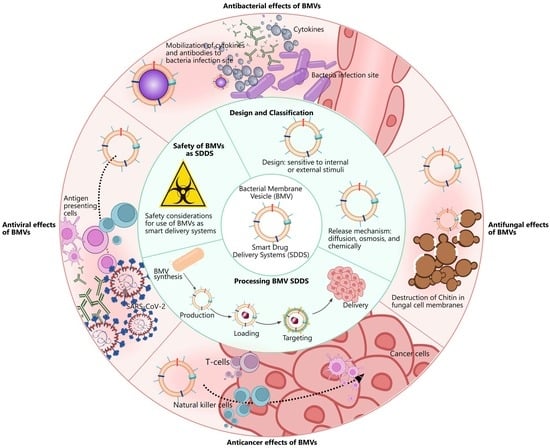
Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy
Abstract
1. Introduction
2. Design and Classification of Smart Drug Delivery Systems
2.1. Design
2.1.1. Internal
2.1.2. External
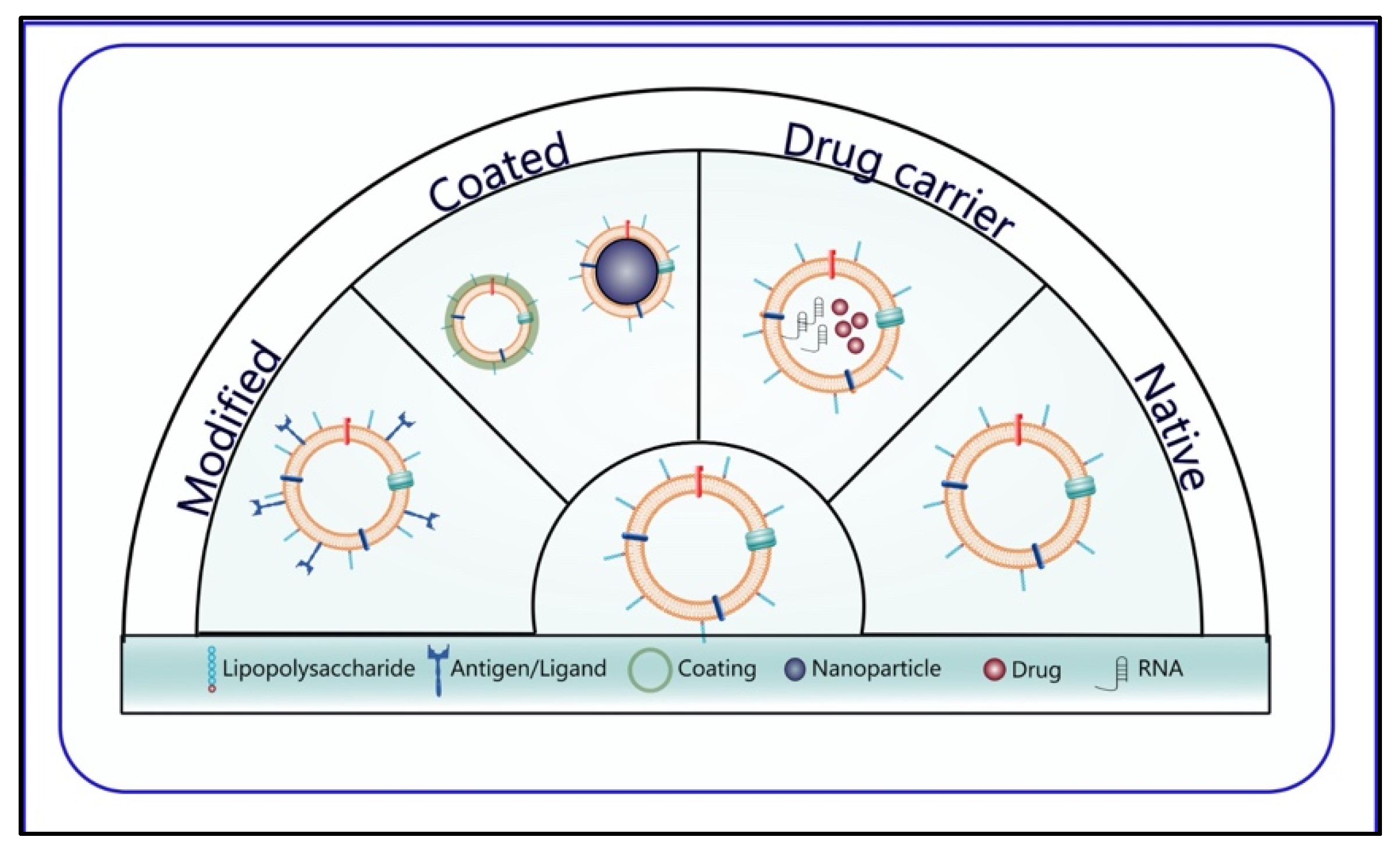
2.2. Classification
3. BMVs as Smart Drug Delivery Systems
3.1. Fabrications of BMVs
3.2. Purification and Characterization Techniques of BMVs
3.3. Properties of BMVs
3.4. Cargo Loading or/and Drug Encapsulation Techniques into BMVs
3.4.1. Active Cargo Loading
3.4.2. Passive Cargo Loading
3.5. Drug Release Mechanisms from BMVs
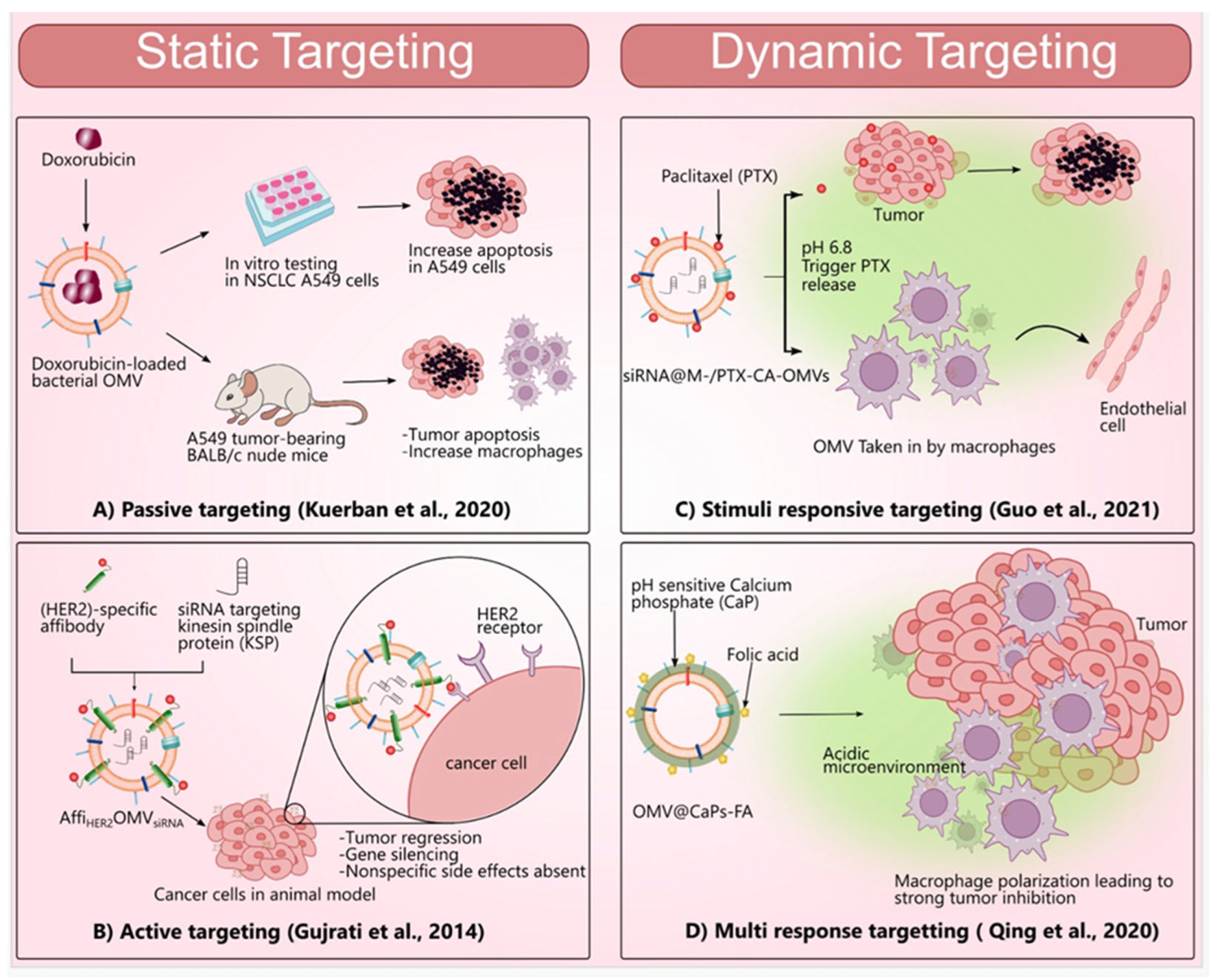
3.6. Strategies Used in BMVs for Targeting
3.6.1. Static Targeting
Passive Targeting
Active Targeting
3.6.2. Dynamic Targeting
Stimuli-Responsive Targeting
Dual/Multi-Responsive Targeting
Inverse Targeting
4. The Role of BMV-Based Smart Drug Delivery Systems in Diagnosis
5. The Potential Role of BMV-Based Smart Drug Delivery Systems in Therapy
5.1. Cancer Therapy
5.1.1. Native OMVs
5.1.2. Cargo or Drug Loading OMVs
5.1.3. Modification of OMVs
5.1.4. OMVs Designed with Coated and Hybrid Membrane Technology
5.2. Antimicrobial Therapy
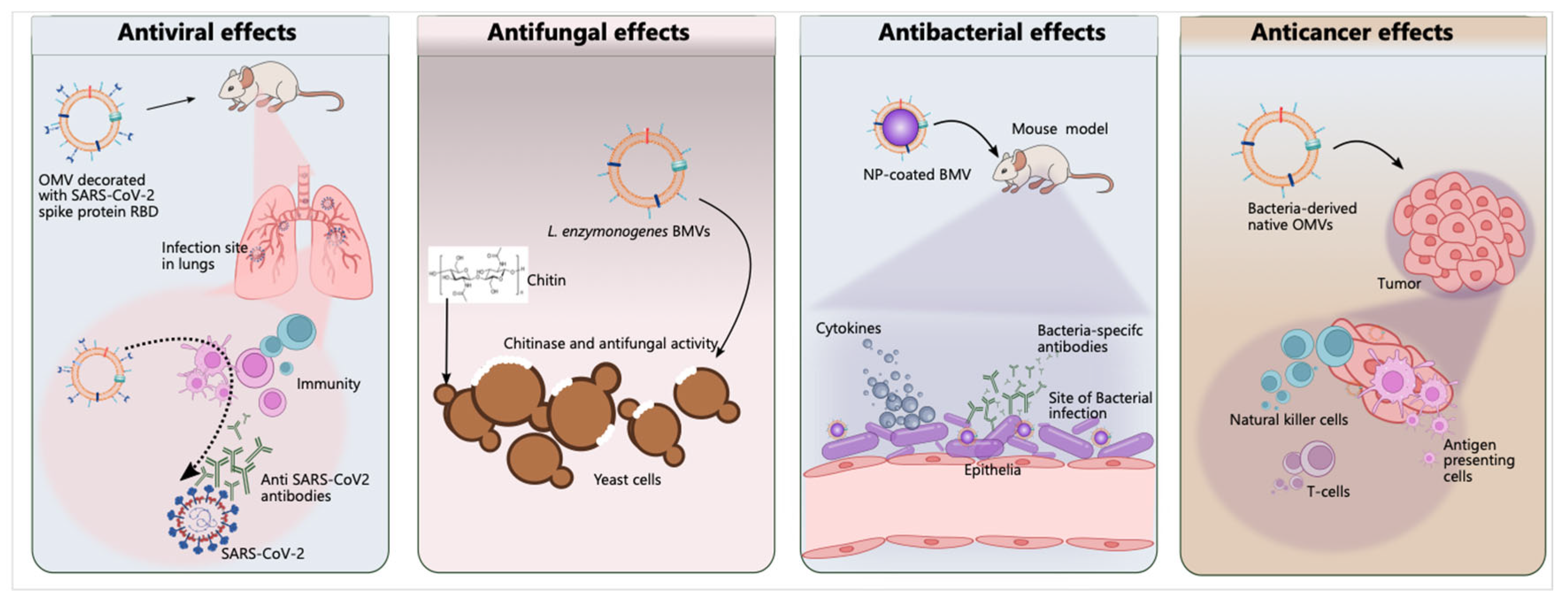
5.2.1. Antibacterial Activity
5.2.2. Antifungal Activity
5.2.3. Antiviral Activity
6. Safety of BMVs as Smart Drug Delivery Systems
7. Obstacles of BMVs for Clinical Use as Smart Drug Delivery Systems
8. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.; Li, B.V.; Uppoor, R.S.; Mehta, M.; Yu, L.X. Pharmaceutical Theory and Practice. In Developing Solid Oral Dosage Forms, 2nd ed.; Bioavailability and Bioequivalence; Academic Press: Cambridge, MA, USA, 2017; pp. 381–397. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. Release 2022, 342, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Calis, S.; Ozturk Atar, K.; Arslan, F.B.; Eroglu, H.; Capan, Y. Nanopharmaceuticals as Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–154. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef]
- Khizar, S.; Alrushaid, N.; Alam Khan, F.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Nanocarriers based novel and effective drug delivery system. Int. J. Pharm. 2023, 632, 122570. [Google Scholar] [CrossRef]
- Wen, J.; Lv, Y.; Xu, Y.; Zhang, P.; Li, H.; Chen, X.; Li, X.; Zhang, L.; Liu, F.; Zeng, W.; et al. Construction of a biodegradable, versatile nanocarrier for optional combination cancer therapy. Acta Biomater. 2019, 83, 359–371. [Google Scholar] [CrossRef]
- Jain, S.; Pillai, J. Bacterial membrane vesicles as novel nanosystems for drug delivery. Int. J. Nanomed. 2017, 12, 6329–6341. [Google Scholar] [CrossRef]
- Fazal, S.; Lee, R. Biomimetic Bacterial Membrane Vesicles for Drug Delivery Applications. Pharmaceutics 2021, 13, 1430. [Google Scholar] [CrossRef]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, H.; Zhang, W.; Zhai, G. Internal stimuli-responsive nanocarriers for drug delivery: Design strategies and applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Rama, A.; Ladani, B.; Verma, N.; Kannan, S.; Naha, A. Temperature and pH-responsive nanogels as intelligent drug delivery systems: A comprehensive review. J. Appl. Pharm. Sci. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, N.; Feng, X. The role of internal and external stimuli in the rational design of skin-specific drug delivery systems. Int. J. Pharm. 2021, 592, 120081. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Shen, J.; Zhang, L.; Wang, L.; Xu, H.; Han, Y.; Jia, J.; Lu, Y.; Yu, R.; Liu, H. Injectable postoperative enzyme-responsive hydrogels for reversing temozolomide resistance and reducing local recurrence after glioma operation. Biomater. Sci. 2020, 8, 5306–5316. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Gasik, M. Smart Hydrogels for Advanced Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 3365. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Advanced drug delivery systems: Nanotechnology of health design A review. J. Saudi Chem. Soc. 2014, 18, 85–99. [Google Scholar] [CrossRef]
- Faheem, A.M.; Abdelkader, D.H. Novel drug delivery systems. In Engineering Drug Delivery Systems; Woodhead Publishing: Sawston, UK, 2020; pp. 1–16. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Ho, E.A. Challenges in the development and establishment of exosome-based drug delivery systems. J. Control. Release 2021, 329, 894–906. [Google Scholar] [CrossRef]
- Wang, S.; Gao, J.; Wang, Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1523. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Cho, H.; Lee, S.; Kim, K.S. Biomimetic Nanoparticles Coated with Bacterial Outer Membrane Vesicles as a New-Generation Platform for Biomedical Applications. Pharmaceutics 2021, 13, 1887. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact. Mater. 2022, 14, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Jorritsma, S.H.T.; Gonzaga, Z.J.; Evert, B.; Chen, S.; Rehm, B.H.A. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials 2021, 268, 120597. [Google Scholar] [CrossRef]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef]
