Redox-Responsive Comparison of Diselenide and Disulfide Core-Cross-Linked Micelles for Drug Delivery Application
Abstract
1. Introduction
2. Materials and Methods
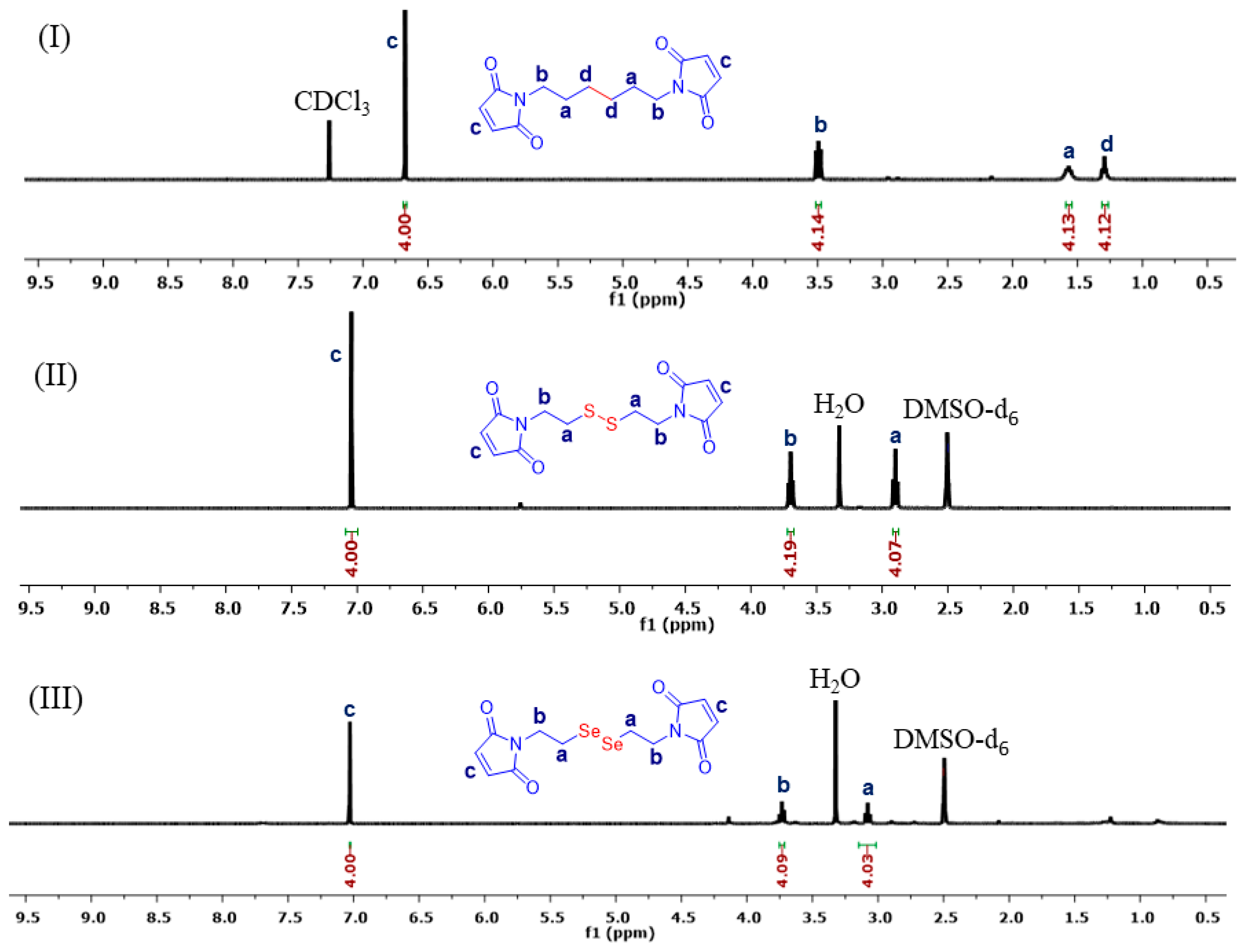
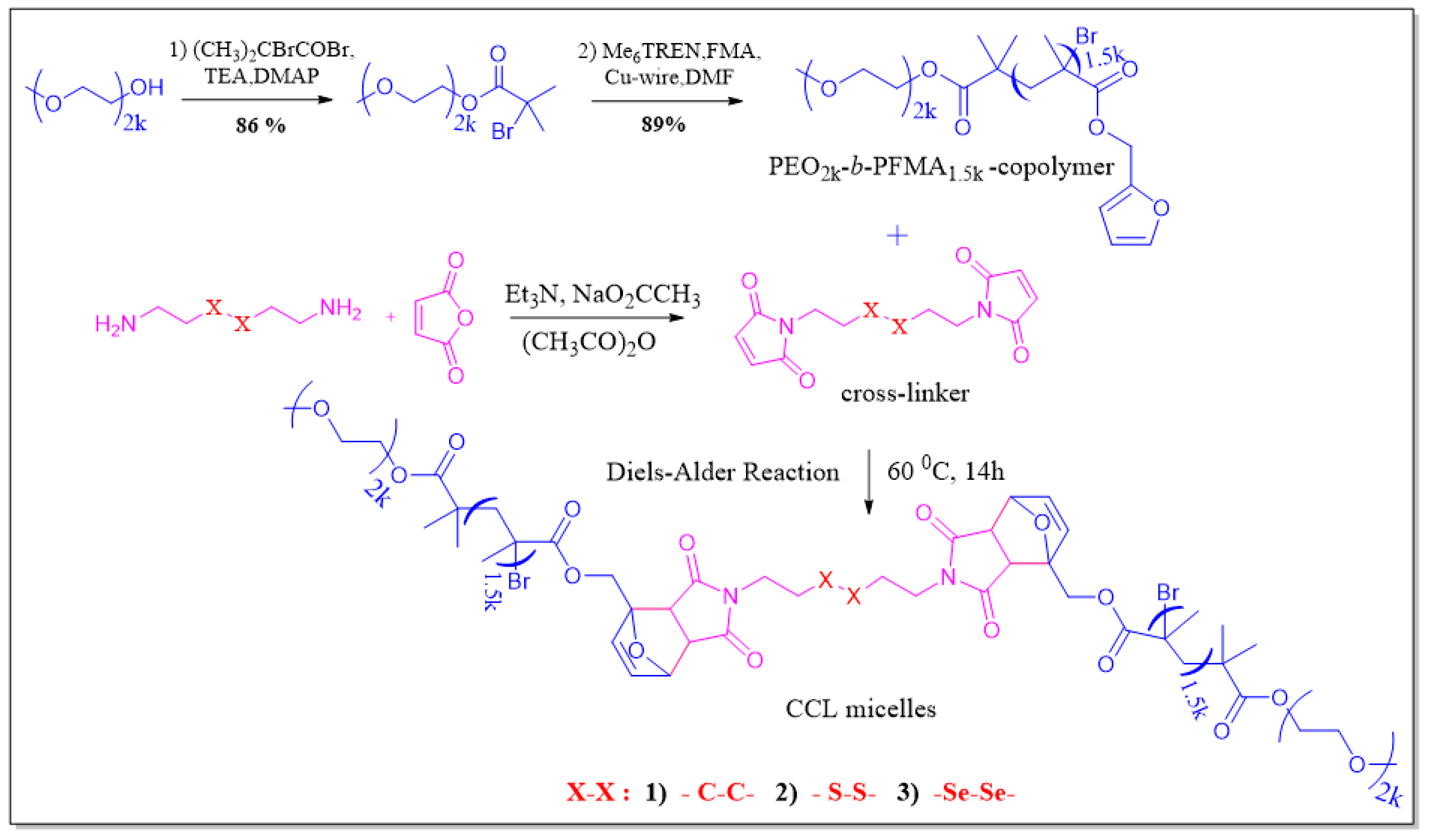
2.1. Synthesis of BisMH, DTME, and DseME Cross-Linkers
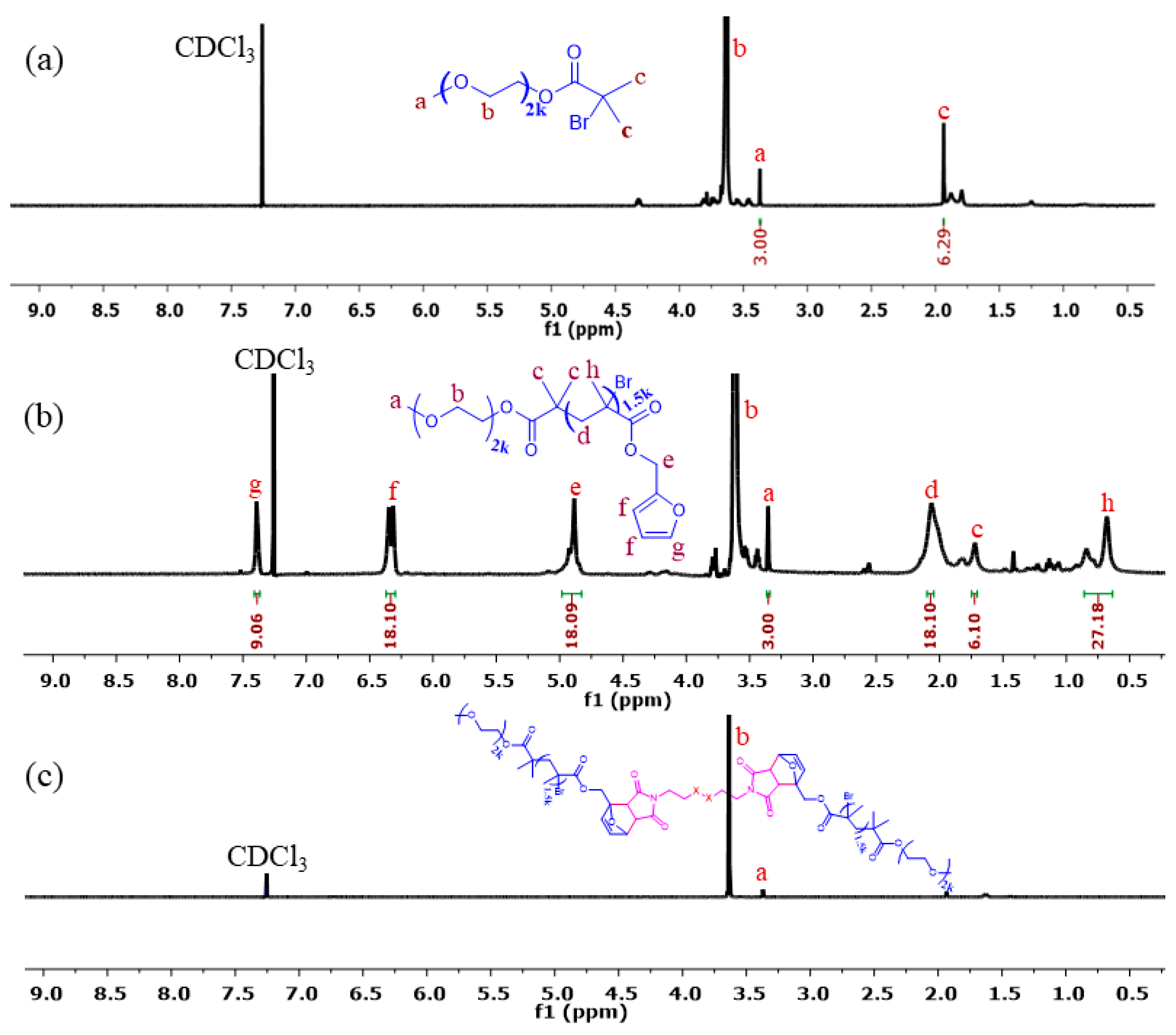
2.2. Synthesis of PEO2k-b-PFMA1.5k
2.3. Critical Micelle Concentration (CMC) Measurement
2.4. The Stability of Non-CCL/DOX and C–C/S–S/Se–Se CCL/DOX Micelles
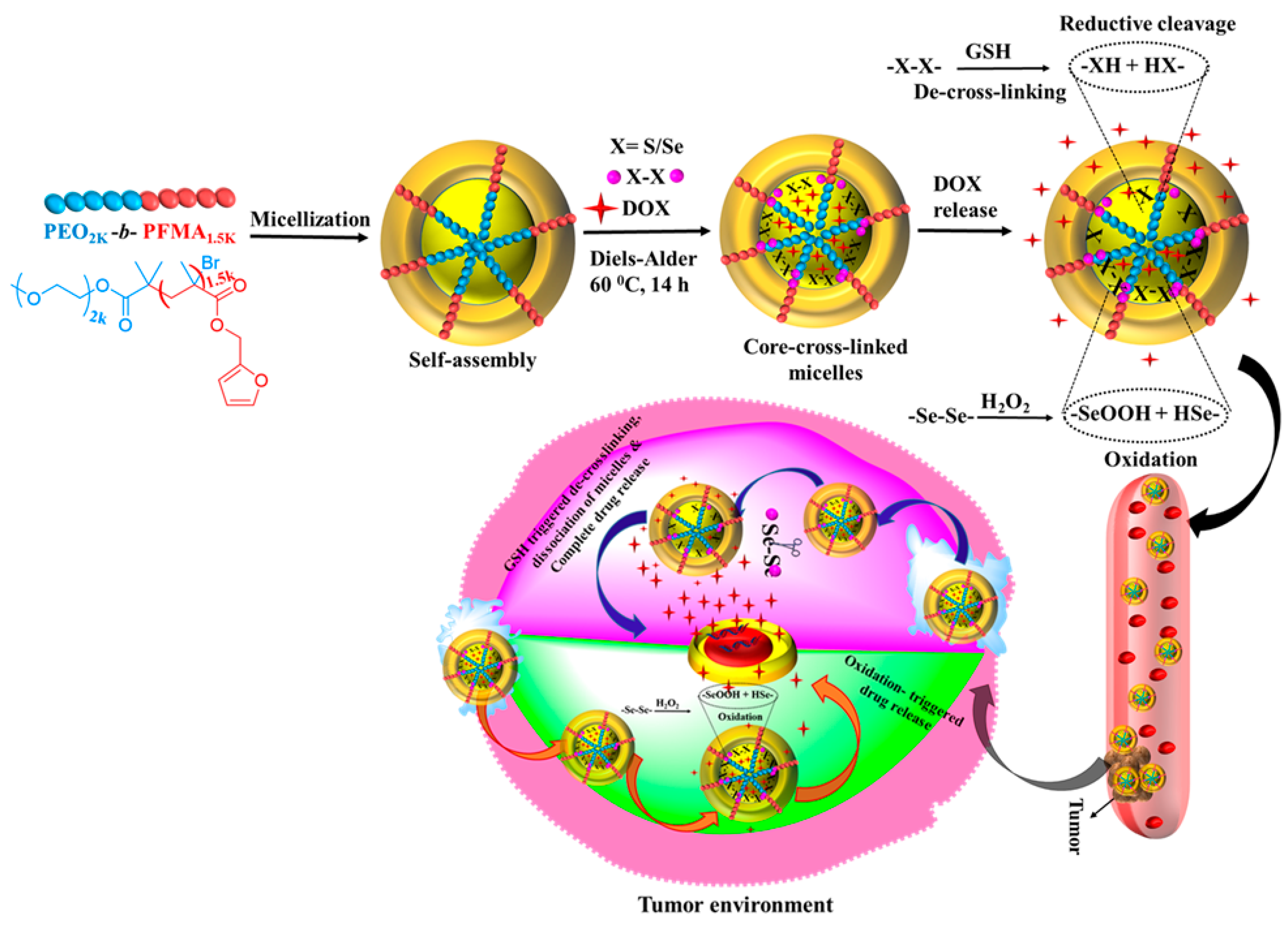
2.5. Synthesis of DOX-Loaded C–C/S–S/Se–Se CCL Micelles of PEO2k-b-PFMA1.5k via DA Reaction
2.6. In Vitro Release Study of DOX from S–S/Se–Se CCL Micelles
3. Results and Discussion
3.1. Synthesis and Characterization of C–C/S–S/Se–Se CCL Micelles
3.2. Stability of C–C/S–S/Se–Se CCL/DOX and Non-CCL/DOX Micelles
3.3. Drug Loading Studies
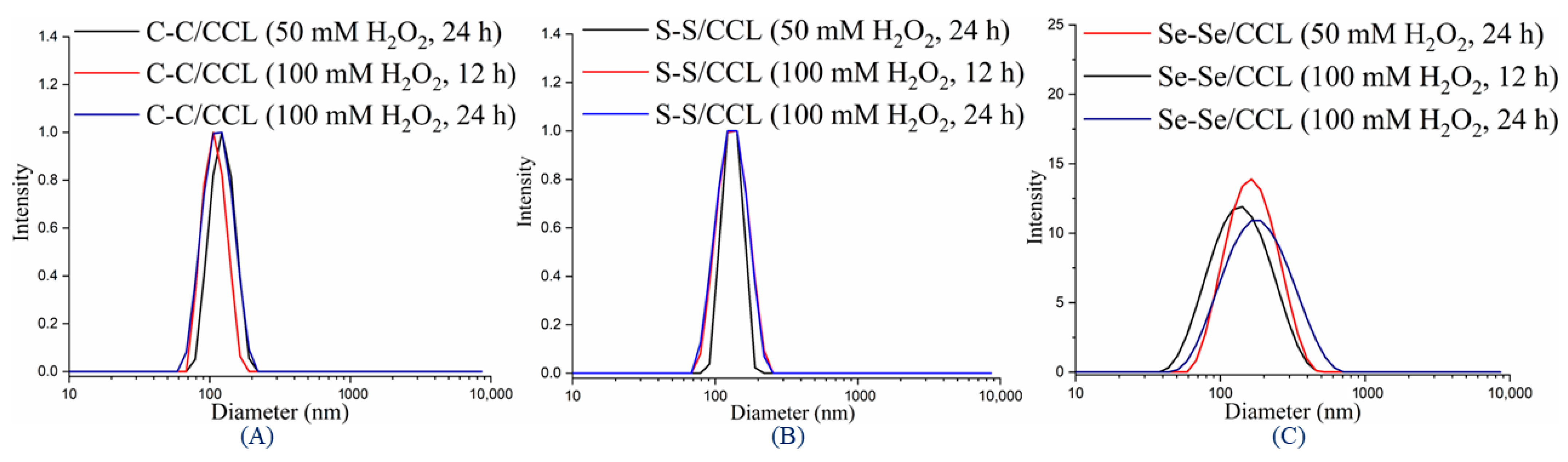
3.4. Redox-Responsive Properties of CCL Micelles
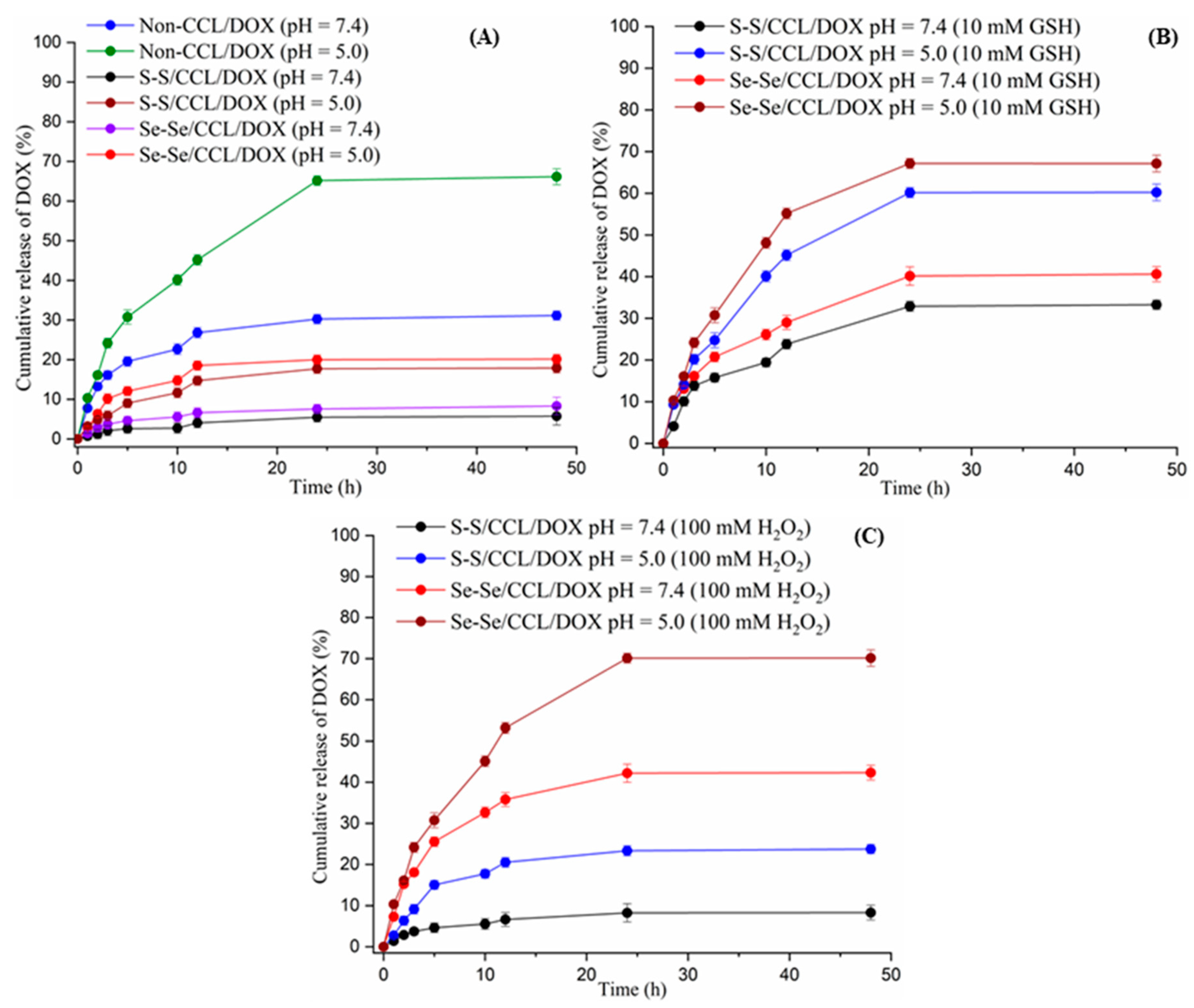
3.5. Redox-Triggered In Vitro DOX Release
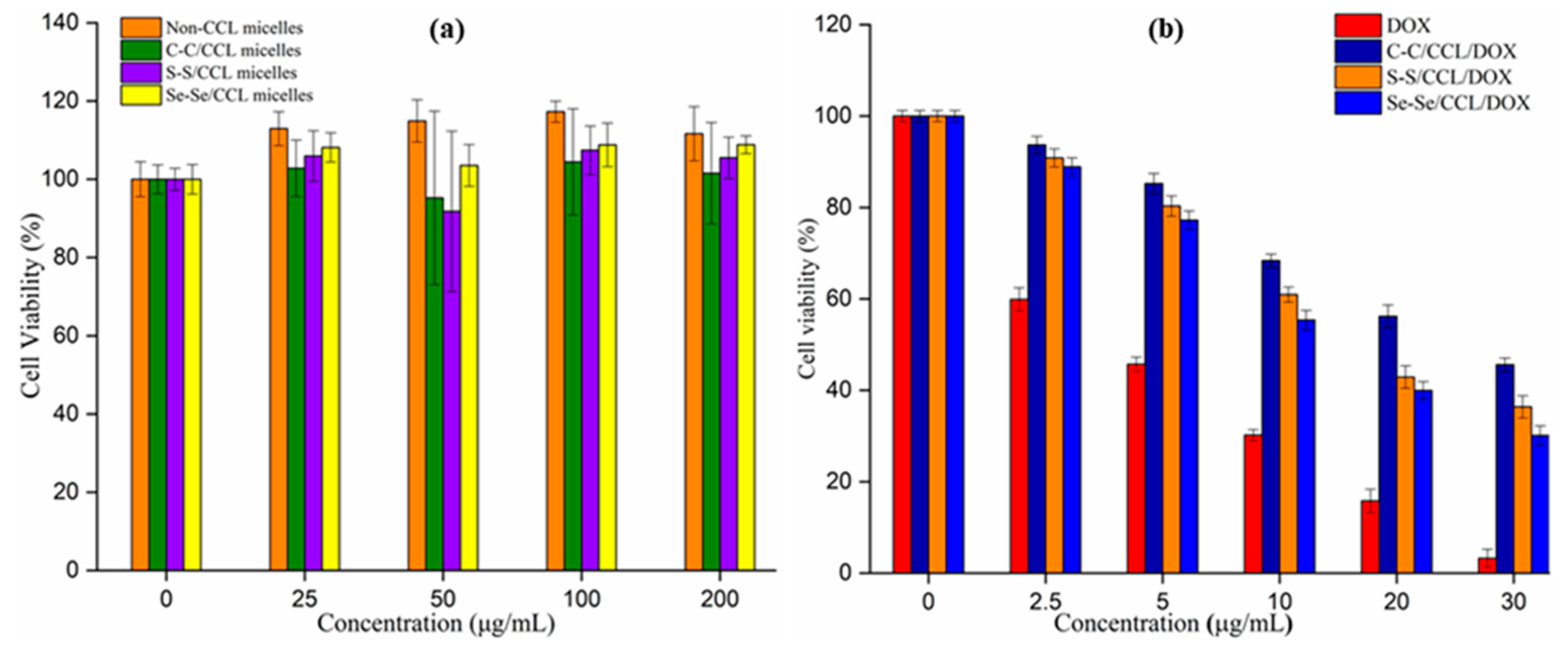
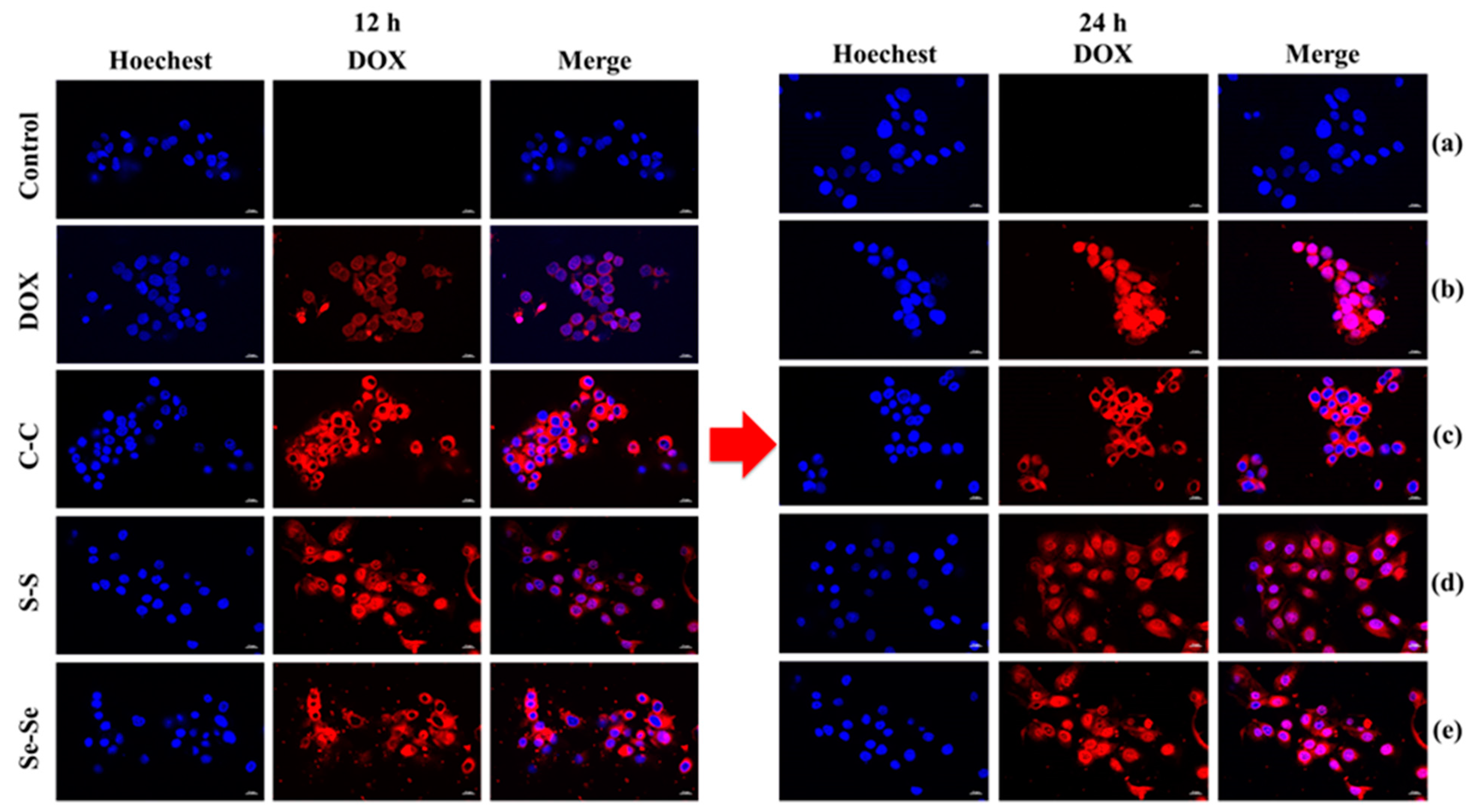
3.6. Cytotoxicity of DOX-Loaded C–C/S–S/Se–Se CCL Micelles and Non-CCL Micelles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; He, J.; Zhang, M.; Ni, P. A polyphosphoester-conjugated camptothecin prodrug with disulfide linkage for potent reduction-triggered drug delivery. J. Mater. Chem. B 2015, 3, 4922–4932. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, P.; Jo, S.-H.; Park, S.-H.; Lee, W.-K.; Yoo, S., II; Lim, K.T. Redox-responsive properties of core-cross-linked micelles of poly(ethylene oxide)-b-poly(furfuryl methacrylate) for anticancer drug delivery application. React. Funct. Polym. 2022, 175, 105271. [Google Scholar] [CrossRef]
- John, J.V.; Uthaman, S.; Augustine, R.; Manickavasagam Lekshmi, K.; Park, I.-K.; Kim, I. Biomimetic pH/redox dual stimuli-responsive zwitterionic polymer block poly(L-histidine) micelles for intracellular delivery of doxorubicin into tumor cells. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2061–2070. [Google Scholar] [CrossRef]
- Carr, C.; Ng, J.; Wigmore, T. The side effects of chemotherapeutic agents. Curr. Anaesth. Crit. Care 2008, 19, 70–79. [Google Scholar] [CrossRef]
