Efficient Delivery of Antimicrobial Peptides in an Innovative, Slow-Release Pharmacological Formulation
Abstract
:1. Introduction
2. Materials and Methods
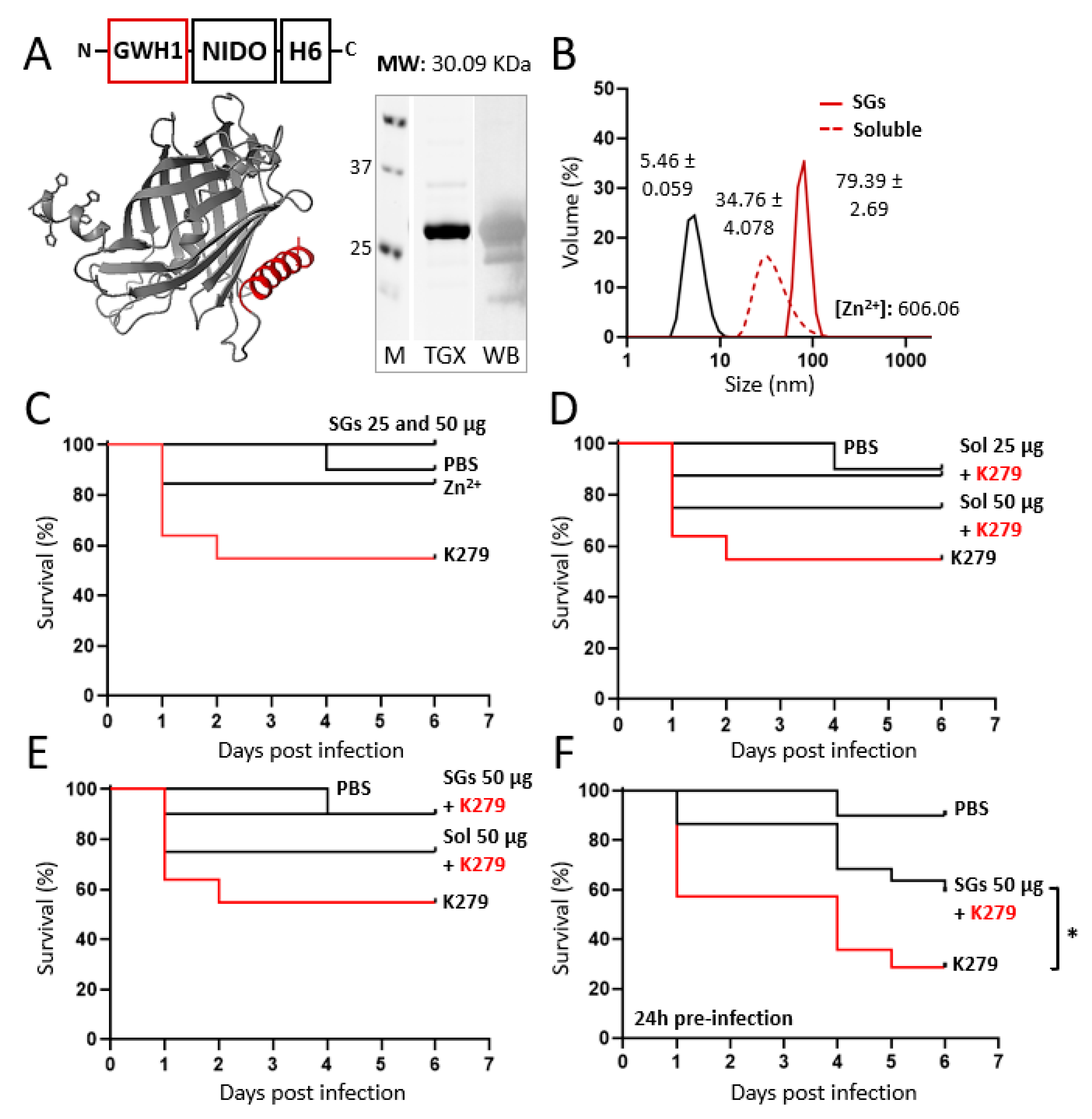
2.1. Genetic Design, Production, and Purification
2.2. Purity, Concentration, and Integrity
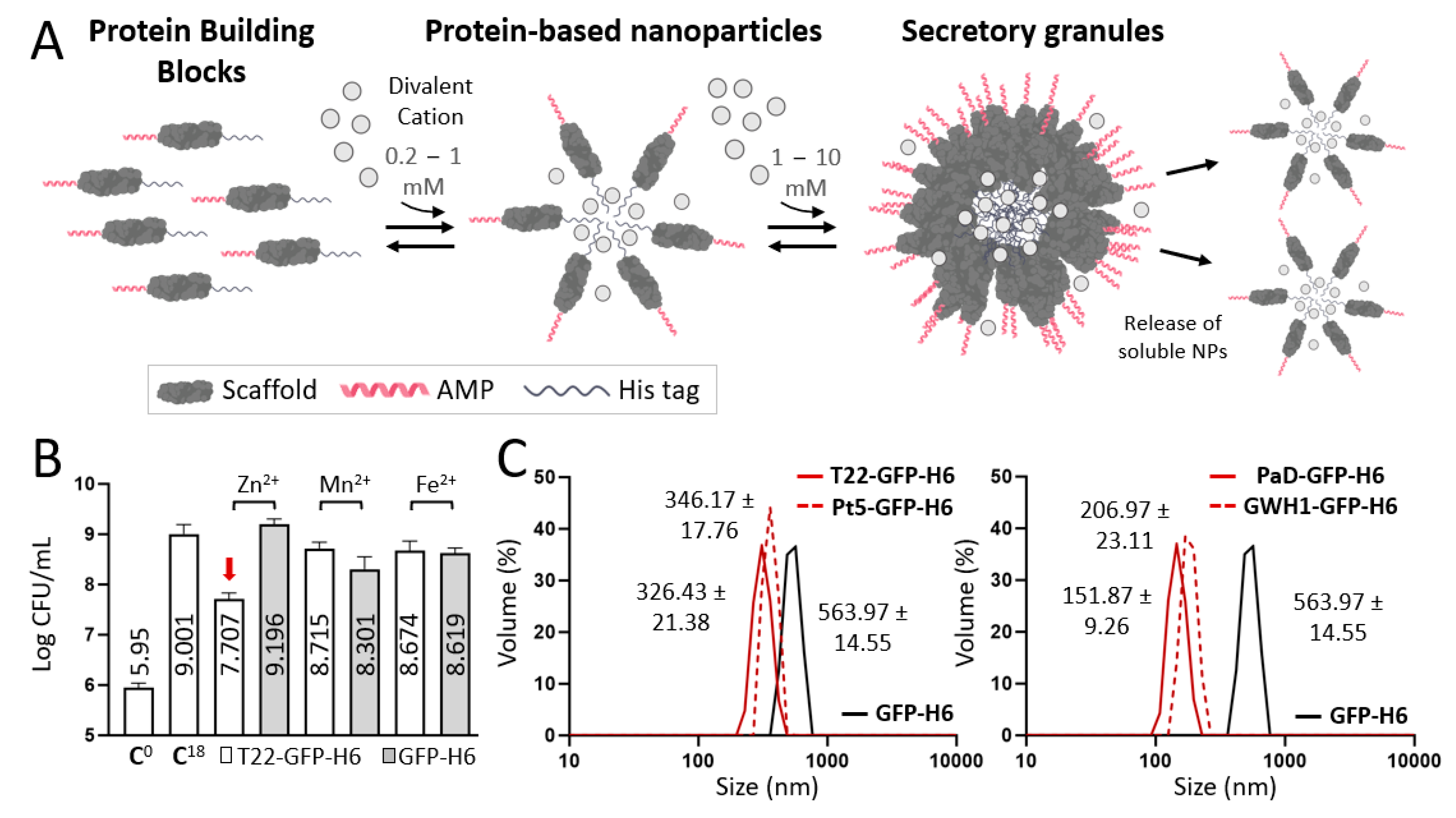
2.3. Size, Ultrastructural Morphometry and Surface Charge Characterization
2.4. Synthesis of Secretory Granules
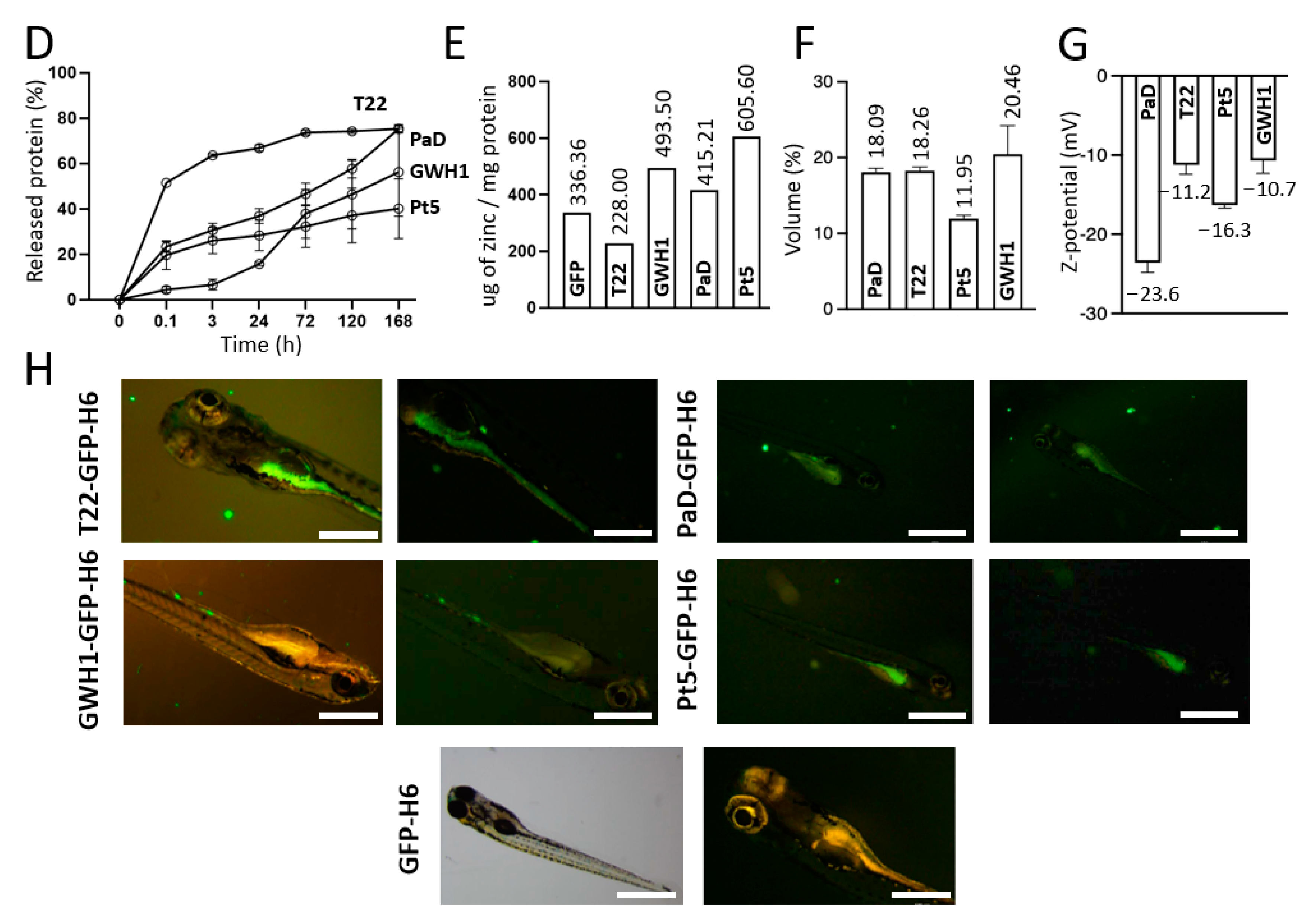
2.5. Chemical Detection of Zinc within Secretory Granules
2.6. Protein Release from Secretory Granules
2.7. In Vitro Bactericidal Assay in Staphylococcus aureus
2.8. Zebrafish Husbandry and Breeding
2.9. In Vivo Biodistribution in Zebrafish Larvae
2.10. In Vivo Model of Infection: Stenotrophomonas maltophilia (K279 Strain) in Adult Zebrafish
2.11. 3D Modelling
2.12. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef]
- Klemm, E.J.; Wong, V.K.; Dougan, G. Emergence of dominant multidrug-resistant bacterial clades: Lessons from history and whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2018, 115, 12872–12877. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Pieper, D.H. Tackling threats and future problems of multidrug-resistant bacteria. Curr. Top Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Laport, M.S.; Santos, O.C.S.; Muricy, G. Marine Sponges: Potential Sources of New Antimicrobial Drugs. Curr. Pharm. Biotechnol. 2009, 10, 86–105. [Google Scholar] [CrossRef]
- Rafał, I.G.; Króliczewski, B.J.; Górniak, I.; Bartoszewski, R.; Króliczewski, Á.J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2018, 18, 241–272. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Pedron, C.N.; Higashikuni, Y.; Kramer, R.M.; Cardoso, M.H.; Oshiro, K.G.N.; Franco, O.L.; Junior, P.I.S.; Silva, F.D.; Junior, V.X.O.; et al. Structure-function-guided exploration of the antimicrobial peptide polybia-CP identifies activity determinants and generates synthetic therapeutic candidates. Commun. Biol. 2018, 1, 221. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Straus, S.K. Design, Engineering and Discovery of Novel α-Helical and β-Boomerang Antimicrobial Peptides against Drug Resistant Bacteria. Int. J. Mol. Sci. 2020, 21, 5773. [Google Scholar] [CrossRef] [PubMed]
