopoulos, D.; et al. A novel metal-based imaging probe for targeted dual-modality SPECT/MR imaging of angiogenesis. Front. Chem. 2018, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhong, Y.; Ji, G.; Lu, Q.; Dai, X.; Guo, Z.; Zhang, P.; Peng, G.; Zhang, K.; Li, Y. Preparation and charac-terization of Fe3O4@Au-C225 composite targeted nanoparticles for MRI of human glioma. PLoS ONE 2018, 13, e0195703. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Z.; Wang, F.; Yang, Y.; Liu, Y.; Wei, G.; Yang, A.; Zhang, R.; Huan, Y.; Cui, Y.; et al. MRI of prostate stem cell antigen expression in prostate tumors. Nanomedicine 2012, 7, 691–703. [Google Scholar] [CrossRef]
- Abdi, N.; Shahbazi-Gahrouei, D. Assessment of superparamagnetic iron oxide nanoparticles conjugated with anti-epidermal growth factor receptor antibody for the detection of lung cancer by Magnetic Resonance Imaging (MRI) in C57BL/6. J. Isfahan. Med. Sch. 2021, 38, 1038–1042. [Google Scholar]
- Chen, L.; Xie, J.; Wu, H.; Zang, F.; Ma, M.; Hua, Z.; Gu, N.; Zhang, Y. Improving sensitivity of magnetic resonance imaging by using a dual-targeted magnetic iron oxide nanoprobe. Colloids Surf. B Biointerfaces 2018, 161, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Paredes, O.K.; Ruiz-Cabello, J.; Martínez-Ruíz, P.; Pingarrón, J.M.; Villalonga, R.; Filice, M. Hybrid Decorated Core@Shell Janus Nanoparticles as a Flexible Platform for Targeted Multimodal Molecular Bioimaging of Cancer. Appl. Mater. Interfaces 2018, 10, 31032–31043. [Google Scholar] [CrossRef] [PubMed]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, Z.; Dong, L.; Zhou, H.; Yang, S.; Wu, W.; Lin, J. A GPC3-specific aptamer-mediated magnetic resonance probe for hepatocellular carcinoma. Int. J. Nanomed. 2018, 13, 4433–4443. [Google Scholar] [CrossRef]
- Wang, G.; Qian, K.; Mei, X. A theranostic nanoplatform: Magneto-gold@fluorescence polymer nanoparticles for tumor targeting T1&T2-MRI/CT/NIR fluorescence imaging and induction of genuine autophagy mediated chemo-therapy. Nanoscale 2018, 10, 10467–10478. [Google Scholar] [CrossRef]
- Kimura, A.; Utsumi, S.; Shimokawa, A.; Nishimori, R.; Hosoi, R.; Stewart, N.J.; Imai, H.; Fujiwara, H. Targeted Imaging of Lung Cancer with Hyperpolarized 129Xe MRI Using Surface-Modified Iron Oxide Nanoparticles as Molecular Contrast Agents. Cancers 2022, 14, 6070. [Google Scholar] [CrossRef]
- Rahmani, R.; Darroudi, M.; Gharanfoli, M.; Chamani, J.; Gholamin, M.; Hashemi, M. Conjugated PNC-27 pep-tide/PEI-Superparamagnetic iron oxide nanoparticles (SPIONs) as a double targeting agent for early cancer diag-nosis: In vitro study. IJBMS 2022, 25, 1234–1242. [Google Scholar] [CrossRef]
- Chen, C.; Ge, J.; Gao, Y.; Chen, L.; Cui, J.; Zeng, J.; Gao, M. Ultrasmall superparamagnetic iron oxide nanoparticles: A next generation contrast agent for magnetic resonance imaging. WIREs Nanomed. Nanobiotechnology 2021, 14, e1740. [Google Scholar] [CrossRef]
- Wei, H.; Bruns, O.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wiśniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef]
- Aires, A.; Fernández-Afonso, Y.; Guedes, G.; Guisasola, E.; Gutiérrez, L.; Cortajarena, A.L. Engineered Pro-tein-Driven Synthesis of Tunable Iron Oxide Nanoparticles as T1 and T2 Magnetic Resonance Imaging Contrast Agents. Chem. Mater. 2022, 34, 10832–10841. [Google Scholar] [CrossRef]
- Mao, Y.; Li, Y.; Zang, F.; Yu, H.; Yan, S.; Song, Q.; Qin, Z.; Sun, J.; Chen, B.; Huang, X.; et al. Contin-uous synthesis of extremely small-sized iron oxide nanoparticles used for T1-weighted magnetic resonance imaging via a fluidic reactor. Sci. China Mater. 2022, 65, 1646–1654. [Google Scholar] [CrossRef]
- Nasrin, S.; Chowdhury, F.-U.-Z.; Hossen, M.M.; Islam, A.; Kumar, A.; Hoque, S.M. Study of the suitability of manganese-substituted cobalt ferrites nanoparticles as MRI contrast agent and treatment by employing hyperther-mia temperature. J. Magn. Magn. Mater. 2022, 564, 170065. [Google Scholar] [CrossRef]
- Saeidi, H.; Mozaffari, M.; Ilbey, S.; Dutz, S.; Zahn, D.; Azimi, G.; Bock, M. Effect of Europium Substitution on the Structural, Magnetic and Relaxivity Properties of Mn-Zn Ferrite Nanoparticles: A Dual-Mode MRI Contrast-Agent Candidate. Nanomaterials 2023, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lai, S.-M.; Li, C.-Z.; Yu, H.-P.; Venkatesan, P.; Lai, P.-S. D-Alpha-Tocopheryl Poly(ethylene Glycol 1000) Succinate-Coated Manganese-Zinc Ferrite Nanomaterials for a Dual-Mode Magnetic Resonance Imaging Contrast Agent and Hyperthermia Treatments. Pharmaceutics 2022, 14, 1000. [Google Scholar] [CrossRef] [PubMed]
- Cheraghali, S.; Dini, G.; Caligiuri, I.; Back, M.; Rizzolio, F. PEG-Coated MnZn Ferrite Nanoparticles with Hier-archical Structure as MRI Contrast Agent. Nanomaterials 2023, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Gas, P. Essential facts on the history of hyperthermia and their connections with electromedicine. Prz. Elektrotechniczny 2011, 87, 37–40. [Google Scholar] [CrossRef]