- Lee, S.C.; Kwon, I.K.; Park, K. Hydrogels for delivery of bioactive agents: A historical perspective. Adv. Drug. Deliv. Rev. 2013, 65, 17–20. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.; Morgovan, C.; Arseniu, A.M.; Rus, L.L.; Butuca, A.; Juncan, A.M.; Totan, M.; Vonica-Tincu, A.L.; Cormos, G.; et al. Applications and Limitations of Dendrimers in Biomedicine. Molecules 2020, 25, 3982. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Veiga, F.; Figueiras, A. Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications. Materials 2019, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems—The current state. Adv. Colloid. Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Amasya, G.; Bakar-Ates, F.; Wintgens, V.; Amiel, C. Layer by layer assembly of core-corona structured solid lipid nanoparticles with beta-cyclodextrin polymers. Int. J. Pharm. 2021, 592, 119994. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2011, 12, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.; Bianco, A.; Prato, M.; Kostarelos, K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv. Drug. Deliv. Rev. 2006, 58, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug. Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Kuchur, O.A.; Tsymbal, S.A.; Shestovskaya, M.V.; Serov, N.S.; Dukhinova, M.S.; Shtil, A.A. Metal-derived nanoparticles in tumor theranostics: Potential and limitations. J. Inorg. Biochem. 2020, 209, 111117. [Google Scholar] [CrossRef]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Campora, S.; Ghersi, G. Recent developments and applications of smart nanoparticles in biomedicine. Nanotechnol. Rev. 2022, 11, 2595–2631. [Google Scholar] [CrossRef]
- Castillo-Romero, K.F.; Santacruz, A.; Gonzalez-Valdez, J. Production and purification of bacterial membrane vesicles for biotechnology applications: Challenges and opportunities. Electrophoresis 2023, 44, 107–124. [Google Scholar] [CrossRef] [PubMed]
- De, S.N. Enterotoxicity of Bacteria-free Culture-filtrate of Vibrio cholerae. Nature 1959, 183, 1533–1534. [Google Scholar] [CrossRef]
- Aytar Celik, P.; Derkus, B.; Erdogan, K.; Barut, D.; Blaise Manga, E.; Yildirim, Y.; Pecha, S.; Cabuk, A. Bacterial membrane vesicle functions, laboratory methods, and applications. Biotechnol. Adv. 2022, 54, 107869. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; An, M.; Zhu, J.; Tan, Z.; Chen, G.Y.; Stidham, R.W.; Lubman, D.M. A Method for Isolation and Proteomic Analysis of Outer Membrane Vesicles from Fecal Samples by LC-MS/MS. J. Proteom. Bioinform. 2019, 12, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Torres Crigna, A.; Fricke, F.; Nitschke, K.; Worst, T.; Erb, U.; Karremann, M.; Buschmann, D.; Elvers-Hornung, S.; Tucher, C.; Schiller, M.; et al. Inter-Laboratory Comparison of Extracellular Vesicle Isolation Based on Ultracentrifugation. Transfus. Med. Hemother 2021, 48, 48–59. [Google Scholar] [CrossRef]
- Qing, G.; Gong, N.; Chen, X.; Chen, J.; Zhang, H.; Wang, Y.; Wang, R.; Zhang, S.; Zhang, Z.; Zhao, X.; et al. Natural and engineered bacterial outer membrane vesicles. Biophys. Rep. 2019, 5, 184–198. [Google Scholar] [CrossRef]
- Klimentova, J.; Stulik, J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Li, R.; Liu, Q. Engineered Bacterial Outer Membrane Vesicles as Multifunctional Delivery Platforms. Front. Mater. 2020, 7, 202. [Google Scholar] [CrossRef]
- De Benedetto, G.; Cescutti, P.; Giannelli, C.; Rizzo, R.; Micoli, F. Multiple Techniques for Size Determination of Generalized Modules for Membrane Antigens from Salmonella typhimurium and Salmonella enteritidis. ACS Omega 2017, 2, 8282–8289. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, M.; Zeng, Y.; Lv, Z.; Wang, P.; Han, L. Recent advances in biomedical applications of bacterial outer membrane vesicles. J. Mater. Chem. B 2022, 10, 7384–7396. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef] [PubMed]
- Tiku, V.; Kofoed, E.M.; Yan, D.; Kang, J.; Xu, M.; Reichelt, M.; Dikic, I.; Tan, M.W. Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii. Sci. Rep. 2021, 11, 618. [Google Scholar] [CrossRef]
- Barut, D.; Enuh, B.M.; Derkuş, B.; Güler, B.; Salih, B.; Çelik, P.A. The relationship between bacterial outer membrane vesicles and halophilic adaptation. Mol. Omics 2023, 19, 174–181. [Google Scholar] [CrossRef]
- Yu, Y.J.; Wang, X.H.; Fan, G.C. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacol. Sin. 2018, 39, 514–533. [Google Scholar] [CrossRef]
- Kulkarni, H.M.; Jagannadham, M.V. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology 2014, 160, 2109–2121. [Google Scholar] [CrossRef]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Briaud, P.; Carroll, R.K. Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria. Infect. Immun. 2020, 88, e00433-20. [Google Scholar] [CrossRef]
- Lima, S.; Matinha-Cardoso, J.; Tamagnini, P.; Oliveira, P. Extracellular Vesicles: An Overlooked Secretion System in Cyanobacteria. Life 2020, 10, 129. [Google Scholar] [CrossRef]
- Haurat, M.F.; Elhenawy, W.; Feldman, M.F. Prokaryotic membrane vesicles: New insights on biogenesis and biological roles. Biol. Chem. 2015, 396, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.K.; Jaganandham, M.V. Vesicles-mediated resistance to antibiotics in bacteria. Front. Microbiol. 2015, 6, 758. [Google Scholar] [CrossRef]
- Azam, A.H.; Tanji, Y. Bacteriophage-host arm race: An update on the mechanism of phage resistance in bacteria and revenge of the phage with the perspective for phage therapy. Appl. Microbiol. Biotechnol. 2019, 103, 2121–2131. [Google Scholar] [CrossRef]
- Tran, F.; Boedicker, J.G. Plasmid Characteristics Modulate the Propensity of Gene Exchange in Bacterial Vesicles. J. Bacteriol. 2019, 201, e00430-18. [Google Scholar] [CrossRef]
- Cao, Y.; Lin, H. Characterization and function of membrane vesicles in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 1795–1801. [Google Scholar] [CrossRef]
