Development of Transdermal Oleogel Containing Olmesartan Medoxomil: Statistical Optimization and Pharmacological Evaluation
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Solubility Study
2.3. Oleogel Formulations Preparation
2.4. Statistical Design
2.5. Characterization of the Prepared Oleogel Formulations
2.5.1. pH Determination
2.5.2. Drug Content
2.5.3. Viscosity Measurement and Determination of Rheological Characteristics
2.5.4. Texture Analysis
2.5.5. Ex Vivo Skin Bioadhesive Properties
2.6. Characterization of the Optimized Oleogel Formulation
2.6.1. In Vitro OLM Release Study
2.6.2. Ex Vivo Permeation Study
2.7. In Vivo Study
2.7.1. Histopathological Study
2.7.2. Pharmacodynamic Study
2.7.3. Blood Sampling for Biochemistry
2.7.4. Pharmacokinetic Study
3. Results and Discussion
3.1. Solubility Study
3.2. Preparation of Oleogel Formulations Loaded with OLM
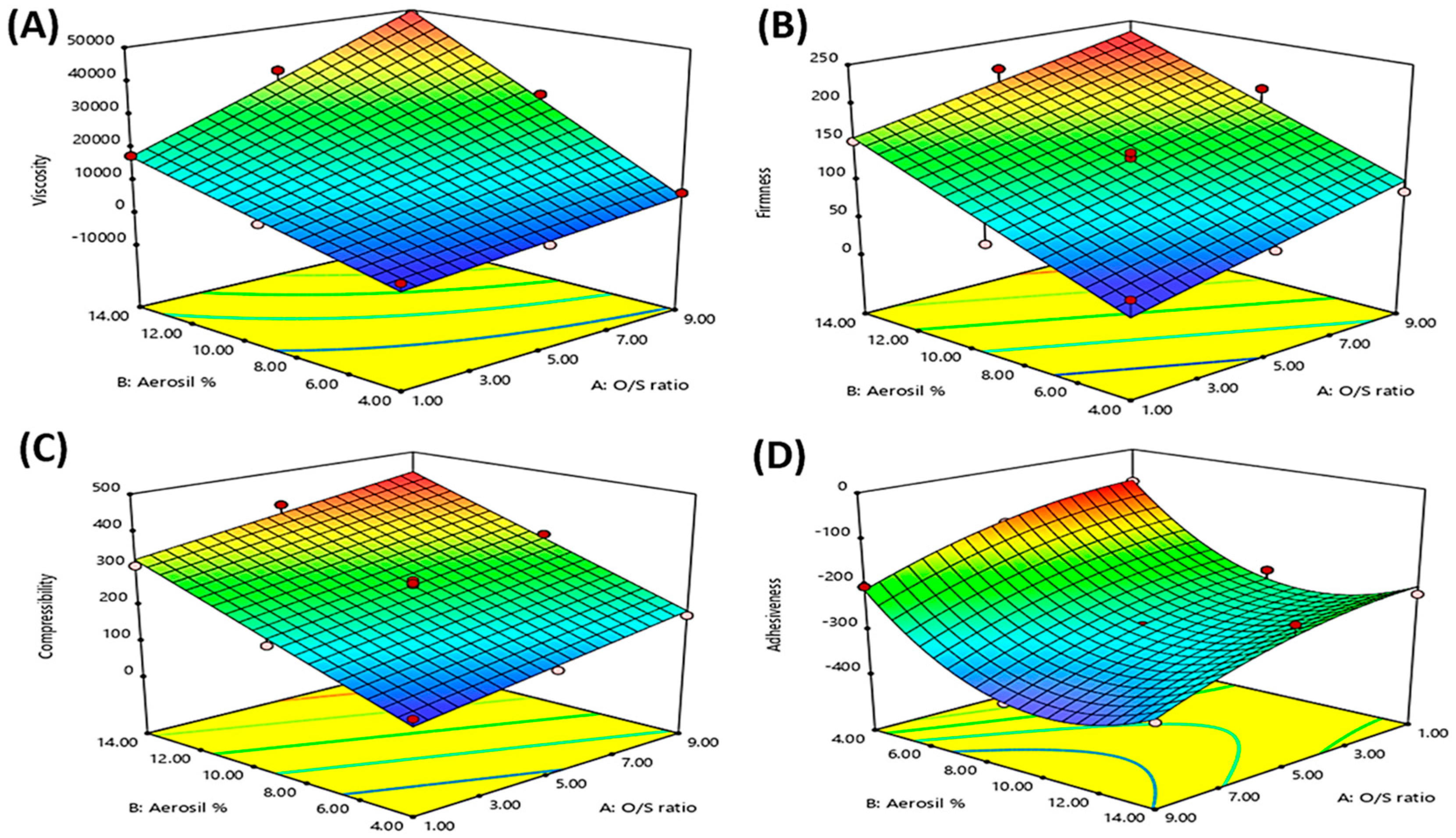
3.3. Statistical Analysis of the Experimental Design
3.4. Characterization of the Prepared Oleogel Formulations Loaded with OLM
3.4.1. pH Determination
3.4.2. Drug Content
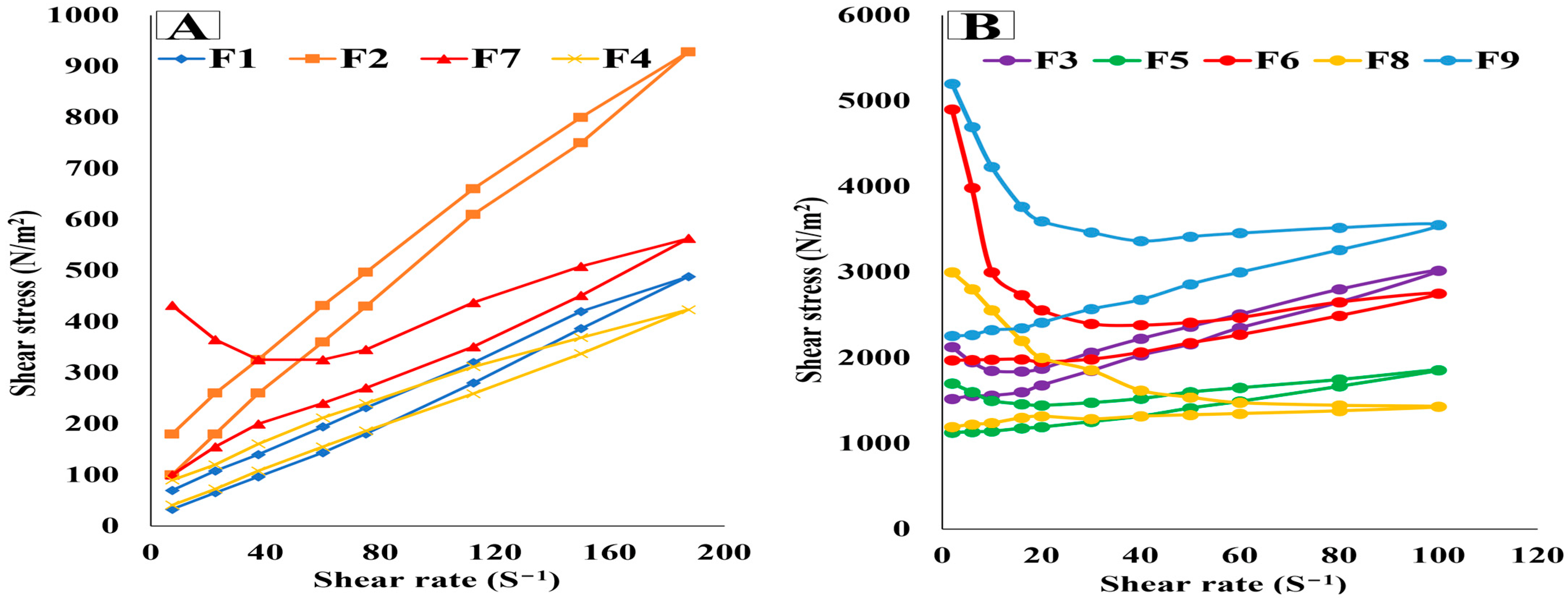
3.4.3. Viscosity Measurement and Rheological Characteristics Determination
3.4.4. Texture Analysis
Firmness
Compressibility
Adhesiveness
3.4.5. Ex Vivo Skin Bioadhesive Properties
3.5. Selection of the Optimized Oleogel Formulation
3.6. Characterization of the Optimized Oleogel Formulation
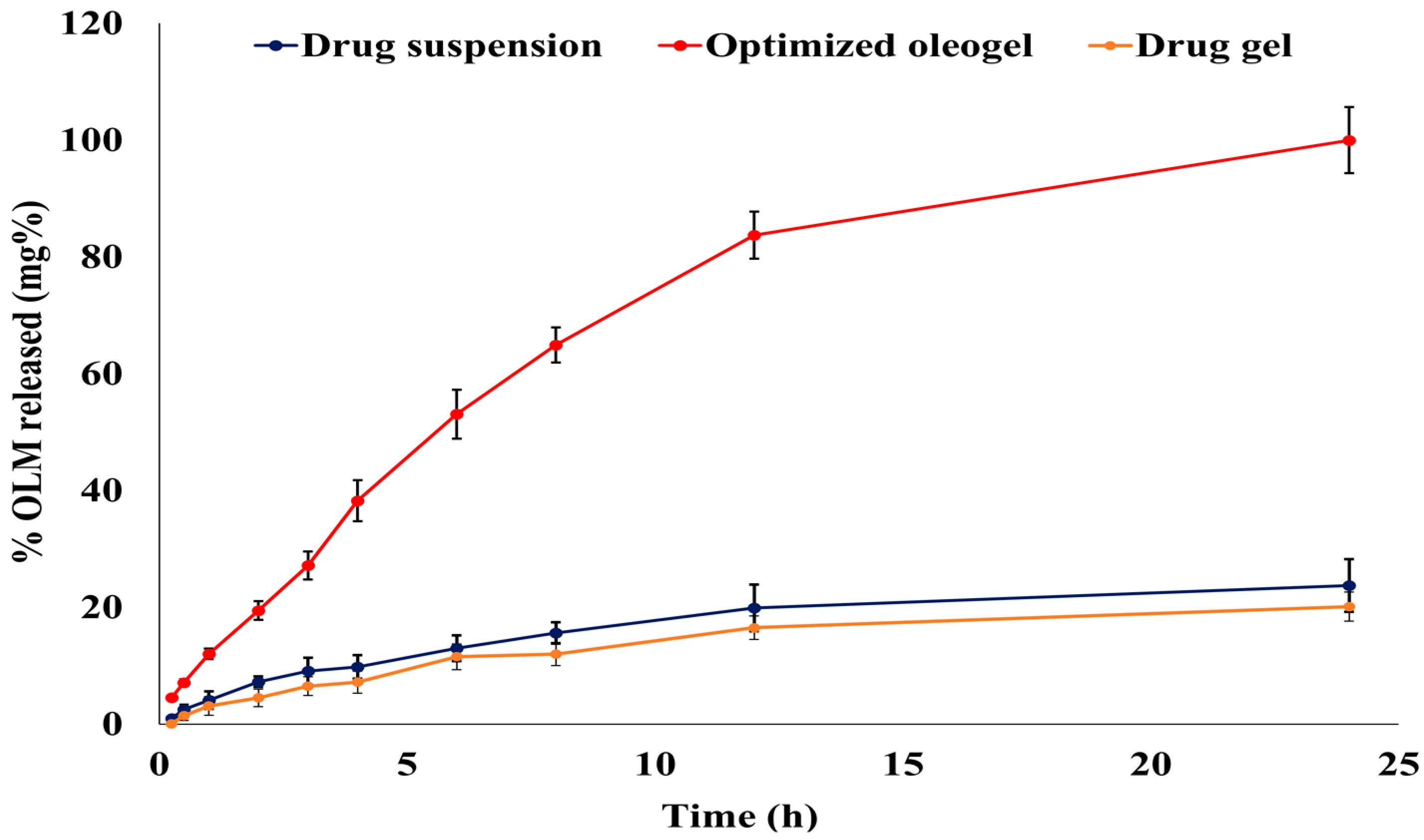
3.6.1. In Vitro OLM Release from the Optimized Oleogel Formulation
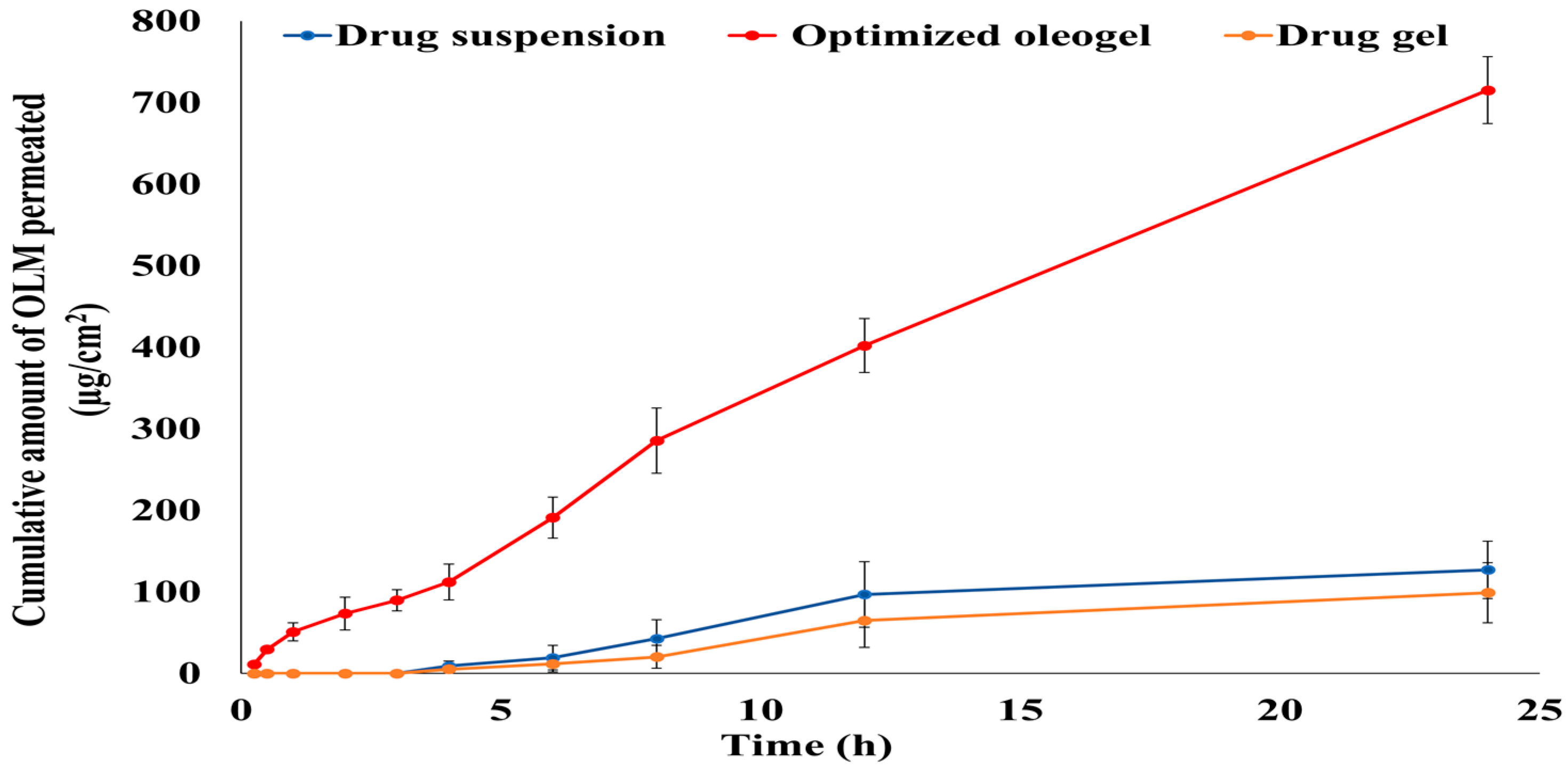
3.6.2. Ex Vivo Permeation Study
3.7. In Vivo Studies
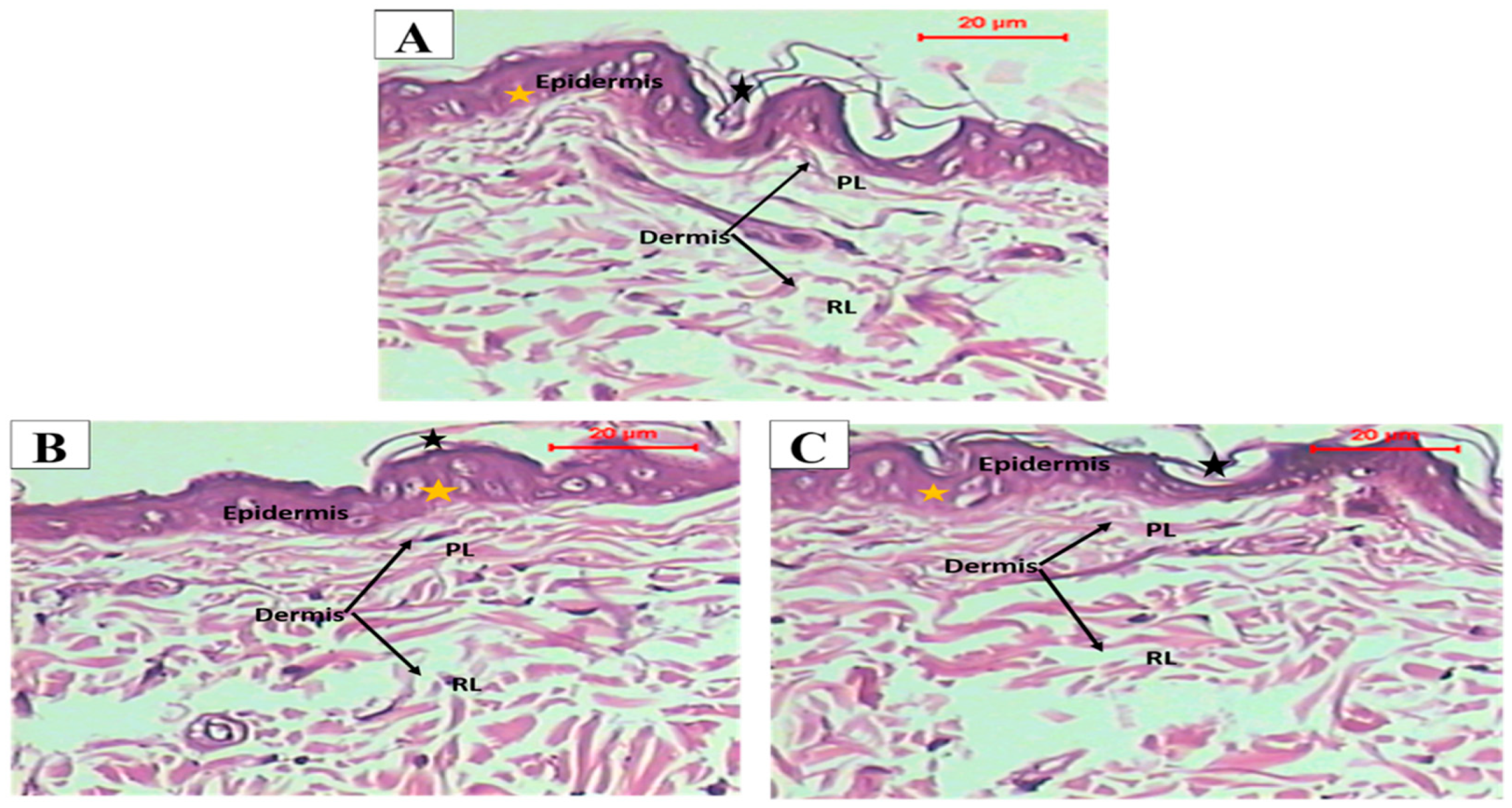
3.7.1. Histopathological Study
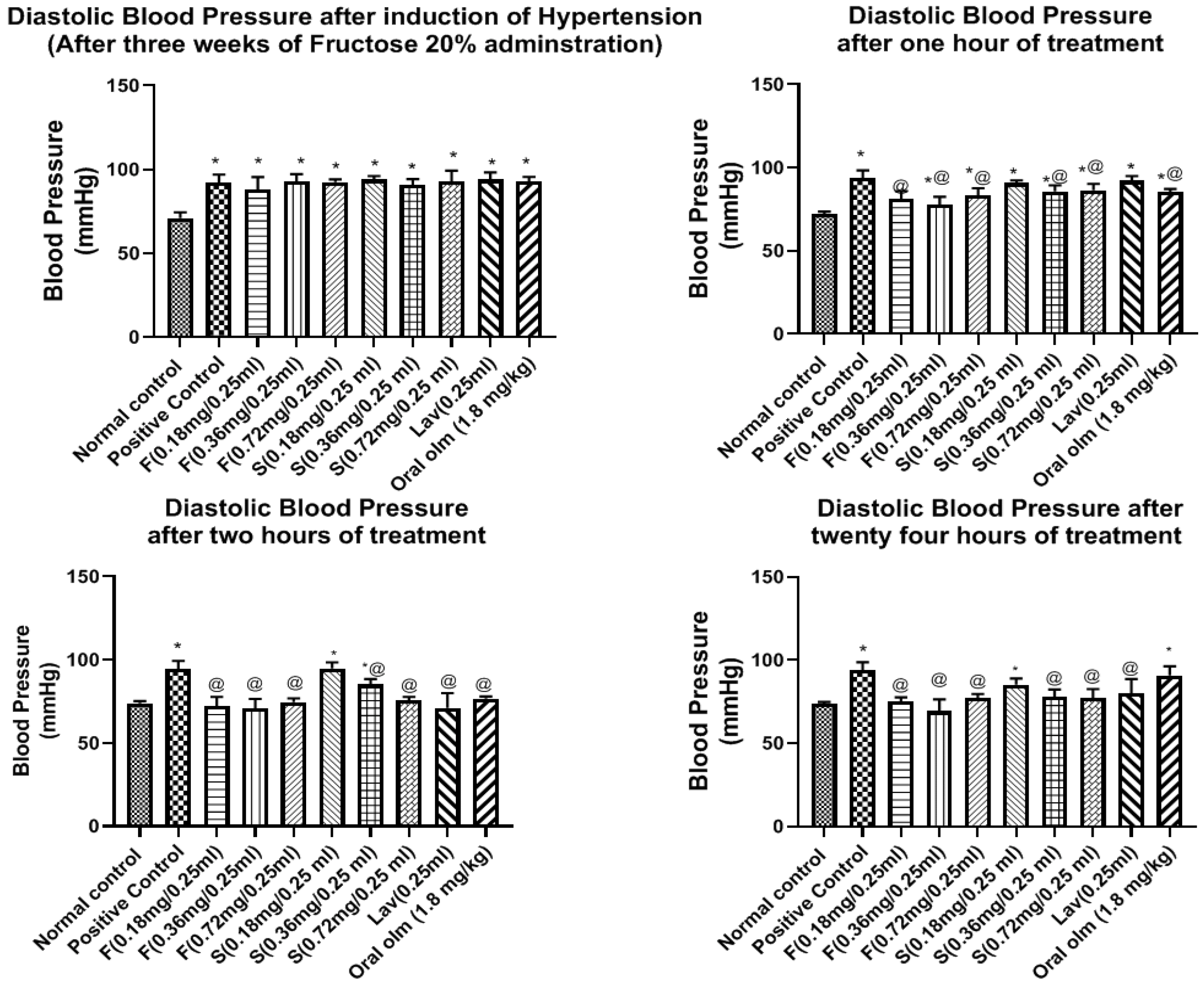
3.7.2. Pharmacodynamic StudyEffect of OLM on Blood Pressure
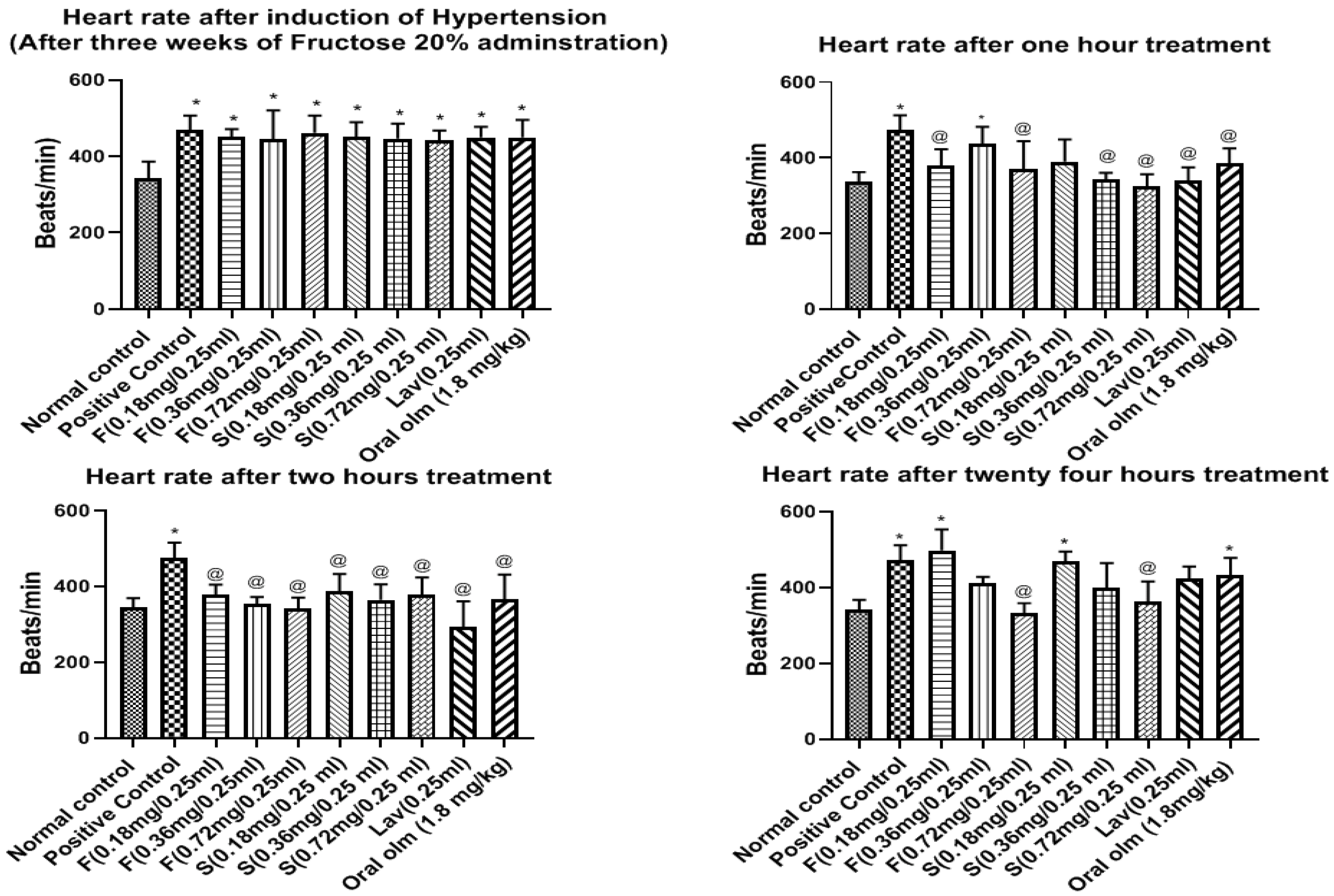
Effect of OLM on Heart Rate
3.7.3. Blood Sampling for Biochemistry