- Smerkova, K.; Dolezelikova, K.; Bozdechova, L.; Heger, Z.; Zurek, L.; Adam, V. Nanomaterials with active targeting as advanced antimicrobials. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1636. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef]
- Casciaro, B.; Cappiello, F.; Verrusio, W.; Cacciafesta, M.; Mangoni, M.L. Antimicrobial Peptides and their Multiple Effects at Sub-Inhibitory Concentrations. Curr. Top. Med. Chem. 2020, 20, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Bhopale, G.M. Antimicrobial Peptides: A Promising Avenue for Human Healthcare. Curr. Pharm. Biotechnol. 2020, 21, 90–96. [Google Scholar] [CrossRef]
- Ashcheulova, D.O.; Efimova, L.V.; Lushchyk, A.Y.; Yantsevich, A.V.; Baikov, A.N.; Pershina, A.G. Production of the recombinant antimicrobial peptide UBI 18-35 in Escherichia coli. Protein Expr. Purif. 2018, 143, 38–44. [Google Scholar] [CrossRef]
- Cao, J.; de la Fuente-Nunez, C.; Ou, W.R.; Torres, T.M.D.; Pande, G.S.; Sinskey, J.A.; Lu, T.K. Yeast-Based Synthetic Biology Platform for Antimicrobial Peptide Production. ACS Synth. Biol. 2018, 7, 896–902. [Google Scholar] [CrossRef]
- Rezaei, S.; Hadadian, S.; Khavari-Nejad, R.A.; Norouzian, D. Recombinant Tandem Repeated Expression of S3 and S∆3 Antimicrobial Peptides. Rep. Biochem. Mol. Biol. 2020, 9, 348–356. [Google Scholar] [CrossRef]
- Dong, B.; Cheng, R.Q.; Liu, Q.Y.; Wang, J.; Fan, Z.C. Multimer of the antimicrobial peptide Mytichitin-A expressed in Chlamydomonas reinhardtii exerts a broader antibacterial spectrum and increased potency. J. Biosci. Bioeng. 2018, 125, 175–179. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, Z.; Zhu, B.; Zheng, R.; Cheng, H.; Nie, Z. Tandem expression and activity determination of antibacterial peptide Spinosan-C from Paa spinosa. Chin. J. Biotechnol. 2018, 34, 132–139. [Google Scholar] [CrossRef]