- Seike, S.; Kobayashi, H.; Ueda, M.; Takahashi, E.; Okamoto, K.; Yamanaka, H. Outer Membrane Vesicles Released From Aeromonas Strains Are Involved in the Biofilm Formation. Front. Microbiol. 2020, 11, 613650. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Tao, Y.; Cao, Y.; Zhou, Y.; Lin, H. Streptococcus mutans Membrane Vesicles Harboring Glucosyltransferases Augment Candida albicans Biofilm Development. Front. Microbiol. 2020, 11, 581184. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rossi, O.; Necchi, F.; Micoli, F. OMV Vaccines and the Role of TLR Agonists in Immune Response. Int. J. Mol. Sci. 2020, 21, 4416. [Google Scholar] [CrossRef]
- Chelakkot, C.; Choi, Y.; Kim, D.K.; Park, H.T.; Ghim, J.; Kwon, Y.; Jeon, J.; Kim, M.S.; Jee, Y.K.; Gho, Y.S.; et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018, 50, e450. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Young, J.L.; Dean, D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Gujrati, V.; Kim, S.; Kim, S.H.; Min, J.J.; Choy, H.E.; Kim, S.C.; Jon, S. Bioengineered Bacterial Outer Membrane Vesicles as Cell-Specific Drug-Delivery Vehicles for Cancer Therapy. ACS Nano 2014, 8, 1525–1537. [Google Scholar] [CrossRef]
- Ayed, Z.; Cuvillier, L.; Dobhal, G.; Goreham, R.V. Electroporation of outer membrane vesicles derived from Pseudomonas aeruginosa with gold nanoparticles. SN Appl. Sci. 2019, 1, 1600. [Google Scholar] [CrossRef]
- Zhang, Q.; Fang, R.H.; Gao, W.; Zhang, L. A Biomimetic Nanoparticle to "Lure and Kill" Phospholipase A2. Angew. Chem. Int. Ed. Engl. 2020, 59, 10461–10465. [Google Scholar] [CrossRef]
- Gao, F.; Xu, L.; Yang, B.; Fan, F.; Yang, L. Kill the Real with the Fake: Eliminate Intracellular Staphylococcus aureus Using Nanoparticle Coated with Its Extracellular Vesicle Membrane as Active-Targeting Drug Carrier. ACS Infect. Dis. 2019, 5, 218–227. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, H.; Wu, W.; Huang, G.; Li, Y.; Wu, M.; Tang, G.; Ping, Y. Bioengineering Bacterial Vesicle-Coated Polymeric Nanomedicine for Enhanced Cancer Immunotherapy and Metastasis Prevention. Nano Lett. 2020, 20, 11–21. [Google Scholar] [CrossRef]
- Kuerban, K.; Gao, X.; Zhang, H.; Liu, J.; Dong, M.; Wu, L.; Ye, R.; Feng, M.; Ye, L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm. Sin. B 2020, 10, 1534–1548. [Google Scholar] [CrossRef]
- Erdogan Gover, K.; Isik, M.; Barut, D.; Sengel-Turk, C.T.; Amasya, G.; Derkus, B.; Cabuk, A.; Aytar Celik, P. Capecitabine-loaded bacterial membrane vesicles derived from Enterococcus faecalis promotes apoptosis in HT-29 colon cancer cells. Biochem. Eng. J. 2022, 189, 108722. [Google Scholar] [CrossRef]
- Allan, N.D.; Beveridge, T.J. Gentamicin delivery to Burkholderia cepacia group IIIa strains via membrane vesicles from Pseudomonas aeruginosa PAO1. Antimicrob. Agents Chemother. 2003, 47, 2962–2965. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Kim, S.R.; Yoon, Y.J.; Park, K.S.; Kim, J.H.; Lee, J.; Kim, O.Y.; Choi, E.J.; Kim, D.K.; Choi, D.S.; et al. In vivo kinetic biodistribution of nano-sized outer membrane vesicles derived from bacteria. Small 2015, 11, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Q.; Li, W.; Yuan, M.; Zhou, J.; Hua, L.; Chen, Y.; Ye, C.; Ma, Y. Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. J. Control. Release 2020, 317, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Zhou, W.; Chu, Y.; Chen, Q.; Zhang, Y.; Li, C.; Chen, H.; Liu, P.; Zhao, Z.; et al. Sequentially Triggered Bacterial Outer Membrane Vesicles for Macrophage Metabolism Modulation and Tumor Metastasis Suppression. ACS Nano 2021, 15, 13826–13838. [Google Scholar] [CrossRef] [PubMed]
- Qing, S.; Lyu, C.; Zhu, L.; Pan, C.; Wang, S.; Li, F.; Wang, J.; Yue, H.; Gao, X.; Jia, R.; et al. Biomineralized Bacterial Outer Membrane Vesicles Potentiate Safe and Efficient Tumor Microenvironment Reprogramming for Anticancer Therapy. Adv. Mater. 2020, 32, e2002085. [Google Scholar] [CrossRef]
- Elkhodiry, M.A.; Momah, C.C.; Suwaidi, S.R.; Gadalla, D.; Martins, A.M.; Vitor, R.F.; Husseini, G.A. Synergistic Nanomedicine: Passive, Active, and Ultrasound-Triggered Drug Delivery in Cancer Treatment. J. Nanosci. Nanotechnol. 2016, 16, 1–18. [Google Scholar] [CrossRef]
- Dutta, B.; Barick, K.C.; Hassan, P.A. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv. Colloid Interface Sci. 2021, 296, 102509. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug. Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef]
- Dadwal, A.; Baldi, A.; Kumar Narang, R. Nanoparticles as carriers for drug delivery in cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 295–305. [Google Scholar] [CrossRef]
- Gujrati, V.; Prakash, J.; Malekzadeh-Najafabadi, J.; Stiel, A.; Klemm, U.; Mettenleiter, G.; Aichler, M.; Walch, A.; Ntziachristos, V. Bioengineered bacterial vesicles as biological nano-heaters for optoacoustic imaging. Nat. Commun. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Dai, L.; Liu, J.; Luo, Z.; Li, M.; Cai, K. Tumor therapy: Targeted drug delivery systems. J. Mater. Chem. B. 2016, 4, 6758–6772. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Gu, T.W.; Wang, M.Z.; Niu, J.; Chu, Y.; Guo, K.R.; Peng, L.H. Outer membrane vesicles derived from E. coli as novel vehicles for transdermal and tumor targeting delivery. Nanoscale 2020, 12, 18965–18977. [Google Scholar] [CrossRef]
- Wang, S.; Huang, W.; Li, K.; Yao, Y.; Yang, X.; Bai, H.; Sun, W.; Liu, C.; Ma, Y. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int. J. Nanomed. 2017, 12, 6813–6825. [Google Scholar] [CrossRef]
- Hirsjärvi, S.; Passirani, C.; Benoit, J.P. Passive and Active Tumour Targeting with Nanocarriers. Curr. Drug. Discov. Technol. 2011, 8, 188–196. [Google Scholar] [CrossRef]
- Biffi, S.; Voltan, R.; Bortot, B.; Zauli, G.; Secchiero, P. Actively targeted nanocarriers for drug delivery to cancer cells. Expert. Opin. Drug. Deliv. 2019, 16, 481–496. [Google Scholar] [CrossRef]