3.7.4. Pharmacokinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, S.; Hsieh, Y.S.; Shin, Y.K.; Kang, P.; Seol, G.H. Linalyl acetate prevents olmesartan-induced intestinal hypermotility mediated by interference of the sympathetic inhibitory pathway in hypertensive rat. Biomed. Pharmacother. 2018, 102, 362–368. [Google Scholar] [CrossRef]
- Kamran, M.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y.; Ali, A. Design, formulation and optimization of novel soft nano-carriers for transdermal olmesartan medoxomil delivery: In vitro characterization and in vivo pharmacokinetic assessment. Int. J. Pharm. 2016, 505, 147–158. [Google Scholar] [CrossRef]
- Hathout, R.M.; Elshafeey, A.H. Development and characterization of colloidal soft nano-carriers for transdermal delivery and bioavailability enhancement of an angiotensin II receptor blocker. Eur. J. Pharm. Biopharm. 2012, 82, 230–240. [Google Scholar] [CrossRef]
- Beg, S.; Katare, O.P.; Saini, S.; Garg, B.; Khurana, R.K.; Singh, B. Solid self-nanoemulsifying systems of olmesartan medoxomil: Formulation development, micromeritic characterization, in vitro and in vivo evaluation. Powder Technol. 2016, 294, 93–104. [Google Scholar] [CrossRef]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: In- vitro characterization, ex-vivo permeation and in-vivo assessment. Int. J. Nanomed. 2019, 14, 6555–6574. [Google Scholar] [CrossRef]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Use of transethosomes for enhancing the transdermal delivery of olmesartan medoxomil: In vitro, ex vivo and in vivo evaluation. Int. J. Nanomed. 2019, 14, 1953–1986. [Google Scholar] [CrossRef]
- Murdan, S. Organogels in drug delivery. Expert Opin Drug Deliv. 2005, 2, 489–505. [Google Scholar] [CrossRef]
- Wroblewska, M.; Szymańska, E.; Szekalska, M.; Winnicka, K. Different types of gel carriers as metronidazole delivery systems to the oral mucosa. Polymers 2020, 12, 680. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Wróblewska, M.; Winnicka, K.; Wieczorek, P.; Majewski, P.; Celínska-Janowicz, K.; Sawczuk, R.; Miltyk, W.; Tryniszewska, E.; Tomczyk, M. Novel gel formulations as topical carriers for the essential oil of bidens tripartita for the treatment of candidiasis. Molecules 2018, 23, 2517. [Google Scholar] [CrossRef]