- Gupta, V.; Sengupta, M.; Prakash, J.; Tripathy, B.C. Production of Recombinant Pharmaceutical Proteins. In Basic and Applied Aspects of Biotechnology; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Pollet, J.; Chen, W.-H.; Strych, U. Recombinant protein vaccines, a proven approach against corona-virus pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.C.; Farinas, C.S.; Miranda, E.A. Liquefied wheat bran as carbon source and inducer in high-solids submerged cultivation of Aspergillus niger for xylanase production. Biocatal. Agric. Biotechnol. 2019, 21, 101346. [Google Scholar] [CrossRef]
- Said, S.D.; Zaki, M.; Asnawi, T.M.; Novita, E. Single cell protein production by a local Aspergillus niger in solid state fermentation using rice straw pulp as carbon source: Effects of fermentation variables. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 012002. [Google Scholar] [CrossRef]
- Yatmaz, E.; Turhan, I. Carob as a carbon source for fermentation technology. Biocatal. Agric. Biotechnol. 2018, 16, 200–208. [Google Scholar] [CrossRef]
- Wollensack, L.; Budzinski, K.; Backmann, J. Defossilization of pharmaceutical manufacturing. Curr. Opin. Green Sustain. Chem. 2022, 33, 100586. [Google Scholar] [CrossRef]
- Lagassé, H.A.D.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Sauna, Z.E.; Kimchi-Sarfaty, C. Recent advances in (therapeutic protein) drug development. F1000Research 2017, 6, 113. [Google Scholar] [CrossRef]
- Agyei, D.; Ahmed, I.; Akram, Z.; Iqbal, H.M.N.; Danquah, M.K. Protein and Peptide Biopharmaceuticals: An Overview. Protein Pept. Lett. 2017, 24, 94–101. [Google Scholar] [CrossRef]
- Thapa, R.K.; Diep, D.B.; Tønnesen, H.H. Nanomedicine-based antimicrobial peptide delivery for bacterial infections: Recent advances and future prospects. J. Pharm. Investig. 2021, 51, 377–398. [Google Scholar] [CrossRef]
- Tran, T.T.D.; Tran, P.H.L. Nanoconjugation and Encapsulation Strategies for Improving Drug Delivery and Therapeutic Efficacy of Poorly Water-Soluble Drugs. Pharmaceutics 2019, 11, 325. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carbone, C.; Sousa, M.C.; Espina, M.; Garcia, M.L.; Sanchez-Lopez, E.; Souto, E.B. Nanomedicines for the Delivery of Antimicrobial Peptides (AMPs). Nanomaterials 2020, 10, 560. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Huang, G.; Gao, J. Cooperativity Principles in Self-Assembled Nanomedicine. Chem. Rev. 2018, 118, 5359–5391. [Google Scholar] [CrossRef]