- Chiriac, H.; Petreus, T.; Carasevici, E.; Labusca, L.; Herea, D.-D.; Danceanu, C.; Lupu, N. In vitro cytotoxicity of fe–cr–nb–b magnetic nanoparticles under high frequency electromagnetic field. J. Magn. Magn. Mater. 2015, 380, 13–19. [Google Scholar] [CrossRef]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nano 2014, 6, 11553–11573. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, G.; Liang, C.; Xu, J.; Dong, Z.; Han, X.; Wang, C.; Liu, Z. Iron Nanoparticles for Low-power Local Magnetic Hyperthermia in Combination with Immune Checkpoint Blockade for Systemic Antitumor Therapy. Nano Lett. 2019, 19, 4287–4296. [Google Scholar] [CrossRef] [PubMed]
- Pham, P.T.T.; Le, X.T.; Kim, H.; Kim, H.K.; Lee, E.S.; Oh, K.T.; Choi, H.-G.; Youn, Y.S. Indocyanine green and curcumin Co-loaded nano-fireball-like albumin nanoparticles based on near-infrared-induced hyperthermia for tumor ablation. Int. J. Nanomed. 2020, 15, 6469–6484. [Google Scholar] [CrossRef]
- Salati, A.; Ramazani, A.; Kashi, M.A. Tuning hyperthermia properties of FeNiCo ternary alloy nanoparticles by morphological and magnetic characteristics. J. Magn. Magn. Mater. 2019, 498, 166172. [Google Scholar] [CrossRef]
- Al Nasir, M.H.; Siddique, S.; Aisida, S.O.; Altowairqi, Y.; Fadhali, M.M.; Shariq, M.; Khan, M.S.; Qamar, M.A.; Shahid, T.; Shahzad, M.I.; et al. Structural, Magnetic, and Magnetothermal Properties of Co100−xNix Nanoparticles for Self-Controlled Hyperthermia. Coatings 2022, 12, 1272. [Google Scholar] [CrossRef]
- Dhawan, U.; Tseng, C.-L.; Wu, P.-H.; Liao, M.-Y.; Wang, H.-Y.; Wu, K.C.-W.; Chung, R.-J. Theranostic doxorubicin encapsulated FeAu alloy@metal-organic framework nanostructures enable magnetic hyperthermia and medical imaging in oral carcinoma. Nanomed. Nanotechnol. Biol. Med. 2023, 48, 102652. [Google Scholar] [CrossRef]
- Sanad, M.F.; Meneses-Brassea, B.P.; Blazer, D.S.; Pourmiri, S.; Hadjipanayis, G.C.; El-Gendy, A.A. Superparamagnetic Fe/Au Nanoparticles and Their Feasibility for Magnetic Hyperthermia. Appl. Sci. 2021, 11, 6637. [Google Scholar] [CrossRef]
- Ting, C.-K.; Dhawan, U.; Tseng, C.-L.; Alex Gong, C.-S.; Liu, W.-C.; Tsai, H.-D.; Chung, R.-J. Hyperthermia-Induced Controlled Local Anesthesia Administration Using Gelatin-Coated Iron–Gold Alloy Nanoparticles. Pharmaceutics 2020, 12, 1097. [Google Scholar] [CrossRef]
- Kawahara, I.; Goto, K.; Kodama, K.; Luo, Y.; Fujiwara-Tani, R.; Mori, T.; Miyagawa, Y.; Tanaka, H.; Kodama, H.; Hosoito, N.; et al. Magnetic Hyperthermia Using Self-Controlled Heating Elements Consisting of Fe-Al Milling Alloy Induces Cancer Cell Apoptosis while Preserving Skeletal Muscle. Pathobiology 2019, 86, 254–262. [Google Scholar] [CrossRef]
- Astefanoaei, I.; Gimaev, R.; Zverev, V.; Stancu, A. Modelling of working parameters of Gd and FeRh nanoparticles for magnetic hyperthermia. Mater. Res. Express 2019, 6, 125089. [Google Scholar] [CrossRef]
- Astefanoaei, I.; Stancu, A.; Chiriac, H. Magnetic hyperthermia with Fe-Cr-Nb-B magnetic particles. AIP Conf. Proc. 2017, 1796, 040006. [Google Scholar] [CrossRef]
- Pan, I.A.Y.; Jamil, Y.; Shah, A.A.; Amir, M.; Al Islam, S.; Fazal, Y.; Chen, J.; Shen, Z. Comparison of copper-based Cu-Ni and Cu-Fe nanoparticles synthesized via laser ablation for magnetic hyperthermia and antibacterial applications. Phys. B Condens. Matter 2023, 650, 414503. [Google Scholar] [CrossRef]
- Briceño, S.; Hernandez, A.C.; Sojo, J.; Lascano, L.; Gonzalez, G. Degradation of magnetite nanoparticles in biomimetic media. J. Nanopart. Res. 2017, 19, 140. [Google Scholar] [CrossRef]
- Zahn, D.; Landers, J.; Buchwald, J.; Diegel, M.; Salamon, S.; Müller, R.; Köhler, M.; Ecke, G.; Wende, H.; Dutz, S. Ferrimagnetic Large Single Domain Iron Oxide Nanoparticles for Hyperthermia Applications. Nanomaterials 2022, 12, 343. [Google Scholar] [CrossRef]
- Ferreira, L.P.; Reis, C.P.; Robalo, T.T.; Melo Jorge, M.E.; Ferreira, P.; Gonçalves, J.; Hajalilou, A.; Cruz, M.M. Assisted Synthesis of Coated Iron Oxide Nanoparticles for Magnetic Hyperthermia. Nanomaterials 2022, 12, 1870. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, O.A.; Zharkov, M.N.; Ageev, V.P.; Yakobson, D.E.; Shlyapkina, V.I.; Zaborovskiy, A.V.; Inchina, V.I.; Balykova, L.A.; Tishin, A.M.; Sukhorukov, G.B.; et al. Magnetic Hyperthermia Nanoarchitectonics via Iron Oxide Nanoparticles Stabilised by Oleic Acid: Anti-Tumour Efficiency and Safety Evaluation in Animals with Transplanted Carcinoma. Int. J. Mol. Sci. 2022, 23, 4234. [Google Scholar] [CrossRef]
- Hermosa, G.C.; Liao, C.-S.; Wu, H.-S.; Wang, S.-F.; Liu, T.-Y.; Jeng, K.-S.; Lin, S.-S.; Chang, C.-F.; Sun, A.-C.A. Green Synthesis of Magnetic Ferrites (Fe3O4, CoFe2O4, and NiFe2O4) Stabilized by Aloe Vera Extract for Cancer Hyperthermia Activities. IEEE Trans. Magn. 2022, 58, 5400307. [Google Scholar] [CrossRef]
- Andrade, R.G.D.; Ferreira, D.; Veloso, S.R.S.; Santos-Pereira, C.; Castanheira, E.M.S.; Côrte-Real, M.; Rodrigues, L.R. Synthesis and Cytotoxicity Assessment of Citrate-Coated Calcium and Manganese Ferrite Nanoparticles for Magnetic Hyperthermia. Pharmaceutics 2022, 14, 2694. [Google Scholar] [CrossRef]
- Liu, N.N.; Pyatakov, A.P.; Saletsky, A.M.; Zharkov, M.N.; Pyataev, N.A.; Sukhorukov, G.B.; Gun’ko, Y.K.; Tishin, A.M. The “field or frequency” dilemma in magnetic hyperthermia: The case of ZnMn ferrite nanoparticles. J. Magn. Magn. Mater. 2022, 555, 169379. [Google Scholar] [CrossRef]