- Wroblewska, M.; Szekalska, M.; Hafner, A.; Winnicka, K. Oleogels and bigels as topical drug carriers for ketoconazole–development and in vitro characterization. Acta Pol. Pharm.-Drug Res. 2018, 75, 777–786. [Google Scholar]
- Lu, Z.; Fassihi, R. Influence of Colloidal Silicon Dioxide on Gel Strength, Robustness, and Adhesive Properties of Diclofenac Gel Formulation for Topical Application. AAPS PharmSciTech 2015, 16, 636–644. [Google Scholar] [CrossRef]
- Patel, A.R.; Mankoč, B.; Bin Sintang, M.D.; Lesaffer, A.; Dewettinck, K. Fumed silica-based organogels and ‘aqueous-organic’ bigels. RSC Adv. 2015, 5, 9703–9708. [Google Scholar] [CrossRef]
- Balakumar, K.; Raghavan, C.V.; Selvan, N.T.; Rahman, S.M.H. Self emulsifying drug delivery system: Optimization and its prototype for various compositions of oils, surfactants and co-surfactants. J. Pharm. Res. 2013, 6, 510–514. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.; Wu, Y.; Liu, P.; Yao, J.; Lu, Q.; Zhang, H.; Duan, J. Potential of Essential Oils as Penetration Enhancers for Transdermal Administration of Ibuprofen to Treat Dysmenorrhoea. Molecules 2015, 20, 18219–18236. [Google Scholar] [CrossRef]
- Lawrence, M.J. Surfactant systems: Their use in drug delivery. Chem. Soci. Rev. 1994, 23, 417–424. [Google Scholar] [CrossRef]
- Weerapol, Y.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P. Self-nanoemulsifying drug delivery system of nifedipine: Impact of hydrophilic-lipophilic balance and molecular structure of mixed surfactants. AAPS PharmSciTech 2014, 15, 456–464. [Google Scholar] [CrossRef]
- Naveed, A.; Rasool, F.; Saeed, T.; Murtaza, G. Penetration Enhancing Effect of Polysorbate 20 and 80 on the In Vitro Percutaneous Absorption of LAscorbic Acid. Trop. J. Pharm. Res. 2011, 10, 281. [Google Scholar]