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int. J. Biol. Macromol. 2020, 143, 297–304. [Google Scholar] [CrossRef]
- Parilti, R.; Caprasse, J.; Riva, R.; Alexandre, M.; Vandegaart, H.; Bebrone, C.; Dupont-Gillain, C.; Howdle, S.M.; Jérôme, C. Antimicrobial peptide encapsulation and sustained release from polymer network particles prepared in supercritical carbon dioxide. J. Colloid Interface Sci. 2018, 532, 112–117. [Google Scholar] [CrossRef]
- Yang, G.; Huang, T.; Wang, Y.; Wang, H.; Li, Y.; Yu, K.; Dong, L. Sustained Release of Antimicrobial Peptide from Self-Assembling Hydrogel Enhanced Osteogenesis. J. Biomater. Sci. Polym. Ed. 2018, 29, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
- Serna, N.; Carratalá, J.V.; Conchillo-Solé, O.; Martínez-Torró, C.; Unzueta, U.; Mangues, R.; Ferrer-Miralles, N.; Daura, X.; Vázquez, E.; Villaverde, A. Antibacterial Activity of T22, a Specific Peptidic Ligand of the Tumoral Marker CXCR4. Pharmaceutics 2021, 13, 1922. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.M.; López-Laguna, H.; Álamo, P.; Serna, N.; Sánchez-Chardi, A.; Nolan, V.; Cano-Garrido, O.; Casanova, I.; Unzueta, U.; Vazquez, E.; et al. Artificial Inclusion Bodies for Clinical Development. Adv. Sci. 2020, 7, 1902420. [Google Scholar] [CrossRef] [PubMed]
- Álamo, P.; Parladé, E.; López-Laguna, H.; Voltà-Durán, E.; Unzueta, U.; Vazquez, E.; Mangues, R.; Villaverde, A. Ion-dependent slow protein release from in vivo disintegrating micro-granules. Drug Deliv. 2021, 28, 2383–2391. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Carratalá, J.V.; Serna, N.; Unzueta, U.; Nolan, V.; Sánchez-Chardi, A.; Voltà-Durán, E.; López-Laguna, H.; Ferrer-Miralles, N.; Villaverde, A.; et al. The Poly-Histidine Tag H6 Mediates Structural and Functional Properties of Disintegrating, Protein-Releasing Inclusion Bodies. Pharmaceutics 2022, 14, 602. [Google Scholar] [CrossRef] [PubMed]
- López-Laguna, H.; Parladé, E.; Álamo, P.; Sánchez, J.M.; Voltà-Durán, E.; Serna, N.; Sánchez-García, L.; Cano-Garrido, O.; Sánchez-Chardi, A.; Villaverde, A.; et al. In Vitro Fabrication of Microscale Secretory Granules. Adv. Funct. Mater. 2021, 31, 2100914. [Google Scholar] [CrossRef]