- Jamir, M.; Borgohain, C.; Borah, J.P. Study of chitosan coated copper substituted nano-ferrites for hyperthermia applications. Phys. E Low-Dimens. Syst. Nanostructures 2023, 146, 115560. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, M.A.; Torres, T.E.; Andrés-Vergés, M.; Costo, R.; de la Presa, P.; Serna, C.J.; Morales, M.P.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Magnetic nanoparticles for power absorption: Optimizing size, shape and magnetic properties. J. Solid State Chem. 2009, 182, 2779–2784. [Google Scholar] [CrossRef]
- Sathya, A.; Guardia, P.; Brescia, R.; Silvestri, N.; Pugliese, G.; Nitti, S.; Manna, L.; Pellegrino, T. CoxFe3–xO4 nanocubes for theranostic applications: Effect of cobalt content and particle size. Chem. Mater. 2016, 28, 1769–1780. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.-C.; Gazeau, F. Size-sorted anionic Iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Gu, H.C.; Yang, Z.Q. The heating effect of magnetic fluids in an alternating magnetic field. J. Magn. Magn. Mater. 2005, 293, 334–340. [Google Scholar] [CrossRef]
- Maksoud, A.; Ahmed, M.I.; Mohamady, G.M.; Ahmad, K.S.; Amer, F.R.; Ahmed, O.I.; Al-Muhtaseb, A.H.; Rooney, D.W.; Norhan, M.M.A.N.; Ahmed, A.H. Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications. Nanotechnol. Rev. 2022, 11, 372–413. [Google Scholar] [CrossRef]
- Khurshid, H.; Masa, J.A.; Nemati, Z.; Phan, M.-H.; Mukherjee, P.; Fdez-Gubieda, M.L.; Barandiarán, J.; Srikanth, H. Anisotropy effects in magnetic hyperthermia: A comparison between spherical and cubic exchange-coupled FeO/Fe3O4 nanoparticles. J. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Porshokouh, Z.N.; Kalappattil, V.; Torres, D.; Phan, M.-H.; Garaio, E.; García, J.Á.; Llamazares, S.J.L.; Srikanth, H. Tunable high aspect ratio Iron oxide nanorods for enhanced hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Kovac, J.; Kubovcikova, M.; Antal, I.; Kopcansky, P.; Bednarikova, M.; Muckova, M. The cytotoxicity of iron oxide nanoparticles with different modifications evaluated in vitro. J. Magn. Magn. Mater. 2015, 380, 85–89. [Google Scholar] [CrossRef]
- Ding, Q.; Liu, D.; Guo, D.; Yang, F.; Pang, X.; Che, R.; Zhou, N.; Xie, J.; Sun, J.; Huang, Z.; et al. Shape-controlled fabrication of magnetite silver hybrid nanoparticles with high performance magnetic hyperthermia. Biomaterials 2017, 124, 35–46. [Google Scholar] [CrossRef]
- Clark, A.J.; Wiley, D.T.; Zuckerman, J.E.; Webster, P.; Chao, J.; Lin, J.; Yen, Y.; Davis, M.E. CRLX101 nanoparticles localize in human tumors and not in adjacent, nonneoplastic tissue after intravenous dosing. Proc. Natl. Acad. Sci. USA 2016, 113, 3850–3854. [Google Scholar] [CrossRef]
- Wu, V.M.; Huynh, E.; Tang, S.; Uskoković, V. Brain and bone cancer targeting by a ferrofluid composed of superparamagnetic iron-oxide/silica/carbon nanoparticles (earthicles). Acta Biomater. 2019, 88, 422–447. [Google Scholar] [CrossRef]
- De Nardo, S.J.; De Nardo, G.L.; Natarajan, A.; Miers, L.A.; Foreman, A.R.; Gruettner, C.; Adamson, G.N.; Ivkov, R. Thermal dosimetry predictive of efficacy of 111In-ChL6 nanoparticle AMF–induced thermoablative therapy for human breast cancer in mice. J. Nucl. Med. 2007, 48, 437–444. [Google Scholar] [PubMed]
- Zhang, J.; Dewilde, A.H.; Chinn, P.; Foreman, A.; Barry, S.; Kanne, D.; Braunhut, S.J. Herceptin-directed nanoparticles activated by an alternating magnetic field selectively kill HER-2 positive human breast cells in vitro via hyperthermia. Int. J. Hyperth. 2011, 27, 682–697. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Wang, J.-Q.; Ashby, C.R.; Zeng, L.; Fan, Y.-F.; Chen, Z.-S. Gold nanoparticles: Synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, F.; Najam-ul-Haq, M.; Javeed, R.; Huck, C.W.; Bonn, G.K. Au-Nanomaterials as a Superior Choice for Near-Infrared Photothermal Therapy. Molecules 2014, 19, 20580–20593. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, R.J.; Karsh, E.H.; Taqi, Z.J.; Jabir, M.S. Biocompatibility of gold nanoparticles: In-vitro and In-vivo study. Mater. Today Proc. 2021, 42, 3041–3045. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Na-noparticles and Their Endocytotic Fate Inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Fan, J.H.; Hung, W.I.; Li, W.T.; Yeh, J.M. Biocompatibility Study of Gold Nanoparticles to Human Cells. In IFMBE Proceedings, Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; Springer: Berlin/Heidelberg, Germany, 2009; Volume 23. [Google Scholar] [CrossRef]
- Tian, Y.; Qiang, S.; Wang, L. Gold Nanomaterials for Imaging-Guided Near-Infrared in vivo Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 398. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Hariharan, K.; Patel, P.; Mehta, T. Surface modifications of gold nanoparticles: Stabilization and recent applications in cancer therapy. Pharm. Dev. Technol. 2022, 27, 665–683. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Q.; Zhu, C.; Luo, Y.; Lu, Q.; Li, H.; Ye, R.; Du, D.; Lin, Y. Mitochondrial-targeted multifunctional mesoporous Au@Pt nanoparticles for dual-mode photodynamic and photothermal therapy of cancers. Nanoscale 2017, 9, 15813–15824. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, M.; Wu, J.; Li, C.; Shen, D.; Yang, D.; Li, L.; Ge, M.; Chang, Z.; Dong, W. Core-Shell Magnetic Gold Nanoparticles for Magnetic Field-Enhanced Radio-Photothermal Therapy in Cervical Cancer. Nanomaterials 2017, 7, 111. [Google Scholar] [CrossRef] [PubMed]