- Cserhati, T. Alkyl Ethoxylated and alkylphenol ethoxylated nonionic surfactants: Interaction with bioactive compounds and biological effects. Environ. Health Perspect. 1995, 103, 358–364. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar]
- Hurler, J.; Engesland, A.; Kermany, P.; Kalko-Basnet, N.S. Improved Texture Analysis for Hydrogel Characterization: Gel Cohesiveness, Adhesiveness, and Hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Carvalho, F.l.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.; Amaral, J.; Ribeiro, M.; Sebastião, E.; Vargas, C.; Franzen, F.; Schneidera, G.; Lorenzo, J.M.; Friesa, L.L.; Cichoski, A.J. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci. 2019, 149, 141–148. [Google Scholar]
- Nasr, M.; Karandikar, H.; Abdel-Aziz, R.T.A.; Moftah, N.; Paradkar, A. Novel nicotinamide skin-adhesive hot melt extrudates for treatment of acne. Expert Opin. Drug Deliv. 2018, 15, 1165–1173. [Google Scholar] [CrossRef]
- Abd-Allah, H.; Abdel-Aziz, R.T.A.; Nasr, M. Chitosan nanoparticles making their way to clinical practice: A feasibility study on their topical use for acne treatment. Int. J. Biol. Macromol. 2020, 156, 262–270. [Google Scholar] [CrossRef]
- Hassan, D.H.; Shohdy, J.N.; El-Setouhy, D.A.; El-Nabarawi, M.; Naguib, M.J. Compritol-Based Nanostrucutured Lipid Carriers (NLCs) for Augmentation of Zolmitriptan Bioavailability via the Transdermal Route: In Vitro Optimization, Ex Vivo Permeation, In Vivo Pharmacokinetic Study. Pharmaceutics 2022, 14, 1484. [Google Scholar] [CrossRef]
- El-Dahmy, R.M.; Elsayed, I.; Elshafeey, A.H.; Abd El Gawad, N.A.; El-gazayerly, O.N. Optimization of long circulating mixed polymeric micelles containing vinpocetine using simple lattice mixture design, in vitro and in vivo characterization. Int. J. Pharm. 2014, 477, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, I.; El-Dahmy, R.M.; El-Emam, S.Z.; Elshafeey, A.H.; Abd El Gawad, N.A.; El-gazayerly, O.N. Response surface optimization of biocompatible elastic nanovesicles loaded with rosuvastatin calcium: Enhanced bioavailability and anticancer efficacy. Drug Deliv. Transl. Res. 2020, 10, 1459–1475. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; El-Dahmy, R.M. Formulation and development of oral fast-dissolving films loaded with nanosuspension to augment paroxetine bioavailability: In vitro characterization, ex vivo permeation, and pharmacokinetic evaluation in healthy human volunteers. Pharmaceutics 2021, 13, 1869. [Google Scholar] [CrossRef]
- Abdelrahman, F.E.; Elsayed, I.; Gad, M.K.; Badr, A.; Mohamed, M.I. Investigating the Cubosomal Ability for Transnasal Brain Targeting: In Vitro Optimization, Ex Vivo Permeation and in Vivo Biodistribution. Int. J. Pharm. 2015, 490, 281–291. [Google Scholar] [CrossRef]
- Elsayed, I.; El-Dahmy, R.M.; Elshafeey, A.H.; El Gawad, A.; Abdelaziz, N.; Gazayerly, E.; Naim, O. Tripling the Bioavailability of Rosuvastatin Calcium Through Development and Optimization of an In-Situ Forming Nanovesicular System. Pharmaceutics 2019, 11, 275. [Google Scholar] [CrossRef]
- El-Dahmy, R.M.; Elshafeey, A.H.; Abd El Gawad, N.A.; El-Gazayerly, O.N.; Elsayed, I. Statistical optimization of nanostructured gels for enhancement of vinpocetine transnasal and transdermal permeation. J. Drug Deliv. Sci. Technol. 2021, 66, 102871. [Google Scholar] [CrossRef]
- Albash, R.; Fahmy, A.M.; Hamed, M.I.; Darwish, K.M.; El-Dahmy, R.M. Spironolactone hyaluronic acid enriched cerosomes (HAECs) for topical management of hirsutism: In silico studies, statistical optimization, ex vivo, and in vivo studies. Drug Deliv. 2021, 28, 2289–2300. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Elnahas, O.S.; Assar, N.H.; Gad, A.M.; Hosary, R. El Nanocrystals of Fusidic Acid for Dual Enhancement of Dermal Delivery and Antibacterial Activity: In Vitro, Ex Vivo and In Vivo Evaluation. Pharmaceutics 2020, 12, 199. [Google Scholar] [CrossRef]