- López-Laguna, H.; Sánchez, J.; Unzueta, U.; Mangues, R.; Vázquez, E.; Villaverde, A. Divalent Cations: A Molecular Glue for Protein Materials. Trends Biochem. Sci. 2020, 45, 992–1003. [Google Scholar] [CrossRef]
- López-Laguna, H.; Voltà-Durán, E.; Parladé, E.; Villaverde, A.; Vázquez, E.; Unzueta, U. Insights on the emerging biotechnology of histidine-rich peptides. Biotechnol. Adv. 2022, 54, 107817. [Google Scholar] [CrossRef]
- Seuring, C.; Verasdonck, J.; Gath, J.; Ghosh, D.; Nespovitaya, N.; Wälti, M.A.; Maji, S.K.; Cadalbert, R.; Güntert, P.; Meier, B.H.; et al. The three-dimensional structure of human β-endorphin amyloid fibrils. Nat. Struct. Mol. Biol. 2020, 27, 1178–1184. [Google Scholar] [CrossRef]
- Jacob, R.S.; Anoop, A.; Maji, S.K. Protein Nanofibrils as Storage Forms of Peptide Drugs and Hormones. Adv. Exp. Med. Biol. 2019, 1174, 265–290. [Google Scholar] [CrossRef]
- Maji, S.K.; Perrin, M.H.; Sawaya, M.R.; Jessberger, S.; Vadodaria, K.; Rissman, R.A.; Singru, P.S.; Nilsson, K.P.R.; Simon, R.; Schubert, D.; et al. Functional Amyloids As Natural Storage of Peptide Hormones in Pituitary Secretory Granules. Science 2009, 325, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Jacob, R.S.; Das, S.; Ghosh, S.; Anoop, A.; Jha, N.N.; Khan, T.; Singru, P.; Kumar, A.; Maji, S.K. Amyloid formation of growth hormone in presence of zinc: Relevance to its storage in secretory granules. Sci. Rep. 2016, 6, 23370. [Google Scholar] [CrossRef] [PubMed]
- Serna, N.; Cano-Garrido, O.; Sánchez, J.M.; Sánchez-Chardi, A.; Sánchez-García, L.; López-Laguna, H.; Fernández, E.; Vázquez, E.; Villaverde, A. Release of functional fibroblast growth factor-2 from artificial inclusion bodies. J. Control. Release 2020, 327, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.-l.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- López-Laguna, H.; Sánchez-García, L.; Serna, N.; Voltà-Durán, E.; Sánchez, J.M.; Sánchez-Chardi, A.; Unzueta, U.; Łoś, M.; Villaverde, A.; Vázquez, E. Engineering Protein Nanoparticles Out from Components of the Human Microbiome. Small 2020, 16, e2001885. [Google Scholar] [CrossRef]