- Cheng, L.; Luan, T.; Liu, D.; Cheng, J.; Li, H.; Wei, H.; Zhang, L.; Lan, J.; Liu, Y.; Zhao, G. Diblock copolymer glyco-nanomicelles constructed by a maltoheptaose-based amphiphile for reduction- and pH-mediated intracellular drug delivery. Polym. Chem. 2018, 9, 1337–1347. [Google Scholar] [CrossRef]
- Tong, R.; Tang, L.; Ma, L.; Tu, C.; Baumgartner, R.; Cheng, J. Smart chemistry in polymeric nanomedicine. Chem. Soc. Rev. 2014, 43, 6982–7012. [Google Scholar] [CrossRef]
- Yan, Y.; Such, G.K.; Johnston, A.P.R.; Best, J.P.; Caruso, F. Engineering Particles for Therapeutic Delivery: Prospects and Challenges. ACS Nano 2012, 6, 3663–3669. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Delivery Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Shi, L.; Jin, Y.; Du, W.; Lai, S.; Shen, Y.; Zhou, R. Diselenide-containing nonionic gemini polymeric micelles as a smart redox-responsive carrier for potential programmable drug release. Polymer 2020, 198, 122551. [Google Scholar] [CrossRef]
- Liu, J.; Pang, Y.; Huang, W.; Huang, X.; Meng, L.; Zhu, X.; Zhou, Y.; Yan, D. Bioreducible Micelles Self-Assembled from Amphiphilic Hyperbranched Multiarm Copolymer for Glutathione-Mediated Intracellular Drug Delivery. Biomacromolecules 2011, 12, 1567–1577. [Google Scholar] [CrossRef]
- Li, M.; Ling, L.; Xia, Q.; Li, X. A reduction-responsive drug delivery with improved stability: Disulfide crosslinked micelles of small amiphiphilic molecules. RSC Adv. 2021, 11, 12757–12770. [Google Scholar] [CrossRef] [PubMed]
- Siboro, S.A.P.; Salma, S.A.; Kim, H.-R.; Jeong, Y.T.; Gal, Y.-S.; Lim, K.T. Diselenide Core Cross-Linked Micelles of Poly (Ethylene Oxide)-b-Poly (Glycidyl Methacrylate) Prepared through Alkyne-Azide Click Chemistry as a Near-Infrared Controlled Drug Delivery System. Materials 2020, 13, 2846. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, S.M.; Azizov, S.; Elmasry, M.R.; Sharipov, M.; Lee, Y.-I. Recent advances in nanomicelles delivery systems. Nanomaterials 2020, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ren, Y.; Dou, Y.; Ding, T.; Fang, X.; Xu, Y.; Xu, H.; Zhang, W.; Xie, Z. Photo-cross-linked poly(ether amine) micelles for controlled drug release. RSC Adv. 2015, 5, 105880–105888. [Google Scholar] [CrossRef]