- Teaima, M.H.; Alsofany, J.M.; El-Nabarawi, M.A. Clove Oil Endorsed Transdermal Flux of Dronedarone Hydrochloride Loaded Bilosomal Nanogel: Factorial Design, In Vitro Evaluation and Ex Vivo Permeation. AAPS PharmSciTech 2022, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Alsofany, J.M.; Hamza, M.Y. Abdelbary AA Fabrication of Nanosuspension Directly Loaded Fast-Dissolving Films for Enhanced Oral Bioavailability of Olmesartan Medoxomil: In Vitro Characterization and Pharmacokinetic Evaluation in Healthy Human Volunteers. AAPS PharmSciTech 2018, 19, 2118–2132. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.M.; Josef, M.; El-Nabarawi, M.A.; Teaima, M. Sertaconazole-Nitrate-Loaded Leciplex for Treating Keratomycosis: Optimization Using D-Optimal Design and In Vitro, Ex Vivo, and In Vivo Studies. Pharmaceutics 2022, 14, 2215. [Google Scholar] [CrossRef]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M.; Ali, A. Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan. Nanomedicine 2012, 8, 237–249. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef]
- Ong, S.; Zhang, Y.; Whitworth, J. Mechanisms of Dexamethasone-Induced Hypertension. Curr. Hypertens. Rev. 2009, 5, 61–74. [Google Scholar] [CrossRef]
- Kaithwas, V.; Dora, C.P.; Kushwah, V.; Jain, S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf. B. Biointerfaces 2017, 154, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, R.E.; Ibrahim, B.M.M.; Abdel Jaleel, G.A. Neuro-protective effects of Ginkgo biloba leaves extract on cerebral ischemia-reperfusion injury induced experimentally in ovariectomized rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 237–242. [Google Scholar]
- Trinder, P. A Rapid Method for the Determination of Sodium in Serum. Analyst 1951, 76, 596–599. [Google Scholar] [CrossRef]
- Sunderman, F.W.J.; Sunderman, F.W. Determination of potassium. Am. J. Clin. Pathol. 1958, 29, 95. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Belgamwar, V.; Gattani, S.; Surana, S.; Tekade, A. Pluronic lecithin organogel as a topical drug delivery system. Drug Deliv. 2010, 17, 38–47. [Google Scholar] [CrossRef] [PubMed]
- The United States Pharmacopeia, USP 41–NF 36; The United States Pharmacopeial Convention: Rockville, MD, USA, 2018.