- Maximenko, A.; Depciuch, J.; Łopuszyńska, N.; Stec, M.; Światkowska-Warkocka, Ż.; Bayev, V.; Zielinski, P.M.; Baran, J.; Fedotova, J.; Weglarz, W.P.; et al. Fe3O4@SiO2@Au nanoparticles for MRI-guided chemo/NIR photothermal therapy of cancer cells. RSC Adv. 2020, 10, 26508–26520. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hsu, C.-H.; Huang, C.-C.; Chang, P.-Y. Development of Therapeutic Au–Methylene Blue Nanoparticles for Targeted Photodynamic Therapy of Cervical Cancer Cells. ACS Appl. Mater. Interfaces 2014, 7, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.W.; Raziq, F.; Fakhar-e-Alam, M.; Aziz, M.H.; Alimgeer, K.S.; Atif, M.; Amir, M.; Hanif, A.; Farooq, A. Tailoring of Au-TiO2 Nanoparticles conjugated with Doxorubicin for their Synergistic Response and Photodynamic Therapy Applications. J. Photochem. Photobiol. A Chem. 2019, 384, 112040. [Google Scholar] [CrossRef]
- Jia, P.; Ji, H.; Liu, S.; Zhang, R.; He, F.; Zhong, L.; Yang, P. Integration of IR-808 and thiol-capped Au-Bi bimetallic nanoparticles for NIR light mediated photothermal/photodynamic therapy and imaging. J. Mater. Chem. B 2021, 9, 101–111. [Google Scholar] [CrossRef]
- Rezaeivala, Z.; Imanparast, A.; Mohammadi, Z.; Najafabad, B.K.; Sazgarnia, A. The multimodal effect of Photothermal/Photodynamic/Chemo therapies mediated by Au-CoFe2O4 @Spiky nanostructure adjacent to mitoxantrone on breast cancer cells. Photodiagnosis Photodyn. Ther. 2023, 41, 103269. [Google Scholar] [CrossRef]
- Guo, D.; Bao, Y.; Zhang, Y.; Yang, H.; Chen, L. Reduction-responsive Au decorated mesoporous silica-based nanoplatform for photodynamic-chemotherapy. Microporous Mesoporous Mater. 2020, 292, 109729. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, W.; Yu, Y.; Zhang, H.; Caia, C.; She, Q. Anisotropic plasmonic Pd-tipped Au nanorods for near-infrared light-activated photoacoustic imaging guided photothermal–photodynamic cancer therapy. J. Mater. Chem. B 2022, 10, 2028–2037. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, H.; Zhao, J.; Cheng, M.; Sun, S. Synthesis of porphyrin-conjugated silica coated Au nanorods for synergistic therapy of PTT and PDT of tumor. Nanotechnology 2019, 20, 265102. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotech. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Sharifi, M.; Cho, W.C.; Ansariesfahani, A.; Tarharoudi, R.; Malekisarvar, H.; Sari, S.; Bloukh, S.H.; Edis, Z.; Amin, M.; Gleghorn, J.P.; et al. An Updated Review on EPR-Based Solid Tumor Targeting Nanocarriers for Cancer Treatment. Cancers 2022, 14, 2868. [Google Scholar] [CrossRef] [PubMed]
- De Maar, J.S.; Sofias, A.M.; Porta Siegel, T.; Vreeken, R.J.; Moonen, C.; Bos, C.; Deckers, R. Spatial heterogeneity of nanomedicine investigated by multiscale imaging of the drug, the nanoparticle and the tumour environment. Theranostics 2020, 10, 1884–1909. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Tietze, R.; Lyer, S.; Dürr, S.; Struffert, T.; Engelhorn, T.; Schwarz, M.; Eckert, E.; Göen, T.; Vasylyev, S.; Peukert, W.; et al. Efficient drug-delivery using magnetic nanoparticles—Biodistribution and therapeutic effects in tumour bearing rabbits. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Gurtler, R.; Hohenberger, P.; Haas, N.; Sohr, R.; Sander, B.; Lemke, A.J.; Ohlendorf, D.; Huhnt, W.; Huhn, D. Clinical experiences with magnetic drug targeting: A phase I study with 4′-epidoxorubicin in 14 patients with advanced solid tumors. Cancer Res. 1996, 56, 4686–4693. [Google Scholar]
- Ziegelberger, G. International Commission on Non-Ionizing Radiation Protection Guidelines on Limits of Exposure to Static Magnetic Fields. Health Phys. 2009, 96, 504–514. [Google Scholar]
- Garello, F.; Svenskaya, Y.; Parakhonskiy, B.; Filippi, M. Micro/Nanosystems for Magnetic Targeted Delivery of Bioagents. Pharmaceutics 2022, 14, 1132. [Google Scholar] [CrossRef]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Lodhi, M.S.; Khalid, F.; Khan, M.T.; Samra, Z.Q.; Muhammad, S.; Zhang, Y.-J.; Mou, K. A Novel Method of Magnetic Nanoparticles Functionalized with Anti-Folate Receptor Antibody and Methotrexate for Antibody Mediated Targeted Drug Delivery. Molecules 2022, 27, 261. [Google Scholar] [CrossRef]
- Hartono, F.; Widiyanti, P.; Zaidan, A.H. In vivo studies of magnetic nanoparticles-folic acid (FA) functionalized for breast cancer targeting drug carrier. AIP Conf. Proc. 2023, 2554, 060005. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Xu, Z.; Wang, Z. Advances in Nanoliposomes for the Diagnostic and Treatment of Liver Cancer. J. Nanomed. 2022, 17, 909–925. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Das, A.; Bal, C.S. Tumor-Targeting Agents. In Nuclear Medicine and Immunology; Harsini, S., Alavi, A., Rezaei, N., Eds.; Springer: Cham, Switzerland, 2022; pp. 55–73. [Google Scholar] [CrossRef]