- Rambarran, T.; Sheardown, H.D. Block copolymer synthesis using free-radical polymerization and thiol–maleimide ‘click’ conjugation. RSC Adv. 2021, 11, 34631–34635. [Google Scholar] [CrossRef]
- Salma, S.A.; Patil, M.P.; Kim, D.W.; Le, C.M.Q.; Ahn, B.-H.; Kim, G.-D.; Lim, K.T. Near-infrared light-responsive, diselenide containing core-cross-linked micelles prepared by the Diels–Alder click reaction for photocontrollable drug release application. Polym. Chem. 2018, 9, 4813–4823. [Google Scholar] [CrossRef]
- Talelli, M.; Barz, M.; Rijcken, C.J.F.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef]
- Yu, S.; Ding, J.; He, C.; Cao, Y.; Xu, W.; Chen, X. Disulfide Cross-Linked Polyurethane Micelles as a Reduction-Triggered Drug Delivery System for Cancer Therapy. Adv. Healthc. Mater. 2014, 3, 752–760. [Google Scholar] [CrossRef]
- Gregoritza, M.; Brandl, F.P. The Diels–Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. [Google Scholar] [CrossRef]
- Le, C.M.Q.; Thi, H.H.P.; Cao, X.T.; Kim, G.-D.; Oh, C.-W.; Lim, K.T. Redox-responsive core cross-linked micelles of poly(ethylene oxide)-b-poly(furfuryl methacrylate) by Diels-Alder reaction for doxorubicin release. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3741–3750. [Google Scholar] [CrossRef]
- Breslow, R.; Maitra, U.; Rideout, D. Selective diels-alder reactions in aqueous solutions and suspensions. Tetrahedron Lett. 1983, 24, 1901–1904. [Google Scholar] [CrossRef]
- Meijer, A.; Otto, S.; Engberts, J.B.F.N. Effects of the hydrophobicity of the reactants on Diels–Alder reactions in water. J. Org. Chem. Res. 1998, 63, 8989–8994. [Google Scholar] [CrossRef]
- Rideout, D.C.; Breslow, R. Hydrophobic acceleration of Diels-Alder reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Ke, W.; Ge, Z. A Near-Infrared Photothermal Effect-Responsive Drug Delivery System Based on Indocyanine Green and Doxorubicin-Loaded Polymeric Micelles Mediated by Reversible Diels–Alder Reaction. Macromol. Rapid Commun. 2015, 36, 1841–1849. [Google Scholar] [CrossRef]