- López-Laguna, H.; Unzueta, U.; Conchillo-Solé, O.; Sánchez-Chardi, A.; Pesarrodona, M.; Cano-Garrido, O.; Voltà, E.; Sánchez-García, L.; Serna, N.; Saccardo, P.; et al. Assembly of histidine-rich protein materials controlled through divalent cations. Acta Biomater. 2019, 83, 257–264. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Chen, Y.L.S.; Li, J.H.; Yu, C.Y.; Lin, C.J.; Chiu, P.H.; Chen, P.W.; Lin, C.C.; Chen, W.J. Novel cationic antimicrobial peptide GW-H1 induced caspase-dependent apoptosis of hepatocellular carcinoma cell lines. Peptides 2012, 36, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Irazazabal, L.N.; Porto, W.F.; Fensterseifer, I.C.M.; Alves, E.S.F.; Matos, C.O.; Menezes, A.C.S.; Felício, M.R.; Gonçalves, S.; Santos, N.C.; Ribeiro, S.M.; et al. Fast and potent bactericidal membrane lytic activity of PaDBS1R1, a novel cationic antimicrobial peptide. Biochim. Biophys. Acta Biomembr. 2019, 1861, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Liu, X.; Bu, L.; Li, H.; Zhang, S. Antimicrobial–immunomodulatory activities of zebrafish phosvitin-derived peptide Pt5. Peptides 2012, 37, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Horzmann, K.A.; Freeman, J.L. Making Waves: New Developments in Toxicology with the Zebrafish. Toxicol. Sci. 2018, 163, 5–12. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Pei, Y.A.; Pei, M. Nidogen: A matrix protein with potential roles in musculoskeletal tissue regeneration. Genes Dis. 2022, 9, 598–609. [Google Scholar] [CrossRef]
- Quast, I.; Tarlinton, D. Time is of the essence for vaccine success. Nat. Immunol. 2022, 23, 1517–1519. [Google Scholar] [CrossRef]
- Pons-Faudoa, F.P.; Ballerini, A.; Sakamoto, J.; Grattoni, A. Advanced implantable drug delivery technologies: Transforming the clinical landscape of therapeutics for chronic diseases. Biomed. Microdevices 2019, 21, 47. [Google Scholar] [CrossRef]
- Abdelkader, H.; Fathalla, Z.; Seyfoddin, A.; Farahani, M.; Thrimawithana, T.; Allahham, A.; Alani, A.W.; Al-Kinani, A.A.; Alany, R.G. Polymeric long-acting drug delivery systems (LADDS) for treatment of chronic diseases: Inserts, patches, wafers, and implants. Adv. Drug Deliv. Rev. 2021, 177, 113957. [Google Scholar] [CrossRef] [PubMed]