- Yang, J.; Lee, H.; Hyung, W.; Park, S.B.; Haam, S. Magnetic PECA nanoparticles as drug carriers for targeted delivery: Synthesis and release characteristics. J. Microencapsul. 2006, 23, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, K.; Sohrabi, N.; Mohammadi, R.; Amini-Fazl, M.S. Preparation and characterization of magnetic nanohydrogel based on chitosan for 5-fluorouracil drug delivery and kinetic study. Int. J. Biol. Macromol. 2022, 202, 191–198. [Google Scholar] [CrossRef]
- Eslami, P.; Albino, M.; Scavone, F.; Chiellini, F.; Morelli, A.; Baldi, G.; Cappiello, L.; Doumett, S.; Lorenzi, G.; Ravagli, C.; et al. Smart Magnetic Nanocarriers for Multi-Stimuli On-Demand Drug De-livery. Nanomaterials 2022, 12, 303. [Google Scholar] [CrossRef]
- Mdlovu, N.V.; Lin, K.-S.; Weng, M.-T.; Lin, Y.-S. Design of doxorubicin encapsulated pH-/thermo-responsive and cat-ionic shell-crosslinked magnetic drug delivery system. Colloids Surf. B Biointerfaces 2022, 209, 112168. [Google Scholar] [CrossRef] [PubMed]
- Demin, A.M.; Vakhrushev, A.V.; Pershina, A.G.; Valova, M.S.; Efimova, L.V.; Syomchina, A.A.; Uimin, M.A.; Minin, A.S.; Levit, G.L.; Krasnov, V.P.; et al. Magnetic-Responsive Doxorubicin-Containing Materials Based on Fe3O4 Nanoparticles with a SiO2/PEG Shell and Study of Their Effects on Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 9093. [Google Scholar] [CrossRef]
- Farmanbar, N.; Mohseni, S.; Darroudi, M. Green synthesis of chitosan-coated magnetic nanoparticles for drug deliv-ery of oxaliplatin and irinotecan against colorectal cancer cells. Polym. Bull. 2022, 79, 10595–10613. [Google Scholar] [CrossRef]
- Ayyanaar, S.; Bhaskar, R.; Esthar, S.; Vadivel, M.; Rajesh, J.; Rajagopal, G. Design and development of 5-fluorouracil loaded biodegradable magnetic microspheres as site-specific drug delivery vehicle for cancer therapy. J. Magn. Magn. Mater. 2022, 546, 168853. [Google Scholar] [CrossRef]
- Novoselova, M.; Chernyshev, V.S.; Schulga, A.; Konovalova, E.V.; Chuprov-Netochin, R.N.; Abakumova, T.O.; German, S.; Shipunova, V.O.; Mokrousov, M.D.; Prikhozhdenko, E.; et al. Effect of Surface Modification of Multifunctional Nanocomposite Drug Delivery Carriers with DARPin on Their Biodistribution In Vitro and In Vivo. ACS Appl. Bio Mater. 2022, 5, 2976–2989. [Google Scholar] [CrossRef]
- Espinoza, M.J.C.; Lin, K.-S.; Weng, M.-T.; Kunene, S.C.; Liu, S.-Y.; Lin, Y.-S. In vivo and in vitro studies of magnetic silica nanocomposites decorated with Pluronic F127 for controlled drug delivery system. J. Ind. Eng. Chem. 2022, 115, 510–520. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Gao, J. Biocompatible Iron Oxide Nanoparticles for Targeted Cancer Gene Therapy: A Review. Nanomaterials 2022, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fu, X.; Jia, J.; Wikerholmen, T.; Xi, K.; Kong, Y.; Wang, J.; Chen, H.; Ma, Y.; Li, Z.; et al. Glioblastoma Therapy Using Codelivery of Cisplatin and Glutathione Peroxidase Tar-geting siRNA from Iron Oxide Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 43408–43421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Li, J.; Zhang, Z.; Huang, C.; Lian, G.; Yang, K.; Chen, S.; Lin, Y.; Wang, L.; et al. Co-delivery of mi-croRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci. 2017, 108, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Alvizo-Báez, C.A.; Peña-Torres, A.A.; Terrazas-Armendáriz, L.D.; Luna-Cruz, I.E.; Uscanga-Palomeque, A.C.; Sampayo-Reyes, A.; Tamez-Guerra, R.S.; Rodríguez-Padilla, C.; Alcocer-González, J.M. Synergic effect between TRAIL gene and curcumin in magnetic chitosan nanoparticles on cancer cells apoptosis enhanced by laser photoactivation. J. Nanopart. Res. 2022, 24, 165. [Google Scholar] [CrossRef]
- Khan, S.; Setua, S.; Kumari, S.; Dan, N.; Massey, A.; Hafeez, B.B.; Yallapu, M.M.; Stiles, Z.E.; Alabkaa, A.; Yue, J.; et al. Superparamagnetic iron oxide nanoparticles of curcumin enhance gemcitabine therapeutic response in pancreatic cancer. Biomaterials 2019, 208, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.M.; Aidoudi-Ahmed, S.; Sharma, S.; Kotamraju, V.R.; Foster, P.J.; Sugahara, K.N.; Ruoslahti, E.; Rutt, B.K. Nanoparticles coated with the tumor-penetrating peptide iRGD reduce experimental breast cancer metastasis in the brain. J. Mol. Med. 2015, 93, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.M.; Jun, Y.W.; Song, H.T.; Kim, S.; Choi, J.S.; Lee, J.H.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. In vivo magnetic resonance detection of cancer by using multifunctional magnetic nanocrystals. J. Am. Chem. Soc. 2005, 127, 12387–12391. [Google Scholar] [CrossRef]
- Kute, T.; Lack, C.M.; Willingham, M.; Bishwokama, B.; Williams, H.; Barrett, K.; Mitchell, T.; Vaughn, J.P. Develop-ment of Herceptin resistance in breast cancer cells. Cytom. A 2004, 57, 86–93. [Google Scholar] [CrossRef]
- Gomes, H.I.O.; Martins, C.S.M.; Prior, J.A.V. Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells. Nanomaterials 2021, 11, 964. [Google Scholar] [CrossRef]
- Ding, J.; Chen, G.; Chen, G.; Guo, M. One-Pot Synthesis of Epirubicin-Capped Silver Nanoparticles and Their Anti-cancer Activity against Hep G2 Cells. Pharmaceutics 2019, 11, 123. [Google Scholar] [CrossRef]
- Khalid, S.; Hanif, R. Green biosynthesis of silver nanoparticles conjugated to gefitinib as delivery vehicle. IJASEAT 2017, 5, 59–63. [Google Scholar]