- Yan, B.; Boyer, J.-C.; Branda, N.R.; Zhao, Y. Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. J. Am. Chem. Soc. 2011, 133, 19714–19717. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Zhong, Z. Bioresponsive polymeric nanotherapeutics for targeted cancer chemotherapy. Nano Today 2015, 10, 656–670. [Google Scholar] [CrossRef]
- Deng, B.; Ma, P.; Xie, Y. Reduction-sensitive polymeric nanocarriers in cancer therapy: A comprehensive review. Nanoscale 2015, 7, 12773–12795. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zhang, K.; Chen, Y.; Luo, X. Redox-responsive comparison of diselenide micelles with disulfide micelles. Colloid Polym. Sci. 2019, 297, 225–238. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.; Liu, M.; Wang, K.; Tao, L.; Wan, Q.; Wei, Y. Recent progress and advances in redox-responsive polymers as controlled delivery nanoplatforms. Mater. Chem. Front. 2017, 1, 807–822. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, A.; Saji, J.; Umapathi, A.; Kumar, S.; Daima, H.K. Smart nanomaterials for cancer diagnosis and treatment. Nano Converg. 2022, 9, 21. [Google Scholar] [CrossRef]
- Choi, Y.S.; Huh, K.M.; Shim, M.S.; Park, I.S.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Disrupting the Redox Balance with a Diselenide Drug Delivery System: Synergistic or Antagonistic? Adv. Funct. Mater. 2021, 31, 2007275. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Xiao, F.; Zhao, D.; Luan, Y. Disulfide bond based polymeric drug carriers for cancer chemotherapy and relevant redox environments in mammals. Med. Res. Rev. 2018, 38, 1485–1510. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Wang, Y.; Zhang, T.; Pu, X.; Zong, L.; Zhu, H.; Zhao, L.; Feng, B. Redox-responsive disulfide bond-bridged mPEG-PBLA prodrug micelles for enhanced paclitaxel biosafety and antitumor efficacy. Front. Oncol. 2019, 9, 823. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, K.; Luo, J.; Xiao, W.; Lee, J.S.; Gonik, A.M.; Kato, J.; Dong, T.A.; Lam, K.S. Well-defined, reversible disulfide cross-linked micelles for on-demand paclitaxel delivery. Biomaterials 2011, 32, 6633–6645. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, P. Synthesis strategies for disulfide bond-containing polymer-based drug delivery system for reduction-responsive controlled release. Front. Mater. Sci. 2015, 9, 211–226. [Google Scholar] [CrossRef]