- Behera, A.; Padhi, S. Passive and active targeting strategies for the delivery of the camptothecin anticancer drug: A review. Environ. Chem. Lett. 2020, 18, 1557–1567. [Google Scholar] [CrossRef]
- Wakaskar, R.R. Passive and Active Targeting in Tumor Microenvironment. Int. J. Drug. Dev. Res. 2017, 9, 37–41. [Google Scholar]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Cui, C.; Guo, T.; Zhang, S.; Yang, M.; Cheng, J.; Wang, J.; Kang, J.; Ma, W.; Nian, Y.; Sun, Z.; et al. Bacteria-derived outer membrane vesicles engineered with over-expressed pre-miRNA as delivery nanocarriers for cancer therapy. Nanomedicine 2022, 45, 102585. [Google Scholar] [CrossRef]
- Peng, L.; Wang, M.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.; Yuan, T.; Jiang, Z.; Gao, J.; Ning, X. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL–programmed therapy against melanoma. Sci. Adv. 2020, 6, eaba2735. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Li, X.; Shao, B.; Xu, F.; Huang, X.; Guo, X.; Zhou, S. Self-Blockade of PD-L1 with Bacteria-Derived Outer-Membrane Vesicle for Enhanced Cancer Immunotherapy. Adv. Mater. 2022, 34, e2106307. [Google Scholar] [CrossRef]
- Jiang, Z.; Guan, J.; Qian, J.; Zhan, C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater. Sci. 2019, 7, 461–471. [Google Scholar] [CrossRef]
- Sepahdar, Z.; Miroliaei, M.; Bouzari, S.; Khalaj, V.; Salimi, M. Surface Engineering of Escherichia coli-Derived OMVs as Promising Nano-Carriers to Target EGFR-Overexpressing Breast Cancer Cells. Front. Pharmacol. 2021, 12, 719289. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Cheng, K.; Zhang, K.; Wang, Y.; Zhang, Y.; Li, Y.; Liu, G.; Xu, J.; Xu, J.; et al. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano 2020, 14, 16698–16711. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; Gonzalez, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regi, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert. Opin. Drug. Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef]
- Moradi Kashkooli, F.; Soltani, M.; Souri, M. Controlled anti-cancer drug release through advanced nano-drug delivery systems: Static and dynamic targeting strategies. J. Control. Release 2020, 327, 316–349. [Google Scholar] [CrossRef]
- Qin, J.; Yang, T.; Li, J.; Zhan, G.; Li, X.; Wei, Z.; Chen, Z.; Zheng, W.; Chen, H.; Yang, X.; et al. Bacterial outer membrane vesicle-templated biomimetic nanoparticles for synergistic photothermo-immunotherapy. Nano Today 2022, 46, 101591. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Liu, L.; Li, L.; Zhang, L.; Wang, Y.; Zhao, J. Self-Assembly Catalase Nanocomplex Conveyed by Bacterial Vesicles for Oxygenated Photodynamic Therapy and Tumor Immunotherapy. Int. J. Nanomed. 2022, 17, 1971–1985. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 2019, 308, 172–189. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Pandey, S.P.; Sudheesh, M.S. Site-specific drug delivery, targeting, and gene therapy. In Nanoarchitectonics in Biomedicine; William Andrew: Norwich, NY, USA, 2019; pp. 473–505. [Google Scholar] [CrossRef]
- Jahromi, L.P.; Fuhrmann, G. Bacterial extracellular vesicles: Understanding biology promotes applications as nanopharmaceuticals. Adv. Drug. Deliv. Rev. 2021, 173, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Rozovsky, S.; Chen, W. Engineering multi-functional bacterial outer membrane vesicles as modular nanodevices for biosensing and bioimaging. Chem. Commun. 2017, 53, 7569–7572. [Google Scholar] [CrossRef]
- Huang, Y.; Beringhs, A.O.; Chen, Q.; Song, D.; Chen, W.; Lu, X.; Fan, T.H.; Nieh, M.P.; Lei, Y. Genetically Engineered Bacterial Outer Membrane Vesicles with Expressed Nanoluciferase Reporter for in Vivo Bioluminescence Kinetic Modeling through Noninvasive Imaging. ACS Appl. Bio Mater. 2019, 2, 5608–5615. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kroll, A.V.; Holay, M.; Fang, R.H.; Zhang, L. Biomimetic Nanotechnology toward Personalized Vaccines. Adv. Mater. 2020, 32, e1901255. [Google Scholar] [CrossRef]
- Aly, R.G.; El-Enbaawy, M.I.; Abd El-Rahman, S.S.; Ata, N.S. Antineoplastic activity of Salmonella Typhimurium outer membrane nanovesicles. Exp. Cell Res. 2021, 399, 112423. [Google Scholar] [CrossRef]
- Zhuang, W.R.; Wang, Y.; Lei, Y.; Zuo, L.; Jiang, A.; Wu, G.; Nie, W.; Huang, L.L.; Xie, H.Y. Phytochemical Engineered Bacterial Outer Membrane Vesicles for Photodynamic Effects Promoted Immunotherapy. Nano Lett. 2022, 22, 4491–4500. [Google Scholar] [CrossRef]
- Behzadi, E.; Mahmoodzadeh Hosseini, H.; Imani Fooladi, A.A. The inhibitory impacts of Lactobacillus rhamnosus GG-derived extracellular vesicles on the growth of hepatic cancer cells. Microb. Pathog. 2017, 110, 1–6. [Google Scholar] [CrossRef]
- Kim, O.Y.; Park, H.T.; Dinh, N.T.H.; Choi, S.J.; Lee, J.; Kim, J.H.; Lee, S.W.; Gho, Y.S. Bacterial outer membrane vesicles suppress tumor by interferon-gamma-mediated antitumor response. Nat. Commun. 2017, 8, 626. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.; Li, R.; Huang, X.; Liu, Q. Design of Outer Membrane Vesicles as Cancer Vaccines: A New Toolkit for Cancer Therapy. Cancers 2019, 11, 1314. [Google Scholar] [CrossRef]
- Long, Q.; Zheng, P.; Zheng, X.; Li, W.; Hua, L.; Yang, Z.; Huang, W.; Ma, Y. Engineered bacterial membrane vesicles are promising carriers for vaccine design and tumor immunotherapy. Adv. Drug. Deliv. Rev. 2022, 186, 114321. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Shu, C.; Hua, L.; Zhao, Y.; Xie, H.; Qi, J.; Gao, F.; Gao, R.; Chen, Y.; Zhang, Q.; et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020, 108, 300–312. [Google Scholar] [CrossRef]