- Benyettou, F.; Rezgui, R.; Ravaux, F.; Jaber, T.; Blumer, K.; Jouiad, M.; Trabolsi, A. Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J. Mater. Chem. B 2015, 3, 7237–7245. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Mohamad, H.; Ghazaly, M.M.; Laith, A.A.; Abdullah, M.A. Cytotoxic effects of Tetraselmis suecica chloroform extracts with silver nanoparticle co-application on MCF-7, 4 T1, and Vero cell lines. J. Appl. Phycol. 2020, 32, 127–143. [Google Scholar] [CrossRef]
- Murawala, P.; Tirmale, A.; Shiras, A.; Prasad, B.L.V. In situ synthesized BSA capped gold nanoparticles: Effective car-rier of anticancer drug Methotrexate to MCF-7 breast cancer cells. Mater. Sci. Eng. C 2014, 34, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Battocchio, C.; Carlini, L.; Amatori, S.; Porchia, M.; Tisato, F.; Bondino, F.; Magnano, E.; Pellei, M.; et al. Highly Hydrophilic Gold Nanoparticles as Carrier for Anticancer Copper(I) Complexes: Loading and Re-lease Studies for Biomedical Applications. Nanomaterials 2019, 9, 772. [Google Scholar] [CrossRef]
- Abdel-Rashid, R.S.; Omar, S.M.; Teiama, M.S.; Khairy, A.; Magdy, M.; Anis, B. Fabrication of gold nanoparticles in absence of surfactant as in vitro carrier of plasmid DNA. Int. J. Nanomed. 2019, 14, 8399–8408. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and metal oxide-based antiviral nanoparticles: Prop-erties, mechanisms of action, and applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Dehghani, F.; Mosleh-Shirazi, S.; Shafiee, M.; Kasaee, S.R.; Mohammad, A. Antiviral and antioxidant properties of green synthesized gold nanoparticles using Glaucium flavum leaf extract. Appl. Nanosci. 2022. ahead of print. [Google Scholar] [CrossRef]
- Abate, C.; Carnamucio, F.; Giuffrè, O.; Foti, C. Metal-Based Compounds in Antiviral Therapy. Biomolecules 2022, 12, 933. [Google Scholar] [CrossRef]
- Rawat, P.; Gupta, S. Dual engineered gold nanoparticle based synergistic prophylaxis delivery system for HIV/AIDS. Med. Hypotheses 2021, 150, 110576. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Hsueh, Y.-H.; Hsieh, C.-T.; Tzou, D.-Y.; Chang, P.-L. Antiviral Activity of Graphene–Silver Nanocompo-sites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, J.; Zheng, M.; Yang, Z.; Zhao, L.; Zhang, W.; Zhong, W.; Chen, Y.-Y.; Lin, J. Metal–Protein Nanoparti-cles Facilitate Anti-VSV and H1N1 Viruses Through the Coordinative Actions on Innate Immune Responses and METTL14. Macromol. Biosci. 2021, 21, 2000382. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Verma, D.; Saini, N.; Arbi, R.; Munir, M.; Jovic, M.; Turak, A. Antiviral nanoparticles for sanitizing surfaces: A roadmap to self-sterilizing against COVID-19. Nano Today 2021, 40, 101267. [Google Scholar] [CrossRef] [PubMed]
- Park, G.W.; Cho, M.; Cates, E.L.; Lee, D.; Oh, B.-T.; Vinjé, J.; Kim, J.-H. Fluorinated TiO2 as an ambient light-activated virucidal surface coating material for the control of human norovirus. J. Photochem. Photobiol. B Biol. 2014, 140, 315–320. [Google Scholar] [CrossRef]
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012, 11, 1293. [Google Scholar] [CrossRef]
- Hajkova, P.; Spatenka, P.; Horsky, J.; Horska, I.; Kolouch, A. Photocatalytic Effect of TiO2 Films on Viruses and Bacte-ria. Plasma Process. Polym. 2007, 4 (Suppl. S1), S397–S401. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Communic. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Du, T.; Zhang, J.; Li, C.; Song, T.; Li, P.; Liu, J.; Du, X.; Wang, S. Gold/Silver Hybrid Nanoparticles with Enduring Inhibition of Coronavirus Multiplication through Multisite Mechanisms. Bioconjugate Chem. 2020, 31, 2553–2563. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral activity of silver nanoparti-cle/chitosan composites against H1N1 influenza A virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celi-chowski, G.; Grobelny, J.; et al. Tannic Acid Modified Silver Nanoparticles Show Antiviral Activity in Herpes Simplex Virus Type 2 Infection. PLoS ONE 2014, 9, e104113. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Abdel-Bar, H.M.; Elmowafy, E.; El-khouly, A.; Mansour, M.; Awad, G.A.S. Investigating the Internaliza-tion and COVID-19 Antiviral Computational Analysis of Optimized Nanoscale Zinc Oxide. ACS Omega 2021, 6, 6848–6860. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Shahzad, K.; Mushtaq, S.; Ali, I.; Rafe, M.H.; Fazal-ul-Karim, S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express 2019, 6, 105409. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Ismail, N.S.M.; McLean, G.R.; Hamdy, N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Ma, R.; Yin, Y.; Miao, X.; Chen, S.; Fan, K.; Xi, J.; Liu, Q.; Gu, Y.; Yin, Y.; et al. Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 2019, 9, 6920–6935. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Zhao, M.; Xu, T.; Wang, C.; Hua, L.; Wang, H.; Xia, H.; Zhu, B. Silver Nanoparticle Based Codelivery of Oseltamivir to Inhibit the Activity of the H1N1 Influenza Virus through ROS-Mediated Signaling Pathways. ACS Appl. Mater. Interfaces 2016, 8, 24385–24393. [Google Scholar] [CrossRef]