- Yue, D.; Cheng, G.; He, Y.; Nie, Y.; Jiang, Q.; Cai, X.; Gu, Z. Influence of reduction-sensitive diselenide bonds and disulfide bonds on oligoethylenimine conjugates for gene delivery. J. Mater. Chem. B 2014, 2, 7210–7221. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, J.; Tian, L.; Li, J.; Gao, Y.; Xing, Y.; Yan, W.; Hua, C.; Xie, X.; Liu, C.; et al. Insights into stimuli-responsive diselenide bonds utilized in drug delivery systems for cancer therapy. Biomed. Pharmacother. 2022, 155, 113707. [Google Scholar] [CrossRef]
- Song, W.; You, J.; Zhang, Y.; Yang, Q.; Jiao, J.; Zhang, H. Recent Studies on Hydrogels Based on H2O2-Responsive Moieties: Mechanism, Preparation and Application. Gels 2022, 8, 361. [Google Scholar] [CrossRef]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef]
- Deepagan, V.G.; Kwon, S.; You, D.G.; Nguyen, V.Q.; Um, W.; Ko, H.; Lee, H.; Jo, D.-G.; Kang, Y.M.; Park, J.H. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials 2016, 103, 56–66. [Google Scholar] [CrossRef]
- Dispinar, T.; Van Camp, W.; De Cock, L.J.; De Geest, B.G.; Du Prez, F.E. Redox-responsive degradable PEG cryogels as potential cell scaffolds in tissue engineering. Macromol. Biosci. 2012, 12, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Milton, M.D.; Khan, S.; Singh, J.D.; Mishra, V.; Khandelwal, B.L. A facile access to chalcogen and dichalcogen bearing dialkylamines and diols. Tetrahedron Lett. 2005, 46, 755–758. [Google Scholar] [CrossRef]
- Chen, R.; Ma, Z.; Xiang, Z.; Xia, Y.; Shi, Q.; Wong, S.C.; Yin, J. Hydrogen Peroxide and Glutathione Dual Redox-Responsive Nanoparticles for Controlled DOX Release. Macromol. Biosci. 2020, 20, 1900331. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.K.; Abdul-Karim, R.; Rahim, S.; Musharraf, S.G.; Malik, M.I. Analysis of individual block length of amphiphilic di-& tri-block copolymers containing poly (ethylene oxide) and poly (methyl methacrylate). RSC Adv. 2017, 7, 41693–41704. [Google Scholar]
- Kim, Y.; Pourgholami, M.H.; Morris, D.L.; Stenzel, M.H. Effect of cross-linking on the performance of micelles as drug delivery carriers: A cell uptake study. Biomacromolecules 2012, 13, 814–825. [Google Scholar] [CrossRef]