- Gao, X.; Feng, Q.; Wang, J.; Zhao, X. Bacterial outer membrane vesicle-based cancer nanovaccines. Cancer Biol. Med. 2022, 19, 1290–1300. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X.; Liu, H.; Wang, Y. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Nieh, M.P.; Chen, W.; Lei, Y. Outer membrane vesicles (OMVs) enabled bio-applications: A critical review. Biotechnol. Bioeng. 2022, 119, 34–47. [Google Scholar] [CrossRef]
- Yang, J.; Kim, E.K.; McDowell, A.; Kim, Y.K. Microbe-derived extracellular vesicles as a smart drug delivery system. Transl. Clin. Pharmacol. 2018, 26, 103–110. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Qiu, X.; Dong, S.; He, J.; Liu, J.; Xu, W.; Huang, S.; Hu, X.; Xiang, D.X. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact. Mater. 2023, 20, 548–560. [Google Scholar] [CrossRef]
- Xie, Y.J.; Huang, M.; Li, D.; Hou, J.C.; Liang, H.H.; Nasim, A.A.; Huang, J.M.; Xie, C.; Leung, E.L.; Fan, X.X. Bacteria-based nanodrug for anticancer therapy. Pharmacol. Res. 2022, 182, 106282. [Google Scholar] [CrossRef]
- Gao, J.; Su, Y.; Wang, Z. Engineering bacterial membrane nanovesicles for improved therapies in infectious diseases and cancer. Adv. Drug. Deliv. Rev. 2022, 186, 114340. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, S.; Zhang, S.; Wang, L.; Yuan, H.; Hu, F. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 3233–3254. [Google Scholar] [CrossRef]
- Krishnan, N.; Kubiatowicz, L.J.; Holay, M.; Zhou, J.; Fang, R.H.; Zhang, L. Bacterial membrane vesicles for vaccine applications. Adv. Drug. Deliv. Rev. 2022, 185, 114294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, A.; Jiang, L.; Gu, Y.; Liu, J. Hybrid Membrane-Coated Biomimetic Nanoparticles (HM@BNPs): A Multifunctional Nanomaterial for Biomedical Applications. Biomacromolecules 2021, 22, 3149–3167. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, Z.; Pan, H.; Liu, Y.; Chu, Y.; Wang, J.; Chen, L. Biofilm-encapsulated nano drug delivery system for the treatment of colon cancer. J. Microencapsul. 2020, 37, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Huang, G.; Wu, W.; Wang, J.; Hu, J.; Mao, J.; Chu, P.K.; Bai, H.; Tang, G. A Hybrid Eukaryotic-Prokaryotic Nanoplatform with Photothermal Modality for Enhanced Antitumor Vaccination. Adv. Mater. 2020, 32, e1908185. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.; You, S.; Zhang, K.; Li, M.; Cao, Y.; Wang, C.; Dong, H.; Zhang, X. Bacterial Vesicle-Cancer Cell Hybrid Membrane-Coated Nanoparticles for Tumor Specific Immune Activation and Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 41138–41147. [Google Scholar] [CrossRef]
- Schmidt, C.K.; Medina-Sanchez, M.; Edmondson, R.J.; Schmidt, O.G. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat. Commun. 2020, 11, 5618. [Google Scholar] [CrossRef]
- Zhou, J.; Karshalev, E.; Mundaca-Uribe, R.; Esteban-Fernandez de Avila, B.; Krishnan, N.; Xiao, C.; Ventura, C.J.; Gong, H.; Zhang, Q.; Gao, W.; et al. Physical Disruption of Solid Tumors by Immunostimulatory Microrobots Enhances Antitumor Immunity. Adv. Mater. 2021, 33, e2103505. [Google Scholar] [CrossRef]
- Collins, S.M.; Brown, A.C. Bacterial Outer Membrane Vesicles as Antibiotic Delivery Vehicles. Front. Immunol. 2021, 12, 733064. [Google Scholar] [CrossRef]
- Huang, W.; Meng, L.; Chen, Y.; Dong, Z.; Peng, Q. Bacterial outer membrane vesicles as potential biological nanomaterials for antibacterial therapy. Acta Biomater. 2022, 140, 102–115. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: Conceptually new antibiotics. J. Bacteriol. 1996, 178, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Rimmer, M.A.; Turner, K.B.; Phillips, D.A.; Caruana, J.C.; Hervey, W.J.t.; Leary, D.H.; Walper, S.A. Lactobacillus acidophilus Membrane Vesicles as a Vehicle of Bacteriocin Delivery. Front. Microbiol. 2020, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, C.; Peng, X.; Wu, S.; Li, Y.; Tan, J.P.K.; Yang, Y.Y.; Yuan, P.; Ding, X. Fight bacteria with bacteria: Bacterial membrane vesicles as vaccines and delivery nanocarriers against bacterial infections. Nanomedicine 2021, 35, 102398. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ji, H.; Guo, X.; Li, Y.; Ren, T.; Dong, H.; Liu, J.; Liu, Y.; Shi, X.; He, B. Nanoparticle reinforced bacterial outer-membrane vesicles effectively prevent fatal infection of carbapenem-resistant Klebsiella pneumoniae. Nanomedicine 2020, 24, 102148. [Google Scholar] [CrossRef]
- Cooke, A.C.; Nello, A.V.; Ernst, R.K.; Schertzer, J.W. Analysis of Pseudomonas aeruginosa biofilm membrane vesicles supports multiple mechanisms of biogenesis. PLoS ONE 2019, 14, e0212275. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Delivery of the Non-Membrane-Permeative Antibiotic Gentamicin into Mammalian Cells by Using Shigella flexneri Membrane Vesicles. Antimicrob. Agents Chemother. 1998, 42, 1476–1483. [Google Scholar] [CrossRef]
- Vasilyeva, N.V.; Tsfasman, I.M.; Suzina, N.E.; Stepnaya, O.A.; Kulaev, I.S. Secretion of bacteriolytic endopeptidase L5 of Lysobacter sp. XL1 into the medium by means of outer membrane vesicles. FEBS J. 2008, 275, 3827–3835. [Google Scholar] [CrossRef]
- Evans, A.G.L.; Davey, H.M.; Cookson, A.; Currinn, H.; Cooke-Fox, G.; Stanczyk, P.J.; Whitworth, D.E. Predatory activity of Myxococcus xanthus outer-membrane vesicles and properties of their hydrolase cargo. Microbiology 2012, 158, 2742–2752. [Google Scholar] [CrossRef]