- Chiodo, F.; Marradi, M.; Calvo, J.; Yuste, E.; Penadés, S. Glycosystems in nanotechnology: Gold glyconanoparticles as carrier for anti-HIV prodrugs. Beilstein, J. Org. Chem. 2014, 10, 1339–1346. [Google Scholar] [CrossRef]
- Shahabadi, N.; Falsafi, M.; Feizi, F.; Khodarahmi, R. Functionalization of γ-Fe2O3@SiO2 nanoparticles using the antiviral drug zidovudine: Synthesis, characterization, in vitro cytotoxicity and DNA interaction studies. RSC Adv. 2016, 6, 73605–73616. [Google Scholar] [CrossRef]
- Hoseininasr, A.S.; Tayebee, R. Synthesis and characterization of superparamagnetic nanohybrid Fe3 O4/NH2 -Ag as an effective carrier for the delivery of acyclovir. Appl. Organomet. Chem. 2018, 32, e4565. [Google Scholar] [CrossRef]
- Ahangarani-Farahani, R.; Bodaghifard, M.A.; Asadbegi, S. Magnetic triazine-based dendrimer as a versatile nanocar-rier for efficient antiviral drugs delivery. Sci. Rep. 2022, 12, 19469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Bhawna, M.; Madhukar, M.; et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemo-Ther. 2019, 25, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Kohzadi, S.; Najmoddin, N.; Baharifar, H.; Shabani, M. Functionalized SPION immobilized on graphene-oxide: An-ticancer and antiviral study. Diam. Relat. Mater. 2022, 127, 109149. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.; Valizadeh, H.; Panahi, Y.; Fatahi, Y.; Chen, M.; Zarebkohan, A.; Gao, H. The impact of protein corona on the biological behavior of targeting nanomedicines. Int. J. Pharm. 2022, 614, 121458. [Google Scholar] [CrossRef]
- Mikelez-Alonso, I.; Aires, A.; Cortajarena, A.L. Cancer Nano-Immunotherapy from the Injection to the Target: The Role of Protein Corona. Int. J. Mol. Sci. 2020, 21, 519. [Google Scholar] [CrossRef]
- Kim, Y.; Mok, H. Citraconylated exosomes for improved internalization into macrophages. Appl. Biol. Chem. 2019, 62, 26. [Google Scholar] [CrossRef]
- Ezzat, K.; Pernemalm, M.; Pålsson, S.; Roberts, T.C.; Järver, P.; Dondalska, A.; Bestas, B.; Sobkowiak, M.J.; Levänen, B.; Sköld, M.; et al. The viral protein corona directs viral pathogenesis and amyloid aggregation. Nat. Commun. 2019, 10, 2331. [Google Scholar] [CrossRef]
- Liu, K.; Salvati, A.; Sabirsh, A. Physiology, pathology and the biomolecular corona: The confounding factors in nano-medicine design. Nanoscale 2022, 14, 2136–2154. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Furman, N.E.T.; Hartman, K.A.; Sherman, M.B.; De Rosa, E.; Kirui, D.K.; Salva-tore, F.; Tasciotti, E. Unveiling the in vivo protein corona of circulating leukocyte-like carriers. ACS Nano 2017, 11, 3262–3273. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Gopalakrishnan, S.; Castellanos-Garcia, L.; Li, G.; Malassiné, M.; Uddin, I.; Huang, R.; Luther, D.C.; Vachet, R.W.; et al. Intracellular Activation of Bioorthogonal Nanozymes through Endosomal Proteolysis of the Protein Corona. ACS Nano 2020, 14, 4767–4773. [Google Scholar] [CrossRef]
- Neagu, M.; Piperigkou, Z.; Karamanou, K.; Engin, A.B.; Docea, A.O.; Constantin, C.; Negrei, C.; Nikitovic, D.; Tsatsakis, A. Protein bio-corona: Critical issue in immune nanotoxicology. Arch. Toxicol. 2017, 91, 1031–1048. [Google Scholar] [CrossRef]
- Ma, S.; Gu, C.; Xu, J.; He, J.; Li, S.; Zheng, H.; Pang, B.; Wen, Y.; Fang, Q.; Liu, W.; et al. Strategy for Avoiding Protein Corona Inhibition of Targeted Drug Delivery by Linking Recombinant Affibody Scaffold to Magnetosomes. Dove Press 2022, 17, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Cai, R.; Wang, J.; Daniyal, M.; Baimanov, D.; Liu, Y.; Yin, D.; Liu, Y.; Miao, Q.; Zhao, Y.; et al. Precision Nano-medicine Development Based on Specific Opsonization of Human Cancer Patient-Personalized Protein Coronas. Nano Lett. 2019, 19, 4692–4701. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensi-tisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Horwood, N.; Nanchahal, J.; Chan, J.K.; Roth, J.; Oppenheim, J.J.; Tracey, K.J.; Vogl, T.; Feldmann, M.; Hor-wood, N.; et al. Alarmins: Awaiting a clinical response. J. Clin. Investig. 2012, 122, 2711–2719. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Roche, K.C.; Tian, S.; Eblan, M.J.; Mckinnon, K.P.; Caster, J.M.; Chai, S.; Herring, L.E.; Zhang, L.; Zhang, T.; et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy HHS Public Access Author manuscript. Nat. Nanotechnol. 2017, 12, 877–882. [Google Scholar] [CrossRef]
- Han, S.; da Costa Marques, R.; Simon, J.; Kaltbeitzel, A.; Koynov, K.; Landfester, K.; Mailänder, V.; Lieberwirth, I. Endosomal sorting results in a selective separation of the protein co-rona from nanoparticles. Nat. Commun 2023, 14, 295. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, T.; Wang, J.; Wang, Q.; Lv, X.; Ke, H.; Guo, Z.; Shen, J.; Wang, Y.; Xing, C.; et al. Size-Tunable Gd2O3@Albumin Nanoparticles Conjugating Chlorin e6 for Magnetic Resonance Imaging-Guided Photo-Induced Therapy. Theranostics. 2017, 7, 764–774. [Google Scholar] [CrossRef]
- Nan, X.; Zhang, X.; Liu, Y.; Zhou, M.; Chen, X.; Zhang, X. Dual-Targeted Multifunctional Nanoparticles for Magnetic Resonance Imaging Guided Cancer Diagnosis and Therapy. ACS Appl. Mater. Interfaces 2017, 9, 9986–9995. [Google Scholar] [CrossRef]