- Alper Isoglu, I.; Ozsoy, Y.; Dincer Isoglu, S. Advances in micelle-based drug delivery: Cross-linked systems. Curr. Top. Med. Chem. 2017, 17, 1469–1489. [Google Scholar] [CrossRef]
- Biancacci, I.; De Lorenzi, F.; Theek, B.; Bai, X.; May, J.N.; Consolino, L.; Baues, M.; Moeckel, D.; Gremse, F.; von Stillfried, S. Monitoring EPR effect dynamics during nanotaxane treatment with theranostic polymeric micelles. Adv. Sci. 2022, 9, 2103745. [Google Scholar] [CrossRef]
- Coelho, J.F. Drug delivery systems: Advanced technologies potentially applicable in personalised treatment, educational measures. EPMA J. 2014, 5, A15. [Google Scholar] [CrossRef]
- Korge, P.; Calmettes, G.; Weiss, J.N. Increased reactive oxygen species production during reductive stress: The roles of mitochondrial glutathione and thioredoxin reductases. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 514–525. [Google Scholar] [CrossRef]
- Cheng, G.; He, Y.; Xie, L.; Nie, Y.; He, B.; Zhang, Z.; Gu, Z. Development of a reduction-sensitive diselenide-conjugated oligoethylenimine nanoparticulate system as a gene carrier. Int. J. Nanomed. 2012, 7, 3991–4006. [Google Scholar]
- Cheng, X.; Jin, Y.; Qi, R.; Fan, W.; Li, H.; Sun, X.; Lai, S. Dual pH and oxidation-responsive nanogels crosslinked by diselenide bonds for controlled drug delivery. Polymer 2016, 101, 370–378. [Google Scholar] [CrossRef]
- Vega-Hissi, E.G.; Andrada, M.F.; Díaz, M.G.; Garro Martinez, J.C. Computational study of the hydrogen peroxide scavenging mechanism of allyl methyl disulfide, an antioxidant compound from garlic. Mol. Divers. 2019, 23, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Siboro, S.A.; Anugrah, D.S.; Jeong, Y.T.; Yoo, S.I.; Lim, K.T. Systematic investigation to the effects of near-infrared light exposure on polymeric micelles of poly (ethylene glycol)-block-poly (styrene-alt-maleic anhydride) loaded with indocyanine green. Polym. Degrad. Stab. 2019, 167, 241–249. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Y.; Wang, X.; Cao, Y.; Liu, J.; Liu, J.; Deng, L.; Dong, A. Balancing the stability and drug release of polymer micelles by the coordination of dual-sensitive cleavable bonds in cross-linked core. Acta Biomater. 2015, 11, 126–136. [Google Scholar] [CrossRef] [PubMed]