- Schulz, E.; Goes, A.; Garcia, R.; Panter, F.; Koch, M.; Muller, R.; Fuhrmann, K.; Fuhrmann, G. Biocompatible bacteria-derived vesicles show inherent antimicrobial activity. J. Control. Release 2018, 290, 46–55. [Google Scholar] [CrossRef]
- Goes, A.; Lapuhs, P.; Kuhn, T.; Schulz, E.; Richter, R.; Panter, F.; Dahlem, C.; Koch, M.; Garcia, R.; Kiemer, A.K.; et al. Myxobacteria-Derived Outer Membrane Vesicles: Potential Applicability Against Intracellular Infections. Cells 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Afoshin, A.S.; Kudryakova, I.V.; Borovikova, A.O.; Suzina, N.E.; Toropygin, I.Y.; Shishkova, N.A.; Vasilyeva, N.V. Lytic potential of Lysobacter capsici VKM B-2533(T): Bacteriolytic enzymes and outer membrane vesicles. Sci. Rep. 2020, 10, 9944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hoffmann, J.P.; Chou, C.W.; Honer Zu Bentrup, K.; Fuselier, J.A.; Bitoun, J.P.; Wimley, W.C.; Morici, L.A. Burkholderia thailandensis outer membrane vesicles exert antimicrobial activity against drug-resistant and competitor microbial species. J. Microbiol. 2020, 58, 550–562. [Google Scholar] [CrossRef] [PubMed]
- da Silva Barreira, D.; Laurent, J.; Lourenco, J.; Novion Ducassou, J.; Coute, Y.; Guzzo, J.; Rieu, A. Membrane vesicles released by Lacticaseibacillus casei BL23 inhibit the biofilm formation of Salmonella Enteritidis. Sci. Rep. 2023, 13, 1163. [Google Scholar] [CrossRef]
- Tashiro, Y.; Hasegawa, Y.; Shintani, M.; Takaki, K.; Ohkuma, M.; Kimbara, K.; Futamata, H. Interaction of Bacterial Membrane Vesicles with Specific Species and Their Potential for Delivery to Target Cells. Front. Microbiol. 2017, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- Camacho, A.I.; Irache, J.M.; de Souza, J.; Sanchez-Gomez, S.; Gamazo, C. Nanoparticle-based vaccine for mucosal protection against Shigella flexneri in mice. Vaccine 2013, 31, 3288–3294. [Google Scholar] [CrossRef]
- Gao, W.; Fang, R.H.; Thamphiwatana, S.; Luk, B.T.; Li, J.; Angsantikul, P.; Zhang, Q.; Hu, C.M.; Zhang, L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015, 15, 1403–1409. [Google Scholar] [CrossRef]
- Adriani, R.; Mousavi Gargari, S.L.; Nazarian, S.; Sarvary, S.; Noroozi, N. Immunogenicity of Vibrio cholerae outer membrane vesicles secreted at various environmental conditions. Vaccine 2018, 36, 322–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Lo, C.; Zhuang, J.; Angsantikul, P.; Zhang, Q.; Wei, X.; Zhou, Z.; Obonyo, M.; Fang, R.H.; et al. Inhibition of Pathogen Adhesion by Bacterial Outer Membrane-Coated Nanoparticles. Angew. Chem. Int. Ed. Engl. 2019, 58, 11404–11408. [Google Scholar] [CrossRef]
- Huang, Y.; Nan, L.; Xiao, C.; Dong, J.; Li, K.; Cheng, J.; Ji, Q.; Wei, Q.; Bao, G.; Liu, Y. Outer Membrane Vesicles Coating Nano-Glycyrrhizic Acid Confers Protection Against Borderella bronchiseptica Through Th1/Th2/Th17 Responses. Int. J. Nanomed. 2022, 17, 647–663. [Google Scholar] [CrossRef]
- Blackburn, S.A.; Shepherd, M.; Robinson, G.K. The polyene antifungal candicidin is selectively packaged into membrane vesicles in Streptomyces S4. Arch. Microbiol. 2022, 204, 289. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Jiang, J.; Taylor, A.J.; Leite, A.L.; Dodds, E.D.; Du, L. Outer Membrane Vesicle-Mediated Codelivery of the Antifungal HSAF Metabolites and Lytic Polysaccharide Monooxygenase in the Predatory Lysobacter enzymogenes. ACS Chem. Biol. 2021, 16, 1079–1089. [Google Scholar] [CrossRef]
- Meers, P.R.; Liu, C.; Chen, R.; Bartos, W.; Davis, J.; Dziedzic, N.; Orciuolo, J.; Kutyla, S.; Pozo, M.J.; Mithrananda, D.; et al. Vesicular Delivery of the Antifungal Antibiotics of Lysobacter enzymogenes C3. Appl. Environ. Microbiol. 2018, 84, e01353-18. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.H.; Seo, S.H.; Kim, C.U.; Jang, M.S.; Song, M.S.; Lee, T.Y.; Jeong, Y.J.; Lee, M.S.; Park, J.H.; Lee, P.; et al. Bacterial Outer Membrane Vesicles Provide Broad-Spectrum Protection against Influenza Virus Infection via Recruitment and Activation of Macrophages. J. Innate Immun. 2019, 11, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.U.; Eo, S.; Lee, P.; Kim, S.H.; Kim, Y.S.; Kim, D.J. Pretreatment of outer membrane vesicle and subsequent infection with influenza virus induces a long-lasting adaptive immune response against broad subtypes of influenza virus. Microbes Infect. 2022, 24, 104878. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Machado, D.; Theizen, T.H.; Guarnieri, J.P.O.; Bernardes, B.G.; Gomide, G.P.; Corat, M.A.F.; Abbehausen, C.; Modena, J.L.P.; Melo, C.; et al. Outer Membrane Vesicles from Neisseria Meningitidis (Proteossome) Used for Nanostructured Zika Virus Vaccine Production. Sci. Rep. 2018, 8, 8290. [Google Scholar] [CrossRef]
- Thapa, H.B.; Muller, A.M.; Camilli, A.; Schild, S. An Intranasal Vaccine Based on Outer Membrane Vesicles Against SARS-CoV-2. Front. Microbiol. 2021, 12, 752739. [Google Scholar] [CrossRef]
- Fu, Y.; Xiong, S. Tagged extracellular vesicles with the RBD of the viral spike protein for delivery of antiviral agents against SARS-COV-2 infection. J. Control. Release 2021, 335, 584–595. [Google Scholar] [CrossRef]
- Jiang, L.; Driedonks, T.A.P.; Jong, W.S.P.; Dhakal, S.; Bart van den Berg van Saparoea, H.; Sitaras, I.; Zhou, R.; Caputo, C.; Littlefield, K.; Lowman, M.; et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J. Extracell. Vesicles 2022, 11, e12192. [Google Scholar] [CrossRef]
- Qiao, L.; Rao, Y.; Zhu, K.; Rao, X.; Zhou, R. Engineered Remolding and Application of Bacterial Membrane Vesicles. Front. Microbiol. 2021, 12, 729369. [Google Scholar] [CrossRef]