- Hakim, L.K.; Yazdanian, M.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Khayatan, D.; Seifalian, A.; Ranjbar, R.; Yazdanian, A. Biocompatible and Biomaterials Application in Drug Delivery System in Oral Cavity. Evidence-Based Complement. Altern. Med. 2021, 2021, 9011226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dou, X.; Zhang, L.; Wang, H.; Zhang, T.; Bai, R.; Sun, Q.; Wang, X.; Yu, T.; Wu, D.; et al. Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact. Mater. 2022, 11, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Soragni, A.; Maji, S.K.; Riek, R. Toward a comprehension of functional aggregation into amyloids in pituitary secretory granules. Amyloid 2010, 17, 41. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna, N.; López-Laguna, H.; Aceituno, P.; Rojas-Peña, M.; Parladé, E.; Voltà-Durán, E.; Martínez-Torró, C.; Sánchez, J.M.; Di Somma, A.; Carratalá, J.V.; et al. Efficient Delivery of Antimicrobial Peptides in an Innovative, Slow-Release Pharmacological Formulation. Pharmaceutics 2023, 15, 2632. https://doi.org/10.3390/pharmaceutics15112632
Serna N, López-Laguna H, Aceituno P, Rojas-Peña M, Parladé E, Voltà-Durán E, Martínez-Torró C, Sánchez JM, Di Somma A, Carratalá JV, et al. Efficient Delivery of Antimicrobial Peptides in an Innovative, Slow-Release Pharmacological Formulation. Pharmaceutics. 2023; 15(11):2632. https://doi.org/10.3390/pharmaceutics15112632
Chicago/Turabian StyleSerna, Naroa, Hèctor López-Laguna, Patricia Aceituno, Mauricio Rojas-Peña, Eloi Parladé, Eric Voltà-Durán, Carlos Martínez-Torró, Julieta M. Sánchez, Angela Di Somma, Jose Vicente Carratalá, and et al. 2023. "Efficient Delivery of Antimicrobial Peptides in an Innovative, Slow-Release Pharmacological Formulation" Pharmaceutics 15, no. 11: 2632. https://doi.org/10.3390/pharmaceutics15112632
APA StyleSerna, N., López-Laguna, H., Aceituno, P., Rojas-Peña, M., Parladé, E., Voltà-Durán, E., Martínez-Torró, C., Sánchez, J. M., Di Somma, A., Carratalá, J. V., Livieri, A. L., Ferrer-Miralles, N., Vázquez, E., Unzueta, U., Roher, N., & Villaverde, A. (2023). Efficient Delivery of Antimicrobial Peptides in an Innovative, Slow-Release Pharmacological Formulation. Pharmaceutics, 15(11), 2632. https://doi.org/10.3390/pharmaceutics15112632