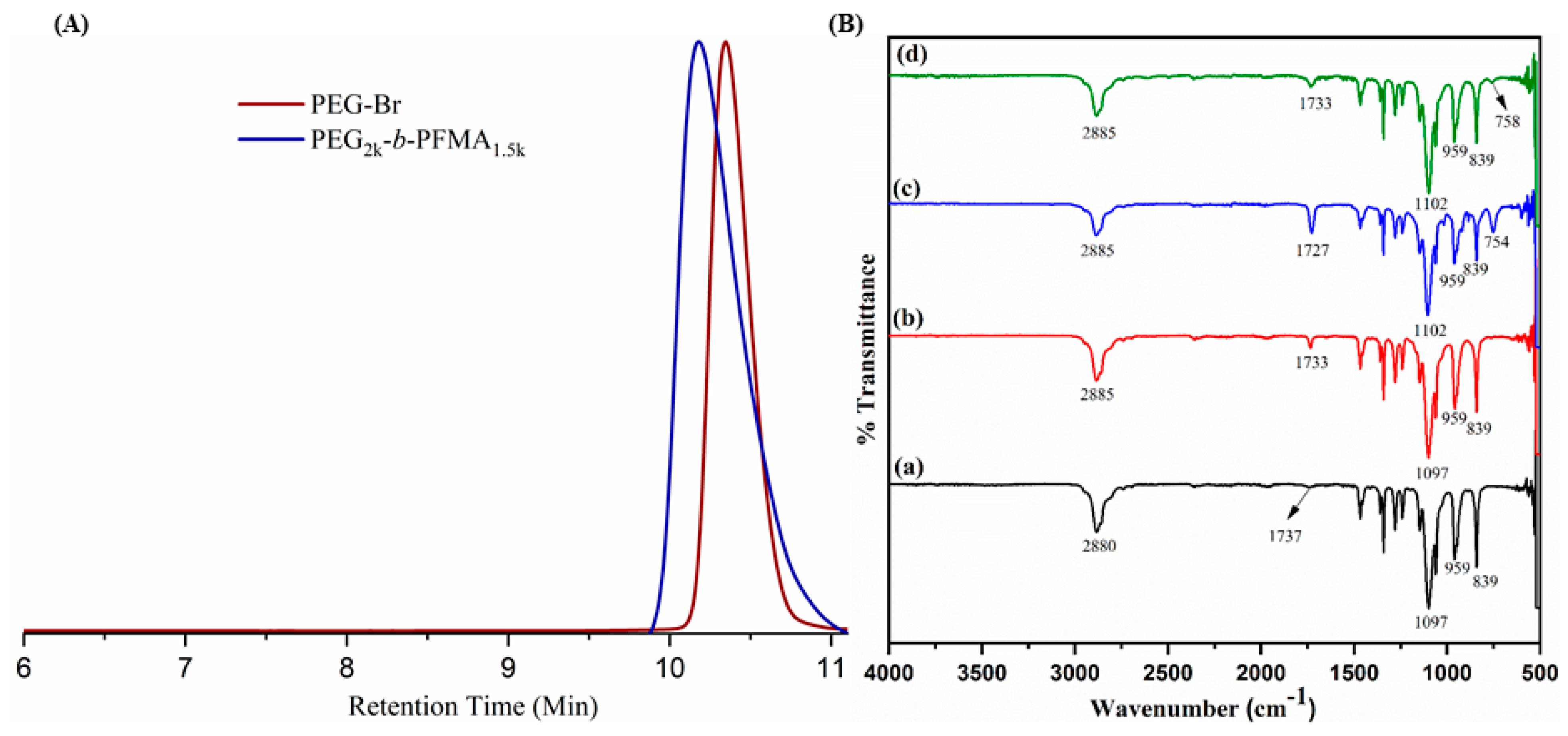
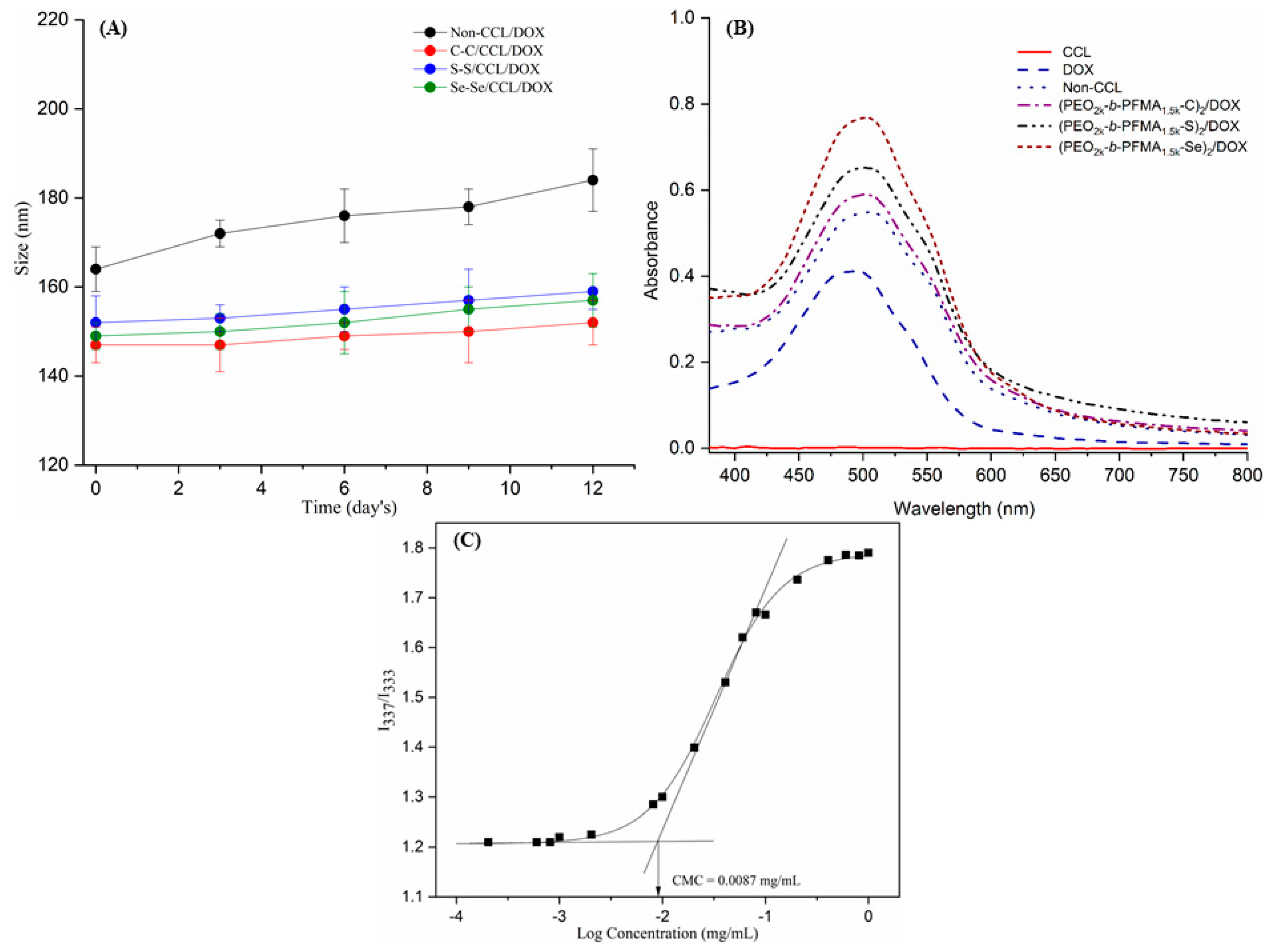
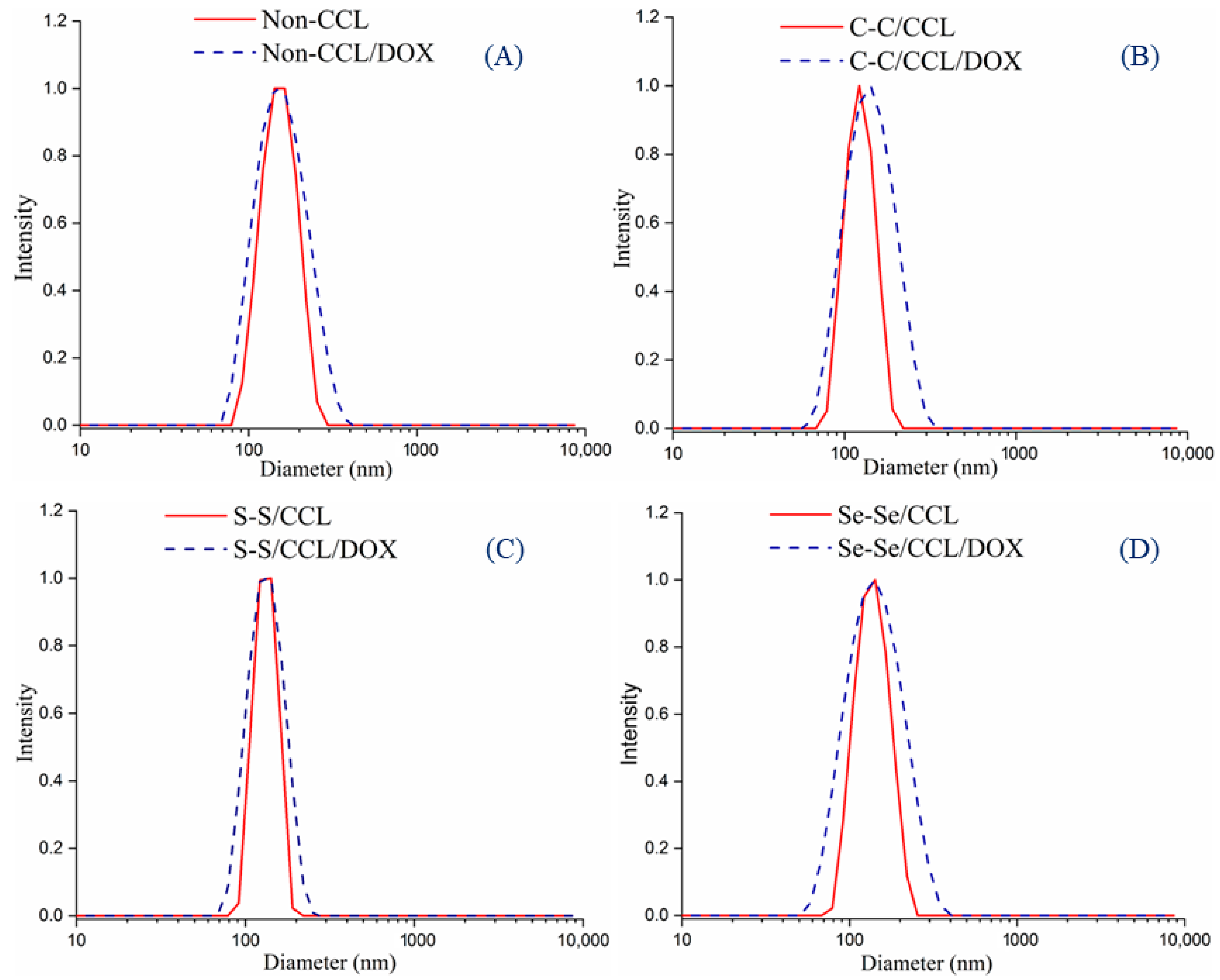
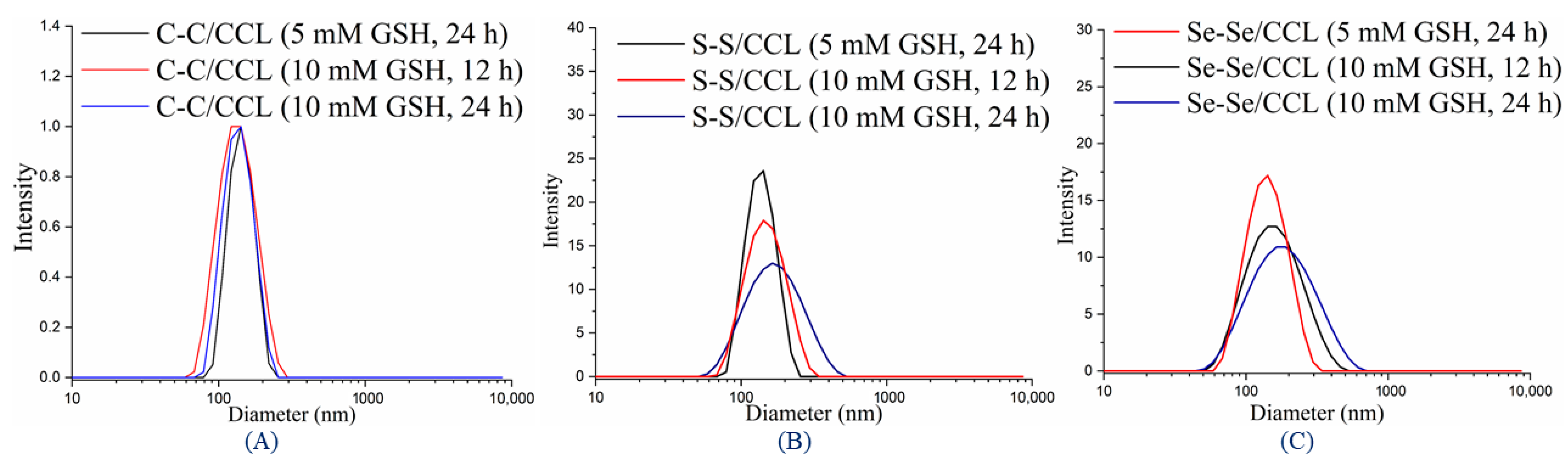
| Run | Polymer | Mn (NMR) a (g/mol) | Mn (GPC) b (g/mol) | Ð |
|---|---|---|---|---|
| 1 | PEO2k-Br | 2150 | 2300 | 1.06 |
| 2 | PEO2k-b-PFMA1.5k | 3645 | 3900 | 1.12 |
| Micelles | Blank Micelles | DOX-Loaded Micelles b | ||||
|---|---|---|---|---|---|---|
| Diameter a (nm) | PDI a | Diameter a (nm) | PDI a | LC (%) | LE (%) | |
| Non-CCL | 155 ± 35 | 0.243 | 164 ± 54 | 0.261 | 60.28 | 6.85 |
| C-C/CCL | 124 ± 35 | 0.243 | 147 ± 53 | 0.237 | 68.36 | 7.76 |
| S-S/CCL | 133 ± 31 | 0.263 | 152 ± 55 | 0.260 | 74.45 | 8.45 |
| Se-Se/CCL | 139 ± 31 | 0.295 | 149 ± 54 | 0.251 | 79.50 | 9.03 |
| Micelles | Time (h) | 5 mM GSH a | 50 mM H2O2 a | ||
|---|---|---|---|---|---|
| Diameter (nm) | PDI | Diameter (nm) | PDI | ||
| C-C/CCL | 24 | 124 ± 54 | 0.253 | 127 ± 54 | 0.277 |
| S-S/CCL | 24 | 141 ± 70 | 0.240 | 135 ± 31 | 0.293 |
| Se-Se/CCL | 24 | 156 ± 65 | 0.255 | 161 ± 54 | 0.327 |
| Micelles | 10 mM GSH a | 100 mM H2O2 a | |||
| Diameter (nm) | PDI | Diameter (nm) | PDI | ||
| C-C/CCL | 0 | 124 ± 35 | 0.243 | -- | -- |
| 12 | 121 ± 57 | 0.248 | 125 ± 53 | 0.236 | |
| 24 | 126 ± 52 | 0.232 | 130 ± 52 | 0.211 | |
| S-S/CCL | 0 | 133 ± 31 | 0.263 | -- | -- |
| 12 | 148 ± 56 | 0.271 | 134 ± 64 | 0.271 | |
| 24 | 150 ± 75 | 0.355 | 137 ± 62 | 0.275 | |
| Se-Se/CCL | 0 | 139 ± 31 | 0.295 | -- | -- |
| 12 | 155 ± 61 | 0.293 | 159 ± 55 | 0.298 | |
| 24 | 158 ± 88 | 0.388 | 164 ± 69 | 0.322 | |
| Code | GSH/H2O2 | pH | Zero-Order | First Order | Higuchi Model | Korsmeyer-Peppas | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| K0 | r2 | K1 | r2 | KH | r2 | n | r2 | |||
| Non-CCL/DOX | NA | 7.4 | 0.932 | 1.558 | 0.013 | 1.036 | 5.966 | 0.475 | 0.403 | 0.959 |
| 5.0 | 1.881 | 0.032 | 0.046 | 0.719 | 11.470 | 0.886 | 0.525 | 0.981 | ||
| S-S/DOX | NA | 7.4 | 0.160 | 0.206 | 0.002 | 0.239 | 0.964 | 0.895 | 0.548 | 0.884 |