- Tran, A.X.; Karbarz, M.J.; Wang, X.; Raetz, C.R.; McGrath, S.C.; Cotter, R.J.; Trent, M.S. Periplasmic cleavage and modification of the 1-phosphate group of Helicobacter pylori lipid A. J. Biol. Chem. 2004, 279, 55780–55791. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Fonseca, S.; Miquel-Clopes, A.; Cross, K.; Kok, K.S.; Wegmann, U.; Gil-Cordoso, K.; Bentley, E.G.; Al Katy, S.H.M.; Coombes, J.L.; et al. Bioengineering commensal bacteria-derived outer membrane vesicles for delivery of biologics to the gastrointestinal and respiratory tract. J. Extracell. Vesicles 2019, 8, 1632100. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Sun, X.; Cimino, J.; Guan, Z.; Sun, W. Recombinant Pseudomonas Bionanoparticles Induce Protection against Pneumonic Pseudomonas aeruginosa Infection. Infect. Immun. 2021, 89, e00396-21. [Google Scholar] [CrossRef]
- Gnopo, Y.M.D.; Watkins, H.C.; Stevenson, T.C.; DeLisa, M.P.; Putnam, D. Designer outer membrane vesicles as immunomodulatory systems–Reprogramming bacteria for vaccine delivery. Adv. Drug. Deliv. Rev. 2017, 114, 132–142. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Zhang, T.; Liu, X.; Zhang, H.; Geng, Z.; Su, J. Engineered bacterial extracellular vesicles for osteoporosis therapy. Chem. Eng. J. 2022, 450, 138309. [Google Scholar] [CrossRef]
- Gao, J.; Wang, S.; Dong, X.; Wang, Z. RGD-expressed bacterial membrane-derived nanovesicles enhance cancer therapy via multiple tumorous targeting. Theranostics 2021, 11, 3301–3316. [Google Scholar] [CrossRef] [PubMed]

| Classification of SDDS | Advantages | Disadvantages | |
|---|---|---|---|
| Organic | Polymeric nanoparticles | Controllable particle and surface properties Enhanced stability of API Tunable release properties API encapsulation both hydrophilic and lipophilic character | Difficulty in adapting production processes on an industrial scale Residual material associated with production process |
| Hydrogels | Ability to absorb water or biological fluids Capacity to mimic biological structures with 3D structure Biodegradable and nontoxic nature Site-specific application | Possibility of drug deactivation during production Limited hydrophobic drug delivery Difficulty in sterilization Mechanically unstable High production costs | |
| Dendrimers | Increased drug solubility High loading efficiency through internal cavities Surface modification with terminal functional groups Enhanced permeation effects and long in vivo circulation lifetime | High nonspecific toxicity Increased cytotoxicity due to an increase in the number of generations Possible hemolytic activity Relatively expensive raw material requirement | |
| Liposomes | Nontoxic, biodegradable, and flexible vesicles cell membrane-like structure Simultaneous entrapment of hydrophilic and lipophilic API prolonging circulation time Possible to formulate sterically stabilized liposomes | Possibility of organic solvent residue Lipid oxidation/hydrolysis problem during shelf life | |
| Lipid nanoparticles | Safe composition of physiological lipids Avoiding the use of organic solvents Economical and low-cost production Possible to scale up Site-specific drug delivery | Low drug-loading capacity for hydrophilic molecules Expulsion of API due to polymorphic transitions during storage Burst release | |
| Inorganic | Carbon nanotubes | Easy to modify and functionalize Sequential structure High mechanical strength Effective for molecules to enter cells | Highly hydrophobic nature The lack of solubility in solvents compatible with biological fluid Biodegradation problem |
| Mesoporous silica system | High surface–volume ratio Presence of nanopores Low-cost complex system design Avoiding the early drug release | Solubility and biodegradability characteristics Presence of silanol groups and their interaction with membrane lipids | |
| Metal nanoparticles | Availability of green synthesis Tunable size, geometry, and surface Suitable for large-scale production Unique optical, electronic physicochemical features Can be used as a diagnostic tool | Not biodegradable Tendency to accumulate non-specifically in the body Environmental toxicity risk | |
| Biologic | Exosomes | Ability to mediate intercellular communication Resistant to digestive enzymes | Challenges related to the isolation and purity Rapid elimination from the bloodstream Limited large-scale production |
| Bacterial membrane vesicles | Easy-to-access raw material source Able to be designed with the help of genetic engineering Surface modification with biological ligands | Limited scalable manufacturing Relatively low BMV yield Lack of standardization in production | |
| Class | Indication | Ligand | Target | References |
|---|---|---|---|---|
| Aptamer | Breast cancer | AS-1411 | Nucleolin | [103] |
| Carbohydrate | M2 macrophages | Mannose | Mannose receptor (MR, CD206+) | [87] |
| Peptide | Melanoma | RGP and RGD | αvβ3 integrin | [96] |
| RGP | [104] | |||
| Breast cancer Melanoma Colon cancer | LyP1 | p32/gC1qR | [105,106] | |
| Protein | Breast cancer Ovarian cancer | HER2 affibody | HER2 receptor | [77] |
| Breast cancer | EGFR affibody | EGFR | [107] | |
| Colon cancer | PD1 | PD-L1 | [108] | |
| Small molecule | Breast cancer | Folic acid (FA) | Folate receptor (FR) | [88] |
| Bacterial Source of OMVs | Modification and/or Guest Molecules | Particle Size (nm) | Modification or Loading Method | Stimulating Factor | Therapy Strategy | Outcomes | References |
|---|---|---|---|---|---|---|---|
| E. coli DH5α | Ce6 | 70–140 | Co-incubation | Photodynamic |
|
| [133] |
| DOX | |||||||
| E. coli BL21 (ΔmsbB) | tRNALys-pre-miRNA-126 | 108.2 | Genetic engineering | - | Targeting breast cancer cells by specific binding of the aptamer to nucleolus proteins on the surface of breast cancer cell membranes. |