- Maheshwari, M.; Miglani, G.; Paradkar, A.; Yamamura, S.; Kadam, S. Development of Tetracycline-Serratiopeptidase-Containing Periodontal Gel: Formulation and Preliminary Clinical Study. AAPS PharmSciTech 2006, 7, 76. [Google Scholar] [CrossRef]
- Jin, S.G.; Yousaf, A.M.; Son, M.W.; Jang, S.W.; Kim, D.W.; Kim, J.O.; Yong, C.S.; Kim, J.H.; Choi, H.G. Mechanical properties, skin permeation and in vivo evaluations of dexibuprofen-loaded emulsion gel for topical delivery. Arch Pharm Res. 2015, 38, 216–222. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; El-Dahmy, R.M. A novel oral medicated jelly for enhancement of etilefrine hydrochloride bioavailability: In vitro characterization and pharmacokinetic evaluation in healthy human volunteers. Saudi Pharm. J. 2022, 30, 1435–1447. [Google Scholar] [CrossRef]
- Coviello, T.; Coluzi, G.; Palleschi, A.; Grassi, M.; Santucci, E.; Alhaique, F. Structural and Rheological Characterization of Scleroglucan/borax Hydrogel for Drug Delivery. Int. J. Biol. Macromol. 2003, 32, 83–92. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Morton, D.A.V. Drug-lactose binding aspects in adhesive mixtures: Controlling performance in dry powder inhaler formulations by altering lactose carrier surfaces. Adv. Drug Deliv. Rev. 2012, 64, 275–284. [Google Scholar] [CrossRef]
- Park, S.; Park, H.; Han, O.H.; Chae, S.A.; Lee, D.; Kim, D. Non-sticky silicate replica mold by phase conversion approach for nanoimprint lithography applications. J. Mater. Sci. 2010, 10, 1039. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Tan, A.; Martin, A.; Prestidge, C.A. Tableting Lipid-Based Formulations for Oral Drug Delivery: A Case Study with Silica Nanoparticle–Lipid–Mannitol Hybrid Microparticles. J. Pharm. Sci. 2013, 102, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Escotet-Espinoza, M.S.; Scicolone, J.V.; Moghtadernejad, S.; Sanchez, E.; Cappuyns, P.; Van Assche, I.; Di Pretoro, G.; Ierapetritou, M.; Muzzio, F.J. Improving Feedability of Highly Adhesive Active Pharmaceutical Ingredients by Silication. J. Pharm. Innov. 2021, 16, 279–292. [Google Scholar] [CrossRef]
- Palacio, M.L.B.; Bhushan, B. Bioadhesion: A review of concepts and applications. Phil. Trans. R. Soc. A. 2012, 370, 2321–2347. [Google Scholar] [CrossRef]
- Martinsa, A.J.; Silvaa, P.; Maciela, F.; Pastranab, L.M.; Cunhac, R.L.; Cerqueirab, M.A.; Vicente, A.A. Hybrid gels: Influence of oleogel/hydrogel ratio on rheological and textural properties. Food Res. Int. 2019, 116, 1298–1305. [Google Scholar] [CrossRef]
- Albash, R.; El-Dahmy, R.M.; Hamed, M.I.A.; Darwish, K.M.; Alahdal, A.M.; Kassem, A.B.; Fahmy, A.M. Repurposing levocetirizine hydrochloride loaded into cationic ceramide/phospholipid composite (CCPCs) for management of alopecia: Central composite design optimization, in- silico and in-vivo studies. Drug Deliv. 2022, 29, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Polli, J.E.; Rekhi, G.S.; Augsburger, L.L.; Shah, V.P. Methods to compare dissolution profiles and a rationale for wide dissolution specifications for metoprolol tartrate tablets. J. Pharm. Sci. 1997, 86, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Arun, B.; Narendar, N.; Veerabrahma, K. Development of olmesartan medoxomil lipid based nanoparticles and nanosuspension: Preparation, characterization and comparative pharmacokinetic evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 126–137. [Google Scholar]
- Kim, M.K.; Lee, C.H.; Kim, D.D. Skin permeation of testosterone and its ester derivatives in rats. J. Pharm. Pharmacol. 2000, 52, 369–375. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B. 2016, 4, 1686–1695. [Google Scholar] [CrossRef]
- Vakilian, K.; Atarha, M.; Bekhradi, R.; Chaman, R. Healing advantages of lavender essential oil during episiotomy recovery: A clinical trial. Complement. Ther. Clin. Pract. 2011, 17, 50–53. [Google Scholar] [CrossRef]
- Soto-Piña, A.E.; Franklin, C.; Rani, C.S.S.; Fernandez, E.; Cardoso-Peña, E.; Benítez-Arciniega, A.D.; Gottlieb, H.; Hinojosa-Laborde, C.; Strong, R. Dexamethasone Causes Hypertension in Rats Even Under Chemical Blockade of Peripheral Sympathetic Nerves. Front. Neurosci. 2019, 13, 1305. [Google Scholar] [CrossRef]
- Sayorwan, W.; Siripornpanich, V.; Piriyapunyaporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Ruangrungsi, N. The effects of lavender oil inhalation on emotional states, autonomic nervous system, and brain electrical activity. J. Med. Assoc. Thai. 2012, 95, 598–606. [Google Scholar] [PubMed]
- Chien, L.W.; Cheng, S.L.; Liu, C.F. The Effect of Lavender Aromatherapy on Autonomic Nervous System in Midlife Women with Insomnia. Evid.-Based Complement. Altern. Med. 2012, 2012, 740813. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Yamanaka, T.; Mizuno, M.; Motokawa, M.; Shirasawa, Y.; Miyagi, S.; Nishio, T.; Yoshida, A.; Kimura, G. Angiotensin II type 1 receptor blocker, olmesartan, restores nocturnal blood pressure decline by enhancing daytime natriuresis. J. Hypertens. 2008, 26, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Li, W.; Webb, R.; Zhao, L.; Wang, Q.; Guo, W. Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: A randomized, double-blind, 8-week study. J. Clin. Hypertens. 2019, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]