| 5.0 | 0.522 | 0.163 | 0.006 | 0.012 | 3.227 | 0.822 | 0.595 | 0.980 | ||
| Se-Se/DOX | NA | 7.4 | 0.239 | 0.648 | 0.003 | 0.568 | 1.497 | 0.734 | 0.476 | 0.955 |
| 5.0 | 0.608 | 0.692 | 0.007 | 0.473 | 3.851 | 0.645 | 0.550 | 0.938 | ||
| S-S/DOX | 10 mM GSH | 7.4 | 0.952 | 0.090 | 0.013 | 0.202 | 5.839 | 0.853 | 0.493 | 0.933 |
| 5.0 | 1.739 | 0.063 | 0.039 | 0.672 | 10.618 | 0.871 | 0.631 | 0.997 | ||
| Se-Se/DOX | 10 mM GSH | 7.4 | 1.173 | 0.426 | 0.018 | 0.049 | 7.270 | 0.809 | 0.432 | 0.994 |
| 5.0 | 1.975 | 0.107 | 0.056 | 0.730 | 12.201 | 0.820 | 0.650 | 0.996 | ||
| S-S/DOX | 100 mM H2O2 | 7.4 | 0.255 | 1.031 | 0.003 | 0.933 | 1.622 | 0.590 | 0.515 | 0.994 |
| 5.0 | 0.709 | 0.383 | 0.009 | 0.155 | 4.451 | 0.723 | 0.608 | 0.947 | ||
| Se-Se/DOX | 100 mM H2O2 | 7.4 | 1.466 | 0.326 | 0.028 | 0.284 | 9.152 | 0.770 | 0.605 | 0.992 |
| 5.0 | 2.026 | 0.049 | 0.056 | 0.805 | 12.380 | 0.869 | 0.620 | 0.993 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Ramesh, K.; Reddy, O.S.; Karthika, V.; Kumar, P.; Jo, S.-H.; Yoo, S.I.; Park, S.-H.; Lim, K.T. Redox-Responsive Comparison of Diselenide and Disulfide Core-Cross-Linked Micelles for Drug Delivery Application. Pharmaceutics 2023, 15, 1159. https://doi.org/10.3390/pharmaceutics15041159
Yadav S, Ramesh K, Reddy OS, Karthika V, Kumar P, Jo S-H, Yoo SI, Park S-H, Lim KT. Redox-Responsive Comparison of Diselenide and Disulfide Core-Cross-Linked Micelles for Drug Delivery Application. Pharmaceutics. 2023; 15(4):1159. https://doi.org/10.3390/pharmaceutics15041159
Chicago/Turabian StyleYadav, Sonyabapu, Kalyan Ramesh, Obireddy Sreekanth Reddy, Viswanathan Karthika, Parveen Kumar, Sung-Han Jo, Seong II Yoo, Sang-Hyug Park, and Kwon Taek Lim. 2023. "Redox-Responsive Comparison of Diselenide and Disulfide Core-Cross-Linked Micelles for Drug Delivery Application" Pharmaceutics 15, no. 4: 1159. https://doi.org/10.3390/pharmaceutics15041159
APA StyleYadav, S., Ramesh, K., Reddy, O. S., Karthika, V., Kumar, P., Jo, S.-H., Yoo, S. I., Park, S.-H., & Lim, K. T. (2023). Redox-Responsive Comparison of Diselenide and Disulfide Core-Cross-Linked Micelles for Drug Delivery Application. Pharmaceutics, 15(4), 1159. https://doi.org/10.3390/pharmaceutics15041159