| [103] |
| aptamer AS1411 | Incubation | ||||||
| Attenuated Salmonella | αPD-L1 | 140.907 | Extrusion | Photodynamic | To increase the amount of O2 in tumor cells by means of negatively charged catalase and Ce-6, thus overcoming the hypoxia barrier in front of the photodynamic effect and obtaining an effective antitumoral effect. |
| [114] |
| Catalase-Ce6 | Co-extrusion | ||||||
| E. coli K12 (ΔmsbB) | LyP1 polypeptide | ~136.9 | Genetic engineering | - |
|
| [105] |
| PD1 plasmid | Electroporation | ||||||
| E. coli Nissle 1917 | CuS | 170.2 ± 0.2 | Incubation | Photothermal | Generating strong hyperthermia in tumors through the photothermal effect. |
| [113] |
| E. coli BL21 (ΔmsbB) | Redd1 siRNA | 130 ± 15.16 | Electroporation | pH-sensitive |
|
| [87] |
| DSPE-PEG-CA-PTX | Co-incubation | ||||||
| E. coli (ΔmsbB/ΔpagP) | GALA | 135.76 ± 30.33 | Genetic engineering | Ensuring targeted binding, specifically to cancer cells overexpressing the EGF receptor through expressed affi-EGFR proteins. |
| [107] | |
| EGFR | |||||||
| E. coli DH5α | BFGF | 166.9 | Genetic engineering | - | To achieve a lasting and effective antitumor effect by inducing the production of anti-BFGF autoantibodies. |
| [128] |
| E. coli | TRAIL | 94.46 ± 5.22 | Genetic engineering | Photothermal |
|
| [96] |
| ICG | Incubation | ||||||
| RGP or RGD peptide | Incubation | ||||||
| E. coli (ΔmsbB) | PD1 | 32.7 ± 10.6 | Genetic engineering | - | Enabling both internalizations of OMVs by binding of PD1 to PD-L1 on the surface of tumor cells as well as preventing inhibition of T cell proliferation by tumor cells through inhibition of PD-L1. |
| [108] |
| Attenuated K. pneumonia ATCC 60095 | DOX | 93.09 | Incubation | - | Enabling the generation of chemoimmunotherapeutic responses by using OMVs as drug delivery systems for chemotherapeutic agents. |
| [82] |
| E. coli | TRAIL | 94.54 ± 1.46 | Genetic engineering | Photothermal |
|
| [104] |
| ICG | Electrostatic interaction | ||||||
| RGP peptide | Incubation | ||||||
| E. coli BL21 | Calcium phosphate (CaP) shells | 100–150 | Incubation | pH-sensitive |
|
| [88] |
| ICG | Photothermal | ||||||
| E. coli K12 (ΔmsbB) | Melanin | 20–100 | Genetic engineering | Photothermal | Both to create contrast for optoacoustic imaging on cancer cells and to achieve an anti-tumoral effect benefiting from the high photothermal conversion effect of melanin. |
| [93] |
| E. coli DH5α | Protein E7 (HPV16E7) | 20–200 | Genetic engineering | - | Enabling antitumoral effects to occur by stimulating cellular immune responses of antigen-presenting recombinant OMVs. |
| [97] |
| E. coli K12 (ΔmsbB) | HER2 | 30–250 | Genetic engineering | - |
|
| [77] |
| KSP siRNA | Electroporation |
| BMV Source | Application Type of BMVs | Active Ingredient | Target Bacteria | References |
|---|---|---|---|---|
| P.aeruginosa | Natural Drug delivery | Autolysin Gentamicin | S. aureus E. coli | [150,151] |
| Lysobacter sp. XL1 | Natural | Endopeptidase L5 | S. aureus Erwinia marcescens | [152] |
| Myxococcus xanthus | Natural | Hydrolase content | E. coli | [153] |
| Cystobacter velatus Cbv34, Cystobacter ferrugineus Cbfe23 | Natural | Cystobactamid | S. aureus E. coli | [154,155] |
| Lysobacter capsici | Natural | Bacteriolytic enzymes | Micrococcus roseus S. aureus Micrococcus luteus Bacillus cereus | [156] |
| Burkholderia thailandensis E264 | Natural | Peptidoglycan hydrolases, 4-hydroxy-3-methyl-2-(2-non-enyl)-quinoline (HMNQ), long-chain rhamnolipid | A. baumannii S. aureus | [157] |
| L. acidophilus | Natural | lactacin B | L. delbrueckii | [147] |
| Lacticaseibacillus casei BL23 | Natural | Antibofilm agent peptidoglycan hydrolases | Salmonella enterica | [158] |
| Buttiauxella agrestis | Drug delivery | Gentamicin | Buttiauxella agrestis | [159] |
| A. baumannii | Drug delivery | Levofloxacin, Amikacin Ciprofloxacin, Norfloxacin | K. pneumoniae E. coli P. aeruginosa | [86] |
| Shigella flexneri | NP-OMV | Poly(anhydride) NP-OMV | Shigella flexneri | [160] |
| E. coli | NP-OMV | Gold nanoparticles (AuNPs)-OMV | Unknown | [161] |
| Vibrio cholerae | NP-OMV | Chitosan-tripolyphosphate NP-OMV | Vibrio cholerae | [162] |
| Helicobacter pylori | NP-OMV | PLGA NP-OMV | Helicobacter pylori | [163] |
| S. aureus | NP-MV | PLGA NP-MV | S. aureus | [80] |
| K. pneumoniae | NP-OMV | - | carbapenem-resistant K. pneumoniae | [149] |
| Bordetella bronchiseptica | NP-OMV | Glycyrrhizic acid-NP | Bordetella bronchiseptica | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aytar Çelik, P.; Erdogan-Gover, K.; Barut, D.; Enuh, B.M.; Amasya, G.; Sengel-Türk, C.T.; Derkus, B.; Çabuk, A. Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy. Pharmaceutics 2023, 15, 1052. https://doi.org/10.3390/pharmaceutics15041052
Aytar Çelik P, Erdogan-Gover K, Barut D, Enuh BM, Amasya G, Sengel-Türk CT, Derkus B, Çabuk A. Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy. Pharmaceutics. 2023; 15(4):1052. https://doi.org/10.3390/pharmaceutics15041052
Chicago/Turabian StyleAytar Çelik, Pınar, Kubra Erdogan-Gover, Dilan Barut, Blaise Manga Enuh, Gülin Amasya, Ceyda Tuba Sengel-Türk, Burak Derkus, and Ahmet Çabuk. 2023. "Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy" Pharmaceutics 15, no. 4: 1052. https://doi.org/10.3390/pharmaceutics15041052
APA StyleAytar Çelik, P., Erdogan-Gover, K., Barut, D., Enuh, B. M., Amasya, G., Sengel-Türk, C. T., Derkus, B., & Çabuk, A. (2023). Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy. Pharmaceutics, 15(4), 1052. https://doi.org/10.3390/pharmaceutics15041052