| X1: Oil/SAA Ratio | X2: Aerosil % (w/v) | pH | Drug Content (%) | Viscosity (cps) | Firmness (N) | Compressibility (mJ) | Adhesiveness (mJ) | Fmax (mN) | Wad (µJ) | |
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 1 | 4 | 5.51 ± 0.13 | 98.46 ± 3.71 | 686.4 ± 13.90 | 42.3 ± 2.98 | 87.7 ± 4.40 | −76.2 ± 5.35 | 131.6 ± 4.79 | 176.4 ± 3.96 |
| F2 | 1 | 9 | 5.26 ± 0.15 | 97.27 ± 5.11 | 6664 ± 42.48 | 61.5 ± 4.02 | 178.2 ± 7.63 | −235.6 ± 11.60 | 539.7 ± 16.11 | 741.7 ± 20.48 |
| F3 | 1 | 14 | 4.91 ± 0.27 | 95.51 ± 2.04 | 17620 ± 79.22 | 151.8 ± 11.68 | 308.5 ± 16.81 | −230.4 ± 8.97 | 445.1 ± 21.04 | 710.2 ± 18.66 |
| F4 | 5 | 4 | 5.17 ± 0.12 | 99.06 ± 4.95 | 982.5 ± 37.09 | 53.1 ± 3.44 | 113.1 ± 6.04 | −119.3 ± 3.41 | 157.3 ± 8.12 | 198.9 ± 7.41 |
| F5 | 5 | 9 | 6.20 ± 0.31 | 97.13 ± 1.84 | 16670 ± 55.24 | 129.6 ± 8.92 | 269.4 ± 18.39 | −301.8 ± 15.08 | 449.1 ± 17.31 | 507.1 ± 11.38 |
| 6.09 ± 0.33 | 96.85 ± 3.61 | 15628 ± 63.28 | 137 ± 6.87 | 261.6 ± 21.40 | −309 ± 10.42 | 438 ± 13.56 | 477.3 ± 9.21 | |||
| 6.18 ± 0.26 | 97.22 ± 1.46 | 16593 ± 80.69 | 126.2 ± 9.46 | 257.3 ± 12.43 | −295.1 ± 7.14 | 451.5 ± 20.63 | 492.1 ± 13.14 | |||
| F6 | 5 | 14 | 5.73 ± 0.40 | 94.86 ± 2.01 | 36670 ± 137.58 | 213.4 ± 10.83 | 408 ± 26.68 | −226.3 ± 13.64 | 393.2 ± 14.53 | 415.6 ± 10.08 |
| F7 | 9 | 4 | 6.11 ± 0.15 | 96.50 ± 2.83 | 6948 ± 53.90 | 84.9 ± 22.64 | 172.1 ± 4.29 | −203.5 ± 6.77 | 314.1 ± 6.16 | 280.9 ± 7.65 |
| F8 | 9 | 9 | 4.99 ± 0.31 | 98.34 ± 1.45 | 29370 ± 94.56 | 186.3 ± 4.91 | 324.4 ± 15.75 | −390.1 ± 11.82 | 460.8 ± 23.74 | 547.5 ± 13.89 |
| F9 | 9 | 14 | 5.87 ± 0.21 | 95.94 ± 3.42 | 48620 ± 183.65 | 221.7 ± 7.64 | 427.6 ± 10.36 | −349.6 ± 12.16 | 415.6 ± 15.73 | 508.2 ± 12.09 |
| Responses | Viscosity (cps) | Firmness (N) | Compressibility (mJ) | Adhesiveness (mJ) | Fmax (mN) | Wad (µJ) |
|---|---|---|---|---|---|---|
| Minimum | 686.4 ± 17.90 | 42.3 ± 2.98 | 87.7 ± 4.40 | −76.2 to ± 5.35 | 131.6 ± 4.79 | 176.4 ± 3.96 |
| Maximum | 48,620 ± 183.65 | 221.7 ± 7.64 | 427.6 ± 10.36 | −390.1 ± 11.82 | 539.7 ± 16.11 | 741.7 ± 20.48 |
| Model | 2 FI | Linear | Linear | Quadratic | Quadratic | Quadratic |
| F value | 170.78 | 61.05 | 189.48 | 52.24 | 18.77 | 18.33 |
| p-value | <0.0001 | <0.0001 | <0.0001 | 0.0003 | 0.0030 | 0.0031 |
| Adequate precision | 40.83 | 23.93 | 40.29 | 23.51 | 11.83 | 13.33 |
| Adjusted R2 | 0.981 | 0.923 | 0.974 | 0.962 | 0.898 | 0.896 |
| Predicted R2 | 0.951 | 0.863 | 0.954 | 0.840 | 0.469 | 0.475 |
| R2 | 0.986 | 0.940 | 0.980 | 0.981 | 0.949 | 0.948 |
| Significant factors | X1, X2, and X1·X2 | X1 and X2 | X1 and X2 | X1, X2, X22 | X2, X1·X2, X22 | X2, X1·X2, X12, X22 |
| Observed values of optimal oleogel | 10,769.4 | 113.19 | 229.97 | −251.63 | 532.12 | 730.86 |
| Predicted values of optimal oleogel | 10,852.9 | 109.43 | 236.91 | −266.72 | 520.58 | 734.85 |
| Group Parameter | Normal Control | Positive Control | F (0.36 mg/0.25 mL) | S (0.36 mg/0.25 mL) | Lavender Oil (0.25 mL) | Oral OLM (1.8 mg/kg) |
|---|---|---|---|---|---|---|
| Na+ | 132.3 ± 1.2 | 146 ± 0.57 | 134 ± 0.7 | 144 ± 0.84 | 145 ± 0.54 | 137.7 ± 0.88 |
| K+ | 4.2 ± 0.1 | 3.33 ± 0.08 | 4.4 ± 0.17 | 4.7 ± 0.11 | 4.5 ± 0.06 | 5.5 ± 0.23 |
| Pharmacokinetics Parameter | Optimized Oleogel | Treatment (Mean ± SD) | |
|---|---|---|---|
| Standard Gel | Market Tablet | ||
| Cmax (µg/mL) a | 41.75 ± 6.84 | 9.14 ± 2.95 | 20.80 ± 5.14 |
| AUC0-48 (ng.h/mL) a | 1098.50 ± 72.41 | 218.85 ± 35.82 | 427.56 ± 57.53 |
| AUC0-∞ (ng.h/mL) a | 1145.19 ± 84.28 | 234.38 ± 41.13 | 429.00 ± 68.91 |
| tmax (h) a | 8 ± 0.00 | 8 ± 0.00 | 8 ± 0.00 |
| t1/2 (h) a | 9.99 ± 1.47 | 10.65 ± 0.94 | 5.39 ± 1.26 |
| K (l/h) a | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.13 ± 0.01 |
| MRT a | 17.60 ± 6.84 | 17.63 ± 7.11 | 13.73 ± 4.92 |
| % Relative bioavailability (%RB) | 488.60% (compared to the standard gel) 266.94% (compared to the market tablet) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Dahmy, R.M.; Elsayed, I.; Hussein, J.; Althubiti, M.; Almaimani, R.A.; El-Readi, M.Z.; Elbaset, M.A.; Ibrahim, B.M.M. Development of Transdermal Oleogel Containing Olmesartan Medoxomil: Statistical Optimization and Pharmacological Evaluation. Pharmaceutics 2023, 15, 1083. https://doi.org/10.3390/pharmaceutics15041083
El-Dahmy RM, Elsayed I, Hussein J, Althubiti M, Almaimani RA, El-Readi MZ, Elbaset MA, Ibrahim BMM. Development of Transdermal Oleogel Containing Olmesartan Medoxomil: Statistical Optimization and Pharmacological Evaluation. Pharmaceutics. 2023; 15(4):1083. https://doi.org/10.3390/pharmaceutics15041083
Chicago/Turabian StyleEl-Dahmy, Rania Moataz, Ibrahim Elsayed, Jihan Hussein, Mohammad Althubiti, Riyad A. Almaimani, Mahmoud Zaki El-Readi, Marawan A. Elbaset, and Bassant M. M. Ibrahim. 2023. "Development of Transdermal Oleogel Containing Olmesartan Medoxomil: Statistical Optimization and Pharmacological Evaluation" Pharmaceutics 15, no. 4: 1083. https://doi.org/10.3390/pharmaceutics15041083
APA StyleEl-Dahmy, R. M., Elsayed, I., Hussein, J., Althubiti, M., Almaimani, R. A., El-Readi, M. Z., Elbaset, M. A., & Ibrahim, B. M. M. (2023). Development of Transdermal Oleogel Containing Olmesartan Medoxomil: Statistical Optimization and Pharmacological Evaluation. Pharmaceutics, 15(4), 1083. https://doi.org/10.3390/pharmaceutics15041083