- Ali, R.; Aziz, M.H.; Gao, S.; Khan, M.I.; Li, F.; Batool, T.; Shaheen, F.; Qiu, B. Graphene oxide/zinc ferrite nanocomposite loaded with doxorubicin as a potential theranostic mediu in cancer therapy and magnetic resonance imaging. Ceram. Int. 2022, 48, 10741–10750. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, M.; Fu, G.; Zhang, L.; Yu, H.; Yan, X.; Liu, F.; Sun, P.; Jia, X.; Liu, X.; et al. Ti3C2 MXene Nanosheets Functionalized with NaErF4:0.5%Tm@NaLuF4 Nanoparticles for Dual-Modal Near-Infrared IIb/Magnetic Resonance Imaging-Guided Tumor Hyperthermia. ACS Appl. Nano Mater. 2022, 5–6, 8142–8153. [Google Scholar] [CrossRef]

| MRI Agent Core Composition | NPs Size/Shape | Type of Contrast Agent | Vector (Targeting Agent) | Application Test | Reference |
|---|---|---|---|---|---|
| Fe3O4-Au | 25 nm octahedral-shaped | T2 | EPR, passive | in vitro (4T1 cancer cell line); in vivo (breast cancer model) | [27] |
| Fe3O4-Au mixed | Au 2 nm; Fe3O4 15 nm | T2 | EPR, passive | in vivo (glioblastoma) | [28] |
| MIONPs | 20 nm | T2 | HER2 single-chain antibody | in vitro (NCI-N87 human gastric cancer cells and human pancreatic cancer cells SUIT2); in vivo (pancreatic cancer) | [29] |
| MIONPs | T2 | BCZM | in vivo (breast cancer cells transfected with the VEGF-165 isoform) | [30] | |
| Au coated Fe3O4 | 46 nm | T2 | C225 | in vitro and in vivo (human glioma) | [31] |
| Au coated Fe3O4 | 50 nm | T2 | Prostate stem cell antigen antibody | in vivo (prostate tumors) | [32] |
| MIONPs | T2 | anti-epidermal growth factor receptor antibody | in vivo (lungs tumor) | [33] | |
| Fe3O4 | 22 nm | T2 | arginine-glycine-asparticacid-tumornecrosis factor-related apoptosis-inducing ligand | in vivo (colorectal cancer) | [34] |
| Fe3O4/mesoporous silica-Au | 16 nm | T1 and T2 | peptide cyclo[Arg-Gly-Asp-D-Phe-Lys] | in vivo (fibro-sarcoma) | [35] |
| MIONPs | 10 nm | T2 | glypican-3 (GPC3)-specific aptamer (AP613-1) | in vitro and in vivo (liver cancer) | [37] |
| Fe3O4-Au | Fe3O4 2 nm Fe3O4-Au 7 nm | T1 and T2 | tumor homing peptide (LyP-1) | in vitro and in vivo (hepatocellular carcinoma) | [38] |
| MIONPS | T2 | folic acid | in vivo (lungs cancer) | [39] | |
| MIONPS | 1–3 nm | T1 | - | in vivo (mice) | [42] |
| Co1−xMnxFe2O4 | 10–50 nm | T2 | - | in vivo (mice) | [45] |
| INPs Core | NPs Size/Shape | Covering | Drug Substance | Application Tests | Reference |
|---|---|---|---|---|---|
| Fe3O4 | 9 nm | PECA | Cisplatin and Gemcitabine | In vitro experiments of drug release and magnetic mobility | [115] |
| Fe3O4 | 6 nm | chitosan-polyacrylic acid | 5-FLU | In vitro drug loading and release studies | [116] |
| Flower-shaped magnetite | 16 nm | poly (N-vinylcaprolactam-co-acrylic acid) | Doxorubicin4 | In vitro drug release (pH and temperature dependent). Cytotoxicity assay and cellular uptake study: MCF-7 (breast cancer cell line) and A375 (melanoma cell line) | [117] |
| MIONPs | 10–20 nm | Pluronic F127 and branched polyethylenimine | Doxorubicin | In vitro cellular uptake studies (HepG2) | [118] |
| Fe3O4 | 13 nm | silica and covalently modified with [(3-triethoxysilyl)-propyl]-succinic acid–polyethylene glycol | Doxorubicin | Cytotoxicity Assay (epithelial, human breast cancer cell—MDA-MB231, HepG2, animal model for stage IV human breast cancer-4T1, colon carcinoma CT26, and melanoma—B16) | [119] |
| Fe3O4 | 25–40 nm | chitosan | Oxaliplatin, Irinotecan | - | [120] |
| MIONPs | PLGA | 5-FLU | pH-dependent release of 5-FLU | [121] | |
| Fe3O4 | 1–10 nm | polyarginine hydrochloride, DEX | Doxorubicin | In vivo and ex vivo biodistribution studies; Flow cytometry (MCF-7) | [122] |
| INPs Core | NPs Size/Shape | Covering | Drug Substance | Virus | Reference |
|---|---|---|---|---|---|
| Ag | 2–3 nm | Oseltamivir | influenza H1N1 | [160] | |
| Au | 3 nm | Glucose | Abacavir and Lamivudine | HIV | [161] |
| γ-Fe2O3 | 25 nm | SiO2 | Zidovudine | - | [162] |
| Fe3O4@SiO2/NH2-Ag | 150–400 nm | - | Acyclovir | - | [163] |
| Fe3O4@SiO2 | 15–35 nm | Dendrimer | Favipiravir and Zidovudine | - | [164] |
| Fe3O4 | 10–15 | Glycine | Glycine | influenza H1N1 | [165] |
| Fe3O4 | 25 nm | Graphene oxide and chitosan | SARS-CoV-2 | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabatina, T.I.; Vernaya, O.I.; Shimanovskiy, N.L.; Melnikov, M.Y. Metal and Metal Oxides Nanoparticles and Nanosystems in Anticancer and Antiviral Theragnostic Agents. Pharmaceutics 2023, 15, 1181. https://doi.org/10.3390/pharmaceutics15041181
Shabatina TI, Vernaya OI, Shimanovskiy NL, Melnikov MY. Metal and Metal Oxides Nanoparticles and Nanosystems in Anticancer and Antiviral Theragnostic Agents. Pharmaceutics. 2023; 15(4):1181. https://doi.org/10.3390/pharmaceutics15041181
Chicago/Turabian StyleShabatina, Tatyana I., Olga I. Vernaya, Nikolay L. Shimanovskiy, and Mikhail Ya. Melnikov. 2023. "Metal and Metal Oxides Nanoparticles and Nanosystems in Anticancer and Antiviral Theragnostic Agents" Pharmaceutics 15, no. 4: 1181. https://doi.org/10.3390/pharmaceutics15041181
APA StyleShabatina, T. I., Vernaya, O. I., Shimanovskiy, N. L., & Melnikov, M. Y. (2023). Metal and Metal Oxides Nanoparticles and Nanosystems in Anticancer and Antiviral Theragnostic Agents. Pharmaceutics, 15(4), 1181. https://doi.org/10.3390/pharmaceutics15041181







